Abstract
Preventive immunotherapy is an attractive strategy for patients at a high risk of having cancer. The success of prophylactic cancer vaccines would depend on the selection of target antigens that are essential for tumour growth and progression. The overexpression of GM3 ganglioside in murine and human melanomas and its important role in tumour progression makes this self antigen a potential target for preventive immunotherapy of this neoplasm. We have previously shown that preventive administration of a GM3-based vaccine to C57BL/6 mice elicited the rejection of the GM3 positive-B16 melanoma cells in most of the animals. Despite the crucial role of cellular immune response in tumour protection, the involvement of T cells in anti-tumour immunity of ganglioside vaccines is not described. Here, we examined the mechanisms by which this immunogen confers tumour protection. We have found that induction of anti-GM3 IgG antibodies correlated with tumour protection. Surprisingly, CD8+ T cells, but not NK1.1+ cells, are required in the effector phase of the antitumour immune response. The depletion of CD4+ T cells during immunization phase did not affect the anti-tumour activity. In addition, T cells from surviving-immunized animals secreted IFNγ when were co-cultured with IFNα-treated B16 melanoma cells or DCs pulsed with melanoma extract. Paradoxically, in spite of the glycolipidic nature of this antigen, these findings demonstrate the direct involvement of the cellular immune response in the anti-tumour protection induced by a ganglioside-based vaccine.
Keywords: Preventive cancer vaccines, Melanoma, GM3 ganglioside, CD8 T cells
Introduction
Most therapeutic cancer vaccines trials have been performed in advanced and heavily treated cancer patients. In this stage of the disease, the vaccination is poorly effective due to that, the immune system is not able to overcome tumour-induced or therapy-induced immunosuppression [1]. Many of the problems that diminish therapeutic effects of cancer vaccines would not need to be considered in the setting of tumour prevention. An immune system that is primed against tumour antigens would be expected to destroy the tumour before it can suppress and evade the immune response. Consequently, preventive immunotherapy is an attractive strategy for patients at a high risk of having cancer, including viral-associated malignancies such as hepatocellular carcinoma [2] and cervical cancer [3], with some studies still in progress. In the case of melanoma, epidemiologic studies have identified environmental, host and genetic risk factors in which are included sun exposure, multiple banal or dysplastic nevi, fair skin, familial melanoma and susceptibility genes [4].
The chances of success of vaccines for cancer prevention depend on the selection of target antigens that are essential for tumour growth and progression. In this sense, GM3 ganglioside is a glycolipid present in normal tissue but overexpressed on tumours like melanomas [5]. The immunosuppressive properties of this molecule and its important role in tumour progression make this self antigen a potential target for preventive immunotherapy of this neoplasm.
For several years, gangliosides were considered as T cell independent antigens because they were not presented to T lymphocytes by the major histocompatibility complex as regular peptide antigens [6]. However, this category became controversial due to the finding of CD1-restricted T cells specific for gangliosides in healthy donors and multiple sclerosis patients [7].
Several studies have shown that tumour-associated gangliosides are immunogenic in melanoma patients and that antibodies against them have a favorable prognostic effect [8]. The role of antibodies in the antitumour effect of ganglioside vaccines has been considered to be predominant, if not exclusive [9, 10]. However, the involvement of T cells in the antitumour protection induced by ganglioside-based vaccines has not yet been described. This is an important issue, since if a robust cellular immune response could be elicited, then immunological memory could be long lasting, eradicating tumour before it becomes clinically obvious.
The GM3-based vaccine is composed by very small size proteoliposomes (VSSP) resulting from the hydrophobic conjugation of GM3 ganglioside with Neisseria meningitidis membrane proteins (VSSP/GM3) [11]. We have previously shown that preventive immunization of C57BL/6 mice with this nanoparticulated immunogen consistently elicited the rejection of B16 melanoma cells [12, 13]. However, the precise mechanisms by which this vaccine confers tumour protection were unknown.
In the present work, we examined the effect of in vivo depletion of lymphocyte populations on tumour protection induced by VSSP/GM3 vaccine in B16 melanoma model. We have also tested the role of anti-GM3 antibodies in the vaccine capacity to reject the tumour. We found that the induction of anti-GM3 IgG antibodies correlated with tumour protection. However, the depletion of CD4+ T cells during immunization phase did not affect the antitumour activity. Surprisingly, CD8+ T cells but not NK1.1+ cells were required in the effector phase of the immune response for antitumour protection. In addition, T cells from surviving-immunized animals recognized the MHC class I high B16 melanoma cells and DC pulsed with melanoma extract and secreted IFNγ.
Altogether, these findings demonstrate the direct involvement of the cellular immune response in the anti-tumour protection induced by a ganglioside-based vaccine.
Materials and methods
Mice and cell lines
Female C57BL/6 aged 7–8 weeks were obtained from CENPALAB (Havana, Cuba) and kept with water and food ad libitum in the animal house facility at the Center of Molecular Immunology (CIM, Havana, Cuba). Every handling and experiments were performed in accordance with institutional guidelines. B16 melanoma cell subline F10 (high metastatic capacity) and metastatic clone of Lewis lung carcinoma cell line (3LL-D122), both syngeneic for the C57BL/6 strain were cultured in RPMI-1640 containing 2 mM glutamine (Gibco BRL, Grand Island, NY) and supplemented with 10% heat-inactivated fetal calf serum (FCS). An antibiotic mixture containing penicillin and streptomycin (final concentrations 100 IU/ml and 100 μg/ml, respectively) was added to the culture medium to prevent contamination. Hybridomas YTS191, PK136 y YTS169 were used for producing anti-CD4, anti-NK1.1 and anti-CD8 MAbs, respectively. All cell lines were purchased from ATCC collection (American Type Culture Collection). Cell viability was assessed using trypan blue exclusion technique.
Vaccine
The VSSP/GM3 vaccine was produced by the CIM (Havana, Cuba). Briefly, GM3 monosialoganglioside containing the N-acetylneuraminic acid was purified from canine erythrocytes and hydrophobically conjugated with outer membrane proteins (OMP) from N. meningitidis strain 385, as described in detail [11]. The method allowed gangliosides and proteins to incorporate into VSSP and conferred high solubility to the conjugate. Each VSSP/GM3 doses contained 120 μg of ganglioside in 0.05 ml of phosphate buffered saline (PBS), mixed with equal volume of the immunological adjuvant Montanide ISA 51 (SEPPIC, France) prior to injection. Vaccines were administered intramuscularly (i.m) in the quadriceps, and control animals received only PBS.
