Abstract
Despite spontaneous or vaccination-induced immune responses, pancreatic cancer remains one of the most deadly immunotherapy-resistant malignancies. We sought to comprehend the spectrum of pancreatic tumor-associated antigens (pTAAs) and to assess the clinical relevance of their immunogenicity. An autologous SEREX-based screening of a cDNA library constructed from a pancreatic T3N0M0/GIII specimen belonging to a long-term survivor (36 months) revealed 18 immunogenic pTAA. RT-PCR analysis displayed broad distribution of the identified antigens among normal human tissues. PNLIPRP2 and MIA demonstrated the most distinct pancreatic cancer-specific patterns. ELISA-based screening of sera for corresponding autoantibodies revealed that although significantly increased, the immunogenicity of these molecules was not a common feature in pancreatic cancer. QRT-PCR and immunohistochemistry characterized PNLIPRP2 as a robust acinar cell-specific marker whose decreased expression mirrored the disappearance of parenchyma in the diseased organ, but was not related to the presence of PNLIPRP2 autoantibodies. Analyses of MIA—known to be preferentially expressed in malignant cells—surprisingly revealed an inverse correlation between intratumoral gene expression and the emergence of autoantibodies. MIAhigh patients were autoantibody-negative and had shorter median survival when compared with autoantibody-positive MIAlow patients (12 vs. 34 months). The observed pTAA spectrum comprised molecules associated with acinar, stromal and malignant structures, thus presenting novel targets for tumor cell-specific therapies as well as for approaches based on the bystander effects. Applying the concept of cancer immunoediting to interpret relationships between gene expression, antitumor immune responses, and clinical outcome might better discriminate between past and ongoing immune responses, consequently enabling prognostic stratification of patients and individual adjustment of immunotherapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0870-9) contains supplementary material, which is available to authorized users.
Keywords: Pancreatic cancer, Immunoediting, SEREX, PNLIPRP2, MIA, Antibody
Introduction
Pancreatic cancer remains one of the most deadly malignancies despite sustained immunocompetence and tumor-specific immune responses detected in patients at various stages of cancer progression (http://www-dep.iarc.fr/) [1–12]. Induction of immune responses to known tumor-associated antigens (TAAs) has also not yielded a clear clinical benefit in patients with pancreatic cancer [13–18].
Poor clinical response despite antitumor immunity is one of the most intriguing paradoxes of cancer [19–21]. Decades of experimental work have laid the groundwork for the concept of cancer immunoediting, which emphasizes dual role of immunity in not only preventing, but also shaping neoplastic disease. A broadened form of the immunosurveillance theory, immunoediting views tumor progression as a dynamic process in which the immune system sculpts the ‘non-edited’ antigenic repertoire of a tumor toward emergence of ‘edited’ antigen-loss variants over an ‘Elimination–Equilibrium–Escape’ continuum [22–24].
In pancreatic cancer, spontaneous humoral responses (assumed to reflect antigen-specific T-cell responses) correlate with both better and worse outcomes [25–29]. The concept of immunoediting could provide a logical explanation for the lack of clinical benefit of immune responses against tumors in which the corresponding antigen is already lost: responses measurable at later stages of the disease reflect past events which are potentially prognostic, but no longer have therapeutic relevance. The presence of antibodies that reflect an immune response which eliminated immunogenic tumor cells with extremely pro-malignant functions—thus allowing expansion of less-aggressive variants—will have a positive prognostic value. Conversely, detection of antibodies reflecting immune responses eliminating less malignant tumor variants—thus favoring expansion of super-aggressive non-immunogenic species—will have negative prognostic value. However, with immune effectors persisting after a target is eliminated, peptide-based vaccination approaches would fail while inducing recall responses without clinical impact. Thus, individual immunogenicity of TAA might enable immunoediting, which in turn might alter the TAA profile in a way which will greatly affect outcome. An immunogenic repertoire of a disease can be determined by different proteomic methods, the most comprehensive being the SEREX (Serological analysis of Recombinant cDNA Expression libraries) [30–33]. To date, the SEREX method for the identification of pancreatic TAA (pTAA) has been restricted to libraries constructed from a cell line or from testis tissue, but not from pancreatic cancer specimens [34–36]. The identity of the detected immunogenic TAA (1) differed among these heterologous approaches, (2) did not match the few findings deposited in the Cancer Immunome Database (http://ludwig-sun5.unil.ch/CancerImmunomeDB) which were obtained by other proteomic methods or reported by the SEREX study conducted in a syngeneic mouse model [37–42], and (3) was not interpreted in relation to antigen expression and clinical outcome.
In the current study, we sought to comprehend the spectrum of pTAA and to determine whether immunogenicity of pTAA is relevant for disease outcome. We applied an autologous SEREX-based screening to establish the profile of immunogenic pTAA in a long-term survivor, reasoning that this patient was more likely to have a protracted equilibrium phase because of tumor displaying immunogens with potentially positive prognostic value. The association of immunogenicity with humoral responses capable of editing the antigenic repertoire of malignant cells in a prognostically relevant way was further explored by analyzing the relationship between pTAA expression, emergence of autoantibodies, and survival time in patients with pancreatic cancer.
Materials and methods
Sample collection
The study was approved by the Medical Ethics Committee of the University of Heidelberg (case numbers 159/2002 and 301/2001). Upon written informed consent from the patients, tissue and sera specimens were collected in accordance with governmental and international regulations. The primary sera screening cohort included 20 healthy donors (4 females, 16 males; median age 60 years) and 34 pancreatic ductal adenocarcinoma patients (8 females, 26 males; median age 65 years; stage IIA, T3N0M0: n = 1; stage IIb, T3N1M0: n = 24; stage IV, T3N0M1: n = 2 and T3N1M1: n = 7; 6th edition AJCC/UICC). For extended assays, additional individuals were recruited to the control or patient cohorts as indicated in the main text.
Construction of the cDNA library from human pancreatic ductal adenocarcinoma tissue and immunoscreening with autologous patient serum (SEREX)
The SEREX screening was performed according to previously established protocols [30, 31, 43]. Briefly, a cDNA expression library was constructed in a ZAPexpress vector (Stratagene; La Jolla, USA) using 5 µg of polyA+-mRNA isolated from a pancreatic specimen from a 68-year-old female with ductal pancreatic adenocarcinoma (Stage IIA, T3N0M0/GIII, survival = 36 months). An established library was screened with pre-absorbed autologous serum diluted at 1:200 followed by alkaline phosphatase-conjugated anti-human IgG antibody (Dianova; Hamburg, Germany). One round of secondary screening was performed. The immunoreactive clones were excised in vivo into pBK-CMV plasmid forms (Stratagene). Plasmid DNA was purified with a plasmid isolation kit (Qiagen; Hilden, Germany) and sequenced (GATC Biotech AG; Konstanz, Germany). The identity of inserts was established by searching Gene Bank and EST databases using the BLAST algorithm.
