Abstract
Dendritic cell (DC)-based immunotherapy has not been as effective as expected in most solid tumors even in the murine model, particularly in renal cell carcinoma (RCC). Our investigation was initiated to identify what causes the limitations of DC-based immunotherapy in solid RCC. We have investigated immunosuppressive factors from tumors and their effects on DC migration, as well as cytotoxic T lymphocyte (CTL) response and lymphocyte infiltration into the tumor mass upon vaccination with mouse renal adenocarcinoma (Renca) cell lysate-pulsed bone marrow (Bm)-derived DC in tumor-bearing mice. We also investigated pulmonary metastasis- and tumor recurrence-inhibitory effects of DC-vaccination in the solid tumor-bearing mice. In these experiments, we found that the limitations of DC-based immunotherapy to solid RCC likely result from tumor-mediated TGF-β hindrance of immune attack rather than insufficient immune induction by DC therapy. In fact, the CTL response induced by DC therapy was quite sufficient and functional for the inhibition of tumor recurrence after surgery or of tumor metastasis induced by additional tumor-challenge to the tumor-bearing mice. Taken together, our present results obtained in mouse model suggest the potential of DC immunotherapy in tumor patients for hindering or blocking disease progression by inhibition of tumor metastasis and/or tumor recurrence after surgery.
Keywords: Dendritic cells, Renal cell carcinoma, T cell proliferation, TGF-β, Metastasis, Tumor recurrence
Introduction
It has been reported that DCs are able to enhance the therapeutic antitumor immune response when vaccinated after being pulsed with tumor lysates in vitro [17, 30, 46]. Indeed, DC therapy has already been found to be somewhat efficacious in the treatment of human prostate cancer, B cell lymphoma, and melanoma, as well as in a number of murine tumor models [3, 8, 12, 13, 41]. Moreover, in parallel with this, basic investigations for different DC subsets have been also performed at the cellular level [1, 32]. However, several reports have shown that a number of obstacles must be overcome before DC-based immunotherapy can be used for general application in tumor therapy [7, 35]. One of the major difficulties in the treatment of advanced tumors seems to be the ability of the tumor cells to suppress the patient immune response against the tumor. It has recently been reported that regulatory T (Treg) cells producing TGF-β, IL-10, and IL-4 play an important role in the control of immune reactivity against self- and/or non-self antigens [14, 24, 36, 40, 42]. Several Treg subsets have been identified that are capable of inhibiting autoimmune and chronic inflammatory responses and maintaining immune tolerance in tumor-bearing hosts.
Recently, a DC vaccination strategy for RCC using a tumor cell-DC hybrid has shown therapeutic potential [22, 23]. Although such trials were promising, several limitations still remain to be resolved in order to generate a more effective antitumor response. A good animal model for developing DC strategies to vaccinate against RCC would be helpful in optimizing and overcoming many technical challenges posed by immunotherapeutic approaches to RCC [6, 44]. We have examined the efficacy of DC vaccination for the treatment of RCC in the murine RCC model established by inoculation with mouse renal adenocarcinoma Renca cells. The Renca model of RCC fulfills many of the requirements for a useful animal tumor model and forms a primary tumor mass within the mouse. [44]. Renca shows progressive disease stages similar to human RCC [21]. We have examined whether Renca cells induce any of the immunosuppressive effects generally reported in human RCC and other solid tumors, which might attenuate T cell-mediated antitumor immunity.
There have been many attempts to treat Renca with some success, such as cytokine therapies with IL-12 and IL-2, and gene therapies with apoptosis-related molecules [34, 37, 43]. These methods are very useful in providing further understanding about Renca, but they are not applicable in humans, mostly because of toxicity or lack of effectiveness. However, a DC vaccine would be the best method to reduce tumor growth in tumor-bearing mice and would be also applicable to humans. Using the Renca model, we attempted to determine whether the DC vaccine could be a strategy of choice to treat RCC and overcome the immune suppression similar to that observed in human RCC. Additionally, we have examined the use of DCs to stimulate the immune response against tumors and found that it is appropriate for use as a therapeutic vaccine against RCC even though some obstacles remain before general application is possible.
Materials and methods
BmDC generation
BmDCs were generated from bone marrow progenitors as described previously [31], with the following modifications. In brief, Bm single-cell suspensions were prepared, the cells were washed, and the washed cells were cultured in RPMI-1640 containing 10% FBS (Life Technologies, Grand Island, NY, USA) (RPMI-10), glutamine, penicillin/streptomycin, rmGM-CSF (10 ng/ml) (Endogene, Woburn, MA, USA), and rmIL-4 (10 ng/ml) (Endogene, Woburn, MA, USA). At day 2, the cultures were vigorously pipetted and non-adherent cells were removed and discarded. Fresh RPMI-10 containing cytokines was added to the adherent cells. At days 4 and 6, half of the culture supernatant was collected and centrifuged, and then the cell pellet was resuspended in RPMI-10 containing cytokines and returned to the original plate. At day 7, non-adherent or loosely attached cells, which included DCs, were collected. For BmDC maturation, Day 6-DCs were incubated with freeze-thawed tumor lysates (from in vitro cultures of Renca tumor cells) at the ratio of three tumor cells to one DC (i.e., 3:1) in RPMI-1640, as described previously [10, 28]. After incubation for 8 h, DCs were further matured in the presence of rmTNF-α (100 U/ml) (Endogene, Woburn, MA, USA) and IFN-γ (100 U/ml) (Endogene, Woburn, MA, USA). After 16 h of incubation, DCs were harvested, washed twice in Hank’s buffered salt solution (HBSS), and resuspended in normal saline or RMPI-1640 for further studies.
FACS analysis
For phenotypic analysis, direct immunofluorescence was used for cell-surface staining of mature BmDCs, which were stained in FACS buffer (0.2 BSA, 0.02 sodium azide in PBS) with 1 × 105 cells per staining. Antibody incubation (CD11b, CD11c, CD40, CD54, MHC class I/II, CD80, CD86, CD4/8, and CD14/19 purchased from Pharmingen, San Diego, CA, USA) was performed at 4°C for 20 min. Data were reported as a histogram or dot plot by using FACSCalibur (BD) with CellQuest software.
Quantitation of antigen uptake
Cells (5 × 105) were equilibrated at 37°C or 0°C for 10 min and then pulsed with fluorescein-conjugated dextran (Molecular Probes, Eugene, OR, USA) at a concentration of 1 mg/ml. After varying periods of incubation at 37°C or 0°C, cold staining buffer (or cold PBS) was added to stop the reaction. Cells were washed three times and analyzed by FACScan. Non-specific binding of dextran to DC, as determined by incubation of DC with fluorescein-conjugated dextran at 0°C, was subtracted. Moreover, for the identification of DC phagocytosis, 7-AAD (20 μg/ml) (7-amino-actinomycin, BD Pharmingen, San Diego, CA, USA)-labeled apoptotic Renca tumor cells (for 30 min at room temperature) were incubated with day 6-BmDCs that were labeled with FITC-conjugated MHC class II antibody for 1 h. After three washes with PBS, cells were visualized under a confocal spectral microscope (LEICA) following excitation with a 600 nm argon-ion laser. A fluorescence image was taken at 545 nm emission (software zoom ×4, original magnification ×1,000). For UV-generated apoptotic tumor cells, labeled Renca cells (1 × 106/ml) were irradiated with a pair of FS40 sunlamps (290–320 emission spectra). Cells received a total dose of 90 J/m2 at 3.8 W/m2 (∼LD50).