Vaccination and tumour challenge
On days 0, 14, 28 and 42, groups of at least ten C57BL/6 mice were immunized i.m, with 120 μg of VSSP/GM3 emulsified with Montanide ISA 51 or PBS (control group) in a total volume of 0.1 ml/dose. No toxicity or morbidity was detected as a consequence of vaccine administration, as reported previously [12]. Three weeks after the last vaccination (day 63), mice were injected in the subcutis with 104 live B16 cells. Animals were monitored for tumour growth every other day by palpation, and diameters were measured using a Vernier caliper. For ethical reasons, animals were sacrificed when the tumour size reached 250 mm2 or when the general condition of the animals was affected. The mouse sera used for antibody response determination were collected on days 56, 70 and 93 after the first vaccination. Sera from control group treated with PBS and inoculated with B16 melanoma cells were also obtained at the same time points. The collection of sera affected neither the antitumour activity of the vaccine nor the tumour growth in the non-vaccinated group.
In vivo depletion experiments
Vaccinated and control mice were injected intraperitoneally (i.p) with 1 mg of total proteins isolated by NH4SO4 protein precipitation from supernatants containing anti-CD4, anti-CD8 or anti NK1.1 MAbs obtained from rat anti-mouse hybridomas YTS191, YTS169 and PK136, respectively.
The anti-CD8 and anti-NK Abs were injected (0.2 ml) the day before tumour challenge, followed by two i.p injections on day 7 and 14 after tumour inoculation. Depletion of CD4+ T cells during the priming phase of the immune response was conducted by i.p administration of anti-CD4 depleting antibody (0.2 ml), beginning on the day 3 before the first immunization and was maintained during the immunization period administering the MAb every 3 days. Optimal conditions for depletion of the target population were determined in a pilot experiment by FACS analysis of spleen, blood and lymph nodes 3 and 6 days after the treatment using FITC-conjugated anti-CD4 (RM4-5), PE-conjugated anti-CD8 (53-6.7) and PE-conjugated anti-NK1.1 (PK136) (BD Pharmingen, San Diego, CA). In all cases, subset depletion higher than 95% was maintained 3 days with 1 mg/dose of protein solution.
Flow cytometry
Cells were stained with appropriate MAbs in PBS containing 2% FCS and 0.01% sodium azide as previously described [14], and analyzed using FACS flow cytometer and CellQuest software package (Becton Dickinson, USA). Necrotic cells and cellular debris were identified by forward scatter (FSC/SSC) profiles and propidium iodide (PI) (Sigma-Aldrich, St Louis, MO) staining, and excluded from the analysis.
Enzyme-linked immunosorbent assay (ELISA)
For anti-GM3 antibodies detection, PolySorp 96-well plates (Nunc, Denmark) were coated with 0.16 nmol/well of ganglioside dissolved in methanol and dried at 37°C for 1.5 h. To reduce background, plates were washed with PBS containing Tween 20 (0.05%, v/v) plus 0.2 M extra of NaCl and then blocked with 1% bovine serum albumin in PBS. Serum samples, biotinylated goat anti-IgM or anti-IgG antibodies and streptavidin-conjugated alkaline phosphatase (Jackson Immunoresearch Laboratories Inc., USA) were diluted in blocking buffer and sequentially incubated on the plates for 2 h at room temperature with extensive washing between each incubation. The enzymatic reaction was visualized with p-nitrophenyl phosphate (PNPP) (Sigma, St Louis, MO), 1 mg/ml, dissolved in 1 M diethanolamine buffer, pH 9.6, plus 1 mM MgCl2 and absorbency was measured at 405 nm with an ELISA plate reader (Organon Teknika, Salzbury, Austria). To eliminate the effect of non-specific recognition, sera were also tested on wells to which no ganglioside had been added. The absorbance at each serum dilution obtained on these wells was subtracted from that of the ganglioside-coated wells. The titer was defined as the highest dilution yielding a corrected absorbance of 0.18 or greater.
Complement-mediated cytotoxicity assay (CMC)
CMC assay were performed by PI staining. B16 melanoma cells were incubated 4 h with the sera from pre- and post-immunization (day 70) at 37°C in a humidified CO2 incubator. Both the complement and serum were used at a final dilution of 1:10 diluted in PBS containing heat-inactivated FCS 1%. After the sera incubation, the cells were washed and a solution of PBS FCS 1% containing propidium iodide at 100 μg/ml was added. The percent of post treatment lysis was calculated as the percent of death cells (positively stained with PI) on day 0 subtracted from the percent of death cells on day 70.
Generation of bone marrow-derived dendritic cells (bmDCs)
As previously described [15], bone marrow cells were harvested from femurs and tibias of normal C57BL/6 mice and washed with PBS. Briefly, cells were resuspended in RPMI-1640 supplemented with 10% FCS, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 μg/ml gentamicin, 50 μM 2-ME (Sigma-Aldrich) and 10 ng/ml recombinant murine GM-CSF (R&D Systems, Abingdon, Oxon) and cultured in six-well plates (Costar, NY, USA). On day 3, the cells were fed with fresh medium. After 6 days of culture, non-adherent and loosely adherent cells were harvested, washed, and used for in vitro experiments. For bmDCs characterization we used FITC-conjugated CD40 (3/23), H-2Kb (AF6-88.5), CD80 (16-10A1), CD86 (GL1) and PE-conjugated CD11c (HL3), I-A/I-E (M5/114.15.2) (all were purchased from PharMingen). Cultures typically contained >90% cells expressing CD11c and MHC class II on the surface, as determined by flow cytometry.
In vitro treatment of B16 melanoma and 3LL-D122 lung carcinoma cells with IFNα
B16 or 3LL-D122 cells (5 × 105) were treated overnight with 1,000 U/ml of culture supernatant-containing IFNα kindly provided by Vaccine Department from CIM (Havana, Cuba). The effect of this cytokine on B16 and 3LL-D122 cells was measured by the expression of MHC class I molecule using FITC-conjugated anti-H-2Kb using flow cytometry. Matching isotype Ab was used as control. Both were purchased from PharMingen
IFNγ production by T cells
Eight weeks after challenge with B16 melanoma cells, T cells were isolated from spleen of surviving-vaccinated or tumour-free untreated C57BL/6 mice using anti-CD90 MACS microbeads (Miltenyi Biotech GmBH, Germany). Purified T cells were subsequently cocultured in 96-well, flat-bottom microtiter plates (Costar, NY, USA) at 2 × 105 cells/well in RPMI-1640 medium containing 10% heat-inactivated FCS in the presence of the following stimulus: irradiated (30 Gy) B16 or 3LL-D122 tumour cell lines pretreated or not with IFNα, bmDCs alone, bmDCs that had been pretreated with GM3 ganglioside (10 μg/ml) or with 1:5 dilution of B16 cell extract. To obtain melanoma extract, 107 tumour cells were lysed by six cycles of freezing and thawing followed by sonication and centrifugation. Culture supernatants were collected after 96 h of culture to measure production of IFNγ by using a commercial ELISA kit (BD Pharmingen, San Diego, CA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was conducted using the computer program SPSS for Windows (Chicago, IL). The Mann–Whitney U method was used as a non-parametric test for pair-wise comparisons. Correlation between anti-GM3 antibodies isotype and rejection of B16 tumours was performed by Spearman correlation. Statistical analysis on survival data was performed by the Kaplan–Meier method and differences were assessed using Log-rank test. Findings were considered significant if p < 0.05.