Cloning of full-length open reading frames (ORFs) and expression of His-fusion proteins
The ORFs of SEREX-defined genes were amplified by PCR from cDNA derived from normal pancreas and testis using primer sets for PNLIPRP2 (pancreatic lipase-related protein 2): forward 5′-aaatttgagctcatgatgctgcccccttg-3′, reverse 5′-aaatttctgcagttaacaagggtaaagagattgca-3′; MIA (melanoma-inhibitory activity): forward 5′-aaagggatccatggcccggtccct-3′, reverse 5′-aaaaagctttcactggcagtagaaatcccattt-3′; and IFITM3 (interferon-inducible transmembrane protein-3): forward 5′-aaaggatccatgaatcacactgtccaaacc-3′, reverse 5′-aaaaagcttctatccataggcctggaagatcag-3′. ORFs were sequentially subcloned into the pGEMTeasy vector (Promega; Mannheim, Germany) and then into the pQE-30 vector encoding His-fusion proteins (Qiagen) with SacI/PstI for PNLIPRP2 and with BamHI/HindIII for MIA and IFITM3.
Protein expression of His-fusion proteins in E. coli M15 bacteria was carried out as described previously [44]. Briefly, protein expression was induced by 1 mM IPTG for 4 h at 37°C in the pQ30-transformed bacteria grown to a density of OD600 = 0.6. For the non-induced negative controls, no IPTG was added. Bacterial lysates and affinity-purified recombinant proteins were analyzed by SDS-PAGE, Coomassie staining and immunoblotting using antibodies directed against PNLIPRP2 (mouse IgG, 1:2500) (Abnova; Teipeh, Taiwan), MIA (goat IgG, 1:1000) (Santa Cruz), IFITM3 (mouse IgG, 1:2500) (Abnova) and His-tag (mouse IgG, 1:3000) (Qiagen). After addition of appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (Dianova and Sigma), the proteins were visualized by the ECL method (GE Healthcare; Freiburg, Germany) (Supplementary Figure 1).
Reactivity of heterologous sera to SEREX-defined antigens
Purified proteins were used in ELISA for the screening of sera for corresponding autoantibodies based on published protocols [45, 46]. Sera were blocked with 100 µg/ml lysate prepared from control bacteria transformed with an empty pQE-30 vector, diluted 1:1000 and incubated with plate-bound His-tagged recombinant proteins. Seropositivity was visualized using HRP-coupled anti-human antibodies. The cut-off was determined by calculating the mean value of absorbance measured at 490 nm for the control cohort plus three times the standard deviation. Recombinant NY-ESO-1 protein and an anti-NY-ESO-1-immunoreactive patient serum as well as anti-His antibody and protein-specific antibodies used for immunoblotting served as positive controls.
Analysis of expression of SEREX-defined antigens in normal human tissues by RT-PCR
Total RNA from 22 normal tissues, each obtained and pooled from five disease-free individuals, was purchased from AMS biotechnology (Abingdon, UK). After cDNA synthesis (Invitrogen; Karlsruhe, Germany), PCR was performed with 35 cycles of amplification using sets of primers specific for 18 SEREX-defined gene products presented in Supplementary Table 1. β-actin served as control for input cDNA. PCR products were analyzed by electrophoretic separation and visualized by ethidium bromide staining.
Quantification of PNLIPRP2, MIA and IFITM3 expression by QRT-PCR
Cryocut normal and diseased pancreatic tissues as well as eight pancreatic cancer cell lines (AsPC1, BxPC3, Capan1, Colo357, MiaPaca2, Panc1, SU 86.86, T3M4) underwent quantitative RT-PCR using RAS technologies (Roche Applied Science; Mannheim, Germany) for MagNAPure-based mRNA isolation, cDNA synthesis and LightCycler-based real-time PCR with the FastStart DNA SYBR Green kit and primers obtained from Search-LC (Heidelberg, Germany), as described previously [47–50]. The expression of each specific gene was normalized to housekeeping gene cyclophilin B (CPB) and presented as the number of transcripts per 10,000 CPB copies.
Immunohistochemistry
The localization of SEREX-defined proteins in pancreatic tissues was assayed by immunohistochemical method based on routine protocols [48–50]. In brief, antigens in 4-µm sections prepared from paraffin-embedded tissues were exposed by boiling in 10 mM citrate buffer (pH 6.0). After blocking with peroxide and beriglobin (ZLB Behring; Hattersheim, Germany), tissue sections were incubated with rabbit anti-human PNLIPRP2 (1:500) (Abnova), mouse anti-human IFITM3 (1:500) (Abnova) or appropriate isotype-specific negative controls diluted in antibody diluent with background-reducing components (DAKO; Carpinteria, CA, USA). On overnight incubation at 4°C, slides were incubated with HRP-labeled anti-rabbit or anti-mouse detection reagents and liquid DAB + Substrate Chromogen System (DAKO) and then counterstained with Mayer’s hematoxylin (Merck; Darmstadt, Germany).
Western blot analysis of pancreatic tissues
On histological evaluation, cryocut pancreatic tissues were pulverized and dissolved in RIPA buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS). 20 µg of protein was resolved by SDS-PAGE using 4–12% NuPAGE Novex gels (Invitrogen). 1 µg of recombinant PNLIPRP2-GST (Abnova) protein was used as a positive control. The proteins were transferred to nitrocellulose membranes and visualized using antibodies to PNLIPRP2 (D01P, Abnova), GAPDH (loading control, Santa Cruz) and corresponding secondary peroxidase-labeled anti-rabbit (GE Healthcare) and anti-goat (Santa Cruz) antibodies and an ECL detection kit (GE Healthcare).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 5 Software (GraphPad Software Inc.; La Jolla, CA, USA). The median and range were calculated and used for graphical presentation of quantitative data. To compare different groups, we used either Fisher’s exact test or the Kruskal–Wallis test followed by Dunn’s test. The Kaplan–Meier method and Gehan–Breslow–Wilcoxon test were employed to analyze survival among patients presenting with different antigen/antibody profiles. Significance was defined at p < 0.05. On graphs, only significant differences between the groups are shown indicating *p < 0.05, **p < 0.01 and ***p < 0.001.
