Abstract
Active immunization against pro-angiogenic growth factors or their receptors is an emerging strategy for controlling tumor growth and angiogenesis. Previous studies in rodent tumor models have indicated that immunization against xenogeneic growth factors is more likely to induce effective anti-tumor responses than immunization against the autologous growth factor. However, the effectiveness or safety of the xenogeneic vaccination approach has not been previously assessed in a clinically relevant outbred, spontaneous tumor model. Therefore, we investigated the safety and anti-tumor and anti-angiogenic effects of a xenogeneic vascular endothelial cell growth factor (VEGF) vaccine in pet dogs with spontaneous cancer. Nine dogs with soft tissue sarcoma were immunized with a recombinant human VEGF vaccine over a 16-week period. The effects of immunization on antibodies to human and canine VEGF, circulating VEGF concentrations, tumor microvessel density (MVD), and tumor growth were assessed. The xenogeneic VEGF vaccine was well-tolerated by all dogs and resulted in induction of humoral responses against both human and canine VEGF in animals that remained in the study long enough to receive multiple immunizations. Three of five multiply immunized dogs also experienced sustained decreases in circulating plasma VEGF concentrations and two dogs had a significant decrease in tumor MVD. The overall tumor response rate was 30% for all treated dogs in the study. We conclude therefore that a xenogeneic VEGF vaccine may be a safe and effective alternative means of controlling tumor growth and angiogenesis.
Keywords: Canine, Cancer, Antibodies, Immunization, Endothelial cell
Introduction
Angiogenesis is critical to the ability of tumors to grow progressively and vascular endothelial cell growth factor (VEGF) is one of the key growth factors regulating the process of angiogenesis. A number of previous studies have demonstrated the importance of VEGF in regulating tumor angiogenesis and growth [1–3]. For this reason, strategies to inhibit the biologic effects of VEGF have become important approaches to the inhibition of tumor angiogenesis [4–9]. A variety of strategies to inhibit VEGF have been evaluated, including the use of mAbs to neutralize VEGF or block its receptors, the use of soluble VEGF receptors, and the use of small molecule inhibitors of VEGF receptor tyrosine kinase activity [7–10]. Notably, the clinical validity of inhibiting tumor angiogenesis by neutralization of VEGF activity was demonstrated in a pivotal study of bevacizumab (anti-VEGF mAb) in humans with colorectal cancer [11, 12]. This and other studies were the first to demonstrate that neutralization of VEGF, in combination with chemotherapy, could produce a significant survival benefit in cancer patients [6, 12–14].
Active vaccination against pro-angiogenic factors or their receptors is an alternative approach to therapeutic inhibition of angiogenesis [15–18]. For example, it was found that immunization against xenogeneic VEGF via the use of plasmid DNA vaccination could elicit cross-reactive antibodies that neutralized endogenous VEGF and inhibited tumor growth and angiogenesis in a mouse tumor model [19]. Immunization against the VEGF receptor was also found in other studies to inhibit tumor angiogenesis in rodent models [20–24]. Vaccination against other pro-angiogenic growth factors, such as fibroblast growth factor (FGF), appears to also inhibit tumor angiogenesis [25]. An alternative method to inhibit tumor angiogenesis is to actively immunize against endothelial cells themselves. For example, immunization with xenogeneic enodothelial cells, or more recently with autologous endothelial cells, was shown to elicit therapeutic inhibition of angiogenesis in several different rodent models [26–28].
One appeal of the immunization approach to angiogenesis inhibition is the ability to achieve sustained inhibition without the necessity of continuous administration of angiogenesis inhibiting drugs [17]. However, the vaccine approach may also hold some risk, in as much as sustained, effective neutralization of endogenous VEGF activity may elicit deleterious side-effects, such as coagulopathy and interference with wound healing [9].
Therefore, to more fully assess the potential effectiveness and safety of VEGF vaccination as a viable treatment option in humans, we conducted a study of a xenogeneic VEGF vaccine in pet dogs with cancer. Dogs spontaneously develop a number of cancers that closely resemble their related tumors in humans [29, 30]. In addition, dogs with cancer have been shown to have elevated circulating VEGF concentrations [31]. Because dogs are a highly outbred species, immunological studies in dogs are likely to more accurately reflect or predict immune responses in humans. For this study, we chose dogs with cutaneous soft tissue sarcoma, which is a relatively slow growing tumor of dogs that is locally invasive tumor and accessible to repeated biopsy [29].
The purpose of the study was to determine whether immunization against a xenogeneic VEGF protein (human VEGF165) could elicit therapeutic anti-VEGF responses. We selected huVEGF as the immunogen because the amino acid sequences of human and canine VEGF (caVEGF) differ by only 4.8%, indicative of a high degree of homology [32]. Moreover, the homology was close enough that canine VEGF bound to and activated the human VEGF receptor [32]. Unlike most previous studies that relied on DNA vaccination, in this study dogs were immunized with recombinant human VEGF protein. The vaccine was administered using a liposome–DNA adjuvant shown recently by our laboratory to be very effective in eliciting immune responses against protein antigens [33–35].
The endpoints of the study were induction of anti-VEGF antibody responses, effects on circulating VEGF concentrations, effects on tumor growth and angiogenesis, and safety. Nine dogs were enrolled in the study and five dogs remained in the study long enough to receive at least five immunizations. None of the dogs enrolled in the study experienced adverse effects that might be associated with excessive neutralization of endogenous VEGF concentrations. In the dogs that remained in the study long enough to receive multiple immunizations, the huVEGF vaccine elicited anti-VEGF antibody responses. Moreover, the induction of VEGF antibody responses was associated with reduction in circulating VEGF concentrations, tumor angiogenesis, and decreased tumor volume. We concluded from these results that immunization against xenogeneic VEGF may be a safe and effective treatment option for the induction of anti-tumor and anti-angiogenic activity in some patients with cancer.
