Abstract
Dendritic cells (DC), genetically modified to express ovalbumin by the retroviral vector GCDNsap, can elicit stronger anti-tumor immunity than those loaded with the peptides. To assess the clinical feasibility of the strategy, such DC were prepared by differentiation of hematopoietic progenitor cells transduced with the human epidermal growth factor receptor 2 (HER2). When inoculated in mice, the DC primed both HER2-specific cytotoxic T lymphocytes and type 1 T helper lymphocytes, resulting in production of HER2-specific antibody. Of importance is that the antibody mediated antibody-dependent cellular cytotoxicity and opsonization. The potent anti-tumor effects were also confirmed by results of experiments using HER2-transgenic mice. Inoculation of HER2-transduced DC resulted in longer disease-free survival of treated mice that showed significant reduction of primary and metastatic tumors. Interestingly, footpad inoculation resulted in stronger anti-tumor effects compared to subcutaneous administration and induced higher levels of the HER2-specific antibody, suggesting that an important role of humoral immunity in anti-tumor effects for malignancies with membrane-type tumor-associated antigens (TAA). Taken together, vaccination of the TAA-transduced DC may represent a promising form of therapy for breast cancers expressing HER2.
Keywords: Dendritic cells, Cancer, Tumor immunology, Immunotherapy, Vaccination
Introduction
Unique features of dendritic cells (DC), that is, they are capable of priming naïve T lymphocytes without additional exogenous adjuvants in the context of major histocompatibility complex (MHC) and immunostimulatory molecules have made them key players in cancer immunotherapy [2, 4, 11, 20, 34]. The establishment of sophisticated culture conditions allowing differentiation into mature DC from either hematopoietic progenitor cells (HPC) or peripheral monocytes has opened the way to clinical use of DC manipulated with loading of tumor-associated antigen (TAA)-derived peptides or tumor lysates in clinical vaccine trials for various cancers [1, 3, 24, 26, 39]. However, only a few cases have proven effective [7, 30], partly because of few available peptides restricted to the MHC class II molecules necessary to induce tumor-specific CD4+ T lymphocytes. To overcome the problems, we have recently proposed that such DC be prepared by differentiation of HPC transduced with the cDNA of TAA using the silencing-resistant retroviral gene transfer vector, GCDNsap [25]. Indeed, DC that were differentiated from HPC transduced with an ovalbumin (OVA) cDNA induced more OVA-specific cytotoxic T lymphocytes (CTL) and type 1 T helper (Th1) lymphocytes in mice bearing OVA-expressing tumors than DC prepared by loading with the OVA peptides [25]. Of importance is that the generation of OVA-specific CD4+ T lymphocytes induced production of antibody against OVA, which was not detected in mice inoculated with OVA peptide-pulsed DC [25].
Having verified the efficacy of the vaccine strategy for tumors bearing TAA with relatively strong immunogenicity, we set out to attempt to assess the capability of the transduced DC to elicit anti-tumor effects against tumors expressing TAA that we often encounter in clinical arena. Human epidermal growth factor receptor 2 (HER2), a member of the epidermal growth factor receptor tyrosine kinase family [5, 22], is frequently amplified and overexpressed in human cancers such as mammary, lung and ovary carcinomas [22, 32, 37]. In particular, it should be noted that the expression level of HER2 is significantly correlated with malignancy and poor prognosis [32]. Recent molecular biology has developed new and effective therapeutic methods as typified by molecular-targeted therapy. Among them, a humanized antibody against HER2, Trastuzumab, has proven effective in patients with advanced breast cancer [33]. On the contrary, clinical trials of vaccination using HER2 peptides or peptide-pulsed DC have reported disappointing clinical responses [8, 16]. In this study, we evaluated the potential of DC retrovirally modified to express the HER2 cDNA as a feasible treatment for breast cancer using a mouse model that develops mammary carcinoma spontaneously.
Materials and methods
Mice
C57BL/6N (B6) and FVB/N mice were purchased from Nihon Clea (Tokyo, Japan). FVB-Tg (MMTVneu) 202 Mul/J (FVB-Tg) mice expressing the rat neu under the control of the murine mammary tumor virus promoter [14] were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed and bred under specific pathogen-free conditions at the Animal Resource Center of the University of Tsukuba. All experiments using mice were approved by the Institutional Review Committee and performed according to the guidelines of the University of Tsukuba.
Retroviral vector construction and preparation
A 715 amino acids long product of the truncated HER2 membrane form void of the cytoplasmic kinase domain referred to as tHER2 (M) was amplified by PCR using the full-length HER2 cDNA (kindly provided by Dr. S. Shibata at Takeda Pharmaceutical Company, Osaka, Japan) as a template, 1.25 U of PfuUltra Hotstart DNA Polymerase (STRATAGENE, La Jolla, CA, USA) and the following primer set: 5′-CTGACGCGGCCGCAGTGAGCACCATG-3′ (forward), and 5′-CTTCACCTTCCTCGAGCTCCGTCTATTTCAGGAT-3′ (reverse). The PCR product was once subcloned into pBlueScript (STRATAGENE) and a NotI-XhoI fragment containing the tHER2 (M) cDNA digested from the vector was inserted into the corresponding sites of the GCDNsap retroviral vector [35]. Infectious retroviral particles packaged in vesicular stomatitis virus G envelope (VSV-G) protein were generated by transduction into 293gpg [27] as described elsewhere [35]. The final titer was 1 × 107 infectious units/ml on Jurkat cells [31]. The retrovirus was named GCDN/tHER2 (M).
Cell cultures
EL4 [13] and its retrovirally transduced derivatives were cultured in RPMI 1640 with 10% fetal calf serum (HyClone, Logan, UT, USA), 2 mM l-glutamine, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate (R10), and 50 μM 2-mercaptoethanol. Jurkat and SK-BR-3 [36] (kindly provided by the Cell Resource Center for Biomedical Research, Tohoku University, Miyagi, Japan) were cultured in R10. 293gpg cells were maintained in a way as described [27]. Primary mammary tumors from female FVB-Tg mice were excised, minced and passed through a 70 μm diameter Cell Strainer (BD Biosciences, Falcon, San Jose, CA, USA) to generate primary mammary carcinoma (PMC) cells that were cultured in R10. All other culture reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Vaccination with HER2-transduced DC
DC pulsed with lysate of EL4 expressing tHER2 (M) (EL4/DN/EGFP & tHER2 (M)) and those transduced with tHER2 (M) were prepared as previously described [25, 26]. In a vaccine study, 5 × 105 of lysate-pulsed or transduced DC were subcutaneously inoculated into the left flank of the B6 mice on Days −21, −14, −7, and 5 × 106 EL4/DN/EGFP & tHER2 (M) cells were injected into the right flank on Day 0. Tumors were measured in two perpendicular diameters every 2 days for 2 weeks and represented as the Tumor Index. For FVB-Tg mice, tHER2 (M)-transduced DC were also inoculated into footpads of female mice. In a therapeutic setting, 1 × 106 of the transduced DC were inoculated into footpads of tumor-bearing FVB-Tg mice three times. Stable disease periods after vaccination were defined as the number of days passed between the time of last tumor occurrence and the appearance of the next mass. Tumor-free periods, stable disease periods, the Tumor Index, and the number of tumors were recorded every 3 days up to the age of 300 days. The number of lung metastases was counted at 300 to 330 days.
