Abstract
Purpose
The receptor responsible for the attachment of bacillus Calmette-Guerin (BCG) to fibronectin, fibronectin attachment protein (FAP), has been cloned. Studies targeting FAP as an inducer of immunity in mycobacterial infections suggest that FAP is a highly immunogenic protein. In light of these findings and the need to find effective alternatives to BCG treatment for bladder cancer, we tested the ability of FAP to induce antitumor activity.
Materials and methods
The ability of FAP to bind to bladder tumor cells and the bladder wall was established using 125I-FAP. For testing antitumor activity in vivo, mice were catheterized and 5 × 104 MB-49 bladder tumor cells were implanted orthotopically on day 0. Test groups were treated with PBS only, FAP, or BCG on day 1 and day 8. A subset of mice was preimmunized with FAP prior to treatment.
Results
FAP was observed to bind to bladder tumor cells in a fibronectin-dependent manner. Attachment of FAP within the bladder followed the pattern established for BCG binding. Antitumor studies showed a significant reduction in tumor growth in FAP-treated mice that had been preimmunized with FAP. Tumor growth was not inhibited in naïve mice treated with FAP. Dose-response studies showed that FAP-induced antitumor activity is dose dependent, and experiments comparing BCG with FAP showed equivalent antitumor effects. In vitro experiments showed antigen-specific lymphocyte proliferation and a cytokine profile indicative of Th-1 polarization of the FAP-induced immune response. CD8+ T cells and natural killer cells were found to be required for the FAP-induced antitumor response.
Conclusions
FAP is an effective antitumor agent that inhibits tumor growth at a level equivalent to that observed for BCG. This protein may thus provide an alternative to BCG for treatment of superficial bladder cancer.
Keywords: Antitumor Activity, Bladder Tumor, Superficial Bladder Cancer, Intravesical Instillation, Naive Mouse
Introduction
Since its discovery in 1976 by Morales and associates, bacillus Calmette-Guerin (BCG) immunotherapy has become the treatment of choice for superficial bladder cancer [22]. In spite of the fact that BCG currently is the most efficacious treatment for superficial bladder cancer, treatments can induce severe toxicity in some patients [17]. Moreover, toxicity increases with multiple treatments, limiting a patient’s tolerance for treatment regimens optimized for anticancer effects [19]. Additionally, long-term follow up of BCG-responsive patients has revealed a high recurrence rate over time—up to 70% over 10 years [7]. Given the toxicities associated with BCG treatment, the post-response recurrence rate and the fact that BCG ultimately has little impact on patient survival, the identification of alternative treatments for superficial bladder cancer is desirable.
Since most superficial bladder tumors readily respond to BCG treatment, the identification of alternative immunotherapy approaches that do not use viable bacteria may yield treatments that are more effective and less toxic. Although the mechanism of action of BCG is incompletely understood, its investigation to date has shown that the elimination of tumors is dependent on T cell immunity, with laboratory and clinical studies having demonstrated a significant correlation between a BCG-activated Type I delayed type hypersensitivity (DTH) response and antitumor activity [15, 24, 29, 30]. Consistent with a role for DTH in antitumor activity, interferon-γ (IFN-γ) levels have been linked to successful therapeutic outcomes, both in murine models and in patients undergoing BCG therapy [10, 24, 28]. The effector mechanisms mediating BCG-induced antitumor activity have not been clearly identified, although the innate immune response has been shown to be an important component [21, 27, 35, 36]. Thus, identifying an immunotherapy regimen that requires T cells and induces Type I immunity is a primary focus of our efforts to improve treatments for superficial bladder cancer.
Here we report on an immunogenic protein that is a component of BCG, i.e. the fibronectin attachment protein (FAP). FAP mediates BCG attachment to bladder tumor cells and bladder wall following intravesical instillation [34, 40, 41]. FAP has unique properties, which include its ability to mediate the internalization of BCG by bladder epithelial cells through the α5-β1 integrin receptor [1, 9, 13, 14]. Moreover, the nucleotide sequence for FAP has been shown to be identical to that of a Mycobacterium tuberculosis protein termed the Apa/45-47 complex, [8, 20, 41] and both are highly immunogenic proteins capable of inducing strong CD8 T cell immune responses [20]. The utility of other immunogenic proteins, such as keyhole-limpet hemocyanin (KLH) and the mycobacterial protein PstS1, in anti-bladder tumor therapy has been well documented, in both animal models and clinical trials, but each has been found to have some important limitations [11, 12, 18, 33, 37, 38]. Since FAP is a highly antigenic protein with the unique properties of binding to, and being internalized by, bladder cancer cells, we hypothesized that FAP would induce an effective T cell-mediated antitumor response. In this paper, recombinant FAP is tested for its ability to induce antitumor activity in an orthotopic bladder tumor model.
Materials and methods
Animals
All studies were carried out on female C57BL/6 mice at 8–10 weeks of age. Mice were given food and water ad libitum. All animal studies were performed in accordance with institutional guidelines.
