Abstract
Background
Recent reports have demonstrated that the enzyme indoleamine 2,3-dioxygenase (IDO) is upregulated in human dendritic cells (DCs) upon in vitro maturation. IDO is supposed to convey immunosuppressive effects by degrading the essential amino acid tryptophan, thereby downregulating T-cell functions. Hence, we evaluated IDO expression in DC preparations used for therapeutic DC vaccination and its in vivo effects.
Patients, methods and results
IDO expression was detected by real-time-PCR in a series of human clinical grade DCs (n = 28) prior to vaccination of advanced melanoma patients (n = 11). These analyses revealed an intra- and interpersonal variation in IDO mRNA levels. IDO was strongly upregulated in human DCs on RNA and on protein level upon in vitro maturation by Interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), Interleukin-6 (IL-6) and Prostaglandin E2 (PGE2) over a time course of 24 h. The enzymatic activity of induced IDO was demonstrated by measuring tryptophan degradation. Moreover, in biopsies obtained 24 h after application of the DC vaccine a prominent infiltrate of IDO-positive cells was observed by immunohistochemistry. The inflammatory infiltrate of these sites stained positive for the transcription factor Forkhead box P3 (FoxP3), suggesting an IDO-mediated induction of regulatory T-cells. All analysed melanoma patients (n = 11) receiving DC based immunotherapy exhibited rapid disease progression with a short overall survival due to advanced tumour stage.
Conclusion
The presented observations suggest a potential clinical relevance of IDO expression in DC-based therapeutic vaccines via the attraction or induction of FoxP3+ T-cells.
Keywords: Indoleamine 2,3-dioxygenase (IDO); Dendritic cell (DC); Immunotherapy
Introduction
Immunological strategies to fight cancer have demonstrated less clinical efficacy than anticipated. Several factors contribute to this status quo, including immune escape mechanisms of tumours or the limited immunogenicity of the antigen delivery systems [1]. One potential key mechanism, leading to immunological tolerance, is the recently recognised immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) [2]. IDO is a heme-containing enzyme which catalyses the initial, rate limiting-step in the degradation of the essential amino acid tryptophan into distinct kynurenine metabolites [3]. Depletion of tryptophan renders T-cells more susceptible to apoptosis [4]. Moreover, various tryptophan downstream metabolites, e.g. kynurenine and quinolinate, themselves are directly toxic for T-cells [5]. IDO-expressing, tolerizing antigen presenting cells (APCs) are furthermore supposed to induce regulatory T-cells (Tregs) [6]. Clinically relevant immunoregulatory functions of IDO include the protection from intracellular pathogens [7], maintenance of maternal tolerance towards the foetus during pregnancy [8], suppression of T-cell responses to MHC-mismatched allografts [9], protection from autoimmune diseases [10] and—most important—tumour resistance to cytotoxic CD8+ T-lymphocytes [11, 12]. In this respect, IDO is expressed by two complementary constituents both in the tumour microenvironment as well as in the regional draining lymph nodes: the malignant cells themselves and subsets of APCs [13]. Thereby, both local and systemic tolerance to neoplastic cells may be generated and maintained.
IDO-expression in APCs and its complex modulation by various cytokines and direct cellular interactions may become an issue for dendritic cell (DC)-based vaccine therapies. Indeed, previous reports have demonstrated that functional IDO is induced upon in vitro generation of mature DCs [14, 15]. Thus, we analysed IDO expression, its activity as well as its in vivo relevance for melanoma patients receiving DC-based vaccinations [16, 17].
Patients, materials and methods
Patient characteristics
Eleven patients suffering from metastasised melanoma, which were treated by DC-based vaccinations, were included into this advanced immunmonitoring. Informed consent was obtained from each patient prior to study inclusion and any subsequent measures. All procedures were approved by the local ethics committee. The study details have been described before [17]; patients received autologous peptide-pulsed monocyte-derived DCs administered subcutaneously every 2 weeks for the first five vaccinations, followed by vaccinations in 4-weekly intervals [16]. Detailed patient characteristics are summarised in Table 1.