Results
Identification of PDAC-specific immunogens by autologous SEREX
A cDNA expression library containing 6 × 105 primary clones was constructed from mRNA of a pancreatic carcinoma specimen and screened with autologous serum diluted at 1:200. After two rounds of screening, 42 immunoreactive clones had been purified and sequenced, yielding 18 distinct gene products (Table 1).
Table 1.
Identity of genes detected by SEREX in pancreatic carcinoma
| Symbol | Identifier | Entrez gene name | Previously detected immunogenicity (presence of autoantibodies) | Extra-pancreatic expression (normal tissues, n = 21) | Level of expression in pancreas | ||
|---|---|---|---|---|---|---|---|
| Freq. (%) | Level | Normal | Cancer | ||||
| ANXA2 | NM_004039.2 | Annexin A2 | Tongue and gingivobuccal complex cancer [51], squamous cell and lung cancer [52] | 100 | ++ | ++ | ++ |
| ATP6V0E1 | NM_003945.3 | ATPase, H+ transporting, lysosomal 9 kDa, V0 subunit e1 | – | 100 | + | + | + |
| COX3 | NC_001807.4 | Cytochrome c oxidase subunit 3 | – | 100 | ++ | ++ | ++ |
| DYNC1I2 | NM_001378.1 | Dynein, cytoplasmic 1, intermediate chain 2 | – | 100 | + | + | ++ |
| GNB2L1 | NM_006098.4 | Guanine nucleotide-binding protein (G protein), beta polypeptide 2-like 1 | – | 100 | ++ | ++ | ++ |
| HDGFRP3 | NM_016073.2 | Hepatoma-derived growth factor, related protein 3 | Ulcerative colitis [53] | 100 | + | + | ++ |
| IFITM3 | NM_021034.1 | Interferon-induced transmembrane protein 3 | – | 100 | + | + | + |
| KALRN | NM_007064.2 | Kalirin, RhoGEF kinase | – | 95 | + | + | ++ |
| KEL | NM_000420.2 | Kell blood group, metalloendopeptidase | – | 52 | + | − | ++ |
| LUC7L | NM_201412.1 | LUC7-like | – | 95 | + | + | ++ |
| MIA | NM_006533.1 | Melanoma-inhibitory activity | – | 43 | + | − | + |
| PNLIPRP2 | NM_005396.3 | Pancreas lipase-related protein 2 | – | 14 | + | ++ | + |
| POSTN | NM_006475.1 | Periostin, osteoblast-specific factor | – | 80 | + | + | ++ |
| RPL13 | NM_000977.2 | Ribosomal protein L13 | – | 100 | + | + | + |
| S100A6 | NM_014624.3 | S100 calcium-binding protein A6 | Liver cancer [54] | 95 | + | + | + |
| SMARCA5 | NM_003601.2 | SWI/SNF related, matrix associated, actin-dependent regulator of chromatin, subfamily a, member 5 | – | 100 | + | + | ++ |
| TAGLN | NM_001001522.1 | Transgelin | Prostate cancer [55], liver cancer [56] | 100 | + | + | ++ |
| TUBA6 | NM_032704.2 | Tubulin, alpha 1c | Tongue and gingivobuccal complex cancer [51], relapsing polychondritis [57], healthy individuals [58, 59] | 95 | + | + | ++ |
Pancreatic specificity of SEREX-defined antigens
A number of genes identified by SEREX in our study were previously described as immunogenic under malignant or inflammatory conditions (Table 1; [51–59]); however, none has been found in pancreatic cancer using heterologous SEREX techniques [34–36]. Data mining revealed that immune responses were not restricted to tumor cell-specific antigens, but developed without any preference for cellular compartment (acini, stroma and ducts).
In the pancreas, a differential expression pattern between cancerous and normal tissues was observed for 11 of 18 genes: 2 were de novo, 8 over-, and one under-expressed (Table 1 and Supplementary Table 2). However, analysis of extra-pancreatic mRNA expression of these SEREX-defined genes in a panel of 21 normal tissues demonstrated broad distribution and abundant expression for most antigens. Two genes, MIA and PNLIPRP2, displayed the most restricted differential pattern of expression: present in few extra-pancreatic tissues and significantly different between normal and cancerous pancreas specimens. mRNA for MIA was observed in 43% of non-pancreatic tissues (9/21: lung, mammary gland, melanocytes, prostate, small intestine, stomach, testis, thymus, trachea). In the pancreas, MIA expression was non-detectable in intact specimens, but up-regulated in the cancerous specimens. The expression of PNLIPRP2 was hardly detectable and rare (14%) in non-pancreatic normal tissues (3/21: small intestine, spleen, testis). In pancreas, PNLIPRP2 expression was abundant in normal, but reduced in diseased tissue. In summary, the antigenic repertoire defined by autologous SEREX was not strictly associated with pancreatic malignancy or with pancreatic tumor cells.
Next, we explored whether the immunogenicity of MIA and PNLIPRP2, which showed the most restrictive differential expression pattern, is a common feature of pancreatic cancer. A ubiquitous and unaltered membranous immunogen—IFITM3—served as control.
Immunogenicity of SEREX-defined antigens and favorable outcome in patients with pancreatic cancer
Coincident autoantibody responses to MIA, PNLIPRP2 and IFITM3 were evaluated by ELISA in sera of 20 healthy donors and 34 patients with pancreatic cancer (Fig. 1a). No control individual had antibodies to more than one antigen: a single case of anti-MIA seroreactivity was observed. Among patients with pancreatic cancer, none were seropositive for all three antibodies and only one showed simultaneous anti-MIA and anti-PNLIRP2 humoral responses. The frequency of single antibody-positive patients ranged from 9 to 15%, yielding totals of 32%, as compared to 6% for the control cohort (p < 0.05).
Fig. 1.
