Abstract
Malignant histiocytosis (MH) is an aggressive cancer derived from myeloid lineage cells in both dogs and humans. In dogs, the tumor is characterized by the rapid development of metastatic tumors in multiple sites, including especially the lungs and lymph nodes. Humans develop an analogous disease known as Langerhans cell histiocytosis, which primarily affects children and young adults. Because these tumors are often resistant to conventional chemotherapy, there is a need for newer therapeutic approaches. Systemic administration of liposomal clodronate (LC) has been shown to effectively deplete phagocytic cells (e.g., macrophages and dendritic cells) in mice. We investigated therefore whether LC could also be used to treat naturally occurring MH in dogs. First, the susceptibility of canine MH cells to LC-mediated killing was assessed in vitro. Then the clinical safety and effectiveness of LC as a treatment for MH was assessed in a pilot study in five pet dogs with spontaneous MH. We found that canine MH cells were very susceptible to LC-induced apoptotic cell death, whereas other tumor cell lines were resistant to killing by LC. Studies using labeled liposomes demonstrated that susceptibility to LC killing was directly related to the efficiency of liposome uptake. In pet dogs with spontaneous MH, we found that a short course of LC treatment elicited significant tumor regression in two of five treated animals. These findings suggest that liposomal delivery of clodronate and possibly other bisphosphonates may offer an effective new approach to treatment of histiocytic neoplasms in dogs and humans.
Keywords: Cancer, Immune, Bisphosphonate, Macrophage, Dendritic cell
Introduction
Malignant histiocytosis (MH) in dogs (also known as histiocytic sarcoma) is a neoplasm of histiocytic cell origin, which is thought to arise from either macrophage or dendritic cell (DC) precursors [2, 25, 31, 40]. A similar malignancy known as Langerhans cell histiocytosis also develops in humans [5, 13, 41]. Malignant histiocytosis in dogs is rapidly metastatic, and often involves multiple organs including lung parenchyma, bone marrow, spleen, liver, and lymph nodes [20]. Thus, MH in dogs serves as a valuable spontaneous tumor model for Langerhans cell histiocytosis in humans [1, 2, 41]. Malignant histiocytosis in dogs also progresses very rapidly and is uniformly fatal, with reported median survival times of 2–4 months [20, 31, 42]. The rapid and aggressive metastases that develop in dogs with MH disease often render conventional treatment modalities such as radiation therapy and surgery ineffective. Chemotherapy has also been typically unrewarding for treatment of MH in dogs, with treatment responses to corticosteroids and lomustine often transient and incomplete [31]. Given the generally aggressive nature of histiocytic malignancies in both dogs and humans, new approaches to treatment are needed.
Liposomal clodronate (LC) has been used extensively to deplete macrophages in mice for investigation of normal and pathological immune responses in animal models of infection, vaccination, and autoimmune diseases [6, 23, 35, 36]. A large body of literature indicates that systemic administration of LC in mice can elicit rapid and effective macrophage depletion [3, 15, 34–36]. Clodronate is a bisphosphonate drug that kills osteoclasts and other macrophages via induction of apoptosis, possibly mediated by competition with ATP as a substrate for intracellular ATPase [9, 17, 26, 27]. When clodronate is incorporated within liposomes, uptake by phagocytic cells such as macrophages is greatly enhanced, resulting in selective targeting of macrophages for killing [22, 23, 36].
Liposomal clodronate has also been used successfully to treat autoimmune hemolytic anemia (AIHA) in a rodent model and more recently in dogs with spontaneous AIHA [15, 21]. In the study in dogs, LC was safely administered i.v. to dogs and induced functional depletion of macrophages that was sufficient to block destruction of opsonized erythrocytes in vivo. LC also induced in vitro killing of splenic macrophages from dogs [21].
Since MH in dogs is a tumor of macrophage and DC origin, we wondered if LC would be an effective agent for killing MH cells, and also whether the drug could be administered systemically to treat dogs with spontaneous MH. Therefore, we investigated the ability of LC to kill canine MH cells in vitro and assessed mechanisms of cell killing. We also conducted a pilot study to assess the safety and efficacy of LC therapy for treatment of five dogs with advanced MH tumors. We found that LC effectively killed canine MH cells in vitro, primarily via delayed induction of apoptotic cell death. Moreover, preliminary results suggested that LC could be safely administered to dogs with MH and induce antitumor activity in some treated dogs. We conclude therefore that LC has promise as a novel agent for treatment of histiocytic tumors in man and dogs.
Materials and methods
Cell culture and tumor cell lines
The canine MH tumor cell line DH82 and the canine osteosarcoma cell line D17 were both obtained from the American Type Tissue Collection (Gaithersburg, MD, USA) [40]. Two other MH cell lines were established from primary cultures of biopsies obtained from dogs with MH and were a kind gift of Dr. Peter Moore (College of Veterinary Medicine, University of California-Davis, Davis, CA, USA). The canine thyroid carcinoma cell line CTAC was a kind gift of Dr. Stuart Helfand (School of Veterinary Medicine, University of Wisconsin, USA). The canine melanoma cell line Mel-J was derived from primary culture of a dog with malignant melanoma [8]. All cell lines were maintained in MEM (minimal essential medium, Invitrogen, Grand Island, NY, USA) supplemented with 10% heat inactivated fetal bovine serum (Hyclone, Logan, UT, USA), non-essential amino acids, l-glutamine, sodium bicarbonate, penicillin and streptomycin. The cell lines were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Preparation of liposomal clodronate and liposomal PBS
Liposomal clodronate was prepared as previously described [21]. Briefly, phosphatidylcholine and cholesterol (both purchased from Avanti Polar Lipids, Alabaster, AL, USA) were dissolved in chloroform and combined at a 5:1 molar ratio in a glass round bottom tube and dried to completeness overnight in a vacuum lyophilizer (VirTis, Gardiner, NY, USA). To prepare mannose-containing liposomes, p-amino phenyl mannopyranoside (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in methanol and added at 1.75 mg per 25 mg of phosphatidylcholine and dried down together with the phosphatidylcholine and cholesterol lipids. Liposomes were prepared by rehydration in a concentrated solution of clodronate (Sigma-Aldrich) as described previously [21]. Liposomal PBS was prepared in a similar fashion, using a 1.5 M stock of PBS instead of clodronate to rehydrate the liposomes.
For the preparation of fluorescent liposomes, 0.5 mL of a 1 mM solution of BODIPY cholesterol (Invitrogen, Eugene, OR, USA) was added to the phosphatidylcholine and cholesterol lipid solution and dried down prior to rehydration.
MTT assay for cell viability
Cell viability was assessed using the MTT assay, as described previously [32]. Briefly, MTT (thiazolyl blue tetrazolium bromide, Sigma-Aldrich. St Louis, MO, USA) was added to wells containing live cells and incubated for 2 h at 37°C. The cells were then dissolved in a 0.1 N HCl solution in isopropanol and the absorbance was determined using an ELISA plate reader (Multiscan Ascent, Thermo Labsystems, Cambridge, MA, USA). Cell viability was calculated as the mean percent absorbance of the treated wells compared to the mean absorbance of the untreated control wells, with the inverse of this value representing the percentage killing. The percentage specific killing was determined as the difference between the percentage killing elicited by LC and the percentage killing elicited by PBS liposomes.