In vivo immunization and tumor challenge
Renca cells (the Balb/c renal adenocarcinoma cell line obtained from Samsung Medical Center, Seoul, South Korea) were trypsinized, washed twice in 1 × HBSS to eliminate traces of serum proteins, and resuspended in normal saline at 1 × 107 cells/ml (for injection). Seven-to-ten-week female Balb/c mice (DaeHan BioLinks, ChungNam, Republic of Korea) were then challenged subcutaneously (sc) in the left flank with 100 μl (1 × 106 cells) of cell suspension. Animals were housed at the Animal Maintenance Facility of the CreaGene Research Institute (Gyeonggi, South Korea). After 3 days, Renca tumor lysate (approximately 100 μg of proteins)-pulsed BmDCs (1 × 106) were immunized sc in the right flank twice, on days 3 and 10, after tumor implantation. The size of each tumor was assessed in a blinded, coded fashion every 3 days using calipers, and the largest perpendicular diameter of the tumor was recorded as the tumor area (in square mm). Data are reported as the average tumor size ± SD. For the treatment of pulmonary metastasis, Balb/c mice were challenged intravenously (i.v.) in the lateral tail vein with 1 × 106 Renca tumor cells. The mice were then immunized sc with 1 × 106 DCs (tumor lysate-pulsed) on days 3 and 10 after tumor injection, and were killed on day 17. The lungs were removed and fixed in Bouin’s fluid (Sigma-Aldrich, St. Louis, MO, USA) for 10 min. The lobes of the lungs were separated, and the total number of superficially visible colonies per lung was counted. Lungs containing tumor nodules were photographed. Additionally, survival of tumor-implanted and DC-vaccinated mice was observed for approximately 60 days. However, 13 days after sc tumor implantation, DC-vaccinated tumor-bearing mice were challenged a second time with Renca cells (1 × 106, 1 × 105 or 1 × 104) through the sc route in order to examine the remaining anti-tumor immunity induced by vaccination with DCs. The results were evaluated 28 days after the first tumor inoculation. In addition, the tumors of mice vaccinated sc with DCs were removed 30 days after sc tumor inoculation, and were then observed for additional 30 days to evaluate tumor recurrence after surgery (including suture). For an additional trial, 21 days after sc tumor implantation, DC-vaccinated tumor-bearing mice were tumorectomized and iv challenged with Renca cells (1 × 106) 7 days after tumor surgery. The mice were then killed on day 50. The lungs were removed as described above, and the total number of tumor colonies per lung was counted.
Reverse transcription (RT)-PCR
To investigate mRNA transcription for the immunosuppressive agents in Renca cells isolated from the tumor mass or passaged in vitro, total RNAs were extracted from Renca cells using TRIZOL reagent (InVitrogen, Carlsbad, CA, USA) and purified using an RNeasy total RNA isolation kit (Qiagen, Valencia, CA, USA) according to the instructions of the manufacturer. One μg of total RNA was mixed with 50 μM oligo-(dT)20 and 10 mM dNTP mix, heated at 65°C for 5 min, and placed on ice for at least 1 min. Then, 10 × RT buffer ([25] mM MgCl2, 0.1 M DTT, RNaseOUT™, 40 U) and 1 μl of SuperScript™ III RT (200 U/μl, InVitrogen, Carlsbad, CA, USA) were added, and the mixture was incubated at 42°C for 1 h. The reaction was terminated by incubation at 75°C for 5 min, followed by chilling on ice. The PCR was performed using the cDNA as a template along with certain gene-specific primers.
The initial cDNA content in each sample was normalized with the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplification reactions were performed in a 20-μl volume using 5 ng or 10 ng of each cDNA on a Perkin-Elmer DNA thermocycler 9600 Prism for 35 cycles. The PCR reactions were separated on 1.2% agarose gels and stained with ethidium bromide. Moreover, RT-PCR was performed as described above in order to examine the effects on the chemokine expression of mature DCs in the presence of TGF-β (30 or 125 ng/ml, for 24 h) (R&D system, Minneapolis, MN, USA).
ELISA
Culture supernatants were prepared from 7-day cultures of Renca cells (1 × 106 cells) from sc tumor masses or in vitro subcultures. Mouse sera were prepared from sc tumor-bearing mice and pulmonary metastatic tumor-bearing mice 17 days after tumor inoculation. The serum of tumorectomized mice was prepared a week after surgery from day-17 sc tumor-bearing mice. The culture supernatants and mouse sera were analyzed for TGF-β expression using a commercially available ELISA kit (BioSource, Camarillo, CA, USA).
DC chemotaxis assay
Chemotactic ability of the DCs treated with TGF-β (30 or 125 ng/ml) was analyzed using the ChemoTx system (96-well ChemoTx Chamber; NeuroProbe, Gaithersburg, MD, USA) as previously described [26]. In brief, mature DCs were resuspended in RPMI-10 and treated with TGF-β. Chemokines (rmMIP-1α, rmMIP-1β, rmMIP-3α, rmMIP-3β, rmRANTES, and rm6C-kine purchased from R&D system, Minneapolis, MN, USA) at 1 μg/ml were placed in the lower chamber, and a filter with a pore size of 5 μm was placed on top. Aliquots of 1 × 106 cells/well were applied to the top surface of the filter, and the plate was incubated at 37°C in 5% CO2 for 4 h. The cells that migrated to the bottom chamber were analyzed for CD11c (Pharmingen, San Diego, CA, USA) expression by FACS. For the marker, 104 cells were counted in the gate.
Immunohistochemistry
To investigate DC migration into regional lymph nodes in pulmonary metastatic or sc tumor-bearing mice (on day 17 after tumor implantation), Renca tumor lysate-pulsed DCs were incubated for 30 min at 37°C in 10 μM CMFDA (5-chloromethyl-fluorescein diacetate, Molecular Probes, Eugene, OR, USA) in serum-free RPMI, and were then pulsed for another 30 min at 37°C in serum-free RPMI. The DCs were washed, counted, and intradermally injected into the left footpads of the mice (1 × 106 cells). After 18 h, inguinal lymph nodes were isolated from each immunized mouse. Frozen tissues were sectioned at a thickness of 25 μm and mounted. The sections were then analyzed by confocal microscopy as described previously [25]. Moreover, tumor masses removed from sc tumor-bearing mice vaccinated with DC were sectioned at a thickness of 10 μm under cryostatic conditions. The sections were then fixed in 3.5% paraformaldehyde for 30 min, blocked with 10% normal mouse serum in PBS for 30 min, stained with FITC or PE-conjugated antibodies (CD3, CD4, CD8, MHC class I/II, IL-12, IFN-γ, CD25, NK1.1, and CD11c purchased from Pharmingen, San Diego, CA, USA) and analyzed by confocal microscopy. To identify the expression of TGF-β, the sections of tumor masses above were deparaffinized, hydrated, and then treated with 3% H2O2 in methanol to block the activity of the endogenous peroxidase. After washing with PBS (0.1 M, pH 7.4) for 5 min, the sections were treated with a 10% protein-blocking agent (Shandon, Waltham, MA, USA) for 1 h at room temperature to block nonspecific antigen-antibody reactions. These sections were incubated with 1:400 anti-TGF-β antibody (R&D system, Minneapolis, MN, USA) diluents for 12 h at 4°C and washed with PBS. They were then incubated with biotinylated secondary antibodies (Shandon) for 60 min at room temperature. After being washed with PBS, the sections were treated with streptavidin peroxidase reagent (Shandon) for 30 min at room temperature. After administering the peroxidase treatment, the tissue sections were washed with PBS and colorized with a 0.05% diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, USA) solution for less than 1 min.