Flowcytometric analysis
Antibodies for surface antigens used are anti-c-ErbB2 (TA-1; Merck Biosciences, Calbiochem, San Diego, CA, USA), phycoerythrin (PE)-conjugated anti-mouse immunoglobulin (Ig) rabbit polyclonal F (ab′)2 (Dako, Produktionsvej, Denmark), CD11c (HL3; BD Bioscience, PharMingen), CD83 (Michel17, eBioscience, San Diego, CA, USA), biotinylated anti-mouse CD40 (HM40-3), CD86 (GL-1), I-A (M5/114.15.2) (eBioscience) and streptavidin-allophycocyanin (PharMingen). For intracellular staining, splenic CD4+ T cells were treated with BD GolgiStop (PharMingen) and stained with fluorescein isothiocyanate-conjugated anti-mouse interleukin (IL)-2 (JES6-5H4; eBioscience) or PE-conjugated anti-mouse interferon (IFN)-γ (XMG1.2; PharMingen). To detect anti-HER2 antibody in mouse sera, the HER2-positive human breast cancer cell line, SK-BR-3 cells were incubated with mouse serum and stained with PE-conjugated anti-mouse immunoglobulin (Ig) rabbit polyclonal F (ab′)2 (Dako), or biotinylated anti-mouse IgG1 (A85-1), IgG2a (R19-15), IgG2b (R12-3), and IgG3 (R40-82) and streptavidin-PE (PharMingen). Cells were analyzed with a FACSCalibur (BD Biosciences).
Lysispot assay
The assay was performed as previously described [25]. EL4 expressing enhanced green fluorescent protein (EGFP) or tHER2 (M) and EGFP were established by infection of EL4 with GCDN/EGFP [35] and GCDN/tHER2 (M), respectively. Splenic CD8+ T cells obtained from B6 mice immunized with the tumor lysate-pulsed, untransduced or tHER2 (M)-transduced DC were co-cultured with 1 × 104 target cells in Multiscreen-IP ELISpot plates (Millipore, Bedford, MA, USA) coated with anti-GFP antibody (Invitrogen Life Technologies, Molecular Probes, Carlsbad, CA, USA) at the indicated effector to target ratios [25].
51Cr-release assay
Inguinal and popliteal lymph nodes (LN) isolated from the non-immunized or immunized FVB-Tg mice were treated with 1 mg/ml of collagenase D (Roche Diagnostics, Basel, Switzerland) at 37°C for 45 min followed by grinding and passage through a Cell Strainer (Falcon), and splenic CD8+ T cells were isolated with mouse CD8-MicroBeads using Midi-MACS (Miltenyi Biotec, Bergisch Gladbach, Germany). PMC cells (1 × 104) were labeled with 100 μl of 2 mCi/ml [Na2 51CrO4] (MP Biomedicals, Solon, OH, USA) and co-cultured at the indicated effector to target ratios in 96-Well Round Bottom TC-Treated Microplates (CORNING, Corning, NY, USA) at 37°C for 4 h with effector cells re-stimulated with tHER2 (M)-transduced DC. Chromium release in each well was determined with an auto well gamma system ARC-380 (ALOKA, Tokyo, Japan).
Antibody-dependent cellular cytotoxicity (ADCC) assay
An ADCC activity induced by the immunized mouse sera was evaluated by JAM test [23]. EL4/DN/EGFP and EL4/DN/EGFP & tHER2 (M) cells as target cells were cultured in R10 containing 5 μCi/ml of [methyl-3H]-thymidine (TRA120; GE Healthcare Biosciences, Little Chalfont, UK) and stained with 1 μl of mouse sera. Labeled target cells were co-cultured at the indicated effector to target ratios in 96-Well V Bottom TC-Treated Microplates (CORNING) at 37°C for 4 h with splenocytes that had been activated with 50 ng/ml mouse IL-2 (PharMingen) and 20 ng/ml mouse IL-12 p70 (eBioscience). DNA-bound [3H] radioactivity was measured with a liquid scintillation counter LS-6500 (Beckman Coulter, Fullerton, CA, USA).
Opsonization assay
Macrophages were prepared as adherent cells in bone marrow cells that were cultured in R10 containing 10 ng/ml human macrophage colony-stimulating factor (R & D systems, Minneapolis, MN, USA) for 4 days. Irradiated EL4/DN/EGFP & tHER2 (M) cells as apoptotic cells were incubated with 1 μl of the mouse sera and then co-cultured with macrophages for 1 to 3 h. The opsonic activity was estimated as the percentages of double Mac-1 (M1/70; PharMingen) and EGFP-positive cells determined by FACSCalibur (BD Biosciences).
Migratory DC into draining LN
Five million EGFP-transduced DC were injected into the flank or footpads of FVB/N mice. At 72 h after inoculation, the number of DC that migrated into draining LN (inguinal and popliteal LN) was evaluated by flowcytometry and immunohistochemistry. In immunohistochemistry, de-paraffinized sections of draining LN were stained with a primary antibody solution containing 2 μg/ml of anti-GFP rabbit IgG (Molecular Probes) or isotype control (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and Histofine Simple Stain Mouse MAX-PO (horseradish peroxidase-conjugated goat anti-rabbit polyclonal IgG Fab′ polymer; Nichirei Bioscience, Tokyo, Japan). EGFP-expressing cells visualized with a DAKO ENVISION+ Kit/HRP (DAB) (Dako) were counted in randomly selected 20 microscope fields magnified by 200× and the average number per field was calculated.
Adoptive transfer of spelnocytes
CD4+ or CD8+ cells were removed from splenocytes of FVB-Tg mice that had been immunized with the transduced DC by the negative selection using anti-mouse CD4 (GK1.5) or CD8α (53-6.7) antibodies (eBioscience). Thirty millions of the CD4- or CD8-depleted splenocytes, or whole splenocytes were infused into non-immunized FVB-Tg mice thorough the tail vein and the tumor-free periods, Tumor Index, and the number of primary or metastatic tumors of the mice were recorded.
Statistical analysis
Kaplan–Meier estimation with a Fischer-type generalized Wilcoxon method was used for statistical analysis of the differences between the tumor-free periods. The correlation between anti-HER2 antibodies titers and tumor-free periods, the number of tumor masses and lung metastases was evaluated with Spearman’s rank-order correlation coefficient. All other statistical analyses were performed with the Mann–Whitney U test. A probability of less than 0.05 (P < 0.05) was considered statistically significant.