Materials and methods
Study design
Client-owned dogs of any age, breed, or gender with spontaneous, initial or recurring, histologically confirmed, cutaneous soft tissue sarcomas measuring <125 cm3 at the time of diagnosis were eligible for entry into the study. Tumor volume was determined as the product of three tumor measurements (length, width, height). Any dog previously treated with radiation therapy or angiogenesis inhibitors and any dog currently receiving steroids or chemotherapy was excluded from entry into the study. Dogs with concurrent disease or evidence of metastatic disease were also excluded from the study. These studies were approved by the Institutional Animal Care and Use Committee at Colorado State University. The study was designed to include a series of six immunizations administered over a course of 16 weeks. Animals were enrolled and treated at the Colorado State University Veterinary Teaching Hospital (Ft Collins, CO, USA) or at the Veterinary Referral Center of Colorado (Englewood, CO, USA).
Preparation of VEGF vaccine
The vaccine was prepared using rhVEGF165, which was manufactured by R&D Systems (Minneapolis, MN, USA) and provided by the NCI Reagent Repository. The liposome–DNA complexes used as the vaccine adjuvant were prepared as described previously [33]. Briefly, liposomes were prepared by dissolving the cationic lipid DOTIM (octadecenolyoxy{ethyl-2-heptadecenyl-3-hydroxyethyl} imidazolinium chloride; Sigma-Aldrich Chemical Co., St Louis, MO, USA) and cholesterol (Avanti Polar Lipids, Alabaster, AL, USA) in chloroform and adding equimolar concentrations to round bottom 15 ml glass tubes. The solution was then dried overnight in a vacuum dessicator to a thin film and the lipids were rehydrated in 5% dextrose in water at 50°C for 50 min, followed by extrusion through a series of 1, 0.45, and 0.20 μm filters to form the final liposomes. To form the liposome–DNA complexes, non-coding plasmid DNA (prepared by the Valentis Corp, Burlingame, CA, USA) was added to liposomes in solution (100 μl liposomes per ml D5W) with gentle pipetting at a final concentration of 100 μg DNA per ml of liposome solution. The vaccine was then prepared by adding rhVEGF directly to the liposome–DNA solution at room temperature, followed by gentle mixing. Dogs were immunized within 15 min of preparing the VEGF vaccine.
Treatment and monitoring
A total of six immunizations with the VEGF vaccine were administered intradermally to dogs. Dogs were initially immunized once every other week for three immunizations, then once every four weeks for three additional immunizations. For the first immunization, dogs received 50 μg rhVEGF in 600 μl adjuvant (administered in two different sites over the lateral thorax), while all subsequent immunizations consisted of 25 μg rhVEGF in 300 μl adjuvant administered in one site over the lateral thorax. Dogs enrolled in the study were monitored for adverse effects by physical examination at the time of each immunization. Complete blood counts and serum biochemical profiles were evaluated prior to starting the study and again at week 6 and week 16. The vaccine site was evaluated at each repeat immunization to assess injection site responses. In addition, dogs were carefully evaluated clinically for evidence of coagulopathy (hemorrhage, petechiation) and delayed wound healing at tumor biopsy sites (see below).
Tumor volume was determined using calipers to measure tumor dimensions prior to treatment and at each vaccination boost. Tumor responses were classified as partial responses if tumor volume decreased by >50% from pre-treatment volume measurements and complete responses if the tumor was no longer detectable. Stable disease was defined as tumor volume that did not increase or decrease by >50% from starting values, whereas progressive disease was defined as tumor volume that increased >50% from starting values. For any dog where there was documented progressive tumor growth, the dog was removed from the study and alternative therapy was implemented.
Assessment of anti-huVEGF antibody responses
Plasma and serum samples were obtained from each dog prior to treatment and immediately prior to vaccination for each of the six treatments and stored at −80°C. Plasma concentrations of anti-huVEGF antibodies were determined by enzyme linked immunosorbent assay (ELISA). Briefly, the anti-huVEGF antibody ELISA was prepared by coating Immunolon-3 plates (Dynatech Laboratories, Chantilly, VA, USA) with a solution of 5 μg/ml rhVEGF (R&D Systems) in 50 mM carbonate/bicarbonate buffer at a pH of 9.6 incubated overnight at 4°C. Wells were blocked with a 5% solution of non-fat dried milk in phosphate buffered saline (PBS). The plasma samples were pre-diluted 1:1,000 in buffer (PBS with 1% BSA) and incubated at room temperature for 90 min. After washing, the plates were incubated with biotinylated-rabbit anti-dog IgG (Jackson ImmunoResearch, West Grove, PA, USA) for 60 min at room temperature. After washing, streptavidin–horseradish peroxidase (HRP) conjugate (Jackson ImmunoResearch) was added for 30 min at room temperature, then washed again and the substrate developed with 3,3′,5,5′-tetramethylbenzadine (Sigma-Aldrich). The colorimetric reaction was stopped with 1 M hydrochloric acid and the optical density was determined by ELISA plate reader (Thermo LabSystems, Franklin, MA, USA). Plasma concentrations of anti-caVEGF antibodies were determined following the same protocol as described above except that the plates were initially coated with a 5 μg/ml solution of recombinant caVEGF. Recombinant caVEGF was purchased from R&D Systems.
Measurement of plasma VEGF concentrations
Plasma VEGF concentrations in dogs were determined using an ELISA kit developed for detection of huVEGF (R&D Systems) in accordance with the manufacturer’s directions. Validation of this kit for detection of caVEGF in plasma has been previously reported [31, 36, 37].
Tumor biopsy and assessment of tumor microvessel density, VEGF expression, and IgG deposition
Tumor biopsies were obtained under local anesthesia by wedge biopsy immediately prior to the first treatment, at week 6, and at week 16. Biopsy specimens were embedded in Tissue-Tek OCT compound (TedPella Inc., Redding, CA, USA), snap frozen in isopentane and dry ice, and stored at −80°C. Tissues were cryosectioned to a thickness of 4 μm and adhered to positively charged glass slides. For identification of tumor microvessels, a mAb specific for human CD146 (clone P1H12, Chemicon, Temecula, CA, USA) which cross reacts with canine endothelial cells was utilized, as described previously [37]. Briefly, tissues were fixed in acetone, then blocked With H2O2 to eliminate endogenous peroxidase activity, then incubated with the primary antibody (mouse anti-human CD146) for 30 min at room temperature, followed by incubation with donkey anti-mouse IgG antiserum (Jackson ImmunoResearch). Slides were then treated with streptavidin–HRP followed by 3-amino-9-ethylcarbazole (AEC) substrate (Vector Laboratories, Burlingame, CA, USA), counterstained with hematoxylin, air dried, and cover slipped with aqueous-based crystal mount. Negative controls for IHC included incubation with irrelevant mAb and omission of the primary antibodies. Normal canine spleen was utilized as a positive control tissue for microvessel density (MVD) assessment.