Table 1.
Patient characteristics of 11 melanoma patient treated with peptide-loaded dendritic cells
| P | Sex | Age | AJCC | Primary tumour | Metastatic sites | Objective response | TTP (months) |
|---|---|---|---|---|---|---|---|
| 1 | f | 40 | M1c | Uvea melanoma | Liver | PD | 3 |
| 2 | m | 52 | M1c | NM, CLIV, 2.5 mm | Lung, kidney, soft tissue | PD | 1 |
| 3 | m | 51 | M0 | NM, CL III, 0.42 mm | |||
| 4 | m | 71 | M1c | SSM, CL IV, 1.3 mm | Liver, lung, LN | PD | 2 |
| 5 | m | 38 | M1c | SSM, CL III, 0.9 mm | LN, kidney | PD | 3 |
| 6 | m | 69 | M1c | NM, CL IV, 6.0 mm | LN, liver | PD | 3 |
| 7 | m | 72 | M1c | NM, CLIV, 3.2 mm | Liver, LN, kidney | PD | 2 |
| 8 | m | 72 | M1c | Soft tissue melanoma | Soft tissue, skin | PD | 1 |
| 9 | m | 40 | M1a | SSM, CL III, 0.6 mm | LN, soft tissue | PD | 4 |
| 10 | m | 52 | M1c | Uvea melanoma | Liver, kidney, muscle, soft tissue | PD | 3 |
| 11 | w | 36 | M1c | SSM, CL III, 0.6 mm | Liver, spleen, LN, lung | PD | Immediately |
Patient 3 was treated with DC vaccine in an adjuvant setting exhibiting no evidence of metastatic disease
Clark level CL, nodular melanoma NM, progressive disease PD, superficial spreading melanoma SSM, time to progression TTP
Dendritic cells
Peptide-pulsed dendritic cells were generated from leukapheresis samples as previously described [17, 18].
Briefly, peripheral blood mononuclear cells (PBMCs) were plated in 85 mm culture dishes (Falcon, Becton Dickinson, Hershey, USA) at a density of 5 × 107 cells per dish in 10 ml of complete culture medium [RPMI-1640 (Cambrex, Vervier, Belgium) with 10% FCS] and incubated at 37°C and 5% CO2 for 1 h. After a microscopic control of adherence, the non-adherent fraction was removed and 10 ml of fresh, prewarmed medium was added (day 0). All adherent fractions were cultured until day 1, then culture medium was taken off carefully so that loosely adherent cells were not removed, and fresh culture medium containing granulocyte monocyte colony stimulating factor (GM-CSF) (800 U/ml final concentration) and Interleukin-4 (IL-4) (1,000 U/ml final concentration) was added. Cytokines were added again on day 5 in 5 ml fresh medium (containing 800 U GM-CSF and 1,000 U IL-4) per dish. On day 6, different stimuli to induce maturation of DCs were added or not [Interleukin-1β (IL-1β) 2 ng/ml, Interleukin-6 (IL-6) 1,000 U/ml, tumour necrosis factor-α (TNF-α) 10 ng/ml (all of R&D Systems, Minneapolis, USA), Prostaglandin E2 (PGE2) 1 μg/ml (Sigma)]. Cells or supernatant were harvested at different time points for further analysis. On day 7, DCs were loaded with different peptides and subsequently injected subcutaneously into the extremities close to the inguinal lymph nodes [16, 17].
Real-time-PCR of IDO
DNase-treated total RNA was isolated from DCs (1 × 105 cells) by means of RNeasy Micro Kit (Stratagene, La Jolla, CA, USA). The cDNA was synthesised from mRNA with poly(dT) primers, dNTPs, random primer and Superscript II reverse transcriptase (Invitrogen, Rockville, MD, USA). Quantification of IDO-expression in dendritic cells was performed by real-time PCR with Taqman technology (Applied Biosystems, Weiterstadt, Germany). Primers and probe for IDO were designed with Primer Express software (Applied Biosystems) and are 5′-TTG-GAG-AAA-GCC-CTT-CAA-GTG-T-3′ (forward primer), 5′-TGC-CTT-TCC-AGC-CAG-ACA-A-3′ (reverse primer) and 5′-CAC-CAA-ATC-CAC-GAT-CAT-GTG-AAC-CCA-3′ (probe) (Eurogentec, Seraing, Belgium). The relative expression of IDO was calculated in relation to the expression of mRNA coding for the housekeeping gene GAPDH (Applied Biosystems). Measurements were performed in triplicates.