Results
Vaccine induced anti-GM3 IgG but not IgM antibodies are present in sera of mice protected against B16 challenge
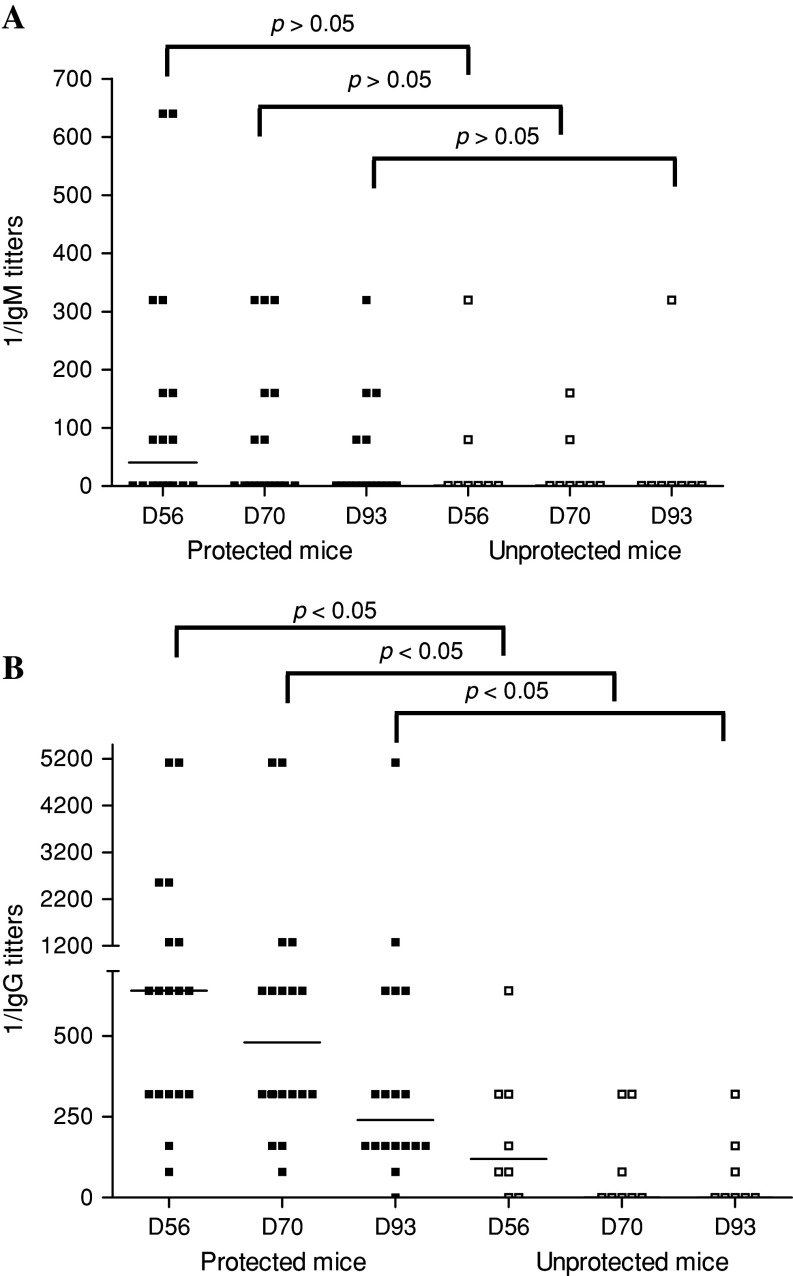
The role in melanoma protection of the anti-ganglioside antibody response, detected in vaccinated mice, was addressed by injecting C57BL/6 mice with four doses of VSSP/GM3 + Montanide ISA 51. The mice were challenged with 104 B16 melanoma cells on day 63. Sera were collected four times until day 93 and the anti-GM3 IgG and IgM titers were measured by ELISA. The frequency of vaccinated mice with serum IgM and IgG antibodies was higher in the group rejecting the melanoma challenge if compared with the unprotected individuals (Table 1). Additionally, a comparison of median anti-GM3 IgM and IgG titers at each time point between protected and unprotected animals is shown in Fig. 1. Before immunization, antibodies against GM3 were absent from animal sera (data not shown). In protected mice, median IgG titers were 1:640, 1:480 and 1:240 for days 56, 70 and 93, respectively, while in unprotected mice these values were 1:120, 0 and 0 for the same time points (p < 0.05, Mann Whitney test) (Fig. 1b). Interestingly, in protected mice, specific IgG antibodies persisted, in almost all animals, at least 50 days after the last vaccination. In contrary, median IgM titers were similar in both groups of mice (Fig. 1a). Noteworthy, a significant direct correlation between anti-GM3 IgG antibody response and the capacity to reject the tumour was observed for each post immunization time points (p < 0.05, Spearman correlation).
Table 1.
Presence of anti-GM3 IgG and IgM antibodies in mice vaccinated with VSSP/GM3 + Montanide ISA 51 and challenged with B16F10 melanoma
| Frequency of respondersa (IgM) | Frequency of responders (IgG) | |||||
|---|---|---|---|---|---|---|
| Day 56 | Day 70 | Day 93 | Day 56 | Day 70 | Day 93 | |
| Protected mice (n = 18) | 9 (50%) | 7 (39%) | 5 (28%) | 18 (100%) | 18 (100%) | 17 (94%) |
| Unprotected mice (n = 8) | 2 (25%) | 2 (25%) | 1 (12%) | 6 (75%) | 3 (37%) | 3 (37%) |
Frequency of responders expressed as percent (positive/total animals)
aResponders (with anti-GM3 antibodies) are mice with titers ≥1:80
Fig. 1.
High anti-GM3 IgG antibody titers in immunized mice correlated with the rejection of B16 melanoma tumours. C57BL/6 mice were immunized with VSSP/GM3 + Montanide ISA 51 on days 0, 14, 28, 42 and challenged with 104 B16 cells on day 63. Anti-GM3 antibody titers were individually determined in sera by ELISA on days 0, 56, 70 and 93. Each dot represents an individual mouse and the horizontal lines the median titers for each day. a Differences in IgM median antibody titers between both groups of immunized mice, at different time points, did not reach statistical significance (p > 0.05, Mann–Whitney test); b IgG median antibody titers were higher in protected mice if compared with unprotected ones at each analyzed day (p < 0.05, Mann–Whitney test). Spearman method was used for statistical correlations. This result is representative of two independent experiments
Finally, to test whether just the implantation of the B16F10 cells in the mice could induce anti-GM3 antibodies, sera from non-immunized tumour-bearing mice were checked by ELISA. All tested sera were negative for the presence of these antibodies.