Results
Characterization of DC genetically modified to express the truncated HER2
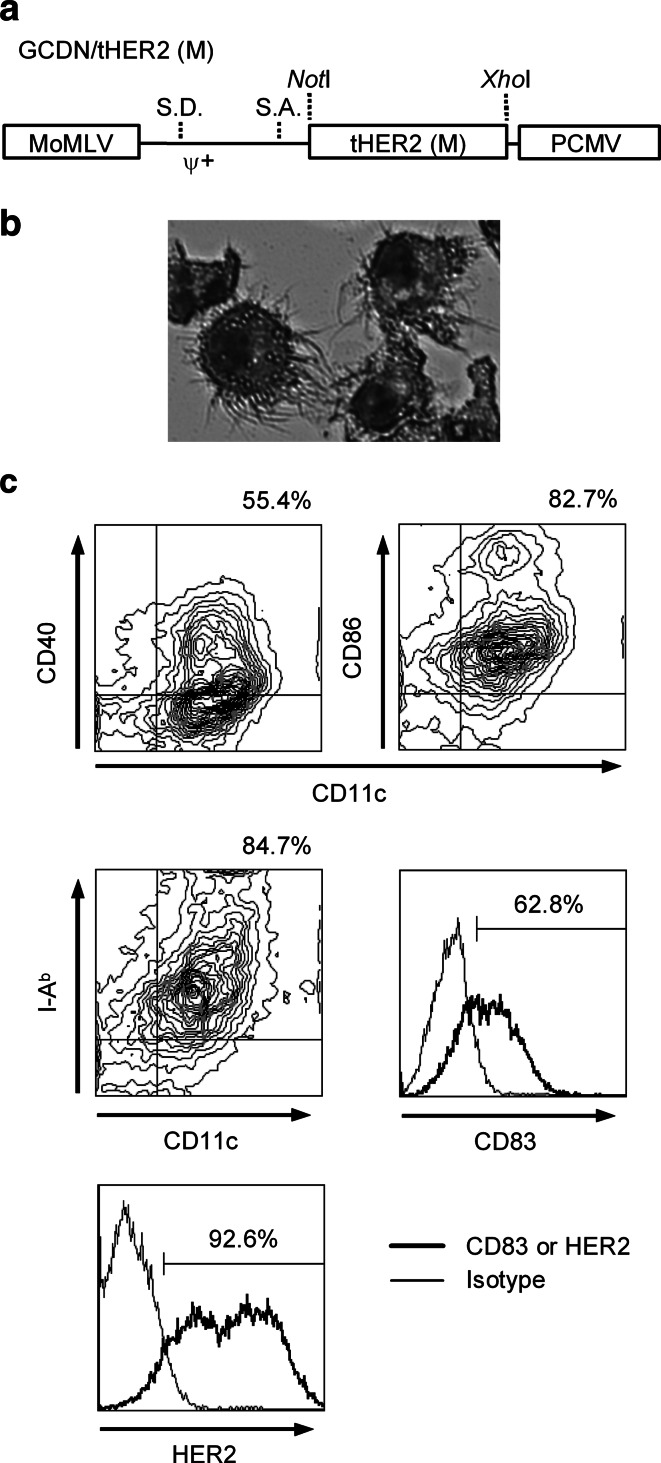
The primary end-point of the present study was to induce strong immune responses against HER2-expressing tumor through vaccination with the HER2-transduced DC. Therefore, to avoid potential negative effects of HER2 expression on DC, we used a truncated membrane form of the receptor (tHER2 (M)) devoid of the intracellular kinase domain that would be suitable for clinical applications. The structure of the gene-silencing-resistant retroviral vector GCDNsap encoding the tHER2 (M) cDNA is shown in Fig. 1a. The virus titer was approximately 1 × 107 infectious units/ml on Jurkat cells. c-KIT+/lineage− cells isolated from B6 mouse bone marrow were transduced with the GCDN/tHER2 (M), expanded (31.2-fold ±13.6), and then differentiated into DC (Fig. 1b). The cells expressed high levels of CD11c, CD40, CD86, MHC class II, and CD83 on their surface (Fig. 1c) and IL-12 p40 mRNA (data not shown), suggesting that they were mature DC. Approximately 5 to 10 × 106 DC were prepared per mouse and more than 90% of the cells highly expressed HER2 (Fig. 1c).
Fig. 1.
Structure of GCDN/tHER2 (M) and characters of tHER2 (M)-transduced DC. a Structure of GCDN/tHER2 (M). The vector contains the PCMV-derived long terminal repeat with intact splice donor and splice acceptor sequences. The tHER2 (M) cDNA was inserted between the NotI and the XhoI sites. Sequences present in the vector labeled as follows: MoMLV Moloney murine leukemia virus; S.D. splice donor; Ψ+ packaging signal; S.A. splice acceptor; PCMV PCC4 cell-passaged myeloproliferative sarcoma virus. b Morphological appearances of transduced DC (May-Gruenwald-Giemsa staining). c Surface markers and transgene expression of transduced DC
Anti-tumor immunity induced by tHER2 (M)-transduced DC
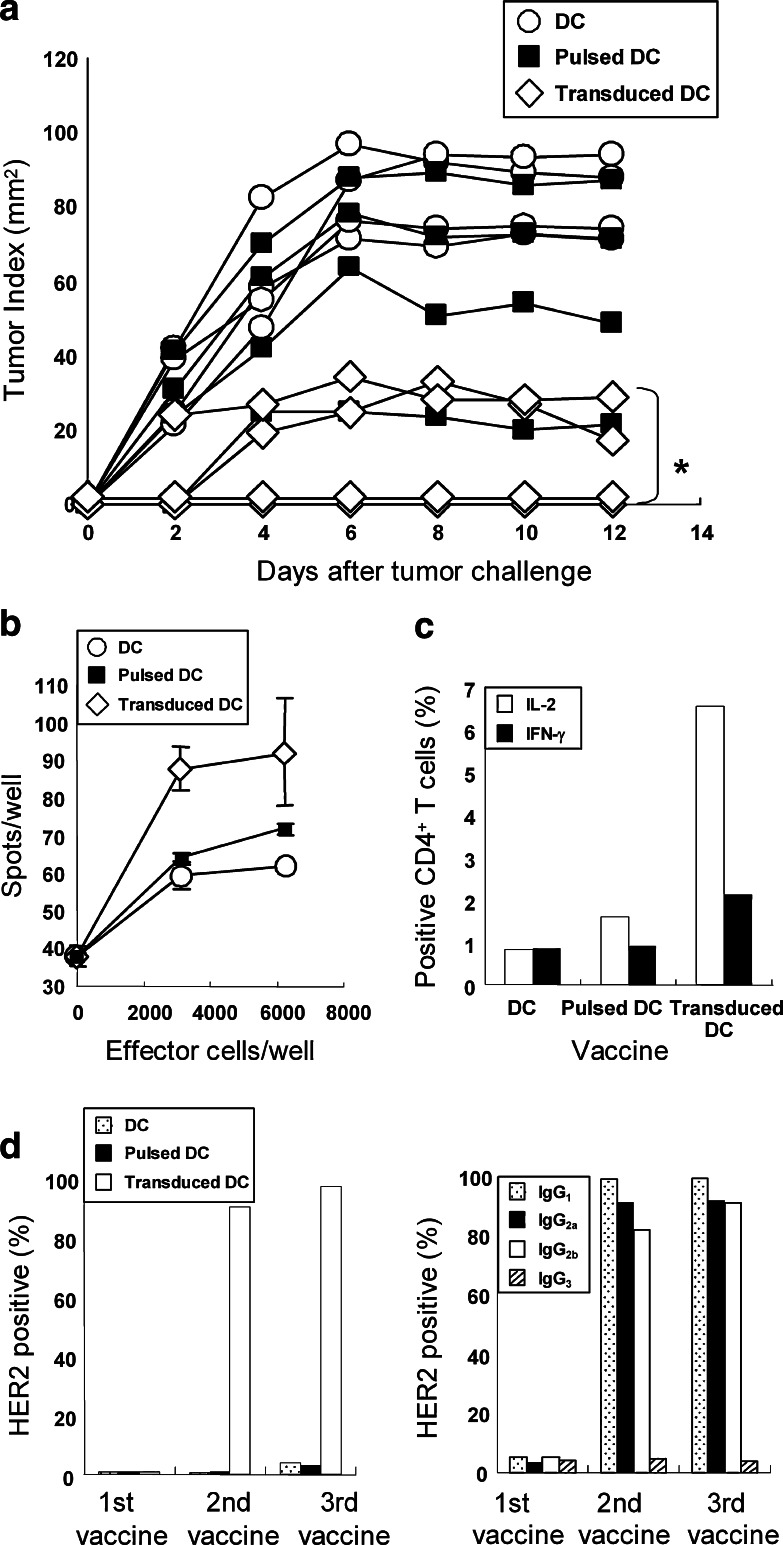
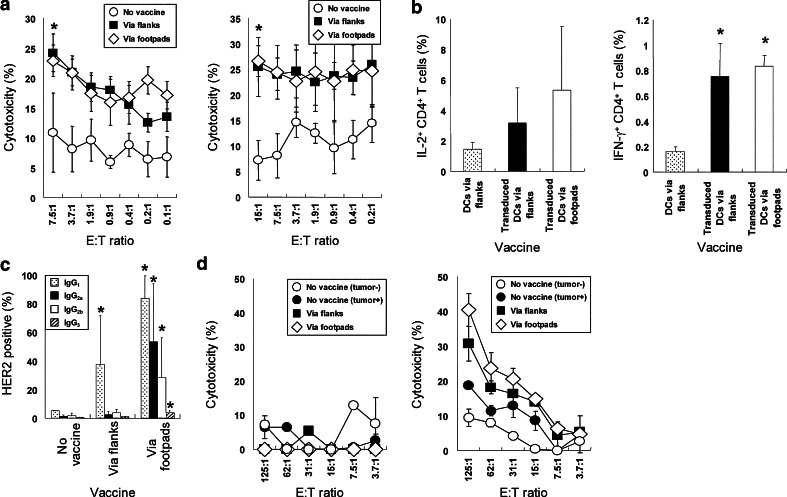
To assess the ability of the cells to induce anti-tumor effects, 5 × 105 tHER2 (M)-transduced DC were subcutaneously inoculated into the left flank of B6 mice on Day −21, −14, and −7, following by graft of 5 × 106 tHER2 (M)-expressing mouse lymphoma cell line EL4 cells (B6 background) into the right flank on Day 0. As the end of the observation periods, two out of four mice inoculated with transduced DC rejected the tumor graft completely and the other two showed significant inhibition of tumor progression (Fig. 2a). In contrast, mice inoculated with tumor lysate-pulsed DC as well as non-transduced ones showed little effects on the tumor growth. The results suggested that the transduced DC induced HER2-specific immunity, which was stronger than that observed in mice inoculated with conventional methods. To account for the difference of anti-tumor effects observed among three groups, the prevalence of HER2-specific CTL in those mice was compared by Lysispot assay (Fig. 2b). While the number of spots observed in mice inoculated with tumor lysate-pulsed DC was similar to that of control mice, mice inoculated with transduced DC provided significantly more spots (Fig. 2b), suggesting that the transduced DC primed HER2-specific CTL more efficiently than did lysate-pulsed DC. In addition, mice immunized with transduced DC also generated HER2-specific Th1 cells that produced IL-2 and IFN-γ when co-cultured with tHER2 (M)-transduced DC, which were not detected in mice inoculated with lysate-pulsed DC (Fig. 2c). As expected, a high amount of total anti-HER2 antibody and IgG1, IgG2a, and IgG2b subclasses (left and right panels of Fig. 2d) were detected in sera from mice immunized with transduced DC after second vaccine. Plausible explanation for these results is that they could prime and activate both CD8+ and CD4+ T cells by presenting HER2-derived epitope peptides in the context of MHC class I and II on their surface, respectively, resulting in establishment of HER2-specific CTL and Th1 cells.