Western Blot analysis
For Western blot analysis cell lysates (1 × 105 cells) were prepared by suspending cultured dendritic cells at indicated time points in Laemmli lysis buffer. Lysates were incubated for 5 min at 96°C and were then loaded on a 10% polyacrylamid gel (Cambrex). Gels were run under reducing conditions. Following electrophoresis, samples were electroblotted on nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Blots were blocked for 30 min in blocking solution (3% milk powder in phosphate-buffered saline (PBS)/0.05% Tween) at room temperature and then incubated overnight with primary monoclonal IDO antibody (1:1,000, 636931A, Chemicon, CA, USA) in blocking solution at 4°C. After washing with PBS/0.05% Tween, membranes were incubated for 2 h at room temperature with secondary antibody rabbit anti mouse peroxidase-labeled immunoglobulin G (1:1,000 dilution) (Dako, Hamburg, Germany) in blocking solution. After additional washings, binding of the peroxidase-labeled antibody was visualised by chemiluminescence (Boehringer Mannheim, Germany). Analysis was performed at least twice for each sample. As loading control β-actin was detected using a monoclonal antibody (Dako).
Chromatography of supernatants for tryptophan concentration
To analyse tryptophan catabolism at day 6 of in vitro maturation 1 × 105 cells/well were plated in 96-well plates (Falcon, Becton Dickinson, Hershey, USA) in Hanks buffered salt solution (HBSS) (Gibco BRL, Gaithersburg, MD, USA) containing 100 μM l-tryptophan (Sigma, Deisenhofen, Germany). Supernatants of matured or immature dendritic cells were harvested at different time points and were analysed for tryptophan concentration by thin layer chromatography (TLC). Chromatography was performed on 20 × 20 cm silica gel 60 precoated plates (Merck, Darmstadt, Germany) with mobile phase containing 20% methanol and 10% Tris–glycine for 12 h. After developing and drying TLC layers, spots on chromatograms were detected by reflectance densitometry at wavelength of 280 nm using a CAMAG TLC Scanner 3 (CAMAG, Muttenz, Switzerland). Data collection and integration were accomplished using CATS Software Version 4.06.
Immunohistochemical analysis of IDO, CD83 and FoxP3
Briefly, cryopreserved specimen of vaccination sites and normal skin were fixed in 4% formalin for 10 min at 4°C and washed in PBS afterwards. Endogenous peroxidase was blocked with peroxidase blocking solution (S2023, Dako) and slides were washed twice with buffer. The tissue was then incubated for 40 min at room temperature with anti-IDO antibody (AB 5969 Rabbit polyclonal, Chemicon), with anti-CD83 antibody (74841E, BD Biosciences, Heidelberg, Germany) or anti-FoxP3 antibody (236A/E7, Chemicon). After washing with PBS, sections were subjected to secondary antibody anti-rabbit HRP (Envision K4003, Dako) for 30 min. After washing in PBS, the sections were placed in AEC-substrate (Multi link Biotin Kit, K5003, Dako). Sections were washed in Aqua bidestilata, mounted and cover-slipped. Evaluation was performed by two independent histologists.