In vitro assessment of MH cell killing
Cells were pipetted into quadruplicate wells of a 96-well flat bottomed plate at a cell density of 4 × 103 cells/well and this cell density was used throughout the in vitro assays. Cells were allowed to adhere for 24 h and were then treated with LC or L-PBS at volumes of 1, 2.5, or 5% v/v in complete tissue culture medium. The LC concentrations used were determined in serial dilution experiments which demonstrated minimal specific killing at LC concentrations less than 1% v/v, as well as no additional increase in activity at LC concentrations greater than 5% v/v (data not shown). Malignant histiocytosis cells were incubated with LC or PBS liposomes for 72 h, as previously described for killing of mouse macrophages with LC in vitro [23]. In some experiments, MH cells and non-phagocytic tumor cells (carcinoma [CTAC], melanoma [Mel-J], and osteosarcoma [D17]) were also incubated with free clodronate at varying concentrations. Finally, DH82 cells were incubated with either free clodronate or LC at equivalent concentrations, and analyzed via the MTT assay after 72 h of incubation. A concentration of 1.7 mM of free clodronate was calculated to be equivalent to the amount of clodronate contained in 5 µl of LC, based on a previous study done using 99mTc labeled clodronate [36].
Generation of canine monocyte-derived macrophages
Blood monocytes were obtained by plastic adherence from blood of normal healthy dogs after separation of peripheral blood mononuclear cells by Ficoll density separation. The cells were incubated for 3 h at 37°C and then all non-adherent cells were discarded. The cells were cultured in DMEM (Dulbecoo’s modified eagle medium, Invitrogen, Grand Island, NY, USA) supplemented with 1% glutamate, 10% heat inactivated fetal bovine serum, non-essential amino acids, l-glutamine, sodium bicarbonate, penicillin and streptomycin, with the addition of 10% v/v of L929 cell conditioned medium, as described previously [24]. Cells were cultured in medium for 10 days prior to treatment with LC and assessment of cell killing.
Measurement of liposome uptake by flow cytometry
BODIPY-labeled liposomes and flow cytometry were used to quantitate liposome uptake by MH and other tumor cell lines. Cells were re-suspended at a concentration of 5.0 × 105 cells/mL and incubated with serial dilutions of BODIPY-labeled PBS liposomes in complete medium for 4 h at 37°C, with periodic shaking to assure even distribution and uptake of liposomes. The cells were washed twice to remove unbound liposomes. In most experiments, incubation with trypan blue was used to quench the fluorescence emitted by surface bound but non-internalized liposomes prior to analysis by flow cytometry. Briefly, trypan blue quenching was accomplished by incubating samples with trypan blue (50 µl of a 0.008% solution of Trypan Blue (Sigma-Aldrich, St. Louis) diluted in 1 ml of cells) in PBS for 15 min. The percentages of internalized and surface-bound liposomes were calculated by analyzing samples before and after blue quenching. Flow cytometry was done using a Cyan-ADP flow cytometry (Beckman-Coulter, Ft Collins, CO, USA) and analysis was done using Summit software (Beckman-Coulter).
Determination of apoptosis by Annexin V and propidium iodide and flow cytometry
Detection of apoptotic cells was done using an Annexin V and propidium iodide (PI) assay and flow cytometry, as previously described [33, 38]. Briefly, cells in triplicate wells were treated with the indicated volume of liposomes for periods of 12–72 h. A positive control for apoptosis was included with each experiment and consisted of cells incubated for 6 h with a 4.5 μM solution of camptothecin (Sigma-Aldrich). After incubation with LC or L-PBS, cells were detached and washed and then stained with FITC-conjugated Annexin V, according to manufacturer’s directions (BD Biosciences, San Jose, CA, USA). Immediately prior to analysis by flow cytometry, PI was added to the cells to identify dead cells. Early apoptotic cells were defined as Annexin+ and PI−, while cells in mid-apoptosis were defined as Annexin+ and PI+, and cells in late apoptosis were defined as Annexin− and PI+.
Clinical evaluation of LC treatment in dogs with MH
A pilot study of LC therapy for treatment of pet dogs with MH was conducted in five dogs with biopsy-confirmed MH tumors. These studies were approved by the Institutional Animal Care and Use Committee at Colorado State University. Dogs were treated by i.v. administration of LC at a dose of 0.5 ml/kg over a 60-min period, as described previously [21]. The LC treatment was repeated once 2 weeks later in four dogs and a third dog received a total of three infusions administered at 2-week intervals. Dogs were monitored for the first 24 h after treatment for changes in body temperature and heart rate and respiratory rate, while complete blood count and serum biochemistry values were determined prior to administration of subsequent LC doses.
Statistical analysis
Comparison between two treatment groups was done by Student’s paired t test. For comparison of multiple treatment groups, ANOVA was used, followed by Bonferroni’s multiple means comparison test. Analysis was done using GraphPad Prism software (GaphPad, San Diego, CA). Differences were considered statistically significant for p values less than 0.05.
Results
Assessment of MH cell susceptibility to LC-induced killing
Previous studies have shown that administration of LC effectively depletes macrophages in mice, both in vitro and in vivo [3, 15, 23, 34–36]. Therefore, we conducted in vitro assays using three different canine MH cell lines (DH82, MH-1, and MH-2) to determine their susceptibility to LC-induced killing. These cell lines were all derived from dogs with spontaneous MH malignancies and all three have been shown to express characteristics typical of both DC and macrophages [4, 40, 42]. LC was prepared for these studies using phosphatidylcholine liposomes, as described previously [21]. However, in our studies the PC liposomes were also modified by the addition of a mannosylated aminophenyl group, since we found that this modification increased uptake and killing of murine macrophages and canine MH cells (data not shown). Cells were incubated with indicated dilutions of LC for 72 h and cell viability was assessed using MTT assay [32]. Non-specific cytotoxic effects of liposomes were controlled for by using liposomes prepared using concentrated PBS instead of clodronate.