Fig. 2.
Induction of HER2-specific immunity by tHER2 (M)-transduced or tumor lysate-pulsed DC. a B6 mice inoculated with tumor lysate-pulsed, untransduced or tHER2 (M)-transduced DC were transplanted with EL4/DN/EGFP & tHER2 (M) cells on Day 0. Results of four mice per group are presented. *P < 0.05 compared with the other groups. b CD8+ T cells obtained from immunized mice were incubated with EL4/DN/EGFP & tHER2 (M) cells. The frequency of HER2-specific CTL was determined by Lysispot assay. Representative results of one experiment out of independent three are shown. c CD4+ T cells obtained from immunized mice were stained with anti-mouse IL-2 or IFN-γ antibody intracellularly. d SK-BR-3 cells were stained with sera from mice immunized 1–3 times with each DC vaccine. The amount of total antibody (left) and IgG subclasses (right) against HER2 are shown as percentages of HER2-positive SK-BR-3 cells
Anti-tumor effects of the HER2-specific antibodies
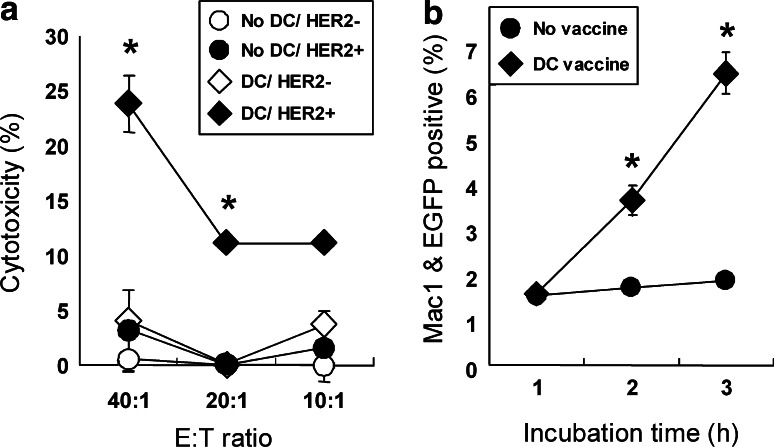
Having observed that mice inoculated with transduced DC produced HER2-specific antibody, we next analyzed anti-tumor effects exerted by such antibody in several immunological assays. ADCC results in perforin- and granzyme-mediated destruction of antibody-coated target cells by cells expressing Fc receptors, such as FcγRIII (CD16) on NK cells. When HER2-expressing EL4 cells were coated with sera obtained from mice immunized with transduced DC, they were efficiently lysed by splenocytes pre-stimulated with IL-2 and IL-12 (closed diamonds; Fig. 3a), while the same sera did not induce ADCC activity on the original EL4 cells that did not express HER2 (open diamonds). The specificity of the antibody against HER2-expressing cells was confirmed by a finding that HER2-expressing EL4 cells were not lysed by splenocytes when they were coated with sera of non-immunized mice (closed circles).
Fig. 3.
Involvement of antibody against HER2 in anti-tumor effects. a EL4/DN (open) or EL4/DN/EGFP & tHER2 (M) cells (closed) were incubated with sera from B6 mice vaccinated with or without tHER2 (M)-transduced DC (diamonds or circles, respectively) and co-cultured with activated splenocytes. *P < 0.05 compared with the other groups. b Apoptotic EL4/DN/EGFP & tHER2 (M) cells stained with sera from mice immunized with or without transduced DC (diamonds or circles, respectively) were co-cultured with macrophages. The opsonic activity was shown as the percentages of EGFP-positive Mac-1+ cells. *P < 0.01 compared with non-treated mice. Results shown are representative in three independent experiments
The antibody can also mediate phagocytosis by neutrophils and macrophages(through a mechanism known as opsonization. We found that sera from mice inoculated with transduced DC enhanced phagocytosis of irradiated HER2-expressing EL4 cells by macrophages (closed diamonds; Fig. 3b) whereas the phagocytosis was not detected for irradiated HER2-expressing EL4 cells coated with sera obtained from non-immunized mice (open circles). These results indicated that the anti-HER2 antibody in sera from immunized mice with transduced DC contributed to anti-tumor effects by ADCC activity and phagocytosis.
