Abstract
The low immunogenicity of malignant cells is one of the causes responsible for the lack of antitumor immune responses. Thus, development of new therapeutic strategies aimed at enhancing presentation of tumor antigens to T cells is a main goal of cancer immunotherapy. With this aim, we studied the efficacy of administering adjuvants poly(I:C) and agonistic anti-CD40 antibody plus a tumor antigen. Joint intravenous immunization with these adjuvants and a model tumor antigen (ovalbumin) was able to synergistically induce potent and long lasting antitumor T-cell responses. These responses protected against challenge with E.G7–OVA tumor cells in prophylactic short- and long-term vaccination. In a therapeutic setting, repeated intratumor administration of adjuvants plus antigen was able to reject established tumors in all treated animals, leading in some cases to the rejection of both locally treated and untreated tumors. Antitumor immune responses induced by these protocols were mediated not only by T-cells but also by NK cells. In conclusion, combined administration of adjuvants poly(I:C) and anti-CD40 plus a tumor antigen is an efficient strategy for prophylactic and therapeutic antitumor vaccination.
Keywords: Adjuvants, Tumor rejection, T-cells
Introduction
Characterization of the role of innate and acquired immunity in antitumor therapy, together with the identification of tumor antigens, has opened the possibility of new strategies for the design of future cancer vaccines. Thus, preclinical models have shown that when potent T-cell responses against a tumor antigen are induced, tumor rejection usually takes place [9, 12, 24]. These findings have prompted many researchers to develop protocols capable of inducing potent antitumor T-cell immunity. It is now well established that T-cells recognize protein antigens in the form of short peptides presented on MHC molecules [10]. Recognition of these MHC/peptide complexes on the surface of a tumor cell may lead to elimination of this cell by the action of lytic mechanisms or by cytokines secreted by CD8 and CD4 cells [1, 14, 42]. However, although tumor cells can be recognized by effector T-cells, they cannot prime naive T-cells. T-cell priming is carried out by professional antigen presenting cells, such as dendritic cells (DC), which after antigen encounter are activated in a process known as maturation [4]. Maturation is usually triggered by PAMPs (pathogen associated molecular patterns) [16] or by proinflammatory cytokines, which are recognized by specific receptors on DC [17, 29, 33]. Tumor cells do not express molecules able to activate DC and, although DC can capture and present tumor antigens, these cells are not usually in the mature state which provides MHC/peptide complexes together with the costimulatory molecules and cytokines necessary for T-cell priming [26, 27]. This explains why tumors are usually poorly immunogenic. To overcome this lack of immunogenicity it has been suggested that administration of tumor antigens with molecules able to activate DC (adjuvants) may be a good strategy to induce potent antitumor T-cell immune responses [28, 37]. Among these molecules, ligands of toll like receptors (TLR) are of special interest. These molecules are usually PAMPs belonging to different microorganisms like viruses, fungi and bacteria [36]. Together with these activating signals provided by exogenous pathogens, endogenous signals such as pro-inflammatory cytokines, or cell-associated molecules like CD40L [7], are also able to activate DC. Each of these activators has a particular receptor and an intracellular signaling pathway, which finally leads to DC maturation. It has been shown that combination of different maturation stimuli, able to trigger different signaling pathways, synergizes on the effector functions of DC [2, 39, 40]. Thus, with the aim of inducing potent antitumor T-cell responses, ligands able to strongly activate DC would facilitate T-cell priming. To achieve this goal, we tested the combined effect of two maturation stimuli: polyinosinic polycytidylic acid (poly(I:C)), a double-stranded RNA which is a ligand of TLR3 [3], and an agonistic anti-CD40 antibody which mimics CD40L functions [30]. Prophylactic and therapeutic antitumor efficacy of this combined immunization protocol was tested using a mouse model where the tumor antigen is ovalbumin (OVA). We describe below the results of these experiments together with an interpretation of the mechanisms responsible for tumor rejection.
Materials and methods
Antigens and reagents
CTL epitopes OVA(257–264), OVA(176–183) and OVA(55–62) (SIINFEKL, NAIVFKLG and KVVRFDKL, respectively) [22, 31] as well as T helper peptide OVA(323–339) (ISQAVHAAHAEINEAGR) [34] belonging to OVA were synthesized by the solid-phase method of Merrifield using the Fmoc alternative and a manual multiple solid-phase peptide synthesizer. Ninhydrin test of Kaiser was used to monitor every step. At the end of the synthesis, peptides were cleaved and deprotected with trifluoroacetic acid and washed with diethyl ether. Purity of the peptides was always above 90% as determined by HPLC. OVA was purchased from Sigma Aldrich (Madrid, Spain). Poly(I:C) was obtained from Amersham (Barcelona, Spain) and agonistic anti-CD40 antibody was obtained from ascytic fluid of nude mice injected with the FGK45.5 hybridoma cells [30] and purified by ammonium sulfate precipitation.
Mice
Six- to 8-week-old female C57BL/6 mice were obtained from Harlan (Barcelona, Spain). Rag1–/– mice in C57BL/6 background were kindly provided by Dr. I. Melero (Center for Applied Medical Research, Pamplona, Spain). All animals were maintained in pathogen-free conditions and treated according to guidelines of our institution, after study approval by the review committee.
Cell lines
EL4 thymoma cells (H-2b) as well as OVA-transfected E.G7–OVA cells [25] were purchased from American Type Culture Collection (Manassas, VA, USA) and used as target cells in chromium release assays and in vivo for tumor protection and treatment experiments. They were cultured in complete medium (RPMI 1640 containing 10% fetal calf serum, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 2 mM glutamine and 50 μM 2-mercaptoethanol). Medium for E.G7-OVA cells also contained 400 μg/ml of G418.
Immunization for cytokine and CTL induction
Groups of three mice were immunized with poly(I:C) (50 μg/mouse), anti-CD40 (50 μg/mouse) and OVA (500 μg/mouse). Poly(I:C) and OVA were administrated intravenously (i.v.) and anti-CD40 antibody was injected intraperitoneally (i.p.). Six or 48 days after immunization, animals were sacrificed to evaluate short- and long-term immune responses, respectively.
Flow cytometry
OVA(257–264) specific cells were enumerated by flow cytometry using OVA(257–264)/H-2Kb tetramers. Spleen cells from immunized or control mice were first treated for 10 min with Fc Block™ (BD-Biosciences; San Diego, CA, ) and then stained with PE-labeled OVA(257–264)/H-2Kb tetramers (Immunomics; Marseille, France) and anti-CD8- FITC antibodies (BD-Biosciences). After 15 min, cells were washed and surface expression of the different markers was analyzed by using a FACSCalibur flow cytometer (Becton Dickinson).
