Abstract
Alpha-Fetoprotein (AFP) is produced principally in fetal liver, gastrointestinal tract and the yolk sac which is temporarily present during embryonic development. AFP is overexpressed in the majority of Hepatocellular Carcinoma (HCC) and thus offers an attractive target for immunotherapy against this neoplasm. Here, we report that anti-HCC effects were achieved in a therapeutic setting with a DNA vaccine encoding mouse AFP and co-expressing Heat Shock Protein 70 (HSP70) gene. We also demonstrated that this vaccine elicited a marked and highly effective AFP specific CTL response against AFP-positive target cells. This vaccine also induced the prolongation of life span in mice bearing the tumor and the eradication of HCC. It is anticipated that vaccine strategies such as this may contribute to the effective future treatment of Hepatocellular Carcinoma.
Keywords: DNA vaccine, Hepatocellular carcinoma, Alpha-fetoprotein, Heat shock protein 70
Introduction
Hepatocellular Carcinoma (HCC) is one of the most common and devastating malignancies in China. This is due in part to its high mutation rate, effective immune escape, poor antigenicity and abundant blood supply. In fact, more than 120,000 people die of HCC every year in China which represents 44% of the annual number of deaths from this disease worldwide. Multiple treatment modalities which have traditionally been applied for the treatment of HCC, include surgery, cryosurgery, percutaneous ethanol injection, radiofrequency thermal ablation, arterial embolization, chemoembolization and systemic chemotherapy; however, unfortunately none of these therapies have significantly improved the poor prognosis of patients with unresectable HCC. Therefore, novel therapeutic strategies for the treatment of HCC are being developed, including gene and immunotherapies which could serve as a prospective auxiliary therapy for unresectable HCC and HCC cells in circulation.
Specific CD8+CTLs play a major role in anti-tumor immunity induced by DNA vaccines that can overcome immunological tolerance against tumor self-antigens. DNA vaccines in both prophylactic and therapeutic settings have made considerable progress in evoking anti-tumor response and some of them are being tested and applied in clinic trials [1]. Since tumor associated antigens (TAAs) can potentially induce immunity, they have been investigated to a considerable extent. There were some successful investigations which demonstrated that immunity induced by targeting TAA could suppress tumor growth and metastases [2].
The majority of HCC overpress α-fetoprotein (AFP), a glycoprotein produced at high levels by fetal liver, which is transcriptionally repressed after birth but produced at low serum levels throughout a lifetime. Measurements of serum AFP play an important role in diagnosis as well as screening for recurrence and monitoring for the prognosis of HCC. Some of these studies suggested that AFP could offer an excellent target for anti-hepatocellular carcinoma therapy since it is frequently expressed at high levels during this disease. Vollmer et al. [3] reported that mAFP can serve as a target for cell-mediated immune responses. Their experiments demonstrated that both AFP-transduced dendritic cells (DCs) and immunization with AFP plasmids could generate effective T-cell immune responses in vivo. Grimm et al. used the gene gun for intramuscular co-immunizations with DNA expression vectors encoding mAFP together with plasmids encoding IL-12, GM-CSF, and IL-18. Their data showed that such DNA vaccines could induce CTL activity against mAFP in different mouse strains with some mice also developing anti-mAFP antibody responses. These findings also suggested that a DNA vaccine against mAFP could break immunological ignorance. Therefore, these results are consistent with observations made with those made with many other self antigens [4–7]. In fact, Bei et al. [8, 9] found that patients’ immunoglobulins could recognize linear protein epitopes that were cryptic in the native protein as demonstrated by their restricted reactivity with denatured, deglycosylated AFP. Butterfield et al. [10] identified some epitopes of human AFP that could be recognized by the human T cell repertoire which also recognized some epitopes of murine AFP. This did indeed amplify immunotherapeutic targeting of AFP. Thus, Hanke et al. [11] reported that DNA vaccination with an AFP-expressing plasmid protected mice from growth of subcutaneous AFP-expressing tumor cells and did not interfere with liver regeneration in mice. These data also suggested that AFP-specific DNA vaccination represents a useful and safe tool to inhibit growth of AFP-expressing tumors in mice. Consequently DNA vaccination targeting AFP could also be developed for the immunotherapy of HCC.