Anti-tumor effects of tHER2 (M)-transduced DC on spontaneous mammary carcinomas
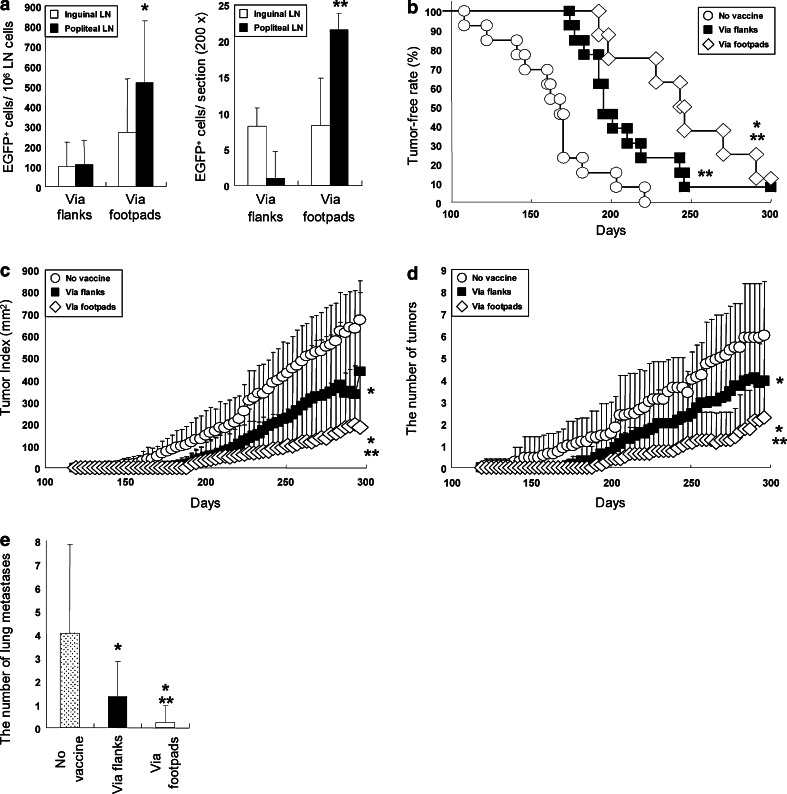
To assess the feasibility of the HER2 (M)-transduced DC in a clinical setting where the vaccine would be conducted for cancer-bearing subjects, we applied the FVB/N-Tg (MMTVneu) 202 Mul/J transgenic mouse model of breast cancer (FVB-Tg mice). Female FVB-Tg mice that spontaneously develop mammary carcinomas expressing the rat HER2 homolog are an ideal tool to assess the efficacy of cancer vaccines targeting HER2 [14]. c-KIT+/lineage− cells obtained from the parental mouse strain FVB/N were transduced with tHER2 (M), expanded (45.4-fold ±22.7) and induced to differentiated into mature DC. The phenotypes of these DC, including surface markers or cytokine expression patterns, the transduction efficiency and the yield of the DC were similar to those of DC obtained from B6 mice. Based on results of preliminary experiments using Balb/c mice that footpad inoculation of the transduced DC gave rise to higher amounts of HER2-specific antibody than did subcutaneous inoculation in flanks or intraperitoneal inoculation (unpublished observation), FVB-Tg mice were immunized with the transduced DC either at the flanks or footpads. Interestingly, the number of DC that migrated to their regional LN (popliteal and inguinal LN) by footpad inoculation was larger than that by subcutaneous inoculation (Fig. 4a). Consistent with the results, stronger anti-tumor effects in terms of the disease-free periods, tumor size, the number of primary and lung metastatic tumors were observed in mice with footpad inoculation (Fig. 4b–e), suggesting that the footpad inoculation induced potent HER2-specific immune responses by facilitating migration of the infused DC into their regional LN.
Fig. 4.
Anti-tumor efficacy of the transduced DC for the FVB-Tg mice. a The number of DC migrating into draining LN was calculated by flowcytometry (left) and immunohisochemisrty (right). *P < 0.05 compared with the other groups. **P < 0.005 compared with the other groups. b Tumor-free periods of untreated FVB-Tg mice (n = 13) or inoculated with tHER2 (M)-transduced DC via flanks (n = 13) or footpads (n = 8). *P < 0.05 compared with via flanks, **P < 0.005 compared with no vaccine. Tumor Index (c), the number of primary tumors (d), and lung metastases (e) in mice left untreated or inoculated with the transduced DC via flanks or footpads are shown. *P < 0.05, flanks versus no vaccine, and footpads versus flanks, and **P < 0.01, footpads versus no vaccine in (c) (d) and (e)
Comparison of immune responses induced by inoculation at flanks and footpads
To determine the main contributor to the difference in anti-tumor effects dependent on the route of inoculation, cellular and humoral immune responses against HER2 induced by inoculation of the tHER2 (M)-transduced DC either at flanks and footpads were further analyzed. When the HER2-specific CTL activity of CD8+ T cells in the spleen and draining LN cells of immunized mice was measured by co-culture with PMC cells that developed in untreated FVB-Tg mice, both mice inoculated via either route showed similar CTL activities (Fig. 5a). Furthermore, the number of HER2-specific Th1 cells in both mice was not significantly different (Fig. 5b). However, footpad inoculation produced a significantly higher amount of all anti-HER2 IgG subclasses than did subcutaneous inoculation at the flanks (Fig. 5c). Interestingly, the subcutaneous inoculation produced a high amount of HER2-specific IgG1 and little, if any, other HER2-specific IgG subclasses. In agreement with results of experiments using B6 mice (Fig. 3a), the anti-HER2 antibody, especially that in footpad inoculation, exerted the strong ADCC activity against HER2-expressing tumors in an antigen-specific manner (Fig. 5d), suggesting that the different levels of humoral immunity against HER2 induced by the inoculation route mainly contributed to the difference of anti-tumor effects observed between mice.
Fig. 5.