T cell proliferation, CTL, and ELISPOT assay
Splenocytes obtained from sc or iv tumor-implanted mice (7 days after the second DC vaccination) were treated with ammonium chloride-potassium lysing buffer for 3 min to deplete erythrocytes, and were then washed twice with HBSS. These splenocytes were incubated in RPMI-10 for 90 min, and nonadherent cells were gently removed and incubated on nylon wool columns for 1 h at 37°C. After elution, the resulting cells were determined to be ∼90% CD3+ by FACS analysis (data not shown), and were used as responder cells. For proliferation assays, purified CD3+ T cells were seeded into round-bottomed microplates in the presence of 1 × 105 Renca lysate-pulsed DCs (at a responder-to-stimulator ratio of 20:1). T cells alone were used as the background control. After 5 days, 1 μCi of tritiated deoxythymidine (PerkinElmer) was added to each well for an additional 16 h. The incorporation of thymidine was determined using a liquid scintillation counter (Beckman). CTL assays were performed by crystal violet absorbance assay [38]. CTLs were prepared by stimulation with Renca tumor lysate-pulsed DC for 5 days, and were incubated with Renca tumor cells as a target for 24 h. After washing, target cells were stained with crystal violet (4 mg/ml in PBS, 100 μl/well) for 30 min at room temperature. The plate was washed with PBS and lysed using methanol. Target cell survival was analyzed by measuring the absorbance at 470 nm. IFN-γ ELISPOT analysis was performed using the spleen obtained after vaccination. Splenocytes from the spleen were cultured overnight in complete RPMI 1640 medium. CD8+ T cells were isolated from splenocytes by negative depletion (Miltenyi Biotec, Auburn, CA, USA). After blocking, 1 × 105 T cells and 1 × 104 Renca lysates-pulsed DCs were added to each well of 96-well nitrocellulose plates (Multiscreen-IP, Millipore, Bedford, MA, USA) precoated with 2 μg/ml IFN-γ capture antibody (Pharmingen, San Diego, CA, USA). Plates were incubated for 20 h at 37°C, and biotinylated IFN-γ detection antibody (Pharmingen, San Diego, CA, USA) was added to each well. Cells were then incubated for an additional 2 h at room temperature, then with streptavidin-alkaline phosphatase (1 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA); plates were developed with substrate (KPL, Gaithersburg, MD, USA). After washing, spots were counted using an automated ELISPOT reader (Carl Zeiss, Berlin, Germany).
Statistics
The Mann–Whitney U test was used for all statistical analysis. A p-value of less than 0.05 was considered significant.
Results
Generation of BmDCs
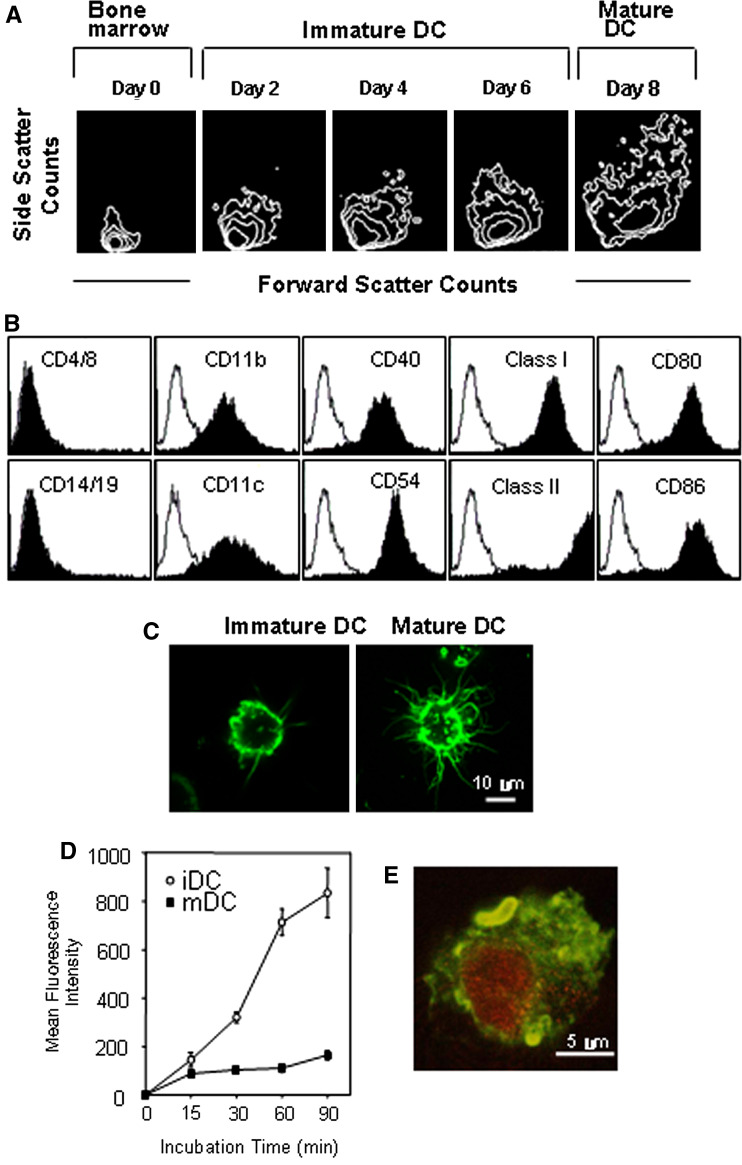
First, we demonstrated that BmDCs had typical morphology and surface phenotypes (Fig. 1a–c), and then assessed the ability of DCs to uptake the soluble fluorescent antigen, FITC-dextran, for various durations of time. It has been well documented that immature DCs were very effective in uptaking FITC-dextran as compared with mature DCs (Fig. 1d). Figure 1e shows that our BmDCs (stained with FITC-MHC class II antibody, green) completely engulfed Renca tumor cells (7-AAD labeled, red). Additionally, tumor lysate-pulsed DCs efficiently primed naïve syngeneic T cells in vitro in comparison with unpulsed DCs (data not shown).
Fig. 1.