Sera from surviving-immunized mice induce complement-mediated cytotoxicity (CMC) on tumour cells
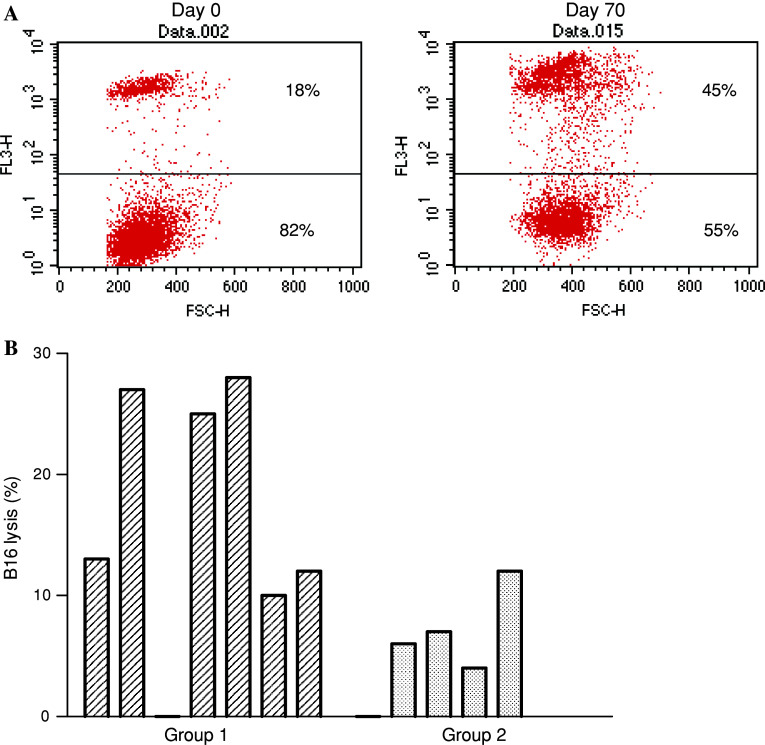
Sera from day 0 and day 70 of seven protected and five unprotected vaccinated mice were employed for CMC assessment. B16 melanoma cells were used as targets (Fig. 2). In five out of seven vaccinated mice rejecting the melanoma challenge, the post vaccination sera showed an increase over 10% in CMC, compared with pretreatment sera. By contrast, only one of the five unprotected mice showed a 12% increase. The differences in CMC between both groups of mice were statistically significant (p < 0.05, Mann Whitney test). The higher lytic potential of sera in the protected group of mice (25, 27 and 28%) corresponded to the higher IgG titers (1:1,280, 1:1,280 and 1:5,120).
Fig. 2.
CMC effect is induced by sera from surviving-immunized animals. C57BL/6 mice were immunized with emulsified VSSP/GM3 on days 0, 14, 28, 42 and challenged with 104 B16 cells on day 63. Sera from protected (n = 7, group 1) and unprotected mice (n = 5, group 2) were incubated with B16 cells in the presence of autologous complement. a Increased cytotoxic capacity versus B16 target cells of day 70 serum from a representative mouse from group 1, as compared with the day 0 sample. The determination consisted in a PI cell viability test evaluated by flow cytometry. b Significant increase of cytolysis (calculated as: postvaccination serum lysis − prevaccination serum lysis) was detected in protected vaccinated mice compared with unprotected counterparts (p < 0.05, Mann–Whitney test). Each bar represents an individual animal. Results are representative of two independent experiments
CD8+ T cells, but not NK1.1+ cells, are required for the anti-B16 melanoma protective effect of VSSP/GM3 + Montanide ISA 51
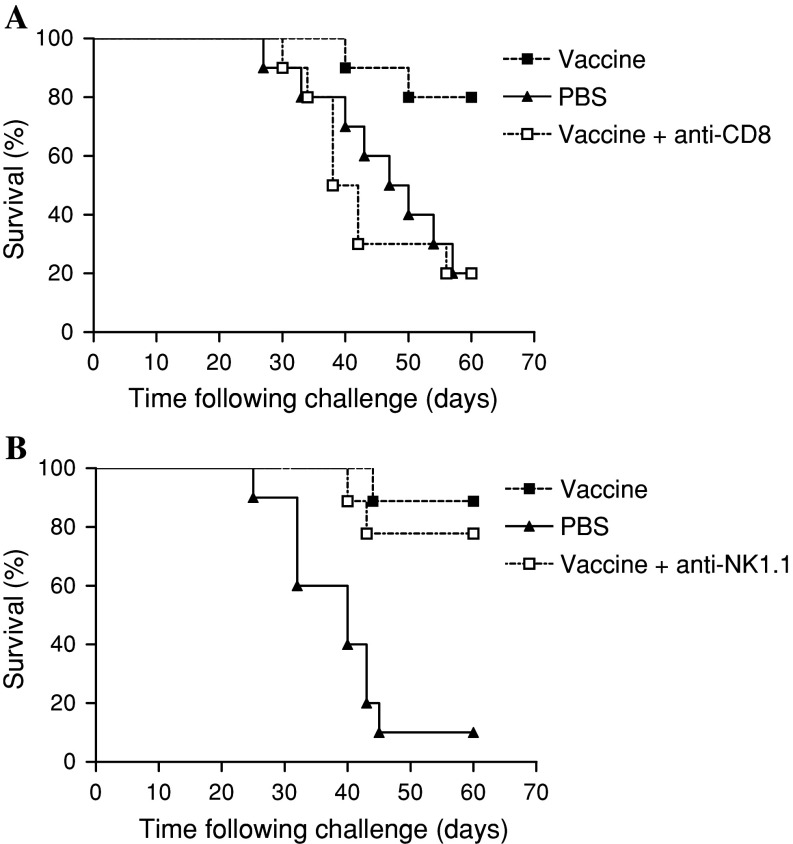
We studied the role of NK1.1+ cells and CD8+ T cells in the rejection of melanoma, observed in mice immunized with VSSP/GM3 + Montanide ISA 51 and challenged with B16F10 tumour cells (see “Materials and methods”). Independent group of C57BL/6 mice received the same vaccination schedule but additionally, depleting amounts of anti-CD8+ or anti-NK1.1+ Abs were administered a day before and 1 and 2 weeks after melanoma inoculation. Depletion of CD8+ T cells completely abrogated the protection from tumour challenge compared with non-depleted vaccinated mice (Fig. 3a). In contrast, administering the anti-NK1.1+ Ab to mice did not affect the anti-tumour activity of the vaccine (Fig. 3b). These results suggest that the anti-melanoma protective effect of this vaccine is strongly mediated by CD8+ T effector cells while is independent of either NK or NKT cells.
Fig. 3.