Results
IDO mRNA expression in mature human DCs
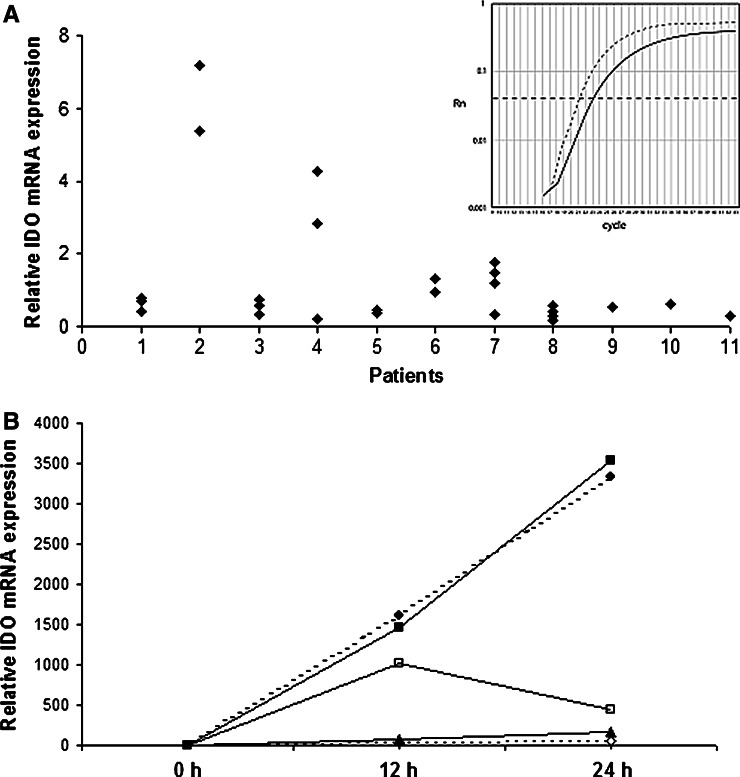
DCs are known to possess both immunostimulatory as well as immunomodulatory properties. Indeed, upregulation of IDO in human DCs under certain culture conditions has already been demonstrated [14, 15]. Consequently, we scrutinised IDO expression in clinical grade DCs generated for therapeutic vaccination of melanoma patients. Leukaphereses were repeatedly performed for individual patients at different time points during immunotherapy for therapeutic and diagnostic purposes; thus a total of 28 DC preparations of 11 patients were analysed. IDO mRNA expression was analysed by real-time-PCR at day 7 of in vitro maturation, i.e. directly prior to vaccine application. A generally strong IDO mRNA expression as compared to the housekeeping gene GAPDH was observed in the majority of the patients (Fig. 1a) with inter- and intrapersonal variation.
Fig. 1.
a Relative IDO mRNA expression profile in multiple DC preprations of 11 melanoma patients at day 7 of in vitro generation, prior to DC vaccine application. An inter- and intrapersonal variation of IDO expression relative to GAPDH is demonstrated. All data are normalized to IDO mRNA expression level of patient 1. Insert Representative example of mRNA levels of IDO (solid line) and GAPDH (interrupted line) of one patient. b IDO mRNA expression in DCs obtained from 5 melanoma patients (filled square, filled circle, thick line, diamond) after in vitro maturation over a time course of 24 h
Expression of IDO mRNA and protein is upregulated in human DCs during in vitro maturation
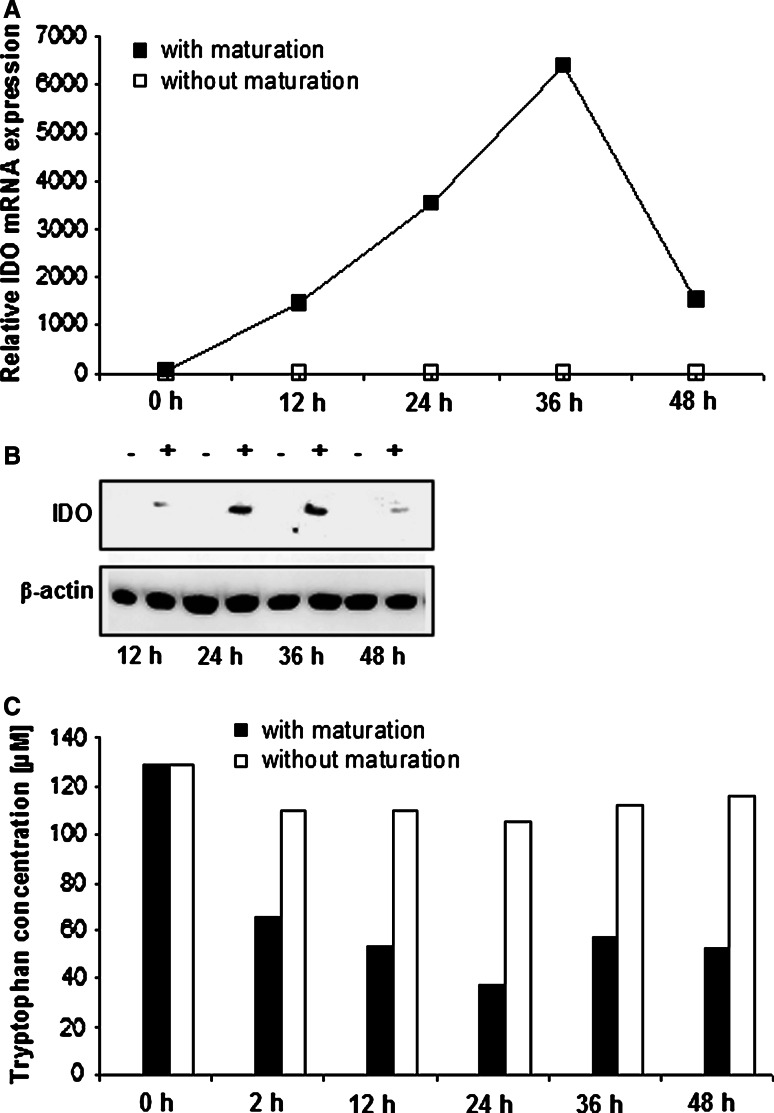
In order to study if the observed IDO mRNA expression in mature DCs at day 7 is a result of the maturation process or a sign for an incomplete maturation, a kinetic expression profile of IDO mRNA was performed in five of the patients. The time course covered 24 h (n = 5) and 48 h (n = 2) after induction of maturation at day 