Research on tumor immunity recently suggested that molecular chaperones may play an important role in antigen presentation [12]. Thus, Todryk et al. reported that HSP70 could induce T cells and stimulated macrophages and DCs to infiltrate tumors and produce cytokines such as IFN-γ, TNF-α and IL-12. This, in turn, enhanced anti-tumor immunity primed by T cells [13]. It has also been demonstrated that HSP strongly enhanced immune reactions against tumor-associated antigens [13, 14] and induced specific CTL responses suppressing tumor growth and virus infection. [15, 16] Moreover, HSP70 was reported to capture immature APC and enhance their ability to capture antigen [17]. In fact, HSP70 secreted by dead tumor cells could be directly absorbed by DC. This finding implied that antigens accompanied by chaperones were taken up by DCs processed, and then effectively presented to the TCR. HSP70 could also be demonstrated on the surface of tumor cells and stimulated antigen to be recognized by TCR or TCRγδ [18–20]. This finding implies that HSP70 could activate anti-tumor immunity against a target antigen. In this regard, many experiments with fusion vaccines of HSP70 with other antigens showed that HSP70 could enhance both cellular and humoral immunity in mice against antigens fused to HSP70. Thus, it is important to realize that HSP70 can enhance specific CTLs and increase their level of IFN-γ [21–23].
Here, we hypothesized that a DNA vaccine encoding mouse AFP could induce specific CTL activity against AFP positive HCC. Furthermore, we also hypothesized that the chaperone of mycobacterium-tuberculosis i.e., heat shock protein 70 (Mt.HSP70), could help to overcome peripheral T cell tolerance and enhance AFP specific T cell immune responses to the point where they could inhibit growth of Hepatocellular carcinoma in mice.
Materials and methods
Animals and cell lines
C57BL/6J mice, 6–8 weeks of age, were purchased from the Animal facility of Chongqing University of Medical Science. All animal experiments were performed according to the Chinese National Academy Guidelines used for the Care and Use of Laboratory Animals
The murine liver cancer cell line Hepa1-6 was a present from Prof. Ya-jun Guo, Institute for Tumor of the Second Military Medical University, P.R.C. The murine lymphoma cell line EL4 was stored at our Institute for Viral Hepatitis, Chongqing Medical University, P.R.C.
Vector construction and protein expression
The full-length cDNA of murine AFP (1.8 Kb) was amplified by RT-PCR (Promega, USA) from total RNA extracted from the Hepal-6 cell line and cloned into the eukaryotic expression plasmid pcDNA3.1 (Invitrogen CA, USA) in which the AFP-cDNA was transcribed under the control of the cytomegalovirus promoter/enhancer. HSP70 was amplified from a plasmid we constructed previously [24], and fused to the AFP gene without a stop codon and with a G-S-G-G-S linker. All plasmids were confirmed by restriction enzyme digestion and DNA sequencing.
To demonstrate protein expression of the recombinant plasmids in eukaryotic cells, the plasmids were transfected into COS-7 cells with Lipofectamine 2000 (Invitrogen CA, USA) and the protein expression of these plasmids was detected by RT-PCR and immunocytochemical staining with mAbs following the manufacturer’s instruction. Control cells were stained with the same antibodies.
Preparation of target cells
The H-2b-positive murine mastocytoma cell line EL4 was cultured in RPMI 1640 medium, supplemented with 10% (V/V) fetal calf serum. EL4 cells were transfected with pcDNA3.1-neo and pcDNA3.1-AFP by electroporation (Amaxa, Germany) and maintained in medium containing 500 μg/ml G418. The AFP expression of these cells was confirmed by immunofluorescence, Western blotting and immunohistochemistry.