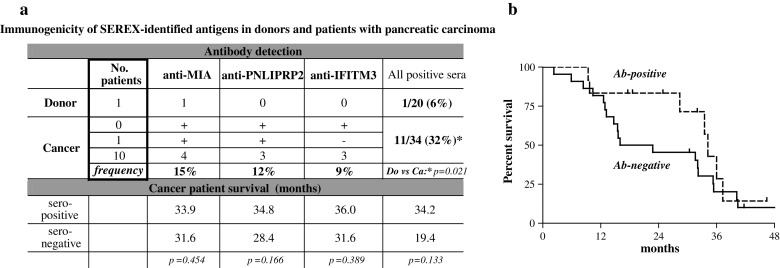
Immunogenicity of SEREX-defined antigens and outcome in patients with pancreatic adenocarcinoma. a The immunogenicity of SEREX-identified MIA, IFITM3 or PNLIPRP2 was determined by ELISA-based detection of circulating autoantibodies serving as a read-out of the humoral immune response. The frequency of antigen-specific and cumulative antibody seropositivity of donors and patients with pancreatic cancer was calculated and related to the survival time. b Kaplan–Meier analysis showed a better outcome trend for the patients carrying autoantibodies against the SEREX-identified antigens
Taken separately, the presence of specific autoantibody was not associated with a better outcome (Fig. 1a). However, a comparison of all seropositive and all seronegative patients (Fig. 1a, b) showed that the 2-year survival rates and the median survival time were doubled for seropositive patients, although overall survival remained unaffected.
The results imply that the immunogenicity of selected antigens defined by autologous SEREX is increased in pancreatic cancer and tends to be associated with increased median survival. The lack of linear correlation between intra-pancreatic expression and antibody presence questioned the relevance of the most common mechanism promoting immunogenicity of cancer antigens—overexpression—as a deciding factor in pancreatic cancer. Thus, we aimed to determine how humoral responses relate to the patterns of intra-pancreatic expression of PNLIPRP2, IFITM3 and MIA.
Sporadic anti-PNLIPRP2 responses and loss of PNLIPRP2-expressing acinar parenchyma in pancreatic cancer
The expression of IFITM3 and PNLIPRP2 in pancreatic diseases has not yet been studied. Our data showed similarly high mRNA expression of IFITM3 in normal (n = 18), inflammatory (chronic pancreatitis, n = 34) and cancerous (n = 53) pancreatic specimens, and in tumor cell lines (n = 8). Immunohistochemical analyses confirmed ubiquitous distribution of IFITM3, whereas ELISA revealed no correlation between anti-IFITM3 seropositivity and the levels of IFITM3 expression (Supplementary Figure 2 and data not shown).
In contrast, QRT-PCR analyses of PNLIPRP2 expression revealed dramatic changes (Fig. 2a). The median level of PNLIPRP2 expression in cancerous lesions was 500 times lower than in normal pancreata (p < 0.001). Notably, the loss of PNLIPRP2 expression varied within a 5-log range and the same pattern was observed in patients with chronic pancreatitis. We first suspected that such pattern might be caused by sporadically arisen autoantibodies eliminating PNLIPRP2-expressing cells (autoimmune attack). However, anti-PNLIPRP2 seropositivity in patients with pancreatic cancer or chronic pancreatitis has not been associated with any particular aberrations in pancreatic PNLIPRP2 expression, such as low QRT-PCR outliers. Next, we supposed that high intra-pancreatic expression of PNLIPRP2 is associated with the emergence of autoantibodies without pathogenic bearing. Yet, we could not detect preferential anti-PNLIPRP2 seropositivity in PNLIPRP2high patients. Moreover, extended screening of 30 additional donors revealed 2 antibody-positive individuals, yielding a total of 4% (2/50) for seropositivity among constitutively PNLIPRP2high donors—in contrast to 14.5% in patients with chronic pancreatitis (11/76) and 9.5% in pancreatic cancer (9/95). Because none of eight analyzed pancreatic tumor cell lines expressed PNLIPRP2 and the most prominent histological hallmark of pancreatic cancer or chronic pancreatitis is the replacement of degenerating/dying exocrine parenchyma by fibrotic or malignant structures, we finally concluded by evaluating whether PNLIPRP2 down-regulation solely reflected altered cellular composition (e.g., disappearance of PNLIPRP2-expressing acini) in studied specimens. Indeed, QRT-PCR analysis of various types of pancreata grouped according to the size of remaining exocrine parenchyma (intact or degenerating; H&E staining of parallel frozen sections) revealed a clear link between PNLIPRP2 level and acinar volume (Fig. 2b). Extremely strong correlation with expression of pancreatic a2-amylase (Spearman’s ρ = 0.9045; p < 0.001) as well as Western blot analysis and a specific pattern of PNLIPRP2 distribution revealed by immunohistochemistry confirmed acinar cells as a sole source of PNLIPRP2 in the pancreas (Fig. 2c, d). PNLIPRP2 becoming an immunogen without pathogenic relevance was further supported by the observation that the degree of acinar degeneration either at the histological level in the studied specimens or at the whole organ level (quantitative computed tomography-assisted imaging, manuscript in preparation) did not correlate with the anti-PNLIPRP2 seropositivity. Thus, we concluded that PNLIPRP2 is a reliable molecular marker of acinar cells present in pancreatic specimens. Emergence of non-pathogenic anti-PNLIPRP2 antibodies in patients with pancreatic diseases does not appear to be a result of aberrant PNLIPRP2 expression, but rather a sporadic event probably associated with the occasional local release of PNLIPRP2 protein by (dying) acini in an immunogenic environment.
Fig. 2.