To quantitate tumor MVD, four digital photomicrographs at 10× magnification were obtained (Leica Microscope equipped with digital camera in conjunction with SPOT Advanced Imaging software), as described previously [37]. Adobe Photoshop and Reindeer Graphics Quantitative Analysis Plug-ins (Asheville, NC, USA) were utilized to convert the photomicrographs to binary images. The number of microvessels per section was then calculated as number of pixels per mm2 tumor tissue. The final MVD value for each time tumor section was calculated as the mean (±SEM) number of vessels for the four 10× fields.
To assess tumor-associated VEGF expression, cryosectioned slides were first fixed in 10% formalin for 5 min rather than fixation in acetone. Prior to staining, slides were treated in target retrieval solution (Dako, Carpinteria, CA, USA) pH 6.0 at 125°C for 1 min. Staining was then performed utilizing a Dako autostainer in conjunction with Dako reagents (LSAB+ Link System) according to manufacturer’s directions. Goat anti-caVEGF antibody (R&D Systems) was utilized as the primary antibody, followed by incubation with HRP-conjugated donkey anti-goat IgG (Dako), followed by incubation with diaminobenzidine as the substrate. After staining, slides were dehydrated through graded alcohol followed by xylene and coverslipped with permanent-based mount. All sections from pre- and post-treatment biopsies were evaluated microscopically to assess the percentage of VEGF-positive cells as well as the relative intensity of VEGF staining.
For assessment of IgG deposition in tumor tissues, cryosectioned biopsy specimens were prepared as described above, except that samples were fixed using acetone. Slides were incubated with peroxidase-conjugated rabbit anti-dog IgG (Jackson ImmunoResearch) for 30 min at room temperature, followed by incubation with AEC substrate for 15 min at room temperature. Incubation with irrelevant primary antibody (rabbit anti-mouse IgG) and omission of the primary antibody were used as negative controls while normal canine splenic tissue was utilized as a positive control. All sections were subjectively evaluated microscopically and assigned a score of 0–3 based on the overall IgG immunoreactivity and intensity of staining.
Statistical analyses
Paired samples were compared statistically using the Student’s t-test. Comparisons between more than two treatment groups or more than two time points were done using one-way analysis of variance (ANOVA), followed by Tukey’s multiple means comparisons test. Analysis was done using GraphPad Prism statistical software (San Diego, CA, USA). For all analyses, P values <0.05 were considered statistically significant.
Results
Effects of vaccination on induction of anti-huVEGF antibodies
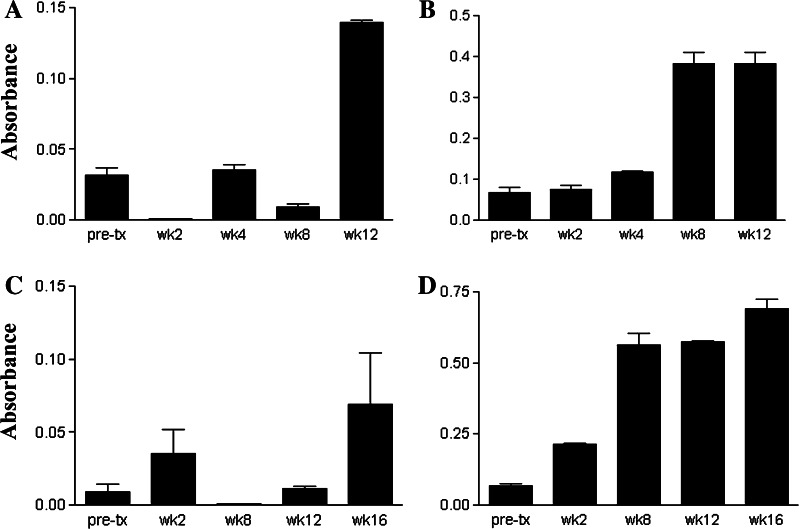
A total of nine dogs with soft tissue sarcoma were enrolled in the study (Table 1). Of these nine dogs, four dogs remained in the study long enough to receive five or more immunizations, while five dogs received three or fewer immunizations. The five dogs that failed to receive greater than three immunizations were removed from the study due to progressive tumor growth (Table 1). For the four dogs that received five or more immunizations, antibody response generally increased over time, with three of the four dogs mounting a marked increase in anti-huVEGF antibody responses (Fig. 1). None of the five dogs receiving three or fewer vaccinations mounted substantial anti-huVEGF antibody response (data not shown and Table 1). Thus, it appeared that immunization with rhVEGF could elicit anti-VEGF antibody responses, but that multiple immunizations (at least three or more) were required to elicit significant increases. Positive antibody responses ranged from four- to tenfold increases in anti-huVEGF antibody increases compared to pre-treatment values.
Table 1.
Clinical response data for nine dogs with soft tissue sarcomas that were immunized with huVEGF
| Dog | Histiotype/grade | Clinical response | VEGF Ab | Plasma VEGF | Tumor MVD | Number of vaccine |
|---|---|---|---|---|---|---|
| 1 | PNST/I | PR | + | Decreased | NC | 6 |
| 2 | PNST/I | PR | + | Decreased | Decreased | 6 |
| 3 | PNST/II | PR | − | Decreased | NC | 6 |
| 4 | PNST/I | PD | + | NC | Decreased/increased | 5 |
| 5 | PNST/I | PD | − | Decreased | NA | 3 |
| 6 | PNST/II | PD | − | NC | NA | 3 |
| 7 | PNST/II | PD | − | NC | NA | 2 |
| 8 | Undiff Src/III | PD | − | NC | NA | 2 |
| 9 | PNST/I | PD | − | NC | NA | 1 |
Nine dogs with soft tissue sarcoma received a series of immunizations with huVEGF. Tumors were classified histologically as peripheral nerve sheath tumors (PNST) or undifferentiated sarcomas (Undiff Src). Tumors were graded on a scale of I to III based on decreasing degree of differentiation, as well as other criteria. Tumor responses were classified as partial response (PR; greater than 50% reduction in tumor volume) or progressive disease (PD; greater than 50% increase in tumor size). Anti-huVEGF antibody responses, circulating VEGF concentrations, and tumor MVD were assessed as described in Sect. “Materials and methods”
NC no significant change, NA not evaluated
Fig. 1.