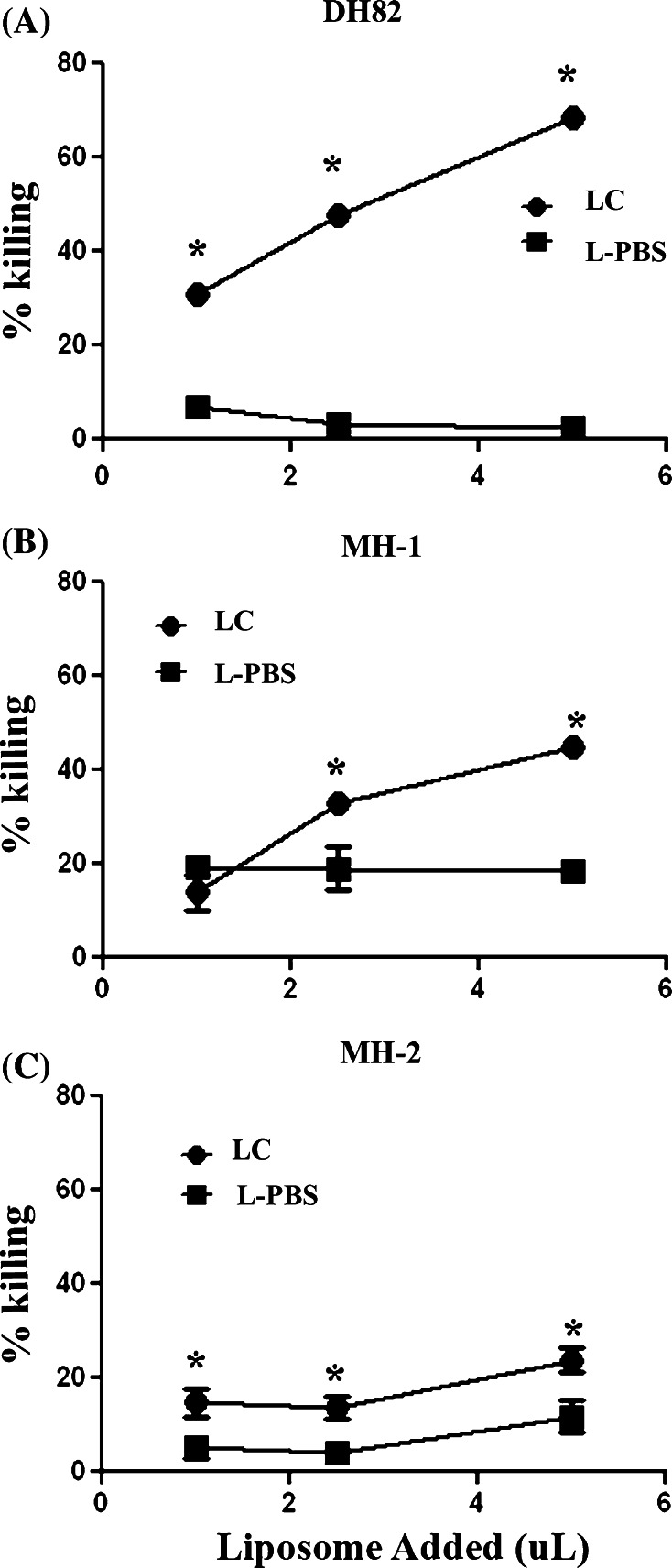
We found that the DH82 cell line was the most susceptibility to killing by LC, compared to the other two MH cell lines (Fig. 1). For example, incubation with 5% LC-induced a 69% loss in cell viability, whereas incubation with 5% L-PBS induced only a 2.5% loss in cell viability. In addition, LC also elicited significant specific killing of the other two MH cell lines, also in a dose-dependent fashion. These data indicated therefore that canine MH cells were highly susceptible to LC killing.
Fig. 1.
Assessment of LC-induced killing of canine MH cells in vitro. Quadruplicate wells containing 4 × 103 MH cells per well were treated with the indicated concentration of liposomal clodronate (LC) or PBS liposomes (L-PBS), incubated for 72 h, then cell viability was assessed by MTT assay, as described in “Methods”. Results are reported as the mean (±SEM) percentage killing. In a–c, the dose-responsiveness of DH82 cells, MH-1 cells, and MH-2 cells, respectively, to incubation with LC and with L-PBS is depicted. Differences in the percentage of killing elicited by treatment with LC and L-PBS were compared at each time point using Student’s t test. (* denotes statistically significant differences of p < 0.05). These data are representative of three independent experiments
Susceptibility of non-phagocytic tumor cells and MH cells to killing by free clodronate
Free clodronate has been shown to kill osteoclasts, which is thought to be the mechanism by which clodronate reduces bone pain associated with skeletal metastases [14, 28, 29]. Moreover, it is also known that free clodronate and other bisphosphonates can elicit cytotoxicity against certain tumor cells [7, 12, 26]. Therefore, we assessed and compared the susceptibility of a variety of non-phagocytic tumor cell lines and MH cell lines to varying concentrations of free clodronate. We also evaluated the susceptibility of MH cells to killing by free clodronate versus LC. For these experiments, we used a free clodronate concentration (1.7 mM) that was calculated to be equivalent to the total amount of clodronate contained in 5 µl of LC, based on determinations from a previous study [36].
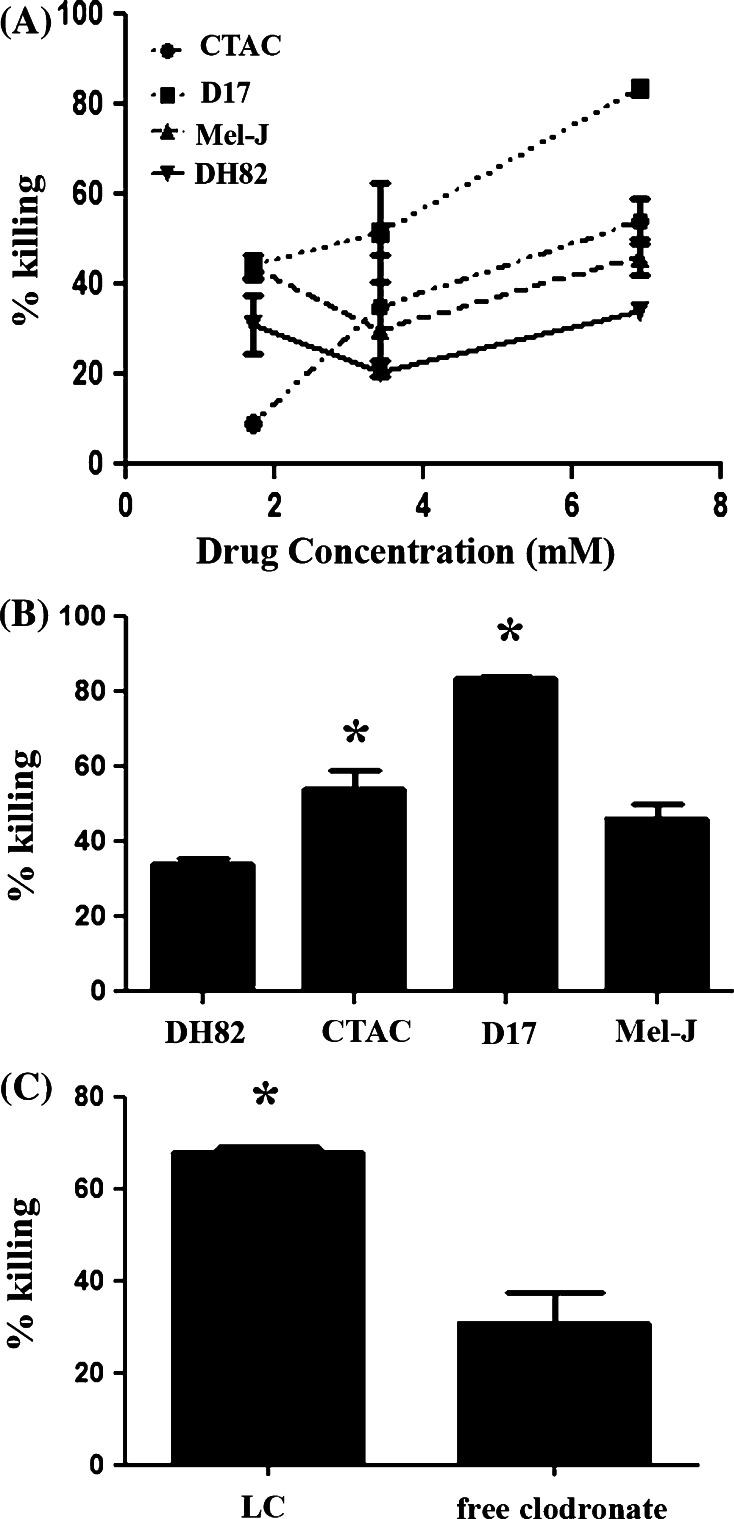
We found that all the tumor cell lines evaluated were susceptible to killing by free clodronate in a dose-dependent manner (Fig. 2a). There were no significant differences in the dose response curves amongst the different cell lines evaluated. Interestingly, two out of the three non-phagocytic cell lines showed significantly more susceptibility to free clodronate than DH82 cells at high concentrations (Fig. 2b). Similar results were obtained for the other two MH cell lines evaluated (MH-1 and MH-2, data not shown).
