Abstract
Head and neck squamous cell carcinoma (HNSCC) is an aggressive malignancy, and despite advances in treatments, the 5-year survival has remained at less than 50%. One treatment strategy is to focus on patients with premalignant oral lesions that carry a high-risk for developing recurrent premalignant lesions and HNSCC disease. As an initial attempt to determine if immune therapy has the potential to be protective in these patients, studies determined if premalignant lesions express tumor antigens that have previously been shown to be expressed on HNSCC. Immunohistochemical analyses showed prominent expression of epidermal growth factor receptor in premalignant lesions, even in lesions with mild dysplasia. MUC-1 and carcinoembryonic antigen were expressed in most patient samples, while NY-ESO-1 was less frequently expressed. Each of these antigens was expressed on HNSCC. This provided the rationale for determining if premalignant oral lesions could be used to stimulate autologous peripheral blood mononuclear leukocytes (PBML) to react against heterologous premalignant lesions and HNSCC. Following sensitization with autologous premalignant lesions, PBML responded to a challenge with either heterologous premalignant oral lesion cells or HNSCC by releasing IFN-γ. In addition, sensitization with autologous premalignant lesion lysates generated cytolytic activity by both PBML and T cells against allogeneic premalignant lesion cells and HNSCC. These studies show the feasibility of using premalignant oral lesions to stimulate immune reactivity against both premalignant oral lesions as well as HNSCC.
Keywords: Cytotoxicity, Head and neck cancer, HNSCC, Oral lesions, Premalignant
Introduction
Head and neck squamous cell carcinoma (HNSCC) is an aggressive epithelial malignancy with a 5-year survival that has remained at less than 50% for the last 30 years. This poor survival rate is mainly due to the high-level of local cancer recurrence, although distant metastases can also occur. The pathogenesis of HNSCC comprises a multistep process which includes damage from carcinogens such as tobacco and alcohol, and genetic alterations [2, 18, 27]. These alterations are seen in premalignant oral lesions and, depending on the site, thickness and type of lesion, they can have a high rate of progression to malignancy [20].
In spite of various treatment approaches, select premalignant oral lesions have a high recurrence rate and patients with these lesions have a high-risk of developing HNSCC. Patients with lesions that exhibit more advanced molecular changes, such as loss of heterozygosity, have a greater risk of developing recurring lesions and HNSCC even following excision of the lesions [8]. Clinical variables that predict development of cancer in patients with oral premalignant lesions include the location of the leukoplakia, with the highest risk sites being the floor of mouth [3, 20]. However, high-risk sites also include the tongue and lip. The thickness of leukoplakias is also directly linked to the risk rate for HNSCC.
Treatment approaches to prevent development of recurrent premalignant oral lesions and HNSCC have included laser surgical resection, photodynamic therapy and retinoid chemoprevention [9, 14, 25]. Even after complete resection, almost one-third of patients have been reported to develop recurrences and carcinomas [14 ]. The failure of current therapies to prevent recurrence of premalignant oral lesions and development of malignant disease supports the need for alternative treatment approaches. One such approach could be immune therapy. Focusing immune therapy on patients having high-risk premalignant oral lesions has an increased likelihood of success in immunization against tumor antigens than once HNSCC disease appears due to the profound immune dysfunction that develops in HNSCC patients [12, 15].
The feasibility of immune therapeutic approaches to prevent development of recurrent premalignant lesions and HNSCC disease is supported by the sharing of antigenic characteristics between premalignant lesions of various organ sites and their malignant counterparts. This includes upregulated expression of tumor antigens. For example, urothelial premalignant lesions are more similar to bladder carcinoma in expression of several tumor antigens, such as carcinoembryonic antigen (CEA), as compared to normal urothelium [23]. MAGE-3 expression is upregulated in intrahepatic cholangiocarcinoma and high-grade precursor lesions, but not in low-grade dysplastic lesions or normal biliary cells [26]. Epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER2) are highly expressed in squamous cell carcinoma and precancerous lesions of the lungs, contrasting with low expression in normal tissues [13, 21]. That these tumor antigens can be used to stimulate anti-tumor immune reactivity has been demonstrated by the protection against mammary carcinogenesis following immunization of the BALB-neuT mouse model with plasmid encoding HER-2/neu [22]. Using HER-2/neu protein as a vaccine for patients having HER-2/neu-overexpressing breast, ovarian, or non-small cell lung cancers stimulates immune reactivity to this protein [7].
Although expression of tumor antigens has been shown for preneoplastic lesions of various sites, less attention has been given to determining the extent to which oral premalignant lesions and HNSCC share tumor antigen expression. HNSCC have been shown to express cancer testis antigens such as NY-ESO-1, the MAGE and RAGE family members [10, 17]. CEA, which is a known tumor antigen for gastrointestinal cancers, is also expressed on cancers of the head and neck [16]. The goal of the present study was to first determine if premalignant oral lesions express tumor antigens that have been shown to be expressed on HNSCC. The demonstration of shared expression of tumor antigens between premalignant oral lesions and HNSCC provided the rationale for determining if premalignant oral lesions could stimulate immune reactivity to heterologous premalignant oral lesions and HNSCC.
Materials and methods
Patient samples
The premalignant lesion tissues used for immunohistochemical analyses were banked tissues consisting of leukoplakias with various levels of dysplasia, ranging from no dysplasia to severe dysplasia. Also used were banked HNSCC tissue specimens. These tissues had been formalin-fixed and paraffin imbedded. Immunological analyses used fresh peripheral blood, premalignant oral lesions, HNSCC and pathologically normal oral tissues adjacent to HNSCC. These fresh specimens and blood were obtained from patients after obtaining informed consent. The tissues used in all of these studies were residual tissues that were excised as part of patient care procedures, but which were no longer needed for clinical purposes. An aliquot of peripheral blood mononuclear cells was immunostained for expression of HLA-A2 and specimens from positive-staining donors were used for the immunological analyses.