CD8+ T cells but not NK1.1+ cells are required in the effector phase of the anti-tumour immune response induced by vaccination. C57BL/6 (ten/group) were vaccinated on days 0, 14, 28 and 42 with VSSP/GM3 + Montanide or PBS and challenged with 104 B16 cells on day 63. Two groups of mice were injected i.p with supernatants containing depleting amounts of anti-CD8 or anti NK1.1 Abs on days 62, 70 and 77. All animals were inspected every other day and scored as positive when tumours became palpable. a Immunized mice lacking effectors CD8+ T cells were unprotected from tumour challenge if compared with their competent littermates (p > 0.05, Log-rank test). b NK cells depleted-immunized animals were significantly protected compared with PBS control mice (p < 0.05, Log-rank test). These results are representative of two and three independent experiments for NK1.1+ and CD8+ T cells depletion, respectively
The absence of CD4+ T cells during vaccination does not affect the anti-tumour activity of the vaccine
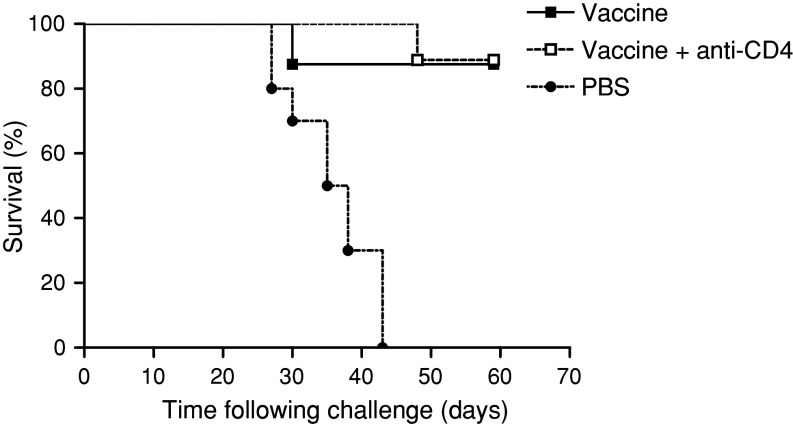
To evaluate whether CD4+ T cell population could be important during the priming of the immune response, C57BL/6 mice were depleted of CD4+ T cells during the immunization period (see “Materials and methods”). The animals were challenged with B16F10 tumour cells, 63 days after the initiation of the vaccination schedule. As shown in Fig. 4, CD4+ T cells depletion did not affect the efficacy of the vaccine in preventing B16 implantation. This result suggests that priming and boosting of the anti-tumour protective effect of the VSSP/GM3 vaccine can be conducted in the absence of CD4+ T cells.
Fig. 4.
CD4+ T cells are not required to induce a vaccine mediated anti-tumour immune response. Mice (ten/group) were vaccinated on days 0, 14, 28 and 42 with VSSP/GM3 + Montanide ISA 51 or PBS. Independent groups of vaccinated mice received i.p supernatant containing anti-CD4 depleting Ab every 3 days, during the immunization period. Mice were further challenged with 104 B16 cells on day 63 and monitored every other day for scoring when the tumours became palpable. Vaccinated animals were significantly protected, in comparison to control mice, independently of the presence or not of CD4+ T cells (p < 0.05, Log-rank test). This result is representative of three independent experiments
Previously exposed bmDCs to B16 lysates stimulate T cells from protected animals to secrete IFNγ
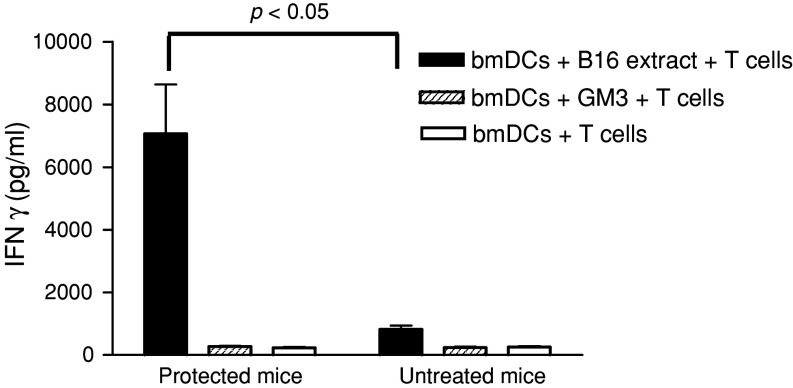
Splenic T cells were obtained from mice remaining free of tumours 8 weeks after the B16 challenge or from normal mice by magnetic beads selection. These cells were further stimulated in vitro during 96 h with bmDCs that had been previously incubated with B16 lysates, GM3 or medium alone. The IFNγ concentration in the supernatant was assessed by ELISA. T cells derived from surviving-immunized mice, stimulated by bmDCs presenting antigens from melanoma cells, secreted seven times more IFNγ than the corresponding cells from normal mice (p < 0.05, Mann–Whitney test) (Fig. 5). On the contrary T cells incubated with GM3-pulsed bmDCs or bmDCs alone did not release IFNγ. Our data suggest that most probably IFNγ-secreting T cells, elicited by vaccination in mice surviving tumour challenge, are directed to melanoma antigens other than GM3 ganglioside.
Fig. 5.
