Abstract
Observations show that humans and animals respond immunologically to most cancers. Why does the immune system then fail to control cancer? We argue from the literature that there is a commonality in the regulation of responses against most murine tumors, and that a major mechanism of escape may be deviation of an effective Th1, cytotoxic T lymphocyte response to a less effective response with a Th2 component. We examined this hypothesis with two well-studied murine tumors. We found, following primary tumor implantation, that resistance correlates with Th1 responses and IgG2a antibody production and progression with mixed Th1/Th2 responses and production of IgG1 and IgG2a antibodies. Resistance is associated with a modulation of the anti-tumor response towards the Th1 pole in both systems. We conclude that the immune responses against these two tumors are in accord with our hypothesis, and argue that this is likely to be true of many human and murine tumors. The correlation of IgG isotype of anti-tumor antibody with the Th1/Th2 nature of the anti-tumor response readily allows one to longitudinally monitor the changing nature of the anti-tumor response. We suggest that such monitoring can guide immunotherapy to maximize the effectiveness of the host’s immune response against cancer.
Keywords: Immune deviation, Antibody IgG isotypes, Th1/Th2, Cancer immunology and correlates of tumor progression/regression
Introduction
Tumor immunology has been transformed in recent decades by the compelling demonstration that the human immune system responds to spontaneous cancers [6]. The development of powerful means to define the antigens recognized by cytotoxic T cells [11], the most potent means of attacking the majority of tumors bearing class I but not class II MHC antigens, and of antibodies recognizing tumor-associated antigens [40], has had a profound impact at two levels. Firstly, these observations leave no doubt that the immune system responds to naturally arising tumors and, in this sense, cancers are subject to immune surveillance [9, 13]. Secondly, the characterization of the antigens recognized by the immune system has implications both for the nature of the oncogenic process and for the possibility of realizing immunological intervention in the form of vaccination and immunotherapy.
Does the existence of diverse cancers reflect a diversity of tumor-escape mechanisms [4–6, 12, 18, 24, 36, 40, 41, 46–48], or are there only a few primary escape mechanisms that account for how most tumors avoid immune attack? This is a critical question. Only if the latter holds will general strategies of vaccination against tumors and of immunotherapy be readily realizable. We believe that studies in animal systems provide grounds for optimism. Thus, the phenomenon of concomitant immunity, evident in many tumor systems, suggests that most tumors are both immunogenic and susceptible to immune attack. Animals bearing a lethal challenge can, shortly after implantation, reject a second and similar tumor challenge at a distinct site [14]. In addition, a number of standard manoeuvers are found to be effective in causing regression of a considerable fraction of the tumors studied. North [30] found that a standard dose of whole body radiation at 6 days after tumor implantation caused regression of three of five tumors examined. A more recent study showed that the administration of the anti-CD25 monoclonal antibody, PC61, that partially depletes CD25+ cells, 4 days before a normally lethal challenge, results in tumor regression in six of eight tumor systems studied [32]. Moreover, one of the tumors, the meth A fibrosarcoma, was included in both this and North’s study and found to be susceptible to both whole body radiation of the host and to pre-treatment of the host with the anti-CD25 antibody. These observations suggest a commonality in the regulation of the response to a considerable proportion of animal tumors. A better understanding of the basis of this commonality in mice should allow one to assess whether a similar situation holds in the context of human cancers.
It has long been recognized that the immune response to diverse antigens, including simple proteins, complex but non-replicating antigens such as red blood cells, and pathogenic and non-pathogenic microbes or parasites, often goes through an exclusive cell-mediated Th1 phase before a Th2 component of the response appears, with the associated production of substantial antibody and a decline in the cell-mediated response in the form, for example, of delayed-type hypersensitivity [7, 8, 23, 33, 42]. This general pattern is reminiscent of North’s findings on the regulation of tumor-specific concomitant immunity. He showed that a challenge with a lethal dose of tumor cells first gave rise to a cell-mediated CTL response, which could be effective against a second lethal challenge of the tumor implanted at a distinct site, but that this concomitant immunity declined as “CD4 T suppressor” cells are generated, which act to down-regulate the anti-tumor cell-mediated response. North’s [29] take-home message from his very extensive studies on the paradox of the expression of concomitant immunity, in animals given a lethal tumor challenge, was “too little (concomitant immunity) too late”.
We were struck by the parallel between North’s findings on the kinetics of expression of concomitant immunity to tumors and on the kinetics of the cell-mediated phase of the immune response to non-tumor antigens. An exclusive cell-mediated phase is often followed by the production of antibody, and the ensuing decay of cell-mediated immunity is associated with the appearance of CD4 T cells that inhibit the cell-mediated response [27, 39]. Although this hypothesis is decades old [19], we believe it is not now being sufficiently considered. It is only mentioned in passing in one [1] of four recent reviews [1, 3, 52, 53], and the possibilities of its pertinence to understanding how the anti-tumor immune response evolves has not been critically evaluated. We decided to examine in two tumor systems studied by North whether there was any substance to this hypothesis. We therefore examined whether tumor resistance/progression correlates with predominant Th1 and mixed Th1/Th2 anti-tumor responses with the P815 mastocytoma and the L5178Y lymphoma, two tumors studied by North.
Materials and methods
Mice
DBA/2J mice were either obtained from Jackson Laboratories (Bar Harbor, ME, USA) or from the University of Saskatchewan animal colony. Mice were housed under specific, pathogen-free conditions and were routinely screened to ensure that they were free of subclinical viral and bacterial infections. The mice employed within each experiment were of the same sex and typically between the ages of 6–12 weeks. All experiments were performed under the guidelines of the Canadian Council on Animal Care.
Tumor cell lines
P815 was obtained as TIB-64, Lot#2310374, from the American Type Culture Collection, Rockville, MD, USA. L5178Y is a thymoma induced by methylcholanthrene in ether, kindly provided by Dr. R. J. North, Trudeau Institute, Saranac Lake, NY, USA. Both tumors were implanted intradermally or subcutaneously in a volume of 0.02 ml.