VEGF antibody responses following immunization of dogs against recombinant huVEGF. Nine dogs with cancer were enrolled in a study to evaluate the effects of vaccination against xenogeneic huVEGF. Plasma samples were collected prior to immunization (week 0) and at several different time points after beginning immunization with rhuVEGF. Plasma samples were assayed for anti-huVEGF antibody responses, as described in Sect. “Materials and methods,” and the mean (±SD) of replicate anti-VEGF antibody determinations was plotted. Anti-huVEGF antibody responses over time were plotted in four dogs (panels a–d and dogs 1–4 in Table 1) that received five or more immunizations
Effects of vaccination on circulating plasma VEGF concentrations
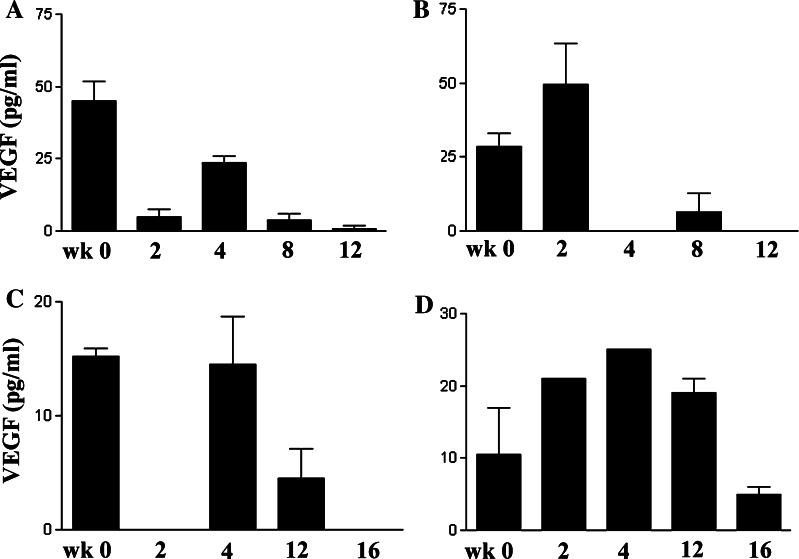
We next assessed the effects of VEGF immunization on circulating VEGF concentrations. A decrease in plasma VEGF concentrations was observed at two or more time points in three of the four dogs that received five or more VEGF immunizations (Fig. 2). In the fourth dog, no changes in circulating VEGF concentrations were noted despite a large increase in anti-huVEGF antibodies (see dog 4, Table 1, Figs. 1, 2). In contrast, VEGF concentrations did not decrease in four of the five dogs that received three or fewer VEGF immunizations (Table 1). The one exception was a dog (dog 5, Table 1) that was immunized three times, where a decrease in VEGF concentrations was noted (data not shown). Thus, in some dogs, repeated immunization with rhVEGF induced a substantial anti-huVEGF antibody response that was associated with a large decline in circulating VEGF concentrations (dogs 1 and 2, Table 1). In two other dogs (dogs 3 and 5, Table 1) there was a decrease in circulating VEGF despite the lack of a anti-huVEGF antibody responses. These results suggest that in some dogs, the decrease in circulating VEGF concentrations may have been mediated by mechanisms other than humoral immune responses.
Fig. 2.
Effects of VEGF vaccination on endogenous VEGF concentrations in dogs. Circulating VEGF concentrations were measured in dogs prior to vaccination (week 0) and at twice weekly intervals following vaccination with huVEGF. VEGF concentrations were determined by VEGF ELISA, as described in Sect. “Materials and methods” and the mean (±SD) of replicate VEGF determinations was plotted. In four dogs (a–d) that received more than five immunizations, VEGF concentrations were plotted over time
Effects of vaccination on generation of anti-canine VEGF antibody responses
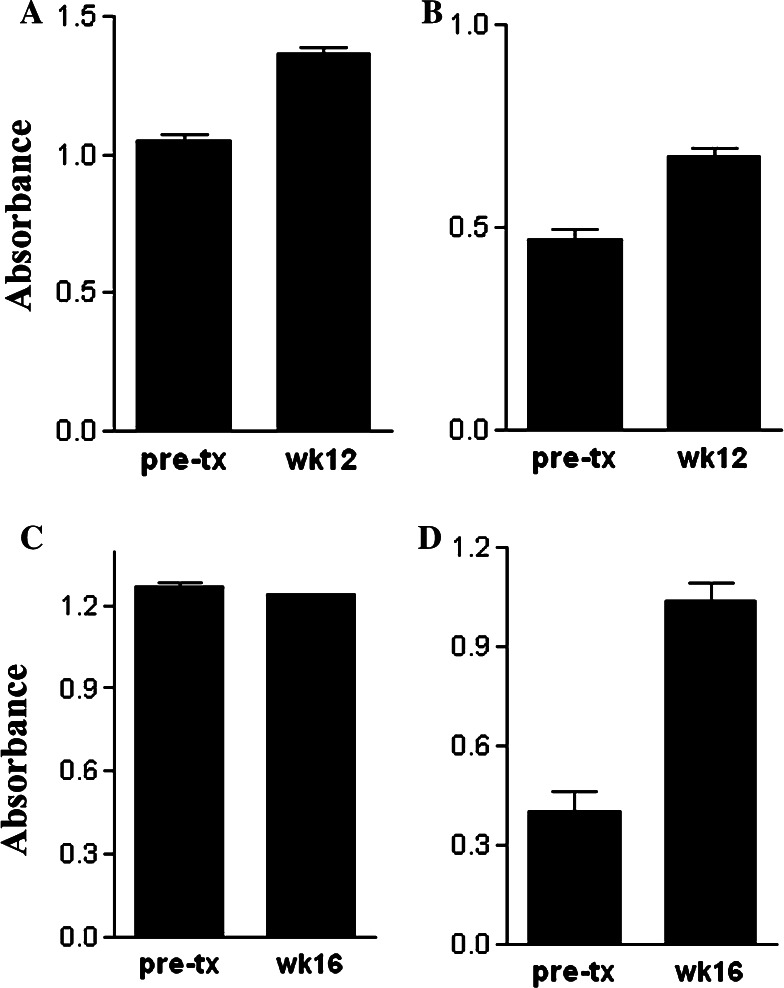
Next, studies were conducted to determine whether vaccination with rhVEGF was capable of eliciting cross-reactive antibodies against endogenous caVEGF. Such a response would be expected if immunization against xenogeneic huVEGF was capable of breaking self-tolerance. Plasma samples from each of the four dogs that received five or more huVEGF immunizations were assessed by ELISA for the presence of antibodies recognizing canine VEGF. We found that anti-caVEGF titers were very modestly increased in three of the four multiply vaccinated dogs that also mounted strong anti-huVEGF responses (Fig. 3). However, the antibody responses against caVEGF were very weak compared to the responses against huVEGF. These findings assume greater importance when one considers the fact that dogs are a highly outbred species with a very diverse MHC repertoire compared to inbred mouse strains [29, 30].