Fig. 2.
Susceptibility of tumor cells to killing by free clodronate versus liposomal clodronate. Killing of a canine MH cell line (DH82) was compared to killing of three non-phagocytic canine tumor cell lines (thyroid carcinoma {CTAC}, malignant melanoma {Mel-J} and osteosarcoma {D17}). The percentage of killing was determined after 72 h of incubation with varying concentrations of free clodronate (a) or at a maximal concentration of 6.7 mM free clodronate (b). Differences amongst the groups were assessed using ANOVA followed by Bonferroni’s post-test. Although there were no significant differences between the dose response curves (a), there was significantly more killing (*, p < 0.05) in the D17 and CTAC cell lines when compared to DH82 cells at the 6.7 mM concentration of clodronate. In (c), DH82 cells were incubated with a 5% solution of LC or with 1.7 mM solution of free clodronate for 72 h and cell viability was assessed by MTT assay, as described in “Methods”. The percentage specific killing of DH82 cells elicited by LC was determined. Killing of DH82 cells elicited by LC and free clodronate was compared statistically using Student’s t test. Significant differences are denoted by * (p < 0.001). The data shown are representative of three independent experiments
We next compared the degree of killing achieved in MH cells between free clodronate and LC. The degree of killing elicited in MH cells by free clodronate was significantly lower than that generated by LC treatment, when adjusted for addition of equivalent amounts of clodronate. In addition, given the rapid distribution of free clodronate into bone following systemic administration, it is very unlikely that such a high, sustained dose of clodronate or other bisphosphonate drug could be attained in vivo [16, 18, 39].
Effects of LC treatment on non-phagocytic tumor cell lines
Experiments were conducted next to determine whether LC was capable of killing non-phagocytic tumor cell lines as effectively as the phagocytic MH cell lines. Therefore, killing of three non-phagocytic canine tumor cell lines by LC, including carcinoma (CTAC cells), sarcoma (D17 cells) and melanoma (Mel-J cells) cell lines was compared to killing of MH cell lines.
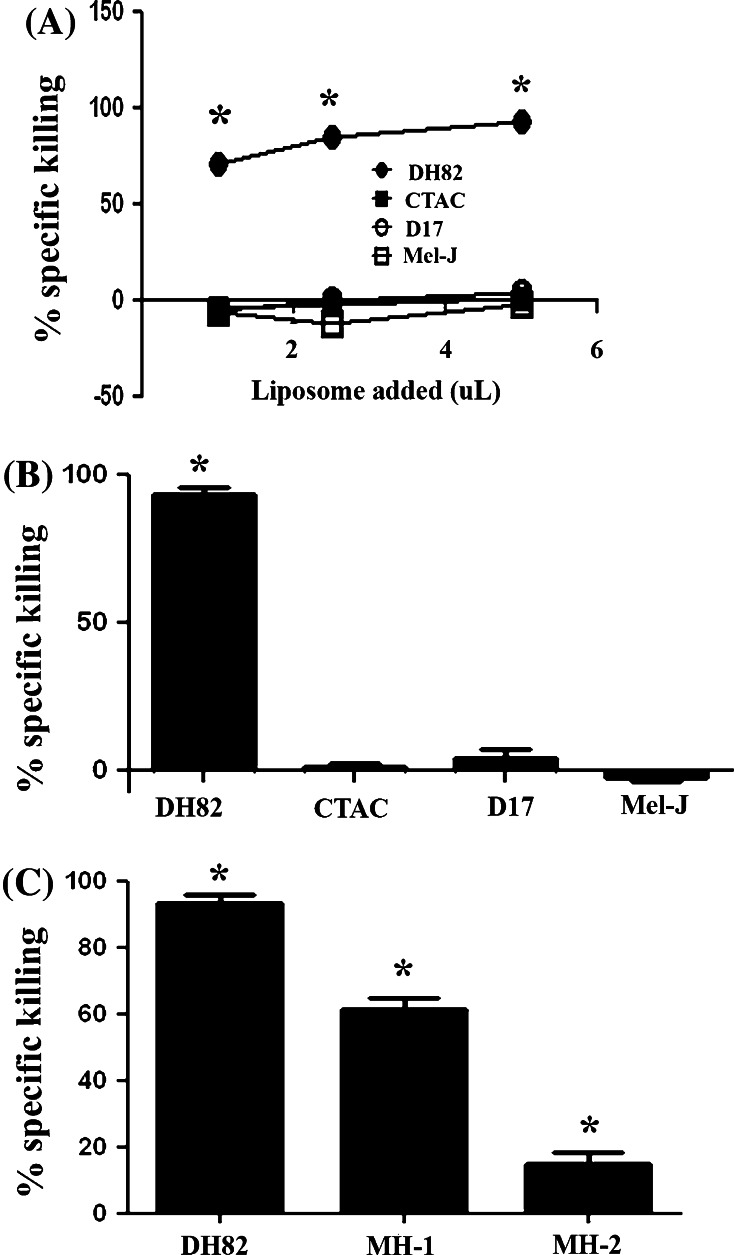
We found that all three non-phagocytic cell lines were relatively refractory to specific killing by LC at all doses evaluated (Fig. 3). However, when incubated with free clodronate, all three of these tumor cell lines were susceptible to cell killing by clodronate (Fig. 2). These results indicated therefore that the resistance of non-phagocytic cells to killing by LC was not mediated by inherent resistance to the cytotoxic effects of clodronate, but was instead more likely related to decreased uptake of LC.
Fig. 3.
Susceptibility of phagocytic and non-phagocytic tumors to killing by LC. Killing of canine MH cell lines (DH82, MH-1, MH-2) by LC was compared to killing of three non-phagocytic canine tumor cell lines (thyroid carcinoma {CTAC}, malignant melanoma {Mel-J} and osteosarcoma {D17}). The percentage specific killing was determined as the difference between the percentage killing elicited by LC and the percentage killing elicited by PBS liposomes for all cell lines shown. In (a), dose response curves of LC percentage specific killing were determined for the DH82 cell line and three non-phagocytic tumor cell lines (CTAC, D17, Mel-J). In (b), the percentage specific killing elicited by 5% LC was plotted for MH cells (DH82) and three non-MH tumor cell lines. Specific killing was significantly greater (*, p < 0.05) for DH82 cells than for the other three tumor cell lines, as assessed by ANOVA, followed by Bonferroni’s post-test. In (c), LC-specific killing of three different MH cell lines was compared, using LC at a concentration of 5%. Killing was significantly greater (*, p < 0.05) for DH82 cells than for the other two MH cell lines, as assessed by ANOVA with Bonferroni’s post-test. The data shown are representative of three independent experiments
We also compared the relative susceptibility of the three different canine MH cell lines to killing by LC and found that there were substantial differences between the three lines (Fig. 3). For example, the DH82 cell line was significantly more susceptible to LC-mediated killing than either the MH-1 or MH-2 cell lines. These findings indicated that there might be substantial tumor-to-tumor heterogeneity in susceptibility to LC killing in dogs with MH tumors, or potentially in humans with Langerhans histiocytosis.