Assessment of the Th1/Th2 nature of the anti-tumor immune response
P815 or L5178Y-dependent IFN-γ and IL-4 production by splenocytes was assessed utilizing an enzyme-linked immunospot (ELISPOT) assay as previously described [35]. Spleen cells harvested from mice exposed to the P815 tumor were prepared and purified on nylon wool columns as per the manufacturer’s instructions (Polysciences Inc. Warrington, PA, USA). This purification enriches the T cells and eliminates the majority of contaminating P815 tumor cells, which had metastasized to the spleen. These primed spleen cells are plated at various dilutions with the addition of spleen cells from normal mice to obtain a total equivalent of 2×106 spleen cells/well. 5×105 γ -irradiated (20,000 Rad) P815 cells (to inhibit the endogenous production of IL-4 by P815 cells) were used as antigen. Plates were allowed to incubate at 37°C in a 5% CO2 atmosphere for 8 hours. Spleen cells harvested from mice exposed to the L5178Y tumor were prepared, and plated at various dilutions with the addition of spleen cells from normal, non-immunized mice to obtain a total of 1×106 spleen cells per well. 1×105 gamma-irradiated L5178Y cells (∼5,000 rad) were used as antigen. Plates were allowed to incubate at 37°C in a 5% CO2 atmosphere for 8 h. Spots were developed as described [35].
Enzyme immunoassay
Mice were tail bled over the course of tumor growth, plasma was collected and kept at −20°C until use. The relative abundance of tumor-specific IgG1 and IgG2a antibodies were assessed by either ELA or Western blotting protocols. High protein-binding polystyrene Immuno-Maxisorp plates (NUNC, Denmark) were coated overnight at 4°C with an equivalent of 3.33×106 freeze-thawed L5178Y cells per well in PBS. The plates were washed, then blocked with 10% heat inactivated calf serum (Gibco Laboratories, Grand Island, NY, USA). Serum samples were diluted, and added to appropriate wells. After waiting overnight, horseradish peroxidase-conjugated goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates Inc., AL, USA) was added in each well of the test plate at a dilution of 1:5,000 in PBS supplemented with 10% calf sera. The plate was incubated at 37°C for 1 h, then washed prior to adding 100 μL ATBS (2,2′-azino-di (3-ethyl-benzthiazoline-6-sulfonate)) 1-component microwell peroxidase substrate (Kirkegaard & Perry Laboratories, Inc, Gaithersburg, MD, USA) to each well. The test plate was incubated for 20 min at 37°C in the dark. The test plate was read using an E-max microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA) at a wavelength of 405 nm, and resulting data was collected and analyzed using the Softmax Professional version 2.2.2 (Molecular Devices Corporation) computer program. The signal from the serum of naïve mice is never above the values obtained from our conjugate blank control wells (an adsorbance of 0.065). The serum titer was determined using a cutoff value of this assay as being an adsorbance two times above the value obtained when using sera from non-immunized animals.
Western blotting
Tumor cells grown in-vitro were re-suspended at 108 cells/ml (for P815) or 3×107 cells/ml (for L5178Y) in PBS. Samples were freeze-thawed, lightly sonicated and diluted 1:1 with Laemmli sample buffer (Bio-Rad Laboratories, CA, USA) and boiled for 4 min. A volume of 200 μL of sample was run on a 4% stacking, and 10% separating sodium dodecyl sulphate polyacrylamide gel. The separated proteins were blotted onto a 0.45 μm nitrocellulose membrane (Bio-Rad Laboratories) using a trans-blot semi-dry transfer cell (Bio-Rad). The membrane was blocked for 1–2 h at room temperature on a rocker with a 5% blotting grade non-fat dry milk blocker in PBST (or PBS when using fluorescent secondary antibodies). After blocking, the membrane was assembled into a mini protean II multi-screen apparatus (Bio-Rad). Mouse serum was diluted as indicated, in our 5% milk in PBST solution, and 400 μL of the sample dilution was run in each lane. The membrane was incubated overnight at 4°C on a rocker. The membrane was then washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG1 or IgG2a (Southern Biotechnology Associates Inc., AL, USA) or IRDye 800TM conjugated goat anti-mouse IgG1 (Rockland Immunochemicals, Gilbertsville, PA, USA) and Alexa-Fluor® 680 conjucated goat anti-mouse IgG2a (Invitrogen) antibodies at a dilution of 1:5,000 in our 5% milk in PBST blocking solution for 1 h at room temperature. The membrane was developed using Immun-Star™ HRP (horseradish peroxidase; Bio-Rad Laboratories) chemiluminescence kit as per the manufacturer’s instructions. When utilizing fluorescent secondary antibodies, bands were visualized using the Licor Odyssey Imaging System (Licor, Lincoin, NE, USA).
Statistical analysis
Error bars represent the standard error observed between triplicate wells. P values were calculated using the two-tailed student’s t test.
Results
Developing valid assays for assessing the Th1/Th2 nature of the anti-tumor immune response
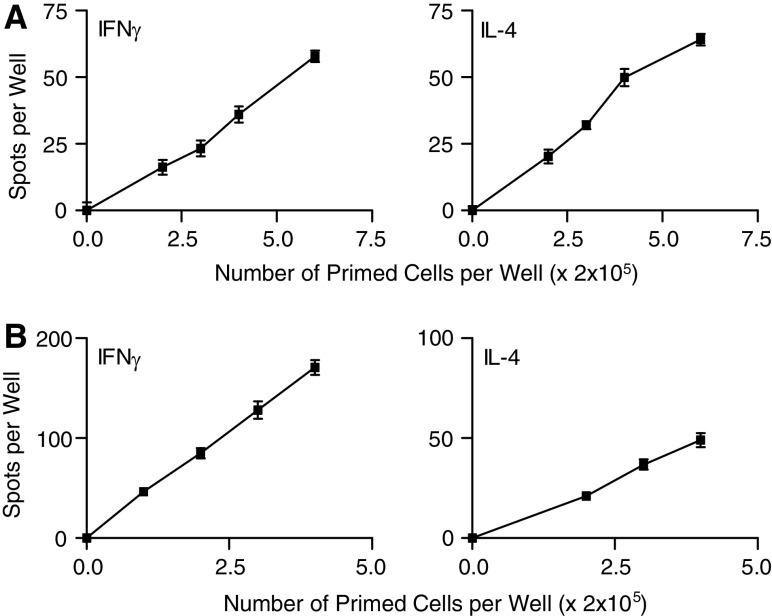
The ELISPOT assay for enumerating single antigen-specific cytokine producing cells is an assay of choice for enumerating cytokine-producing cells, due to its sensitivity and the demonstrable antigen-specificity of the cells it detects. The observations of Fig. 1 show that the number of antigen-dependent spots detected, reflecting both IFN-γ- or IL-4-producing cells, depends linearly, in both the P815 and the L5178Y systems, on the number of sensitized spleen cells plated (Fig. 1). This shows that such spots are a valid way of enumerating antigen-specific cytokine-producing cells.