T lymphocytes from protected mice secrete IFNγ after stimulation with bmDCs incubated with B16 lysates. T cells derived from spleens taken from surviving mice 8 weeks after B16 inoculation or from normal mice were purified using MACS beads. These cells were further stimulated with bmDCs previously incubated with: B16 cell lysates, GM3 ganglioside or empty bmDCs, as described in “Materials and methods”. After 96 h culture, supernatants were harvested and IFNγ release was measured by ELISA. No IFNγ was produced by T cells from normal mice. The mean ± SD of IFNγ determinations from five mice in the vaccinated tumour-free animals and three mice in the reference group are shown. Statistical analysis was performed using the Mann–Whitney test (p < 0.05). One representative experiment of three is shown
CD8+ T cells from surviving-immunized animals secrete IFNγ following stimulation with MHC class I high B16 melanoma cells
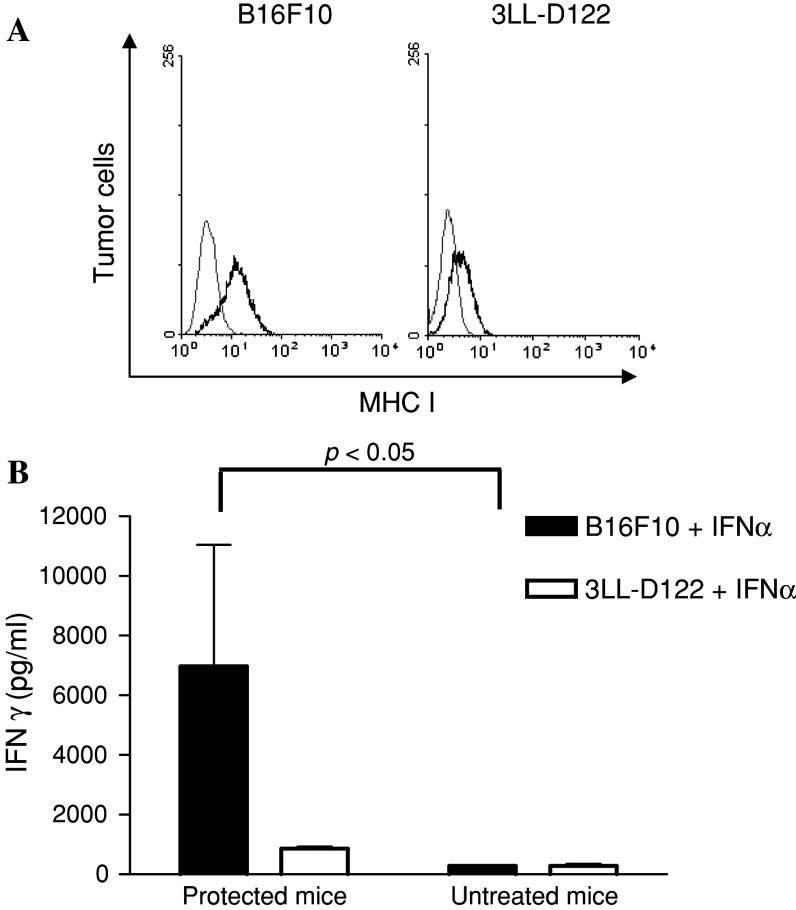
Poorly-immunogenic murine B16 melanoma cells express low levels of MHC class I molecules and this expression is modulated by type 1 interferons [16]. Addition of IFNα to B16F10 and syngeneic 3LL-D122 Lewis lung carcinoma cells increased the amount of MHC I molecules in both cellular membranes, as confirmed by flow cytometry (Fig. 6a).
Fig. 6.
T cells from protected mice secrete IFNγ after stimulation with MHC class I high B16 melanoma cells. Spleen T cells from vaccinated mice surviving at least 8 weeks after B16 inoculation or from normal mice were purified using MACS beads. Lymphocytes were further stimulated with irradiated B16 melanoma cells or syngeneic 3LL-D122 Lewis lung carcinoma cells, treated with IFNα. After 96 h culture supernatants were harvested and IFNγ release measured by ELISA. a About 5 × 105 cells from B16F10 or 3LL-D122 Lewis lung carcinoma were treated with 1,000 U/ml of IFNα for 16 h. Enhancement of MHC class I molecule expression was monitored by flow cytometry with a FITC-conjugated anti-MHC I (H-2Kb) antibody. Gray lines show staining with isotype-matched control Ab. b Mean ± SD of IFNγ determinations in samples from five mice in the vaccinated tumour-free group and three mice in the reference group are shown. Spleen T cells, obtained from untreated mice, were unable to secrete IFNγ independently of the provided stimuli. Statistical analysis was performed using the Mann–Whitney test (p < 0.05). One representative experiment of three is shown
Eight weeks after tumour challenge, purified spleen T cells from surviving-vaccinated or from untreated mice, were stimulated in vitro during 96 h with irradiated B16 or 3LL-D122 cells, previously treated with IFNα or with culture medium. The production of IFNγ in supernatants was quantified by ELISA. T cells derived from vaccinated mice rejecting B16 tumour challenge were able to secrete significant amounts of IFNγ only if they were co-cultured with MHC class I high B16 melanoma cells (p < 0.05, Mann Whitney test). When the stimulus came from 3LL-D122 cells (also pre-treated with IFNα) IFNγ was almost absent from the supernatants (Fig. 6b). T cells did not release IFNγ in the presence of MHC I low counterparts cell lines (data not shown). These results indicate how the vaccination process induced, in protected mice, effectors CD8+ T cells specific for melanoma antigens.
Discussion
The incidence of malignant melanoma is rising faster than any other malignancy. A large number of patients go on to develop metastatic disease, in which therapeutic options are very limited [17]. The therapeutic vaccine effort that has accumulated the largest amount of clinical results has been the development of vaccines for melanoma patients. Unfortunately, a lack of benefit has been seen in most of the clinical studies [18]. One of the reasons for this poor effectiveness is that the antitumour vaccination trials have included patients in late stages of the disease in which the development of tumour-induced immunosuppression significantly interferes with immunization. It is likely that active immunotherapy could operate more efficiently early during tumour progression or could prevent the disease in patients at a high risk of having malignant lesions. In the case of melanoma, a complex interaction of genetics, host and environmental factors contribute to the development of the disease [4].
In previous studies, we have shown that prophylactic administration of the VSSP/GM3 vaccine, produced a significant long-lasting tumour protection in mice challenged with B16 melanoma cells [13]. Recently, we have published the induction of a potent anti-tumour effect of this vaccine administered in a minimal residual disease B16 melanoma model [19]. The precise mechanism by which VSSP/GM3 vaccine conferred tumour protection was unknown.
Here, we reported the cellular and humoral requirements for the antitumour activity of VSSP/GM3 vaccine in the prophylactic B16 melanoma model. It was published that this vaccine consistently induced high titers of IgM- and IgG-specific anti-GM3 antibodies [13, 20]. However, the persistence of this antibody response after the tumour challenge and its correlation with the capacity to reject the tumour was not studied. In the present work, we found that the titers of IgG antibodies specific to GM3 and the frequency of responder animals were higher in the group of surviving-vaccinated animals as compared to the vaccinated animals developing tumours. Additionally, the anti-GM3 IgG antibody response correlated with the capacity of immunized animals to reject B16 melanoma challenge. In line with these findings, our clinical results suggest a positive correlation between antiganglioside IgG antibodies titers and prolonged survival of advanced breast cancer patients immunized with a ganglioside-based vaccine (manuscript in preparation). In contrast, previous papers have shown an association between vaccine-induced anti-GM3 IgM antibodies and tumour protection when C57BL/6 mice were immunized with a melanoma cell-based vaccine [21]. These results have been also found in clinical studies, in which a positive correlation between elevated antiganglioside IgM antibody titers and improved survival for melanoma and soft tissue sarcoma patients has been reported [10, 22, 23]. It has been speculated that anti-ganglioside IgM antibodies are effective against tumours because the capacity of these immunoglobulins to neutralize and clear immunosuppressive gangliosides, eliminating tumour cells by CMC [24].
Regarding the functional properties of the anti-GM3 IgG antibodies induced by the VSSP/GM3 vaccine, we previously reported their capacity to evoke complement mediated cytotoxicity against B16F10 cells in BALB/c mice using heterologous complement. In the present work, we found that anti-GM3 antibodies that persisted after tumour challenge in surviving-vaccinated mice, were able to induce CMC. In this case, autologous complement was used. However, this result should be confirmed in further experiments with larger amount of mice, the CMC activity by IgG antibodies may play an important role in melanoma eradication.