Susceptibility of canine monocyte-derived macrophages to killing by LC
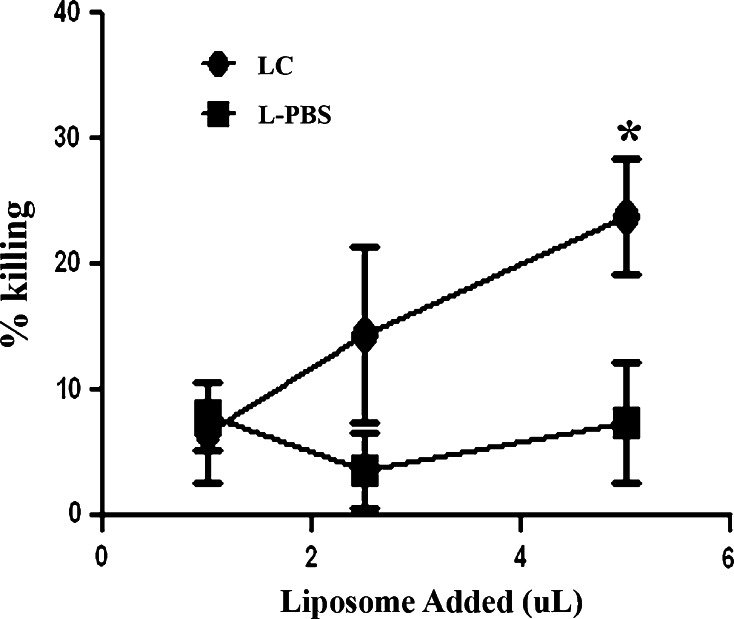
The preceding experiments established that MH cells were very susceptible to killing by LC. However, we also wished to compare the relative LC susceptibility of malignant MH cells (derived from macrophages and/or DC) and non-transformed canine macrophages. Therefore, primary cultures of canine monocyte-derived macrophages were established and the cells were incubated with LC for 72 h and cell killing was assessed. We found that LC-induced significant specific killing of canine monocyte-derived macrophages (Fig. 4). Interestingly, two of the three canine MH cell lines were much more susceptible to LC killing than monocyte-derived macrophages. For example, at a 5% concentration of LC, 32% specific killing of monocyte-derived macrophages was observed, whereas there was 69% killing of DH82 cells and 45% killing of MH-1 cells. Thus, MH cells may be inherently more susceptible to LC killing than normal macrophages.
Fig. 4.
Killing of primary monocyte-derived macrophages by LC. Monocyte-derived macrophages (MDM) were generated from the blood of healthy dogs as described in “Methods”. After 7 days in culture, the MDM were treated with LC or PBS liposomes (L-PBS) and cell viability was analyzed 72 h later by MTT assay. Cell viability following incubation with 5% LC or 5% L-PBS at each time point was statistically compared using Student’s t test. Significant differences (p < 0.05) were denoted by *. The data shown are representative of three independent experiments
Assessment of liposome uptake by MH and non-MH cells
In the preceding experiments, heterogeneity in MH cell susceptibility to killing by LC was observed (see Fig. 1). In addition, we also found that the resistance of non-phagocytic cells to killing by LC was not due to inherent resistance to the cytotoxic effects of free clodronate (data not shown). Therefore, the observed differences in susceptibility to LC killing could likely be accounted for in part by differences in liposome uptake. To address this question, fluorescently labeled liposomes were used to compare liposome uptake by MH cell lines and by non-MH tumor cell lines.
Cells were incubated in suspension at a concentration of 5.0 × 105 cells/mL with PBS liposomes labeled with the fluorescent dye BODIPY, as described in “Methods”. After 4 h of incubation, the cells were washed and analyzed for uptake of labeled liposomes by flow cytometry. To distinguish surface-bound (i.e., non-internalized) liposomes from internalized liposomes, trypan blue was used to quench fluorescence by surface-bound liposomes, as noted previously [19].
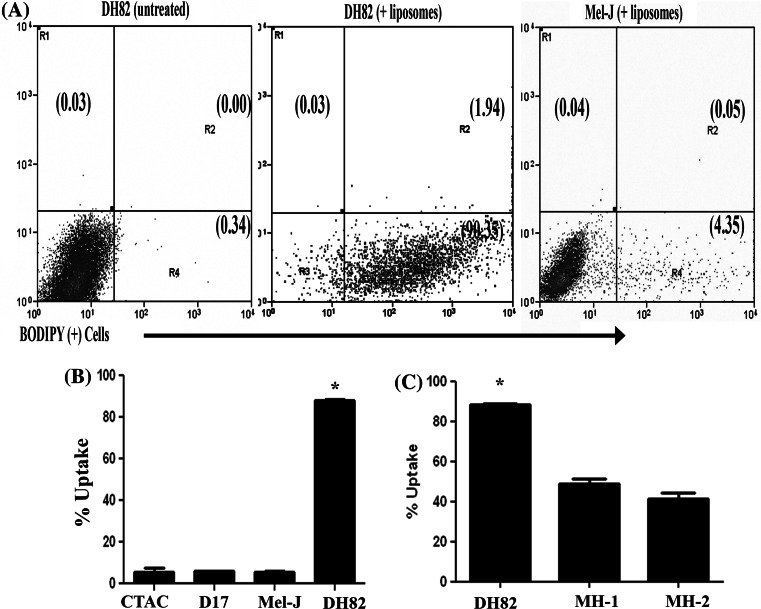
Liposome uptake by all three MH cells was significantly higher than uptake by the three non-MH cell lines (Fig. 5). For example, the percentage of cells that contained internalized liposomes after 4 h incubation was almost 90% in DH82 cells, whereas uptake by each of the three non-MH cell lines was less than 10% of the total cell population. These results suggested therefore that difference in susceptibility to LC-induced cell killing could be explained almost entirely by differences in the efficiency of phagocytosis and liposome uptake.
Fig. 5.
Comparison of liposome uptake by MH and non-MH tumor cell lines. Flow cytometry and fluorescently labeled liposomes were used to assess liposome uptake and internalization. Cells were incubated with BODIPY-conjugated PBS liposomes for 4 h at 37°C, then washed and quenched with trypan blue to exclude surface-bound liposomes, followed by evaluation by flow cytometry, as described in “Methods”. In (a), representative dot plots of untreated DH82 cells (left panel), DH82 cells incubated with labeled liposomes (middle panel), and canine melanoma cells (Mel-J) incubated with liposomes (right panel) are shown. In (b), the mean percentages (±SEM) of liposome+ tumor cells were plotted. The percentage of DH82 cells containing internalized liposomes was significantly higher than for either of the three canine non-MH tumor cell lines, as assessed by ANOVA with Bonferroni’s post-test (*; p < 0.05). In (c), the mean percentage of DH82 cells containing internalized liposomes was significantly higher than for the other two canine MH tumor cell lines (MH-1 and MH-2), as assessed by ANOVA with Bonferroni’s post-test (*; p < 0.05). These data shown are representative of three independent experiments
The efficiency of liposome uptake was also significantly different within the three MH cell lines. For example, the DH82 line exhibited significantly greater uptake of labeled liposomes than did either of the other two MH cell lines (Fig. 5). The efficiency of liposome uptake also correlated directly with the efficiency of LC killing in the three MH cell lines (see Fig. 1). The decreased susceptibility of the MH-1 and MH-2 cells to LC killing was not, however, due to inherent resistance to the effects of free clodronate itself, as the MH1 and MH2 cell lines were actually more susceptible to free clodronate than the DH82 cells (Fig. 2 and data not shown). Therefore, the relative ability to phagocytose liposomes appeared to be a primary determinant of the susceptibility of different MH cell lines to LC-mediated cell killing. This result suggests that highly phagocytic MH tumors would be more susceptible to treatment with LC treatment than less phagocytic tumors.