Fig. 3.
Antibody responses against canine VEGF in dogs vaccinated against huVEGF. Dogs were immunized with rhuVEGF and plasma was collected for analysis of anti-caVEGF antibody responses by ELISA, as described in Sect. “Materials and methods” and the mean (±SD) of replicate anti-caVEGF antibody determinations was plotted. For the four dogs (a–d) that received five or more VEGF vaccines, anti-caVEGF antibody responses were measured prior to treatment and again at the completion of the immunization period (week 12 or week 16)
Effects of VEGF vaccination on tumor angiogenesis
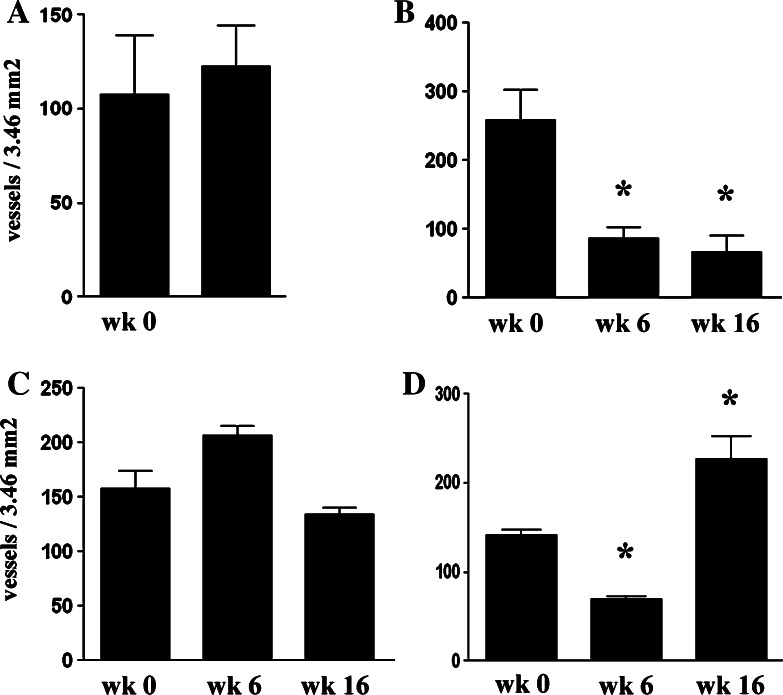
Tumor MVD was evaluated in biopsy specimens obtained pre-treatment, on week 6, and on week 16 of the study from the four dogs that received multiple VEGF immunizations. Due to the design of the study, post-treatment tumor biopsies could not be obtained from any the five dogs that received three or fewer immunizations as they did not remain in the study long enough to reach the first biopsy time point. Two of the four multiply vaccinated dogs demonstrated a significant decrease in tumor MVD at one or more time points when pre- and post-treatment biopsies were compared (Figs. 4, 5). It should be noted however that in one of these dogs tumor MVD actually increased at a later time point, coincident with progressive growth of the tumor (see dog 4, Table 1, Fig. 4). In the other two dogs, tumor MVD remained relatively constant after VEGF immunization was initiated. Thus, it appeared that repeated VEGF immunization was capable of inhibiting tumor angiogenesis in at least half of the dogs.
Fig. 4.
Effects of VEGF vaccination on tumor MVD in dogs with soft tissue sarcomas. Tumor biopsies were obtained from four dogs (a–d) before treatment, at 6 weeks, and again at 16 weeks after beginning VEGF immunization. Tumor MVD was quantitated using CD146 immunohistochemistry, as described in Sect. “Materials and methods” and the mean MVD (±SD) for each tumor was plotted. Pre- and post-treatment tumor MVDs were compared by ANOVA and significant differences (P < 0.05) were denoted by asterisk
Fig. 5.

Determination of tumor MVD by immunohistochemistry. Biopsies were obtained from the tumor of a dog prior to VEGF vaccination and again at week 16 after six VEGF immunizations had been administered. The tumor MVD was assessed by immunostaining for CD146 expression, using a cross-reactive antibody to human endothelial cells, as described in Sect. “Materials and methods.” The tumor MVD was markedly decreased in the post-vaccination tumor biopsy, compared to the MVD in the pre-treatment tumor biopsy
Effects of vaccination on intratumoral VEGF expression and immunoglobulin deposition
We also investigated the possible effects of repeated VEGF vaccination on intratumoral VEGF concentrations. Immunohistochemistry was used to evaluate intracellular expression of VEGF by tumor cells and infiltrating leukocytes in tumor biopsy specimens from four dogs that received more than five immunizations. A consistent difference in the extent or intensity of tumor VEGF staining was not noted when pre- and post-vaccination biopsies were compared (data not shown). The deposition of IgG complexes within the tumor was also assessed, as deposition of IgG complexes has been noted in tumors of mice immunized with xenogeneic VEGF previously [21]. However, we did not find evidence of a vaccine effect when pre- and post-vaccination tumor biopsies were evaluated for the degree of IgG deposition (data not shown). These results suggested therefore that the major effects of immunization on canine VEGF probably occurred systemically, rather than within the tumor itself.
