Abstract
Several examples have shown that C3d3, when fused to a corresponding antigen, had a strong adjuvant effect on certain specific antibody production. In a previous study, we attempted to prove that this was the case of the human chorionic gonadotrophin β chain (hCGβ)-induced immunity following DNA vaccination. However, we found that C3d3 when fused to hCGβ inhibited rather than enhanced the antigen-specific immune response. In the present study, using hCGβ DNA vaccine preparations, we demonstrated that C3d3 inhibited the antigen-specific humoral antibody response and several other immune responses, such as the hCGβ specific lymphoproliferation. Such inhibitory effects of C3d3 were not related to the expression level of the target protein, the gender of the test mice, or the vector used. Contrastingly, C3d3 fused with the envelope protein of hepatitis B virus (PreS2/S) used as a control system resulted in the enhancement of both humoral and cell-mediated antigen-specific immune responses against HBV-preS2/S, which was consistent with other groups’ adjuvant-effect findings. We further showed that the mechanisms involved in the inhibitory effect of C3d3 might be possible due to impairing the function of antigen presenting B lymphocytes and reducing the expression of transcription factors (T-bet and GATA-3) and cytokine IL-4. Collectively, unlike its usual expected adjuvant function, the fusion of C3d3 with the tumor-associated antigen hCGβ was found to inhibit both humoral and cell-mediated antigen-specific immune responses. These findings indicate that research concerning tumor immune escapes and vaccine designs require further extensive attention.
Keywords: C3d3, Inhibition, Immune response, Tumor-associated antigen, DNA vaccination
Introduction
Human chorionic gonadotrophin (hCG) is a heterodimeric molecule consisting of a β-chain common to all members of the glycoprotein hormone family noncovalently associated with a hormone specific β-chain [1]. hCG is normally expressed at detectable levels during pregnancy, but it is also ectopically expressed in trophoblastic and non-trophoblastic carcinomas of the colon, prostate, bladder, breast and lung [2]. The hCGβ dimer secreted by tumors performs the function of growth factors that enhance and regulate proliferation, differentiation and growth of tumor cells [3, 4]. Therefore, hCGβ could be used as a target tumor antigen for the development of a novel tumor vaccine for clinical tumor immunotherapy [5–7].
C3d is a degradation product of C3 complement molecule. Dempsey et al. [8] reported that mice inoculated with hen egg lysozyme (HEL)-mC3d3 (mouse three copies of C3d) obtained a 1,000-fold enhancement of anti-HEL antibody compared to those vaccinated with HEL only. Immunizations with DNA expressing C3d3 conjugated to many different antigens [9–16] could enhance the antigen-specific humoral immune responses, which suggested that C3d3 molecule could serve as a molecular adjuvant. However, there are several recent reports [17–20] which suggested that C3d3 might have inhibitory effects on humoral immune response, although the mechanisms are yet to be determined.
Initially, we fused C3d3 with hCGβ and found that, unlike many antigens, hCGβ fused with C3d3 resulted in the inhibition of both humoral and cell-mediated antigen-specific immune. Conversely, C3d3 fused to the antigen HBV-preS2/S, was found to enhance both humoral and cell-mediated antigen-specific immune responses. The evidence indicated that co-expression of C3d3 molecule with the tumor-associated antigen (TAA) hCGβ down-regulated rather than enhanced the antigen-specific immune responses. Possible mechanisms involved in such inhibition of immune responses were further investigated.
Materials and methods
Mice
Female and male 6- to 8-week-old BALB/c mice were purchased from the Center of Experimental Animal, Fudan University (Shanghai, China). They were bred and raised under the specific pathogen-free conditions. The housing and handling of the experimental animals were performed in accordance with the guidelines of the Chinese Council for Animal Care.
Construction of plasmid DNA
The eukaryotic expression vectors TR421 and TR421-C3d3 (containing three copies of murine C3d) were kindly provided by Dr. T.M. Ross (University of Pittsburgh School of Medicine, USA). The plasmid pSG5.C3d3.YL (containing three copies of murine C3d) was kindly gifted by Prof. Q. Shen (Shanghai Institute of Planned Parenthood Research, Shanghai, China). The plasmids pVAON33-hCGβ and pVAON33-preS2/S were cloned as described [21, 22].
hCGβ encoding sequence was digested from the plasmid pVAON33-hCGβ using HindIII and BamHI restriction endonuclease sites and cloned into vectors TR421 and TR421-C3d3 separately using unique restriction endonuclease sites. The plasmid pcDNA3-hCGβ was constructed by subcloning of a HindIII-XbaI hCGβ fragment from the plasmid pVAON33-hCGβ. The plasmid pcDNA3-hCGβ-C3d3 was constructed by subcloning of a BamHI-XbaI C3d3 fragment from pSG5.C3d3.YL. The HBV-preS2/S encoding sequence was digested from the plasmid pVAON33-preS2/S by using BglII restriction endonuclease enzyme and subcloned into vectors TR421 and TR421-C3d3, respectively, by BamHI restriction endonuclease site. These plasmids were purified by QIAGEN Plasmid Mega Kit according to the manufacturer’s instruction (Qiagen, Hilden, Germany).
DNA immunization
The quadricepses of mice were first injected with a total of 100 μl of 0.25% bupivacaine (Sigma-Aldrich Co., Saint Louis, MO) to enhance the cellular uptake of plasmid DNA. One day later, different doses of each plasmid DNA with a final volume of 100 μl in 0.9% NaCl were injected into the same region of mice, respectively. Blood samples were obtained at indicated times for antibody analyses. Each group of the mice was subsequently boosted twice, at 3-week intervals with the same amount of DNA.
Expression of vaccine constructs in vitro and in vivo
SMMC7721 cells (5 × 105 cells per transfection), a human hepatocellular carcinoma cell line (The cell bank of Chinese Academy of Science, Shanghai, China), were transfected with 2 μg of plasmid TR421, TR421-hCGβ or TR421-hCGβ-C3d3 using 10% lipofectamine 2000 (Invitrogen Co., California) according to the manufacture guidelines. Supernatants were collected and stored at −20°C.
BALB/c mice were immunized with an individual dose of 1, 10 and 100 μg of plasmids TR421, TR421-hCGβ or TR421-hCGβ-C3d3, respectively. Blood samples of the mice were obtained on the second day after DNA immunization for expression analyses. The supernatants and sera were assayed for immunoreactive hCGβ by quantitative radioimmunologic assays (RIA) following the manufacturer’s instruction (Diagnostic Products Co., Shanghai, China).