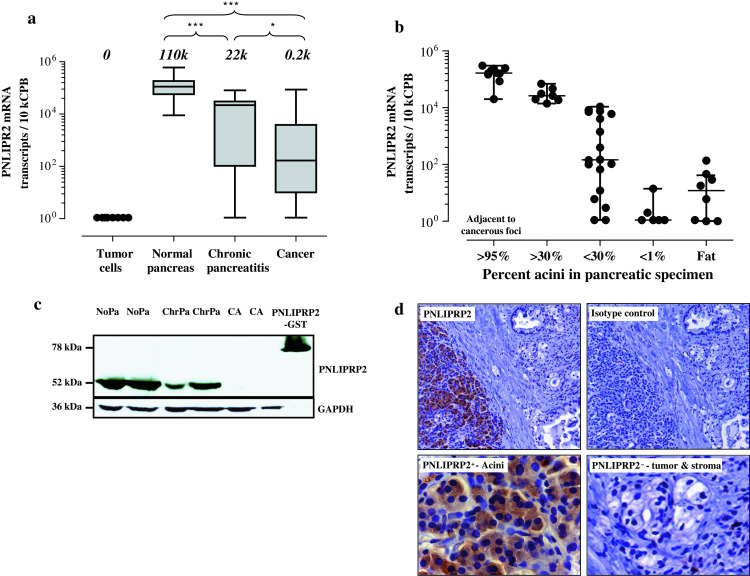
PNLIPRP2 as an indicator of the acinar loss in pancreatic diseases. a QRT-PCR showed that PNLIPRP2 mRNA expression was absent in the cultured tumor cells, high in normal pancreatic tissues and gradually lost in inflammatory and cancerous pancreatic lesions. b PNLIPRP2 mRNA levels in relation to the proportion of the acinar parenchyma presented in the analyzed specimens. c Western blot analysis showed the loss of PNLIPRP2 protein in pancreatic specimens with various degrees of parenchymal loss. d Immunohistochemistry revealed acinar cells as a single source of PNLIPRP2 in pancreatic tissues
Prognostic relevance of differential expression and immunogenicity of MIA
An elevated expression and frequent presence of MIA in tumor cells, vessels and tubular complexes located in inflammatory altered parenchyma adjacent to cancerous foci have been previously described by our group [60]. The analysis of new cohorts comprising 14 donors, 8 chronic pancreatitis and 26 pancreatic adenocarcinoma patients confirmed up-regulation of MIA mRNA expression in cancerous tissues (tenfold; p < 0.001) and also revealed a tendency of MIA transcripts to accumulate in chronic pancreatitis tissues (Fig. 3a). The overexpression of TAAs is one of the most common reasons for immunogenicity. Surprisingly, no MIA-overexpressing patients with pancreatic cancer presented an anti-MIA humoral immune response (Fig. 3b; MIAhigh = upper quartile [Q4] >380 transcripts per 10 kCPB according to our QRT-PCR assay), whereas in the seronegative group, both MIAhigh and MIAlow patients were present. Furthermore, median survival time of MIAhigh patients had a clear tendency to be reduced by half (12 vs. 25 months, p = 0.076; Fig. 3c). This trend gained magnitude and significance once expression levels were viewed in the context of anti-MIA seropositivity (Fig. 3d). The median survival of Ab-positive MIAlow patients reached 34 months, as compared to 18 months in Ab-negative MIAlow patients and 12 months in MIAhigh patients (p < 0.05). Figure 3d shows that Ab-positive MIAlow patients have a much better chance of surviving 2 years after diagnosis; however, long-term survival (>36 months) was similar among the studied groups. Thus, MIA represents a novel immunogen in the repertoire of pancreatic TAA and the immunogenicity of MIA taken in the context of expression patterns and pro-malignant features [60–62] may represent a prognostic marker for pancreatic cancer.
Fig. 3.
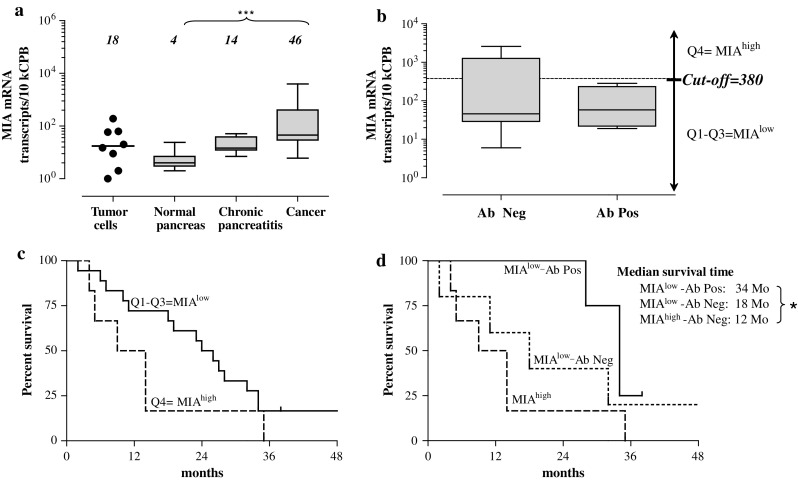
Association of MIA expression levels with autoantibody response and clinical outcome. The overexpression of MIA mRNA in cancerous tissues (a) did not coincide with anti-MIA antibody response (b), but tended to be associated with shorter survival (p = 0.076; Kaplan–Meier analysis, (c). In contrast, median survival time was significantly (p < 0.05) longer in the low-expressing group carrying autoantibodies to MIA (MIAlow-Ab Pos, (d)
Discussion
The ability of the immune system to recognize tumor-associated proteins can be used to define targets for cancer immunotherapy and to identify molecules of relevance to carcinogenesis. Our autologous SEREX-based study identified a spectrum of novel pancreatic TAA (Table 1) and also stressed the importance of a potentially antigenic molecule becoming a factual immunogen. The correlation between MIA mRNA expression, antibody presence, and patient survival suggests that the altering of the immunogenic profiles of tumor cells by the immune system during disease progression may crucially impact outcome. Poor outcome of malignant diseases despite manifest immune responses—paradoxical at first glance—may be explainable in the context of cancer immunoediting [22–24].
Indeed, immunosilent overexpression of pro-malignant MIA in pancreatic cancer as well as the prognosis-relevant imbalance between expression and immunogenicity makes perfect sense if viewed as a product of immunoediting—failed or completed (Fig. 4). Detection of autoantibodies to MIA in a proportion of pancreatic cancer patients means that this protein is intrinsically antigenic and may become immunogenic under certain circumstances, such as a pro-inflammatory environment, an appropriate HLA background, or specific post-translational modification. At the moment, we can only speculate as to which extrinsic factors were missing in a group of anti-MIA-seronegative patients; however, the theory of immunoediting allows prediction of preferential expansion of MIA-expressing variants in the absence of anti-MIA response. Because expression of MIA promotes invasive growth, poor outcome (median survival of 12 months) in such patients would be a foreseeable consequence. Whether a similar process occurs in other types of cancer—in which overexpression of MIA was found to correlate with a poor prognosis (melanoma, gastric cancer and glioma)—also remains to be determined [62–64].
Fig. 4.