To establish primary cultures, the fresh premalignant oral lesions and HNSCC tissues from HLA-A2+-staining donors were cleaned with multiple washes in antibiotic and antimycotic solutions containing 100 μg/ml penicillin, 100 μg/ml streptomycin and 200 μg/ml neomycin. Tissues were finely minced and then seeded into culture in RPMI-1640 medium containing 10% endotoxin-free defined fetal bovine serum, 50 μg/ml penicillin, 50 μg/ml streptomycin, 100 μg/ml neomycin, 0.02 M Hepes buffer, 5 × 10−5 M 2-mercaptoethanol and 2 mM l-glutamine. Once cells from the tissue fragments started to adhere, the fragments were discarded and the adherent cells were washed. Premalignant lesion cells were separated from fibroblasts by selective trypsinization. Either primary premalignant lesion cultures or the dysplastic oral keratinocyte (DOK) cell line was used as challenging or target premalignant lesion cells. These cells have previously been characterized [5]. All cells were passaged by trypsinization, washed and resuspended in fresh medium.
Normal keratinocytes were used as a specificity control. Primary human keratinocyte cultures were purchased from Invitrogen (Carlsbad, CA) and maintained in Defined Keratinocyte-SFM, as recommended by the manufacturer.
The premalignant lesion cells, HNSCC and keratinocytes were assessed for expression of HLA-A2 by flow cytometric analysis of immunostained cells. Cells were immunostained by 30 min incubation on ice with fluorescein isothiocyanate (FITC)-conjugated antibodies to HLA-A2 (clone BB7.2, BD Biosciences, San Jose, CA). After washing, the extent of staining was enumerated by flow cytometry (FACSCanto, BD Biosciences).
Immunohistochemical staining of tissue for expression of tumor antigens
Sections (5 μM) from paraffin blocks of pathologically normal tissue, premalignant lesions, and HNSCC tissues were prepared from the oral tissue bank. Sections were de-paraffinized with xylene and methanol. Tissue sections underwent epitope retrieval with EDTA or mild enzyme digestions, as recommended by manufacturer of the primary antibodies (Zymed-Invitrogen, Carlsbad, CA). Nonspecific antibody binding was blocked by incubating tissue sections with 10% normal rabbit serum for 30 min. Tissues were then incubated for 30 min with antibodies to EGFR (clone 31G7), CEA (clone Col-1), MUC-1 (clone VU-4-H5) or NY-ESO-1 (clone E978) (Zymed-Invitrogen). Positive staining was detected with the Histostain-Plus kit using horseradish peroxidase and AEC chromogen (Zymed-Invitrogen). Staining was visualized on an AxioVision-Zeiss digital imaging system.
Sensitization of peripheral blood mononuclear leukocytes (PBML) to premalignant lesions or HNSCC
To collect PBML, blood was layered over Ficoll-Hypaque (1.077 g/l) and centrifuged. An aliquot of the PBML was immunostained for HLA-A2. PBML from patients that were HLA-A2+ were suspended in the supplemented RPMI-1640 culture medium described above. To these PBML was added 25 μg/ml lysate from autologous premalignant oral lesions or autologous HNSCC. When available, an aliquot of the PBML was also incubated with 25 μg/ml lysate from autologous pathologically normal tissue that was adjacent to HNSCC. The lysate were prepared by course homogenization followed by sonication. Each culture was also supplemented with 5 U/ml IL-2 and 5 ng/ml IL-7. One week later, cells were collected, washed and challenged for 24 h with HLA-A2+ premalignant lesion cells or HNSCC cells established from separate donors than the source of the PBML donors. As controls, cultures were challenged with HLA-A2+ normal keratinocytes or left unchallenged. After 24 h, cell culture supernatants were collected and used to measure levels of released IFN-γ. IFN-γ levels in the supernatants were measured by ELISA (OptEIA ELISA, BD Biosciences, San Jose, CA).
The capacity of the sensitized PBML to kill premalignant lesion cells or HNSCC was also measured. This was accomplished by using as target cells HLA-A2+ allogeneic DOK premalignant lesion cells or HNSCC cultures. Control targets were HLA-A2+ allogeneic normal human keratinocytes. Cytolytic capability was measured with a 4 h calcein-acetoxymethyl cytotoxicity assay (Invitrogen). HLA-A2+ target cells were labeled with Calcein AM, which is then converted intracellularly to green-fluorescent calcein. After 30 min of labeling at 37°C, cells were washed and plated in round-bottom microtiter wells at a density of 2 × 104 cells per well together with increasing numbers of sensitized PBML. Some of the target cells were lysed with detergent to determine the maximal release of fluorescent calcein. Other wells containing only target cells were used to measure the spontaneous calcein release. After 4 h of incubation, 100 μl supernatant was collected to measure calcein release by the fluorescence intensity at 490 nm excitation and 540 nm emission. Cytotoxic activity was calculated by:
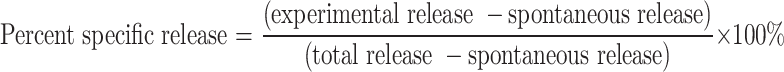 |
To determine if cytotoxicity by premalignant lesion-sensitized PBML was T cell mediated, sensitized cultures were subjected to a pan-T cell isolation column for negative selection of T cells (Miltenyi Biotech, Auburn, CA). They were then washed and tested for their cytolytic activity toward premalignant lesion cells or HNSCC as described above.
Analysis of data
The Student’s t test was used to determine the significance of differences between control and experimental values. Data shown are mean values of multiple experiments or representative results of these analyses.
