Abstract
Background: Dendritic cells (DCs) are important for an immune surveillance. Myeloid DCs (DC1) are important for an effective antitumor immune system. The function and count of circulating DC1 (cDC1) in hosts with a malignant tumor would be defective. This study focused on analyzing the immunological features of cDC1 in patients with pancreatic cancer during the perioperative period. Materials and methods: Thirty-two pancreatic cancer patients who underwent pancreatectomy and 18 age-matched healthy individuals as controls were enrolled in this study. The perioperative cDC count, the stimulatory capacity of cDC1 against allogeneic T cells and TGF-β1 level in the serum were measured. The cDC count was measured at 12 months after the operation. Results: The preoperative cDC1/cDC2 ratio, cDC1 count, and stimulatory capacity of cDC1 were impaired in patients in comparison to controls (P<0.05). The serum TGF-β1 level was significantly higher in patients than controls (P<0.001). The stimulatory capacity of cDC1 recovered after pancreatectomy (P<0.05). The serum TGF-β1 level significantly decreased after the operation (P<0.05); however, they were still significantly higher than controls (P<0.05). Although the cDC1/cDC2 ratio and the cDC1 count did not increase after the pancreatectomy, they recovered as the controls’ level at 12 months after the pancreatectomy in disease-free patients (P<0.05) and the serum TGF-β1 level in those patients at 12 months after the operation significantly decreased compared with those at the postoperative period (P<0.05). Conclusion: Surgical resection of pancreatic cancer could be associated with improved cDC1 function. When a patient remained disease free, the recovery of cDC1 counts was observed approximately 12 months after pancreatectomy. Further strategy will be needed to improve immune function in patients with pancreatic cancer.
Keywords: Pancreatic Cancer, Natural Killer Cell, Natural Killer Cell Activity, Pancreatic Cancer Patient, Stimulatory Capacity
Introduction
Pancreatic cancer is a highly aggressive disease with a poor prognosis and it is widely accepted that surgical resection provides the only chance for the long-term survival of patients with pancreatic cancer [1]. In Japan, pancreatic cancer is the fifth leading cause of death from cancer in men and the seventh in women, and ≈20,000 people die from pancreatic cancer every year [2]. In spite of improved perioperative management and reduced operative mortality, there has been little improvement in the long-term survival of patients with this disease over the last decade [3].
In general, the immune function of hosts with malignant tumors is considered defective, which allows malignant cells to evade the host’s antitumor defenses. It has also been shown that patients with pancreatic cancer have dysfunction of the immune system [4]. An inefficient immune response may contribute to the poor prognosis of patients with pancreatic cancer. Induction of an effective antitumor immune response requires the activation of effector cells by antigen-presenting cells (APCs) that are responsible for the presentation of tumor-specific antigens.
Dendritic cells (DCs) are the most potent APCs originating from bone marrow precursors which express higher MHC and co-stimulatory molecules. DCs play a central role in the initiation and modulation of immune system responses. Recently, techniques for isolating human DCs from peripheral blood have been established, and DCs can be divided into two distinct subsets which are functionally heterogenous: DC1, CD11c+DCs (myeloid DC population); and DC2, CD11c−DCs (lymphoid DC population) [5, 6]. Both subsets express high levels of HLA-DR and lack the lineage markers CD3, CD14, CD15, CD16 and CD19. It has been shown that these two DC subsets regulate immune responses via the polarization of Th1, Th2 or even Th3/Tr1 differentiation through the production of cytokines [7]. DC1 contribute to an essential part of protection against cancer through the strong stimulation of naïve T lymphocytes. When tissues are damaged by malignant transformation, cDCs migrate to these sites. After capturing antigens there, DCs produce a high amount of IL-12 as they mature and migrate into the draining lymph nodes, where they present processed antigens to T lymphocytes to initiate an immune response against the tumor [8].
While cancer cells frequently express antigens that can be recognized by the immune system, they manage to evade defence by immunoeffector cells. It has been suggested that one reason for this is that soluble molecules and cytokines released from tumor cells inhibit the function of immunoeffector cells, which are considered to be mainly T lymphocytes [9]. However, since T lymphocytes are activated by DCs, functional abnormalities of DCs may be the predominant reason, and the malfunction of T lymphocytes may be a secondary reason.
We have recently reported that patients with pancreatic cancer were in an immunosuppressive state in respect to the function and count of cDC1 but that chemoradiotherapy could improve the impaired cDC1 function [10]. In this study, we hypothesized that the function and count of cDC1 would improve after pancreatectomy in patients with pancreatic cancer. We performed immunological monitoring focused on cDCs in patients with pancreatic cancer during the perioperative period.
Materials and methods
Patients and study design
Eighty-eight patients with pancreatic cancer (50 males and 38 females) were hospitalized in the department of surgery of Kansai Medical University from May 2001 to December 2004. Thirty-two of the 88 patients, who had undergone pancreatectomy and in whom the tumor had been histologically confirmed as ductal adenocarcinoma of the pancreas, were enrolled into this study. None of the patients had acute biliary tract infection or other acute inflammation at the time of surgery. Bile duct decompression was given preoperatively to patients who had obstructive jaundice due to tumor invasion of the bile duct.
Eighteen age-matched healthy volunteers with no history of malignant or severe disease served as controls. A written informed consent was obtained from each individual in accordance with the provisions of the Declaration of Helsinki. This study protocol was approved by the Institutional Review Board of Kansai Medical University. Peripheral venous blood was collected into a heparinized syringe from each patient in the morning after overnight fasting, a few days before the operation and before discharge. Each control individual also provided a blood sample at a single point using the same protocol as the patients. The number of circulating DC1 (cDC1), DC2 (cDC2), natural killer (NK) cells and CD4+/CD8+ T lymphocytes were measured in each sample by flow cytometric analysis. As an assessment of immune function, allogeneic mixed-leukocyte reaction (MLR) was carried out. NK cell activity and the proliferative response of T lymphocytes towards mitogen (PHA) were measured. We also measured the serum transforming growth factor beta1 (TGF-β1) concentration to assess changes in the immunoinhibitory cytokine.