Mechanisms of cell death induced by LC treatment
Previous studies using murine macrophages have shown that LC-induced cell death was mediated primarily by induction of apoptosis, though with delayed kinetics relative to apoptosis induced by many chemotherapy agents [26, 37]. Therefore, we investigated whether apoptosis might also account for LC-induced cell death in canine MH cells. For these assays, MH cells were incubated with LC for varying lengths of time, then stained with Annexin V and PI and analyzed via flow cytometry to identify apoptotic cells, as described in “Methods”.
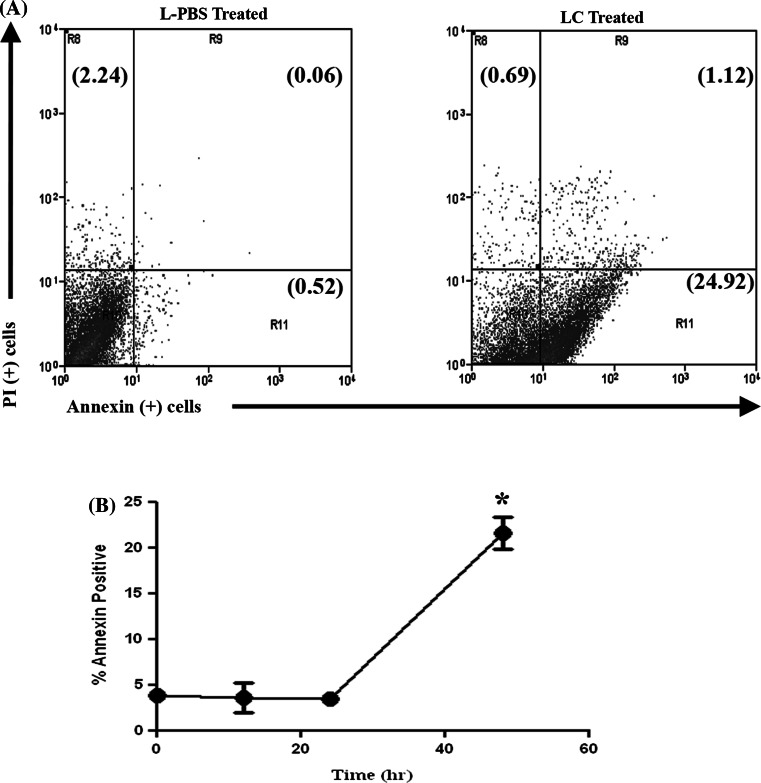
Treatment with LC-induced a significant increase in the percentage of early apoptotic and mid-apoptotic cells (Fig. 6). For example, the percentage of early apoptotic cells increased from 3.5% (±0.6%) of cells prior to treatment to 21.6% (±1.7%) of cells following 48 h of incubation with LC. The MH-1 and MH-2 cell lines also underwent apoptosis following LC treatment, with similar kinetics as for DH82 cells (data not shown). Thus, the majority of cell death induced in MH cells by LC treatment appeared to be mediated by induction of apoptosis.
Fig. 6.
Induction of apoptosis in MH cells following treatment with LC. DH82 cells were incubated with 5% LC or L-PBS for the indicated time period and induction of apoptosis was assessed using flow cytometry and Annexin V and propidium iodide (PI), as noted in “Methods”. In (a), representative dot plot at 48 h of DH82 MH cells treated with LC or L-PBS. In (b), the time course of induction of apoptosis was assessed, using DH82 cells. The mean percentage of Annexin V positive cells (which included both early and mid-apoptosis cells) at varying time points after treatment with LC was determined. The percentage of apoptotic cells at each time point was compared by Student’s t test with values for the 0 h control; (*, p = 0.001). These data are representative of three independent experiments
The kinetics of induction of apoptosis by LC in MH cells was examined next. These studies were prompted by the fact that induction of cell death (as assessed by cell viability assay) was relatively slow following incubation with LC and did not become apparent in the first 12–24 h of incubation. For example, classical inducers of apoptosis in macrophages such as staurosporine or camptothecin induced large increases in apoptosis within 6–8 h of incubation. In contrast, maximal induction of apoptosis in MH cells was not observed until 48 h after treatment with LC (Fig. 6). The delay in induction of apoptosis by LC in MH cells could be related the mechanism of action of clodronate, which involves competitive inhibition for ATP binding, a process that would be expected to induce cell death relatively slowly [14]. In addition, uptake and release of the contents of the clodronate containing liposomes into the MH cell cytoplasm may have also been a rate limiting factor.
Clinical evaluation of LC as a therapeutic for treatment of histiocytic cancer
The preceding experiments indicated that LC was an effective agent for killing MH cells in vitro. Clinically, MH in dogs is typically refractory or only moderately susceptible to treatment with conventional chemotherapy drugs [2, 25]. As a consequence, most dogs with MH are euthanized within weeks of diagnosis. Our in vitro studies indicated that LC had significant activity against MH cell lines. Thus, it was reasonable to propose that systemic administration of LC might be used therapeutically in dogs with spontaneous MH tumors. Fortunately, a safe and effective dose for i.v. administration of LC to healthy dogs and dogs with spontaneous autoimmune hemolytic anemia had been established by our group recently [21]. Therefore, we conducted a pilot study to evaluate the safety and potential efficacy of LC administration as a new approach to treatment of MH and potentially other histiocytic neoplasms.
Five pet dogs MH were enrolled in a clinical trial to evaluate the use of LC for treatment of MH. All of the dogs had previously failed conventional chemotherapy, including treatment with predisone and lomustine. Dogs enrolled in the LC study were treated every other week by i.v. infusion of LC, using a dose of 0.5 ml/kg established in an earlier study [21]. The infusion was administered slowly over a 60-min period through a peripheral intravenous catheter. Animals were monitored during the infusion for acute adverse effects (respiratory, heart rate, blood pressure) and for the next 8 h for side-effects such as fever and respiratory and cardiovascular signs. Additional treatments were administered at 2-week intervals, using the same LC dose and delivery schedule.
Dog 1
A spayed female mixed-breed dog was diagnosed with a large subcutaneous MH tumor on the shoulder. The tumor had not responded to two prior treatments with lomustine. The dog received two i.v. infusions of LC administered 2 weeks apart. The dog developed significant fever within 12 h of administration of each of the two LC treatments and the febrile episodes lasted for approximately for 24 h. Other adverse effects were not noted. The dog was evaluated for a period of a month following the two LC treatments, but no objective tumor response was noted.
Dog 2
A castrated, mixed-breed dog developed a cutaneous MH located on the flank. The tumor did not respond to prior treatment with prednisone. The dog received two i.v. infusions of LC, given 2 weeks apart, with no evidence of treatment related adverse effects. During the 4-week treatment period, objective tumor responses were not noted and treatment was therefore discontinued. However, when the dog was re-examined 10 months later, the previously noted cutaneous MH tumor was observed to have completely regressed. This dog also had a large solitary pulmonary mass that was present at the time of initial MH diagnosis, though the lung tumor was not biopsied. The lung tumor did not respond to treatment and continued to grow slowly. Based on the tumor location, solitary nature, and slow growth rate it was considered to most likely be a primary lung tumor, though this was not confirmed by histopathology.