Stimulation of spleen cells to measure cytokine production in supernatants
Spleen cells from immunized animals were resuspended in complete medium and plated (8 × 105 cells/well) in 0.2 ml in U-bottomed 96-well plates in the absence or in the presence of peptides or OVA. Two days later, supernatants were harvested and IFN-γ and IL-4 were measured by ELISA (BD-Biosciences), according to manufacturer’s instructions.
ELISPOT
ELISPOT assays for IFN-γ were carried out using a kit from Mabtech (Sweden) according to manufacturer instructions. Briefly, plates (Multiscreen HTS; Millipore, Bedford, MA, USA) were coated with purified anti-IFN-γ AN18 antibody (15 μg/ml). After overnight incubation, plates were washed with PBS and blocked for 3 h with DMEM containing 10% fetal calf serum. Then, 1–4 × 105 splenocytes were cultured in triplicate in the absence or in the presence of CTL peptides (1–10 μg/ml), T helper epitope OVA(323–339) (10 μg/ml) or OVA (10 μg/ml). One day later, plates were washed with PBS and incubated with biotinylated anti-IFN-γ R4–6A2 antibody (1 μg/ml). After 4 h, plates were washed and incubated with a 1/500 dilution of streptavidin-peroxidase. One hour later, plates were washed and developed with freshly prepared DAB solution. The reaction was stopped with distilled water and spots were counted using an automated ELISPOT reader (CTL; Aalen, Germany).
Measurement of CTL activity
To measure CTL responses against peptides, splenocytes from immunized animals were incubated with peptides (1–10 μg/ml) for 2 h at 37°C, washed twice and cultured in 24 well plates at 7.5 × 106 cells/well. To measure CTL responses against E.G7-OVA tumor cells, splenocytes (7.5 × 106 cells/well) were cultured in 24 well plates with 7.5 × 105 E.G7-OVA tumor cells, previously treated with mitomycin C. In both cases, 2 days later, IL-2 (Boehringer-Mannhein GmbH, Germany) (2.5 U/ml) was added to the wells, and on days 5–7, cells were harvested for chromium release assays. Lytic activity was measured by incubating for 4 h different numbers of effector cells with 3,000 51Cr-labeled target cells: EL4 cells with or without peptide or E.G7-OVA cells. Percentage of specific lysis was calculated according to the formula: 100 × (cpm experimental − cpm spontaneous)/(cpm maximum − cpm spontaneous), where spontaneous lysis corresponds to target cells incubated in the absence of effector cells and maximum lysis is obtained by incubating target cells with 5% Triton × 100.
Tumor protection experiments
Groups of 5–6 mice were injected with adjuvants with or without OVA protein as described before, and 6 days later, they were challenged by subcutaneous (s.c.) injection with 5 × 105 E.G7-OVA tumor cells. To assess long lasting protection, mice were immunized with OVA and adjuvants twice in a 14-day interval. Thirty days after the last immunization, animals were challenged as explained above. A group of non-immunized mice was always included as a positive control of tumor growth. Tumor volume was calculated according to the formula: V = (length × width2)/2. Mice were killed when tumor diameter reached 18 mm. In some short-term protection experiments, animals were depleted of CD8 cells by injecting 300 μg of anti-CD8 antibodies (obtained from H35.17.2 hybridoma) on days 5, 6 and 7 after immunization.
Tumor treatment experiments
C57BL/6 or Rag1–/– mice were injected s.c. with 5 × 105 E.G7-OVA tumor cells and when the tumor diameter reached 5–6 mm, different treatment protocols were applied. Treatment protocols consisted of one or seven administrations of adjuvants with or without OVA by intratumor (i.t.) and/or i.v. route. In some experiments, to assess systemic effects of treatment, animals received a s.c. administration of E.G7-OVA cells in both flanks. When one of the tumors reached 5–6 mm of diameter, it was treated, maintaining the contralateral tumor without treatment. For NK cell depletion, mice were injected i.p. with rabbit anti-AsGM1 (40 μl/mouse) (Wako Chemicals; Neuss, Germany) on days -2 and 0, 0 being the day of treatment. An equivalent amount of rabbit IgG (Sigma) was injected in the control group. As in protection experiments, untreated mice challenged s.c. with E.G7-OVA cells were used as positive controls of tumor growth.
Statistical analysis
Survival curves of animals treated with different protocols were plotted according to the Kaplan–Meier method. Statistical significance in different treatment groups was compared using the log-rank test. P < 0.05 was taken to represent statistical significance.
Results
Immunization with poly(I:C) and anti-CD40 plus OVA protein induces CD8 and CD4 T-cell responses
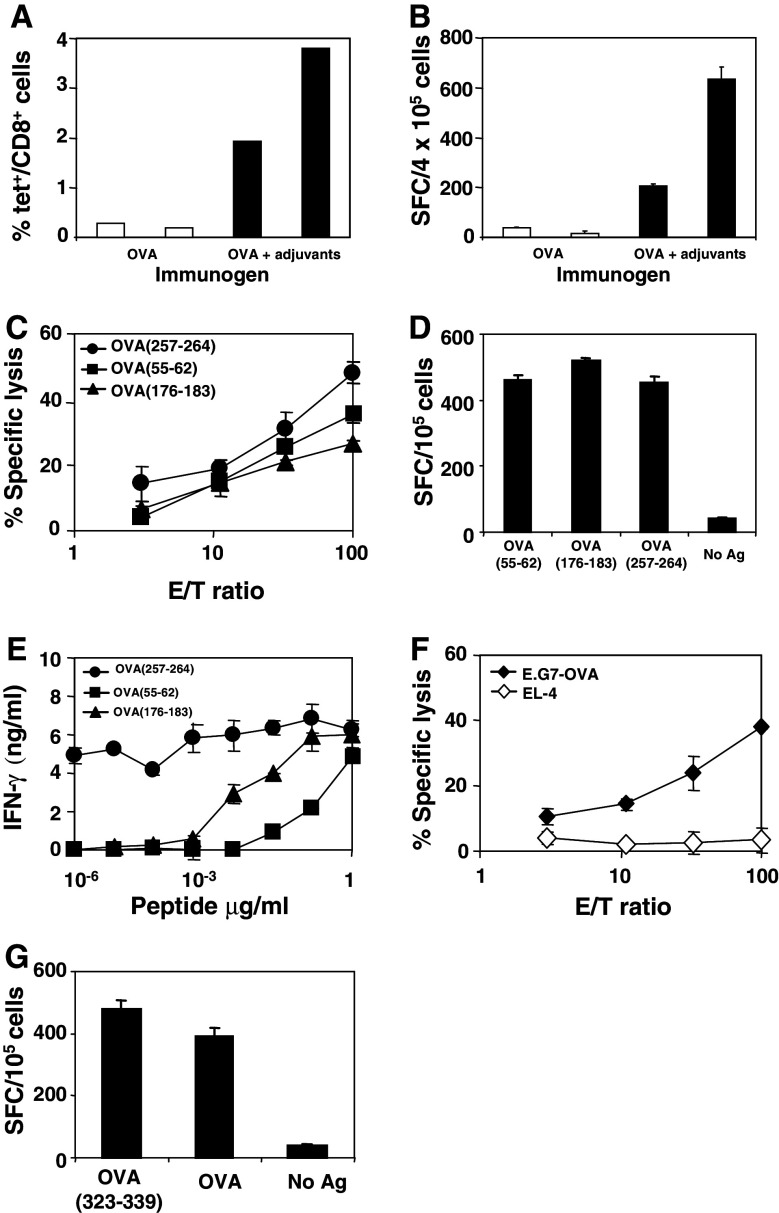