FAP
Recombinant FAP was produced and purified as previously described [40]. Lipopolysaccharide (LPS) was removed with DeToxi gel (Pierce Biotechnology, Rockford, IL) and tested for LPS using the E-TOXATE reagent from Limulus polyphemus (Sigma Chemicals, St Louis, MO).
125I-labeled FAP
Purified FAP was labeled with 125I using the IODO-GEN reagent from Pierce Biochemicals (Rockford, IL) as described in the reagent information.
In vitro 125I-FAP binding studies
Approximately 5 × 104 cells (MB-49 and T-24) were plated in flat-bottom 96-well plates and incubated overnight. Cells were washed and 5 μCi 125I-FAP added to appropriate wells. Cells were incubated 4 h, washed, detached with 0.01 M EDTA and counted in a gamma counter. Wells were visually inspected to assure removal of all cells. When used, anti-FAP was added to the 125I-FAP immediately before addition to cells, and anti-FN was added to the cells 15 min prior to 125I-FAP addition.
In vivo 125I-FAP binding studies
Mice were anesthetized with 100 μl of a 17.5 mg/ml ketamine, 2.5 mg/ml xylazine solution before catheterization with a standard 24-gauge catheter. Some mice were cauterized immediately after catheterization as previously described [41]. Ten micro curie 125I-FAP was added to each bladder in a total volume of 100 μl. Mice were sacrificed 2 h later, and bladders were removed, washed and counted in a gamma counter.
FAP pre-immunizations
Two weeks prior to tumor implantation a subset of mice was immunized subcutaneously with 200 μg FAP in complete Freud’s adjuvant (CFA). Naïve mice served as the control.
Tumor implantation
Mice were anesthetized with 100 μl of a 17.5 mg/ml ketamine, 2.5 mg/ml xylazine solution before catheterization with a standard 24 gauge catheter. Bladders were treated with 4 μl of a 0.3 M silver nitrate solution and immediately rinsed twice with PBS. 100 μl of a 2.5 × 105 cell/ml solution of MB49 tumor cells in RPMI was instilled into the bladder (day 0). The catheter was removed and mice were allowed to recover for 24 h before therapy was initiated. MB49 tumor cells were prepared as a single cell suspension from a subcutaneous tumor, following which they were implantated into the bladder as previously described [30].
FAP therapy
Therapy began 24 h after tumor implantation (day 1). Mice were again anesthetized with 100 μl of a 17.5 mg/ml ketamine, 2.5 mg/ml xylazine solution before catheterization with a standard 24 gauge catheter. Treatments included PBS, FAP (25–100 μg), BCG (107 cfu). All instillations were at a total volume of 100 μl. Therapy was repeated on day 8. Mice with blood in their urine after day 14 were sacrificed and examined for urothelial tumors. Ten mice were used per group.
In vitro proliferation/ELISA assay
Mice were immunized with 200 μg FAP in CFA or CFA alone 2 weeks prior to sacrifice. The spleen from each mouse was harvested and a single-cell suspension was prepared. In vitro proliferation assay: Spleen cells (2 × 105 cells per well) were incubated with the indicated concentration of FAP or anti-CD3 for 48 h. Cell cultures were subsequently incubated with 1μCu of 3H-thymidine for 18 h before cells were collected and counted. ELISA: spleen cells were plated at 5 × 106 cells/well and incubated with 100 μg/ml FAP, 100 μg/ml BSA or 5 μg/ml anti-CD3 for 24 h. Supernatants were collected and frozen at −20°C until assayed for IFNγ or IL-4 by ELISA (BD Biosciences, San Jose, CA). Each data point is an average of the values obtained from 3 mice.
Depletion studies
Three days prior to tumor implantation (day −3), mice were i.p. injected with 100 μg of one of the following depleting antibodies: GK1.5 (CD4+ T-cells), 2.43 (CD8+ T-cells), PK136 (NK1.1 cells) or SFR8 (isotype control). Antibody injections were repeated on days −2 and −1. Designated mice were sacrificed before tumor implantation on day 0 to confirm specific cell depletions. Maintenance antibody injections of 100 μg were administered i.p. on day 7 and continued 2 times per week for the entire experimental period. Ten mice per were used for each group.