6. This kinetic study revealed, that in all specimen IDO mRNA expression was increased within 12–24 h by stimulation with IL-1β, TNF-α, IL-6 and PGE-2 (Fig. 1b). This elevation in IDO mRNA expression was absent in DCs generated from the same leukapheresis sample if no maturation cocktail was added (Fig. 2a). However, maximal expression levels varied substantially between the different DC preparations, and the time course of maximal IDO expression was heterogeneous. Notably, induced IDO gene expression correlated with an enhanced protein expression, as analysed by Western blot using β-actin for normalization of protein loading (Fig. 2b).
Fig. 2.
a IDO expression in correlation to the maturing process with IL-1β, TNF-α, IL-6 and PGE-2 of human DCs (representative example). Expression of IDO mRNA relative to the housekeeping-gene GAPDH is depicted. Filled square Mature DCs, open square Immature DCs. b Western blot analysis of protein lysates of immature (−) and mature (+) human dendritic cells. c Parallel measurement of tryptophan concentration in supernatant, obtained at the given time points after the induction of maturation (filled bars) or not (open bars). Similar results were obtained on repetitive experiments
Overexpression of IDO in mature human DCs is associated with tryptophan catabolism in vitro
IDO exerts its immunoregulatory functions via degradation of the essential amino acid tryptophan together with the concomitant production of directly toxic metabolites. To test the notion that upregulation of IDO correlates with enzymatic activity, we measured tryptophan catabolism in supernatant of immature and matured DCs at different time points during the maturation process. Indeed, this analysis revealed that in parallel with the observed increase in IDO mRNA and protein levels, tryptophan concentration decreased. In contrast, in supernatants of immature DCs, which did not upregulate IDO, no such decline in the tryptophan concentration was observed (Fig. 2c).