Clinical responses in vaccinated dogs
Tumor responses were also assessed by means of tumor volume measurements in all dogs enrolled in the study. A partial tumor response (>50% decrease in tumor volume) was noted in three of the nine enrolled dogs (Table 1). In the other six dogs, progressive tumor growth was observed and all were dropped from the study before completing their full series of VEGF immunizations. The three dogs in which tumor regression was noted were also those that received the full course of six immunizations. For dog 1 (Table 1), the tumor size remained stable over a 1-year period of follow-up. In dog 2, progressive tumor growth developed within 1 month of completing the vaccine trial. Tumor size remained stable in dog 3 for 8 months, then progressive tumor growth developed.
One possible explanation for these results is that receiving the full course of VEGF immunizations may have been associated with tumor regression. For example, spontaneous tumor regressions are very rare in dogs with soft tissue sarcomas. However, selection bias may have also played a role, inasmuch as dogs with inherently less aggressive tumors were those most likely to complete the vaccination regime.
Finally, all treated dogs were also carefully evaluated for adverse effects associated with repeated VEGF immunization. Vaccine site reactions were not observed in any of the vaccinated dogs. In addition, evidence of coagulopathies (e.g., excessive bleeding from venipuncture or biopsy sites) or changes in complete blood counts or serum biochemistries were not observed (data not shown). We also did not observe any interference in wound healing at the tumor biopsy sites in the four dogs that had repeated tumor biopsies performed. Thus, it appeared based on this preliminary clinical investigation that dogs immunized repeatedly against xenogeneic VEGF did not develop serious adverse effects.
Discussion
In this study we found that repeated immunization of dogs with spontaneous tumors with xenogeneic VEGF was associated with induction of anti-VEGF antibody responses and decreases in circulating VEGF concentrations and tumor angiogenesis. Moreover, vaccination was also associated with an overall tumor response rate (partial response) of 30%, in a tumor that is not typically responsive to chemotherapy or other medical treatments. These results, which to our knowledge represent the first evaluation of xenogeneic VEGF vaccination in a large animal spontaneous tumor model, indicate that the approach is technically feasible and safe and is capable of inhibiting tumor angiogenesis and stimulating anti-tumor activity in animals with large, spontaneous tumors.
Not surprisingly, the study revealed that the ability to generate anti-VEGF immune responses was associated with number of immunizations an animal received. Dogs that received three or fewer immunizations generally failed to produce significant anti-huVEGF antibody responses, whereas significant anti-huVEGF antibody responses were observed in all dogs that received five or six immunizations (see Fig. 1). In two of three dogs with high levels of anti-huVEGF antibodies, significant reductions in circulating plasma VEGF concentrations were also observed (see Table 1). Circulating VEGF concentrations were also significantly reduced in two dogs that did not mount significant anti-huVEGF antibody responses. We postulate that the VEGF vaccine was the most likely explanation for the observed decreases in VEGF concentration. This is based in part on the fact that in a previous study we completed of 13 dogs also with soft tissue sarcoma, we did not observe any significant change in circulating VEGF concentration during repeated sampling over a 12-week period, despite the fact that significant tumor responses were observed during that time [37].
Evaluation of pre- and post-treatment tumor MVD in the four dogs that received five or more VEGF vaccinations revealed a significant decrease in MVD in one dog (dog 2, Table 1), no change in MVD in two dogs, and a transient decrease in tumor MVD in one dog followed by an increase (dog 4, Fig. 4). In the one dog that had a subsequent increase in tumor MVD following the initial decrease, it is possible that under the selective pressure of anti-VEGF antibodies, the tumor may have upregulated production of alternative pro-angiogenic growth factors, such as bFGF or IL-8 [25, 38].
We also evaluated tumor responses clinically by serial determinations of tumor volume. From analysis of these data (Table 1), it was apparent that number of vaccinations correlated closely with clinical tumor responses. For example, three of four dogs that received five or more immunizations had objective clinical tumor responses. In two of the dogs with tumor responses, the responses were durable (8 months and >12 months). In contrast, all five dogs that had three or fewer vaccinations experienced progressive tumor growth. It is possible that tumor responses and the number of vaccinations each dog received may have also been influenced by tumor grade. Dogs with low-grade (grade I or II) soft tissue sarcoma in general tend to have a better outcome (less aggressive tumors, less local recurrence) than dogs with grade III tumors [29]. In our study, of the four dogs that received five or more immunizations, three dogs had grade I tumors and one dog had a grade II tumor. Of the five dogs that received fewer than three VEGF vaccinations and had progressive tumor growth, there were two dogs with grade I tumors, two dogs with grade II tumors, and one dog with a grade III tumor.
It is important to note that circulating anti-VEGF antibodies were evaluated in plasma rather than in serum. When blood samples are clotted, the activated platelets release large quantities of VEGF into serum [39–41]. This high level of platelet-released VEGF could then potentially react with any anti-VEGF antibodies present in circulation, thereby falsely lowering the anti-VEGF antibody concentrations detected. In fact, we observed this effect when we initially compared VEGF antibody results from paired serum and plasma samples from some dogs in the study (data not shown). Therefore, we used plasma, which contains relatively low concentrations of platelet-derived VEGF, for assessment of both antibody and circulating VEGF concentration in this study.
One of the premises underlying use of the xenogeneic VEGF vaccine approach is that this technique has the potential to break self-tolerance against endogenous VEGF. Though some dogs in this study (i.e., those that received more than five immunizations) mounted strong antibody responses against huVEGF, we did not observe substantial antibody responses against caVEGF (see Figs. 1, 3), suggesting that immune tolerance was not effectively broken. However, it is also possible that anti-caVEGF antibodies were in fact elicited, but were rapidly eliminated due to complexing with circulating caVEGF. For example, in three of the four multiply vaccinated dogs there was a significant reduction in circulating caVEGF concentrations (Figs. 1, 2) despite the low levels of anti-caVEGF antibodies elicited (Fig. 3). Differences in antibody affinity or specificity for huVEGF versus caVEGF may also help explain the difference in levels of anti-huVEGF and anti-caVEGF antibodies noted in this study. For example, antibodies elicited against unique determinants on huVEGF not expressed on caVEGF would still be detected using the huVEGF ELISA, but would not be detected using the caVEGF ELISA. Also, if higher affinity antibodies were elicited against huVEGF than against caVEGF, this might be reflected by detecting higher antibody concentrations against huVEGF than against caVEGF.