As discussed earlier in Introduction, since tumors are usually poorly immunogenic we decided to test the combined effect of two immunostimulatory reagents, poly(I:C) and anti-CD40, on the induction of antitumor immune responses in a mouse model where the tumor antigen is OVA. It is well known that CTL are important effector cells with antitumor activity and for this reason, we evaluated CD8 responses induced after intravenous immunization with poly(I:C) and anti-CD40 plus OVA. Six days after immunization, animals were sacrificed and the number of OVA(257–264)-specific CD8 T-cells was quantified using OVA(257–264)/H-2Kb tetramers. As shown in Fig. 1a, 1.93 and 3.8% of CD8+ T-cells were tetramer+, as compared to 0.28 and 0.19% obtained in two mice immunized with OVA alone. In order to study the functional ability of CD8 cells, splenocytes from the same mice were stimulated in vitro with OVA(257–264) and IFN-γ spot forming cells were enumerated. This experiment (Fig. 1b) showed a close correlation with results obtained using tetramers, suggesting that inclusion of poly(I:C) and anti-CD40 in the immunization mixture has a quantitative effect on CD8 cells by increasing their number. Splenocytes from animals immunized with poly(I:C) and anti-CD40 plus OVA were also stimulated in vitro with CTL epitopes OVA(257–264), OVA(176–183) and OVA(55–62) to measure lytic activity against these peptides. As shown in Fig. 1c, clear responses were induced against all epitopes. In order to quantify the magnitude of CD8 responses against these three epitopes, ex vivo ELISPOT assays measuring IFN-γ were carried out. These assays confirmed that similar strong responses against all three epitopes could be measured (Fig. 1d). Finally, the avidity of lymphocytes specific for these peptides was also assessed by measuring IFN-γ after stimulation with different peptide concentrations. As shown in Fig. 1e, lymphocytes specific for peptide OVA(257–264) were able to recognize minimal amounts of this epitope, in agreement with its immunodominant role in the context of OVA protein [22]. In order to study whether this immunization protocol inducing peptide-specific responses was also able to induce CTL responses capable of recognizing endogenously expressed antigens, splenocytes were stimulated with E.G7-OVA tumor cells and lytic activity was measured. As shown in Fig. 1f, immune splenocytes recognized E.G7-OVA cells but not parental non-transfected EL4 cells.
Fig. 1.
Immunization with adjuvants poly(I:C) and anti-CD40 plus OVA induces CD8 and CD4 T cells responses. a C57BL/6 mice (two per group) were immunized with 500 μg of OVA (i.v.) with or without 50 μg of poly(I:C) (i.v.) and with 50 μg of anti-CD40 antibody (i.p.) per mouse. Six days after immunization, mice were sacrificed and the percentage of OVA(257–264)/Kb tetramer+/CD8+ cells was calculated in their spleens. b Splenocytes from mice shown in a were stimulated with OVA(257–264) and IFN-γ spot forming cells were enumerated by ELISPOT. c Splenocytes from mice immunized with OVA plus poly(I:C) and anti-CD40 were pooled and stimulated during 5 days with CTL peptides OVA(257–264) (1 μg/ml), OVA(176–183) (10 μg/ml) or OVA(55–62) (10 μg/ml). Five days later, CTL activity was measured against EL4 cells loaded with the corresponding CTL peptides. Responses against EL4 cells incubated in the absence of peptides were always below 3%. d Splenocytes were also cultured in ELISPOT plates with the same peptides for 24 h and IFN-γ-secreting cells were enumerated. e Avidity of T-cells recognizing CTL peptides was assessed by stimulating splenocytes with different peptide concentrations. Two days later, supernatants were harvested and IFN-γ levels were measured by ELISA. IFN-γ produced in the absence of peptide was <0.1 ng/ml. f CTL responses against E.G7-OVA tumor cells were evaluated in vitro by incubating splenocytes with mitomycin C-treated E.G7-OVA tumor cells. Five days later, lytic activity was measured against E.G7-OVA cells or control EL4 cells. g CD4 responses induced by immunization were assessed as IFN-γ production in ELISPOT assays by stimulating splenocytes with OVA (10 μg/ml) or with OVA(323–339) T helper peptide (10 μg/ml). Results are representative of two or three independent experiments
Since CD4 responses are of great interest to activate and maintain effective CD8 antitumor responses [15], we also evaluated the induction of CD4 responses by this immunization protocol. This was carried out in ELISPOT assays by stimulating splenocytes with OVA or with the well-characterized T helper epitope OVA(323–339). Figure 1g shows that in both cases, a clear IFN-γ production was observed. No IL-4 was detected in the same supernatants, indicating that Th1 responses were induced after poly(I:C) and anti-CD40 plus OVA administration (data not shown). Blocking experiments using anti-CD4 and anti-CD8 antibodies showed that IFN-γ production after OVA stimulation was mediated not only by CD4 cells but also by CD8 cells (data not shown). Thus, immunization with OVA plus adjuvants poly(I:C) and anti-CD40 induces CD8 and CD4 responses.
Immunization with poly(I:C) and anti-CD40 plus OVA elicits long lasting CTL immune response
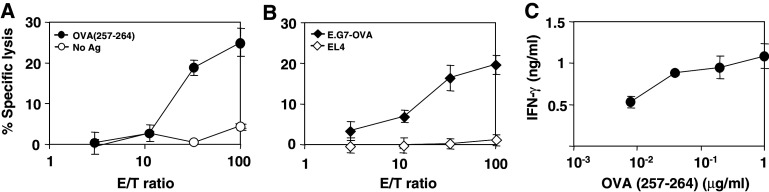
Since strong CTL responses were detected 6 days after immunization with poly(I:C) and anti-CD40 plus OVA, we decided to study if memory CTL responses could be induced by this protocol. Thus, mice were sacrificed 48 days after immunization, and we measured CTL responses against immunodominant OVA(257–264) epitope and against E.G7-OVA tumor cells. In both cases, consistent CTL memory responses were found (Fig. 2a, b). In agreement with this result, high IFN-γ levels were detected in supernatants of splenocytes incubated with different dilutions of OVA(257–264) (Fig. 2c).
Fig. 2.