DCs maintain IDO expression at vaccination sites in situ
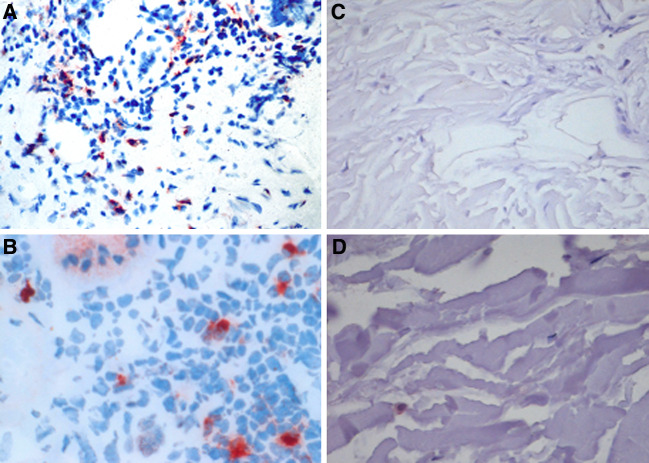
A crucial question is, if the observed IDO expression in human DCs is merely an in vitro culture phenomenon or may have indeed in vivo implications. Therefore, we obtained biopsies from vaccination sites of melanoma patients 24 h after DCs had been injected subcutaneously. These vaccination sites contained mature DCs as visualised by CD83 reactivity of the inflammatory infiltrate (Fig. 3a). Immunohistochemical analysis for IDO expression revealed a diffuse infiltrate of IDO-positive cells present in the subcutaneous compartment at the vaccination site (Fig. 3b). These IDO-expressing cells were detected with different frequencies among individual patients. In normal skin, however, no CD83 positive cells were detected (Fig. 3c) and only rare, single IDO-positive dendritic cells were scattered in the dermal compartment (Fig. 3d).
Fig. 3.
Immunohistochemical detection of CD83 (×20) (a) and IDO (×40) (b) in representative biopsies of DC vaccination sites. CD83 (×20) (c) and IDO (×40) (d) expression in normal skin are shown as negative control
FoxP3+ cells are present at the vaccination sites in situ
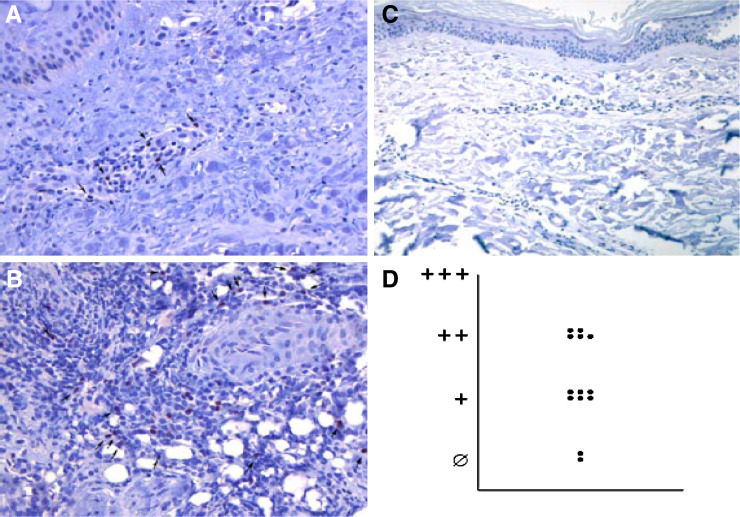
As there is strong evidence that IDO-competent APCs are able to induce regulatory T-cells in a self-reinforcing network, we additionally scrutinised vaccination sites for Forkhead box P3 (FoxP3) expressing cells (Fig. 4). To this end, FoxP3+ cells were observed at higher frequencies at DC vaccination sites (Fig. 4a, b) as compared to normal skin (Fig. 4c). The amount of FoxP3 positive cells varied between the different patients (Fig. 4a, b, d).