Dog 3
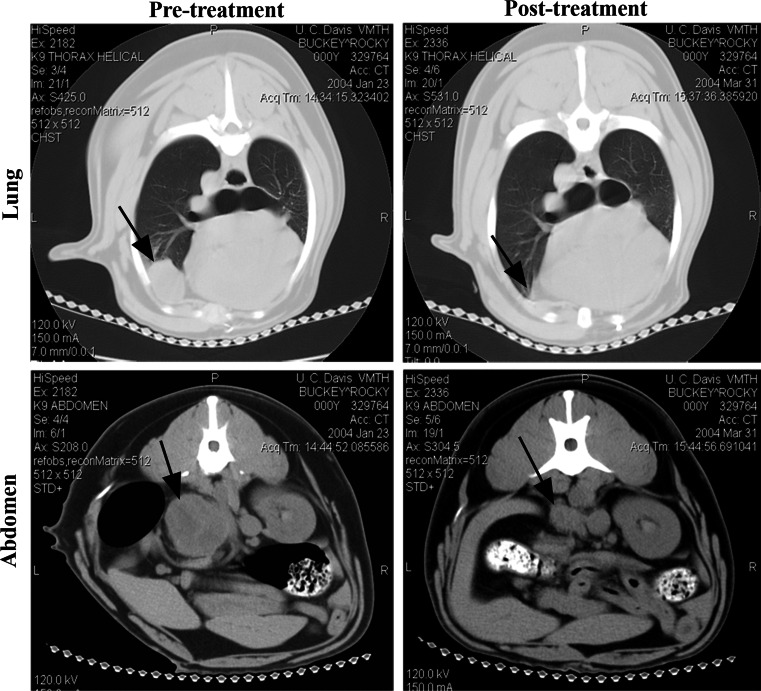
A castrated male Golden Retriever dog developed metastatic MH involving the lungs, adrenal glands, and liver, which was noted on CT scan (Fig. 7). Prior treatment with prednisone and lomustine had not produced objective tumor responses. The dog then received two LC infusions, 2 weeks apart. Adverse effects related to the LC infusions were not noted.
Fig. 7.
Tumor responses following LC treatment in a dog with malignant histiocytosis. A dog with metastatic MH was evaluated by CT imaging. Prior to treatment (left panels), there were large metastases in the lungs (upper panel, arrow) and the adrenal glands (bottom panel, arrow). The dog was then treated with two i.v. infusions of LC 2 weeks apart, as described in “Methods”. When CT imaging was repeated 3 months later (right panels), there was marked reduction in the size of both lung and adrenal metastases. At necropsy 5 months later, there was no evidence of MH in either the lung or adrenal sites
On follow-up CT scan taken 10 weeks after the last LC treatment, significant tumor regression at multiple sites was noted (Fig. 7). Additional treatments were not administered and the dog was followed up with routine rechecks. Five months after completion of LC treatment, the dog developed fatal cardiac arrhythmias and was humanely euthanized. On post-mortem examination of the lungs and adrenal glands, there was no histologic evidence of the prior biopsy-confirmed MH tumors. However, MH was found in the left ventricle of the heart, which was presumed to be the cause of the fatal arrhythmia.
Dog 4
A spayed female Bernese Mountain Dog developed enlarged hilar lymph nodes, a caudal lung mass and a cranial mediastinal lung mass. Cytologic examination of aspirates of the masses were consistent with a diagnosis of MH. Prior treatment with carboplatin and prednisone had elicited only minimal tumor responses. The dog received two LC treatments, given 2 weeks apart. During the 4-week treatment period, adverse effects were not observed. However, at the end of this period, an objective tumor response was not noted and the treatment was therefore discontinued.
Dog 5
A castrated male Bernese Mountain Dog was diagnosed by thoracic radiographs and biopsy with pulmonary metastatic MH. Prior treatment with prednisone and lomustine had not produced a significant tumor response. The dog was therefore treated with two LC infusions, administered 2 weeks apart. During the 4-week treatment period, adverse effects were not observed. However, at the end of the treatment period, an objective tumor response was not noted on repeat thoracic radiographs and the treatment was therefore discontinued.
Discussion
Previous studies have shown that LC is an effective macrophage depleting agent in rodents, following either systemic or local injection [6, 23, 35, 36]. In addition, the clinical potential for LC treatment to be used as a treatment of autoimmune diseases has been evaluated in rodent models and in a study in pet dogs with autoimmune hemolytic anemia [3, 15, 21]. There are also several relatively recent reports evaluating the use of LC treatment in mouse tumor models [11, 43]. However, the current study is the first to our knowledge to investigate the potential for systemically administered LC therapy to be used for treatment of histiocytic neoplasms, particularly inasmuch as the studies were conducted in a large animal spontaneous tumor model.
Notably, we found that systemic LC therapy was well-tolerated in dogs with MH, even those with advanced disease and large tumor burdens. In addition, these preliminary studies demonstrated that LC administration was capable of inducing significant tumor responses in some treated animals. Thus, these results suggest that liposome-encapsulated bisphosphonate therapy warrants further evaluation as a potential treatment for histiocytic malignancies such as Langerhans cell histiocytosis in humans and dogs.
In vitro, LC was found to be very effective at killing canine MH cells. Liposome uptake studies also revealed that only phagocytic tumors were susceptible to the effects of LC-induced killing, even though non-phagocytic cells were in some cases more susceptible to the non-liposome encapsulated drug. Thus, selective targeting of MH cells for killing might explain in part the antitumor activity we observed in our pilot study of LC therapy for treatment of MH in dogs. However, it should be noted that the antitumor activity we observed could also be attributed to indirect effects of LC therapy on the MH tumors. For example, recent studies have demonstrated that repeated LC administration is capable of depleting both tumor associated macrophages and myeloid suppressor cells [11, 30, 43]. We have also observed antitumor activity following i.v. administration of LC in several different non-histiocytic mouse tumor models (manuscript in preparation). Thus, the antitumor activity elicited following LC administration to dogs with MH may have been mediated by a combination of both direct and indirect tumor effects.
The variability in MH susceptibility to LC treatment observed in the three canine MH cells lines in our study also suggested that tumor heterogeneity may have an important impact on response to treatment. We have observed similar heterogeneity in responsiveness to LC in several different mouse macrophage cell lines, with more differentiated macrophages appearing to have greater susceptibility to LC killing (S. Hafeman et al., unpublished data). The liposome uptake studies suggested that variability in susceptibility to LC killing could be largely attributed to variations in efficiency of liposome uptake. Though only small numbers of dogs were evaluated, it is noteworthy that none of the two Bernese Mountain dogs with MH responded to treatment with LC, suggesting that perhaps the tumor in this breed of dogs may be more resistant to the effects of LC treatment.
The mechanism of LC-induced cell death in phagocytic cells is also relevant to the design of more effective clodronate analogues and to the safety of such drugs in vivo. Our results indicated that LC killed MH cells primarily by inducing apoptosis, but with unusual delayed kinetics. For example, evidence of apoptosis and cell death did not become evident until after 48 h of incubation with LC (see Fig. 6), which is much longer than required for induction of apoptosis by camptothecin (6 h). Others have also observed delayed macrophage apoptosis and cell death following LC treatment [37]. The reasons for this delay in in vitro apoptosis are not immediately apparent, but are probably related to the mechanisms of action of clodronate [10]. Curiously, administration of LC to mice elicits substantial elimination of splenic macrophages within 18–24 h of administration [21, 34–36]. Thus, there are important and as yet unexplained differences between the in vitro and in vivo behavior of LC with respect to induction of macrophage apoptosis.