Results
FAP attachment to cells in vitro and in vivo
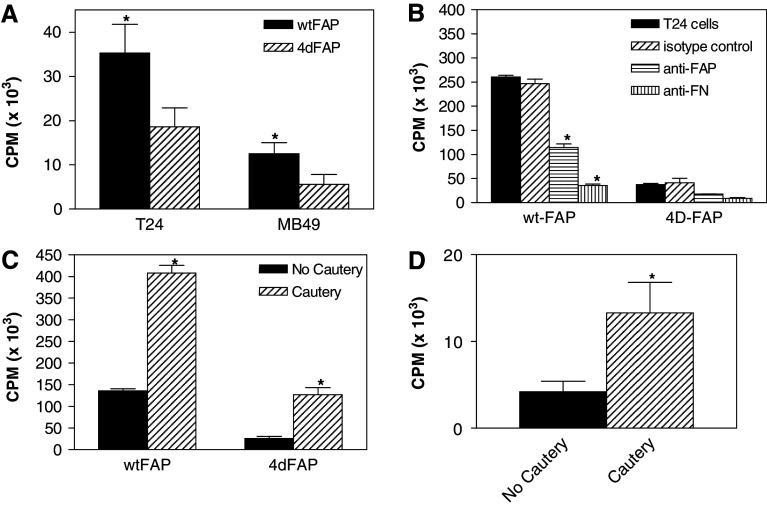
Previous studies have shown that FAP effectively blocks both BCG attachment to bladder tumor cells in vitro and binding to the bladder wall in vivo [41]. One interpretation of the blocking experiments is that under the experimental conditions, FAP acts as a competitive inhibitor of BCG for binding sites. To determine whether FAP actively binds to bladder cancer cells, binding of 125I-labelled FAP was followed. In order to assess whether FAP binding utilizes the previously identified fibronectin-binding motif, RWFV, this experiment was performed with two different FAP constructs: one contained the RWFV amino acid-binding sequence (wtFAP), and another lacking this sequence (4dFAP) [41]. Data in Fig. 1a show that although FAP binds to the bladder tumor lines T-24 and MB-49 in a manner that is not completely dependent on the presence of the RWFV domain, this fibronectin-binding motif is required for a significant proportion of the binding that was observed. This finding is consistent with what has been observed for BCG previously [41]. As reported for BCG binding to T-24 bladder tumor cells, FAP binding to this same line was inhibited by a rabbit polyclonal antibody to FAP. In addition, the binding was inhibited by a rabbit polyclonal antibody to the FAP ligand, fibronectin (FN; Fig. 1b) [14, 41]. These in vitro studies demonstrate that FAP binds to bladder tumor cells and suggest that, like BCG, FAP applied by intravesical instillation would result in binding to the bladder. To directly test this hypothesis, in vivo binding studies were performed with 125I-FAP. Mice were anesthetized, catheterized and either subjected to electrocautery or left untreated. Subsequently, either 125I-wtFAP or 125I-4dFAP was instilled via the inserted catheter. Thirty minutes after instillation, mice were euthanized and their bladders removed, washed and tested for 125I-FAP binding. The data show that 125I-wtFAP is preferentially retained in the bladder (Fig. 1c). Further, as observed for BCG, electrocautery increased 125I-wtFAP retention (Fig. 1c, d). Taken together, these data show that FAP binds to bladder tumor cells in vitro and that it is retained within the bladder after intravesical instillation, suggesting that it has the potential to become a useful tool for inducing an antitumor response.
Fig. 1.
Attachment of FAP to bladder cancer cells and retention within the bladder after intravesical instillation. FAP was purified and labeled with 125I. a Binding of 125I-FAP to T-24 and MB-49 bladder tumor cells; b fibronectin dependence of 125I-FAP binding to T-24 bladder tumor cells; c retention of 125I-FAP within the bladder after intravesical instillation; d retention of 14C-labelled BCG within the bladder after intravesical instillation. * Statistical significance at P ≤ 0.05 using the two-tailed Wilcoxon Rank Sum test
FAP-induced antitumor activity
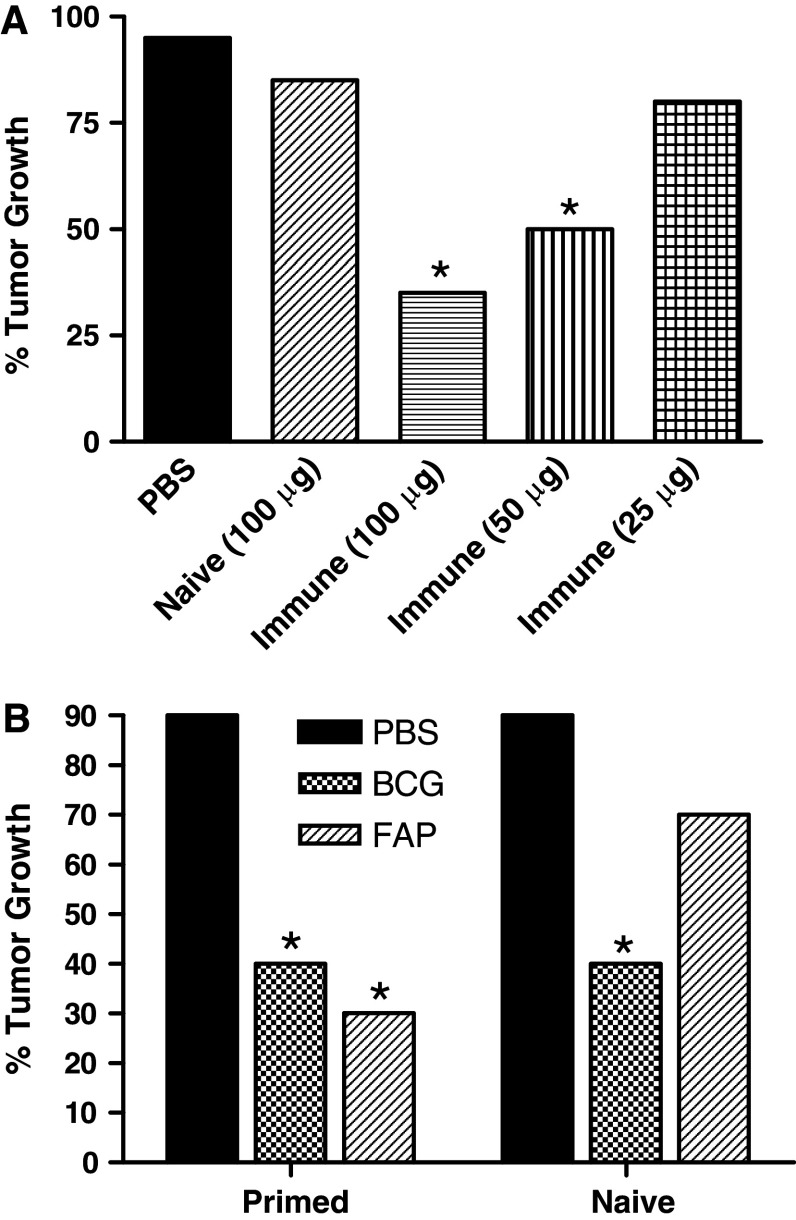