Animal model of tumor growth
Two weeks after the final immunization with the various plasmid DNAs, 1 × 106 Sp2/0-hCGβ cells were incubated subcutaneously following inoculation into the flank of mock-DNA (n = 6), TR421-hCGβ (n = 6) or TR421-hCGβ-C3d3 (n = 6) immunized mice, respectively. The weight of tumors was subsequently evaluated.
Detection of antibody levels and avidity
An indirect ELISA was performed to assay anti-hCGβ-specific IgG and anti-preS2/S-IgG in immune serum. The 0.1 μg/well hCGβ (Shanghai Healthdigit Co., Shanghai, China) or 0.5 μg/well hepatitis B virus surface antigen (HbsAg) (Beijing Hepatitis Reagent Research and Production Research, Beijing, China) were used to coat plates as described [7, 21]. Avidity ELISAs were performed similarly [9]. Plates were washed three times with 0.05% PBS-Tween20. NaSCN (Shanghai Reagent Co., Shanghai, China), a chaotropic compound that interfered with the antigen–antibody reaction, was added subsequently in concentrations ranging from 0.5 to 3.5 M. The plates were washed six times with PBS-Tween20 after they were kept at room temperature for 15 min. After washing, a horseradish peroxidase-labeled goat anti-mouse antibody (Southern Biotech, Alabama) was added at a dilution of 1:6,000. After 60 min incubation, the plates were washed. The absorbance was read at 490 nm. The avidity of antibody was expressed as the effective concentration of NaSCN required to release 50% of antiserum (ED50) and affinity index (AI). AI = ED50 (target antigen-C3d3)/ED50 (target antigen). If AI > 1.0, the number was an indication that C3d3 can accelerate the antibody avidity maturation; if AI < 1.0, it indicated that C3d3 can inhibit the antibody avidity maturation.
Lymphocyte proliferation assay
Splenocytes were harvested 8 weeks after the first immunization. Single-cell suspensions were prepared and the cells were incubated with purified protein (hCGβ 5 μg/ml or HBsAg 12.5 μg/ml) for 72 h and for the last 18 h 3H-labeled thymidine (TdR) was added. A liquid scintillator was used to measure the uptake of radioactive TdR. Proliferative capacity is shown as stimulatory index (SI) calculated according to the following equation: SI = (cpm of stimulated cultures − cpm of control cultures)/cpm of control cultures [22, 23].
Cytotoxicity assay
Target cells were obtained from Sp2/0 myeloma cell lines stably transfected with plasmid pcDNA3-hCGβ or pcDNA3-preS2/S. Splenocytes from immunized mice were obtained and resuspended in RPMI 1640 medium (Gibco Laboratories, USA) containing 10% FBS, antibiotics, and l-glutamine. CTLs were initially made by culturing 5 × 106 immune splenocytes with purified protein in flat-bottom 24-well tissue culture plates for 5–6 days and the medium was changed at 2–3-day intervals supplemented with 20 U/ml mouse IL-2 (Jingmei Biotech Co., Shenzhen, Guangdong China). CTL activity was assayed in a 6-h 3H thymidine-release assay [24] by mixing effector lymphocytes with 5 × 103-labeled target cells at 80:1, 40:1, 20:1, and 10:1 E:T ratios in round-bottom, 96-well plates in triplicate. Values were expressed as percentages of specific lysis.
Antigen presentation function assay
A total of 6 × 106 macrophages or B lymphocytes obtained from female BALB/c mice were cultured with the supernatants containing protein hCGβ or hCGβ-C3d3 for 5 h at 37°C. APC were inactivated by mitomycin C for 60 min at 37°C. Splenocytes were harvested from the mice immunized with plasmid TR421, TR421-hCGβ or TR421-hCGβ-C3d3. A total of 5 × 105 cells/well splenocytes were added to a 96-well flat-bottom microtiter plates in a final volume of 200 μl/well, and co-cultured with mitomycin C-treated APC for 72 h at different E:T ratios. Proliferation was determined by [3H]-thymidine incorporation into DNA and expressed as SI.
mRNA analyses
The total RNA was extracted from the same number (1 × 107 cells) of the re-stimulated splenocytes derived from the immunized mice using TRIzol Reagent system (Life Technologies, USA). RT-PCR was performed as described [25]. Upstream and downstream primers of GAPDH, IL-4, IFN-γ, T-bet and GATA-3 were used as described [25]. Experiments were performed on three independent pools and the mean ± SEM were compared. mRNA expression was calculated as the ratio of the intensity of the corresponding band to the GAPDH band by densitometry, respectively [26].
Statistical analyses
Statistical analyses of the data were performed with the aid of analysis programs in SPSS10.0 software. Differences between the treatment groups were examined by one-way ANOVA. Differences were considered statistically significant when P < 0.05.
Results
C3d3 inhibited hCGβ-specific humoral and cellular immune responses
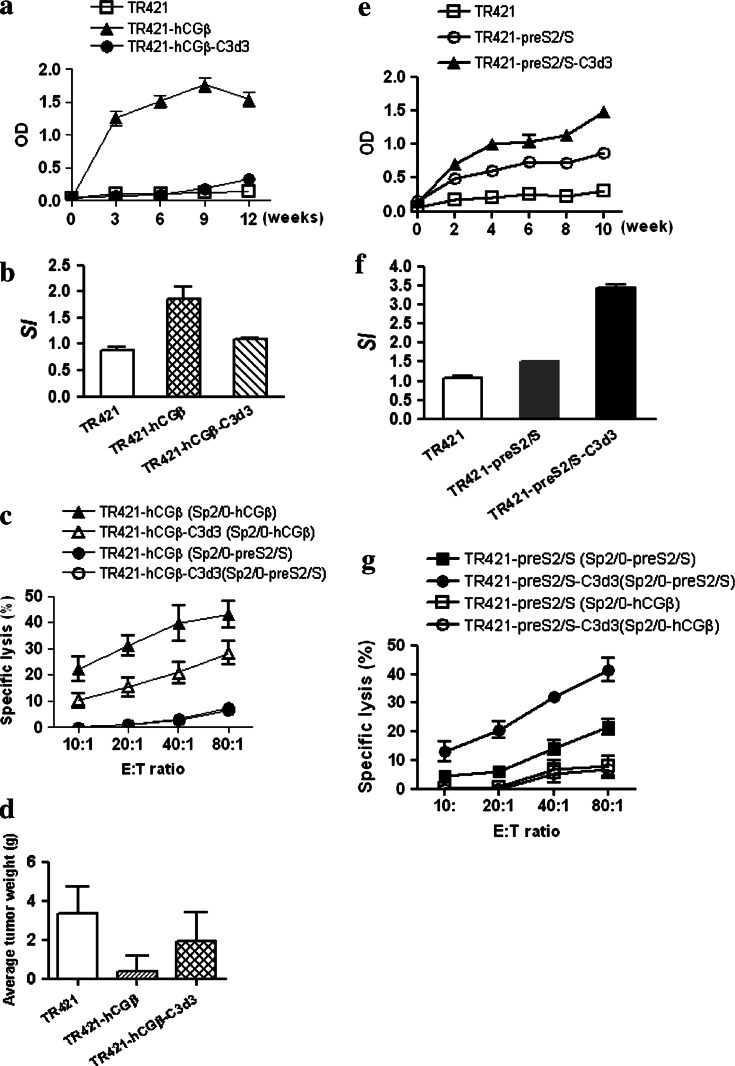
To investigate the specific anti-tumor immunity induced by DNA vaccine encoding hCGβ cDNA, female BALB/c mice were immunized with TR421-hCGβ or mock DNA for three times at three weekly intervals. It was found that TR421-hCGβ elicited high levels of anti-hCGβ antibody response (Fig. 1a) as well as a strong antigen-specific lymphoproliferation and CTL responses (Fig. 1b, c). The dilution for the sera of BALB/c mice immunized with plasmid encoding hCGβ was 1:4,000 at week 3, 1:8,000 at week 6, 1:20,000 (the peak titer) at week 9 and declined to 1:10,000 at week 12 (data not shown). The immune response induced by TR421-hCGβ plasmid was found to inhibit the growth of tumor cells harboring hCGβ in vivo (Fig. 1d).