Generation and characterization of BmDCs from Balb/c mice. a Increased cell size and granulity depend on the differential stages. b Surface phenotype of BmDC matured in the presence of INF-γ and TNF-α after tumor-lysate pulsing. Closed histograms show staining with test antibodies. c Representative morphology of immature (day 6) and mature (day 8) BmDCs. Cells were stained with FITC-conjugated anti-MHC class II antibody at the indicated time point. Representative images were obtained by confocal microscopy with a magnification of ×2,000 (software zoom ×2, original magnification ×1,000). d FITC-dextran uptake by immature and mature BmDCs. Cells were equilibrated at 37°C or 0°C prior to pulsing, and were then pulsed with fluorescein-conjugated dextran (40,000 m.w.) at a concentration of 1 mg/ml. After different durations of incubation, cold buffer was added to stop the reaction. Cells were analyzed using FACS, and non-specific binding was subtracted (incubation at 0°C). iDC immature DC, mDC mature DC. e Uptake of apoptotic Renca tumor cells by BmDCs (day 6). 7-AAD labeled apoptotic Renca tumor cells (red) were incubated with day 6-BmDCs (green), which were labeled with FITC-conjugated MHC class II antibody for 1 h. After washes with PBS, cells were visualized under a confocal spectral microscope with a magnification of ×4,000 (software zoom ×4, original magnification ×1,000)
DC vaccination was highly functional against pulmonary metastatic tumor, but not against sc tumor
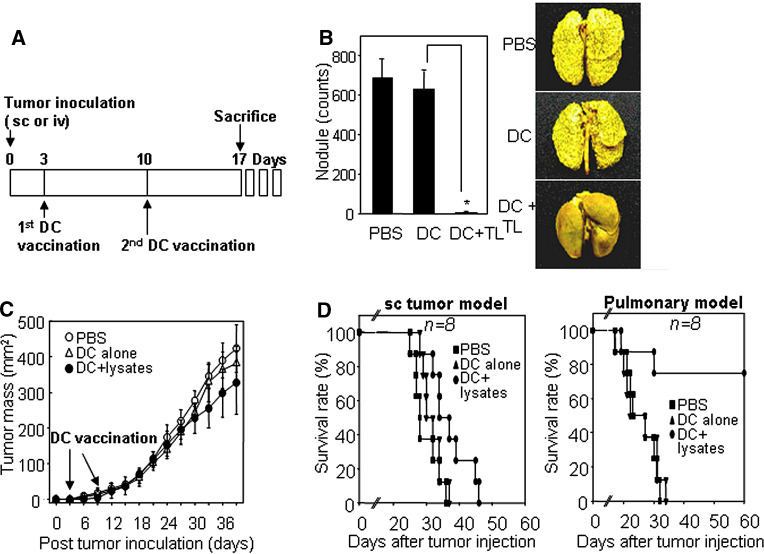
We next investigated whether tumor lysate-pulsed DCs could induce antitumor immune responses in mice bearing pulmonary metastatic or sc tumors. Pulmonary metastasis and sc tumors were established by iv or sc injection, respectively, using mouse renal adenocarcinoma Renca cells; it was previously reported that these cells can grow readily and cause metastasis in the lung, liver, and spleen when transplanted into syngeneic hosts, Balb/c mice [19]. Three days after iv or sc injection of mice with Renca cells, those mice were vaccinated with Renca lysate-pulsed DCs twice during a week interval (Fig. 2a). As shown in Fig. 2b, those mice treated with Renca lysate-pulsed DC showed little or no pulmonary metastatic tumors, while the lungs of control mice treated only with PBS or unpulsed DC were filled with metastatic tumor nodules. In contrast, those mice bearing sc tumors were not as susceptible to the DC therapy as shown in pulmonary metastatic model. Vaccination with Renca lysate-pulsed DC caused only partial inhibition of sc tumor growth as compared with the controls treated with PBS or unpulsed DCs (Fig. 2c). The different efficacies of DC therapy for the sc tumor and the pulmonary metastatic tumor were consistent with the survival rates of the vaccinated mice (Fig. 2d). Over 75% of the pulmonary metastatic tumor-bearing mice survived for over 60 days after treatment with Renca lysated-pulsed DCs, while most of the sc tumor-bearing mice were dead within 50 days after the same treatment, even though DC vaccination enhanced the life span of the tumor-bearing mice by a week or so.
Fig. 2.
DC vaccination was highly effective for survival and tumor regression in pulmonary metastatic tumor-bearing mice, but was not so effective for those of sc tumor-bearing mice. a A vaccination schedule. b Inhibition of pulmonary metastasis by vaccination with Renca tumor lysate-pulsed DCs. Balb/c mice received 1 × 106 viable Renca tumor cells in the lateral tail vein (i.v.) to establish pulmonary metastasis. Mice were then inoculated sc in the right flank with 1 × 106 DCs (Renca tumor lysate-pulsed) twice on days 3 and 10 after tumor injection, and were euthanized on day 17. Tumor nodules on the lungs were visualized by staining with Bouin’s fixative for 10 min (right image). Tumor nodules (shown in right panel) were enumerated, and the values represent the mean number of nodules ± SD of twenty mice per group (left panel). DC + TL tumor lysate-pulsed DC. Asterisk P < 0.01. PBS PBS control, DC alone mature DC, DC + lysates tumor lysate-pulsed DC. c Sc tumor progression after immunization with Renca tumor lysate-pulsed DC. Mice were inoculated (sc) with 1 × 106 viable Renca tumor cells in the left flank and immunized (sc) with Renca tumor lysate-pulsed DC in the right flank, as described above. The size of the tumor mass was recorded every 3 days as the tumor area (in square mm), and was determined by measuring the largest perpendicular diameter with calipers. Data shown are representative of five experiments and are reported as the average tumor size ± SD of eight mice per group. d Survival percentage of both pulmonary metastatic tumor-bearing mice and sc tumor-bearing mice after DC vaccination. Mice were evaluated daily until death. Data are representative of at least two independent experiments with eight mice per group
Renca tumor cells expressed high TGF-β1 titer when passaged in vivo
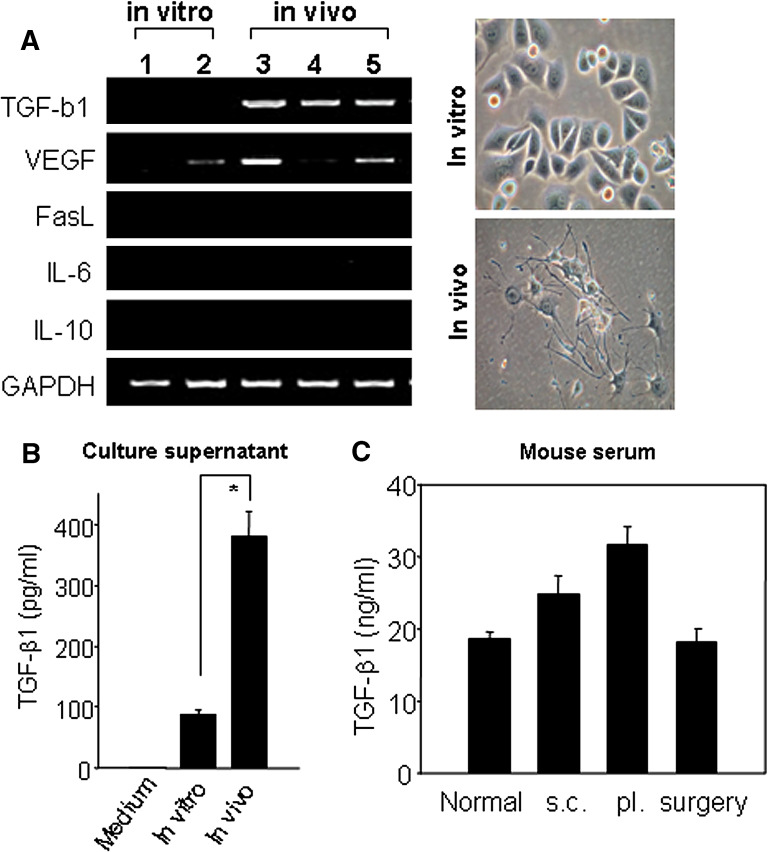
In order to figure out the reasons for the limited success of DC-based immunotherapy in sc tumors, we first examined the expression of immunosuppressive agents in sc tumor-bearing mice. We isolated tumor cells from sc tumor-bearing mice that had received two rounds of DC therapy or had received PBS or unpulsed DCs alone, and tested for the expressions of immunosuppressive molecules such as TGF-β1, vascular endothelial growth factor (VEGF), Fas ligand (CD95L), IL-6, and IL-10, which suppress T cell responses through various mechanisms. RT-PCR analysis indicated that TGF-β1 transcript was highly expressed in all Renca cells isolated from the primary sc tumors in three different mice, but it was undetectable in Renca cells passaged in vitro (Fig. 3a). VEGF expression, however, was not consistent even among the primary Renca cells. The level of TGF-β1 present in the culture supernatants of both in vitro cultured and in vivo passaged Renca tumor cells was determined by ELISA. On day 7 after culture, the level of TGF-β1 secreted from the primary Renca tumor cells (382.1 ± 39.3 pg/ml) was substantially and significantly higher than that secreted from Renca cells cultured in vitro (89.9 ± 7.0 pg/ml) (Fig. 3b). Importantly, these results strongly suggest that TGF-β1 may be related to a less severe antitumor immune response of DC vaccination in sc tumor-bearing mice. However, the systemic TGF-β1 concentration was found to be approximately 25 ∼ 30 ng/ml in the serum of mice bearing subcutaneous (sc) as well as pulmonary (pl) metastatic tumors; this concentration was slightly higher than those of control or tumorectomized mice (∼20 ng/ml; Fig. 3c).