Previous studies have shown that some bacterial proteins, such as KLH, require prior immunization to provide effective antitumor activity, whereas others, such PstS1, do not [33, 37]. Thus, studies were initiated to assess the antitumor potential of FAP in immune and non-immune mice. Mice were preimmunized with FAP as described in “Materials and methods”, and 2 weeks later MB-49 tumor cells were implanted in the bladder (day 0). Subsequently, FAP was instilled intravesically at concentrations of 25, 50 and 100 μg, on both day 1 and day 8. The data show a dose-dependent antitumor response, with FAP significantly inhibiting tumor growth at 50 and 100 μg but not at 25 μg (Fig. 2a). As was previously reported for KLH treatment of bladder tumors, FAP-induced antitumor activity required prior immunization [18, 33, 37].
Fig. 2.
Antitumor activity of FAP in an orthotopic bladder tumor model. a Effect of varying concentrations of FAP on MB-49 bladder tumor growth; b comparison of the antitumor activity of FAP and BCG. * Statistical significance at P ≤ 0.05 using the Fisher’s Exact Chi Square test
Further studies were performed to compare the antitumor activity of FAP with that of BCG. FAP-immunized and naive mice carrying MB-49 tumors were treated with FAP (100 μg) or BCG (107 colony forming units) as outlined in “Materials and methods”. Preimmunization with FAP followed by treatment (intravesical instillation) with FAP was found to be as effective as BCG therapy in inhibiting MB-49 tumor growth (Fig. 2B). Consistent with the results in the dose-response experiment (Fig. 2a), no significant FAP-mediated antitumor activity was observed in naive mice. In contrast, BCG was as effective at inducing antitumor activity in naive mice as in FAP preimmunized mice.
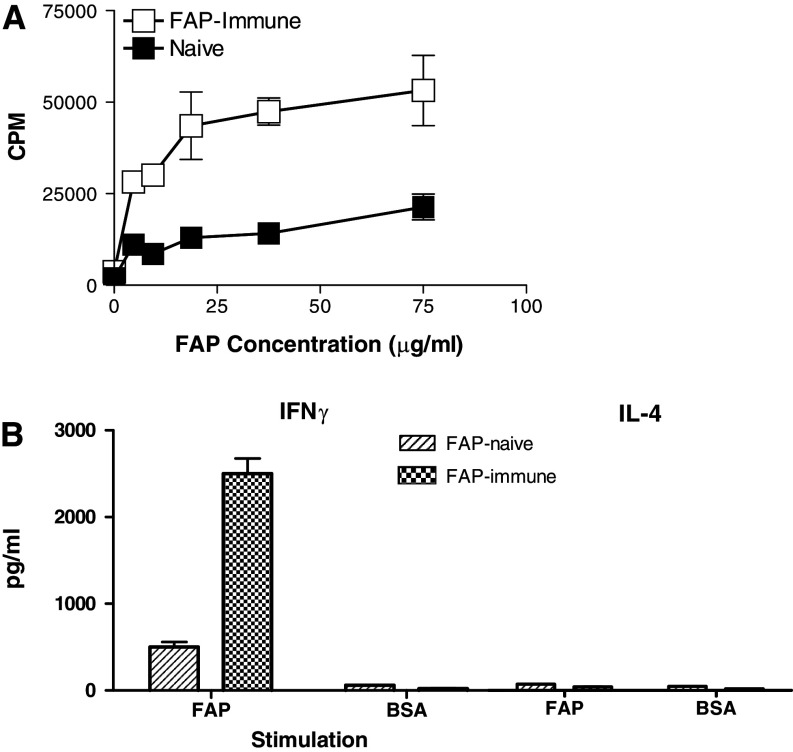
The requirement of FAP preimmunization for effective antitumor activity suggests that T cells participate in its stimulation. To test this theory, we performed in vitro experiments assessing levels of antigen-specific T-cell proliferation in response to FAP administration. Mice were first primed with FAP, and 2 weeks later spleen cells were isolated and assessed for their recognition of FAP in vitro using a 3H-Thymidine proliferation assay. The data show that FAP exposure leads to immunization-specific and dose-dependent proliferation (Fig. 3a). For a positive control, we stimulated both cultures to proliferate in response to anti-CD3 (data not shown). To determine whether FAP induces a Type 1 or Type 2 response, we measured IFNγ (indicative of Type 1 cytokine response) and IL-4 (indicative of Type 2 cytokine response) levels. Antigen-specific IFNγ production was observed in splenocytes isolated from mice that had been both preimmunized and stimulated with FAP (Fig. 3b, c), and these levels were 5-fold higher than those observed in FAP-stimulated naive mice. The data for IL-4, on the other hand, did not reveal an increase in response to FAP exposure. Taken together these in vitro studies show that FAP immunization induces an antigen-specific Type 1 immune response. Furthermore, they formally demonstrate that FAP preimmunization primes mice for this reaction. However, they do not assess the contribution of immunity to the antitumor response.