Our tumor response data suggest that the reduction in circulating VEGF concentrations following vaccination had a functional effect on tumor growth. For example, three of the four dogs that had a reduction in circulating plasma VEGF concentrations also had objective tumor responses (Table 1). However, we also noted that reductions in circulating VEGF concentrations, as well as overt tumor responses, occurred in some dogs before a noticeable increase in anti-VEGF antibody levels. We also observed decreased circulating VEGF concentrations in one dog that did not have detectable anti-VEGF antibodies (Table 1). These findings suggest that other immunologic mechanisms, such as induction of T cell responses against tumor cells expressing high levels of VEGF, may have also contributed to the reduction in circulating VEGF concentrations as well as anti-tumor activity. One also cannot exclude the possibility that a reduction in tumor mass and local production of VEGF was a primary cause of the observed decreases in circulating VEGF concentrations.
The study was also designed to determine whether neutralization of VEGF by vaccination was a safe approach clinically. Since dogs closely resemble humans physiologically and immunologically, a VEGF vaccine study in dogs would be more likely than rodent studies to provide a realistic assessment of safety in humans. Obvious adverse effects were not observed in any of the dogs treated in this study. In particular, we did not observe interference with normal angiogenic processes, such as wound healing. For example, some dogs underwent multiple tumor biopsies over the course of the study and did not develop problems with healing of the tumor biopsy sites. In addition, obvious clotting disturbances were not noted on physical examination or following blood collection by venipuncture. Thus, by clinical criteria vaccination against xenogeneic VEGF did not appear to inhibit normal angiogenesis or clot formation, though these processes were not evaluated by laboratory testing. It is not possible to comment on the possible effects of VEGF vaccination on fertility, as all of the female dogs in this study were neutered.
One of the major determinants of whether or not a treatment effect was observed in this study was how many vaccines could be administered before progressive tumor growth developed. The inherent growth rate and/or biological aggressiveness of the tumor may have been one of the primary determinants of time to tumor progression. To overcome this effect, it might be possible in future studies to augment or accelerate immune responses to the VEGF vaccine. For example, immunization with larger amounts of VEGF antigen or by a different route might have elicited more rapid antibody responses of higher magnitude. Of note, prior to this study we completed a smaller pilot study of xenogeneic VEGF vaccination in four dogs that were immunized with rhVEGF165 plasmid DNA, delivered by intramuscular injection. However, in that study only very weak anti-huVEGF antibody responses were observed and no objective tumor responses occurred (S. W. Dow, unpublished data). Therefore, we predict that use of conventional plasmid DNA immunization in dogs is unlikely to offer a significant improvement over the current immunization protocol. We also did not evaluate cellular immune responses to VEGF and therefore do not know what role cellular immunity might play in some of the responses observed. However, we did not observe any consistent increases in mononuclear cell infiltration when pre- and post-vaccination tumor biopsy specimens were compared (data not shown).
In summary, we show here that repeated vaccination with xenogeneic VEGF protein is well-tolerated clinically in dogs with cancer. Moreover, induction of strong anti-huVEGF antibody responses was associated in most animals with a significant decline in circulating VEGF concentrations, reduction in tumor MVD, and reduction in tumor volume. These data, generated in an outbred species, suggest that xenogeneic vaccination against pro-angiogenic growth factors has potential as a safe adjunct to standard therapies for controlling tumor growth and angiogenesis.
Acknowledgments
The authors wish to acknowledge the excellent technical assistance provided by Julie Willer and Jessica Bushanam. We are also grateful to Drs E. J. Ehrhart and Chris Orton, and Mr Brad Charles for assistance with VEGF immunohistochemistry. These studies were supported by a grant from the NIH (CA86224-01).
References
- 1.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl. 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 2.Millanta F, Silvestri G, Vaselli C, Citi S, Pisani G, Lorenzi D, et al. The role of vascular endothelial growth factor and its receptor Flk-1/KDR in promoting tumour angiogenesis in feline and canine mammary carcinomas: a preliminary study of autocrine and paracrine loops. Res Vet Sci. 2006;81:354–357. doi: 10.1016/j.rvsc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Parikh AA, Ellis LM. The vascular endothelial growth factor family and its receptors. Hematol Oncol Clin North Am. 2004;18(5):951–971. doi: 10.1016/j.hoc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Benouchan M, Colombo BM. Anti-angiogenic strategies for cancer therapy (review) Int J Oncol. 2005;27(2):563–571. [PubMed] [Google Scholar]
- 5.Cao Y. Antiangiogenic cancer therapy. Semin Cancer Biol. 2004;14(2):139–145. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Cardones AR, Banez LL. VEGF inhibitors in cancer therapy. Curr Pharm Des. 2006;12(3):387–394. doi: 10.2174/138161206775201910. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl. 3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 8.Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3(2):263–276. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- 9.Verheul HM, Pinedo HM. Inhibition of angiogenesis in cancer patients. Expert Opin Emerg Drugs. 2005;10(2):403–412. doi: 10.1517/14728214.10.2.403. [DOI] [PubMed] [Google Scholar]