Fig. 1.
a Linearity of the P815-dependent generation of IFN-γ and IL-4-producing and spot-forming cells as detected by our ELISPOT assay. Spleen cells from P815-bearing mice were plated at various dilutions, together with spleen cells from normal mice, to keep the total number of spleen cells constant at 2×106 cells per well with or without 5×105 γ-irradiated (20,000 rads) P815 cells as antigen. Antigen-dependent IFN-γ and IL-4-producing cells were enumerated. b Linearity of the L5178Y-dependent generation of IFN-γ and IL-4 producing, spot-forming cells as detected by our ELISPOT assay. Spleen cells from L5178Y-primed mice were plated at various dilutions, together with spleen cells from normal mice to keep the total number of spleen cells constant at 1×106 cells per well. As antigen, 105 γ-irradiated (5,000 rads) L5178Y cells were added to appropriate wells. Antigen-dependent IFN-γ and IL-4 producing cells were enumerated
Identifying immune correlates of progression and regression in the P815 tumor system
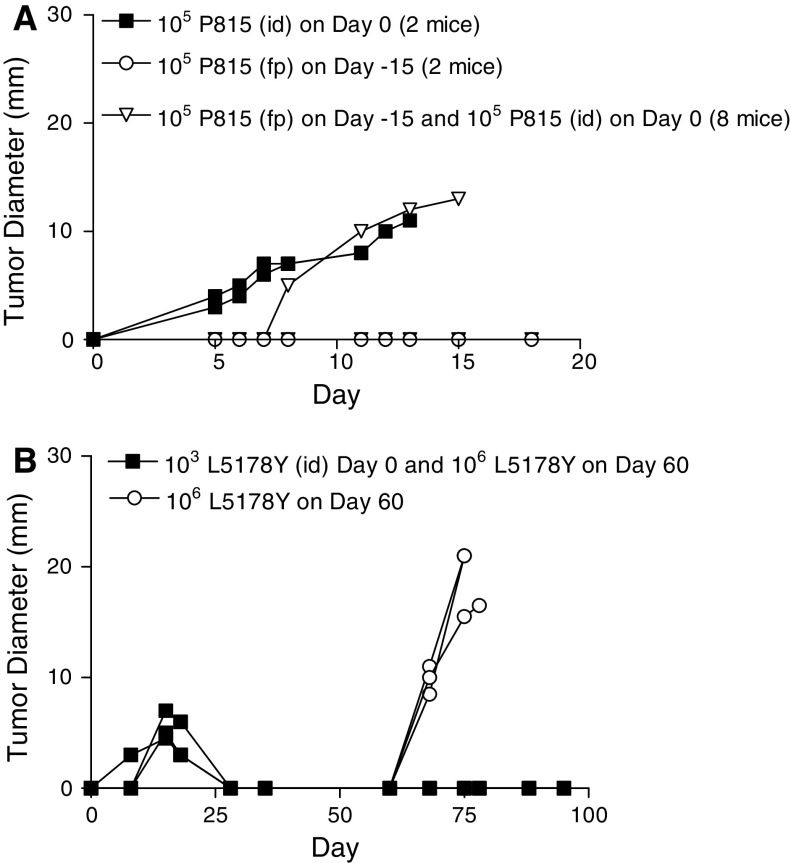
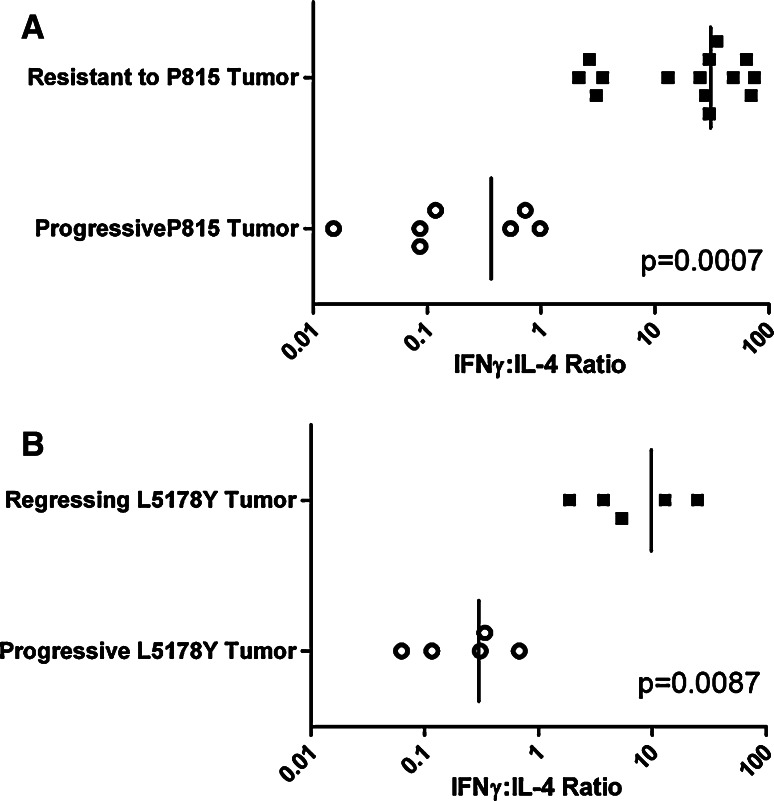
We set out to assess the Th1/Th2 nature of the anti-P815 immune response in mice either resisting a tumor challenge or suffering progressive tumor growth. We first attempted to find a challenge of the tumor that did not cause progressive tumor growth, in the hope that such a challenge would also make the mice resistant to a normally lethal challenge. We found that mice injected with 105 P815 cells subcutaneously into their right hind footpad rarely suffered progressive tumor growth. Moreover, most of these mice resist a subsequent, normally lethal challenge of 2 × 106 P815 cells implanted intradermally into their belly, when given a minimum of 2 weeks post priming. This priming strategy has been performed on more than 100 mice, and consistently renders over 80% of the mice resistant to a normally lethal challenge of the P815 tumor. An example of such resistance is shown in Fig. 2a. We therefore used this system to compare the immune responses of mice resisting or succumbing to a normally lethal challenge. Mice were primed in the footpad, and they and aged-matched control mice were given a normally progressive tumor challenge 1 month after priming. The mice were killed 10 days post challenge and spleen cells enumerated for P815-specific IFN-γ- and IL-4-producing cells. Progressive tumor growth is associated with a mixed IFN-γ/IL-4 response, while mice rendered resistant have a predominant IFN-γ response to P815 antigens. When one plots the ratio of the number of IFN-γ to IL-4 producing cells, we observe a very clear, non-overlapping difference in the type of immunity expressed in mice with regressing or progressing tumors. This striking finding will be extended to more times post-challenge once we have described how we longitudinally follow the nature of the anti-tumor response.