Immunization with poly(I:C) and anti-CD40 plus OVA elicits long lasting CTL immune responses. a C57BL/6 mice (three per group) were immunized as in Fig. 1 and 48 days later they were sacrificed and their splenocytes were pooled and stimulated with the immunodominant CTL peptide OVA(257–264) (1 μg/ml). Five days later, lytic activity was measured against EL4 cells pulsed with 1 μg/ml of OVA(257–264) peptide. Unloaded EL4 cells were used as negative control of lytic activity. b CTL responses against E.G7-OVA tumor cells were also determined in vitro incubating splenocytes with these tumor cells treated with mitomycin C during 5 days, and then measuring their lytic activity against E.G7-OVA. EL4 cells were used as negative control. c IFN-γ produced by splenocytes after a two-day stimulation with different concentrations of OVA(257–264) peptide. IFN-γ produced in the absence of peptide was 0.3 ng/ml. Results are representative of two independent experiments
Immunization with poly(I:C) and anti-CD40 plus OVA induces short- and long-term protection against challenge with E.G7-OVA tumor cells
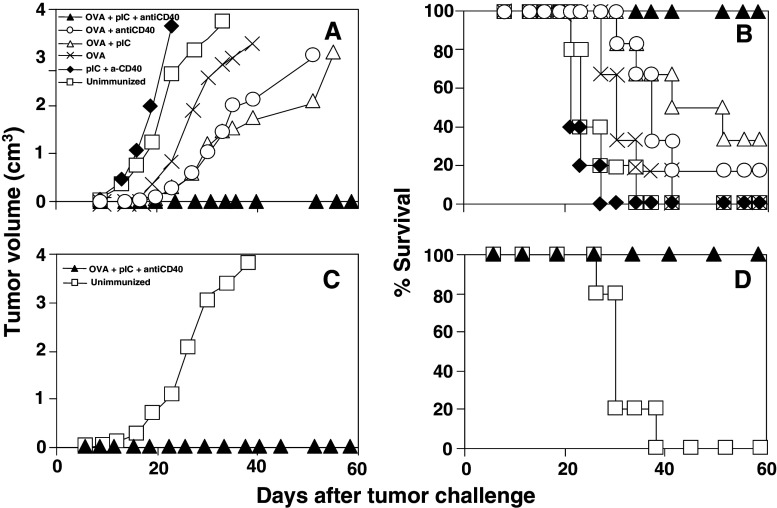
To test the in vivo efficacy of this immunization protocol, tumor protection experiments were carried out using E.G7-OVA tumor cells. In a first set of experiments, short-term protection was evaluated by injecting mice with tumor cells 6 days after immunization. These experiments (Fig. 3a) showed that 94% (15 out of 16) of animals immunized with poly(I:C) and anti-CD40 plus OVA were protected against tumor growth. By contrast, control animals which received adjuvants only or were left unimmunized, developed tumors in all cases. In order to study whether the protection obtained after combined adjuvant administration was due to a synergistic or additive effect of poly(I:C) and anti-CD40, animals immunized with OVA alone or with OVA plus single adjuvants were also included. A delay in tumor growth was observed in animals immunized with OVA alone, and this delay was clearer in those groups immunized with single adjuvants plus OVA. However, only 16 and 33% of mice immunized with OVA+ poly(I:C) and with OVA + anti-CD40 respectively survived (Fig. 3b). These results suggest a synergistic effect between adjuvants, confirming the potent immunostimulatory action of the adjuvant mixture versus the tumor antigen alone.
Fig. 3.
Immunization with poly(I:C) and anti-CD40 plus OVA induces short- and long-term protection against the challenge with E.G7-OVA tumor cells. a C57BL/ 6 mice (five–six per group) were immunized i.v. with OVA, with adjuvants poly(I:C) and anti-CD40, with OVA plus single adjuvants, with OVA plus adjuvant combination or left unimmunized. Six days later they were challenged s.c. with 5 × 105 E.G7-OVA tumor cells and evolution of tumor growth was monitored twice a week. Tumor volume was calculated according to the formula: V = (length × width2)/2. Graph represents the average tumor volume per group of animals studied. b Survival of mice shown in a. c C57BL/6 mice (fiver per group) were immunized twice in a 14-day interval with poly(I:C) and anti-CD40 plus OVA or left unimmunized and 30 days after the last immunization, animals were challenged s.c. with E.G7-OVA tumor cells. Evolution of tumor growth was assessed as above. d Survival of mice shown in c. Results are representative of three independent experiments
Long-term protection induced by immunization with poly(I:C) and anti-CD40 plus OVA was also studied. In this case, mice were immunized twice at a 14-day interval and challenged 30 days after the last immunization. As shown in Fig. 3c, d, all immunized animals were protected against tumor challenge, as opposed to control unimmunized animals where no protection was found. (P = 0.002; poly(I:C) + antiCD40 plus OVA versus untreated animals).
Poly(I:C) and anti-CD40 administration has therapeutic efficacy on established tumors
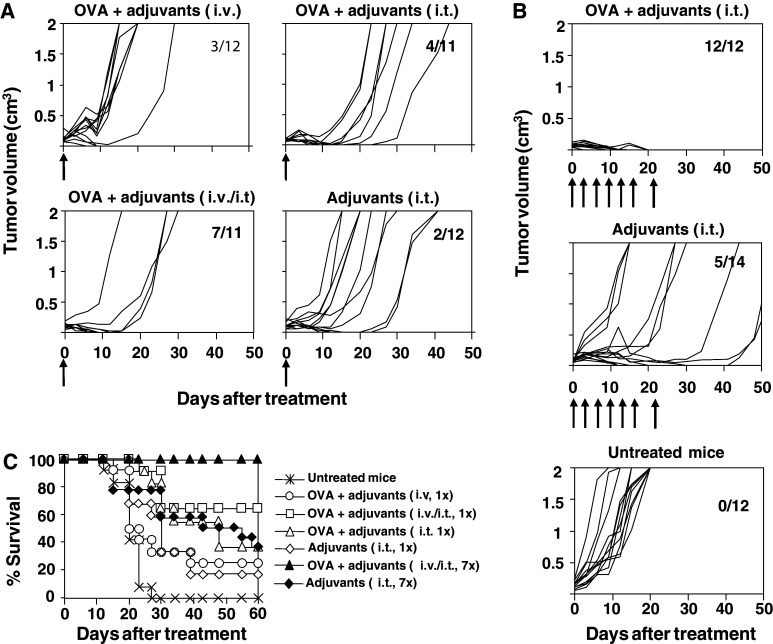
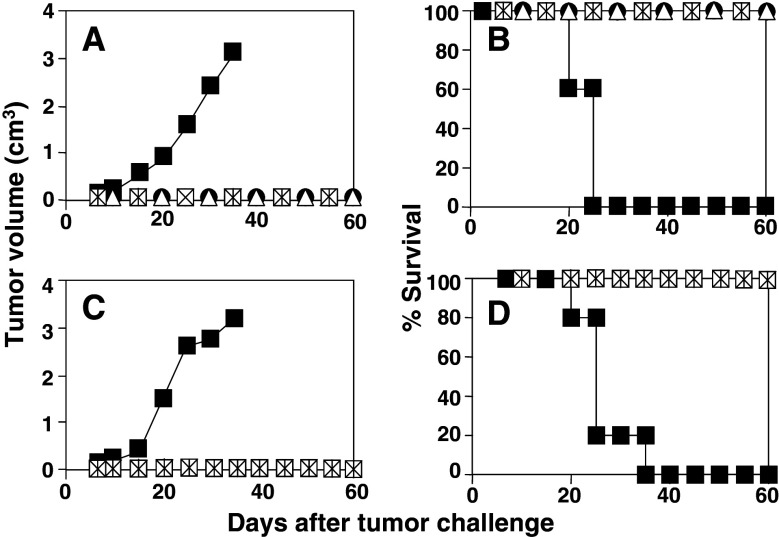
Since immunization with poly(I:C) and anti-CD40 in conjunction with antigen OVA was able to induce potent CD4 and CD8 responses which protected mice against tumor challenge in short- and long-term experiments, the efficacy of this immunization protocol was tested in mice with established tumors. Figure 4a shows that when mice bearing a 5–6 mm tumors were given a single i.v. dose of adjuvants and OVA, 3 out of 12 animals (25%) were able to reject the tumor. In order to enhance the local effects of adjuvants, a new group received the same immunization schedule but directly intratumor (i.t.). Slightly better results were obtained in this case, with 4 out of 11 (36%) mice rejecting their tumors, which did not reach statistical differences (P = 0.16). Finally, when i.v. and i.t. administration routes were combined, results were clearly more effective, eradicating tumors in 7 out of 11 animals (63%) (P = 0.02; i.v./i.t. administration versus i.v. administration). In the case of control animals receiving only adjuvants i.t., 2 out of 12 mice (17%) rejected the tumor.