Elicitation of HER2-specific immune responses dependent on inoculation pathways. a Splenic CD8+ T cells (left) or LN cells (right) from FVB-Tg mice left untreated or inoculated with tHER2 (M)-transduced DC via flanks or footpads were co-cultured with PMC cells. Results of four independent experiments are shown. *P < 0.05, flanks, footpads versus no vaccine. b CD4+ T cells from mice immunized the indicated DC vaccine were stained with anti-mouse IL-2 (left) or IFN-γ antibody (right) intracellularly. Results of three independent experiments are shown. *P < 0.05 compared with DC via flanks. c The amount of antibodies against HER2 was estimated by the percentages of HER2-positive SK-BR-3. *P < 0.01, flanks versus no vaccine, and footpads versus the others. d EL4/DN (left) or EL4/DN/EGFP & tHER2 (M) cells (right) were incubated with sera from immunized mice and co-cultured with activated splenocytes
Anti-tumor effects of humoral immunity against spontaneous mammary carcinomas
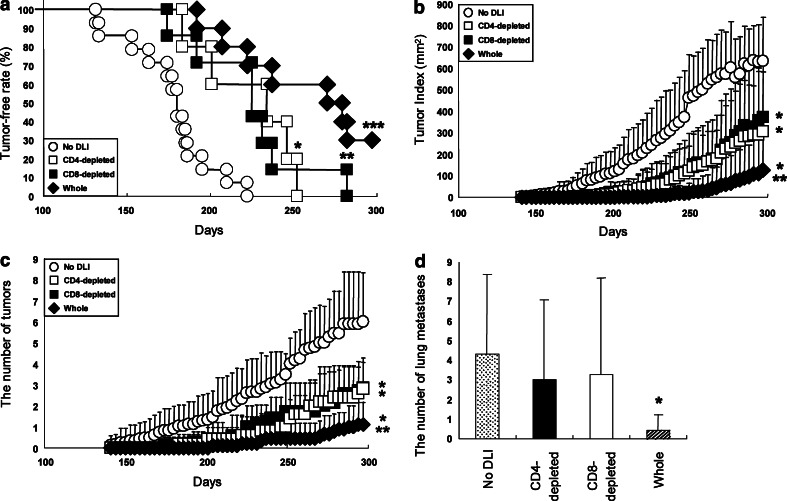
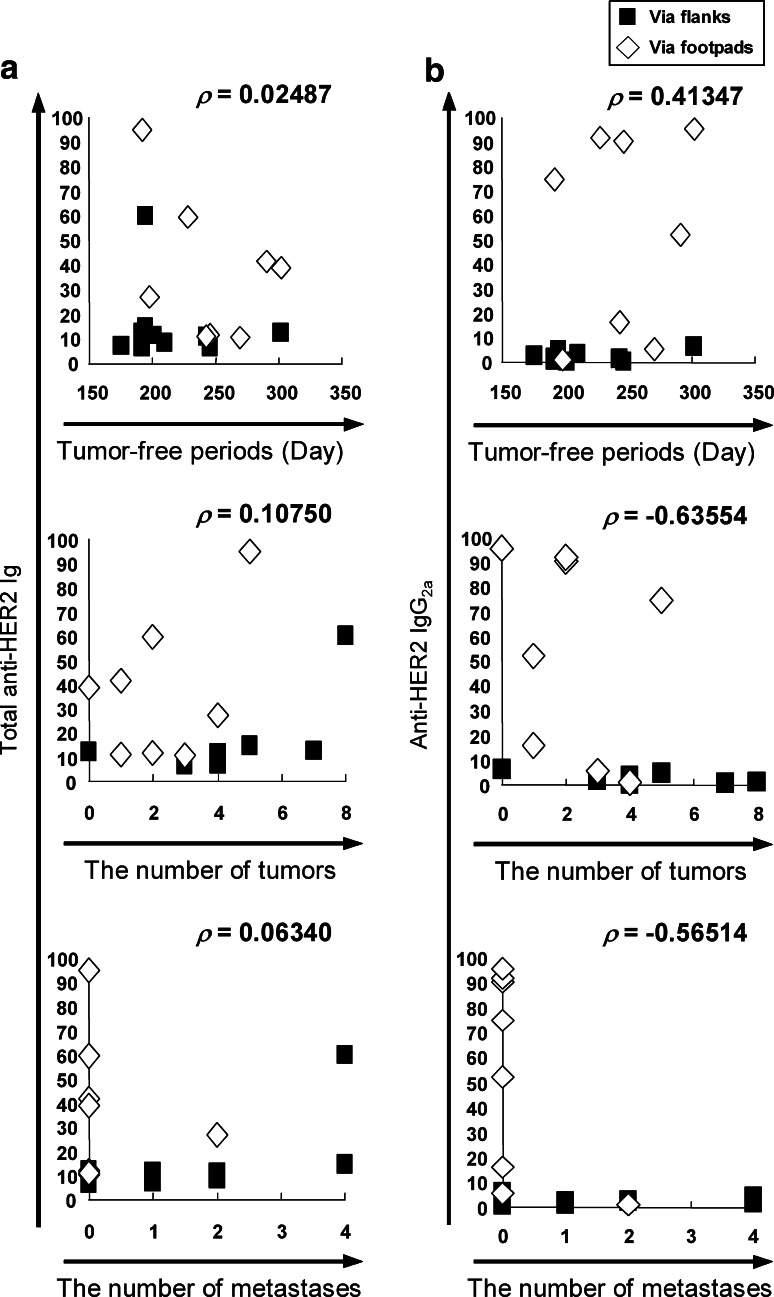
To assess a role of humoral immunity in anti-tumor effects against spontaneous mammary carcinomas in FVB-Tg mice, we infused CD4+-depleted, CD8+-depleted or whole splenocytes prepared from the spleen of mice that had been vaccinated with tHER2 (M)-transduced DC into non-immunized FVB-Tg mice. In contrast to mice infused with whole splenocytes where the potent anti-tumor effects were observed comparable to those observed in mice inoculated with transduced DC, neither group infused with CD4- nor CD8-depleted splenocytes showed anti-tumor effects in terms of the tumor-free periods, tumor progression, and the number of tumor masses (P < 0.05, Fig. 6a–d). Furthermore, the level of HER2-specific IgG2a antibody, not a total amount of HER2-specific antibody, positively correlated with anti-tumor effects represented as tumor-free periods, the number of tumor masses and lung metastases (Fig. 7a, b). Taken together, it is likely that the potent anti-tumor effects observed here were sustained by both cellular and humoral immunity against HER2.
Fig. 6.
Adoptive transfer of splenocytes from immunized FVB-Tg mice. a Tumor-free periods of untreated FVB-Tg mice (No DLI, open circles, n = 14), or mice infused with whole (closed diamonds), CD4- (open squares) or CD8-depleted (closed squares) splenocytes (n = 10, 5, 7, respectively). *P < 0.05, **P < 0.01, and ***P < 0.005 compared with untreated mice. Tumor Index (b), the number of primary tumors (c), and lung metastases (d) in mice with or without adoptive transfer. *P < 0.05, CD4-, CD8-depleted compared to No DLI or Whole, and **P < 0.005, Whole compared to No DLI in (b) and (c). *P < 0.05 compared to other groups in (d)
Fig. 7.