Intramuscular vaccination and tumor cell challenge
A total of 4 × 106 Hepa1-6 cells was collected, washed three times with PBS and suspended in 100 μL PBS. This cell suspension was injected s.c. into the right axilla of mice and developing tumors were measured with a slide gage. When tumors reached an average volume that was comparable within mice of experimental groups, DNA immunizations were performed. Tumor volume was estimated by using the formula VT (mm3) = L×W 2/2, where VT is the estimated tumor volume, and L and W represent the length (mm) and width (mm) of transplanted tumor, respectively. Recombinant plasmids were purified with the plasmid Maxi Kits (Qiagen, Valencia, CA, USA), solubilized in PBS and adjusted to a concentration of 1 mg/μl. DNA immunizations were performed by injecting plasmids intramuscularly at five different sites of C57BL/J mice (n = 8) in a final volume of 100 μl 0.25% Bupivacaine and 0.1% Methyl p-hydroxybenzoate. Naked DNA (100 μg) was injected into the same intramuscular sites 3 days after pretreatment. Booster immunizations were performed 1 month after the first immunization with the same amount of DNA plasmid.
In vitro cytotoxicity assay
Splenocytes obtained from mice 14 days after the last of three immunizations were isolated and purified by liquid density gradient centrifugation in a lymphocyte separating medium. Both, EL4 and EL4(AFP) cells were inactivated by Mitomycin C and added to splenocytes at a ratio of 1:4. This mixture of effector cells was cultivated for 1 week in a medium containing 50 μM 2-ME 5 μg/ml Con A and 10 U/ml of rhIL-2. This culture medium was supplemented daily with 10 U/ml rhIL-2. Effector cells were incubated with EL-4 or EL4(AFP) target cells at an E/T ratio of 50:1 or 25:1. Twelve hours later, the supernatant of these cell suspensions was collected and assayed for LDH as recommended by the manufacturer (Boehringer Mannheim). Absorbance of the supernatant was measured at 490 and 630 nm. Percent of cytotoxicity was calculated by substituting the absorbance of supernatants in tissue culture wells into the following equation: [(effector-target cell mix−effector control)−low control]/(High control−low control) × 100.
ELISA
Splenocytes (5 × 105/well) derived from immunized mice were re-stimulated for 48 h by admixture with either 105 EL4 or EL4(AFP) cells per well which had been previously inactivated by ultrasound and the supernatants collected for detection of IL-4 or IFN-γ by ELISA as per instructions of the manufacturer (Dilon). The regression equation for IFN-γ was obtained by ANOVA of standard samples. IFN-γ content (pg/ml) of all groups of mice was calculated by this regression equation.
Statistical analysis
The statistical significance of differential finding between experimental groups of mice and controls was determined by Student’s t test and considered significant if two-tailed P values were P < 0.005
Results
Plasmid construction and protein expression
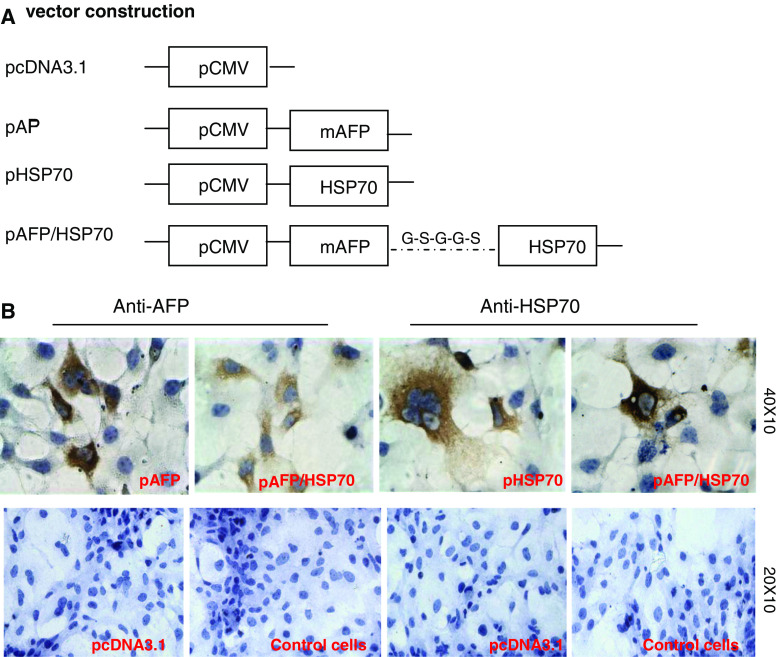
To test our hypothesis that AFP and HSP70 fusion vaccine can induce anti-hepatocellular carcinoma effects, we constructed three eukaryotic expression vectors based on the pcDNA3.1 vector backbone encoding either pcDNA3.1-AFP, pcDNA3.1-HSP70 or pcDNA3.1-AFP-HSP70 (Fig. 1a). All plasmids were coincident with the corresponding sequences in the Genebank by DNA sequencing. Immunocytochemical analyses indicated that there was AFP or HSP70 protein expression as indicated by brown particles in COS-7 cells transfected with pcDNA3.1-AFP-HSP70 by using either anti-AFP or anti-HSP70 as the first antibody. However, these analyses proved negative in COS-7 cells treated only with pcDNA3.1 devoid of plasmids (Fig. 1b). These data demonstrate that plasmids harboring the AFP and HSP70 genes could be expressed in eukaryotic cells in vivo.