Potentially curative pancreatectomy was scheduled in each patient. Lymph node dissection was performed extensively beyond the area of the regional nodes (including the nodes around the common hepatic, celiac and superior mesenteric arteries, the nodes in the hepatoduodenal ligament and the paraaortic nodes [for carcinoma of the head], and the nodes around the common hepatic, celiac, splenic, and superior mesenteric arteries [for carcinoma of the body or tail]). Dissection of the right-sided nerve plexus surrounding the superior mesenteric artery, celiac artery, and the celiac ganglia was performed. When macroscopic invasion of the portal vein or superior mesenteric vein was encountered, the vein was resected and reconstructed. After pancreatectomy, patients were discharged when the signs of acute inflammation (high-grade fever and elevated white blood cells [WBC] and C reactive protein [CRP] levels) disappeared and sufficient oral intake was obtained.
Patients underwent abdominal ultrasonography, contrast enhanced CT or MRI, and blood tests every 3 months to check for local recurrences or distant metastases. Local recurrence or distant metastasis was diagnosed after detection of either a mass formation on the radiological findings or continuously increasing levels of a tumor marker (carbohydrateantigen 19–9 [CA 19-9], DUPAN-II or carcinoembryonic antigen [CEA]). “The cDC count and the serum TGF-β1 level were measured at 12 months after pancreatectomy.” Pathological staging was performed in accordance with the TNM classification of malignant tumors, sixth edition [11].
Antibodies
The phenotypes of peripheral blood mononuclear cells (PBMCs) were determined by two- and three-color flow cytometric analysis using monoclonal antibodies (mAbs) that were directly conjugated to fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), or PE cyanin 5.1 (PE-Cy5).
Cells were stained with the following mAbs: PE-Cy5-conjugated anti-HLA-DR, a mixture of FITC-conjugated anti-CD3, CD14, CD15, CD16, CD19 so-called “ lineage cocktail (Lin)” and PE-conjugated anti-CD11c mAbs for DCs; PE-Cy5-conjugated anti-CD3, FITC-conjugated anti-CD4 and PE-conjugated anti-CD8 for T lymphocytes; PE-conjugated anti-CD14 and FITC-conjugated anti-CD56 for NK cells.
Flow cytometric analysis
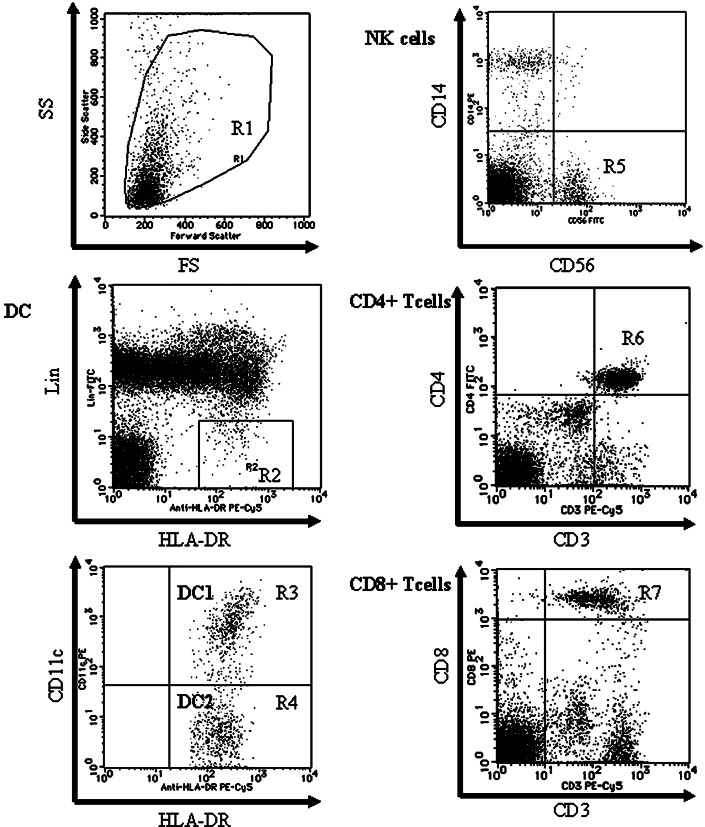
PBMCs were prepared by Lymphoprep (Nycomed Pharma, Oslo, Norway) gradient centrifugation of heparinized peripheral blood and then washed in PBS supplemented with 1% fetal bovine serum and 0.1% NaN3. Cells were incubated for 30 min at 4°C with the mAbs and were analyzed using a FACScan® (Becton Dickinson, Sunnyvale, CA). At least 100,000 events were counted for each mononuclear fraction by FACScan. Each cell population determined by flow cytometry is shown in Fig. 1. Region R1 includes lymphocytes and monocytes but excludes debris. DCs were detected in region R2 as the population of Lin−/HLA-DR+ cells. Two subsets of DCs were identified within the Lin−/HLA-DR+ population, which were based on differential expression of CD11c: DC1 (CD11c+ population; region R3) and DC2 (CD11c− population; region R4). The absolute number (per ml) in each subset of cDCs was calculated by multiplying the percentage of each region of the cDCs by the PBMC count. The number of NK cells (CD14−/CD56+ population; region R5), CD4+/CD3+ T lymphocytes (region R6), and CD8+/CD3+ T lymphocytes (region R7) were calculated in the same manner.
Fig. 1.