Fig. 4.
Therapeutic effects of poly(I:C) and anti-CD40 plus OVA administration in tumor-bearing mice. C57BL/6 mice were injected s.c. with 5 × 105 E.G7-OVA tumor cells and when tumor diameter reached 5–6 mm, different treatment protocols were applied. In a first set of experiments (a), treatment protocols consisted of a single administration of adjuvants plus OVA i.v., adjuvants plus OVA i.t., adjuvants plus OVA i.v. and i.t., and only adjuvants i.t.. In the second set of experiments (b), mice were given adjuvant plus OVA i.t. or only adjuvants i.t. twice a week until a total of seven administrations. Arrows indicate the days of administration. A group of untreated mice was also included as a control. Figures represent evolution of tumor growth per individual mouse. Numbers in each graph indicate tumor-free mice with respect to treated mice. c Survival of mice shown in a and b. Data correspond to global results obtained in two different experiments using 5–7 mice per group in each experiment
It is interesting to note that, although in many cases tumors were not eradicated by the different treatment protocols tested, tumor masses reduced their volume during a period of time, and in some cases this reduction continued until tumors were not palpable for some time, although they rebounded later. These results suggested that a single immunization had some effect but was not enough for tumor eradication. For these reasons, we decided to use the same protocols but giving seven administrations at a 20-day interval. These experiments (Fig. 4b) showed that repeated i.t. administration of poly(I:C) and anti-CD40 plus OVA enhanced its therapeutic efficacy, inducing tumor rejection in 12 out of 12 mice (100%) (P = 0.001; adjuvants plus OVA seven i.t. administrations versus adjuvants plus OVA single i.t. administration). After i.t. administration of seven doses of adjuvants without antigen, slightly better results than with a single adjuvant injection were obtained, with 5 out 14 animals cured (35%), although it did not reach statistical differences. These results also showed that co-administration of antigen with adjuvants has a higher efficacy than administration of adjuvants alone (P = 0.001; OVA + adjuvants i.t. versus adjuvants i.t.). Repeated administration of adjuvants + OVA by i.v. or i.v./i.t routes showed a toxic effect, and although it was able to induce tumor rejection in some cases, mice suffered a shock after the third injection which was fatal in most cases (data not shown), so therapeutic antitumor efficacy of these protocols could not be evaluated in these groups. None of the untreated mice was able to reject the tumor. Survival of mice belonging to the different experimental groups is shown in Fig. 4c.
Since therapeutic immunity was induced as a consequence of treatment, it was interesting to study whether it conferred long lasting protection in re-challenge experiments. Thus, mice which were cured after different treatment protocols were again challenged with E.G7-OVA tumor cells. These experiments showed that all mice which had rejected the tumor after a single administration of OVA plus adjuvants (i.t., i.v., or i.t/i.v. routes) or after administration of adjuvants (i.t.), were protected against a second tumor challenge given 45 days after treatment (Fig. 5a, b). In a similar manner, all mice which rejected their tumor after repeated administration of OVA plus adjuvants (i.t.) or after administration of adjuvants (i.t.), were also protected against the second tumor challenge, given 60 days after treatment (Fig. 5c, d). Overall, 14 out of 14 mice were totally protected, suggesting that memory T-cell responses were induced by treatment with poly(I:C) and anti-CD40.
Fig. 5.