Fig. 1.
a Schematic diagram depicting vaccination vectors. Three expression vectors were constructed encoding either full-length murine AFP, HSP70, or both based on the pcDNA3.1 plasmid backbone. b Protein expressions of mAFP and HSP70 were verified by immunocytochemical staining. The positive cells that were transfected with pAFP, pHSP70 or pAFP/HSP70 plasmids are shown in brown
Immunization with the mAFP/HSP70 vaccine evoked AFP specific CTLs
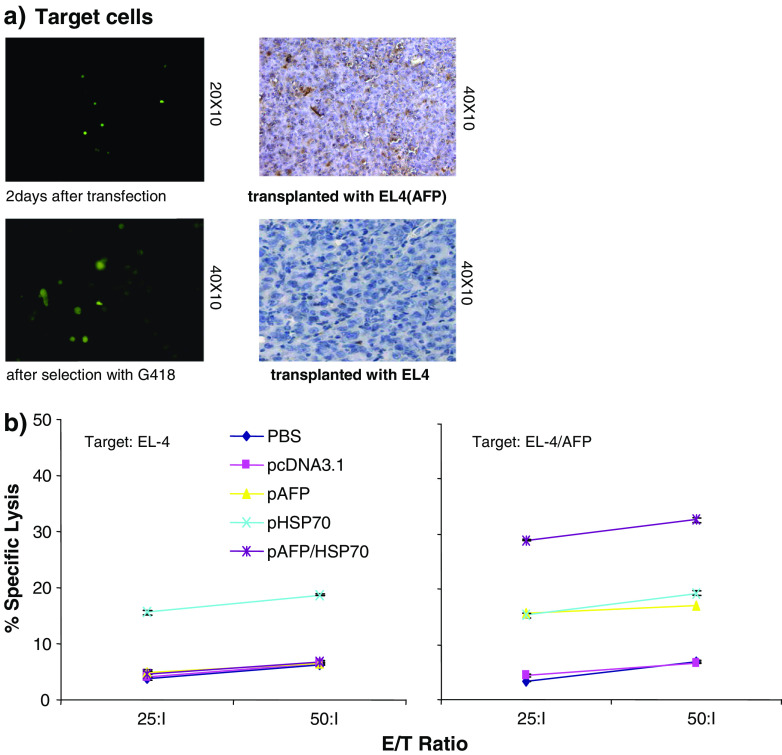
In order to assess whether CD8 T cells are able to specifically react against AFP positive cells, we generated AFP-positive EL4 target cells—EL4(AFP). These were prepared by transfecting pcDNA3.1-AFP into EL4 cells. Such transfected cells were propagated in medium with G418 and confirmed by immunofluorescence (Fig. 2a, left panel). Simultaneously, EL4(AFP) cells were injected s.c. into mice to establish a solid tumor. Immunohistochemical analysis of EL4(AFP) tumor section was performed to detect AFP expression and the results indicated that AFP expression was positive in the majority of EL4(AFP) cells (Fig. 2a, right panel).