Glycolipids are generally categorized as T lymphocyte independent because they are not presented by classical presentation molecules [6]. In the last years, this category became controversial due to the finding of CD1-restricted human T cells specific for GM1 ganglioside in healthy donors and multiple sclerosis patients [7]. These activated lymphocytes were able to secrete cytokines such as IFNγ and TNFα. Additionally, GD3 ganglioside can be presented by CD1d to mouse NKT cells [25]. Despite these findings, no ganglioside-specific T cell induction by a ganglioside-based vaccine has been described so far. There were some evidences of T cell involvement in VSSP/GM3 immunized mice that elicited antibodies of IgG2b subclass [11, 13] but this finding needed to be directly confirmed. We reported here, for the first time, the cellular requirements for the induction of tumour protection in animals immunized with this type of vaccine. In our in vivo depletion experiments, we found that CD8+ T cells, but not NK1.1+ cells were essential for tumour protection. These activated CD8+ T lymphocytes are generated in vaccinated mice after the inoculation of the tumour and seem to be specific for other melanoma antigens different from gangliosides, as it was suggested by in vitro T cells assays. In those experiments, T cells from surviving-immunized mice were primed to produce IFNγ after co-culture with DCs loaded with B16 tumour extract but not GM3-pulsed DC. Moreover, these T cells secreted IFNγ when were stimulated specifically with MHC I upregulated B16 cells after treatment with IFNα. It is known that B16 melanoma is a tumour with well-established mechanisms of tolerance induction and immune escape. One of these mechanism is the downregulation of class I MHC molecules and antigen processing machinery [26, 27]. Moreover, it has been extensively shown that IFNγ is required in vaccine-induced antitumour immunity, specifically it is effective against B16 melanoma cells [28, 29]. Considering these findings, we can hypothesize that the vaccine could induce cytokine release such as interferons able to upregulate the MHC I expression on tumour cells, which could be then eliminated by CTL specific for melanoma antigens. By contrasting, upregulation of MHC I expression prevents recognition of tumour cells by NK cells which, in our data set, were not necessary in the effector phase of the antitumour response of the vaccine.
CD4+ T cells are thought to have a broader role in providing help to CTL and B cells, as well as a direct role in tumour rejection [30, 31]. An important role of CD4+ T cells has been reported in previous studies using vaccination with DCs loaded with peptides eluted from whole tumour cells [32] or with irradiated, whole tumour vaccines [33]. In the case of B16 tumours protection, a clear involvement of CD4+ T cells has been demonstrated in a prophylactic setting, when a DC loaded with melanoma cells has been used as vaccine [34, 35]. In contrast, in our in vivo depletion experiments the elimination of CD4+ T helper cells during the induction phase of the immune response abrogated neither the antitumour activity against B16 melanoma nor the induction of anti-GM3 IgG antibodies. However, the mechanism by which antibodies are generated in the absence of CD4+ help is not clear, it might be that other cells such as NK or NKT could function as helpers. NK cells fulfill essential accessory function for the priming of Ag-specific CTL [36] and for the activation of B cells to produce IgG [37, 38]. On the other hand, it was published that NKT cells cooperate with B cells to induce a specific antibody responses in mice immunized with a protein subunit vaccine [39]. The role of NK or NKT cells during the induction phase of immune response with our vaccine remains to be elucidated in further depletion experiments.
Experiments in BALB/c mice that are transgenic for activated rat Erbb2 have demonstrated that effective protection requires not only CTL response but also antibody response. In addition, adoptive-transfer experiments show that better protection is transferred when the recipient mice receive both antibodies and T cells from the immunized donor mice [40, 41]. This finding indicates that effective vaccines should be designed to elicit both humoral and cellular immunity which, in the case of preventive vaccine, should persist for very long periods. In our vaccine, the protection against B16 melanoma depends on humoral and cellular response. Our results suggested that the mechanism of protective immunity associated to the vaccine could start with the induction of T-independent anti-GM3 antibodies. Once tumour cells are inoculated, specific IgG kill tumour cells by complement-mediated mechanism. Tumour debris creates an inflammatory environment where new tumour antigens are taken up by resident and peripheral APC, particularly DCs. These cells could have been additionally activated by “danger signals” present in the vaccine, since VSSP has been reported to strongly provoke DC maturation [42]. In the following step of the effector phase, CD4+ T cells may be crucial for the induction of the antitumour immunity. In this sense, properly activated DCs are able to present tumour antigens to CD4+ T cells, which can function as helper for CTL response. IFNγ-secreting CD8+ cytotoxic lymphocytes, specific for melanoma antigens, could efficiently destroy tumour cells, leading to tumour rejection.
Based of these findings, is feasible propose the VSSP/GM3 vaccine as a therapy designed to elicit antitumour immunity in subjects with premalignant lesions or in healthy individuals with high genetic risk to develop malignant melanoma.
Acknowledgments
The authors thank Dr. Carmen Viada for statistical advice, Armando López and Johan González for excellent technical assistance and Dr. Tania Crombet for her careful English revision.
Abbreviations
- VSSP
Very small size proteoliposomes
- FCS
Fetal calf serum
- OMP
Outer membrane proteins
- MAbs
Monoclonal antibodies
- PI
Propidium iodide
- PNPP
p-Nitrophenylphosphate
- CMC
Complement-mediated cytotoxicity
- bmDCs
Bone-marrow-derived dendritic cells
Footnotes
Grant support: Center of Molecular Immunology, Elea Laboratories and Recombio.