Results
Tumor antigen expression on oral premalignant lesions
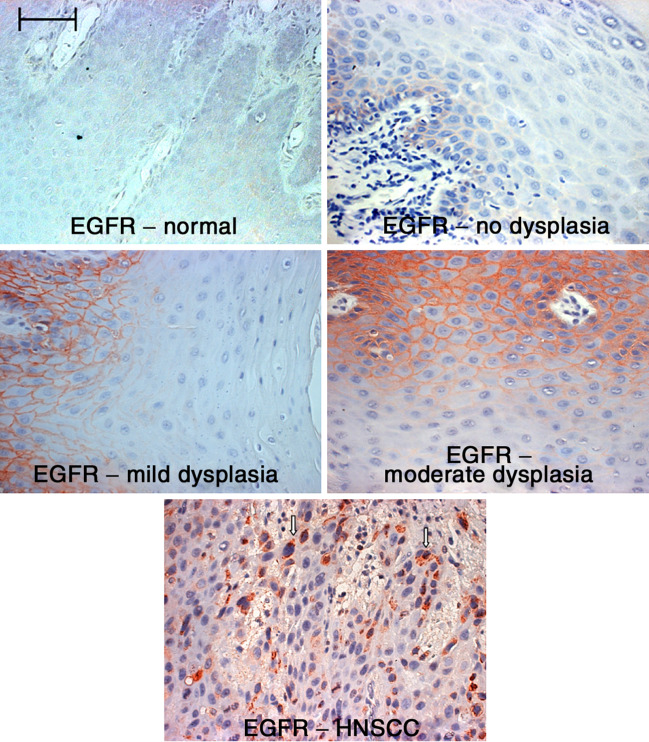
Premalignant lesions of various sites have been shown to share expression of several tumor antigens with their malignant counterparts. However, very little has been done to determine the extent to which oral premalignant lesions express tumor antigens that are on HNSCC. Because of the recent interest in EGFR expression and how its signaling could contribute to progression of HNSCC [4, 6], EGFR expression on pathologically normal oral tissue, premalignant lesions, and HNSCC was first examined. The premalignant lesions that were examined were leukoplakias having different levels of dysplasia (Fig. 1). Immunohistochemical staining showed that EGFR staining was localized to the basal cell layer of the non-dysplastic premalignant oral lesion. However, EGFR staining was more prominent in dysplastic leukoplakias. Furthermore, staining was not localized to the basal cell layer in tissues with dysplasia. In tissues with any level of dysplasia, EGFR was expressed within the lower spinous layer. EGFR expression extended to the upper spinous layer in areas of tissue with moderate or severe dysplasia. In contrast, only low levels of EGFR were visualized in pathologically normal oral tissue that was adjacent to HNSCC (Fig. 1).
Fig. 1.
Increased expression of EGFR on dysplastic premalignant oral lesions. Tissue sections from premalignant oral lesions with various levels of dysplasia or HNSCC tissue were immunostained for EGFR. Shown are representative microscopic images of immunostained tissue. Scale bar for all panels = 50 μm
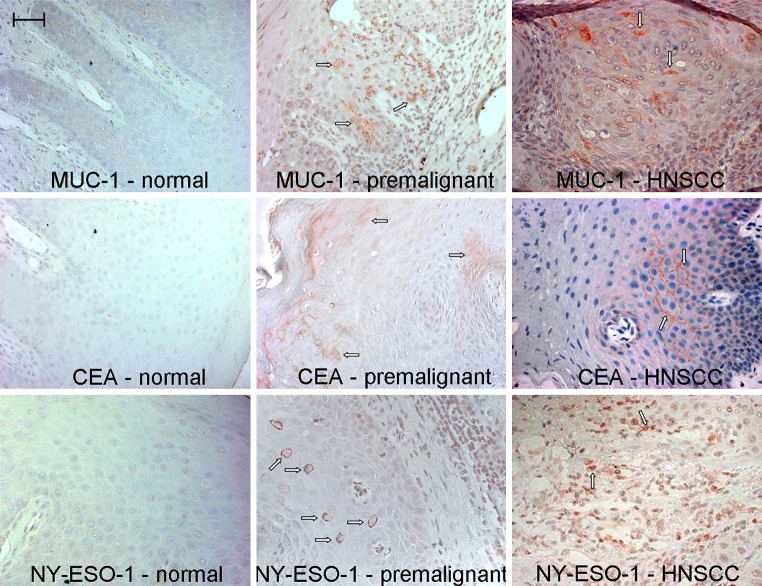
Lesions with moderate to severe dysplasia were further analyzed for expression of additional tumor antigens. These included MUC-1, NY-ESO-1 and CEA. In Fig. 2, representative immunostained sections of normal, premalignant lesion and HNSCC tissues are shown. In addition to expression of EGFR (Fig. 1), HNSCC specimens showed prominent staining for MUC-1 (Fig. 2). Cells staining positive for CEA and NY-ESO-1 were also present in HNSCC lesions. Dysplastic premalignant oral lesions also contained cells that stained positive for CEA, MUC-1 and NY-ESO-1. Expression of CEA and MUC-1 was more prominently visible on a greater number of patient specimens than was staining for NY-ESO-1. Of the eight different premalignant lesion specimens examined, EGFR was expressed on all lesions, and expression of CEA and MUC-1 was readily detectable in seven and six specimens, respectively. Expression of NY-ESO-1 was detectable on occasional cells in premalignant oral lesions and on a lower proportion of tissue specimens (three of eight). Contrasting with premalignant oral lesions, normal tissue showed light expression of MUC-1, but CEA and NY-ESO-1 staining was not readily apparent.
Fig. 2.