Therapeutic administration of poly(I:C) and anti-CD40 induces protective memory immune responses. Mice which had rejected their tumors after a single (a, b) or repeated (c, d) therapeutic administration of OVA plus adjuvants i.v. (closed circles), OVA plus adjuvants i.t. (open squares), OVA plus adjuvants i.v./i.t. (open triangles) or adjuvants i.t. (asterisks) were given a second s.c. challenge with 5 × 105 E.G7-OVA tumor cells. In the groups of mice cured after a single administration, challenge was carried out 45 days after initial treatment, whereas in those mice cured after repeated administrations, challenge was carried out 60 days after treatment. Experiments also included a control group (closed squares) of previously untreated mice. In all cases, average of tumor volume per group (a, c) and survival (b, d) is shown. Results are representative of two independent experiments
T cells and NK cells are involved in the antitumor effect induced by immunization with poly(I:C) and anti-CD40 plus OVA
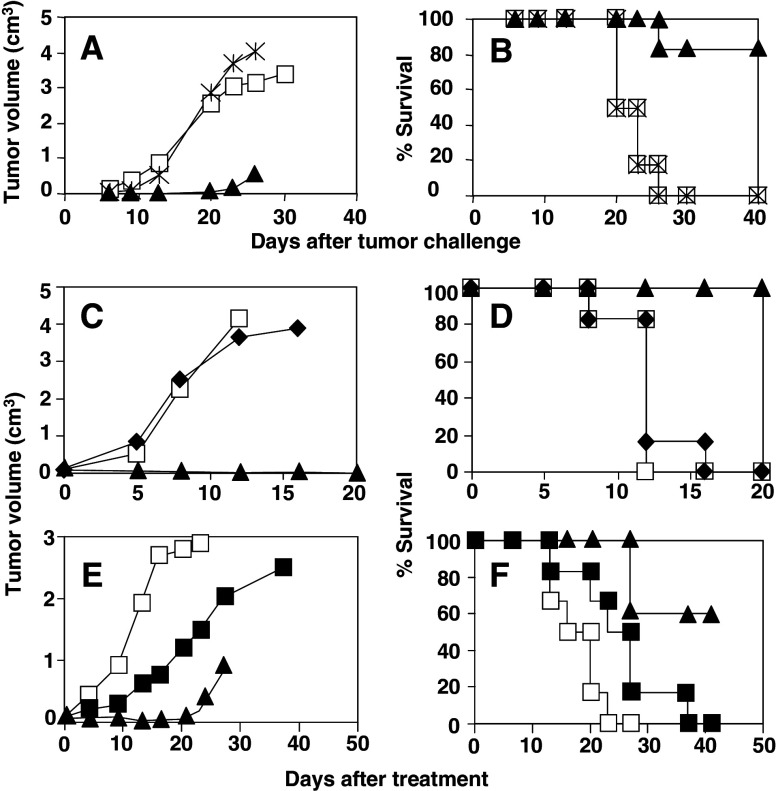
Adjuvant administration induces activation of cells belonging to the innate immune system (DC, monocytes, NK cells, etc) which in turn activate acquired immunity. Administration of poly(I:C) and anti-CD40 alone does not induce prophylactic antitumor immunity, thus, it was interesting to characterize which lymphocyte population (B cells, CD4 or CD8 T-cells) was responsible for these protective responses. Since E.G7-OVA cells express OVA intracellularly, we discarded a major implication of B cells as effector cells with direct antitumor activity, although they could be involved in antigen capture and presentation. The importance of CD8 T-cells was studied by treating immunized mice with anti-CD8 antibodies on days −1, 0 and 1 after tumor challenge. In these conditions, all animals depleted of CD8 cells, developed tumors as untreated animals (Fig. 6a, b), showing that CD8 T-cells elicited after immunization are the principal effector cells responsible for protection against tumor challenge.
Fig. 6.
T cells and NK cells are involved in the antitumor effect induced by immunization with poly(I:C) and anti-CD40 plus OVA. a C57BL/6 mice (six per group) were immunized i.v. with poly(I:C) and anti-CD40 plus OVA and on days 5, 6 and 7 after immunization, animals were depleted of CD8 cells by i.p. injection with an anti-CD8 antibody (asterisks) or left untreated (closed triangles). A third group of unimmunized mice was also included (open squares). Mice were then challenged s.c. with 5 × 105 E.G7-OVA tumor cells and tumor volume was calculated according to the formula described in Materials and methods. b Survival of mice shown in a. c Rag1–/– mice (six per group) were injected s.c. with 5 × 105 E.G7-OVA tumor cells and when tumor diameter reached 5–6 mm, a group was treated with seven i.t. doses of poly(I:C) and anti-CD40 (closed diamonds) whereas the control group was left untreated (open squares). Also, a group of wild type mice treated with seven i.t. doses of poly(I:C) and anti-CD40 plus OVA (closed triangles) was included as a control. Average of tumor volume per group of animals is represented. d Survival of mice shown in C. e Three groups of C57BL/6 mice (six per group) were injected s.c. with 5 × 105 E.G7-OVA tumor cells. When tumor diameter reached 5–6 mm a first group was left untreated (open squares) and two groups received a single administration of OVA plus poly(I:C) and anti-CD40 by i.v./i.t. routes. Treated animals were depleted of NK cells by injection of anti-AsGM1 (closed squares) on days -2 and 0, 0 being the day of treatment, or received an equivalent amount of rabbit IgG (closed triangles). f Survival of mice shown in e. Results are representative of two independent experiments
An interesting finding in therapeutic immunization experiments was that repeated administration of poly(I:C) and anti-CD40, in the absence of exogenously added antigen, was able to reject tumors in 35% of mice (Fig. 4b). Thus, to characterize whether innate or adaptive immunity was responsible for eradication of established tumors, treatment experiments were carried out in Rag1–/– mice. Tumor bearing mice were treated with poly(I:C) and anti-CD40 only, without including OVA, because Rag1–/– mice do not have T-cells able to recognize antigens. This experiment showed that tumors grew at the same rate in treated and untreated Rag1–/– mice (Fig. 6c, d), suggesting that T-cells are implicated in the mechanism of tumor eradication when poly(I:C) and anti-CD40 are administered.
The role of NK cells in therapeutic experiments was analyzed by depleting these cells in tumor bearing C57BL/6 mice which received a single administration of OVA + adjuvants by i.v./i.t. routes, protocol with the highest efficacy in single administration experiments. As shown in Fig. 6e, NK cell depletion increased the tumor growth rate, impairing the therapeutic efficacy of OVA + adjuvants administration, as compared to mice treated with OVA + adjuvants which received a control IgG. Moreover, none of the mice depleted of NK cells and receiving OVA + adjuvants rejected the tumor (Fig. 6f), whereas three out of five mice receiving OVA + adjuvants which had been previously administered with control IgG were able to eliminate the tumor.