The correlation of anti-HER2 antibodies with prognoses of FVB-Tg. Correlation of total anti-HER2 Ig (a) or IgG2a (b) with tumor-free period (upper) and the number of primary tumors (middle) or lung metastases (lower) was evaluated with Spearman’s rank-order correlation coefficient and represents ρ values
Anti-tumor effects of transduced DC in a therapeutic setting
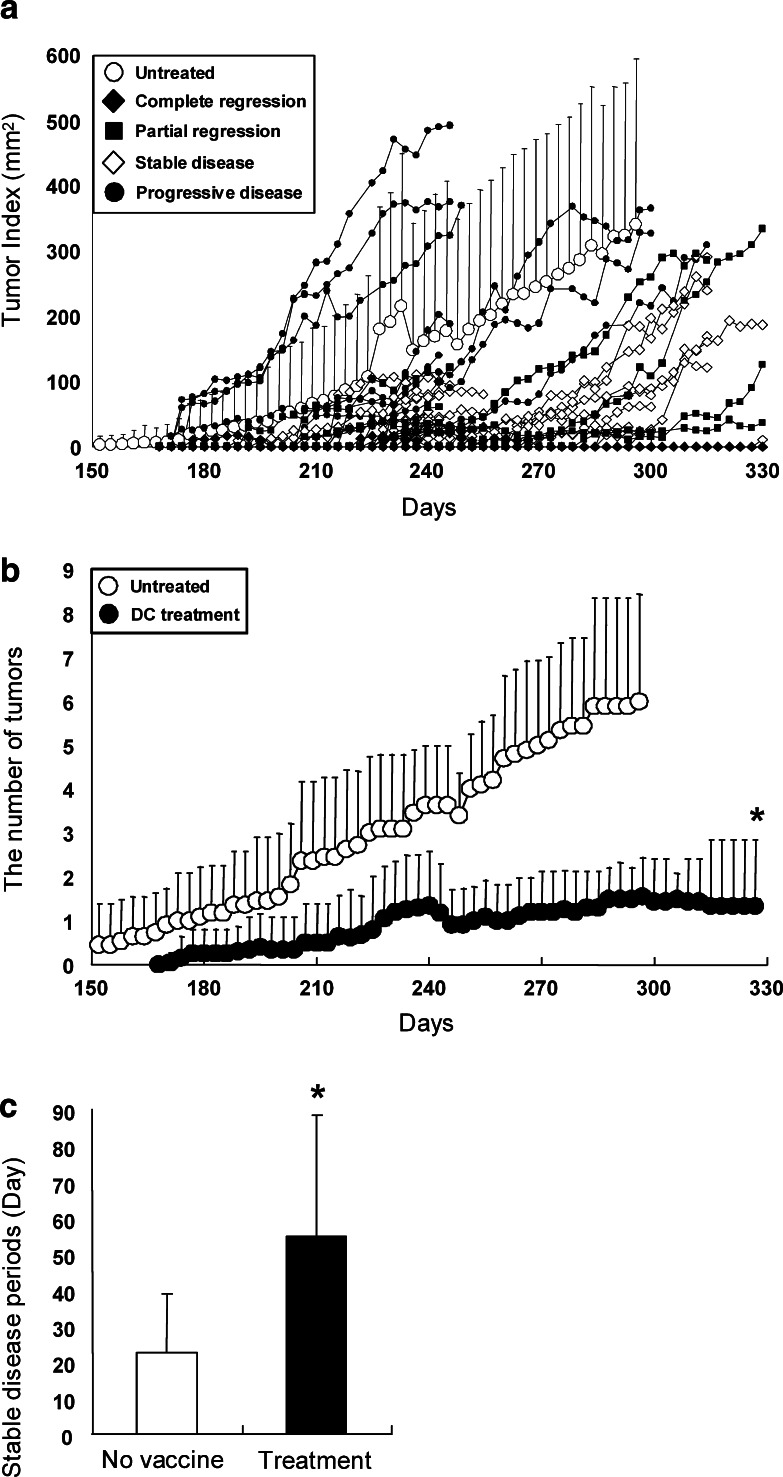
Finally, to evaluate anti-tumor effects of vaccine with tHER2 (M)-transduced DC in a therapeutic setting, FVB-Tg mice that had already developed mammary tumors were inoculated three times with 1 × 106 transduced DC via footpad route. In the result, 4 out of 41 tumors present in the mice at the vaccine were completely eradicated and six partially regressed (Fig. 8a). Of note, the eradicated tumors did not relapse over 60 days of observation. Although nine tumors kept increasing in size, 22 tumors remained stable. Compared with untreated mice, mice inoculated with transduced DC showed significantly fewer tumors and longer stable disease periods (Fig. 8b, c). The results suggested that vaccine with tHER2 (M)-transduced DC elicited potent anti-tumor effects against pre-existing mammary carcinomas in a situation very similar to clinical arena.
Fig. 8.
Treatment with the transduced DC for tumor-bearing FVB-Tg mice. a Kinetics of tumor progression of tumor-bearing FVB-Tg mice inoculated with transduced DC (n = 20) or without treatments (n = 11) are shown. These mice are classified as complete regression, partial regression, stable disease, and progressive disease. The number of tumors (b) and stable disease periods (c) in mice treated with transduced DC or untreated mice. *P < 0.005 compared with untreated
Discussion
In the present study, we demonstrate the potent anti-tumor effects of the tHER2 (M)-transduced DC in vivo with induction of both cellular and humoral immunity against tumors expressing HER2. Of importance is that the effects were confirmed by experiments using FVB-Tg mice in which multiple mammary carcinomas expressing HER2 developed spontaneously. Mice inoculated with transduced DC showed less primary and metastatic tumors and longer disease-free periods compared to non-treated mice. In addition to these observations, we also observed the superiority of footpad inoculation over subcutaneous one in facilitation of DC migration into their draining LN, resulting in induction of strong humoral immune responses. Finally, the vaccine strategy with the transduced DC via footpad route induced strong immune responses against the tumors even in mice with pre-existing mammary carcinomas.
Although DC genetically modified to express TAA by a retroviral vector are likely to be more beneficial than those pulsed with TAA-derived epitope peptides or tumor lysates because of continuous and stable presentation of the TAA through the both MHC class I and II on their surfaces [12, 15, 17, 18, 29, 38], no clinical trials using such DC have been reported, partly because of low transduction efficiency and gene silencing of conventional retroviral vectors when used for HPC and mature DC [17, 19, 38]. We have developed the gene-silencing-resistant retroviral vector, GCDNsap [35] and demonstrated the ability of the vector to express the transgene in mature DC [25]. Indeed, DC transduced with OVA cDNA using the retroviral vector elicited potent anti-tumor immune responses against tumors expressing OVA [25] similar to what observed in the present study for HER2 antigen, suggesting the general versatility of the strategy described here in term of TAA-derived epitope presentation.
While the present study demonstrated that vaccination with tHER2 (M)-transduced DC was superior to that with tumor lysate-pulsed DC, it also showed the importance of vaccine sites, with footpad inoculation providing more potent anti-tumor effects than subcutaneous immunization at the flanks by producing higher levels of anti-HER2 antibody, especially IgG2a subclass. The result may suggest that both inoculation pathways are sufficient to fully activate HER2-specific CTL, resulting in the maximal plateau of anti-HER2 cellular immunity in vivo. On the contrary, anti-HER2 humoral immunity remains insufficient in the case of subcutaneous inoculation that is unlikely to be a proper route for DC migration into their draining LN. Although the reason why subcutaneous inoculation at the flanks induced less humoral immunity while eliciting strong cellular immunity comparable to footpad inoculation should be reconciled by additional experiments, effective anti-tumor effects against tumors expressing TAA on their surfaces may necessitate potent humoral immunity against the tumors as demonstrated in clinical studies using Trastuzumab (humanized antibody against HER2) for breast cancer patients.
It should be noted that the HER2 cDNA used for treatment of FVB-Tg mice expressing rat HER2 homolog is the human HER2/ErbB2. Unfortunately, we cannot deny that use of the xenoantigen may jeopardize the promising results observed in the present study, but it is also true that orthologous proteins for “self” antigens is commonly used to break self-tolerance due to its higher immunogenicity [9, 10, 21, 28]. Therefore, use of the orthologous HER2 antigen derived from other species except for human, such as mouse or rat and direct injection of DC into patient’s axillary or inguinal LN with ultrasound echo guidance may be worth considering to enhance anti-tumor immunity in clinical trials.