Flow cytometric analyses of PBMCs by FACS can. The figures are representative of a pancreatic cancer patient. Region R1 includes lymphocytes and monocytes but excludes debris. DCs are detected in region R2 as the population of Lin−/HLA-DR+ and divided into two fractions by the expression of CD11c (region R3; CD11c+ DC (DC1) and region R4; CD11c− DC (DC2)). The NK cell fraction is gated in the CD14−/CD56+ population (region R5). CD3+/CD4+ T lymphocytes are detected in region R6 and CD3+/CD8+ T lymphocytes are detected in region R7
Isolation of circulating DCs from peripheral blood
The cDCs from peripheral blood were enriched according to a method described previously [5]. Briefly, PBMCs were incubated with anti-CD3 and anti-CD14 mAbs for 30 min on ice. Cells that were bound to these mAbs were removed using sheep antimouse Ig-coated magnetic beads (M-450; Dynal, Oslo, Norway). The CD3−/CD14− cells were further incubated with CD4-conjugated microbeads (Miltenyi Biotec., Bergisch Gladbach, Germany). The CD4+ cells were then enriched by passing them through a Mini MACS® magnetic separation column (Miltenyi Biotec.). By using this protocol, the percentage of cDCs (originally <1% of total PBMCs) was increased by 20–50%, which was dependent on the individual. The resultant DC-enriched population (CD4+/CD3−/CD14− cells) was stained with PE-conjugated anti-CD11c mAb, FITC-conjugated lineage cocktail, and PE-Cy5-conjugated anti-HLA-DR mAb. The stained cells were analyzed and sorted using an EPICS ELITE® flow cytometer (Coulter, Hialeah, FL). Consequently, two purified and phenotypically distinct fractions of cDC1 and cDC2 were collected and used in the experiments.
Allogeneic mixed-leukocyte reaction (MLR) of circulating DC1
The cDC1, isolated from peripheral blood, were examined for their stimulating capacity against allogeneic T lymphocytes in a standard MLR [12]. cDC1 were irradiated at 15 Gy (Gamma Cell, Nordion, Ontario, Canada). Graded doses of cDC1 were co-cultured with 2×105 allogeneic T lymphocytes (collected by magnetic beads as CD3+ cells) in 200 μl of culture medium in 96-well culture plates for 4 days. “The following culture medium was used throughout the experiments: RPMI 1640 supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-mercaptoethanol (2-ME; Sigma, St Louis, MO), and heat-inactivated 10% fetal bovine serum.” GM-CSF was added to the culture medium for the maintenance of cDC1. The cells were pulsed with 1 μCi of [3H]-thymidine during the last 16 h of the culture period. The cells were harvested onto glass fiber filter papers using an automated harvester. Cell-bound radioactivity was counted in a liquid scintillation counter. The allostimulatory capacity was calculated by dividing the counts per minute (c.p.m.) of patients or controls by the c.p.m. of healthy third party volunteers.
Natural killer (NK) cell activity
Natural killer cell activity was measured using the standard 4 h 51Cr-release assay. Each 100 μl of PBMCs (effector) and K562 cells (target) were combined at an effector-to-target (E:T) ratio of 50:1 in 96-well microtiter plates. Maximum release was determined by the addition of detergent to K562 cells. Spontaneous release was measured by culturing K562 cells without PBMCs. After 4 h incubation at 37°C in air with 5% CO2, supernatants were harvested and radioactivity was assessed using a gamma counter (Aloka, Japan). All assays were performed in triplicate and the value was calculated as the mean of triplicate cultures. The percentage cytotoxicity was determined as follows: (experimental c.p.m. − spontaneous c.p.m.)/(maximum c.p.m. − spontaneous c.p.m.) × 100. The values of spontaneous release were less than 10% of the maximum release in all experiments. Since the amount of samples was limited, only an E/T ratio of 50:1 was used in this experiment.
Proliferative response of T lymphocytes towards mitogen (PHA)
The in vitro proliferative capacity of lymphocytes towards mitogen (phytohemagglutinin) was quantified using standardized assay formats (BAG, Germany). T lymphocytes were incubated with 1 μCi of 3H-thymidine during the last 6.5 h of the culture period. Cell-bound radioactivity was counted in a liquid scintillation counter. Results are presented as the mean c.p.m. of triplicate cultures.
Serum concentration of TGF-β1
TGF-β1 is one of the cytokines that inhibits the upregulation of critical T lymphocytes, co-stimulatory molecules on the surface of DCs and antigen-presenting capacity of DCs [13, 14]. TGF-β1 levels in serum were measured by ELISA using a commercial kit (BioSource International, Inc., Camarillo, CA) according to the manufacturer’s instructions.
Statistical analysis
The data were expressed as median and range. Mann–Whitney U test and Wilcoxon signed-rank test were used for the statistical analyses with the Stat View statistical program (Abacus Concepts, Berkeley, CA). The differences were considered significant when tied P values were less than 0.05.
Results
Patients’ characteristics
The clinical characteristics of patients with pancreatic cancer and the control group are summarized in Tables 1 and 2. There were not any significant differences in age or gender between the two groups. Serum albumin and hemoglobin levels in patients were significantly lower than in controls (P<0.05) (Table 1). Pancreatoduodenectomy (PD) was performed in 19 patients, 4 underwent pylorus preserving pancreatoduodenectomy (PpPD), 8 underwent distal pancreatectomy (DP), and 1 underwent total pancreatectomy (TP). The median postoperative hospitalization term was 45 days (range 17–93). Most tumors developed in the pancreatic head (n=23) with up to T3 tumor status (n=30) and lymph node metastases (n=22). Eight patients had no residual tumor (R0), 19 had microscopic residual tumors (R1), and 5 had macroscopic residual tumors (R2) after pancreatectomy. Twenty patients had a clinical diagnosis of Stage 2b. None of the patients in this study died whilst in hospital. Each patient was followed up in the out-patient clinic. At least 12 months passed after pancreatectomy in 26 patients. Twelve of the 26 patients did not develop any obvious recurrence or metastasis over 12 months after pancreatectomy. In contrast, 14 patients relapsed within 12 months after the operation (6 liver metastases, 7 local recurrences, and 1 paraaorta lymph node metastasis). Six of the fourteen patients died from the relapsed disease within 12 months after pancreatectomy. Twenty patients remained alive over 12 months after the operation.
Table 1.