Fig. 2.
AFP specific cytotoxic T cell response is induced by the AFP/HSP70 vaccine. a AFP positive EL4 target cells were transfected with pcDNA3.1-AFP, and selected with G418 (right panel). AFP expression on EL4 cells was confirmed by immunohistochemical staining (left panel). b A T cell-specific cytotoxity assay against AFP-positive EL4 target cell was performed. Splenocytes, enriched for CD8+ cells were isolated from vaccinated mice 14 days after immunization. A LDH release assay was performed at different effector-to-target cell ratios with splenocytes being re-stimulated with either inactivated EL4 or AFP+EL4 cells for 1 week, while EL4 or AFP+EL4 cells served as target cells
An in vitro cytotoxicity assay was performed to ascertain the CTL activity of splenocytes isolated from immunized mice. This CTL activity was found to be significantly different (P < 0.001) between groups of mice immunized with pcDNA3.1-AFP-HSP70 when targeting EL4(AFP) or EL4 when compared to control groups (PBS, pcDNA3.1, pcDNA3.1-AFP or pcDNA3.1-HSP70). Moreover, the CTL activity increased with increasing E/T ratio (P < 0.001) (Fig. 2b). These results demonstrate that the CTL activity obtained was AFP specific and that HSP70 could enhance their efficacy.
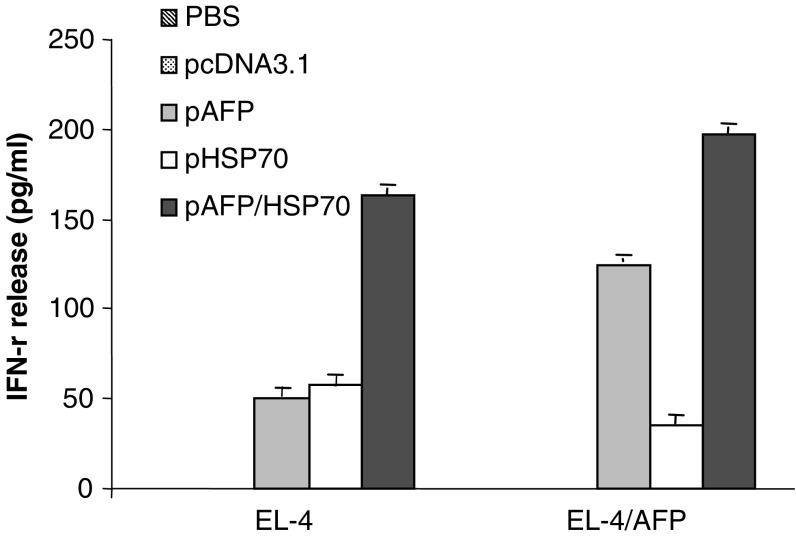
In addition, ELISA assays were performed to detect cytokine- IL-4 and IFN-γ release from splenocytes derived from immunized mice which were re-stimulated by either EL4 or EL4(AFP). The IFN-γ release was markedly up-regulated in the pcDNA3.1-AFP-HSP70 immunized group of mice when compared with mice immunized only with pcDNA3.1-AFP or pcDNA3.1-HSP70 (P < 0.05) (Fig. 3). There was no IL-4 release observed in the supernatant of splenocytes obtained from all experimental groups (data not shown). Together, these data indicate that the specific IFN-γ release was more strongly induced in mice immunized with a plasmid harboring AFP than in mice immunized with a plasmid lacking AFP expression. Moreover, HSP70 could markedly enhance the release of IFN-γ.
Fig. 3.