Fig. 2.
a Generation of resistance to P815 in DBA/2J mice. Eight DBA/2J mice were injected with 105 P815 cells subcutaneously into their right hind footpad (fp). 15 days later, these mice and two naïve mice were implanted with 105 P815 cells given id on their belly. Tumor progression of individual mice was assessed. 7/8 of the mice primed with the P815 resisted the challenge. b Generation of resistance to L5178Y in DBA/2J mice. Three mice were implanted with 103 L5178Y cells id on the belly on day 0. Sixty days after the initial implantation, these mice (closed squares), and three naïve age-matched control mice (open circles), were challenged with a normally lethal dose of 106 L5178Y tumor cells id on the belly
Identifying immune correlates of progression/regression in the L5178Y tumor system
We obtained the L5178Y lymphoma from North in order to test our working hypothesis with a second tumor employed in his studies. When we first received the L5178Y tumor, we had some difficulty in consistently obtaining progressive tumor growth. The majority of mice, given what was reported to be a normally lethal tumor challenge of 106 cells implanted id, spontaneously resolved their tumors. We decided to first compare the Th1/Th2 nature of the immunity being generated in mice challenged with the same tumor dose, but whose tumor took a different course. We gave all mice 106 L5178Y cells id on the belly and sacrificed them on day 30 pi, by which time it was apparent which of the mice were rejecting their tumor and which were suffering from progressive tumor growth. A significant predominance of tumor-specific IL-4-producing cells was evident in the spleen of mice suffering from progressive tumor growth, while IFN-γ-producing cells dominated the immune response in mice that had successfully contained the tumor (see Fig. 3b). We passaged the L5178Y tumor in vivo to increase its aggressiveness. After several passages, we found that a challenge of 106 L5178Y tumor cells given intradermally on the belly grew progressively in virtually 100% of animals. All challenges referred to in the text as “lethal challenges” were made with this more aggressive lymphoma line.
Fig. 3.
Lymphocytes from mice rendered resistant secrete IFN-γ, while mice suffering from progressive tumor growth predominately secrete IL-4, in response to tumor antigens. a Normal mice and mice made resistant to P815 by priming with 105 P815 cells sc into their hind footpad were challenged 1 month after the sc priming with 106 P815 cells id into their belly. The immune response was assessed 10 days post challenge. All normal mice had progressive tumor growth, and all primed mice appeared to be resistant at the time of killing. b Mice were implanted with 106 L5178Y cells id into their belly on day 0. On day 30 of tumor growth when it became apparent which animals were successfully containing the tumor or suffering from progressive L5178Y growth, the spleens of the animals were collected and the immune response generated against the L5178Y tumor was assessed employing the ELISPOT assay
Correlation of tumor-specific antibody subclass with tumor resistance or tumor progression
We often observed, in the P815 system, that the magnitude of the anti-tumor immune responses, as detected by ELISPOT at a particular time, varied among animals in the same experimental groups, and were even not readily measurable in some mice. We thought this variability might reflect kinetic differences in the development of the anti-tumor immune response in different animals, making it difficult to find a time when all animals had readily measurable responses. We considered that it might be possible to overcome this problem if we could quantitate the relative levels of IgG1 and IgG2a anti-tumor antibody to indirectly assess the Th1/Th2 nature of the anti-tumor immune response, as we had previously done in assessing the nature of immune responses to the intracellular parasite Leishmania major [8, 25, 26]. High amounts of pathogen-specific IgG1 antibodies correlate with a response to a large Th2 component, while predominance of pathogen-specific IgG2a antibodies are associated with a predominant Th1 response. We thought such an antibody-dependent assay, if it could be validated, would have two advantages. Firstly, the relative amounts of these two subclasses of antibody would not reflect the instantaneous immune response at the time of assay, but an integrated response over the last few days, and so responses might well be more consistently detected among mice belonging to the same group, as assessed by this assay, compared to responses detected by the ELISPOT assay. In addition, if such an assay could be validated, it would allow one to assess how the immune response changes in individual mice longitudinally with time. We felt this could be a very valuable asset in understanding the regulation of the immune response to tumors.
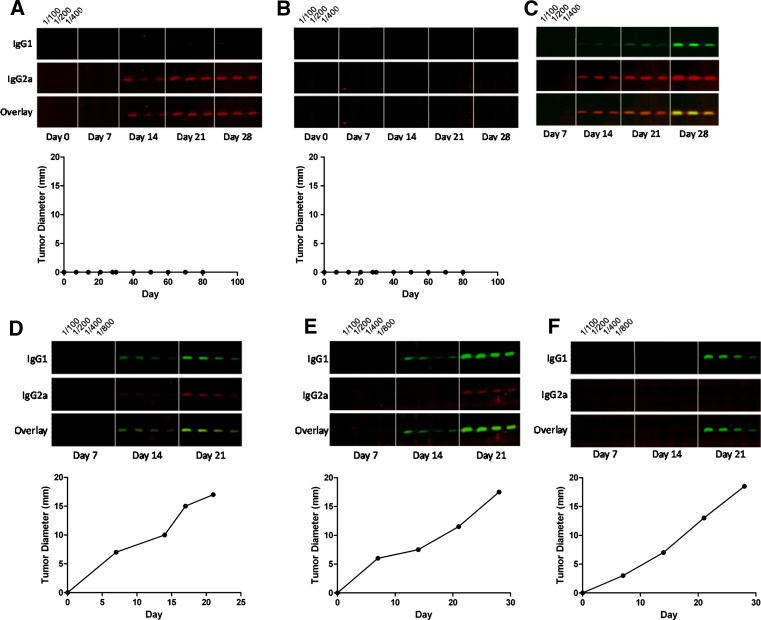
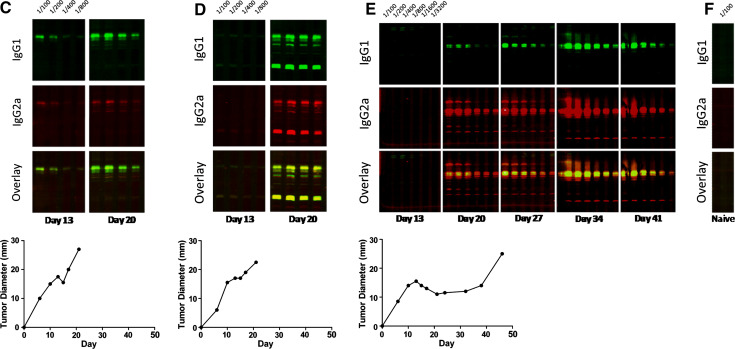
We utilized Western blots to assess the relative predominance of tumor-specific IgG1 and IgG2a antibodies present in the sera of tumor-challenged mice. This protocol very consistently detects anti-P815 antibodies in the serum of P815-bearing mice beyond those present in the sera of P815 non-exposed mice. We examined the relative abundance of P815-specific IgG1 and IgG2a antibodies in the sera of individual mice given a normally non-progressive challenge of the P815 tumor that renders most mice resistant to a subsequent normally lethal challenge. In each animal, we observed one of three scenarios, the two most common being represented in Fig. 4a and b. In the first of these situations, we observed a predominance of P815-specific IgG2a antibodies, and little or no detectable P815-specific IgG1 antibodies. In the second, we could not observe detectable P815-specific antibodies. We argue in the “Discussion” that this most probably reflects a very predominant Th1 response. On the other hand, mice given a normally non-progressive dose very occasionally suffer progressive growth. In this case, a predominance of tumor-specific IgG1 antibodies is found in the mouse’s serum (Fig. 4c). This Ig isotype pattern is very similar to that seen in mice given a standard lethal id challenge on the belly (Fig. 4d–f). These observations correlate with the data on the relative number of IFN-γ- and IL-4-producing cells as assessed by the ELISPOT assay. Using this Western blotting methodology, we were able to infer that the priming strategy, which renders DBA/2J mice resistant to a normally lethal tumor challenge, induces a stable Th1 response to tumor antigens (Fig. 4a, b).