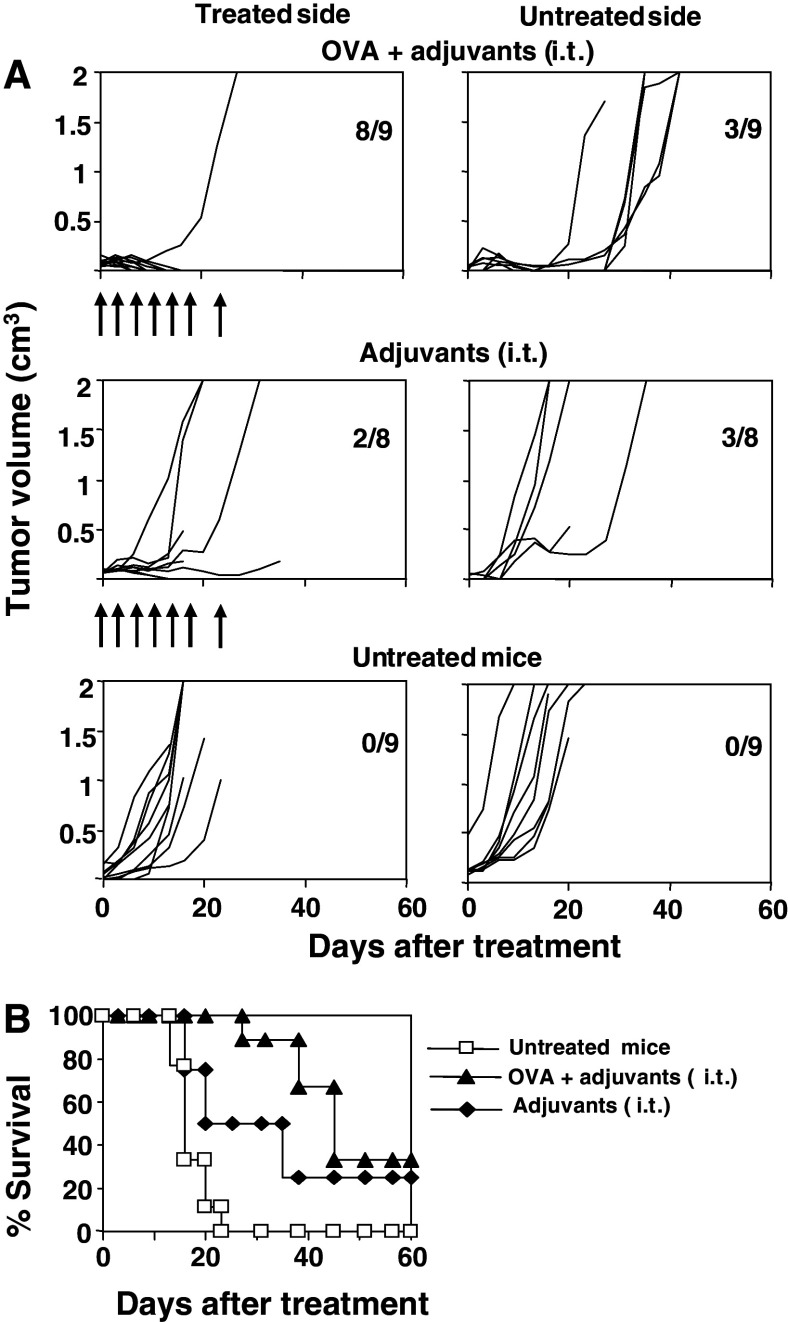
Finally, due to the role played by T- cells in tumor eradication, it was interesting to analyze whether successful therapeutic protocols could induce systemic immunity able to reject distant tumors. For this purpose, a two-tumor model was used (one tumor in each flank of the animal). Only one tumor was treated, but tumor growth was analyzed in both flanks. When a tumor reached 5–6 mm, it was treated by seven administrations of poly(I:C) and anti-CD40 with or without OVA through i.t. route. Eight out of nine mice (88%) treated by repeated i.t. administration with poly(I:C) and anti-CD40 plus OVA rejected the treated tumor (Fig. 7a), confirming previous data. Moreover, three out of these nine mice (33%) rejected untreated tumor. When adjuvants alone were administered i.t. in the absence of exogenously added antigen, only two out of eight mice (25%) and three out of eight mice (37%) rejected treated and untreated tumors, respectively. Finally, all tumors grew in control, untreated mice. Survival of animals belonging to all groups is represented in Fig. 7b.
Fig. 7.
Induction of local and systemic antitumor immunity by administration of poly(I:C) and anti-CD40 plus OVA. a C57BL/6 mice were injected s.c. with 5 × 105 E.G7-OVA tumor cells in both flanks and when a tumor reached a diameter of 5–6 mm, this tumor was treated seven times with adjuvants plus OVA i.t. or with adjuvants i.t., but the tumor from the opposite flank was left untreated. Arrows indicate the days of administration. A control group including untreated mice was also followed. Graphs represent the evolution of tumor growth per mouse in the treated and untreated side. Numbers in each graph indicate tumor-free mice with respect to treated mice. b Survival of mice shown in a
Discussion
Characterization of immunostimulatory molecules able to trigger potent T-cell responses is an important goal in the field of antitumor immunology. With this purpose, two DC maturation stimuli, poly(I:C) and an anti-CD40 agonistic antibody, have been combined with a model tumor antigen to study their ability to induce efficient antitumor immune responses. It is known that DC are activated by many different molecules which behave as adjuvants, and that combination of molecules using different signaling pathways leads in many cases to a synergistic effect on DC activation [2, 39, 40]. Poly(I:C) is a double-stranded RNA able to trigger DC activation through TLR3 [3]. It has been recently shown that it can also activate type I IFN production through recognition by intracellular receptor MDA5 [11, 18]. On the other side, anti-CD40 binds to CD40 molecules present on the DC surface. Separate administration of these molecules induces different functions of DC, but combined administration synergizes on CD8 T-cell activation [2]. In this work, we have seen that this adjuvant combination, when administered with the model antigen OVA, enhances the number of CD8 cells producing IFN-γ. Moreover, CD8 responses detected in vitro after administration of these adjuvants with OVA, not only recognized CTL epitopes, but also tumor cells. Accordingly, the synergistic effect reported in vitro correlates with the in vivo antitumor effect, as shown in prophylactic experiments. It is interesting to note that after using this adjuvant combination clear CD4 responses were also detected. CTL responses were long lasting, probably due to direct action of the adjuvants as well as to CD4 T helper cells. It has been demonstrated that IFN-α, a cytokine induced after stimulation through TLR3, has important effects on the maintenance of memory responses, through the induction of IL-15 [23, 41]. Also, activation through CD40 has been implicated on the induction of memory responses [5, 20, 21]. Finally, activation of CD4 T helper cells, as shown in the present work, is another important requisite for the priming of memory CTL responses. Thus, combined action of these mechanisms is probably responsible for the induction of potent memory responses.
Antitumor effect of this adjuvant combination plus the antigen OVA was studied in vivo using E.G7-OVA tumor cells. It was found that immunization with these components was able to fully protect mice in prophylactic vaccination experiments, not only in the short-term but also in the long-term. Depletion experiments also showed that the protective effect was mainly mediated by CD8 T-cells, since in the absence of these cells, protection was completely abolished. No CD4 depletion experiments were carried out at the time of challenge, because in our experience, depletion of CD4 cells under these circumstances depletes not only CD4+CD25− effector Th cells but also CD4+CD25+ regulatory T-cells [6]. This leads to a full tumor protection due to the elimination of the immunosuppressive effect of Tregs, and underestimates the role of effector CD4 T-cells. Thus, although CD4 T-cells do not seem to be directly implicated in tumor rejection, its role in the activation of CD8 T-cells cannot be discarded.