The present study showed promising results from the use of transduced DC-mediated anti-tumor immunity against pre-existing tumors in FVB-Tg mice, which encourages us to take a step to apply the strategy in a clinical setting. Furthermore, considering that the considerable number of clinical trials for primary immunodeficiency where the patient CD34+ cells are genetically modified by retroviral vectors has proven beneficial [6], our strategy that patient CD34+ cells transduced with HER2 by GCDNsap are differentiated into mature DC in vitro followed by infusion into patients’ LN directly has sufficient possibility for clinical applications. In a situation where few vaccine trials using HER2 peptides or peptide-pulsed DC proved beneficial [7, 8, 16], the HER2-transduced DC described here may present attractive and promising therapy for mammary carcinomas.
Acknowledgments
We thank Drs. Fabio Candotti (NHGRI, NIH) for providing a critical review of the manuscript, Sachio Shibata (Takeda Pharmaceutical Company) for providing full-length HER2 cDNA, Richard C. Mulligan (Harvard Medical School) for providing 293gpg cells, and Flaminia Miyamasu for proofreading. This work was supported by the Grant-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science and Technology (to M.O.), JSPS Research Fellowships for Young Scientists and Cancer Research Fellowships of the YASUDA Medical Foundation for Graduate Students (to T.N.).
Abbreviations
- DC
Dendritic cell(s)
- TAA
Tumor-associated antigen(s)
- HER2
Human epidermal growth factor receptor 2
- MHC
Major histocompatibility complex(es)
- HPC
Hematopoietic progenitor cell(s)
- OVA
Ovalbumin
- CTL
Cytotoxic T lymphocyte(s)
- Th1
Type 1 T helper
- B6
C57BL/6N
- FVB-Tg
FVB-Tg (MMTVneu) 202 Mul/J
- tHER2 (M)
Truncated membrane form of HER2
- PMC
Primary mammary carcinoma
- PE
Phycoerythrin
- Ig
Immunoglobulin
- IL
Interleukin
- IFN
Interferon
- EGFP
Enhanced green fluorescent protein
- LN
Lymph node(s)
- ADCC
Antibody-dependent cellular cytotoxicity
References
- 1.Ashley DM, Faiola B, Nair S, Hale LP, Bigner BD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce anti-tumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Bargmann CI, Hung M, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986;319:226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- 6.Bordignon C. Stem-cell therapies for blood diseases. Nature. 2006;441:1100–1102. doi: 10.1038/nature04962. [DOI] [PubMed] [Google Scholar]
- 7.Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004;53:275–306. doi: 10.1007/s00262-003-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 9.Disis ML, Shiota FM, Cheever MA. Human HER-2/neu protein immunization circumvents tolerance to rat neu: a vaccine strategy for ‘self’ tumor antigen. Immunology. 1998;93:192–199. doi: 10.1046/j.1365-2567.1998.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 11.Gallucci S, Loloema M, Martinzer P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1995;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 12.Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46:82–87. doi: 10.1007/s002620050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorer PA. Studies in antibody response of mice to tumour inoculation. Br J Cancer. 1950;4:372–379. doi: 10.1038/bjc.1950.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;9:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson RA, Nimgaonkar MT, Watkins SC, Robbins PD, Ball ED, Finn OJ. Human dendritic cells genetically engineered to express high level of the human epithelial tumor antigen mucin (MUC-1) Cancer Res. 1996;56:3763–3770. [PubMed] [Google Scholar]
- 16.Ikuta Y, Katayama N, Wang L, Okugawa T, Takahashi Y, Schmitt M, Gu X, Watanabe M, Akiyoshi K, Nakamura H, Kuribayashi K, Sunamoto J, Shiku H. Presentation of a major histocompatibility complex class 1-binding peptide by monocyte-derived dendritic cells incorporating hydrophobized polysaccharide-truncated HER2 protein complex: implications for a polyvalent immuno-cell therapy. Blood. 2002;99:3717–3724. doi: 10.1182/blood.V99.10.3717. [DOI] [PubMed] [Google Scholar]
- 17.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102–107. doi: 10.1016/S1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 18.Lapointe R, Royal RE, Reevesb ME, Altomare I, Robbins PF, Hwu P. Retrovirally transduced human dendritic cells can generate T cells recognizing multiple MHC class I and class II epitopes from the melanoma antigen glycoprotein 100. J Immunol. 2001;167:4758–4764. doi: 10.4049/jimmunol.167.8.4758. [DOI] [PubMed] [Google Scholar]
- 19.Lindemann C, Schilz AJ, Emons B, Baum C, Löw R, Fauser AA, Kuehlcke K, Eckert H. Down-regulation of retroviral transgene expression during differentiation of progenitor-derived dendritic cells. Exp Hematol. 2002;30:150–157. doi: 10.1016/S0301-472X(01)00778-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/S0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wei Y, Tian L, Zhao X, Yang L, Hu B, Kan B, Wen Y, Liu F, Deng H, Li J, Mao Y, Lei S, Huang M, Peng F, Jiang Y, Zhou H, Zhou L, Luo F. Immunogene therapy of tumor with vaccine based on xenogeneic epidermal growth factor receptor. J Immunol. 2003;170:3162–3170. doi: 10.4049/jimmunol.170.6.3162. [DOI] [PubMed] [Google Scholar]
- 22.Maguire HC, Jr, Greene MI. The neu (c-erbB-2) oncogene. Semin Oncol. 1989;16:148–155. [PubMed] [Google Scholar]
- 23.Matzinger P. The JAM test: a simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-A. [DOI] [PubMed] [Google Scholar]
- 24.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumor peptides elicit protective and therapeutic anti-tumor immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 25.Nabekura T, Otsu M, Nagasawa T, Nakauchi H, Onodera M. Potent vaccine therapy with dendritic cells genetically modified by the gene-silencing-resistant retroviral vector GCDNsap. Mol Ther. 2006;13:301–309. doi: 10.1016/j.ymthe.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 27.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves ME, Royal RE, Lam JS, Rosenberg SA, Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996;56:5672–5677. [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang IC, Restifo MP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider U, Schwenk HU, Bornkamm G. Characterization of EBV-genome negative “null” and “T” cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1997;15:621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- 32.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 33.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Obi K, Urabe T, Hayakawa H, Yamada M, Kaneko S, Onodera M, Mizuno Y, Mochizuki H. Feasibility of ex vivo gene therapy for neurological disorders using the new retroviral vector GCDNsap packaged in the vesicular stomatitis virus G protein. J Neurochem. 2002;82:953–960. doi: 10.1046/j.1471-4159.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 36.Trempe GL. Human breast cancer in culture. Recent Results Cancer Res. 1976;57:33–41. doi: 10.1007/978-3-642-81043-5_5. [DOI] [PubMed] [Google Scholar]
- 37.van de Vijver MJ, Peterse JL, Mooi WJ, Wisman P, Lomans J, Dalesio O, Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319:1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 38.Veerman MD, Heirman C, Meirvenne SV, Devos S, Corthals J, Moser M, Thielemans K. Retrovirally transduced bone marrow-derived dendritic cells require CD4+ T cell help to elicit protective and therapeutic antitumor immunity. J Immunol. 1999;162:144–151. [PubMed] [Google Scholar]
- 39.Wen Y, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate–specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–3285. doi: 10.1182/blood.V99.9.3280. [DOI] [PubMed] [Google Scholar]