Fig. 3.
In vitro immune response to FAP. a proliferation of spleen cells from mice previously immunized with FAP monitored by the uptake of 3H-thymidine; b IL4 produced after antigen-specific stimulation of FAP immune spleen cells; c IFNγ produced after antigen-specific stimulation of FAP immune spleen cells
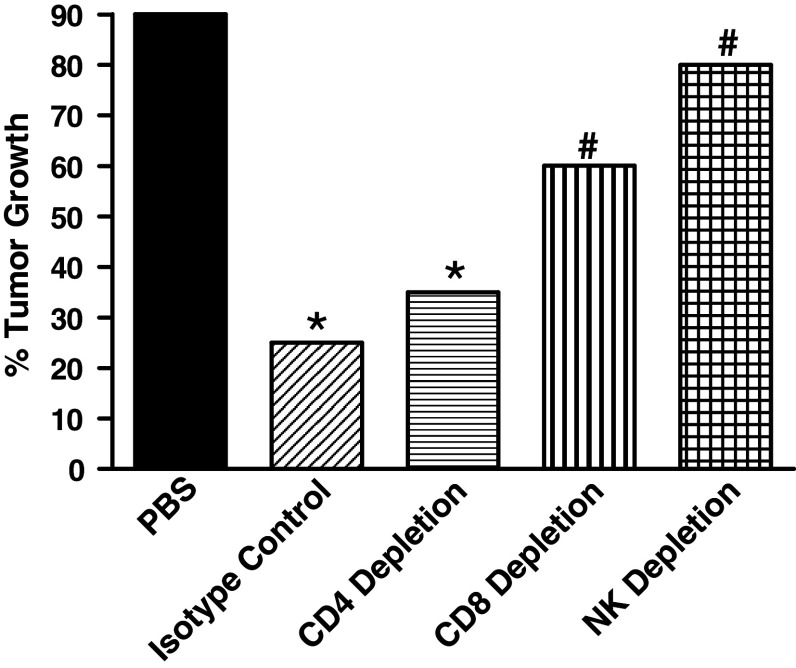
To dissect the relative contributions of T cells and NK cells to FAP-induced antitumor activity, we performed experiments in which CD4+, CD8+, and NK cells were individually depleted in vivo. Mice were first primed with FAP, and 12 days later they were treated with the appropriate lymphocyte-depleting antibody. MB-49 tumors were implanted on day 14, and FAP treatment was initiated 1 day later. We found that the depletion of either CD8+ T cells or NK cells abrogated FAP-induced antitumor activity (Fig. 4), whereas the depletion of CD4+ T cells had no significant effect on the FAP-induced antitumor response.
Fig. 4.
Identification of immune effector population mediating FAP-induced antitumor activity. Mice were depleted of CD4+, CD8+, and NK cells as described in “Materials and methods”. * Statistical significance comparing treatment groups to PBS control at P ≤ 0.05 using the Fisher’s Exact Chi Square test. # statistical significance comparing isotype control with effector cell-depleted groups at P ≤ 0.05 using the Fisher’s Exact Chi Square test
Discussion
Data reported herein demonstrate that the BCG-derived fibronectin attachment protein (FAP) has an effective antitumor activity. Specifically, FAP was shown to bind directly to bladder tumor cells and to be effectively retained in the bladder after intravesical instillation. Antitumor activity was found to be dependent on the immune system, requiring both CD8+ and NK cells for its elimination of tumors. Surprisingly, CD4+ effector T cells do not appear to contribute to the FAP-induced antitumor response. Notably, the FAP-induced antitumor activity observed was found to be equivalent to that of live BCG in terms of its potency, suggesting that it may be possible to use FAP as an alternative to BCG in therapeutic applications.