Immunostaining for tumor antigens on premalignant oral lesions and HNSCC. Sections of pathologically normal tissue adjacent to excised HNSCC, premalignant oral lesions with moderate to severe dysplasia, and HNSCC were immunostained for CEA, MUC-1 and NY-ESO-1. Shown are microscopic images from each category of stained tissue. Scale bar for all panels = 50 μm
In vitro stimulation of PBML against HNSCC and premalignant lesion cells
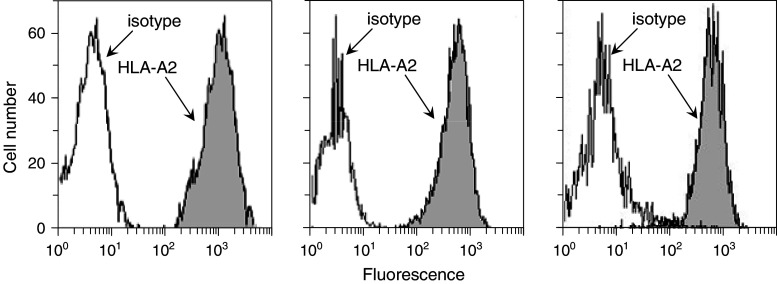
The above studies showing that both HNSCC and premalignant lesions share expression of tumor antigens provided the rationale for testing if premalignant oral lesions could stimulate autologous PBML reactivity to a heterologous premalignant lesion cell and HNSCC cell challenge. As target cells or challenge cells, these studies used allogeneic HLA-A2+ premalignant lesion cells, HNSCC and keratinocytes. Histograms showing expression of HLA-A2 by premalignant lesion cells, HNSCC and keratinocytes that were used as challenging cells or target cells are in Fig. 3. These analyses showed comparable levels of HLA-A2 expression by each of these challenging or target cell populations. This experimental design was used to determine the feasibility of using premalignant oral lesions to generate immune reactivity against newly arising lesions or HNSCC that can develop following excision of the original lesions.
Fig. 3.
Comparable levels of expression of HLA-A2 on premalignant lesion cells, HNSCC and normal keratinocytes that were used as challenging cells or targets. Cells were immunostained for HLA-A2 and levels of expression were measure by flow cytometry. Shown are representative histograms of keratinocytes (left panel), premalignant lesion cells (center panel) and HNSCC (right panel) that were used to challenge or as targets for sensitized BPML or T cells
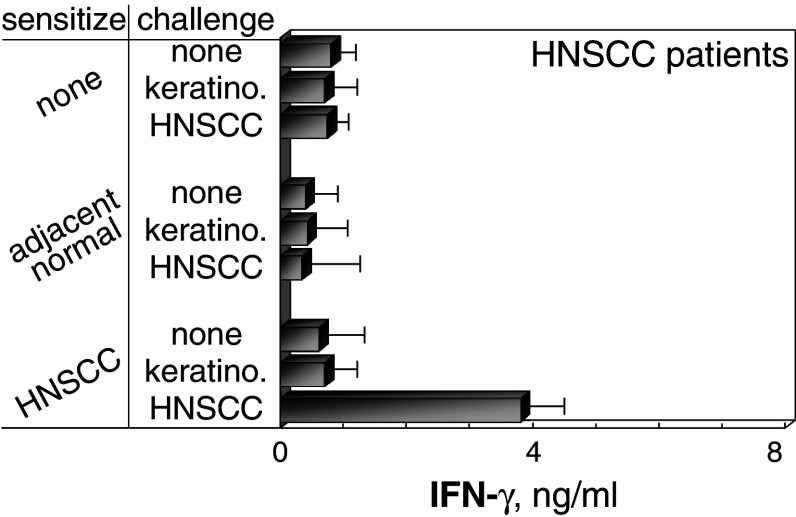
First explored was the feasibility of generating immune reactivity to HNSCC. HLA-A2+ PBML from HNSCC patients were sensitized with lysates of autologous HNSCC and then challenged with HLA-A2+ HNSCC from a separate donor. Cultures that were sensitized with HNSCC lysates showed increased IFN-γ production in response to HNSCC challenge as compared to the response by cultures that were either unsensitized or sensitized with autologous pathologically normal tissue adjacent to HNSCC (P < 0.001; Fig. 4). This production of IFN-γ was not induced in HNSCC-sensitized cultures in response to challenge with normal HLA-A2+ keratinocytes from a separate donor.
Fig. 4.
Stimulation of IFN-γ release from PBML that were sensitized with autologous HNSCC and challenged with allogeneic HNSCC. PBML of HNSCC patients were cultured with lysates of autologous HNSCC, autologous adjacent normal tissue, or no lysate. After 7 days, they were washed, challenged for 24 h with allogeneic HLA-A2+ HNSCC, normal allogeneic keratinocytes or unchallenged. Levels of IFN-γ released during these 24 h was measured by ELISA. Shown are mean ± SEM from at least five cultures
Studies were next conducted to determine if dysplastic premalignant oral lesions could be used to stimulate autologous PBML reactivity toward premalignant lesions or to HNSCC from separate donors. HLA-A2+ PBML cultures that were sensitized with lysates of autologous premalignant lesions were stimulated to secrete IFN-γ in response to the HLA-A2+ premalignant lesion challenge (P < 0.02; Fig. 5). These cultures also responded to a HNSCC challenge by producing IFN-γ. By comparison, cultures that were unsensitized were not stimulated to secrete IFN-γ in response to either premalignant lesion or HNSCC cells. Also, cells that were sensitized to autologous premalignant lesions were unresponsive to a challenge by normal allogeneic keratinocytes.
Fig. 5.