Fig. 1.
The regulations of C3d3 on the immune responses against hCGβ or HBV-preS2/S. a, e BALB/c mice were primed with 100 μg DNA at day 0 and boosted at weeks 3 and 6. Sera were obtained from mice at the indicated weeks and diluted 1:20 for determination of specific IgG levels by ELISA. b, f Splenocytes of the immunized mice were re-stimulated with certain concentration of purified proteins. After a 56-h incubation, [3H]-thymidine (0.5 μCi/well) incorporation was measured for an additional 18 h. Proliferation was determined by [3H]-thymidine incorporation into DNA and expressed as SI. c, g Splenocytes of the immunized mice were stimulated with certain concentration of purified protein for 5 days, then harvested to analyze the CTL activity against Sp2/0-hCGβ or Sp2/0-preS2/S cells by 6-h 3H-thymidine release assay at the indicated E:T. d Two weeks later after BALB/c mice were primed with 100 μg DNA at day 0 and boosted at weeks 3 and 6, they were given injections i.p. of 1 × 106 Sp2/0-hCGβ cells. Tumor growing in recipient mice was observed and tumor weight was evaluated
Initially, the introduction of C3d3 into the vector expression system was to enhance the anti-tumor efficacy of the previously described hCGβ DNA vaccine. However, the mice immunized with plasmid encoding the chimeric hCGβ-C3d3 had significantly lower serum antibody levels than those immunized with plasmid encoding hCGβ alone (P < 0.001; Fig. 1a). The dilution for the sera of BALB/c mice immunized with plasmid encoding hCGβ-C3d3 was very low, i.e., 1:400 (data not shown). In addition, splenocytes isolated from TR421-hCGβ-C3d3-DNA immunized mice exhibited approximately 65% lower proliferative activities than those isolated from TR421-hCGβ-DNA immunized mice (P < 0.05; Fig. 1b). The former further exhibited less specific cytotoxic lysis than the latter (P < 0.05; Fig. 1c). When hCGβ was fused to C3d3, its ability in inhibiting tumor growth in vivo had significantly diminished (P < 0.05; Fig. 1d). These results indicated that C3d3 could inhibit the humoral and cellular immune responses against hCGβ following DNA immunization.
To rule out the possible influence of antigens applied on the inhibitory effects of C3d3 molecule, HBV-preS2/S was selected as the control antigen. When C3d3 was fused with antigen HBV-preS2/S, increased specific antibody response against HBsAg was observed (P < 0.05; Fig. 1e), as well as the production of HBV specific lymphocyte proliferation activity (P < 0.001; Fig. 1f) and CTL activities (P < 0.01; Fig. 1g) using the same vector system and immunization dose. These results suggested that C3d3 had different influences on the immune responses against different antigens.