- 10.Zhang HT, Bicknell R. Therapeutic inhibition of angiogenesis. Mol Biotechnol. 2003;25(2):185–200. doi: 10.1385/MB:25:2:185. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Kabbinavar F. Bevacizumab combined with standard fluoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology. 2005;69(Suppl. 3):17–24. doi: 10.1159/000088480. [DOI] [PubMed] [Google Scholar]
- 13.Salesi N, Bossone G, Veltri E, Di Cocco B, Marolla P, Pacetti U, et al. Clinical experience with bevacizumab in colorectal cancer. Anticancer Res. 2005;25(5):3619–3623. [PubMed] [Google Scholar]
- 14.Sanborn RE, Sandler AB. The safety of bevacizumab. Expert Opin Drug Saf. 2006;5(2):289–301. doi: 10.1517/14740338.5.2.289. [DOI] [PubMed] [Google Scholar]
- 15.Rafii S. Vaccination against tumor neovascularization: promise and reality. Cancer Cell. 2002;2(6):429–431. doi: 10.1016/S1535-6108(02)00208-8. [DOI] [PubMed] [Google Scholar]
- 16.Moniz M, Yeatermeyer J, Wu TC. Control of cancers by combining antiangiogenesis and cancer immunotherapy. Drugs Today (Barc) 2005;41(7):471–494. doi: 10.1358/dot.2005.41.7.893623. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Bohlen P, Hicklin DJ. Vaccination against angiogenesis-associated antigens: a novel cancer immunotherapy strategy. Curr Mol Med. 2003;3(8):773–779. doi: 10.2174/1566524033479438. [DOI] [PubMed] [Google Scholar]
- 18.Reisfeld RA, Niethammer AG, Luo Y, Xiang R. DNA vaccines designed to inhibit tumor growth by suppression of angiogenesis. Int Arch Allergy Immunol. 2004;133(3):295–304. doi: 10.1159/000077009. [DOI] [PubMed] [Google Scholar]
- 19.Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA. 2001;98(20):11545–11550. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8(12):1369–1375. doi: 10.1038/nm794. [DOI] [PubMed] [Google Scholar]
- 21.Liu JY, Wei YQ, Yang L, Zhao X, Tian L, Hou JM, et al. Immunotherapy of tumors with vaccine based on quail homologous vascular endothelial growth factor receptor-2. Blood. 2003;102(5):1815–1823. doi: 10.1182/blood-2002-12-3772. [DOI] [PubMed] [Google Scholar]
- 22.Lu F, Qin ZY, Yang WB, Qi YX, Li YM. A DNA vaccine against extracellular domains 1–3 of flk-1 and its immune preventive and therapeutic effects against H22 tumor cell in vivo. World J Gastroenterol. 2004;10(14):2039–2044. doi: 10.3748/wjg.v10.i14.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keke F, Hongyang Z, Hui Q, Jixiao L, Jian C. A combination of flk1-based DNA vaccine and an immunomodulatory gene (IL-12) in the treatment of murine cancer. Cancer Biother Radiopharm. 2004;19(5):649–657. doi: 10.1089/cbr.2004.19.649. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Wen YJ, Ding ZY, Fu CH, Wu Y, Liu JY, et al. Immunotherapy of tumors with protein vaccine based on chicken homologous Tie-2. Clin Cancer Res. 2006;12(6):1813–1819. doi: 10.1158/1078-0432.CCR-05-1990. [DOI] [PubMed] [Google Scholar]
- 25.Plum SM, Holaday JW, Ruiz A, Madsen JW, Fogler WE, Fortier AH. Administration of a liposomal FGF-2 peptide vaccine leads to abrogation of FGF-2-mediated angiogenesis and tumor development. Vaccine. 2000;19(9–10):1294–1303. doi: 10.1016/S0264-410X(00)00210-3. [DOI] [PubMed] [Google Scholar]
- 26.Scappaticci FA, Nolan GP. Induction of anti-tumor immunity in mice using a syngeneic endothelial cell vaccine. Anticancer Res. 2003;23(2B):1165–1172. [PubMed] [Google Scholar]
- 27.Okaji Y, Tsuno NH, Kitayama J, Saito S, Takahashi T, Kawai K, et al. Vaccination with autologous endothelium inhibits angiogenesis and metastasis of colon cancer through autoimmunity. Cancer Sci. 2004;95(1):85–90. doi: 10.1111/j.1349-7006.2004.tb03175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med. 2000;6(10):1160–1166. doi: 10.1038/80506. [DOI] [PubMed] [Google Scholar]
- 29.Vail DM, MacEwen EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18(8):781–792. doi: 10.3109/07357900009012210. [DOI] [PubMed] [Google Scholar]
- 30.Porrello A, Cardelli P, Spugnini EP. Oncology of companion animals as a model for humans. An overview of tumor histotypes. J Exp Clin Cancer Res. 2006;25(1):97–105. [PubMed] [Google Scholar]
- 31.Clifford CA, Hughes D, Beal MW, Mackin AJ, Henry CJ, Shofer FS, et al. Plasma vascular endothelial growth factor concentrations in healthy dogs and dogs with hemangiosarcoma. J Vet Intern Med. 2001;15(2):131–135. doi: 10.1892/0891-6640(2001)015<0131:PVEGFC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Scheidegger P, Weiglhofer W, Suarez S, Kaser-Hotz B, Steiner R, Ballmer-Hofer K, et al. Vascular endothelial growth factor (VEGF) and its receptors in tumor-bearing dogs. Biol Chem. 1999;380(12):1449–1454. doi: 10.1515/BC.1999.187. [DOI] [PubMed] [Google Scholar]
- 33.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 34.Mueller RS, Veir J, Fieseler KV, Dow SW. Use of immunostimulatory liposome–nucleic acid complexes in allergen-specific immunotherapy of dogs with refractory atopic dermatitis—a pilot study. Vet Dermatol. 2005;16(1):61–68. doi: 10.1111/j.1365-3164.2005.00426.x. [DOI] [PubMed] [Google Scholar]
- 35.Walter CU, Biller BJ, Lana SE, Bachand AM, Dow SW. Effects of chemotherapy on immune responses in dogs with cancer. J Vet Intern Med. 2006;20(2):342–347. doi: 10.1892/0891-6640(2006)20[342:EOCOIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Wergin MC, Kaser-Hotz B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occurring tumours. In Vivo. 2004;18(1):15–19. [PubMed] [Google Scholar]
- 37.Kamstock D, Guth A, Elmslie R, Kurzman I, Liggitt D, Coro L, et al. Liposome–DNA complexes infused intravenously inhibit tumor angiogenesis and elicit antitumor activity in dogs with soft tissue sarcoma. Cancer Gene Ther. 2006;13(3):306–317. doi: 10.1038/sj.cgt.7700895. [DOI] [PubMed] [Google Scholar]
- 38.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11(9):992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 39.Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, Broxterman HJ, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3(12 Pt 1):2187–2190. [PubMed] [Google Scholar]
- 40.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77(6):956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 1998;94(4):395–404. doi: 10.1042/cs0940395. [DOI] [PubMed] [Google Scholar]