ELISA analyses detect IFN- γ release. The supernatants of splenocytes obtained from mice of either treatment group or control groups of mice were collected after using EL4 or AFP+EL4 as stimulator cells. The mean concentration (ng/ml) of each group is shown (mean + SD)
The mAFP/HSP70 vaccine suppresses growth of Hepal-6 hepatocellular carcinoma
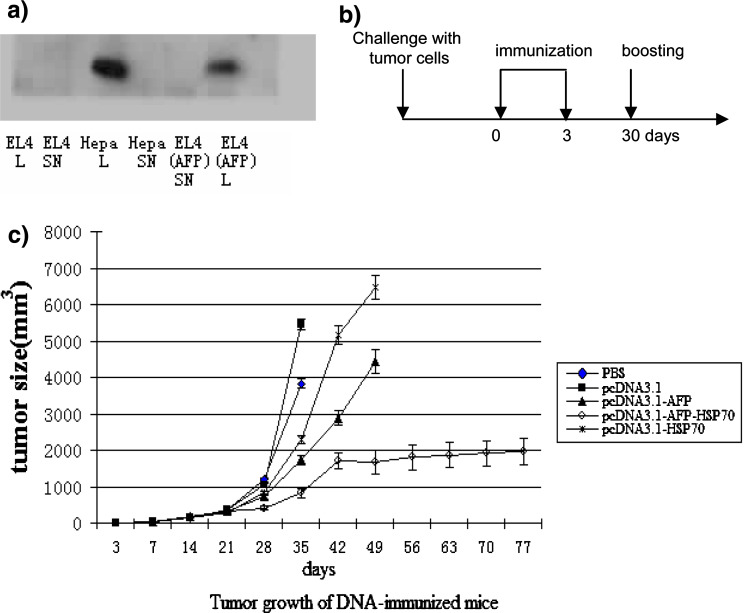
Groups of mice (n = 7) developed tumors in the right auxiliary 4 days after being injected s.c. with Hepal-6 tumor cells that strongly expressed AFP (Fig. 4a, left panel). All such mice were immunized with either recombinant plasmids or PBS 7d after this tumor cell challenge. At this time point the tumors had reached an average volume of 32 mm3 and were comparable in size in mice within all experimental groups. Immunizations were administered twice on days 10 and 40 and boosted once on day 30 after tumor cell challenge and tumor sizes were measured by a slide gage (Fig. 4a, right panel).
Fig. 4.
a The strong expression of AFP on Hepal-6 hepatocellular carcinoma cells was detected by Western blotting (L cell lyste and SN culture supernatant). b The immunization schedule consisted of three vaccinations after tumor cell challenge in a therapeutic model. c Tumor growth was analyzed in mice challenged s.c. with 4 × 106 hepal-6 tumor cells prior to immunization in each treatment or control groups
There was no significant difference (P > 0.05) in tumor size observed among all groups of mice until after day 21. This lack of difference in tumor size was also observed in mice administered on day 28 with either PBS or pcDNA3.1. However, tumor volume of mice immunized with pcDNA3.1-AFP-HSP70 was markedly reduced on day 35 (P < 0.05) when compared with groups of mice immunized with either pcDNA3.1-AFP, pcDNA3.1-HSP70 or PBS. On day 42, only mice immunized with pcDNA3.1-AFP or pcDNA3.1-HSP70 or pcDNA3.1-AFP-HSP70 were able to survive. Furthermore, the group of mice immunized with pcDNA3.1-AFP-HSP70 did survive until day 80 after tumor cell challenge. In contrast, mice immunized with only PBS began to die on day 35 and none of these animals survived beyond day 42. Mice immunized with the empty vector (pcDNA3.1) began to die on day 35 and all the mice within this group were dead by day 60. The group of mice immunized with pcDNA3.1-AFP began to die on day 49 and all animals in this group were dead on day 60. Mice injected with pcDNA3.1-HSP70 started to die on day 35 and the last animal expired on day 49. In contrast, mice immunized with pcDNA3.1-AFP-HSP70 began to die on day 49, but there were still 57% of these mice alive on day 80. A tendency of prolonged survival time is evident from these data which indicate that DNA vaccines harboring AFP and/or HSP70 could prolong the life span of tumor bearing mice and HSP70 indeed could indeed markedly enhance this anti-tumor efficacy.