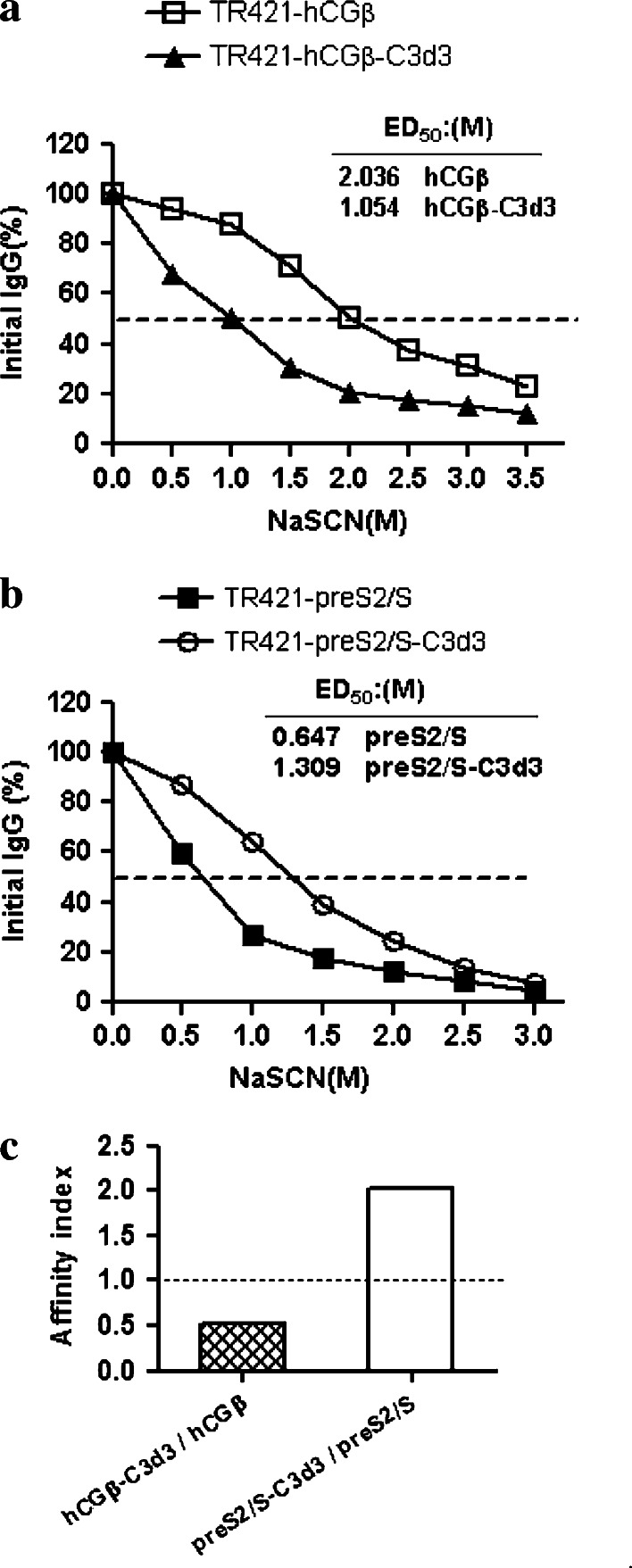
C3d3 resulted in the generation of low-affinity anti-hCGβ IgG antibodies
Since C3d3 had synchronous regulations on the humoral and cellular immune responses induced by hCGβ or HBV-preS2/S, we further investigated the regulations of C3d3 on the humoral immune responses. To evaluate the specific antibody maturation and binding affinity, the anti-hCGβ antibody avidity was measured by sodium thiocyanate (NaSCN)-displacement ELISA. NaSCN is a chaotropic agent which can disrupt the antigen–antibody interaction [9]. The binding of antibodies with greater avidity to the antigen is disrupted at higher concentrations of NaSCN than that of antibodies with less avidity to the antigen. Figure 2a demonstrated that the effective concentration of NaSCN required releasing 50% of antiserum (ED50) collected from hCGβ-DNA immunized mice at 12 weeks after vaccination was ∼2.036 M, while the ED50 from mice immunized with hCGβ-C3d3-expressing DNA was only ∼1.054 M. When pre-S2/S was fused with C3d3, an enhanced antibody avidity was observed (ED50 = 1.309 M NaSCN vs. ED50 = 0.647 M NaSCN; AI > 1.0) (Fig. 2b, c). These results indicated that C3d3 could inhibit the avidity maturation of antibodies against hCGβ but had an opposite effect on the maturation of antibodies against HBsAg.
Fig. 2.
Specific antibody avidity in various DNA immunizations. Sera were obtained from the mice immunized with plasmid TR421-hCGβ (open square), TR421-hCGβ-C3d3 (closed triangle), TR421-preS2/S (closed square) or TR421-preS2/S-C3d3 (open circle), and analyzed from week 12 after immunization in a NaSCN-displacement ELISA. Data are represented as the average of two independent experiments. a The avidity of anti-hCGβ IgG, b the avidity of anti-HBsAg IgG and c the affinity index of anti-hCGβ IgG or anti-HBsAg IgG
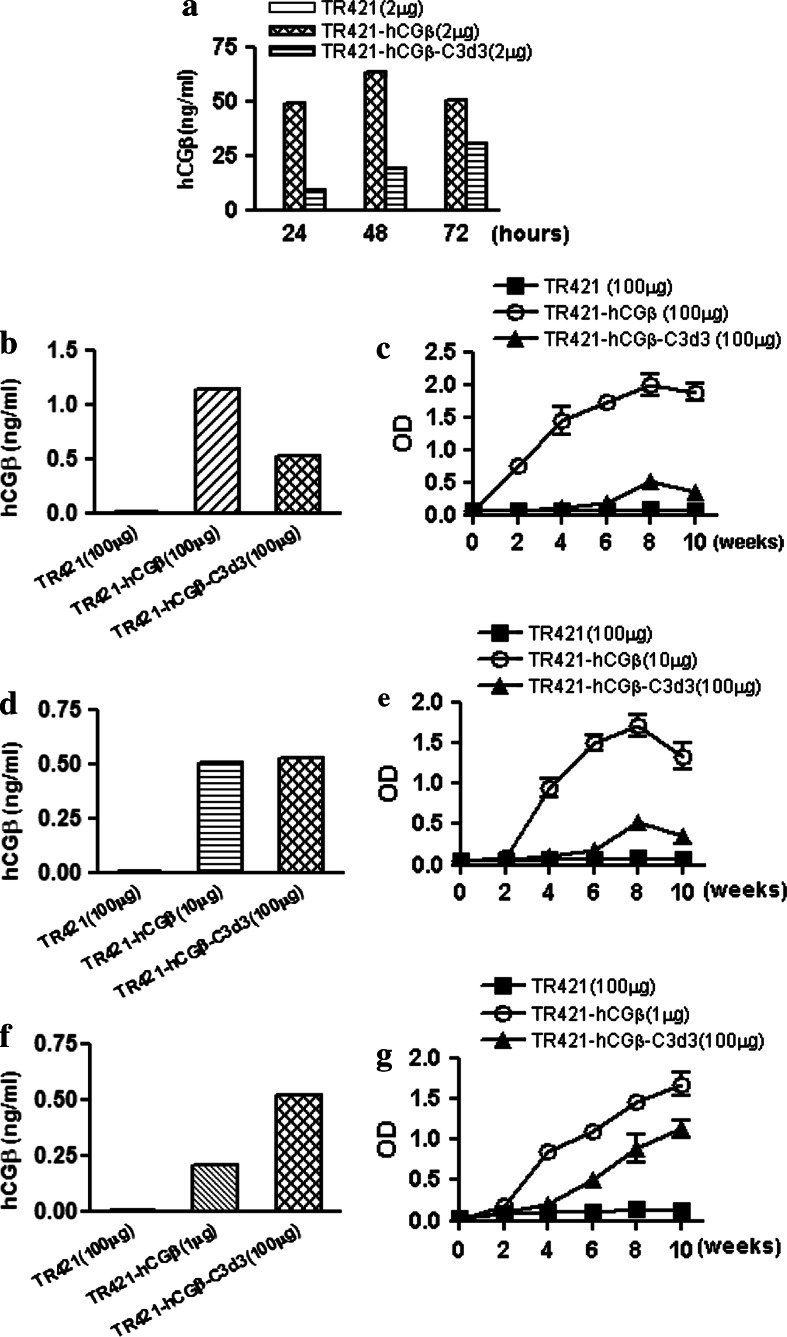
The inhibitory effects of C3d3-hCGβ was not related to the low expression of proteins of interest, and was not due to the vector used or the gender of the test mice
The results from others have shown that the attachment of three copies of C3d decreased the expression amount of antigens to some extent [9–15]. Therefore, it is unknown if the inhibitory efficacy of C3d3 on hCGβ could be due to the low expression of target proteins. The expressions of protein hCGβ in the supernatants were analyzed by RIA. As suspected, C3d3 decreased the expression of hCGβ conjugated, and the expression level of plasmid TR421-hCGβ was more than twice that of plasmid TR421-hCGβ-C3d3 at 48 h after transfection (Fig. 3a).