Due to the potent antitumor effect of poly(I:C) and anti-CD40 as adjuvants in prophylactic vaccination experiments, their ability to cure established tumors was then tested. It was found that a single dose of adjuvants plus OVA, either by i.v. or i.t. administration only cured 25 and 36% of mice, respectively, whereas combination of both routes increased the level of tumor rejection to 63%. A transient reduction in tumor volume was detected in some mice that finally could not reject the tumor. Thus, we tried to enhance this insufficient effect by repeated administration. In this case, repeated i.t. administration was able to reject tumors in all mice. However, repeated administration by i.v. or by i.v. and i.t routes had a toxic effect which did not allow us to evaluate the efficacy of these treatment protocols. Similar results regarding toxicity of repeated i.v. administration of anti-CD40 have been reported [38], favoring local i.t. administration as strategy of treatment when using this adjuvant molecule. An interesting finding was the therapeutic effect of i.t. administration of adjuvants in the absence of exogenously added antigen. These results suggest that these adjuvant molecules stimulate innate immune cells infiltrating the tumor through activation of TLR3 and CD40 molecules present not only in DC, but also in NK cells [35] and macrophages [3], providing a rapid mechanism for tumor rejection. This would also explain the higher efficacy of i.t. versus i.v. administration. As demonstrated in experiments using Rag1–/– mice, T-cells are the main effector cells for tumor rejection. However, as shown in Figs. 6e, f, innate immune cells such as NK cells collaborate in tumor rejection, probably by killing tumor cells and providing tumor antigens for their capture and presentation by DC and also by helping in the development of a proinflammatory milieu which would facilitate T-cell functions [8]. According to this, it has been recently reported that the adjuvant effect of poly(I:C) is dependent on the induction of cytokines produced by NK cells [32]. This is reinforced by the finding that i.v. administration of adjuvant plus OVA has a low therapeutic efficacy, probably because the tumor infiltrate and environment is not as activated as in i.t. administration. The role played by T-cells and NK cells in tumor rejection when using poly(I:C) and anti-CD40 as adjuvants is similar to that played when using CpG as adjuvants. In this last case, NK cells are also in part directly responsible for tumor rejection, because in animals depleted of CD8 cells there is still some therapeutic effect [13, 19]. In line with the role of T-cells when using our adjuvants, animals cured after treatment with poly(I:C) and anti-CD40 without any exogenous OVA, are protected against a second challenge, demonstrating that even in the absence of exogenous antigen, long-lasting T-cell responses are induced. Finally, inclusion of antigen in the therapeutic setting increases the efficacy of treatment to 100%, reinforcing the idea that a full activation of T-cells facilitates tumor rejection.
Another important feature of the immunostimulatory properties of this adjuvant mixture is its ability to induce systemic immunity able to eradicate both treated and untreated tumors. As shown in Fig. 7, i.t. administration of adjuvants plus OVA is able to reject a high number of treated tumors, and in some cases it also rejects untreated tumors. Thus, the use of this adjuvant mixture in combination with a known tumor antigen is a promising strategy to treat tumors, and at the same time would help eliminating metastases originated from the main tumor without the need of treating every tumor.
In summary, combination of two DC activating stimuli like poly(I:C) and anti-CD40 with a tumor antigen is able to induce strong antitumor responses, which have clear in vivo effects on prophylactic and therapeutic vaccination settings. We believe that this strategy could be useful for the treatment of tumors and metastasis.
Acknowledgments
This work was supported by grants from Ministerio de Ciencia y Tecnología (SAF2003–04751) to F.B.-C., and by “UTE project CIMA” and Instituto de Salud Carlos III (C03/02) to all authors. A. Zabaleta is recipient of a scholarship from Gobierno de Navarra. The authors thank Dr. I. Melero for his kindly gift of Rag1–/– mice.
Abbreviations
- OVA
Ovalbumin
- TLR
Toll like receptors
- poly(I:C)
Polyinosinic polycytidylic acid
- i.v.
Intravenous
- i.t.
Intratumor
- s.c.
Subcutaneous
References
- 1.Abrams SI, Hodge JW, McLaughlin JP, Steinberg SM, Kantor JA, Schlom J. Adoptive immunotherapy as an in vivo model to explore antitumor mechanisms induced by a recombinant anticancer vaccine. J Immunother. 1997;20:48–59. doi: 10.1097/00002371-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 6.Casares N, Arribillaga L, Sarobe P, Dotor J, de Lopez-Diaz Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 7.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garbi N, Arnold B, Gordon S, Hammerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–5869. doi: 10.4049/jimmunol.172.10.5861. [DOI] [PubMed] [Google Scholar]
- 9.Gerard CM, Baudson N, Kraemer K, Bruck C, Garcon N, Paterson Y, Pan ZK, Pardoll D. Therapeutic potential of protein and adjuvant vaccinations on tumour growth. Vaccine. 2001;19:2583–2589. doi: 10.1016/S0264-410X(00)00486-2. [DOI] [PubMed] [Google Scholar]
- 10.Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Res. 2001;61:4497–4505. [PubMed] [Google Scholar]
- 13.Heckelsmiller K, Rall K, Beck S, Schlamp A, Seiderer J, Jahrsdorfer B, Krug A, Rothenfusser S, Endres S, Hartmann G. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 14.Hock H, Dorsch M, Kunzendorf U, Qin Z, Diamantstein T, Blankenstein T. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA. 1993;90:2774–2778. doi: 10.1073/pnas.90.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway CA, Medzhitov R., Jr Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 17.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 19.Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hammerling GJ. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001;167:5247–5253. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 20.Koschella M, Voehringer D, Pircher H. CD40 ligation in vivo induces bystander proliferation of memory phenotype CD8 T cells. J Immunol. 2004;172:4804–4811. doi: 10.4049/jimmunol.172.8.4804. [DOI] [PubMed] [Google Scholar]
- 21.Lefrancois L, Altman JD, Williams K, Olson S. Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells. J Immunol. 2000;164:725–732. doi: 10.4049/jimmunol.164.2.725. [DOI] [PubMed] [Google Scholar]
- 22.Lipford GB, Hoffman M, Wagner H, Heeg K. Primary in vivo responses to ovalbumin. Probing the predictive value of the Kb binding motif. J Immunol. 1993;150:1212–1222. [PubMed] [Google Scholar]
- 23.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 24.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 25.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/S0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 26.Ochsenbein AF. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002;9:1043–1055. doi: 10.1038/sj.cgt.7700540. [DOI] [PubMed] [Google Scholar]
- 27.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 28.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Adv Cancer Res. 2006;95:115–145. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 29.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/S1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 31.Rotzschke O, Falk K, Stevanovic S, Jung G, Walden P, Rammensee HG. Exact prediction of a natural T cell epitope. Eur J Immunol. 1991;21:2891–2894. doi: 10.1002/eji.1830211136. [DOI] [PubMed] [Google Scholar]
- 32.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimonkevitz R, Colon S, Kappler JW, Marrack P, Grey HM. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984;133:2067–2074. [PubMed] [Google Scholar]
- 35.Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 37.Tartour E, Ciree A, Haicheur N, Benchetrit F, Fridman WH. Development of non-live vectors and procedures (liposomes, pseudo-viral particles, toxin, beads, adjuvantsellipsis) as tools for cancer vaccines. Immunol Lett. 2000;74:45–50. doi: 10.1016/S0165-2478(00)00248-0. [DOI] [PubMed] [Google Scholar]
- 38.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, Melief CJ, Toes RE. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 40.Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64:5850–5860. doi: 10.1158/0008-5472.CAN-04-0063. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 42.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]