Stimulation of IFN-γ release from PBML that were sensitized with autologous premalignant lesions and challenged with allogeneic premalignant lesion cells or HNSCC. HLA-A2+ PBML were cultured with lysates of autologous premalignant lesions or without lysates. After 7 days, they were washed, challenged with allogeneic HLA-A2+ normal keratinocytes, premalignant lesion cells, HNSCC or left unchallenged for 24 h. The amount of IFN-γ released during these 24 h was measured by ELISA. Shown are mean ± SEM from at least five cultures
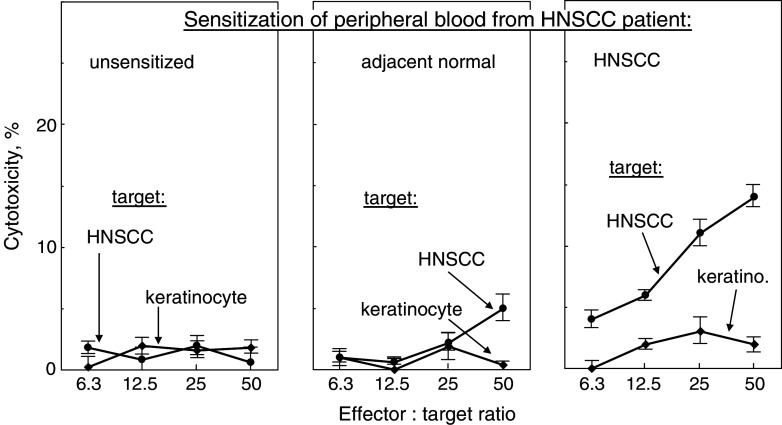
Whether or not sensitization of PBML with lysates of autologous HNSCC or premalignant lesions could stimulate cytolytic activity against premalignant lesion cells or HNSCC was assessed (Figs. 6–8). The exposure to alloantigens was limited to the 4 h duration of the cytotoxicity assay. HLA-A2+ PBML that were sensitized with the patient’s own HNSCC lysates demonstrated cytolytic activity toward allogeneic HLA-A2+ HNSCC (results from representative patient in Fig. 6; cumulative summary of cytotoxicity at a 25:1 effector to target ratio in Fig. 8). By contrast, cytotoxicity toward HNSCC was not induced in cultures that were sensitized with lysates of autologous pathologically normal oral tissue (P < 0.005 vs. HNSCC-sensitized PBML).
Fig. 6.
Generation of cytolytic activity against HNSCC by PBML that were sensitized with autologous HNSCC. HLA-A2+ PBML were cultured with no lysates, or lysates from autologous pathologically normal tissue or autologous HNSCC. After 7 days, they were washed and plated in increasing numbers with 2 × 104 HLA-A2+ allogeneic normal keratinocytes or HNSCC as target cells. The extent of cytotoxicity was measured 4 h later. Shown are mean values from a representative HNSCC patient ± SD from six replicates at each effector to target ratio
Fig. 8.
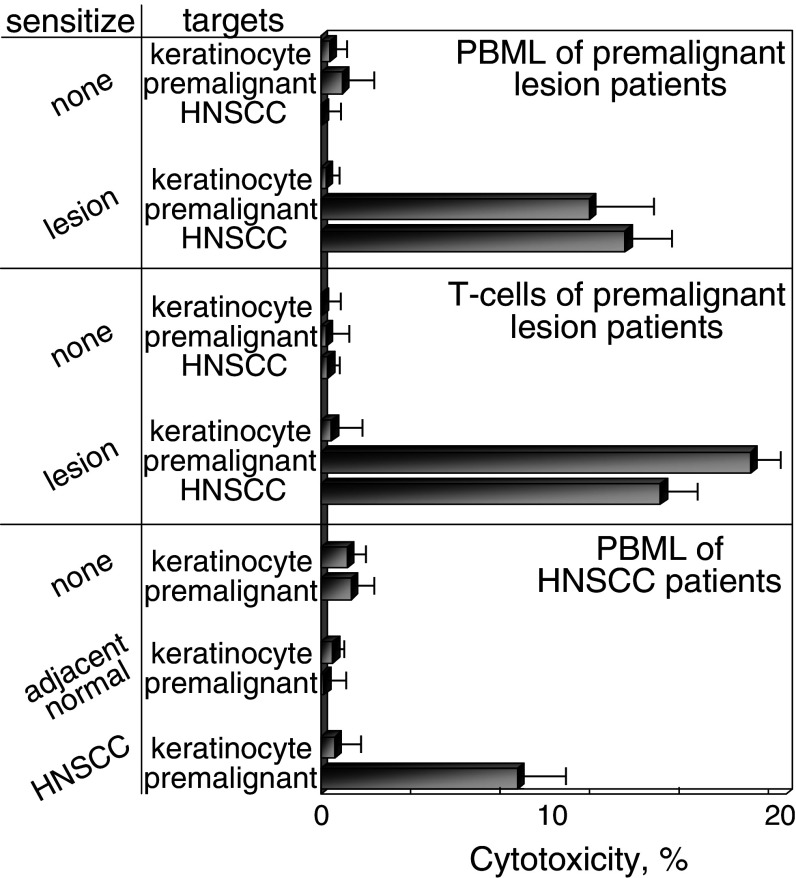
Generation of cytolytic activity against premalignant lesion cells and HNSCC by PBML and T cells that were sensitized with autologous premalignant lesions. HLA-A2+ PBML were cultured with no lysates, or lysates from autologous premalignant lesions or autologous HNSCC. After 7 days, the sensitized bulk PBML or immunomagnetically isolated T cells were tested for cytolytic activity, using allogeneic HLA-A2+ normal keratinocytes, premalignant lesion cells or HNSCC as targets. Shown are mean values of percent cytotoxicity from 25:1 effector to target ratios. Values are from at least five bulk PBML cultures and two isolated T cell effector cell cultures from premalignant lesion patients or HNSCC patient ± SEM
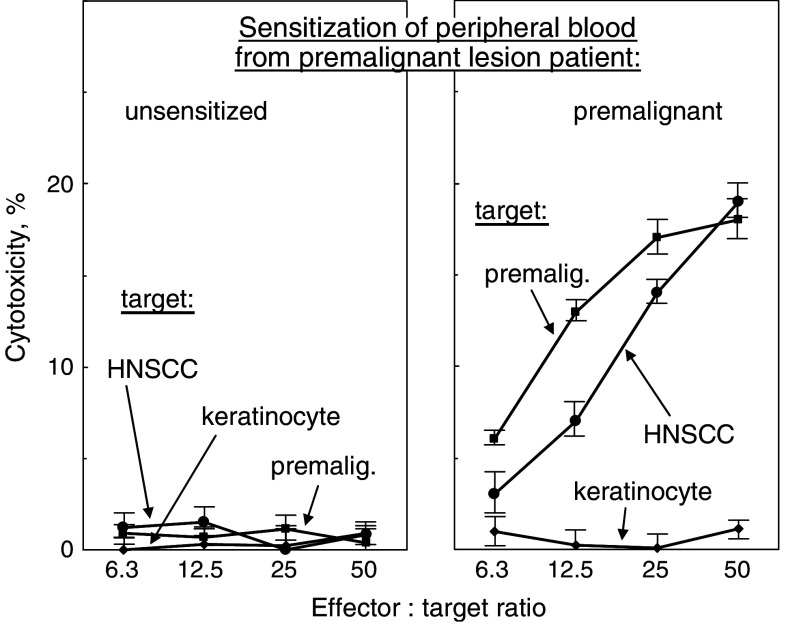
PBML that were sensitized by culture with the patients’ own premalignant lesion lysates were cytolytic toward allogeneic premalignant lesion cells (P < 0.002 vs. unsensitized PBML). Of significance is that these PBML that were sensitized against autologous premalignant lesions were also able to kill HNSCC established from a separate donor (Figs. 7, 8; P < 0.005 vs. keratinocytes as targets). In contrast, cultures that were unsensitized did not show cytolytic activity toward either premalignant lesion or HNSCC target cells.
Fig. 7.