Fig. 3.
Influence of the expression level of target antigen on the down-regulation efficacy of C3d3. a SMMC7721 cells were transfected with TR421, TR421-hCGβ and TR421-hCGβ-C3d3 at indicated quantity by lipofectamine 2000, respectively. The supernatants were obtained at indicated times for detection of the antigens by RIA kit. b, d, f BALB/c mice were immunized with a dose of 1, 10 or 100 μg plasmids TR421, TR421-hCGβ or TR421-hCGβ-C3d3 separately. Sera were obtained from mice at the second day after immunization and detected by RIA kit. c, e, g BALB/c mice were primed with 1, 10 or 100 μg DNA at day 0 and boosted at weeks 3 and 6. Sera were obtained from mice at the indicated weeks and made certain dilution for determination of specific IgG levels by ELISA
As analogous with the results of antigen expression in vitro, the expression of sera hCGβ in mice induced by 100 μg of TR421-hCGβ-C3d3 was lower than that of 100 μg of TR421-hCGβ, and the level of total anti-hCGβ IgG induced by the former was significantly lower than that induced by the latter (P < 0.001, Fig. 3b, c). The expression of hCGβ induced by 100 μg of TR421-hCGβ-C3d3 was similar to that conducted by 10 μg of TR421-hCGβ (Fig. 3d). The antibody level induced by 100 μg of TR421-hCGβ-C3d3 was lower than that induced by 10 μg of TR421-hCGβ (P < 0.001, Fig. 3e). Moreover, it was found that even the expression of hCGβ induced by TR421-hCGβ-C3d3 (100 μg) was significantly higher than that of TR421-hCGβ (1 μg) (P < 0.001, Fig. 3f, the anti-hCGβ immune response induced by the former was still significantly lower than that induced by the latter (P < 0.01, Fig. 3g). Therefore, we concluded that the down-regulation effect of C3d3 on the TAA hCGβ was not caused by the low expression of hCGβ protein.
On the other hand, the level of total anti-hCGβ IgG observed in the hCGβ-immunized mice was significantly higher than that of hCGβ-C3d3 immunized mice regardless of the vector system utilized, i.e. either pcDNA3 vector system or TR421 vector system (data not shown). To confirm that the inhibitory effect of C3d molecule was not ascribed to the gender of mice immunized, male and female BALB/c mice were immunized with TR421, TR421-hCGβ or TR421-hCGβ-C3d3, respectively. Immunization with hCGβ alone had raised significantly higher levels of anti-hCGβ antibody than that with hCGβ-C3d3 in both male and female mice (data not shown). The results suggested that C3d3 inhibited the humoral immune responses against hCGβ, and the immune inhibition by C3d3 molecule was not caused by the vector and gender of the mice applied in this study.
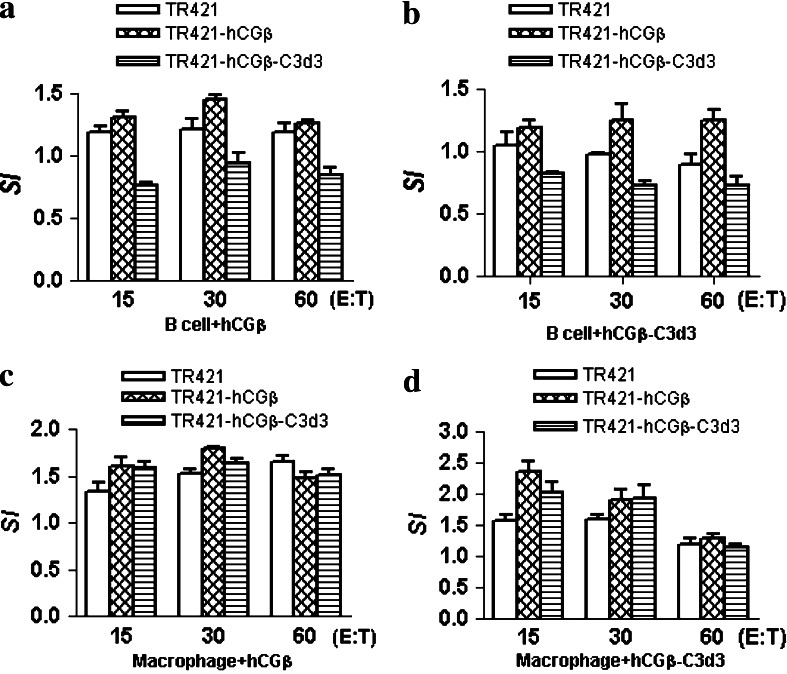
The influence of C3d3 on the antigen presentation of hCGβ by B lymphocyte or macrophage
To evaluate the influence of C3d3 on the antigen presentation, B lymphocytes or macrophages were incubated with the supernatants containing protein hCGβ or hCGβ-C3d3 for 5 h. The splenocytes from the immunized mice were stimulated at different ratios of effector cells and APCs. The proliferation was determined by [3H]-thymidine incorporation into DNA and expressed as SI. Figure 4a, b showed that the proliferation of TR421-hCGβ-C3d3 immunized splenocytes was significantly less than that of TR421-hCGβ immunized splenocytes under the stimulation with B lymphocytes (P < 0.05), no matter whether B lymphocytes were incubated with hCGβ or hCGβ-C3d3. However, there was no significant difference in the proliferation of splenocytes between TR421-hCGβ and TR421-hCGβ-C3d3 group under the stimulation with macrophages, regardless of whether macrophages were incubated with hCGβ or hCGβ-C3d3 (Fig. 4c, d). These findings implied that C3d3 might down-regulate the antigen presentation of hCGβ by B cells, but not macrophages.
Fig. 4.