Clinical characteristics of pancreatic cancer patients and controls
| Controls (n=18) | Patients (n=32) | P value | ||
|---|---|---|---|---|
| Gender | Male:female | 10:8 | 17:15 | 0.869 |
| Age | Years | 63 (45–77) | 64 (47–83) | 0.867 |
| WBC | (×106/ml) | 5.0 (3.6–6.8) | 5.5 (2.4–7.5) | 0.770 |
| Hb | (g/dl) | 13.0 (11.2–15.3) | 11.0 (8.5–14.6) | 0.008 |
| Cr | (mg/dl) | 0.7 (0.3–1.0) | 0.6 (0.3–1.0) | 0.238 |
| Alb | (g/dl) | 4.3 (3.8–5.0) | 3.8 (2.3–4.5) | 0.003 |
| AST | (IU/l) | 19 (16–34) | 25 (15–87) | 0.197 |
| ALT | (IU/l) | 20 (8–31) | 28 (13–237) | 0.183. |
| T.Bil | (mg/dl) | 0.5 (0.2–0.8) | 0.6 (0.3–2.8) | 0.092 |
| Amy | (IU/l) | 93 (53–176) | 59 (8–399) | 0.006 |
| CRP | (mg/dl) | 0.1 (0.0–0.6) | 0.2 (0.0–2.8) | 0.112 |
The data are expressed as median and range
WBC, white blood cells;Hb, hemoglobin; Cr, creatinine; TP, total protein; Alb albumin; AST aspirate transaminase; ALT, alanine transaminase; T.Bil total bilirubin; Amy, amylase; CRP, C reactive protein
Statistical significance was determined using the Mann–Whitney U test
Table 2.
Clinicopathological characteristics of patients with pancreatic cancer included in the study
| Characteristics | Patients (n=32) | Operation time (min) | Bleeding (ml) |
|---|---|---|---|
| Type of surgerya | |||
| PD (PpPD) | 19 (4) | 590 (480–815) | 1180 (400–7250) |
| DP | 8 | 375 (265–490) | 1095 (506–1540) |
| TP | 1 | 900 | 5030 |
| Tumor site in pancreas | |||
| Head | 23 | ||
| Body or tail | 9 | ||
| Tumor status | |||
| T1 | 2 | ||
| T2 | 0 | ||
| T3 | 25 | ||
| T4 | 5 | ||
| Nodal status | |||
| No | 10 | ||
| N1 | 22 | ||
| Residual tumorb | |||
| R0 | 8 | ||
| R1 | 19 | ||
| R2 | 5 | ||
| Tumor differentiation | |||
| Well | 5 | ||
| Moderate | 21 | ||
| Poor | 2 | ||
| Other | 4 | ||
| Stage (pTNM)c | |||
| 1a | 2 | ||
| 2a | 6 | ||
| 2b | 20 | ||
| 3 | 4 | ||
aType of surgery: (Pp)PD, (pylorus-preserving) pancreatoduodenectomy; DP, distal pancreatectomy; TP total pancreatectomy
bResidual tumor: R0, no residual tumor; R1 microscopic residual tumor; R2 macroscopic residual tumor
cPathological staging was based on the TNM classification of malignant tumors, sixth edition
Comparisons of immunological parameters between pancreatic cancer patients before pancreatectomy and controls
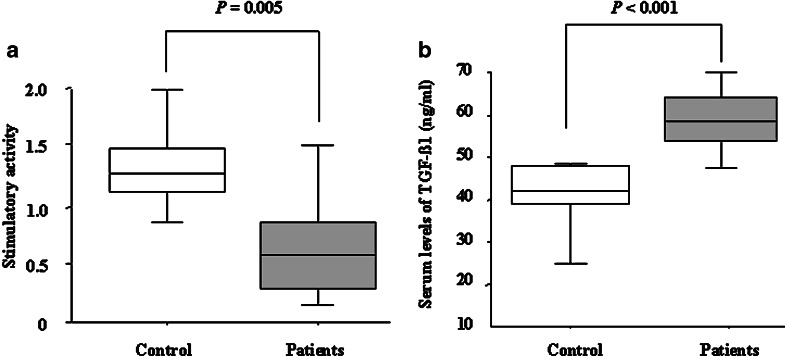
As shown in Table 3, the cDC1 count in pancreatic cancer patients was significantly lower than in the controls (P=0.010). The cDC1/cDC2 ratio in pancreatic cancer patients was also significantly lower than in the controls (P<0.001). However, there was no significant difference between pancreatic cancer patients and controls in the PBMC, total cDC or cDC2 count. In addition, there were no significant differences in other immunological parameters (Table 3). The stimulatory capacity of cDC1 from patients with pancreatic cancer was significantly impaired compared to that of controls (patients: 0.43 (0.11–1.00), controls: 1.25 (0.50–2.66); P=0.005; Fig. 2a). As shown in Fig. 2b, the serum levels of TGF-β1 in patients were significantly higher than in the controls (patients: 59 ng/ml (47–80), controls: 42 ng/ml (23–46); P<0.001).
Table 3.