Discussion
Fluctuation in AFP levels of mice is similar to that observed among humans. In fact, the murine immune system was exposed throughout life to AFP at even higher levels than that in humans. The question arises whether the T-cell repertoire of AFP has been deleted of such self-reactive clones just as predicted by Burnet’s clonal deletion theory. In this regard, Grimm et al. [25] reported that mAFP-specific mRNA was not detectable in the thymus of newborn mice. These data exclude the possibility that peptides derived from mAFP synthesized in the fetal liver are presented to the immune system in the thymus in a tolerogenic manner. This, in turn, suggests that T cells with receptors of low to intermediate affinity for mAFP have evaded central thymic deletion. Although T-cell clones can be activated under suitable conditions, it is not easy to activate T cell receptors for AFP. Thus, it is essential to develop novel, effective and economical strategies that can break immunologic tolerance. It is indeed worthwhile to investigate how one can enhance the antitumor effects of DNA vaccines.
In this regard, we demonstrated that a chimeric DNA vaccine encoding mouse AFP and chaperon HSP70 could overcome peripheral T cell tolerance to AFP and induce a robust AFT specific CTL response that inhibited Hepatocellular carcinoma (Hepal-6) growth and increased life-span of tumor bearing, syngeneic C57BL/6J mice in a therapeutic setting. Our data suggest that IFN-γ secreted by splenocytes of mice that were immunized with the plasmids encoding pcDNA3.1-AFP-HSP70 was markedly increased after two vaccinations and one boost when compared to IFN-γ release by splenocytes of mice immunized only with control vectors. More importantly, IFN-γ secreted by splenocytes stimulated by inactivated EL4 cells with AFP expression was increased dramatically compared to splenocytes being stimulated by inactivated EL4 cells that strongly express AFP induced by transfection with an AFP expression vector. However, there was no difference between splenocytes that were stimulated by either inactivated EL4 or EL4 (AFP) in mice immunized with pcDNA3.1-HSP70. In fact, this finding of an AFP specific IFN-γ release indicated that AFP specific T cells were indeed activated by our fusion vaccine. Furthermore, it has been demonstrated that the capacity to specifically kill tumor cells was induced in splenocytes isolated from mice that had been immunized with fusion plasmids. Cytotoxicity of T cells which originated from splenocytes of mice immunized with pcDNA3.1-AFP or pcDNA3.1-AFP-HSP70 against AFP+ EL4 was achieved, but no cytotoxic activity was observed against AFP- EL4 target cells. These data suggest that an immune response was activated by a recombinant DNA vaccine encoding AFP and HSP70, which assisted in the T cell priming phase, Furthermore, DNA vaccination with a plasmid encoding AFP could break immunologic tolerance against AFP. Consequently, the AFP and HSP70 DNA fusion vaccines could suppress growth of AFP positive tumor cells and prolong the survival time of mice bearing such tumors. Therefore, the DNA vaccine encoding both AFP and HSP70 is likely the best of these vaccine strategies.
In summary, a DNA fusion vaccine encoding both AFP and HSP70 did break immunologic tolerance against AFP and induced both AFP specific IFN-γ release and cytotoxic activity of T cell obtained from immunized mice. This combination vaccine produced a robust immunotherapeutic effect which suppressed the growth of transplanted tumors in syngeneic mice. Our finding strongly suggests that DNA vaccination targeting AFP, boosted by HSP70 as an adjuvant could be a useful prospective strategy for the treatment of HCC.
Abbreviations
- HCC
Hepatocellular carcinoma
- AFP
α-Fetoprotein
- TAAs
Tumor associated antigens
- HSP70
Heat shock protein 70
Footnotes
Ying-hua Lan and Yong-guo Li contributed equally to this work.