Generation of cytolytic activity against allogeneic premalignant lesion cells and HNSCC by PBML that were sensitized with autologous premalignant lesions. HLA-A2+ PBML were cultured with no lysates, or lysates from autologous premalignant lesions. After 7 days, they were washed and plated in increasing numbers with 2 × 104 HLA-A2+ allogeneic normal keratinocytes, premalignant lesion cells or HNSCC as target cells. The extent of cytotoxicity was measured 4 h later. Shown are mean values from a representative premalignant lesion patient ± SD from six replicates at each effector to target ratio
To determine whether T cells were mediating the cytotoxicity toward premalignant lesion cells or HNSCC, T cells were isolated from the sensitized PBML cultures by negative selection prior to their addition to target cells. The HLA-A2+ T cell fraction that was sensitized to autologous premalignant lesions retained the capacity to kill both the allogeneic HLA-A2+ premalignant lesion cells as well as the HNSCC (Fig. 8). As was seen with the bulk culture of premalignant lesion-sensitized PBML, the sensitized T cells showed no cytotoxic activity toward normal allogeneic keratinocytes. These results demonstrate that premalignant oral lesions can be used to stimulate autologous T cell reactivity against a challenge by allogeneic premalignant lesion cells and HNSCC, but not normal keratinocytes.
Discussion
Premalignant lesions have been shown in a variety of tissue types to share tumor antigen expression with their malignant counterparts. However, much less attention has been given to the extent to which this occurs in premalignant oral lesions. Furthermore, studies have not assessed the possibility of capitalizing on these antigenic similarities by using excised premalignant oral lesions to prevent development of additional premalignant oral lesions or to prevent development of HNSCC. This study aimed to determine if there is overlap in tumor antigen expression among premalignant oral lesions and HNSCC. This would provide the rationale for determining if premalignant oral lesions could be used to sensitize autologous T cells to react against either premalignant oral lesions or HNSCC. The rationale for using as challenge and target cells, premalignant lesion cells and HNSCC, is that even when premalignant lesions are surgically excised, new lesions or HNSCC can develop at the excision site or distally. The rationale for using allogeneic premalignant lesion cells or HNSCC to challenge sensitized PBML or as target cells was that neither recurrent premalignant lesions nor HNSCC had yet developed in these patients.
The results of these studies showed that premalignant oral lesions express a number of tumor antigens in common with HNSCC. Of those examined, most prominently expressed was EGFR. As reported by others [1], EGFR staining in non-dysplastic lesions was localized to the basal cell layer. However, expression was less localized and more pronounced in dysplastic tissues. In addition to EGFR, CEA and MUC-1 were expressed in most patient samples. NY-ESO-1 was also expressed, but on a lower proportion of patient samples. While the intent of this study was to determine whether or not there was sharing of tumor antigens so as to consider the feasibility of using premalignant oral lesions to sensitize autologous PBML against premalignant lesions or HNSCC, the intent was not to conduct a comprehensive analysis of the extent to which tumor antigens are shared.