The counts of peripheral blood mononuclear fractions, NK cell activity, and T lymphocytes proliferation in controls and pancreatic cancer patients
| Controls | Patients | |||
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| PBMCs | (×106/ml) | 2.6 (1.3–4.8) | 2.2 (0.9–4.9) | 2.4 (1.3–4.9) |
| cDC1 | (×103/ml) | 8.6 (3.2–26.9) | 5.4 (1.2–21.5)* | 5.9 (0.9–18.9) |
| cDC2 | (×103/ml) | 3.6 (1.7–8.2) | 4.6 (1.6–16.2) | 4.0 (1.9–29.4) |
| Total cDCs | (×103/ml) | 14.7 (5.6–33.6) | 10.4 (3.4–32.0) | 9.3 (4.3–48.3) |
| cDC1/cDC2 | (Ratio) | 2.7 (1.0–5.0) | 1.1 (0.2–5.3)** | 1.3 (0.2–3.8) |
| NK cells | (×105/ml) | 1.8 (0.9–4.2) | 2.2 (0.5–6.5) | 3.4 (0.8–11.7) |
| CD4+ T lymphocytes | (×105/ml) | 7.6 (1.3–22.0) | 5.0 (0.4–12.0) | 4.4 (1.3–10.9) |
| CD8+ T lymphocytes | (×105/ml) | 2.3 (0.2–7.0) | 2.0 (0.6–4.5) | 1.7 (0.4–13.1) |
| CD4+/CD8+ | (Ratio) | 3.0 (0.8–9.7) | 2.3 (0.4–6.0) | 2.1 (0.5–12.7) |
| NK cell activity | (%) | 29.3 (5.4–58.2) | 26.0 (7.2–78.0) | 27.7 (11.4–45.7) |
| PHA | (×104 c.p.m.) | 4.8 (3.5–6.3) | 4.6 (3.9–6.0) | 4.8 (2.8–6.3) |
The data are expressed as median and range
PBMCs, peripheral blood mononuclear cells; DCs, dendritic cells; NK cells, natural killer cells; PHA, Proliferative response of T lymphocytes towards mitogen
Statistical significance was determined using the Mann–Whitney U test and the Wilcoxon signed-rank test. *P=0.01, **P<0.001 versus controls
Fig. 2.
Comparison of stimulatory capacity from cDC1, and serum levels of TGF-β1 between controls and pancreatic cancer patients before operation. a The stimulatory capacity of cDC1 in patients with pancreatic cancer was significantly lower than that of controls (P=0.005). b Serum TGF-β1 levels of pancreatic cancer patients were significantly higher than controls (P<0.001). The data are expressed as the median (interquartile interval). Statistical significance was determined using the Mann–Whitney U test
Comparison of immune parameters in pancreatic cancer patients during the perioperative period
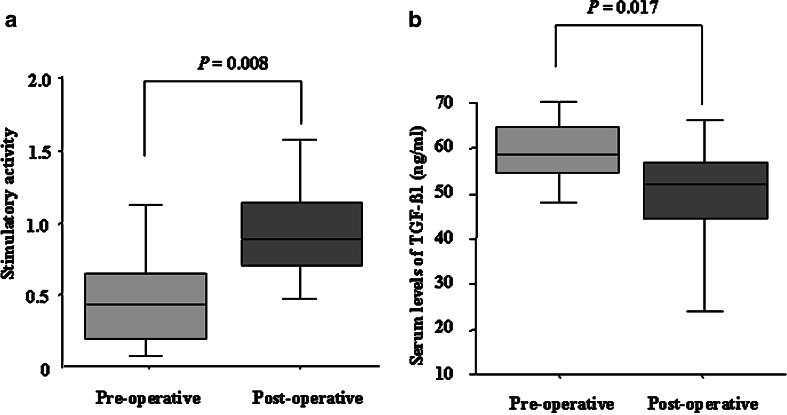
The stimulatory capacity of cDC1 was significantly greater in patients postoperatively than preoperatively (preoperative; 0.43(0.11–1.00), postoperative; 1.00 (0.47–1.90); P=0.008; Fig. 3a). In addition, the serum levels of TGF-β1 in patients were significantly decreased after pancreatectomy (preoperative: 59 ng/ml (47–80), postoperative: 51 ng/ml (9–65); P=0.017; Fig. 3b), although they were still significantly higher than controls (P=0.034). There were no significant changes in NK cell activity or PHA levels during the perioperative period (Table 3). There were also no significant changes in the count of each fraction of PBMC, CD4+/CD8+ ratio or cDC1/cDC2 ratio during the perioperative period (Table 3).
Fig. 3.
Perioperative changes in the stimulatory capacity of cDC1 and serum levels of TGF-β1. a The stimulatory capacity of cDC1 significantly increased after pancreatectomy (P=0.008). b The significant decrease in serum levels of TGF-β1 in cancer patients was observed during the postoperative period compared with the preoperative period (P=0.017). The data are expressed as the median (interquartile interval). Statistical significance was determined using the Wilcoxon signed rank test
Changes in the cDC count and serum TGF-β1 concentration during the postoperative follow-up period
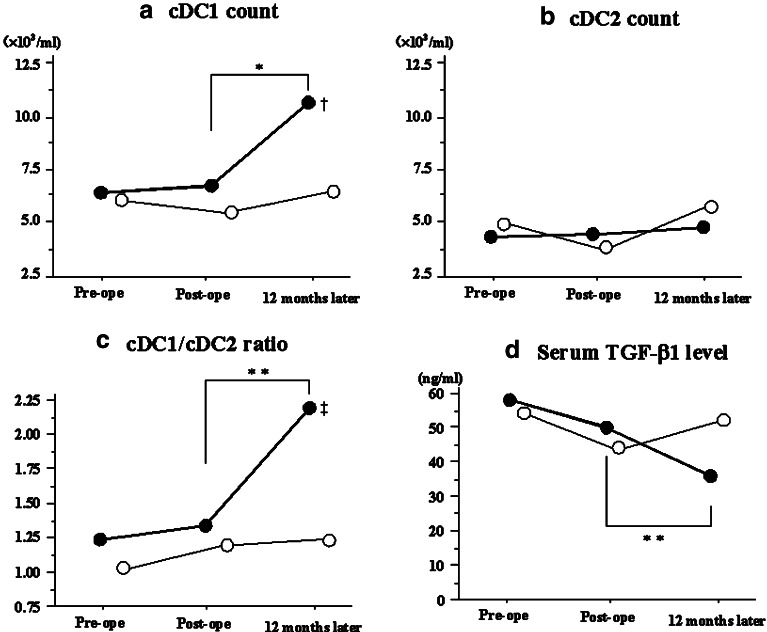
Flow cytometric assay and the serum TGF-β1 level at 12 months after pancreatectomy were performed in 20 patients (12 disease-free patients and 8 relapsed patients). As shown in Fig. 4a and c, the cDC1 count and cDC1/cDC2 ratio in disease-free patients were significantly increased at 12 months after pancreatectomy compared to those at the postoperative period, and reached control levels (cDC1 counts: postoperative, 5.9×103/ml (0.9–18.9); 12 months later, 10.0×103/ml (4.7–27.0); P=0.003), (cDC1/cDC2 ratio: postoperative, 1.3 (0.2–3.8); 12 months later, 2.2 (0.8–5.9); P=0.028). However, they remained stable in those patients with recurrence or metastasis (cDC1 counts: postoperative, 5.1×103/ml (1.8–12.9); 12 months later, 6.0×103/ml (2.7–9.1); P=0.889), (cDC1/cDC2 ratio: postoperative, 1.1 (0.6–2.4); 12 months later, 1.2 (0.5–2.2); P=0.834). The cDC1 count as well as cDC1/cDC2 ratio in disease-free patients was significantly higher than those in relapsed patients at 12 months after the pancreatectomy (cDC1count: P=0.005, cDC1/cDC2 ratio: P=0.012) (Fig. 4a, c). The cDC2 count appeared stable regardless of recurrent or metastatic status (Fig. 4b). The cDC2 count did not change during the 12-month follow-up period (postoperative, 4.1×103/ml (1.9–29.4); 12 months later, 4.5×103/ml (1.7–13.7); P=0.814). In addition, a further decrease in the serum TGF-β1 level was observed in patients that were disease free after 12 months (n=10; postoperative: 50 ng/ml (35–65), 12 months later: 40 ng/ml (19–56); P=0.013) to reach control levels (Fig. 4d). Conversely, the counts of cDC1 and cDC2, the cDC1/cDC2 ratio, and also the serum TGF-β1 levels in patients with recurrence or metastasis did not change with any statistical significance during the postoperative period (Fig. 4a–d). We did not see either any significant change in the counts of NK cells, CD4+ or CD8+ T lymphocytes during the follow-up period after pancreatectomy, regardless of the recurrent or metastatic status (data not shown).