Influence of C3d3 on the antigen presentation of hCGβ by B lymphocytes and macrophages. B lymphocytes or macrophages were incubated with the supernatants containing protein hCGβ (a, c) or hCGβ-C3d3 (b, d) for 5 h. The splenocytes from the DNA vaccinated mice were co-cultured with mitomycin C-treated B lymphocytes (a, b) or macrophages (c, d) for 72 h at different E:T ratios. Proliferation was determined by [3H]-thymidine incorporation into DNA and shown as SI. Results are expressed as the mean ± SD of triplicate measurements
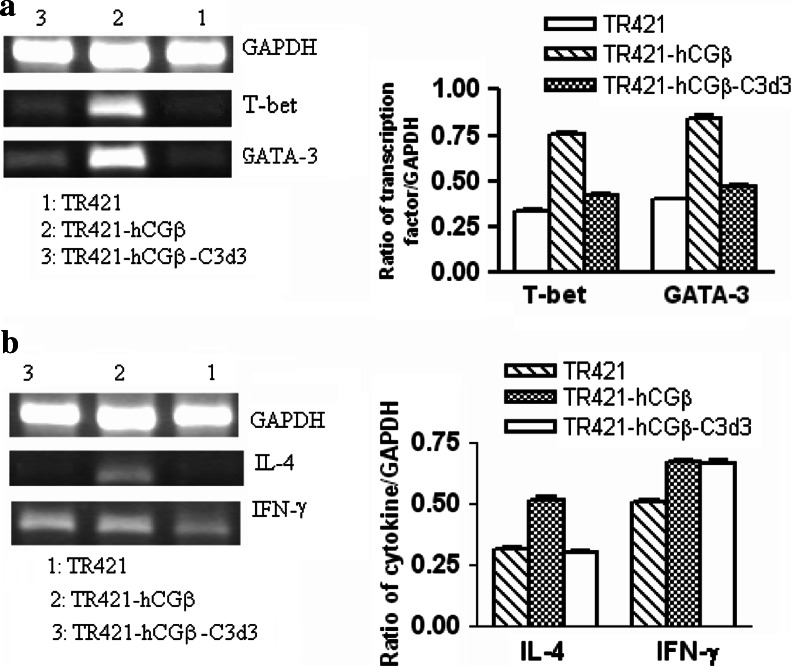
C3d3 limited the production of transcription factors (T-bet and GATA-3) and cytokine IL-4 in splenocytes of the mice immunized with hCGβ DNA vaccine
From the aforementioned results, we observed that co-expression of C3d3 molecule with hCGβ could inhibit both Th1-type and Th2-type antigen-specific immune responses. Transcription factors T-bet and GATA-3, and cytokines IFN-γ and IL-4 played an important role in Th1 and Th2 cells development, respectively [27, 28]. We further explored if the possible mechanisms of the C3d3 mediated immune down-regulation was related to the influence on these transcription factors and cytokines. After the re-stimulation with hCGβ protein in vitro, the splenocytes of the mice immunized with TR421-hCGβ-C3d3 produced significantly lower mRNA levels of T-bet and GATA-3 than those of the mice vaccinated with TR421-hCGβ (Fig. 5a). Stimulation with recombinant hCGβ also produced significantly less IL-4-producing splenocytes of mice vaccinated with TR421-hCGβ-C3d3 than that of mice vaccinated with TR421-hCGβ (Fig. 5b). No difference was found in IFN-γ responses between TR421-hCGβ and TR421-hCGβ-C3d3 groups (Fig. 5b). Taken together, these results indicated that C3d3, when fused to hCGβ, could down-regulate the expression of transcription factors such as T-bet, GATA-3 and cytokine IL-4.
Fig. 5.
Influence of C3d3 on the expression of transcription factors and cytokines in splenocytes. Splenocytes of DNA vaccinated mice were re-stimulated with 5 μg/ml hCGβ for 72 h. mRNA was isolated and equal amounts of total mRNA were reverse transcribed, and the cDNA was subjected to RT-PCR. Values represent the mean ± SE of the ratios of the intensity of the bands representing the RT-PCR products of T-bet, GATA-3, IL-4 or IFN-γ to GAPDH. a The levels of T-bet and GATA-3 mRNA and b the levels of IL-4 and IFN-γ mRNA
Discussion
The present study found that the plasmid coding hCGβ-C3d3 chimeras suppressed the specific immune responses and inhibited the avidity maturation of anti-hCGβ IgG antibody. Moreover, the inhibitory effects of C3d3 molecule on the immune responses against hCGβ were not related to the vector used, the gender of the test mice, or the expression level of the target protein. On the contrary, the fusion of C3d3 with HBV-preS2/S enhanced both humoral and cell-mediated HBV-specific immune responses following DNA immunization and accelerated the avidity maturation of anti-HBsAg IgG antibody.
The inhibitory effect of antigen-specific immune responses following immunization with plasmid coding C3d3 with target antigens possibly depends on the nature of the immunogen fused to C3d3. The previous studies about the molecular adjuvant C3d3 molecule focused on many antigens, including hemagglutinin from influenza virus [9–11], measles virus [12], and the envelope of human immunodeficiency virus [13–15]. Suradhat et al. [17] originally described the possible inhibitory effect of C3d. When one or two copies of C3d fused to rotovirus VP7 or bovine herpesvirus gD protein, the humoral immune response was inhibited. Other reports have shown that C3d3 when fused to circumsporozoite proteins of Plasmodium berghei [18] and diphtheria toxin [19] also inhibited the production of antibodies.
Terrazzini et al. [20] indicated that hCGβ-C3d3 chimera decreased the antibody levels in the immunized animals. We confirmed the inhibitory effects of C3d3 on hCGβ. We found that C3d3 when fused to hCGβ, inhibited not only the antigen-specific humoral antibody response, but also the cellular immune response. We excluded the influence of the expression level of the target protein, the gender of the test mice, or the vector used. We further showed that the mechanisms involved in the inhibitory effect of C3d3 might be due to impairing the function of antigen presenting B lymphocytes and reducing the expression of transcription factors (T-bet and GATA-3) and cytokine IL-4. However, contrary to Terrazzini’s reports and ours, one group [29, 30] has observed an enhanced immune response with hCGβ-C3d3 DNA and protein vaccine. The difference in the findings cannot be explained so far.