The immunohistochemical analyses showing that premalignant oral lesions share expression of tumor antigens with HNSCC provided the rationale for determining if premalignant lesions could be used to stimulate immune reactivity to premalignant lesions and to HNSCC. These immunological analyses showed the feasibility of using autologous premalignant oral lesions or autologous HNSCC to stimulate immune reactivity toward heterologous premalignant oral lesions or HNSCC, respectively. This was demonstrated by the expression of IFN-γ and generation of cytolytic activity toward premalignant oral lesions. In addition, these results showed the feasibility of using premalignant oral lesions to stimulate immune reactivity toward HNSCC. This reactivity was not generated by sensitization with normal tissue that was adjacent to HNSCC. Not yet determined, but of interest, would be whether the immune reactivity that is stimulated by autologous premalignant lesion cells is toward the tumor antigens that were shown in immunohistochemical analyses to be expressed on both premalignant oral lesions and on HNSCC. The stimulation of reactivity toward challenging premalignant lesion or HNSCC cells was not due to alloreactivity since exposure to alloantigens was limited to the last 24 h of culture. Also, no such reactivity was observed against normal allogeneic keratinocytes, which were also established from donors that were separate than the PBML donors. Finally, the results of the cytotoxicity assays were not due to alloreactivity since sensitization was to autologous tissue and these sensitized PBML had no exposure to allogeneic cells until the 4 h exposure with the target cells during the cytotoxicity assay. Differing levels of HLA-A2 expression on the challenging or target cells was also not likely to contribute to the selective cytotoxicity of premalignant lesion-sensitized PBML and T cells toward premalignant lesion cells and HNSCC. The cytolytic activity was mediated by sensitized T cells since those that were isolated by negative immunomagnetic fractionation from the sensitized bulk PBML culture also exhibited selective toxicity toward allogeneic premalignant lesion cells and HNSCC, but not to normal allogeneic keratinocytes.
This study was designed to determine if premalignant oral lesions could be used to induce immune reactivity toward premalignant oral lesions or HNSCC to address the clinical condition in which lesions are excised, but new lesions or HNSCC arise at the same site or distally. The ideal experimental design would be to determine if oral lesions could stimulate autologous T cell reactivity toward a subsequently arising autologous oral lesions or HNSCC. This, however, was not possible due to the absence of the not yet developed lesion or HNSCC with which to test the ideal experimental design. Consequently, the study was designed to use an alternative approach of having HLA-A2+ lesion and HNSCC cells as challenging or target cells for HLA-A2+ PMBL that were sensitized with autologous lesions.
Although immunohistochemical analyses showed expression of several tumor antigens by both premalignant oral lesions and HNSCC, this does not demonstrate that these antigens are responsible for the stimulation of immune reactivity toward either premalignant lesion cells or HNSCC. While prior studies have used individual tumor antigens to generate reactivity toward cancers, the use of premalignant lesion lysates for sensitization has the potential of stimulating a more robust immune response without the need to define the antigens against which reactivity is being generated. The design of the present study was to stimulate bulk PBML cultures with premalignant lesions. An alternate design that would likely induce a stronger response to the lesions would be to generate and pulse patient monocyte-derived dendritic cells with lysates of autologous lesions for sensitization of their T cells. Such an experimental approach could be more applicable when testing the in vivo effectiveness of vaccination against premalignant oral lesions.
The present study showing the feasibility of using premalignant oral lesions to stimulate immune reactivity against premalignant oral lesions and HNSCC lays a foundation for subsequent studies to determine if this reactivity is protective against development of additional lesions or HNSCC. Vaccination with premalignant oral lesions would be most relevant to patients whose premalignant oral lesions carry a high-risk of developing additional lesions or HNSCC. These include patients with thick highly dysplastic lesions and lesions on the floor of mouth, tongue or lip [20]. Also, vaccination of patients having premalignant oral lesions would likely generate a more effective vaccination response than immunotherapeutic approaches for patients that have already developed HNSCC due to the immune dysfunction that is prominent in HNSCC patients. For example, lymph nodes of HNSCC patients are reduced in size and have diminished T cell content [19]. T cells from about one-third of HNSCC patients have been shown to be unresponsive to stimulation through the CD3/T cell receptor [24]. The impact of this immune depression in HNSCC patients, on the clinical course of disease, is indicated by the association between reduced T cell function and poorer disease-specific survival [11]. In comparison, patients with premalignant oral lesions would more likely be immunologically healthy and more responsive to the vaccination.
Acknowledgments
Supported in part by the Medical Research Service of the Department of Veterans Affairs, and by Grants CA85266 and CA97813 from the National Institutes of Health.
References
- 1.Araujo CS, Graner E, Almeida OP, Sauk JJ, Coletta RD. Histomorphometric characteristics and expression of epidermal growth factor and its receptor by epithelial cells of normal gingiva and hereditary gingival fibromatosis. J Periodontal Res. 2003;38:237–241. doi: 10.1034/j.1600-0765.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee AG, Bhattacharyya I, Vishwanatha JK. Identification of genes and molecular pathways involved in the progression of premalignant oral epithelia. Mol Cancer Ther. 2005;4:865–875. doi: 10.1158/1535-7163.MCT-05-0033. [DOI] [PubMed] [Google Scholar]