Fig. 4.
a, b Immunohistochemical analysis of FoxP3 expression on vaccination site infiltrating cells (indicated by arrows) (×20). Representative examples of specimen with different frequencies of FoxP3-positive cells are shown. c Normal skin as negative control (×20). d Graph demonstrating a low (+), medium (++) and high (+++) frequency of FoxP3-expressing cells at 13 analysed vaccination sites of 8 different patients
Clinical course of melanoma patients receiving DC based cancer vaccine
All patients (n = 11) included in this analysis demonstrated rapid disease progression with a median time to progression of only 3 months (Table 1). Due to the collective lack of therapeutic activity—with respect to objective responses or even disease stabilization—no correlation between IDO expression or FoxP3 induction with the clinical course was drawn.
Discussion
The clinical efficacy of DC based vaccines for cancer immunotherapy is influenced by various interplaying, immunomodulating factors. Interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) are well known to counteract the inherent, immunostimulatory capacity of APCs allowing the generation of immunosuppressive regulatory T-cells. A recently recognised additional immunoregulatory determinant is indoleamine 2,3-dioxygenase, which attenuates cytotoxic T-lymphocyte responses inter alia by promoting tryptophan degradation.
Here, we demonstrate that functionally active IDO is expressed in DC preparations used for therapeutic vaccination. IDO mRNA and functional protein was induced upon in vitro maturation of human DCs with standard cytokine cocktail containing IL-1β, TNF-α, IL-6 and PGE-2, but was absent in immature DCs. Previous reports have suggested that immature and especially fully mature antigen presenting cells gain the capacity to express IDO [14, 15]. Indeed, a subtype of IDO-positive dendritic cells, i.e. CD123+ CCR6+ cells, was defined [19]. Prostaglandins, together with additional signalling through TNF receptor or toll like receptors, have been identified as critical determinants for IDO induction and its functional activation [14, 15]. To scrutinise the in vivo relevance of IDO for DC vaccinations, we extended our in vitro observations to in situ immunohistochemical studies of DC injection sites. Biopsies of subcutaneous vaccination sites, i.e. after injection of antigen-pulsed, in vitro matured DCs, revealed a diffuse, dermal infiltrate of CD83+ and IDO+ cells. Notably, a pronounced infiltrate of FoxP3+ cells were present at the vaccination sites. FoxP3 is a lineage specific transcription factor, bearing crucial roles for Treg development and function [20]. It is possible, that FoxP3+ Tregs traffick from the thymus, bone marrow, lymph nodes and peripheral blood under the influence of cytokines to DC vaccination sites [21, 22]. Alternatively, IDO+ DCs may promote Treg-development conveying both local and systemic immunosuppressive properties beyond tryptophan catabolism. The latter scenaria has been suggested by several studies [6, 23, 24]. In this context, it has been very recently demonstrated that human DCs, which have undergone maturation induced by inflammatory stimuli, are able to efficiently expand Tregs both in vitro and in vivo in cancer patients [25]. All patients in our cohort, receiving DC vaccination therapy for advanced melanoma, were characterised by a rapid tumour progression. The detailed immune monitoring of these patients is currently performed within the clinical trial and will provide additional information in this respect. With regard to clinical relevance, several lines of evidence suggest an impact of IDO-expression on the clinical course and a possible role for modulating IDO-acitivity in cancer therapy: (I) Inhibition of IDO activity by competitive antagonists, e.g. 1-methyl-tryptophan (1MT) [19] or antioxidants [26, 27], results in rejection of allogenic foetus [8] and—most important—(II) a modified tumour growth in murine models [28]. In addition, constitutive IDO expression in mouse tumour draining lymph nodes by plasmacytoid dendritic cells has been suggested as one mechanism of inducing systemic immunological unresponsiveness towards the tumour; this unresponsiveness can be reverted by administration of 1MT [29]. Consequently, IDO-inhibitors are considered as novel complements in DC maturation cocktails or adjuvants for therapeutic vaccinations [14]. It should be noted, however, that immunostimulatory cytokines induced in DCs upon maturation may abrogate tolerogenic effects of IDO upregulation [30]. We observed a decline in IDO expression at 24 h after induction of maturation, implying that IDO expression is a complex, regulated process. Interestingly, IDO enzyme activity and tryptophan metabolism may be also essential for DC maturation, activation [31] and chemotactic responsiveness [32].