The inhibitory effect of C3d3 against hCGβ may also be explained by the biological properties of the CD21 and possibly an interaction between C3d molecule and B lymphocytes. Through the binding of C3d-tagged antigens, CD21 functions to coligate the BCR and CD19. Studies have suggested that CD21 may play a critical role in the immune response to foreign antigens and maintain tolerance to self-antigens as well [31, 32]. Also it may be related to self-antigen capture or presentation, or to enhance signaling of auto-reactive B lymphocytes that normally results in deletion [33]. Since hCGβ is TAA, which are “almost self-antigens” or “self-like antigens” to mice, we suspected that the down-regulation of C3d3 might be mediated by influencing the antigen presentation of B lymphocytes. We investigated the influence of C3d3 on the antigen presentation of B lymphocytes and macrophages and found the down-regulation of C3d3 was possibly mediated by down-regulating the antigen presentation of B lymphocytes. That phenomenon was indirectly confirmed by Lee et al. [34].
The inhibitory efficacy of C3d3 against hCGβ may also be explained by the effect of C3d3 on the expression of IL-4 [6, 35]. Previous studies have shown that the cytokine-mediated signaling pathways and the C3 fragment-influenced signaling pathways had important synergy for enhancing B cell response [36, 37]. The enhancing effect of BCR:CD21 colligation (especially for S phase entry) appears to rely on accessory cytokine signaling pathways. IL-4 is the most effective cytokine in the synergy with C3d-bound Ag in eliciting S phase entry of naive human B cells. Moreover, IL-4 is the only cytokine which can lower the minimal BCR:ligand affinity threshold for BCR-triggered S phase entry by C3d-bound Ag. The collaboration between BCR:CD21 colligation and IL-4R signaling in promoting B cell cycle progression appears to involve two mechanisms: promoting the progression of resting B lymphocytes past an early G1 restriction point; and IL-4R-mediated signaling for progression from G1 phase to S phase. IL-4 can decrease the expression of inhibitory receptors CD22, CD72, PIR-B and FcγRII on B lymphocytes and abolish CD22 and FcγRII-mediated B lymphocyte suppression [38]. Our results showed that C3d3 when fused with hCGβ, down-regulated the expression of IL-4 in splenocytes from the mice immunized with DNA vaccine. The down-regulation of IL-4 expression implied that the expressions of inhibitory receptors on B cells were increased and the synergy of BCR:CD21 colligation and IL-4R in promoting B cell cycle progression were decreased.
In conclusion, the present study demonstrated that the immunization with hCGβ fused with C3d3 could result in the inhibition of both humoral and cell-mediated antigen-specific immune responses. Furthermore, the inhibitory effect of specific immune responses by C3d3 might function through influencing the antigen presentation of B lymphocytes and the expression of IL-4. The function of C3d3 found in the present study might further provide the understanding of the mechanisms involved in tumor immune escape and clues to vaccine designs.
Acknowledgments
We sincerely thank Dr. T.M. Ross (University of Pittsburgh School of Medicine, USA) for kindly offering the plasmids TR421 and TR421-C3d3, and Prof. Q. Shen (Shanghai Institute of Planned Parenthood Research, China) for kindly providing the plasmid pSG5.C3d3.YL. We thank Dr. Steve Chu for proof reading the manuscript.
Abbreviations
- hCGβ
The β chain of human chorionic gonadotrophin
- HbsAg
Hepatitis B virus surface antigen
- TAA
Tumor-associated antigen
Footnotes
Qing Dong Guan and Ying Wang have equally contributed to this work.
Grand support: The Programs of STCSM (04XD14003, 04DZ14902), Program for Outstanding Medical Academic Leader and The Major State Basic Research Development Program of People’s Republic of China (2001CB510005) to S. Xiong.
References
- 1.Isaacs NW, Cystine knots Curr Opin Struct Biol. 1995;5:391–395. doi: 10.1016/0959-440X(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 2.Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhomme C, Rougier P. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901–3907. [PubMed] [Google Scholar]
- 3.Rau CS, Lin JW, Liang CL, Lee TC, Chen HJ, Lu K. Production of human chorionic gonadotropin-beta subunit associated with an osteolytic meningioma. Case report. J Neurosurg. 2002;97:197–199. doi: 10.3171/jns.2002.97.1.0197. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S, Braunstein GD. Human chorionic gonadotropin stimulates proliferation of Nb 2 rat lymphoma cells. J Clin Endocrinol Metab. 1983;56:1068–1070. doi: 10.1210/jcem-56-5-1068. [DOI] [PubMed] [Google Scholar]
- 5.Triozzi PL, Stevens VC. Human chorionic gonadotropin as a target for cancer vaccines. Oncol Rep. 1999;6:7–17. [PubMed] [Google Scholar]
- 6.Dangles V, Halberstam I, Scardino A, Choppin J, Wertheimer M, Richon S, Quelvennec E, Moirand R, Guillet JG, Kosmatopoulos K, Bellet D, Zeliszewski D. Tumor-associated antigen human chorionic gonadotropin beta contains numerous antigenic determinants recognized by in vitro-induced CD8+ and CD4+ T lymphocytes. Cancer Immunol Immunother. 2002;50:673–681. doi: 10.1007/s00262-001-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissler M, Wands G, Gesien A, de la Monte S, Bellet D, Wands JR. Genetic immunization with the free human chorionic gonadotropin beta subunit elicits cytotoxic T lymphocyte responses and protects against tumor formation in mice. Lab Invest. 1997;76:859–871. [PubMed] [Google Scholar]
- 8.Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 9.Ross TM, Xu Y, Bright RA, Robinson HL. C3d enhancement of antibodies to Hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1:127–131. doi: 10.1038/77802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JA, Green TD, Bright RA, Ross TM. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine. 2003;21:902–914. doi: 10.1016/S0264-410X(02)00539-X. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe I, Ross TM, Tamura S, Ichinohe T, Ito S, Takahashi H, Sawa H, Chiba J, Kurata T, Sata T, Hasegawa H. Protection against influenza virus infection by intranasal administration of C3d-fused hemagglutinin. Vaccine. 2003;21:4532–4538. doi: 10.1016/S0264-410X(03)00510-3. [DOI] [PubMed] [Google Scholar]