Fig. 3.
TGF-β1 was enhanced for its expression among the immunosuppressive agents in Renca cells isolated from the tumor mass, but was not detected in Renca cells subcultured in vitro. a Screening of the expression of immunosuppressive agents by RT-PCR and their morphology from in vitro-cultured Renca tumor cells or in vivo-passaged tumor cells (subcutaneous). First-strand cDNA was synthesized from total RNA using reverse transcriptase and amplified with primers specific for TGF-β1, VEGF, Fas ligand, IL-6, IL-10 and GAPDH. Phase-contrast photographs were taken directly from the day 7 cultures of in vivo-passaged Renca tumor cells. Original magnification is ×400. 1, 2 In vitro-passaged Renca cells; 3, 4, 5 Renca cells obtained from three different tumor-bearing mice without any treatment, vaccinated with tumor lysate-pulsed DC, and treated with unpulsed DC, respectively. b Quantification of TGF-β1 in culture supernatants. Culture supernatants were prepared from 7-day cultures of Renca cells (1 × 106 cells) from sc tumor masses or in vitro subcultures. ELISA was performed as described in protocol of the manufacturer. Values shown are means ± SD of two experiments. Asterisk P < 0.01. In vitro in vitro-passaged tumor cells, in vivo cells from sc tumor. c Quantification of TGF-β1 in mouse sera. Mouse sera were obtained from sc tumor-bearing mice (s.c.), pulmonary metastatic tumor-bearing mice (pl.) on day 17 after tumor inoculation. The serum of tumor-rectomized mice (surgery) was prepared a week after surgery of day 17 sc tumor-bearing mice. Normal serum (normal) was prepared from 8-week-old Balb/c mice. ELISA was performed as described in protocol of the manufacturer. Values shown are means ± SD of 3 to 5 mice per group
Even in the sc tumor-bearing mice, DC migration and CTL induction were not attenuated when the mice were vaccinated with tumor lysate-pulsed DCs
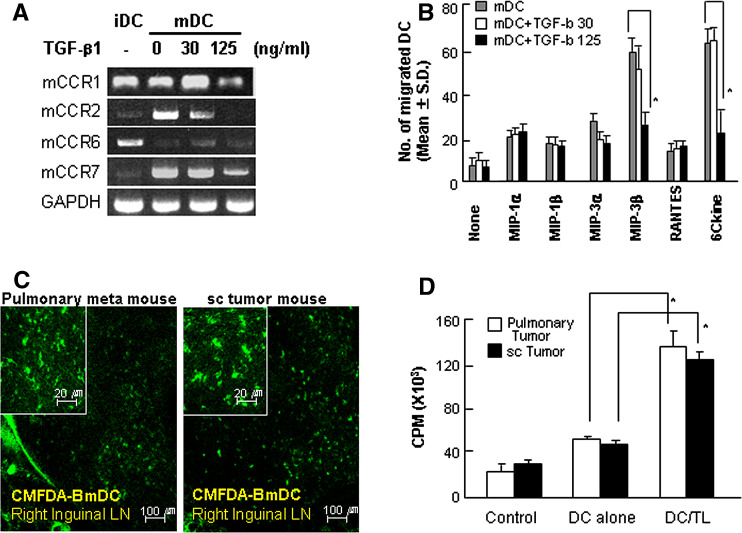
To determine the influence of TGF-β1 on DC migration in vivo, Renca tumor lysate-pulsed DCs were incubated in the presence of TGF-β1 at a concentration of 30 ng/ml or 125 for 24 h, and were then examined by chemotaxis assay and RT-PCR. Once matured, DCs expressed large amounts of CCR7, a major DC chemotactic receptor [27], which was maintained even in the presence of TGF-β1 at a concentration of 30 ng/ml (Fig. 4a). However, DCs incubated with 125 ng/ml TGF-β1 markedly lost their ability to migrate in response to MIP-3β and 6Ckine in a chemotaxis assay in vitro, whereas DCs incubated with 30 ng/ml TGF-β1 or untreated DCs migrated effectively in response to MIP-3b and 6Ckine (Fig. 4b). On the other hand, when using in vivo migration analysis, we were unable to detect any differences in the efficiency of DC migration to regional lymph nodes after DC vaccination between the mice bearing sc tumors and pulmonary metastatic tumors (Fig. 4c). These data indicated that tumor lysate-pulsed DCs are able to migrate effectively into regional lymph nodes even in the sc tumor-bearing mice, although the migratory capacities of DCs decreased when treated with high concentrations of TGF-β1 in vitro. In addition, no clear difference in tumor-specific T cell proliferation was detected between those different tumor-bearing mice on day 7 after the second DC vaccination (Fig. 4d).
Fig. 4.
No difference in the capacity of DC migration and T cell proliferation after DC vaccination between the mouse groups bearing pulmonary metastatic tumors and sc tumors, respectively. a Effect of TGF-β on the chemokine expression of mature DCs. Renca tumor lysate-pulsed DCs were incubated in the presence of 30 or 125 ng/ml of TGF-β1 for 24 h. First-strand cDNA was synthesized and amplified with primers for mCCR1, mCCR2, mCCR6, mCCR7, and GAPDH. iDC immature DC, mDC mature DC. b Effect of TGF-β on DC migration in chemotactic assay. Mature DCs were resuspended in RPMI-10 and treated with TGF-β. Chemokines at 1 μg/ml were placed in the lower chamber, and a filter with 5-μm pore size was placed on top. An aliquot of 1 × 106 cells/well were applied to the top surface of the filter, and the plate was incubated at 37°C for 4 h. Migrated cells were analyzed for CD11c expression by FACS. Each assay was performed in triplicate, and the results were expressed as the mean number of cells that migrate to the lower chamber ± SD. Asterisk P < 0.01. mDC mature DCs, mDC + TGF-b 30 mature DCs treated with 30 ng/ml TGF-β, mDC + TGF-b 125 mature DCs treated with 125 ng/ml TGF-β. c Immunohistochemistry of DC migration into regional lymph nodes in pulmonary metastatic tumor- and sc tumor-bearing mice. Renca tumor lysate-pulsed DCs were labeled with CMFDA and intradermally injected into the left foot pads of the mice. After 18 h, inguinal lymph nodes were isolated from each immunized mouse (on day 17 after tumor implantation). Frozen tissues were sectioned at a thickness of 25 μm and mounted. The sections were then analyzed by confocal microscopy at a magnification of × 400 (inserted images). LN