Fig. 4.
Effective protection against P815 is associated with predominant IgG2a antibodies, while progressive tumor growth is associated with predominant IgG1 antibodies generated against P815 antigens a–c The mice were implanted with 105 P815 cells sc into their hind footpad on day 0. The mice were serially bled over a course of 28 days, and the relative abundance of P815-specific IgG1 and IgG2a antibodies were assessed by Western blot. (a, b) represent sera from mice, which subsequently resisted a normally lethal challenge of the P815 tumor 35 days post-vaccination. Note, day 0 on the graphs in (a) and (b) represent the day of vaccination and c represents the sera of a rare mouse suffering progressive tumor growth at the site of implantation in the footpad. d–f Antibodies in the sera of unprimed mice implanted with a lethal dose of 106 P815 cells id on their belly, as assessed by Western blot. The rate of tumor growth from each animal is shown below the Western blot
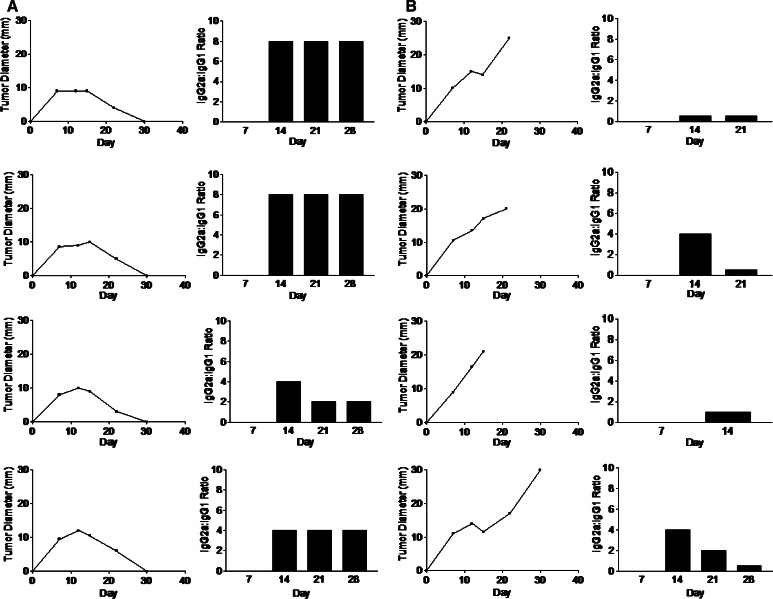
We developed an enzyme immunoassay to assess the relative predominance of L5178Y-specific IgG1 and IgG2a antibodies in L5178Y-primed mice. This assay allowed us to demonstrate a clear correlation between a predominance of IgG2a antibodies with tumor regression, and between a mixed IgG1/IgG2a or a predominant IgG1 antibody response to L5178Y antigens and tumor progression (Fig. 5). These results demonstrate the relatively dynamic nature of the anti-L5178Y immune response. The ratio of L5178Y-specific IgG2a to IgG1 antibodies can change over time. This ratio correlates well with the rate of tumor growth. Mice with spontaneously regressing tumors maintain a relatively high ratio of tumor-specific IgG2a:IgG1 antibodies, while in mice with progressively growing tumors this ratio can initially be high, but subsequently decreases as the tumor progressively grows (see “Discussion”). These results further illustrate the importance of being able to longitudinally assess the development of an anti-tumor immune response.
Fig. 5.