Fig. 4.
Normalized cDC1 count, cDC1/cDC2 ratio and Serum TGF- β1 level at 12 months after pancreatectomy in disease-free patients. These figures are expressed as the changes in parameters between the time of discharge and 12 months after pancreatectomy. a The cDC1 count in patients who remained disease free after 12 months significantly increased at 12 months after pancreatectomy compared to that of the postoperative period (P=0.001), however, that in patients with recurrence or metastasis did not increase. The cDC1 count in disease-free patient at 12 months after pancreatectomy was significantly higher than that in relapsed patients. (P=0.005). b The cDC2 count remained stable during the monitored period, regardless of the disease status (P=0.824). c The cDC1/cDC2 ratio in patients who remained disease free after 12 months significantly increased at 12 months after pancreatectomy compared to that of the postoperative period (P=0.011), however that in patients with recurrence or metastasis did not increase. ‡ The cDC1/cDC2 ratio in disease-free patient at 12 months after pancreatectomy was significantly higher than that in relapsed patients. (P=0.019). d The serum levels of TGF- β1 in patients who remained disease free after 12 months significantly decreased at 12 months after pancreatectomy compared to those of the postoperative period (P=0.013), whereas those in patient with recurrence slightly increased at 12 months after pancreatectomy. Disease-free patients: closed circles, Relapsed patients: Open circles. Symbols represent the median. *P<0.01, ** P<0.03 compared to postoperative period in disease-free patients. † P<0.01, ‡ P<0.03 versus relapsed patients. Statistical significance was determined using the Wilcoxon signed-rank test and the Mann–Whitney U test
Discussion
In the present study, we investigated the numerical and functional changes in cDCs during the perioperative period in patients with pancreatic cancer. The stimulatory capacity of cDC1 in patients with pancreatic cancer was significantly decreased compared to that in controls. The cDC1 count and cDC1/cDC2 ratio were also significantly lower in patients than controls. Although the cDC1 count and cDC1/cDC2 ratio did not change during the perioperative period, the stimulatory capacity of cDC1 clearly improved after pancreatectomy for pancreatic cancer. Interestingly, in patients who remained disease free at postoperative 12 months, the cDC1 count and cDC1/cDC2 ratio were significantly increased and their serum TGF-β1 level was significantly decreased than the perioperative values, reaching control levels.
Pancreatic cancer is one of the most aggressive malignancies and it has been reported that patients with pancreatic cancer have defective immune function [4, 15, 16]. An ineffective immune response may contribute to the poor prognosis of patients with pancreatic cancer. It has been suggested that, in patients with malignant tumors, soluble molecules and cytokines such as TGF-β1 and vascular endothelial growth factor (VEGF) released from cancer cells inhibit the function of immunoeffector cells, which have been considered to be mainly T lymphocytes [9, 17]. Since T lymphocytes are activated by DCs, functional abnormalities of DCs may be the predominant reason for immune dysfunction, and the malfunction of T lymphocytes may be a secondary reason. Defective function of DC1 could impair in vitro tumor antigen presentation to T lymphocytes [18]. As one mature DC stimulates 300–1,000 T lymphocytes, a reduction in the DC1 count could lead to defective tumor antigen presentation in vivo resulting in the declined function of DC1 and might be associated with an impaired immune response. There are some reports that the count and/or function of DCs from peripheral blood and/or sentinel lymph nodes were defective in patients with malignant tumors compared with healthy individuals [15, 19–23]. It has been also suggested that cancer cells prevent the differentiation of stem cells into DCs [24], induce apoptosis of DCs [25, 26], and also decrease the number of circulating or intratumoral DCs during tumor progression [20, 27]. In our patients, the cDC1 count was likely to be more defective as the stage worsened, although statistical significances were not observed (data not shown). Patients enrolled into this study might have had relatively severe defective immune function because most of them had highly advanced disease such as T3 or T4 tumor status (30 of 32 patients, 94%) and positive nodal involvement (22 of 32 patients, 69%). Taking these factors into consideration, the immunosuppressive status and tumor toxicity of advanced cancers may cause exhaustion of DC stores. It has been reported that the count and/or function of cDCs recovered several weeks after surgical resection of cancer [19, 21]. In this study, the functional improvement of cDC1 was observed after pancreatectomy and the numerical recovery of cDC1 and DC1/2 was observed in pancreatic cancer patients who remained disease free at 12 months after pancreatectomy. These results suggest that patients with pancreatic cancer did not have sufficient ability to induce effective antitumor immune responses, but