Currently, BCG is the treatment of choice for superficial bladder cancer [7]. Whereas BCG is effective in most patients, approximately 30% of patients fail to respond to the initial treatment with BCG [2, 7]. Moreover, over time tumors reappear in up to 70% of the patients who are initially responsive [7]. While many individuals who fail to respond to BCG initially do respond to further treatment with either BCG alone or BCG plus interferon, others never become responsive, which creates a dilemma concerning patient management. Furthermore, systemic toxicity can become an issue and, although rare, has resulted in death [16]. Several other factors limit the usefulness of BCG treatment. For example, the potential for systemic infection with BCG precludes its use in immunocompromised patients, including transplant patients as well as individuals positive for human immunodeficiency virus [17]. Also, the fact that treating recurrent low-grade bladder tumors with a higher-risk treatment such as BCG shifts the risk: benefit ratio, preventing some clinicians from proceeding with this approach [4]. Finally, local toxicities can impose limits on sequential BCG treatments—to lower doses in some cases, and to the cessation of BCG treatment altogether in some others [7, 25]. Taken together, these issues highlight the need for new and less toxic anti-bladder tumor agents. Since, in the studies reported herein, FAP appeared to be as effective as BCG at inhibiting tumor growth, FAP may represent a lower-risk alternative to BCG.
Previous studies have reported the use of mycobacterial products to treat bladder cancer [3, 23, 33, 37]. Cell-wall extracts from Mycobacterium phlei (MCWE) inhibit bladder tumor growth in both a murine model and clinical trials [3, 23]. However, in contrast to the observations for FAP-induced antitumor activity, where comparative studies showed FAP to be as potent as BCG in pre-immunized mice, MCWE was significantly less potent [3]. Although the mechanism whereby MCWE induces antitumor activity has not been clearly defined, it appears to be distinct from that of FAP. Like FAP, MCWE modulates immunity through cytokine induction. However, the mechanism of action relevant to its antitumor activity was reported to be an induction of apoptosis specifically in bladder tumor cells [5, 31]. Our findings that FAP-induced antitumor activity depends on both CD8+ T cells and NK cells, and our failure to detect any evidence for cell death in T-24 cells treated with FAP, even at FAP concentrations of up to 100 μg/ml, indicate that antitumor activity induced by FAP is mechanistically distinct from that induced by MCWE.
Other efforts to identify therapies to combat bladder tumors have focused on the soluble mycobacterial protein PstS1, for which Sanger and associates reported significant antitumor activity in a bladder tumor model [33]. Similar to our observations with FAP, these investigators demonstrated a Type I response, with PstS1 also inducing IL12 and IFNγ production in normal human peripheral blood lymphocytes. These data suggest that a Type I response is required for antitumor activity. However, cytokine analyses in the animal model were not performed, leaving the physiological relevance of these findings unclear. Unlike our observations with regard to FAP, effective tumor control with PstS1 was observed only in naive mice. Surprisingly, PstS1 was not active in preimmunized mice. The reason for this is not clear. However, given that the T-cell effector population for FAP-induced antitumor activity is CD8+ T cells, it is possible that PstS1 priming primarily activates CD4+ T cells instead. It is also possible that, in the animal model, PstS1 preimmunization induced a Type II response rather than a Type I response, and the latter appears to be important in bladder tumor therapy [10, 24, 28]. Mitigating against the explanation of a Type II response is data from other investigators, showing that PstS1 induces a strong Type I response [39].
Yet another set of studies has evaluated KLH as an anti-bladder tumor agent [12, 18, 37]. Early clinical studies suggested that the systemic administration of this protein mediates antitumor activity in bladder cancer patients [26, 32]. Subsequent clinical studies, as well as studies in animal models, have clearly demonstrated effective antitumor activity when KLH is administered intravesically [18, 37]. Animal model studies have further shown that, as for FAP, KLH requires preimmunization for optimal antitumor activity, and that it is as potent as BCG in inducing antitumor activity [18, 37]. However, whereas some clinical studies indicated that KLH treatment is more effective than chemotherapy, others failed to confirm this [6, 12]. The reason(s) for the variability in antitumor activity are not clear, but may relate to variations in KLH preparations. With regard to this possibility, it should be noted that KLH exists in multiple forms of varying molecular weight [38]. In addition, studies in an orthotopic bladder cancer model have suggested that the disassociated form of KLH is less effective in inhibiting bladder tumor growth than the associated form [37]. The production of FAP as a recombinant protein eliminates the potential for such variations in the final product, while maintaining effective antitumor activity. As in the case of PstS1, the mechanism whereby KLH induces antitumor activity has not been entirely established. As with FAP, augmentation of NK cells has been reported and suggested to contribute to the KLH antitumor effect, but direct evidence for the participation of this lymphocyte population in KLH-induced antitumor activity is lacking [18]. Furthermore, a role for T-cell immunity in KLH-induced antitumor activity has not been studied. Thus, the potential of KLH for remains limited.
In conclusion, the experiments reported herein investigate the usefulness of FAP in the treatment of bladder cancer. Our findings show that FAP inhibits bladder tumor growth in a dose-dependent manner, and that the antitumor activity in FAP-preimmunized mice is equivalent to that of BCG. In conjunction with the fact that FAP is easy to produce and, more importantly, its application is unlikely to result in severe adverse side effects such as those associated with the instillation of live BCG, they validate further investigation into the use of FAP in the treatment of superficial bladder cancer.