Relative abundance of L5178Y-specific IgG1 and IgG2a antibodies in mice either with progressive or spontaneously regressing L5178Y tumors. a, b Mice were implanted with 2×106 L5178Y cells id on day 0. Four of the 20 mice injected suffered from progressive tumor growth. Four mice resistant and four mice suffering progressive tumor growth were serially bled and the ratio of L5178Y-specific IgG2a to IgG1 antibodies were assessed by EIA. c–e Mice were implanted 106 L5178Y cells id on day 0. Differing rates of tumor growth were observed, and the relative abundance of L5178Y-specific IgG1 and IgG2a antibodies were assessed by Western blot over the course of tumor progression. f Western blot using sera from naïve mice have no detectable L5178Y-reactive antibodies in their sera
Variability in the rate of tumor growth is associated with differences in the phenotype of the anti-tumor immune response
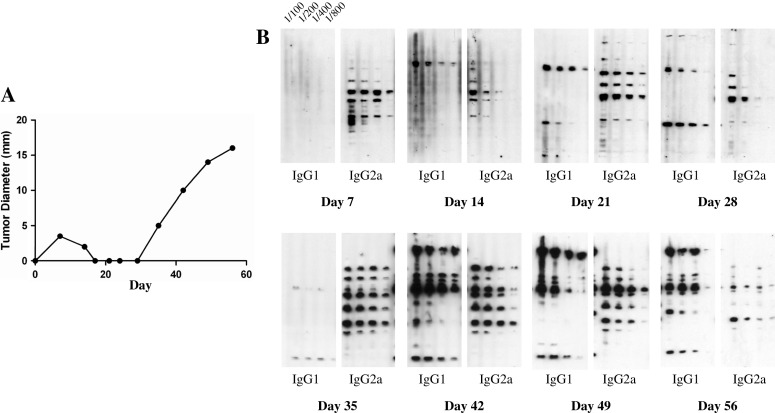
Our observations reflect many reports in the literature that mice, implanted with the same and often lethal dose of tumor cells, display variation in the rate of tumor growth. There could be several explanations for such variability. Our results suggest that mice suffering a more protracted course of tumor growth might be mounting a more effective anti-tumor immune response than mice suffering from rapid tumor growth. Our Western blotting and EIA protocols provide us with the opportunity to indirectly assess the Th1/Th2 nature of the immune response being generated over time and correlate it with the corresponding rates of tumor growth. One feature of this antibody assay is that antibodies have a half-life in the circulation measured in terms of several days, such that the Th1/Th2 nature of the anti-tumor immune response inferred from the nature of the antibodies collected at any particular time represents something like the average immunity expressed the previous week. We have examined longitudinally the antibody response in many mice. Firstly, in both tumor systems, mice given a challenge that grows progressively very often have a higher ratio of IgG2a to IgG1 antibody when the antibody first appears, and this decreases as the tumor progresses (see Fig. 6 for an example). This pattern is just what is expected on our interpretation of North’s picture of how concomitant immunity is established and then decays (see “Discussion”). Sometimes, however, there is insufficient antibody detectable at early time points and, when the antibody is first detected, the IgG1 isotype is already predominant (see Figs. 4, 5). A comparison of antibody responses in mice with rapidly growing tumors, and ones in which there is a phase of slower net or flat tumor growth, shows the expected differences in antibody responses (see Figs. 5, 6).
Fig. 6.
Differing rates of P815 growth is associated with differing relative abundance of tumor-specific IgG1 and IgG2a antibodies. DBA mice were implanted with 105 P815 id on their abdomen on day 0. The rate of tumor growth was assessed as was the relative abundance of tumor-specific IgG1 and IgG2a antibodies by Western blot. A 100-fold dilution of serum was employed in the Western blot
Tumor regression, and subsequent re-emergence, is associated with a complex evolution in the Th1/Th2 nature of the anti-tumor immune response as assessed by IgG isotype
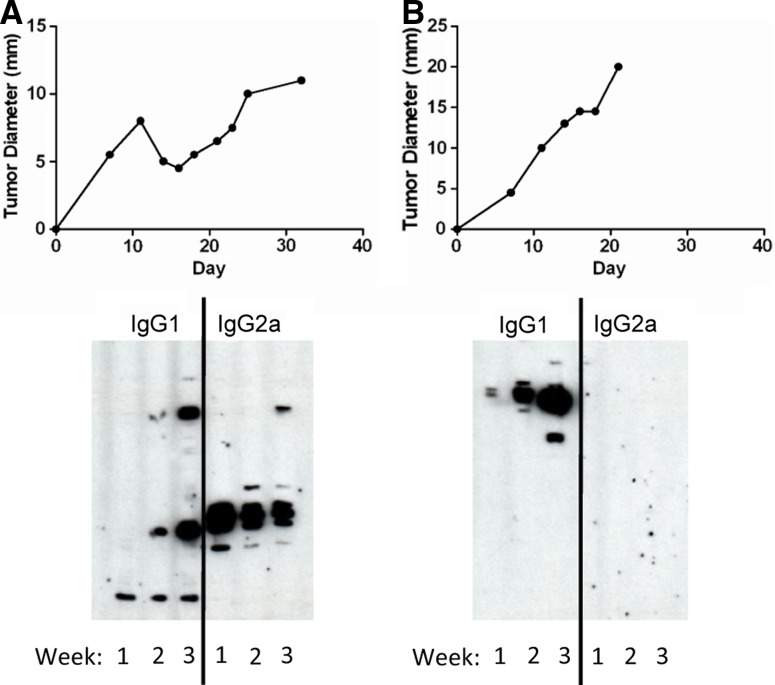
We observed a very rare occurrence in which the spontaneous regression and reappearance of a P815 tumor occurred in a mouse implanted with a normally lethal challenge. This mouse, after the first appearance and apparent disappearance of the tumor, showed no signs of tumor for 10 days, but the tumor subsequently reemerged at the site of implantation and grew progressively. The mouse had been bled over the 10 week course of tumor growth, and the Th1/Th2 nature of the anti-tumor immune response was indirectly assessed by our Western blotting protocol. We were able to demonstrate in this particular case four phases of the anti-tumor antibody response. The first antibody detectable at day 7 pi was predominantly of the IgG2a isotype, reflecting a predominant Th1, cell-mediated response. The antibody response evolved to have a mixture of IgG2a/IgG1 isotypes between day 14 and 28, a time interval that included peak tumor formation and regression of this first phase of tumor growth. Most interestingly, there was a subsequent change to the predominant expression of the IgG2a isotype, as seen in the serum collected at day 35, reflecting a time when the tumor was not evident. Tumor re-emergence and progression involved a rapid switch in the isotypes expressed, the IgG1 isotype becoming ever more dominant. We comment in “Discussion” on what might control these shifts in isotype expression, evident in Fig. 7. Although this represents an analysis of the immune response of a single mouse, undergoing a very uncommon and protracted growth pattern of the P815 tumor, it very clearly demonstrates the utility of our antibody assays in longitudinally following the changing nature of the anti-tumor immune response.
Fig. 7.