In summary, our present data corroborate with former studies, which have raised concern about IDO upregulation in mature human DCs by confirming the in situ relevance of IDO induction in DCs applied for cancer immunotherapy. The presence of FoxP3 positive cells at the site of DC injection suggests possible immunoregulatory effects of IDO expressing DCs. This may contribute to systemic immunological unresponsiveness and thus to therapeutic failure of DC based cancer immune therapies. Future studies will have to evaluate if modulating IDO activity may help to improve the clinical outcome of therapeutic vaccinations.
Acknowledgments
We greatly thank Eva Baumann and Claudia Siedel, Department of Dermatology, University of Wuerzburg, Germany, for their excellent technical assistance and Claudia S. Kauczok for help with the immunohistochemical analysis. Furthermore, we thank Ulrich R. Hengge, University of Duesseldorf, Department of Dermatology, Germany, for providing the IDO antibody for immunohistochemistry.
Abbreviations
- APC
Antigen presenting cell
- CTL
Cytotoxic T-lymphocyte
- DC
Dendritic cell
- FoxP3
Forkhead box P3
- GM-CSF
Granulocyte monocyte colony stimulating factor
- IDO
Indoleamine 2,3-dioxygenase
- IL
Interleukin
- PBMCs
Peripheral blood mononuclear cells
- PGE2
Prostaglandin E2
- Treg
Regulatory T-cell
- TGF-β
Transforming growth factor-β
- TNF-α
Tumour necrosis factor-α
- 1MT
1-Methyl-tryptophan
Footnotes
David Schrama and Juergen C. Becker contributed equally.
References
- 1.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumour cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 2.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 3.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/S1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;19(196):447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faunce DE, Terajewicz A, Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol. 2004;172:1991–1995. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SL, Carlin JM, Pyati P, Dai W, Pfefferkorn ER, Murphy MJ., Jr Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immunol. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 9.Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 10.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–196. doi: 10.1016/S0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 11.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Harlin H, Kuna TV, Peterson AC, Meng Y, Gajewski TF. Tumour progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55:1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, Morton DL, Essner R. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res. 2005;11:107–112. [PubMed] [Google Scholar]
- 14.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, Fiore F, Roth U, Beyer M, Debey S, Wickenhauser C, Hanisch FG, Schultze JL. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by associated-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 16.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, Bock M, Brocker EB, Straten PT, Kampgen E, Becker JC. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, Zimpfer-Rechner C, Berger T, Kamarashev J, Burg G, Jonuleit H, Tuttenberg A, Becker JC, Keikavoussi P, Kampgen E, Schuler G. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 18.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/S0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 21.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S, David O, Curiel TJ, Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 24.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas SR, Salahifar H, Mashima R, Hunt NH, Richardson DR, Stocker R. Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-gamma-activated human macrophages: posttranslational regulation by pyrrolidine dithiocarbamate. J Immunol. 2001;166:6332–6340. doi: 10.4049/jimmunol.166.10.6332. [DOI] [PubMed] [Google Scholar]
- 27.Terentis AC, Thomas SR, Takikawa O, Littlejohn TK, Truscott RJ, Armstrong RS, Yeh SR, Stocker R. The heme environment of recombinant human indoleamine 2,3-dioxygenase. Structural properties and substrate–ligand interactions. J Biol Chem. 2002;277:15788–15794. doi: 10.1074/jbc.M200457200. [DOI] [PubMed] [Google Scholar]
- 28.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumour cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in draining-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI200421583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Bubnoff D, Bausinger H, Matz H, Koch S, Hacker G, Takikawa O, Bieber T, Hanau D, de la SH. Human epidermal langerhans cells express the immunoregulatory enzyme indoleamine 2,3-dioxygenase. J Invest Dermatol. 2004;123:298–304. doi: 10.1111/j.0022-202X.2004.23217.x. [DOI] [PubMed] [Google Scholar]
- 31.Hwang SL, Chung NP, Chan JK, Lin CL. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;15:167–175. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- 32.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/S1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]