References
- 1.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Kirk GD, Bah E, Montesano R. Molecular epidemiology of human liver cancer: insights into etiology, pathogenesis and prevention from The Gambia, West Africa. Carcinogenesis. 2006;27:2070–2082. doi: 10.1093/carcin/bgl060. [DOI] [PubMed] [Google Scholar]
- 3.Roden RB, Monie A, Wu TC. Opportunities to improve the prevention and treatment of cervical cancer. Curr Mol Med. 2007;7:490–503. doi: 10.2174/156652407781387127. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenblith MR, Goldstein AM, Tucker MA, Fraser MC. Genetic testing for melanoma predisposition: current challenges. Cancer Nurs. 2007;30:452–459. doi: 10.1097/01.NCC.0000300165.98391.e7. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton WB, Helling F, Lloyd KO, Livingston PO. Ganglioside expression on human malignant melanoma assessed by quantitative immune thin-layer chromatography. Int J Cancer. 1993;53:566–573. doi: 10.1002/ijc.2910530407. [DOI] [PubMed] [Google Scholar]
- 6.Ishioka GY, Lamont AG, Thomson D, Bulbow N, Gaeta FC, Sette A, Grey HM. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992;148:2446–2451. [PubMed] [Google Scholar]
- 7.Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29:1667–1675. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 9.Livingston PO, Ragupathi G. Carbohydrate vaccines that induce antibodies against cancer. 2. Previous experience and future plans. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravindranath MH, Muthugounder S, Hannah MR, Morton DL. Significance of endogenous augmentation of antiganglioside IgM in cancer patients: potential tool for early detection and management of cancer therapy. Ann N Y Acad Sci. 2007;1107:212–222. doi: 10.1196/annals.1381.023. [DOI] [PubMed] [Google Scholar]
- 11.Estevez F, Carr A, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP) Vaccine. 1999;18:190–197. doi: 10.1016/S0264-410X(99)00219-4. [DOI] [PubMed] [Google Scholar]
- 12.Alonso DF, Gabri MR, Guthmann MD, Fainboim L, Gomez DE. A novel hydrophobized GM3 ganglioside/Neisseria meningitidis outer-membrane-protein complex vaccine induces tumor protection in B16 murine melanoma. Int J Oncol. 1999;15:59–66. doi: 10.3892/ijo.15.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Carr A, Mazorra Z, Alonso DF, Mesa C, Valiente O, Gomez DE, Perez R, Fernandez LE. A purified GM3 ganglioside conjugated vaccine induces specific, adjuvant-dependent and non-transient antitumour activity against B16 mouse melanoma in vitro and in vivo. Melanoma Res. 2001;11:219–227. doi: 10.1097/00008390-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Harris N, Campbell C, Le Gros G, Ronchese F. Blockade of CD28/B7 co-stimulation by mCTLA4-Hgamma1 inhibits antigen-induced lung eosinophilia but not Th2 cell development or recruitment in the lung. Eur J Immunol. 1997;27:155–161. doi: 10.1002/eji.1830270123. [DOI] [PubMed] [Google Scholar]
- 15.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 16.Hofbauer GF, Geertsen R, Laine E, Burg G, Dummer R. Impact of interferons on the expression of melanoma-associated antigens in melanoma short-term cell cultures. Melanoma Res. 2001;11:213–218. doi: 10.1097/00008390-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Koon HB, Atkins MB. Update on therapy for melanoma: opportunities for patient selection and overcoming tumor resistance. Expert Rev Anticancer Ther. 2007;7:79–88. doi: 10.1586/14737140.7.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabri MR, Mazorra Z, Ripoll GV, Mesa C, Fernandez LE, Gomez DE, Alonso DF. Complete antitumor protection by perioperative immunization with GM3/VSSP vaccine in a preclinical mouse melanoma model. Clin Cancer Res. 2006;12:7092–7098. doi: 10.1158/1078-0432.CCR-06-1075. [DOI] [PubMed] [Google Scholar]
- 20.Gabri MR, Ripoll GV, Alonso DF, Gomez DE. Role of cell surface GM3 ganglioside and sialic acid in the antitumor activity of a GM3-based vaccine in the murine B16 melanoma model. J Cancer Res Clin Oncol. 2002;128:669–677. doi: 10.1007/s00432-002-0385-7. [DOI] [PubMed] [Google Scholar]
- 21.Ravindranath MH, Brazeau SM, Morton DL. Efficacy of tumor cell vaccine after incorporating monophosphoryl lipid A (MPL) in tumor cell membranes containing tumor-associated ganglioside. Experientia. 1994;50:648–653. doi: 10.1007/BF01952865. [DOI] [PubMed] [Google Scholar]
- 22.Perez CA, Ravindranath MH, Soh D, Gonzales A, Ye W, Morton DL. Serum anti-ganglioside IgM antibodies in soft tissue sarcoma: clinical prognostic implications. Cancer J. 2002;8:384–394. doi: 10.1097/00130404-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Ravindranath MH, Hsueh EC, Verma M, Ye W, Morton DL. Serum total ganglioside level correlates with clinical course in melanoma patients after immunotherapy with therapeutic cancer vaccine. J Immunother. 2003;26:277–285. doi: 10.1097/00002371-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ragupathi G, Liu NX, Musselli C, Powell S, Lloyd K, Livingston PO. Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol. 2005;174:5706–5712. doi: 10.4049/jimmunol.174.9.5706. [DOI] [PubMed] [Google Scholar]
- 25.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]
- 27.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Immunosuppression in melanoma immunotherapy: potential opportunities for intervention. Clin Cancer Res. 2006;12:2359s–2365s. doi: 10.1158/1078-0432.CCR-05-2537. [DOI] [PubMed] [Google Scholar]
- 28.Prevost-Blondel A, Neuenhahn M, Rawiel M, Pircher H. Differential requirement of perforin and IFN-gamma in CD8 T cell-mediated immune responses against B16.F10 melanoma cells expressing a viral antigen. Eur J Immunol. 2000;30:2507–2515. doi: 10.1002/1521-4141(200009)30:9<2507::AID-IMMU2507>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 29.Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Immunotherapy of melanoma: a dichotomy in the requirement for IFN-gamma in vaccine-induced antitumor immunity versus adoptive immunotherapy. J Immunol. 2001;166:7370–7380. doi: 10.4049/jimmunol.166.12.7370. [DOI] [PubMed] [Google Scholar]
- 30.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 31.Perales MA, Wolchok JD. CD4 help and tumor immunity: beyond the activation of cytotoxic T lymphocytes. Ann Surg Oncol. 2004;11:881–882. doi: 10.1245/ASO.2004.08.911. [DOI] [PubMed] [Google Scholar]
- 32.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol. 2003;171:5940–5947. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 35.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+ CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Zhang J, Tian Z. The regulatory effect of natural killer cells: do “NK-reg cells” exist? Cell Mol Immunol. 2006;3:241–254. [PubMed] [Google Scholar]
- 37.Gray JD, Horwitz DA. Activated human NK cells can stimulate resting B cells to secrete immunoglobulin. J Immunol. 1995;154:5656–5664. [PubMed] [Google Scholar]
- 38.Gao N, Dang T, Dunnick WA, Collins JT, Blazar BR, Yuan D. Receptors and counter receptors involved in NK–B cell interactions. J Immunol. 2005;174:4113–4119. doi: 10.4049/jimmunol.174.7.4113. [DOI] [PubMed] [Google Scholar]
- 39.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croci S, Nicoletti G, Landuzzi L, De Giovanni C, Astolfi A, Marini C, Di Carlo E, Musiani P, Forni G, Nanni P, Lollini PL. Immunological prevention of a multigene cancer syndrome. Cancer Res. 2004;64:8428–8434. doi: 10.1158/0008-5472.CAN-04-2341. [DOI] [PubMed] [Google Scholar]
- 41.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 42.Mesa C, De Leon J, Rigley K, Fernandez LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine. 2004;22:3045–3052. doi: 10.1016/j.vaccine.2004.02.010. [DOI] [PubMed] [Google Scholar]