- 3.Bouquot JE, Whitaker SB. Oral leukoplakia—rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994;25:133–140. [PubMed] [Google Scholar]
- 4.Bruzzese F, Di Gennaro E, Avallone A, Pepe S, Arra C, Caraglia M, Tagliaferri P, Budillon A. Synergistic antitumor activity of epidermal growth factor receptor tyrosine kinase inhibitor gefitinib and IFN-α in head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2006;12:617–625. doi: 10.1158/1078-0432.CCR-05-1671. [DOI] [PubMed] [Google Scholar]
- 5.Chang SE, Foster S, Betts D, Marnock WE. DOK, a cell line established from human dysplastic oral mucosa, shows a partially transformed non-malignant phenotype. Int J Cancer. 1992;52:869–902. doi: 10.1002/ijc.2910520612. [DOI] [PubMed] [Google Scholar]
- 6.Chen JS, Pardo FS, Wang-Rodriguez J, Chu TS, Lopez JP, Aguilera J, Altuna X, Weisman RA, Ongkeko WM. EGFR regulates the side population in head and neck squamous cell carcinoma. Laryngoscope. 2006;116:401–406. doi: 10.1097/01.mlg.0000195075.14093.fb. [DOI] [PubMed] [Google Scholar]
- 7.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2002;68:617–621. [PubMed] [Google Scholar]
- 9.Gorsky M, Epstein JB. The effect of retinoids on premalignant oral lesions: focus on topical therapy. Cancer. 2002;95:1258–1264. doi: 10.1002/cncr.10874. [DOI] [PubMed] [Google Scholar]
- 10.Gotte K, Usener D, Riedel F, Hormann K, Schadendorf D, Eichmuller S. Tumor-associated antigens as possible targets for immune therapy in head and neck cancer: comparative mRNA expression analysis of RAGE and GAGE genes. Acta Otolaryngol. 2002;122:546–552. doi: 10.1080/00016480260092381. [DOI] [PubMed] [Google Scholar]
- 11.Heimdal JH, Aarstad HJ, Klementsen B, Olofsson J. Peripheral blood mononuclear cell (PBMC) responsiveness in patients with head and neck cancer in relation to tumour stage and prognosis. Acta Otolaryngol. 1999;119:281–284. doi: 10.1080/00016489950181828. [DOI] [PubMed] [Google Scholar]
- 12.Heimdal JH, Aarstad HJ, Olofsson J. Peripheral blood T lymphocyte and monocyte function and survival in patients with head and neck carcinoma. Laryngoscope. 2000;110:402–407. doi: 10.1097/00005537-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Scagliotti GV, Langer CJ, Varella-Garcia M, Franklin WA. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer. 2003;41(Suppl 1):S29–S42. doi: 10.1016/S0169-5002(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 14.Ishii J, Fujita K, Munemoto S, Komori T. Management of oral leukoplakia by laser surgery: relation between recurrence and malignant transformation and clinicopathological features. J Clin Laser Med Surg. 2004;22:27–33. doi: 10.1089/104454704773660949. [DOI] [PubMed] [Google Scholar]
- 15.Kacani L, Wurm M, Schennach H, Braun I, Andrle J, Sprinzl GM. Immunosuppressive effects of soluble factors secreted by head and neck squamous cell carcinoma on dendritic cells and T lymphocytes. Oral Oncol. 2003;39:672–679. doi: 10.1016/S1368-8375(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 16.Kass ES, Greiner JW, Kantor JA, Tsang KY, Guadagni F, Chen Z, Clark B, De Pascalis R, Schlom J, Van Waes C. Carcinoembryonic antigen as a target for specific antitumor immunotherapy of head and neck cancer. Cancer Res. 2002;62:5049–5057. [PubMed] [Google Scholar]
- 17.Kienstra MA, Neel HB, Strome SE, Roche P. Identification of NY-ESO-1, MAGE-1, and MAGE-3 in head and neck squamous cell carcinoma. Head Neck. 2003;25:457–463. doi: 10.1002/hed.10223. [DOI] [PubMed] [Google Scholar]
- 18.Kraunz KS, McClean MD, Nelson HH, Peters E, Calderon H, Kelsey KT. Duration but not intensity of alcohol and tobacco exposure predicts p16INK4A homozygous deletion in head and neck squamous cell carcinoma. Cancer Res. 2006;66:4512–4515. doi: 10.1158/0008-5472.CAN-05-3748. [DOI] [PubMed] [Google Scholar]
- 19.Meneses A, Verastegui E, Barrera JL, de la Garza J., Hadden JW. Lymph node histology in head and neck cancer: impact of immunotherapy with IRX-2. Int Immunopharmacol. 2003;3:1083–1091. doi: 10.1016/S1567-5769(03)00017-1. [DOI] [PubMed] [Google Scholar]
- 20.Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215. doi: 10.3322/canjclin.52.4.195. [DOI] [PubMed] [Google Scholar]
- 21.Piyathilake CJ, Frost AR, Manne U, Weiss H, Bell WC, Heimburger DC, Grizzle WE. Differential expression of growth factors in squamous cell carcinoma and precancerous lesions of the lung. Clin Cancer Res. 2002;8:734–744. [PubMed] [Google Scholar]
- 22.Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL, Landuzzi L, Colombo MP, Giovarelli M, Musiani P, Forni G. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 23.de Sanchez-Fernandez Sevilla MC, Morell-Quadreny L, Gil-Salom M, Perez-Bacete M, Llombart-Bosch A. Morphometric and immunohistochemical characterization of bladder carcinoma in situ and its preneoplastic lesions. Eur Urol. 1992;21(Suppl 1):5–9. doi: 10.1159/000474878. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya TY, Wei WZ, Zormeier M, Ensley J, Sakr W, Mathog RH, Meleca RJ, Yoo GH, June CH, Levine BL, Lum LG. Anti-CD3/anti-CD28 bead stimulation overcomes CD3 unresponsiveness in patients with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126:473–479. doi: 10.1001/archotol.126.4.473. [DOI] [PubMed] [Google Scholar]
- 25.Sieron A, Adamek M, Kawczyk-Krupka A, Mazur S, Ilewicz L. Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of oral leukoplakia. J Oral Pathol Med. 2003;32:330–336. doi: 10.1034/j.1600-0714.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsuneyama K, Sasaki M, Shimonishi T, Nakanuma Y. Expression of MAGE-A3 in intrahepatic cholangiocarcinoma and its precursor lesions. Pathol Int. 2004;54:181–186. doi: 10.1111/j.1440-1827.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- 27.Turatti E, da Costa NA, de Magalhaes MH, de Sousa SO. Assessment of c-Jun, c-Fos and cyclin D1 in premalignant and malignant oral lesions. J Oral Sci. 2005;47:71–76. doi: 10.2334/josnusd.47.71. [DOI] [PubMed] [Google Scholar]