In summary, LC was found to be an effective agent for inducing cell death in histiocytic tumor cells of dogs. Preliminary pilot studies in dogs with spontaneous MH, including animals with advanced tumor metastases, also suggested in vivo efficacy of LC against histiocytic malignancies. We concluded therefore that additional studies for treatment of cancer were warranted and that liposomal delivery of bisphosphonate drugs may represent a promising approach to treatment of certain histiocytic neoplasms.
Acknowledgments
The authors wish to acknowledge the assistance of Dr. Amanda Guth with flow cytometry. This work was supported by grants from the Canine Health Foundation and by an NIH Training Grant (T32 RR00707).
References
- 1.Affolter VK, Moore PF. Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol. 2000;22:40–48. doi: 10.1097/00000372-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- 3.Alves-Rosa F, Stanganelli C, Cabrera J, van Rooijen N, Palermo MS, Isturiz MA. Treatment with liposome-encapsulated clodronate as a new strategic approach in the management of immune thrombocytopenic purpura in a mouse model. Blood. 2000;96:2834–2840. [PubMed] [Google Scholar]
- 4.Bird RC, Deinnocentes P, Lenz S, Thacker EE, Curiel DT, Smith BF. An allogeneic hybrid-cell fusion vaccine against canine mammary cancer. Vet Immunol Immunopathol. 2008;123:289–304. doi: 10.1016/j.vetimm.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Bucsky P, Egeler RM. Malignant histiocytic disorders in children. Clinical and therapeutic approaches with a nosologic discussion. Hematol Oncol Clin North Am. 1998;12:465–471. doi: 10.1016/S0889-8588(05)70523-2. [DOI] [PubMed] [Google Scholar]
- 6.Cecchini MG, Felix R, Fleisch H, Cooper PH. Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J Bone Miner Res. 1987;2:135–142. doi: 10.1002/jbmr.5650020209. [DOI] [PubMed] [Google Scholar]
- 7.Clezardin P, Fournier P, Boissier S, Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Curr Med Chem. 2003;10:173–180. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 8.Dow SW, Elmslie RE, Willson AP, Roche L, Gorman C, Potter TA. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest. 1998;101:2406–2414. doi: 10.1172/JCI510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44:2201–2210. doi: 10.1002/1529-0131(200109)44:9<2201::AID-ART374>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 11.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 12.Green JR. Antitumor effects of bisphosphonates. Cancer. 2003;97:840–847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 13.Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278–2290. doi: 10.1002/(SICI)1097-0142(19990515)85:10<2278::AID-CNCR25>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MB, van Rooijen N, Izui S, Kappler J, Marrack P. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood. 2003;101:594–601. doi: 10.1182/blood-2001-11-0061. [DOI] [PubMed] [Google Scholar]
- 16.Lauren L, Osterman T, Karhi T. Pharmacokinetics of clodronate after single intravenous, intramuscular and subcutaneous injections in rats. Pharmacol Toxicol. 1991;69:365–368. doi: 10.1111/j.1600-0773.1991.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, Azhayev A, Vaananen HK, Hassinen IE. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 18.Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 1996;18:75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 19.Loike JD, Silverstein SC. A fluorescence quenching technique using trypan blue to differentiate between attached and ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J Immunol Methods. 1983;57:373–379. doi: 10.1016/0022-1759(83)90097-2. [DOI] [PubMed] [Google Scholar]
- 20.MacEwen EG, Withrow SJ. Small animal clinical oncology. Philadelphia, PA: Saunders Co; 1996. [Google Scholar]
- 21.Mathes M, Jordan M, Dow S. Evaluation of liposomal clodronate in experimental spontaneous autoimmune hemolytic anemia in dogs. Exp Hematol. 2006;34:1393–1402. doi: 10.1016/j.exphem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Monkkonen J, Heath TD. The effects of liposome-encapsulated and free clodronate on the growth of macrophage-like cells in vitro: the role of calcium and iron. Calcif Tissue Int. 1993;53:139–146. doi: 10.1007/BF01321893. [DOI] [PubMed] [Google Scholar]
- 23.Monkkonen J, Taskinen M, Auriola SO, Urtti A. Growth inhibition of macrophage-like and other cell types by liposome-encapsulated, calcium-bound, and free bisphosphonates in vitro. J Drug Target. 1994;2:299–308. doi: 10.3109/10611869409015910. [DOI] [PubMed] [Google Scholar]
- 24.Moore KJ, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 25.Moore PF, Affolter VK, Vernau W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006;43:632–645. doi: 10.1354/vp.43-5-632. [DOI] [PubMed] [Google Scholar]
- 26.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12:6222s–6230s. doi: 10.1158/1078-0432.CCR-06-0843. [DOI] [PubMed] [Google Scholar]
- 27.Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Selander K, Lehenkari P, Vaananen HK. The effects of bisphosphonates on the resorption cycle of isolated osteoclasts. Calcif Tissue Int. 1994;55:368–375. doi: 10.1007/BF00299317. [DOI] [PubMed] [Google Scholar]
- 29.Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK. Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol. 1996;50:1127–1138. [PubMed] [Google Scholar]
- 30.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Skorupski KA, Clifford CA, Paoloni MC, Lara-Garcia A, Barber L, Kent MS, LeBlanc AK, Sabhlok A, Mauldin EA, Shofer FS, Couto CG, Sorenmo KU. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Int Med. 2007;21:121–126. doi: 10.1892/0891-6640(2007)21[121:CFTTOD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(SICI)1097-0320(19980101)31:1<1::AID-CYTO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.van Rooijen N, Kors N. Effects of intracellular diphosphonates on cells of the mononuclear phagocyte system: in vivo effects of liposome-encapsulated diphosphonates on different macrophage subpopulations in the spleen. Calcif Tissue Int. 1989;45:153–156. doi: 10.1007/BF02556058. [DOI] [PubMed] [Google Scholar]
- 35.van Rooijen N, Kors N, ter Hart H, Claassen E. In vitro and in vivo elimination of macrophage tumor cells using liposome-encapsulated dichloromethylene diphosphonate. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54:241–245. doi: 10.1007/BF02899217. [DOI] [PubMed] [Google Scholar]
- 36.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 37.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 38.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 39.Villikka K, Perttunen K, Rosnell J, Ikavalko H, Vaho H, Pylkkanen L. The absolute bioavailability of clodronate from two different oral doses. Bone. 2002;31:418–421. doi: 10.1016/S8756-3282(02)00841-4. [DOI] [PubMed] [Google Scholar]
- 40.Wellman ML, Krakowka S, Jacobs RM, Kociba GJ. A macrophage-monocyte cell line from a dog with malignant histiocytosis. In Vitro Cell Dev Biol. 1988;24:223–229. doi: 10.1007/BF02623551. [DOI] [PubMed] [Google Scholar]
- 41.Willis B, Ablin A, Weinberg V, Zoger S, Wara WM, Matthay KK. Disease course and late sequelae of Langerhans’ cell histiocytosis: 25-year experience at the University of California, San Francisco. J Clin Oncol. 1996;14:2073–2082. doi: 10.1200/JCO.1996.14.7.2073. [DOI] [PubMed] [Google Scholar]
- 42.Zavodovskaya R, Liao AT, Jones CL, Yip B, Chien MB, Moore PF, London CA. Evaluation of dysregulation of the receptor tyrosine kinases Kit, Flt3, and Met in histiocytic sarcomas of dogs. Am J Vet Res. 2006;67:633–641. doi: 10.2460/ajvr.67.4.633. [DOI] [PubMed] [Google Scholar]
- 43.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]