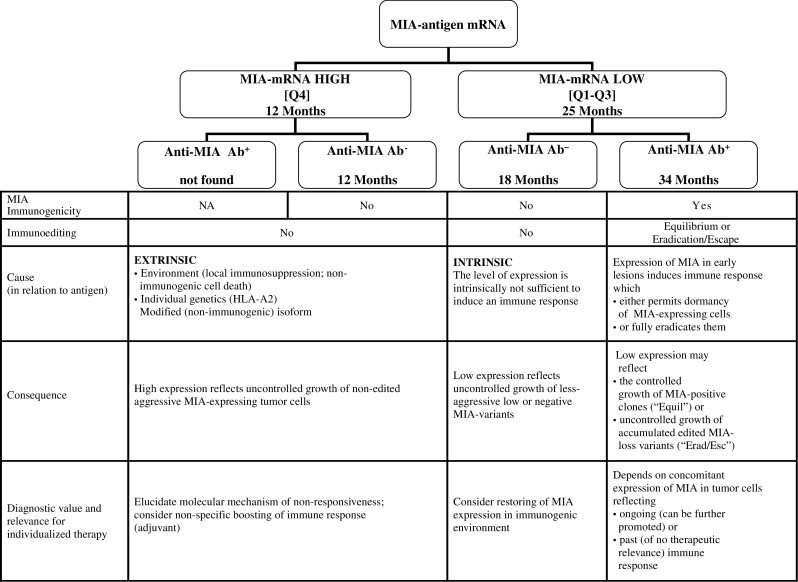
MIA-based prognostic algorithm and concept of immunoediting. This flow chart shows how viewing MIA mRNA expression levels in the context of immunogenicity (as determined by anti-MIA autoantibodies detected in sera) may enable prognostic stratification of the pancreatic cancer patients. An immunoediting-based interpretation of each antigen–antibody outcome signature is consequently attempted, pointing to the individualized therapeutic options
Failure to mount an anti-MIA response can also result from the intrinsic inability of tumor cells to express MIA in an immunogenic form or at a sufficient level for mounting an immune response. Indeed, tumor cell lines showed two-log differences in endogenous MIA expression (Fig. 3a). Clinically, low or modified expression of MIA conveys a less invasive phenotype, and therefore, patients bearing such variants might have higher chances of prolonged survival (median survival of 18 months).
The most intriguing scenario can be offered for the group of anti-MIA-seropositive patients. The fact that this group does not contain MIA overexpressers indicates completed immunoediting, e.g., possible eradication of aggressive MIAhigh tumor cells with accumulation and consequent escape of MIA-loss variants and such a track will explain a longer median, but not overall survival time by a lag phase in growth of edited MIA-loss variants prior to ultimate escape. Alternatively, sustained levels of low to moderate MIA expression may reflect a protracted equilibrium stage, meaning that immune control is not fully eradicating MIA expressers, but keeping them in a dormant state—a situation with the most promising therapeutic perspective. Although both situations yield better outcome (median survival of 34 months), the clinical relevance of detectable antibodies would differ: MIA antibodies would reflect past humoral response in the former case, but an ongoing response in the latter. Consequently, peptide vaccination in the equilibrium situation might promote immune control and further prolong survival. However, this strategy will be of no benefit for patients with MIA-loss variants.
A similar approach can be applied to explain data of a recently published study demonstrating that decreased expression of transgelin—also identified as an immunogen by our autologous SEREX screening—was associated with elevated levels of anti-transgelin antibodies, particularly in later stages, and with lower survival in patients with colorectal cancer [65]. By viewing tumor progression as a process in which immune responses alter the ‘non-edited’ antigenic repertoire of a tumor toward emergence of ‘edited’ antigen-loss variants, the postulate of immunoediting would allow two conclusions to be drawn from these data. First, transgelin is a potent early immunogen inducing a strong immune response leading to the eradication of transgelin-positive cells. Second, because this elimination is associated with shorter survival, transgelin might have antitumor properties, and accumulation of transgelin-loss variants would allow outgrowing of more aggressive tumor cells, thus worsening outcome. Indeed, recent studies identified transgelin as a tumor suppressor [66–68].
Thus, immunogenicity of tumor cell-associated molecules taken in the context of expression patterns might represent a suitable marker to monitor immunoediting in pancreatic cancer. To follow MIA (or annexin A2, or transgelin) expression during disease progression and to ascertain its location in early PanIN lesions and late malignant structures is necessary to deliver the final proof that immunoediting takes place in pancreatic cancer. As for therapeutic consequences, stimulating antigen-specific responses might profit non-edited patients, whereas restoring antigen expression in tumor cells or induction of bystander effects should be considered as a treatment option for immunoedited patients.
In this context, the composition of the immunogenic profile defined by SEREX deserves special attention. Some of the identified gene products have already been shown to be immunogenic in association with other malignant diseases (Table 1; [51–59]). Notably, proteins become immunogenic without any particular preference regarding cellular origin (tumor, acinar, stellate cells). We already stated that appearance of anti-PNLIPRP2 antibodies in patients with pancreatic diseases might reflect occasional release of PNLIPRP2 by dying acini in an immunogenic environment. Emergence of a pro-inflammatory milieu in association with acinar cell death was demonstrated in a previous study [47]. It is possible that immunogenic cell death (ICD) occurs in all patients, but leads to accidental generation of antibodies to different acini-specific molecules in different individuals. This hypothesis can also be extended to explain the emergence of antibodies against IFITM3 or other non-restrictive antigens. Humoral immune responses of this kind (in particular to intracellular or secreted antigens) will not be pathogenic, but indicative of ICD and of a pro-inflammatory environment that might boost immune responses against tumor cell-specific antigens [11, 69]. This would mean that the observed increase in the positive prognostic value achieved by combination of seropositivity against non-related antigens reflects a gain in overall immunogenicity of the tumor by a yet-unidentified mechanism.
The immunogenicity of stroma-associated antigens, for example periostin (Table 1, [50]) might similarly reflect ICD and additionally represent a novel target either for treating edited tumor antigen-loss variants by means of bystander eradication or for reducing the pro-malignant and therapy-inhibiting impact of stroma [49, 70–72].
In conclusion, our results indicate that the immunogenicity of potential antigens is increased, but a single immunogenic profile defined by autologous SEREX is not a common feature of pancreatic cancer. However, creation of signatures comprising antigens specific for acinar, stromal and tumor cells might provide a reliable antigen/antibody-based tool reflecting processes of ICD or immunoediting. Upon incorporation of knowledge concerning antigen function and relation to outcome, this procedure should allow prognostic stratification of patients and individual adjustment of immunotherapeutic approaches (Fig. 4). Further steps toward this goal include large-scale identification of potential immunogens using high-throughput seromics similar to those described by our recent study [41], studying the ability of these antigens to serve as T-cell (CTL and Th) targets, and a deeper understanding of molecular mechanisms driving cancer immunoediting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are very grateful to B. Bentzinger, I. Gosch, M. Meinhardt, K. Schneider and E. Soyka for excellent technical support. This work was supported in part by the Deutsches Bundesministerium für Bildung und Forschung (Grant 01GS08114 to N.G., who is responsible for the contents of this publication).