lymph node, Pulmonary meta mouse metastatic tumor-bearing mice, sc tumor mouse sc tumor-bearing mice. d Proliferation of T cells from sc or pulmonary metastatic tumor-bearing mice in response to Renca tumor lysate-pulsed DCs. Spleens were prepared from mice that were immunized as described in “Materials and methods” and stimulated with Renca tumor lysate-pulsed DCs for 5 days. Then, 1 μCi of tritiated deoxythymidine was added to each well for an additional 16 h. Incorporation of thymidine was determined using a liquid scintillation counter (see "Materials and methods"). Data shown are representative of two independent experiments. Asterisk P < 0.01. Control not stimulated, DC alone stimulated with mature DCs, DC/TL stimulated with Renca tumor lysate-pulsed DCs, Pulmonary tumor metastatic tumor-bearing mice, sc tumor sc tumor-bearing mice
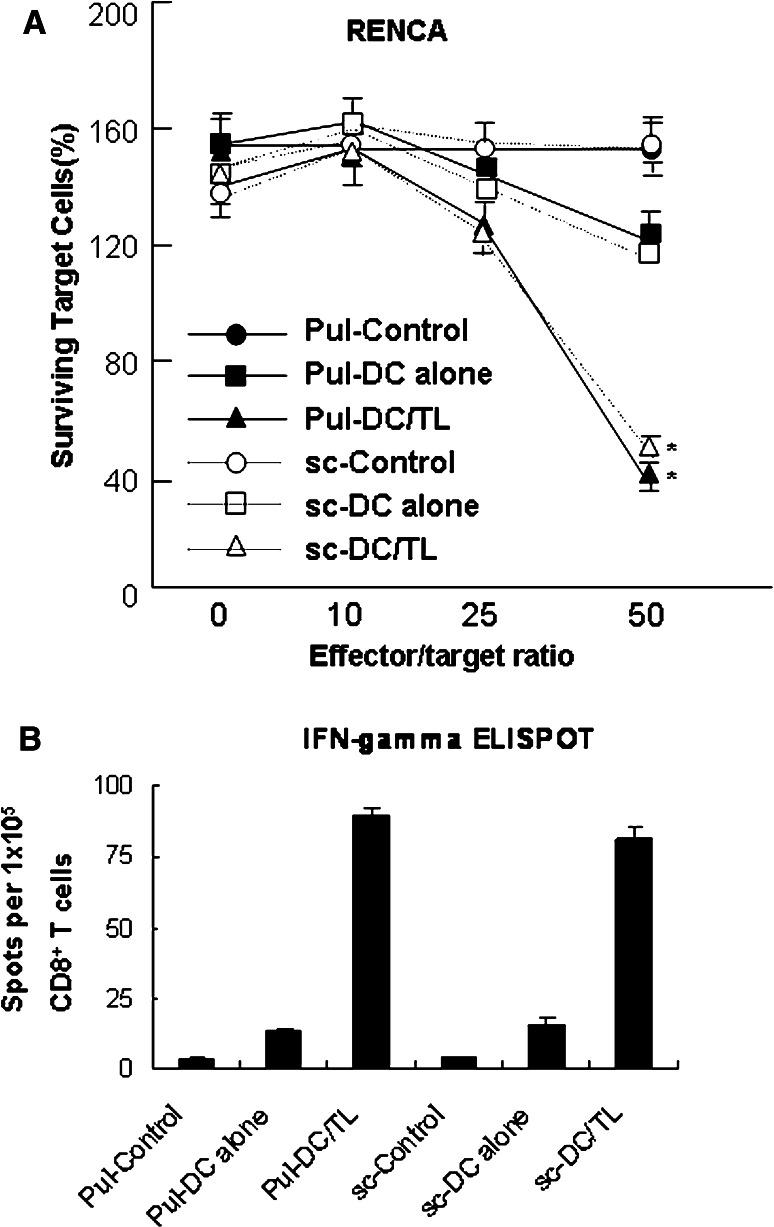
We next investigated Renca-specific CTL in DC-vaccinated mice. Splenic T cells, obtained from DC-vaccinated sc tumor-bearing mice and pulmonary metastatic tumor-bearing mice, were stimulated in vitro with Renca cell lysate-pulsed DCs. As shown in Fig. 5a, even in the tumor-bearing mice, DC vaccination induced effective tumor-specific CTL, and no difference between the CTLs induced in the sc tumor model and in the pulmonary metastatic tumor model was detected. Additionally, the vaccination by Renca lysate-pulsed DCs resulted in potent stimulation of IFN-γ-secreting activated CD8+ T cells (Fig. 5b). These data were in line with the above-mentioned result. These data suggest that the resistance of sc tumors to DC therapy, as compared with pulmonary metastatic tumors, is not likely due to dysfunctional DC migration or insufficient immune induction assumed for the immune-suppressive agents secreted from the solid tumor.
Fig. 5.
DC vaccination induced similar intensities of tumor-specific CTLs and an increase of IFN-γ-secreting CD8 + T cells in both pulmonary metastatic tumor- and sc tumor-bearing mouse groups. a The induction of effective CTL responses by vaccination with Renca tumor lysate-pulsed DCs. CTLs were prepared by stimulation with Renca tumor lysate-pulsed DCs for 5 days and incubated with Renca tumor cells as a target for 24 h. After washing, target cells were stained with crystal violet and lysed using methanol. Surviving target cells were analyzed by measuring the absorbance at 470 nm. Data shown were representative of two independent experiments. Asterisk P < 0.01 compared with the control. b In vivo induction of IFN-γ-secreting CD8+ T cell responses. CD8+ T cells were isolated from post-vaccination spleen samples of mice that received the vaccination with Renca lysate-pulsed DCs (2 cycles of 1 × 106 cells per treatment). Cells were stimulated for 20 h with Renca lysate-pulsed DCs. IFN-γ-expressing T cells were enumerated using an automated ELISPOT reader, and activated T cell frequencies were expressed as the number of spot-forming cells per 1 × 105 CD8+ T cells. Pul-Control: unstimulated CTLs generated from metastatic tumor-bearing mice, Pul-DC alone mature DC-stimulated CTLs generated from metastatic tumor-bearing mice, Pul-DC/TL Renca tumor lysate-pulsed DC-stimulated CTLs generated from metastatic tumor-bearing mice, sc-control unstimulated CTLs generated from sc tumor-bearing mice, sc-DC alone mature DC-stimulated CTLs generated from sc tumor-bearing mice, sc-DC/TL Renca tumor lysate-pulsed DC-stimulated CTLs generated from sc tumor-bearing mice
Many infiltrating immune cells have little effector function in the tumor mass, where the TGF-β1 concentration was remarkable
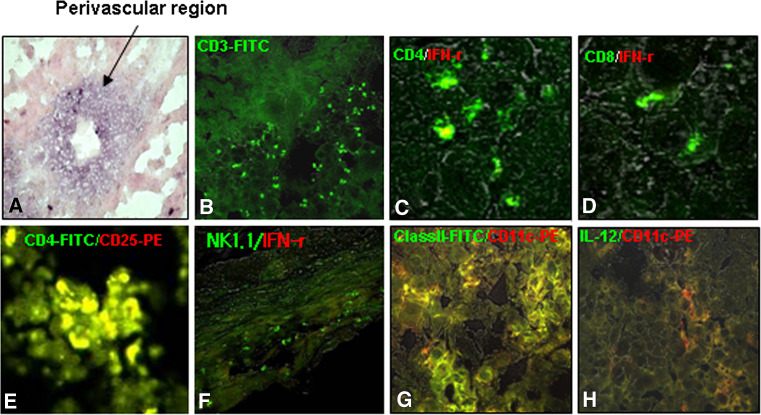
We then turned our attention to the tumor mass itself in order to find clues regarding the resistance of sc tumors to DC immunotherapy. We obviously found that TGF-β1 was highly expressed around the perivascular region of the sc tumor mass using immunohistochemical analysis (Fig. 6a). This information was in line with the results shown in Fig. 3. Additionally, substantial numbers of T cells (Fig. 6b) and DCs (Fig. 6g) were observed in the tumor mass. However, IFN-γ-producing CD4+, CD8+, or NKT cells were rarely observed in the tumor-infiltrating lymphocyte (TIL) population (Fig. 6c, d, and f, respectively). Most of the DCs detected in the tumor masses (Fig. 6g) were immature DEC205+ (data not shown) or IL-12-undetectable inactivated forms (Fig. 6h). Particularly noteworthy is the fact that the CD4+CD25+ cells were densely detected in the tumor mass (Fig. 6e). However, these cells were not stained by FoxP3 or IL-10 (data not shown), which suggests that these cells are probably Tr2-type regulatory T cells as reported previously [47]. Taken together, our results strongly suggest that the tumor-specific CTL and anti-tumor immunity, normally induced by DC vaccination in the sc tumor-bearing mice, were no longer functional in the tumor mass following infiltration, probably by the highly concentrated TGF-β1 secreted from the tumor mass.