References
- 1.Becich MJ, Carroll S, Ratliff TL. Internalization of bacille Calmette-Guerin by bladder tumor cells. J Urol. 1991;145:1316–1324. doi: 10.1016/s0022-5347(17)38622-6. [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL. Risks and benefits of repeated courses of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer. J Urol. 1987;137:220–224. doi: 10.1016/s0022-5347(17)43959-0. [DOI] [PubMed] [Google Scholar]
- 3.Chin JL, Kadhim SA, Batislam E, Karlik SJ, Garcia BM, Nickel JC, Morales A. Mycobacterium cell wall: an alternative to intravesical bacillus Calmette Guerin (BCG) therapy in orthotopic murine bladder cancer. J Urol. 1996;156:1189–1193. doi: 10.1016/S0022-5347(01)65748-3. [DOI] [PubMed] [Google Scholar]
- 4.Donat SM, North A, Dalbagni G, Herr HW. Efficacy of office fulguration for recurrent low grade papillary bladder tumors less than 0.5 cm. J Urol. 2004;171:636–639. doi: 10.1097/01.ju.0000103100.22951.5e. [DOI] [PubMed] [Google Scholar]
- 5.Filion MC, Lepicier P, Morales A, Phillips NC. Mycobacterium phlei cell wall complex directly induces apoptosis in human bladder cancer cells. Br J Cancer. 1999;79:229–235. doi: 10.1038/sj.bjc.6690038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flamm J, Donner G, Bucher A, Holtl W, Albrecht W, Havelec L. Topical immunotherapy (KLH) vs. chemotherapy (Ethoglucid) in prevention of recurrence of superficial bladder cancer. A prospective randomized study. Urologe A. 1994;33:138–143. [PubMed] [Google Scholar]
- 7.Herr HW, Wartinger DD, Fair WR, Oettgen HF. Bacillus Calmette-Guerin therapy for superficial bladder cancer: a 10-year followup. J Urol. 1992;147:1020–1023. doi: 10.1016/s0022-5347(17)37452-9. [DOI] [PubMed] [Google Scholar]
- 8.Horn C, Namane A, Pescher P, Riviere M, Romain F, Puzo G, Barzu O, Marchal G. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J Biol Chem. 1999;274:32023–32030. doi: 10.1074/jbc.274.45.32023. [DOI] [PubMed] [Google Scholar]
- 9.Hudson MA, Brown EJ, Ritchey JK, Ratliff TL. Modulation of fibronectin-mediated bacillus Calmette-Guerin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Res. 1991;51:3726–3732. [PubMed] [Google Scholar]
- 10.Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD, James K. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guerin (BCG) immunotherapy. Clin Exp Immunol. 1995;99:369–375. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jurincic-Winkler C, Engelmann U, Beuth J, Klippel KF. Efficacy of local bacillus Calmette-Guerin treatment in superficial bladder cancer relapsing under Keyhole-Limpet Hemocyanin immunotherapy. Zentralbl Bakteriol. 1995;282:409–415. doi: 10.1016/s0934-8840(11)80712-7. [DOI] [PubMed] [Google Scholar]
- 12.Jurincic-Winkler CD, Metz KA, Beuth J, Klippel KF. Keyhole limpet hemocyanin for carcinoma in situ of the bladder: a long-term follow-up study. Eur Urol. 2000;37(Suppl 3):45–49. doi: 10.1159/000052392. [DOI] [PubMed] [Google Scholar]
- 13.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–67. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda K, Brown EJ, Telle WB, Russell DG, Ratliff TL. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J Clin Invest. 1993;91:69–76. doi: 10.1172/JCI116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamm DL, Reichert DF, Harris SC, Lucio RM. Immunotherapy of murine transitional cell carcinoma. J Urol. 1982;128:1104–1108. doi: 10.1016/s0022-5347(17)53354-6. [DOI] [PubMed] [Google Scholar]
- 16.Lamm DL. Complications of bacillus Calmette-Guerin immunotherapy. Urol Clin North Am. 1992;19:565–572. [PubMed] [Google Scholar]
- 17.Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, Soloway MS, Steg A, Debruyne FM. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. [DOI] [PubMed] [Google Scholar]
- 18.Lamm DL, DeHaven JI, Riggs DR, Ebert RF. Immunotherapy of murine bladder cancer with keyhole limpet hemocyanin (KLH) J Urol. 1993;149:648–652. doi: 10.1016/s0022-5347(17)36172-4. [DOI] [PubMed] [Google Scholar]
- 19.Lamm DL. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology (Williston Park) 1995;9:947–952. [PubMed] [Google Scholar]
- 20.Laqueyrerie A, Militzer P, Romain F, Eiglmeier K, Cole S, Marchal G. Cloning, sequencing, and expression of the apa gene coding for the Mycobacterium tuberculosis 45/47-kilodalton secreted antigen complex. Infect Immun. 1995;63:4003–4010. doi: 10.1128/iai.63.10.4003-4010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O’Donnell MA, Griffith TS. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 22.Morales A, Eidinger D. Bacillus Calmette-Guerin in the treatment of adenocarcinoma of the kidney. J Urol. 1976;115:377–380. doi: 10.1016/s0022-5347(17)59210-1. [DOI] [PubMed] [Google Scholar]