Spontaneous regression, and subsequent reemergence of the P815 tumor, is associated with a switch from a predominant Th1 to a Th2 immune response against tumor antigens. The mouse was implanted with 105 P815 cells id on day 0. a The P815 tumor spontaneously resolved, and subsequently reappeared and grew progressively at the site of primary injection. b The mouse had been bled over a period of 10 weeks, and the relative predominance of P815-specific IgG1 and IgG2a antibodies were assessed by Western blot
Discussion
The idea we explored here, that the immune correlates of tumor regression and progression are often predominant, cell-mediated (Th1) immunity and immunity with a substantial humoral, Th2 component, is not novel. Such a hypothesis, formulated in the contemporary terms of that time, was widely held in the 1960s [19]. However, this hypothesis has somewhat fallen out of favor. For example, in four recent reviews on regulation of the anti-tumor immune response [1, 3, 52, 53], deviation of the immune response into a mixed Th1/Th2 mode, as a possible mechanism of tumor evasion, was mentioned as a relatively minor possibility in only one of the reviews [1]. We have been struck by the parallel between the picture North [30] developed on the generation of concomitant, cell-mediated immunity and its decay, during progressive tumor growth, and of other studies on the nature of the response to non-tumor antigens [7, 8, 22, 23, 33, 39, 42]. These latter studies show that the immune response often first goes through an exclusive cell-mediated phase, before antibody is produced. As antibody is produced, the expression of cell-mediated immunity becomes suppressed by the generation of antigen-specific CD4 T cells [37, 38, 39, 44]. This parallelism led us to test our working hypothesis in two tumor systems employed by North for his analysis of concomitant immunity. Our observations support our hypothesis at two levels. First, we find a correlation between the predominant generation of IFN-γ-producing T cells and containment of the tumor, and of mixed IFN-γ- and IL-4-, or predominant IL-4 producing T cells, with tumor progression. Second, and more tellingly, we found that the response of mice made resistant to a normally lethal challenge had been modulated towards the Th1 pole. We consider that these observations provide strong support to the idea that the tumor progression, seen in unprimed mice implanted with a normally lethal challenge, is a consequence of the response evolving into one with a mixed Th1/Th2 or predominant Th2 mode. Our observations also provide a rationale for the protocol by which resistance is established. This implantation with a number of tumor cells, below the number which would give rise to progressive tumor growth and an immune response with a significant Th2 component, is expected, according to a low-dose vaccination strategy, to generate a Th1 response and “Th1 imprint” upon the immune system [8]. Such an imprint guarantees a Th1 response on subsequent challenge.
We would like to address some points on the relationship between IgG isotypes and the Th1/Th2 phenotype of the response, before discussing the potential use of IgG isotypes to monitor the nature of anti-tumor immune responses. There is compelling evidence that the relative preponderance of different IgG isotypes reflect the Th1/Th2 nature of the immune response [20, 34, 43, 45, 51]. The predominant presence of IgG2a antibodies is sometimes said to reflect an exclusive Th1 response, and an exclusive Th1 response to reflect a state of DTH. However, classical studies in both people and animals show that there is a tendency for exclusiveness between the expression of DTH and production of IgG antibody. It would thus seem that there could be a state of DTH, presumably mediated by Th1-like cells, without the production of measurable IgG antibody. Indeed, we found in our studies on responses by BALB/c mice to infection with very low numbers of Leishmania major parasites that such an infection could generate an exclusive DTH, Th1 response, as assessed by the expression of DTH and presence of parasite-specific IFN-γ-producing, but not of IL-4-producing, CD4 T cells, with undetectable production of IgG2a antibody [49]. Infection with low, but slightly higher numbers of parasites, resulted in predominant production of IFN-γ and IgG2a antibodies and some IL-4 production [8, 25, 26]. These observations show that the direct equating of Th1 responses with IgG2a production is invalid. These considerations might well be pertinent to an understanding of the murine antibody immune response to tumors. Thus, we do find situations in which mice resist a tumor upon exposure to a normally lethal challenge and where the inferred Th1 response following priming is not associated with detectable production of IgG2a antibody (see Fig. 4b for an example). There clearly are different reasons for progressive tumor growth even in the two tumor systems we studied. Thus, we would agree with North that the initial increase in tumor growth, evident in the first few days after implantation of a lethal challenge, was due to a lag period before sufficient immunity had been generated. This is clearly different from the later phase of progressive tumor growth associated with a mixed Th1/Th2 response that often occurs after a period of slower or of little net tumor growth. This semi-stationary phase of tumor growth, when it is evident, is, we would suggest, due to a less rapid switch of the response from a predominant Th1 to a mixed Th1/Th2 or predominant Th2 response, than in mice in which this semi-stationary phase is less evident (see Figs. 5, 6).
Three interconnected questions are raised by the observations reported here. First, are our observations reflective of immune responses to a significant proportion of other animal tumors? This question can only be answered by appropriate observation; however, as we suggested in “Introduction”, the common features of concomitant immunity and its regulation, as well as the rather general applicability of strategies of tumor prevention or treatment, lead us to suggest that there indeed may be only a few major mechanisms of tumor escape. Our findings in these two tumor systems make us eager to explore whether they might bear on immune responses to a significant fraction of other murine tumors. If this proves to be the case, it leads to the second question: how relevant might our findings be to human cancer? Some reports would favor such a possibility. There are observations in the literature suggesting that containment and progression of cancer are associated in humans with predominant Th1 and mixed Th1/Th2 responses in some cases [10, 17, 28]. Furthermore, it appears that cancer patients very often have tumor-specific antibodies [40]. Studies on the Th1/Th2 nature of the anti-tumor response of cancer patients would appear to be both very interesting, but quite difficult to realize. We think an examination of the prevalence of different IgG isotypes in anti-tumor antibody might provide a very simple way of examining whether such immune correlates of progression/containment hold more widely. Moreover, as patients with the same type of cancer often respond to the same antigens [6], it may be possible to carry out such assessments without extracting antigens from the patient’s own cancer cells but with antigens from tumor cell lines of the same type of cancer. The third question is related to why treatments, known to be effective in preventing or causing the regression of murine tumors, work. It would appear possible, for those tumors whose anti-tumor immune responses conform to our working hypothesis, that the net effect of such treatments is to modulate the long-term immune response against a normally lethal challenge from a mixed Th1/Th2 or predominant Th2 to a cell-mediated, Th1 mode. North et al. [30] showed that a standard dose of whole body radiation of mice bearing established tumors could cause tumor regression in three of five tumor systems studied. The regression of established L5178Y could be achieved in this manner. We have confirmed that whole body radiation of DBA mice bearing L5178Y can cause tumor regression. We also found that this regression resulted in a modulation of the response towards the Th1 pole, with the expected change in expression of the IgG isotype of the anti-tumor antibody (Hamilton and Bretscher, unpublished observation).
Our observations led us to speculate that, in those tumor systems where Th2- deviation is associated with tumor progression, it may be possible to monitor the changes in IgG isotype expression of the anti-tumor antibody during treatment. Cancer treatment usually involves a reduction in tumor burden and therefore antigen load, and often the depletion of dividing cells, including CD4+ T cells. Both maneuvers are known, when optimal, to switch mixed Th1/Th2 responses to a Th1 mode [2, 15, 16, 21, 31, 49, 50]. Could such monitoring of the relative prevalence of IgG isotypes be useful in guiding immunotherapy to optimize the effectiveness of the host’s immune response against the tumor? In this case, though the IgG antibodies associated with cancer may not be very effective in directly containing the tumor, they could become an essential clinical tool in optimizing treatment by optimally harnessing the host’s immune response against the tumor.