Footnotes
A. Heller and I. Zörnig contributed equally to this work.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Wong HH, Lemoine NR. Biological approaches to therapy of pancreatic cancer. Pancreatology. 2008;8(4–5):431–461. doi: 10.1159/000151536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz-Winnenthal FH, et al. High frequencies of functional tumor-reactive T-cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65(21):10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 4.Patwa TH, et al. The identification of phosphoglycerate kinase-1 and histone H4 autoantibodies in pancreatic cancer patient serum using a natural protein microarray. Electrophoresis. 2009;30(12):2215–2226. doi: 10.1002/elps.200800857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong SH, et al. Identification of a specific vimentin isoform that induces an antibody response in pancreatic cancer. Biomark Insights. 2006;1:175–183. [PMC free article] [PubMed] [Google Scholar]
- 6.Tomaino B, et al. Autoantibody signature in human ductal pancreatic adenocarcinoma. J Proteome Res. 2007;6(10):4025–4031. doi: 10.1021/pr070281a. [DOI] [PubMed] [Google Scholar]
- 7.Kubuschok B, et al. Naturally occurring T-cell response against mutated p21 ras oncoprotein in pancreatic cancer. Clin Cancer Res. 2006;12(4):1365–1372. doi: 10.1158/1078-0432.CCR-05-1672. [DOI] [PubMed] [Google Scholar]
- 8.Ho M, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11(10):3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 9.Tseng JF, et al. Patients undergoing treatment for pancreatic adenocarcinoma can mount an effective immune response to vaccinations. Pancreatology. 2005;5(1):67–74. doi: 10.1159/000084492. [DOI] [PubMed] [Google Scholar]
- 10.Hong SH. Identification of clp36 as a tumor antigen that induces an antibody response in pancreatic cancer. Cancer Res Treat. 2005;37(1):71–77. doi: 10.4143/crt.2005.37.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SH, et al. An autoantibody-mediated immune response to calreticulin isoforms in pancreatic cancer. Cancer Res. 2004;64(15):5504–5510. doi: 10.1158/0008-5472.CAN-04-0077. [DOI] [PubMed] [Google Scholar]
- 12.Johnston FM, et al. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin Cancer Res. 2009;15(21):6511–6518. doi: 10.1158/1078-0432.CCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer. 2005;5(6):459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 14.Laheru D, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepisto AJ, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–964. [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan RK, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–264. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto K, et al. Muc1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25(5):3575–3579. [PubMed] [Google Scholar]
- 18.Kyte JA. Cancer vaccination with telomerase peptide gv1001. Expert Opin Investig Drugs. 2009;18(5):687–694. doi: 10.1517/13543780902897631. [DOI] [PubMed] [Google Scholar]
- 19.Anichini A, et al. The paradox of T-cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma. Cancer Immunol Immunother. 2004;53(10):855–864. doi: 10.1007/s00262-004-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 21.Kyte JA, et al. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer Immunol Immunother. 2009;58(10):1609–1626. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koebel CM, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 23.Teng MW, et al. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol. 2008;84(4):988–993. doi: 10.1189/jlb.1107774. [DOI] [PubMed] [Google Scholar]
- 24.Dunn GP, et al. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 25.Reuschenbach M, et al. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58(10):1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saffroy R, et al. Clinical significance of circulating anti-p53 antibodies in European patients with hepatocellular carcinoma. Br J Cancer. 1999;79(3–4):604–610. doi: 10.1038/sj.bjc.6690095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gansauge S, et al. The role of anti-p53-autoantibodies in pancreatic disorders. Int J Pancreatol. 1996;19(3):171–178. doi: 10.1007/BF02787365. [DOI] [PubMed] [Google Scholar]
- 28.Jager E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187(2):265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller M, et al. Testing for anti-p53 antibodies increases the diagnostic sensitivity of conventional tumor markers. Int J Oncol. 2006;29(4):973–980. [PubMed] [Google Scholar]
- 30.Sahin U, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92(25):11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tureci O, et al. Identification of tumor-associated autoantigens with SEREX. Methods Mol Med. 2005;109:137–154. doi: 10.1385/1-59259-862-5:137. [DOI] [PubMed] [Google Scholar]
- 32.Jager D, et al. Identification of tumor antigens as potential target antigens for immunotherapy by serological expression cloning. Cancer Immunol Immunother. 2004;53(3):144–147. doi: 10.1007/s00262-003-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scanlan MJ, et al. Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immunol. 2001;1:4. [PubMed] [Google Scholar]
- 34.Nakatsura T, et al. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281(4):936–944. doi: 10.1006/bbrc.2001.4377. [DOI] [PubMed] [Google Scholar]
- 35.Okada T, et al. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin Cancer Res. 2006;12(1):191–197. doi: 10.1158/1078-0432.CCR-05-1206. [DOI] [PubMed] [Google Scholar]
- 36.Okada T, et al. Immune responses to DNA mismatch repair enzymes hMSH2 and hPMS1 in patients with pancreatic cancer, dermatomyositis and polymyositis. Int J Cancer. 2005;116(6):925–933. doi: 10.1002/ijc.21118. [DOI] [PubMed] [Google Scholar]
- 37.Antwi K, et al. Proteomic identification of an MHC-binding peptidome from pancreas and breast cancer cell lines. Mol Immunol. 2009;46(15):2931–2937. doi: 10.1016/j.molimm.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Antwi K, et al. Analysis of the plasma peptidome from pancreas cancer patients connects a peptide in plasma to overexpression of the parent protein in tumors. J Proteome Res. 2009;8(10):4722–4731. doi: 10.1021/pr900414f. [DOI] [PubMed] [Google Scholar]
- 39.Kubuschok B, et al. Expression of cancer testis antigens in pancreatic carcinoma cell lines, pancreatic adenocarcinoma and chronic pancreatitis. Int J Cancer. 2004;109(4):568–575. doi: 10.1002/ijc.20006. [DOI] [PubMed] [Google Scholar]