- 23.Morales A, Chin JL, Ramsey EW. Mycobacterial cell wall extract for treatment of carcinoma in situ of the bladder. J Urol. 2001;166:1633–1637. doi: 10.1016/S0022-5347(05)65642-X. [DOI] [PubMed] [Google Scholar]
- 24.Nadler R, Luo Y, Zhao W, Ritchey JK, Austin JC, Cohen MB, O’Donnell MA, Ratliff TL. Interleukin 10 induced augmentation of delayed-type hypersensitivity (DTH) enhances Mycobacterium bovis bacillus Calmette-Guerin (BCG) mediated antitumour activity. Clin Exp Immunol. 2003;131:206–216. doi: 10.1046/j.1365-2249.2003.02071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol. 2006;24(5):481–487. doi: 10.1007/s00345-006-0112-0. [DOI] [PubMed] [Google Scholar]
- 26.Olsson CA, Chute R, Rao CN. Immunologic reduction of bladder cancer recurrence rate. J Urol. 1974;111:173–176. doi: 10.1016/s0022-5347(17)59919-x. [DOI] [PubMed] [Google Scholar]
- 27.Pang AS, Morales A. BCG induced murine peritoneal exudate cells: cytotoxic activity against a syngeneic bladder tumor cell line. J Urol. 1982;127:1225–1229. doi: 10.1016/s0022-5347(17)54303-7. [DOI] [PubMed] [Google Scholar]
- 28.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol. 1990;144:1248–1251. doi: 10.1016/s0022-5347(17)39713-6. [DOI] [PubMed] [Google Scholar]
- 29.Ratliff TL, Gillen D, Catalona WJ. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. 1987;137:155–158. doi: 10.1016/s0022-5347(17)43909-7. [DOI] [PubMed] [Google Scholar]
- 30.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–1023. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 31.Reader S, Menard S, Filion B, Filion MC, Phillips NC. Pro-apoptotic and immunomodulatory activity of a mycobacterial cell wall-DNA complex towards LNCaP prostate cancer cells. Prostate. 2001;49:155–165. doi: 10.1002/pros.1130. [DOI] [PubMed] [Google Scholar]
- 32.Recker F, Rubben H. Variation of the immunosystem by ciclosporin and keyhole-limpet hemocyanin—are there effects on chemically induced bladder carcinoma? Urol Int. 1989;44:77–80. doi: 10.1159/000281474. [DOI] [PubMed] [Google Scholar]
- 33.Sanger C, Busche A, Bentien G, Spallek R, Jonas F, Bohle A, Singh M, Brandau S. Immunodominant PstS1 antigen of mycobacterium tuberculosis is a potent biological response modifier for the treatment of bladder cancer. BMC Cancer. 2004;4:86. doi: 10.1186/1471-2407-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schorey JS, Holsti MA, Ratliff TL, Allen PM, Brown EJ. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol. 1996;21:321–329. doi: 10.1046/j.1365-2958.1996.6381353.x. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro A, Ratliff TL, Oakley DM, Catalona WJ. Reduction of bladder tumor growth in mice treated with intravesical Bacillus Calmette-Guerin and its correlation with Bacillus Calmette-Guerin viability and natural killer cell activity. Cancer Res. 1983;43:1611–1615. [PubMed] [Google Scholar]
- 36.Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stockle M, Jocham D, Bohle A, Brandau S. Neutrophil granulocytes are required for effective bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–8257. doi: 10.1158/0008-5472.CAN-06-1416. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow RD, Ratliff TL, La Regina M, Ritchey JK, Ebert RF. Immunotherapy with keyhole limpet hemocyanin: efficacy and safety in the MB-49 intravesical murine bladder tumor model. J Urol. 1994;151:1718–1722. doi: 10.1016/s0022-5347(17)35352-1. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow RD, Ebert RF, Lee P, Bonaventura C, Miller KI. Keyhole limpet hemocyanin: structural and functional characterization of two different subunits and multimers. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:537–548. doi: 10.1016/0305-0491(95)02091-8. [DOI] [PubMed] [Google Scholar]
- 39.Vordermeier HM, Coombes AG, Jenkins P, McGee JP, O’Hagan DT, Davis SS, Singh M. Synthetic delivery system for tuberculosis vaccines: immunological evaluation of the M. tuberculosis 38 kDa protein entrapped in biodegradable PLG microparticles. Vaccine. 1995;13:1576–1582. doi: 10.1016/0264-410X(95)00084-E. [DOI] [PubMed] [Google Scholar]
- 40.Zhao W, Schorey JS, Groger R, Allen PM, Brown EJ, Ratliff TL. Characterization of the fibronectin binding motif for a unique mycobacterial fibronectin attachment protein, FAP. J Biol Chem. 1999;274:4521–4526. doi: 10.1074/jbc.274.8.4521. [DOI] [PubMed] [Google Scholar]
- 41.Zhao W, Schorey JS, Bong-Mastek M, Ritchey J, Brown EJ, Ratliff TL. Role of a bacillus Calmette-Guerin fibronectin attachment protein in BCG-induced antitumor activity. Int J Cancer. 2000;86:83–88. doi: 10.1002/(SICI)1097-0215(20000401)86:1<83::AID-IJC13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]