Fig. 6.
TGF-β was clearly observed in sc tumor masses, and effector T cells were rarely observed. CD4+CD25+ Treg cells, inactivated T and NK cells, and immature DCs comprised the major population in the TILs. a TGF-β1 was highly concentrated around the perivascular regions in tumor masses (black arrow). b–h Confocal immunohistochemistry. b Total T cells, c IFN-γ-producing CD4 T cells, d IFN-γ-producing CD8 T cells, e CD4+CD25+ Treg cells, f IFN-γ-producing NK/NKT cells, g MHC class II/CD11c-postive DCs, h IL-12-secreting DCs. See “Materials and methods” for the experimental procedures
DC vaccination was sufficient to inhibit further spread of metastatic tumors in the sc tumor-bearing mice and tumor recurrence after surgery
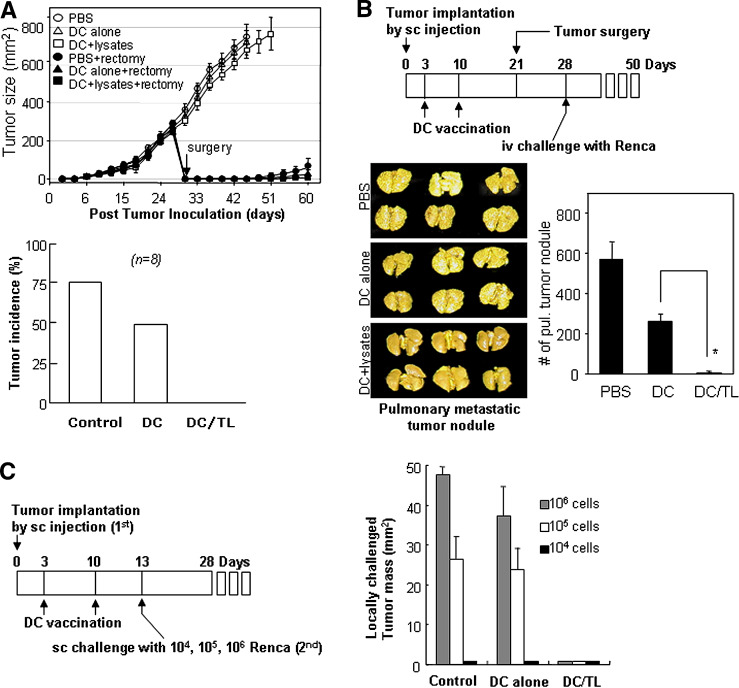
In our next step, we have examined antitumor immunity in DC-vaccinated tumor-bearing mice. Even though the sc tumors were resistant to DC therapy because of their own immunosuppressive mechanisms, we expected that those mice might still have systemic anti-tumor immunity against Renca tumor cells. We examined tumor recurrence after the surgery of DC-vaccinated sc tumor-bearing mice. None of the mice vaccinated with tumor lysate-pulsed DCs showed tumor recurrence for over 30 days after surgery, while most of the untreated mice or mice injected with DCs alone exhibited tumor recurrence (Fig. 7a). DC-vaccinated sc tumor-bearing mice were tumorectomized and then re-challenged i.v. with Renca cells. Pulmonary metastasis was markedly inhibited or completely blocked in those mice vaccinated with tumor lysate-pulsed DCs, while it was not significantly blocked in control mice treated with PBS or unpulsed DCs alone (Fig. 7b). Additionally, DC-vaccinated sc tumor-bearing mice were re-challenged with three different dosages of Renca cells through the sc route around the primary sc tumor mass. None of the mice vaccinated with tumor lysate-pulsed DCs showed any detectable tumor growth even at the highest dosage (1 × 106 Renca cells per injection; Fig. 7c). These findings imply that the DC vaccination, even in the tumor-bearing mice, would be effective in inducing substantial antitumor immunity. In other words, DC vaccination may not be sufficient for causing pre-existing sc tumors to regress, but be sufficient for inhibiting further spreading of metastatic tumors or tumor recurrence after surgery.
Fig. 7.
DC vaccination was sufficient to inhibit further spread of metastatic tumors or tumor recurrence after surgery. a The upper panel shows inhibition of tumor recurrence after surgery in DC-vaccinated mice. The 3-day sc tumor-bearing mice were vaccinated twice, at a 1-week interval, with tumor lysate-pulsed DCs or unpulsed DCs. On day 30, the primary sc tumor of each mouse was surgically removed, and tumor recurrence was then assessed every three days. Data are reported as the average tumor area ± SD of eight mice per group. The lower panel shows the tumor recurrence rate after surgery in three different groups of mice. Control PBS-vaccinated group, DC alone mature DC alone-vaccinated group, DC/TL Renca tumor lysate-pulsed DC-vaccinated group. See “Materials and methods” for the experimental procedures. b Upper panel A schedule for DC vaccination, tumor surgery and tumor re-challenge in the sc tumor-bearing mice. Lower panels Pulmonary metastasis was inhibited in the tumor re-challenge experiment in DC-vaccinated sc tumor-bearing mice. Data are reported as the mean number of tumor nodules ± SD of six mice per group. Asterisk P < 0.01. PBS unvaccinated, DC mature DC-vaccinated, DC/TL Renca tumor lysate-pulsed DC-vaccinated. c Inhibition of sc tumors in the tumor re-challenge experiment with three different doses (left panel an experimental schedule). Data are reported as the average tumor area ± SD of four mice per group (right panel). Three different doses of Renca cells were used for re-challenge (sc) at the abdominal regions of the mice. Control PBS-vaccinated, DC alone mature DC-vaccinated, DC/TL Renca tumor lysate-pulsed DC-vaccinated. Data shown above are representative of two experiments
Discussion
To our knowledge this is only one of a few demonstrations of the ability of DC vaccination to induce systemic antitumor immunity in RCC tumor-bearing hosts, without being present in the sc-implanted local tumor at least due to TGF-β secreted by the tumor itself. Our results show over-secretion of an immunosuppressive mediator, TGF-β, derived from sc tumors, and also show inhibition of tumor metastasis and recurrence by DC vaccination, and suggest that DC vaccination, even in mice with pre-existing sc tumors, would be effective in inducing antitumor immune response, which is sufficient to inhibit further spread of metastatic tumors and tumor recurrence after surgery.
It is well known that tumor cells produce various immunosuppressive factors, including TGF-β [2, 4, 11], VEGF [16, 45], and IL-10 [45]. Among these, overexpression of TGF-β is closely associated with a poor prognosis in patients with malignant tumors [18, 20, 39]. TGF-β suppresses the differentiation of BmDCs, as well as their capacity to secrete the cytokine, IL-12 [33], to present antigen, to stimulate tumor-sensitized T lymphocytes, and to migrate into tumor-draining lymph nodes [29]. In this study, we also found that TGF-β showed remarkable expression and secretion from sc tumors, presumably in order to induce tumor escape from TILs, thereby diminishing antitumor immunity by DC vaccination. Actually, in our experiments, the migratory abilities of DCs decreased when treated with a high dose of TGF-β in vitro. However, our additional results showed that tumor lysate-pulsed DCs effectively migrated into regional lymph nodes and high enough levels of functional CTL in both sc tumor-bearing mice and metastatic tumor-bearing mice. Consequently, we firmly believe that immunity with tumor lysate-pulsed DCs may not be restricted to peritumor tissue in vivo. On the other hand, immunohistochemistry data from sc tumor masses indicated that effector T cells exist at very low levels in TILs, possibly being inhibited by tumor-derived TGF-β. These data could be explained by the fact that naive T cells (particularly, like CD4+CD25−) in sc tumor masses may be primed into Treg (CD4+CD25+) cells by tumor-derived TGF-β as reported previously [9], followed by the inactivation of tumor-infiltrating immune effector cells. Therefore sc tumors showed resistance to the antitumor immunity induced by DC vaccination in sc tumor-bearing mice. During tumor progression, Treg cells accumulate in tumors and secondary lymphoid organs of humans. It has also been suggested that chemokines produced by tumor cells or tumor-infiltrating macrophages recruit Treg cells into the tumor bed [5, 9, 15]. In parallel with these results, we also confirmed the CD4+CD25+ Treg cell population using histopathology (Fig. 6e) and TIL analysis (data not shown).