pancreatectomy for pancreatic cancer could improve the function of cDC1. In our previous report, the cDC2 count was significantly higher in pancreatic cancer patients than controls [10], although there was no statistical significance in the cDC2 count between the patients and controls in this study. Vakkila [28] reported that the prognosis of the children with cancer who had higher level of cDC2 was significantly worse than those who had lower level of cDC2. We examined MLR from freshly isolated cDC2 as well as cDC1 from both pancreatic cancer patients and healthy individuals, and we demonstrated that cDC2 failed to stimulate allogeneic T lymphocytes (data not shown). A few earlier reports also suggest that cDC2 freshly isolated from normal human blood did not induce the proliferation in allogeneic T lymphocytes [29, 30]. Thus, DC2 that fail to induce the proliferation can promote the hyporesponsiveness of antigen-specific T lymphocytes and the generation of T regulatory lymphocytes [31–33]. In this study, pancreatic cancer patients had significantly lower cDC1 count and relatively higher cDC2 count compared to controls. As a result, cDC1/cDC2 ratio would be a marked gap between patients and controls. In addition, Mazariegos [34] reported that patients who have successfully withdrawn from immunosuppression after liver transplantation had significantly lower DC1/DC2 ratio compared to those who have failed to withdraw from immunosuppression and kept on maintenance immunosuppression. The results give a suggestion that the lower cDC1/cDC2 ratio is associated with the immunosuppression of a host. From the immunological significance of DC1 and DC2, the lower cDC1 and higher cDC2 count in our study can inevitably be of lower cDC1/cDC2 ratio and there appears to be a significant relationship between immunosuppression and the cDC/cDC2 ratio.
NK cells and T lymphocytes would also participate in the antitumor immune system. We also examined the proliferative response of T cells towards mitogen (PHA) and NK cell activity as common examinations to assess the function of T lymphocytes and NK cells. Impaired NK activity or PHA was observed in patients with lung, esophageal, head and neck or breast cancer [35–37]. However, there were no significant differences in the count of NK cells or CD4/CD8 T lymphocytes, NK cell activity or PHA between pancreatic cancer patients and controls. The counts of NK cells and CD4/CD8 T lymphocytes were also stable during the monitoring period, regardless of the recurrent status of patients. Although more profound investigation would be required to evaluate the immunological significance of NK cells or T lymphocytes, at least the count of NK cells or CD4/CD8 T lymphocytes might not be so useful compared to cDC1 count or cDC1/cDC2 ratio to prospect the immunological condition after the resection of pancreatic cancer.
Although there were significant decreases in hemoglobin and serum albumin levels in patients compared to controls, there were no differences in the count or function of PBMCs or other effector cells. It is unlikely that the decreased hemoglobin or albumin was the reason for the decreased cDC1 count because DCs were obtained from PBMCs.
We also measured the serum levels of TGF-β1 during the perioperative period. TGF-β1 is a multifunctional and pleiotropic cytokine. Immunosuppression is one of the major biological effects of TGF-β1, and it has the ability to inhibit generations of cytotoxic T lymphocytes. It has been reported that tumor cells could be an important source of TGF-β1 [38–40] and that immunosuppression in cancer patients was associated with enhanced TGF-β1 production [41]. Furthermore, it has been reported that TGF-β1 suppressed the antigen presentation and maturation of DCs [13, 14, 42]. The serum levels of TGF-β1 in patients with pancreatic cancer were significantly elevated compared to controls and significantly decreased after pancreatectomy. In the disease-free patients, further decreases in serum TGF-β1 levels were observed at 12 months after pancreatectomy. These results suggested that the alterations in TGF-β1 levels were reversed by surgical resection of the tumor and might be associated with recovery of cDC1 count and function.
In conclusion, surgical resection of pancreatic cancer could be associated with improved cDC1 function. The cDC1 count and cDC1/cDC2 ratio normalized approximately 12 months after pancreatectomy when patients did not develop any obvious local recurrence or distant metastasis. Since we are able to evaluate the cDC1 count and cDC1/cDC2 ratio using a simple method by flow cytometry, the immunological monitoring of cDCs may be a useful practice in the therapeutic strategy of pancreatic cancer. A more profound study of immunological dynamics, principally on the DCs that surround pancreatic cancer, may lead to immunological therapeutic approaches for improving immune function and clinical outcome.
Acknowledgments
We greatly thank Dr. M. Inaba (First Department of Pathology, Kansai Medical University) for his skillful technical assistance, Ms. S. Miura (First Department of Pathology, Kansai Medical University) for sorting cells on a FACStar, and Ms. A. Kihara (Department of Surgery, Kansai Medical University) for manuscript preparation.