References
- 1.Miller AM, Ozenci V, Kiessling R, Pisa P. Immune monitoring in a phase 1 trial of a PSA DNA vaccine in patients with hormone-refractory prostate cancer. J Immunother. 2005;28:389–395. doi: 10.1097/01.cji.0000165353.19171.41. [DOI] [PubMed] [Google Scholar]
- 2.Qin H, Zhou C, Wang D, Ma W, Liang X, Lin C, Zhang Y, Zhang S. Enhancement of antitumour immunity by a novel chemotactic antigen DNA vaccine encoding chemokines and multiepitopes of prostate-tumour-associated antigens. Immunology. 2006;117:419–430. doi: 10.1111/j.1365-2567.2006.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vollmer CM, Jr, Eilber FC, Butterfield LH, Ribas A, Dissette VB, Koh A, Montejo LD, Lee MC, Andrews KJ, McBride WH, Glaspy JA, Economou JS. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064–3067. [PubMed] [Google Scholar]
- 4.Brasseur F, Marchand M, Vanwijck R, Herin M, Lethe B, Chomez P, Boon T. Human gene MAGE-1, which codes for a tumor-rejection antigen, is expressed by some breast tumors. Int J Cancer. 1992;52:839–841. doi: 10.1002/ijc.2910520528. [DOI] [PubMed] [Google Scholar]
- 5.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- 6.Hodge JW. Carcinoembryonic antigen as a target for cancer vaccines. Cancer Immunol Immunother. 1996;43:127–134. doi: 10.1007/s002620050313. [DOI] [PubMed] [Google Scholar]
- 7.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bei R, Budillon A, Reale MG, Capuano G, Pomponi D, Budillon G, Frati L, Muraro R. Cryptic epitopes on alpha-fetoprotein induce spontaneous immune responses in hepatocellular carcinoma, liver cirrhosis, and chronic hepatitis patients. Cancer Res. 1999;59:5471–5474. [PubMed] [Google Scholar]
- 9.Bei R, Masuelli L, Moriconi E, Visco V, Moretti A, Kraus MH, Muraro R. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene. 1999;18:1267–1275. doi: 10.1038/sj.onc.1202442. [DOI] [PubMed] [Google Scholar]
- 10.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134–3142. [PubMed] [Google Scholar]
- 11.Hanke P, Serwe M, Dombrowski F, Sauerbruch T, Caselmann WH. DNA vaccination with AFP-encoding plasmid DNA prevents growth of subcutaneous AFP-expressing tumors and does not interfere with liver regeneration in mice. Cancer Gene Ther. 2002;9:346–355. doi: 10.1038/sj.cgt.7700445. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Curr Opin Immunol. 1994;6:728–732. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 13.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 14.Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/S0264-410X(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JJ. Therapeutic cancer vaccines: using unique antigens. Proc Natl Acad Sci U.S.A. 2004;101(Suppl 2):14653–14656. doi: 10.1073/pnas.0404839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoos A, Levey DL. Vaccination with heat shock protein-peptide complexes: from basic science to clinical applications. Expert Rev Vaccines. 2003;2:369–379. doi: 10.1586/14760584.2.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 18.Wei YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother. 1995;40:73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massa C, Guiducci C, Arioli I, Parenza M, Colombo MP, Melani C. Enhanced efficacy of tumor cell vaccines transfected with secretable hsp70. Cancer Res. 2004;64:1502–1508. doi: 10.1158/0008-5472.CAN-03-2936. [DOI] [PubMed] [Google Scholar]
- 20.Wang MH, Grossmann ME, Young CY. Forced expression of heat-shock protein 70 increases the secretion of Hsp70 and provides protection against tumour growth. Br J Cancer. 2004;90:926–931. doi: 10.1038/sj.bjc.6601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- 22.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, Wu TC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
- 23.Kim JW, Hung CF, Juang J, He L, Kim TW, Armstrong DK, Pai SI, Chen PJ, Lin CT, Boyd DA, Wu TC. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther. 2004;11:1011–1018. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 24.Peng M, Chen M, Ling N, Xu H, Qing Y, Ren H. Novel vaccines for the treatment of chronic HBV infection based on mycobacterial heat shock protein 70. Vaccine. 2006;24:887–896. doi: 10.1016/j.vaccine.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 25.Grimm CF, Ortmann D, Mohr L, Michalak S, Krohne TU, Meckel S, Eisele S, Encke J, Blum HE, Geissler M. Mouse alpha-fetoprotein-specific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology. 2000;119:1104–1112. doi: 10.1053/gast.2000.18157. [DOI] [PubMed] [Google Scholar]