- 40.Wadle A, et al. Serological immune response to cancer testis antigens in patients with pancreatic cancer. Int J Cancer. 2006;119(1):117–125. doi: 10.1002/ijc.21744. [DOI] [PubMed] [Google Scholar]
- 41.Gnjatic S, et al. Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad Sci USA. 2010;107(11):5088–5093. doi: 10.1073/pnas.0914213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao F, et al. Identification of a novel murine pancreatic tumour antigen, which elicits antibody responses in patients with pancreatic carcinoma. Immunology. 2009;128(1):134–140. doi: 10.1111/j.1365-2567.2009.03090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jager D, et al. Identification of a tissue-specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res. 2001;61(5):2055–2061. [PubMed] [Google Scholar]
- 44.Jager E, et al. Identification of NY-ESO-1 epitopes presented by human histocompatibility antigen (HLA)-DRB4*0101–0103 and recognized by CD4(+) T lymphocytes of patients with NY-ESO-1-expressing melanoma. J Exp Med. 2000;191(4):625–630. doi: 10.1084/jem.191.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stockert E, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998;187(8):1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sehr P, et al. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253(1–2):153–162. doi: 10.1016/S0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 47.Bartel M, et al. Abnormal crosstalk between pancreatic acini and macrophages during the clearance of apoptotic cells in chronic pancreatitis. J Pathol. 2008;215(2):195–203. doi: 10.1002/path.2348. [DOI] [PubMed] [Google Scholar]
- 48.Ceyhan GO, et al. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136(1):177–186. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Koninger J, et al. Overexpressed decorin in pancreatic cancer: potential tumor growth inhibition and attenuation of chemotherapeutic action. Clin Cancer Res. 2004;10(14):4776–4783. doi: 10.1158/1078-0432.CCR-1190-03. [DOI] [PubMed] [Google Scholar]
- 50.Erkan M, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132(4):1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Shukla S, et al. Immunoproteomics reveals that cancer of the tongue and the gingivobuccal complex exhibit differential autoantibody response. Cancer Biomark. 2009;5(3):127–135. doi: 10.3233/CBM-2009-0604. [DOI] [PubMed] [Google Scholar]
- 52.Brichory FM, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci USA. 2001;98(17):9824–9829. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura H, et al. Antibodies against hepatoma-derived growth factor and mucosal repair in ulcerative colitis. J Gastroenterol. 2002;37(Suppl 14):8–14. doi: 10.1007/BF03326407. [DOI] [PubMed] [Google Scholar]
- 54.Zhong X, et al. Construction of human liver cancer vascular endothelium cDNA expression library and screening of the endothelium-associated antigen genes. World J Gastroenterol. 2004;10(10):1402–1408. doi: 10.3748/wjg.v10.i10.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mooney CJ, et al. Identification of autoantibodies elicited in a patient with prostate cancer presenting as dermatomyositis. Int J Urol. 2006;13(3):211–217. doi: 10.1111/j.1442-2042.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 56.Shi YY, et al. Identification and analysis of tumour-associated antigens in hepatocellular carcinoma. Br J Cancer. 2005;92(5):929–934. doi: 10.1038/sj.bjc.6602460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka Y, et al. Proteomic surveillance of autoantigens in relapsing polychondritis. Microbiol Immunol. 2006;50(2):117–126. doi: 10.1111/j.1348-0421.2006.tb03776.x. [DOI] [PubMed] [Google Scholar]
- 58.Avrameas S, et al. Natural antibodies against tubulin, actin myoglobin, thyroglobulin, fetuin, albumin and transferrin are present in normal human sera, and monoclonal immunoglobulins from multiple myeloma and Waldenstrom’s macroglobulinemia may express similar antibody specificities. Ann Immunol (Paris) 1981;132C(2):231–236. doi: 10.1016/0769-2625(81)90031-3. [DOI] [PubMed] [Google Scholar]
- 59.Servettaz A, et al. Identification of target antigens of antiendothelial cell antibodies in healthy individuals: a proteomic approach. Proteomics. 2008;8(5):1000–1008. doi: 10.1002/pmic.200700794. [DOI] [PubMed] [Google Scholar]
- 60.El Fitori J, et al. Melanoma inhibitory activity (MIA) increases the invasiveness of pancreatic cancer cells. Cancer Cell Int. 2005;5(1):3. doi: 10.1186/1475-2867-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt J, Bosserhoff AK. Processing of MIA protein during melanoma cell migration. Int J Cancer. 2009;125(7):1587–1594. doi: 10.1002/ijc.24508. [DOI] [PubMed] [Google Scholar]
- 62.Hau P, et al. Expression levels of melanoma inhibitory activity correlate with time to progression in patients with high-grade glioma. Oncol Rep. 2004;12(6):1355–1364. [PubMed] [Google Scholar]
- 63.Bosserhoff AK, Buettner R. Expression, function and clinical relevance of MIA (melanoma inhibitory activity) Histol Histopathol. 2002;17(1):289–300. doi: 10.14670/HH-17.289. [DOI] [PubMed] [Google Scholar]
- 64.Aung PP, et al. Systematic search for gastric cancer-specific genes based on SAGE data: melanoma inhibitory activity and matrix metalloproteinase-10 are novel prognostic factors in patients with gastric cancer. Oncogene. 2006;25(17):2546–2557. doi: 10.1038/sj.onc.1209279. [DOI] [PubMed] [Google Scholar]
- 65.Peng J, et al. A rat-to-human search for proteomic alterations reveals transgelin as a biomarker relevant to colorectal carcinogenesis and liver metastasis. Electrophoresis. 2009;30(17):2976–2987. doi: 10.1002/elps.200900203. [DOI] [PubMed] [Google Scholar]
- 66.Zhao L, et al. Transgelin as a suppressor is associated with poor prognosis in colorectal carcinoma patients. Mod Pathol. 2009;22(6):786–796. doi: 10.1038/modpathol.2009.29. [DOI] [PubMed] [Google Scholar]
- 67.Prasad PD, et al. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res. 2010;339(2):337–347. doi: 10.1007/s00441-009-0902-y. [DOI] [PubMed] [Google Scholar]
- 68.Assinder SJ, et al. Transgelin: an actin-binding protein and tumour suppressor. Int J Biochem Cell Biol. 2009;41(3):482–486. doi: 10.1016/j.biocel.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9(5):353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olive KP, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang B, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang B, et al. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118(4):1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