Although it was not found to be very functional against sc tumors in sc tumor-bearing mice, DC vaccination was sufficient to inhibit further spread of metastatic tumors or tumor recurrence in mice after surgery, indicating that DC vaccination is still effective for inducing long-lasting systemic antitumor immunity after surgery. We definitely expect that these results would form an important basis for the use and success of DC-based immunotherapy in clinical trials.
In conclusion, our present data obtained from mouse model strongly suggest the potential of DC immunotherapy in tumor patients for hindering or blocking disease progression by inhibition of tumor metastasis and/or tumor recurrence after surgery, even though DC immunotherapy has not yet been found to be very effective against solid tumors.
References
- 1.Ahn JH, Lee Y, Jeon C, Lee SJ, Lee BH, Choi KD, Bae YS. Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood. 2002;100:1742–1754. [PubMed] [Google Scholar]
- 2.Amoils KD, Bezwoda WR. TGF-beta 1 mRNA expression in clinical breast cancer and its relationship to ER mRNA expression. Breast Cancer Res Treat. 1997;42:95–101. doi: 10.1023/A:1005785421815. [DOI] [PubMed] [Google Scholar]
- 3.Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer: a review of clinical data. Curr Opin Investig Drugs. 2005;6:592–596. [PubMed] [Google Scholar]
- 4.Asselin-Paturel C, Echchakir H, Carayol G, Gay F, Opolon P, Grunenwald D, Chouaib S, Mami-Chouaib F. Quantitative analysis of Th1, Th2 and TGF-beta1 cytokine expression in tumor, TIL and PBL of non-small cell lung cancer patients. Int J Cancer. 1998;77:7–12. doi: 10.1002/(SICI)1097-0215(19980703)77:1<7::AID-IJC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97–106. doi: 10.1016/j.cytogfr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Chagnon F, Thompson-Snipes L, Elhilali M, Tanguay S. Murine renal cell carcinoma: evaluation of a dendritic-cell tumour vaccine. BJU Int. 2001;88:418–24. doi: 10.1046/j.1464-410X.2001.02255.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang DH, Dhodapkar MV. Dendritic cells and immunotherapy for cancer. Int J Hematol. 2003;77:439–443. doi: 10.1007/BF02986611. [DOI] [PubMed] [Google Scholar]
- 8.Chen V. Dendritic-cell vaccination for metastatic melanoma? Lancet Oncol. 2006;7:368. doi: 10.1016/S1470-2045(06)70678-7. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen PJ, Cohen PA, Rosenberg SA, Katz SI, Mule JJ. Murine epidermal Langerhans cells and splenic dendritic cells present tumor-associated antigens to primed T cells. Eur J Immunol. 1994;24:315–319. doi: 10.1002/eji.1830240206. [DOI] [PubMed] [Google Scholar]
- 11.Conrad CT, Ernst NR, Dummer W, Brocker EB, Becker JC. Differential expression of transforming growth factor beta 1 and interleukin 10 in progressing and regressing areas of primary melanoma. J Exp Clin Cancer Res. 1999;18:225–232. [PubMed] [Google Scholar]
- 12.Dao T, Gomez-Nunez M, Antczak C, Kappel B, Singh Jaggi J, Korontsvit T, Zakhaleva V, Scheinberg DA. Natural killer cells license dendritic cell cross-presentation of B lymphoma cell–associated antigens. Clin Cancer Res. 2005;11:8763–8772. doi: 10.1158/1078-0432.CCR-05-0975. [DOI] [PubMed] [Google Scholar]
- 13.DeMatos P, Abdel-Wahab Z, Vervaert C, Hester D, Seigler H. Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice. J Surg Oncol. 1998;68:79–91. doi: 10.1002/(SICI)1096-9098(199806)68:2<79::AID-JSO3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 14.Fehervari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209–17. doi: 10.1172/JCI200423395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4:1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 17.Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, Chang AE, Braun TM, Mule JJ. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 18.Ghellal A, Li C, Hayes M, Byrne G, Bundred N, Kumar S. Prognostic significance of TGF beta 1 and TGF beta 3 in human breast carcinoma. Anticancer Res. 2000;20:4413–4418. [PubMed] [Google Scholar]
- 19.Gregorian SK, Battisto JR. Immunosuppression in murine renal cell carcinoma. I. Characterization of extent, severity and sources. Cancer Immunol Immunother. 1990;31:325–334. doi: 10.1007/BF01741403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–71. doi: 10.1002/1097-0142(20010301)91:5<964::AID-CNCR1086>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Hillman GG, Droz JP, Haas GP. Experimental animal models for the study of therapeutic approaches in renal cell carcinoma. In Vivo. 1994;8:77–80. [PubMed] [Google Scholar]
- 22.Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–70. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 24.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1 + CD115 + immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 25.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162:3859–3864. [PubMed] [Google Scholar]
- 28.Kobayashi H, Omiya R, Sodey B, Yanai M, Oikawa K, Sato K, Kimura S, Senju S, Nishimura Y, Tateno M, Celis E. Identification of naturally processed helper T-cell epitopes from prostate-specific membrane antigen using peptide-based in vitro stimulation. Clin Cancer Res. 2003;9:5386–5383. [PubMed] [Google Scholar]
- 29.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 30.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 31.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/S0022-1759(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 32.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 33.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83 + dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 34.Mitrus I, Missol-Kolka E, Plucienniczak A, Szala S. Tumour therapy with genes encoding apoptin and E4orf4. Anticancer Res. 2005;25:1087–1090. [PubMed] [Google Scholar]
- 35.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 37.Pan J, Burdick MD, Belperio JA, Xue YY, Gerard C, Sharma S, Dubinett SM, Strieter RM. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176:1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 38.Peng BG, He Q, Liang LI, Xie BH, Hua YP, Chen ZB, Zhou F. Induction of cytotoxic T-lymphocyte responses using dendritic cells transfected with hepatocellular carcinoma mRNA. Br J Biomed Sci. 2006;63:123–128. doi: 10.1080/09674845.2006.11732731. [DOI] [PubMed] [Google Scholar]
- 39.Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20:4489–4493. [PubMed] [Google Scholar]
- 40.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4 + CD25 + T regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 41.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Sakaguchi S. Naturally arising CD25 + CD4 + regulatory T cells in maintaining immunologic self-tolerance and preventing autoimmune disease. Curr Mol Med. 2003;3:693–706. doi: 10.2174/1566524033479429. [DOI] [PubMed] [Google Scholar]
- 43.Tamura T, Nishi T, Goto T, Takeshima H, Dev SB, Ushio Y, Sakata T. Intratumoral delivery of interleukin 12 expression plasmids with in vivo electroporation is effective for colon and renal cancer. Hum Gene Ther. 2001;12:1265–1276. doi: 10.1089/104303401750270922. [DOI] [PubMed] [Google Scholar]
- 44.Wiltrout RH, Gregorio TA, Fenton RG, Longo DL, Ghosh P, Murphy WJ, Komschlies KL. Cellular and molecular studies in the treatment of murine renal cancer. Semin Oncol. 1995;22:9–16. [PubMed] [Google Scholar]
- 45.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 46.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 47.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]