Acknowledgments
This work was supported by grants from the Ralston Brothers’ Fund and from an NCIC-regional partnership program to Peter A. Bretscher. We thank Dr. C. Havele and G. Wei for help in the early phases of this work. The authors report no competing financial interests.
Abbreviations
- sc
Subcutaneous
- id
Intradermal
References
- 1.Assudani DP, Horton RB, Mathieu MG, McArdle SE, Rees RC. The role of CD4+ T cell help in cancer immunity and the formulation of novel cancer vaccines. Cancer Immunol Immunother. 2007;56(1):70–80. doi: 10.1007/s00262-006-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49(7):1649–1654. [PubMed] [Google Scholar]
- 3.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 4.Biddison WE, Palmer JC. Development of tumor cell resistance to syngeneic cell-mediated cytotoxicity during growth of ascitic mastocytoma P815Y. Proc Natl Acad Sci USA. 1977;74(1):329–333. doi: 10.1073/pnas.74.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur J Immunol. 1996;26(11):2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 6.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 7.Bretscher PA, Ismail N, Menon JN, Power CA, Uzonna J, Wei G. Vaccination against and treatment of tuberculosis, the leishmaniases and AIDS: perspectives from basic immunology and immunity to chronic intracellular infections. Cell Mol Life Sci. 2001;58(12–13):1879–1896. doi: 10.1007/PL00000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 9.Burnet F. Cancer a biological approach. BMJ. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22(3):371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.De Plaen E, Lurquin C, Lethe B, van der Bruggen P, Brichard V, Renauld JC, Coulie P, Van Pel A, Boon T. Identification of genes coding for tumor antigens recognized by cytolytic T lymphocytes. Methods. 1997;12(2):125–142. doi: 10.1006/meth.1997.0462. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273. [Google Scholar]
- 14.Gorelik E. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv Cancer Res. 1983;39:71–120. doi: 10.1016/s0065-230x(08)61033-7. [DOI] [PubMed] [Google Scholar]
- 15.Hahn H, Kaufmann SH, Miller TE, Mackaness GB. Peritoneal exudate T lymphocytes with specificity to sheep red blood cells. I. Production and characterization as to function and phenotype. Immunology. 1979;36(4):691–698. [PMC free article] [PubMed] [Google Scholar]
- 16.Hailu A, Menon JN, Berhe N, Gedamu L, Hassard TH, Kager PA, Olobo J, Bretscher PA. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J Infect Dis. 2001;184(1):112–115. doi: 10.1086/320994. [DOI] [PubMed] [Google Scholar]
- 17.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein G. Tumor-specific transplantation antigens: G.H.A. Clowes Memorial Lecture. Cancer Res. 1968;28:625–635. [PubMed] [Google Scholar]
- 20.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254(5032):707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 21.Lagrange PH, Mackaness GB. A stable form of delayed-type hypersensitivity. J Exp Med. 1975;141(1):82–96. doi: 10.1084/jem.141.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagrange PH, Mackaness GB, Miller TE. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackaness GB, Lagrange PH, Miller TE, Ishibashi T. Feedback inhibition of specifically sensitized lymphocytes. J Exp Med. 1974;139(3):543–559. doi: 10.1084/jem.139.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 25.Menon JN, Bretscher PA. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26(1):243–249. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 26.Menon JN, Bretscher PA. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28(12):4020–4028. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190(5):617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North RJ. Down-regulation of the antitumor immune response. Adv Cancer Res. 1985;45:1–43. doi: 10.1016/S0065-230X(08)60265-1. [DOI] [PubMed] [Google Scholar]
- 30.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986;164(5):1652–1666. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. 1990;71(1):90–95. [PMC free article] [PubMed] [Google Scholar]
- 32.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128–3133. [PubMed] [Google Scholar]
- 33.Parish CR. The relationship between humoral and cell-mediated immunity. Transplant Rev. 1972;13:35–66. doi: 10.1111/j.1600-065x.1972.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 34.Paul WE, Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- 35.Power CA, Grand CL, Ismail N, Peters NC, Yurkowski DP, Bretscher PA. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFNgamma-producing T cells. J Immunol Methods. 1999;227(1–2):99–107. doi: 10.1016/S0022-1759(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramshaw IA, Bretscher PA, Parish CR. Regulation of the immune response. I. Suppression of delayed-type hypersensitivity by T cells from mice expressing humoral immunity. Eur J Immunol. 1976;6(10):674–679. doi: 10.1002/eji.1830061003. [DOI] [PubMed] [Google Scholar]
- 38.Ramshaw IA, Bretscher PA, Parish CR. Regulation of the immune response. II. Repressor T cells in cyclophosphamide-induced tolerant mice. Eur J Immunol. 1977;7(3):180–185. doi: 10.1002/eji.1830070313. [DOI] [PubMed] [Google Scholar]
- 39.Ramshaw IA, McKenzie IF, Bretscher PA, Parish CR. Discrimination of suppressor T cells of humoral and cell-mediated immunity by anti-Ly and anti-Ia sera. Cell Immunol. 1977;31(2):364–369. doi: 10.1016/0008-8749(77)90038-7. [DOI] [PubMed] [Google Scholar]
- 40.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92(25):11810–3. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065X.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 42.Salvin SB. Occurrence of delayed hypersensitivity during the development of Arthus type hypersensitivity. J Exp Med. 1958;107(1):109–124. doi: 10.1084/jem.107.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Severinson E, Fernandez C, Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990;20(5):1079–1084. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]
- 44.Sher A, Gazzinelli RT, Oswald IP, Clerici M, Kullberg M, Pearce EJ, Berzofsky JA, Mosmann TR, James SL, Morse HC., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065X.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 45.Snapper CM, Peschel C, Paul WE. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140(7):2121–2127. [PubMed] [Google Scholar]
- 46.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983;157(3):1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 49.Uzonna JE, Bretscher PA. Anti-IL-4 antibody therapy causes regression of chronic lesions caused by medium-dose Leishmania major infection in BALB/c mice. Eur J Immunol. 2001;31(11):3175–3184. doi: 10.1002/1521-4141(200111)31:11<3175::AID-IMMU3175>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 50.Uzonna Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. J Immunol. 2001;167(12):6967–6974. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 51.Wolf ML, Weng WK, Stieglbauer KT, Shah N, LeBien TW. Functional effect of IL-7-enhanced CD19 expression on human B cell precursors. J Immunol. 1993;151(1):138–148. [PubMed] [Google Scholar]
- 52.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16(2):115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]