- 12.Green TD, Newton BP, Rota PA, Xu Y, Robinson HL, Ross TM. C3d enhancement of neutralizing antibodies to measles hemagglutinin. Vaccine. 2001;20:242–248. doi: 10.1016/S0264-410X(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 13.Ross TM, Xu Y, Green TD, Montefiori DC, Robinson HL. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res Hum Retroviruses. 2001;17:829–835. doi: 10.1089/088922201750252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Mboudjeka I, Shen S, Chou TH, Wang S, Ross TM, Lu S. Independent but not synergistic enhancement to the immunogenicity of DNA vaccine expressing HIV-1 gp120 glycoprotein by codon optimization and C3d fusion in a mouse model. Vaccine. 2004;22:1764–1772. doi: 10.1016/j.vaccine.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Green TD, Montefiori DC, Ross TM. Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J Virol. 2003;77:2046–2055. doi: 10.1128/JVI.77.3.2046-2055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Sunyer JO, Bello LJ. Fusion to C3d enhances the immunogenicity of the E2 glycoprotein of type 2 bovine viral diarrhea virus. J Virol. 2004;78:1616–1622. doi: 10.1128/JVI.78.4.1616-1622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suradhat S, Braun RP, Lewis PJ, Babiuk LA, van den Drunen Littel-van Hurk S, Griebel PJ, Baca-Estrada ME. Fusion of C3d molecule with bovine rotavirus VP7 or bovine herpesvirus type 1 glycoprotein D inhibits immune responses following DNA immunization. Vet Immunol Immunopathol. 2001;83:79–92. doi: 10.1016/S0165-2427(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 18.Bergmann-Leitner ES, Scheiblhofer S, Weiss R, Duncan EH, Leitner WW, Chen D, Angov E, Khan F, Williams JL, Winter DB, Thalhamer J, Lyon JA, Tsokos GC. C3d binding to the circumsporozoite protein carboxy-terminus deviates immunity against malaria. Int Immunol. 2005;17:245–255. doi: 10.1093/intimm/dxh205. [DOI] [PubMed] [Google Scholar]
- 19.Gor DO, Ding X, Li Q, Greenspan NS. Genetic fusion of three tandem copies of murine C3d sequences to diphtheria toxin fragment B elicits a decreased fragment B-specific antibody response. Immunol Lett. 2006;102:38–49. doi: 10.1016/j.imlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Terrazzini N, Hannesdottir S, Delves PJ, Lund T. DNA immunization with plasmids expressing hCGβ-chimeras. Vaccine. 2004;22:2146–2153. doi: 10.1016/j.vaccine.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Wang LX, Xu W, Guan QD, Chu YW, Wang Y, Xiong SD. Contribution of C3d-P28 repeats to enhancement of immune responses against HBV-preS2/S induced by gene immunization. World J Gastroenterol. 2004;10:2072–2077. doi: 10.3748/wjg.v10.i14.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Summer B, Sander CA, Przybilla B, Thomas P. Molecular analysis of T-cell clonality with concomitant specific T-cell proliferation in vitro in nickel-allergic individuals. Allergy. 2001;56:767–770. doi: 10.1034/j.1398-9995.2001.056008767.x. [DOI] [PubMed] [Google Scholar]
- 23.McKay PF, Barouch DH, Santra S, Sumida SM, Jackson SS, Gorgone DA, Lifton MA, Letvin NL. Recruitment of different subsets of antigen-presenting cells selectively modulates DNA vaccine-elicited CD4+ and CD8+ T lymphocyte responses. Eur J Immunol. 2004;34:1011–1020. doi: 10.1002/eji.200324840. [DOI] [PubMed] [Google Scholar]
- 24.Giovarelli M, Santoni A, Forni G. Alloantigen-activated lymphocytes from mice bearing a spontaneous “non-immunogenic” adenocarcinoma inhibit its growth by recruiting host immunoreactivity. J Immunol. 1985;135:3596–3603. [PubMed] [Google Scholar]
- 25.Yu S, Xia M, Xu W, Chu Y, Wang Y, Xiong S. All-trans retinoic acid biases immune response induced by DNA vaccine in a Th2 direction. Vaccine. 2005;23:5160–5167. doi: 10.1016/j.vaccine.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Lobell A, Weissert R, Eltayeb S, de Graaf KL, Wefer J, Storch MK, Lassmann H, Wigzell H, Olsson T. Suppressive DNA vaccination in myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis involves a T1-biased immune response. J. Immunol. 2003;70:1806–1813. doi: 10.4049/jimmunol.170.4.1806. [DOI] [PubMed] [Google Scholar]
- 27.Farrar JD, Ouyang W, Lohning M, Assenmacher M, Radbruch A, Kanagawa O, Murphy KM. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. J Exp Med. 2001;193:643–649. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 29.Li DJ, Wang HM, Li L, Zhao XR, Wang MY, Zhu Y, Meng Y, Yuan MM. Gene fusion of molecular adjuvant C3d to hCGbeta enhances the anti-hCGbeta antibody response in DNA immunization. J Reprod Immunol. 2003;60:129–141. doi: 10.1016/j.jri.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang XL, Li DJ, Yuan MM, Yu M, Yao XY. Enhancement of humoral immunity to the hCG beta protein antigen by fusing a molecular adjuvant C3d3. J Reprod Immunol. 2004;63:97–110. doi: 10.1016/j.jri.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Tedder TF, Inaoki M, Sato S. The CD19–CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/S1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 32.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 33.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/S1074-7613(00)80669-X. [DOI] [PubMed] [Google Scholar]
- 34.Lee Y, Haas KM, Gor DO, Ding X, Karp DR, Greenspan NS, Poe JC, Tedder TF. Complement component C3d-antigen complexes can either augment or inhibit B lymphocyte activation and humoral immunity in mice depending on the degree of CD21/CD19 complex engagement. J Immunol. 2005;175:8011–8023. doi: 10.4049/jimmunol.175.12.8011. [DOI] [PubMed] [Google Scholar]
- 35.Suradhat S, Braun RP, Lewis PJ, Babiuk LA, van den Drunen Littel-van Hurk S, Griebel PJ, Baca-Estrada ME. Fusion of C3d molecule with bovine rotavirus VP7 or bovine herpesvirus type 1 glycoprotein D inhibits immune responses following DNA immunization. Vet Immunol Immunopathol. 2001;83:79–92. doi: 10.1016/S0165-2427(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 36.Mongini PKA, Highet PF, Inman JK. Human B cell activation. Effect of T cell cytokines on the physicochemical binding requirements for achieving cell cycle progression via the membrane IgM signaling pathway. J Immunol. 1995;155:3385–3400. [PubMed] [Google Scholar]
- 37.Mongini PKA, Inman JK. Cytokine dependency of human B cell cycle progression elicited by ligands which coengage BCR and the CD21/CD19/CD81 costimulatory complex. Cell Immunol. 2001;207:127–140. doi: 10.1006/cimm.2001.1758. [DOI] [PubMed] [Google Scholar]
- 38.Rudge EU, Cutler AJ, Pritchard NR, Smith KG. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and Fc gamma RII-mediated B cell suppression. J Exp Med. 2002;195:1079–1085. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]