References
- 1.Lillemoe KD, Cameron JL, Yeo CJ, Sohn TA, Nakeeb A, Sauter PK, Hruban RH, Abrams RA, Pitt HA. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg. 1996;223:718–728. doi: 10.1097/00000658-199606000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health and Welfare Statistics Association J Health Welfare Stat. 2004;51:45–56. [Google Scholar]
- 3.Takai S, Satoi S, Toyokawa H, Yanagimoto H, Sugimoto N, Tsuji K, Araki H, Matsui Y, Imamura A, Kwon AH, Kamiyama Y. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: a retrospective, single-institution experience. Pancreas. 2003;26:243–249. doi: 10.1097/00006676-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 4.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925–932. [PubMed] [Google Scholar]
- 5.Ito T, Inaba M, Inaba K, Toki J, Sogo S, Iguchi T, Adachi Y, Yamaguchi K, Amakawa R, Valladeau J, Saeland S, Fukuhara S, Ikehara S. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–1419. [PubMed] [Google Scholar]
- 6.O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 7.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immun. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 9.Klein L, Trautman L, Psarras S, Schnell S, Siermann A, Liblau R, Boehmer H, Khazaie K. Visualizing the course of antigen-specific CD8 and CD4 T cell responses to a growing tumor. Eur J Immunol. 2003;33:806–814. doi: 10.1002/eji.200323800. [DOI] [PubMed] [Google Scholar]
- 10.Yanagimoto H, Takai S, Satoi S, Toyokawa H, Takahashi K, Terakawa N, Kwon AH, Kamiyama Y. Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol. 2005;114:52–60. doi: 10.1016/j.clim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Sobin LH, Wittekind CH, editors. TNM classificeation of malignant tumors. 6. New York: Wiley; 2002. [Google Scholar]
- 12.Toyokawa H, Inaba M, Takai S, Satoi S, Beuth J, Ko HL, Matsui Y, Kwon AH, Kamiyama Y, Ikehara S. Enhancement of circulating dendritic cell activity by immunomodulators (OK432 and KP-40) Anticancer Res. 2002;22:2137–2145. [PubMed] [Google Scholar]
- 13.Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL, Akporiaye ET. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 15.Dallal RM, Christakos P, Lee K, Egawa S, Son YI, Lotze MT. Paucity of dendritic cells in pancreatic cancer. Surgery. 2002;131:135–138. doi: 10.1067/msy.2002.119937. [DOI] [PubMed] [Google Scholar]
- 16.Ungefroren H, Voss M, Bernstorff W, Schmid A, Kremer B, Kalthoff H. Immunological escape mechanisms in pancreatic cancinoma. Ann Ny Acad Sci. 1999;880:243–251. doi: 10.1111/j.1749-6632.1999.tb09529.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 18.Dong R, Cwynarski K, Entwistle A, Marelli-Berg F, Dazzi F, Simpson E, Goldman JM, Melo JV, Lechler RI, Bellantuono I, Ridley A, Lombardi G. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood. 2003;101:3560–3567. doi: 10.1182/blood-2002-06-1841. [DOI] [PubMed] [Google Scholar]
- 19.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 21.Hoffmann TK, Muller-Berghaus J, Ferris RL, Johnson JT, Storkus WJ, Whiteside TL. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinomas of the head and neck. Clin Cancer Res. 2002;8:1787–1793. [PubMed] [Google Scholar]
- 22.Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323–331. doi: 10.1016/S0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 23.Satthaporn S, Robins A, Vassanasiri W, El-Sheemy M, Jibril JA, Clark D, Valerio D, Eremin O. Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother. 2004;53:510–518. doi: 10.1007/s00262-003-0485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourkova IL, Yamabe K, Foster B, Chatta G, Perez L, Shurin GV, Shurin MR. Murine prostate cancer inhibits both in vivo and in vitro generation of dendritic cells from bone marrow precursors. Prostate. 2004;59:203–213. doi: 10.1002/pros.10369. [DOI] [PubMed] [Google Scholar]
- 25.Esche C, Gambotto A, Satoh Y, Gerein V, Robbins PD, Watkins SC, Lotze MT, Shurin MR. CD154 inhibits tumor-induced apoptosis in dendritic cells and tumor growth. Eur J Immunol. 1999;29:2148–2155. doi: 10.1002/(SICI)1521-4141(199907)29:07<2148::AID-IMMU2148>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Kiertscher SM, LUO J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte derived dendritic cell. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 27.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte derived dendritic cells. Prostate. 2001;46:68–75. doi: 10.1002/1097-0045(200101)46:1<68::AID-PROS1010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Vakkila l, Thomson AW, Vettenranta K, Sariola H, Saarinen-Pihkala UM. Dendritic cell subsets in childhood and in children with cancer: relation to age and disease prognosis. Clin Exp Immunol. 2004;135:455–461. doi: 10.1111/j.1365-2249.2003.02388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 30.Kuwana M, Kaburaki J, Wright TM, Kawakami Y, Ikeda Y. Induction of antigen-specific human CD4(+) Tcell anergy by peripheral blood DC2 precursors. Eur J Immunol. 2001;31:2547–2557. doi: 10.1002/1521-4141(200109)31:9<2547::AID-IMMU2547>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7–1dim, B7–2) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–1545. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, Thomson AW. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3:689–696. doi: 10.1034/j.1600-6143.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 35.Nozoe T, Korenaga D, Ohga T, Futatsugi M, Maehara Y. Suppression of the phytohemagglutinin response to lymphocytes is an independent prognosticator in patients with squamous cell carcinoma of the esophagus. Ann Thorac Surg. 2003;76:260–265. doi: 10.1016/S0003-4975(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 36.Farinas MC, Rodriguez-Valverde V, Zarrabeitia MT, Parra-Blanco JA, Sanz-Ortiz J. Contribution of monocytes to the decreased lymphoproliferative response to phytohemagglutinin in patients with lung cancer. Cancer. 1991;68:1279–1284. doi: 10.1002/1097-0142(19910915)68:6<1279::AID-CNCR2820680617>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 37.Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33:119–124. doi: 10.1002/immu.200390014. [DOI] [PubMed] [Google Scholar]
- 38.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 39.Shim KS, Kim KH, Han WS, Park EB. Elevated serum levels of transforming growth factor-beta1 in patients with colorectal carcinoma: its association with tumor progression and its significant decrease after curative surgical resection. Cancer. 1999;85:554–561. doi: 10.1002/(SICI)1097-0142(19990201)85:3<554::AID-CNCR6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 40.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM. Preoperative plasma levels of transforming growth factor beta1 (TGF-beta1) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 41.Young MR, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–338. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 42.Lin CM, Wang FH, Lee PK. Activated human CD4+ T cells induced by dendritic cell stimulation are most sensitive to transforming growth factor-beta: implications for dendritic cell immunization against cancer. Clin Immunol. 2002;102:96–105. doi: 10.1006/clim.2001.5151. [DOI] [PubMed] [Google Scholar]