Abstract
Modified vaccinia Ankara (MVA) encoding the tumor antigen 5T4 (TroVax®) has been evaluated in an open label phase II study in metastatic colorectal cancer patients. The primary objective was to assess the safety and immunogenicity of TroVax injected before, during and after treatment with 5-fluorouracil, leukovorin and irinotecan. TroVax was administered to 19 patients with metastatic colorectal cancer. Twelve patients had blood samples taken following each of the six injections and were considered to be evaluable for assessment of immunological responses. Both antibody and cellular responses specific for the tumor antigen 5T4 and the viral vector MVA were monitored throughout the study. Administration of TroVax alongside chemotherapy was safe and well tolerated with no SAEs attributed to the vaccine and no enhancement of chemo-related toxicity. Of the 12 patients who were evaluable for assessment of immune responses, ten mounted 5T4-specific antibody responses with titers ranging from 10 to >5,000. IFNγ ELISPOT responses specific for 5T4 were detected in 11 patients with frequencies exceeding one in 1,000 PBMCs in five patients. Eight patients presented with elevated circulating CEA concentrations, six of whom showed decreases in excess of 50% during chemotherapy and four had CEA levels which remained stable for >1 month following completion of chemotherapy. Of the 19 intention to treat (ITT) patients, one had a CR, six had PRs and five had SD. Potent 5T4-specific cellular and/or humoral immune responses were induced in all 12 evaluable patients and were detectable in most patients during the period in which chemotherapy was administered. These data demonstrate that TroVax can be layered on top of chemotherapy regimens without any evidence of enhanced toxicity or reduced immunological or therapeutic efficacy.
Keywords: Colorectal cancer, Vaccine, Chemo-immuno therapy, Tumor antigen, Immune response
Introduction
Therapies for patients with metastatic cancers, while rarely curative, continue to improve and yield incremental increases in survival. However, it is unlikely that a single compound will emerge as a treatment for all metastatic cancers. Therefore, the ability to layer new treatment modalities on to existing therapies without increasing toxicity may provide the opportunity to enhance clinical benefit; cancer vaccines represent one potential therapeutic approach.
The tumor associated antigen 5T4 is a 72 kDa membrane glycoprotein which is expressed at high levels on the placenta and also on a wide range of human carcinomas including colorectal, renal, gastric and ovarian [4, 10, 16]. Overexpression of 5T4 is associated with metastatic spread and/or poor prognosis in patients with colorectal [18], gastric [17] and ovarian carcinoma [22]. The restricted expression of 5T4 on normal tissues and high prevalence on many common human carcinomas (greater than 80% on colorectal tumors; [17]) make 5T4 an attractive target for cancer immunotherapy. Furthermore, its surface expression means that it could potentially be a target for both cytotoxic T cell (CTL) and antibody-mediated effector responses.
A number of tumor-associated antigens have been engineered into vaccinia virus vectors and the recombinant vaccines shown to induce TAA specific immune responses in cancer patients [2, 11, 14, 15, 19]. We selected MVA as an appropriate system with which to deliver the 5T4 tumor antigen. TroVax (MVA encoding 5T4) has been tested in a phase I/II trial in colorectal cancer patients which showed the product to be safe, well tolerated and to induce 5T4-specific immune responses in the majority of patients [7]. Furthermore, 5T4-specific antibody responses were shown to correlate with clinical benefit.
The use of chemotherapy regimens alongside immunotherapy approaches has been viewed as counter intuitive since chemotherapy can have deleterious effects on cells of the immune system such as the induction of lymphopaenia [11]. Therefore, few clinical trials have investigated the relationship between the two treatment modalities. However, a number of studies have shown that cyclophosphamide might modify the T cell compartment to enable expansion of specific T cells in a lymphopenic compartment or may modify effector responses by promoting TH1 rather than TH2 responses or depleting regulatory T cells [3, 9, 13]. Despite such results, many conclusions are based on data derived from animal models and it is important that such concepts are proven and applied in human trials. Recently, we have shown that TroVax is safe, well tolerated and remains highly immunogenic when administered alongside 5-fluorouracil, leukovorin and oxaliplatin [8]. Previously, Weihrauch and colleagues [21] demonstrated proof of principle that a carcinoembryonic antigen (CEA) peptide vaccine was immunogenic when administered alongside 5-fluorouracil, leukovorin and irinotecan, another commonly used chemotherapy regimen for metastatic colorectal cancer.
Given the encouraging safety and efficacy data obtained when TroVax was administered alongside 5-fluorouracil, leukovorin and oxaliplatin [8] and the promising results reported by Weihrauch and colleagues [21] using the irinotecan regimen, we felt that it was important to test TroVax in combination with 5-fluorouracil, leukovorin and irinotecan. The primary objective of the trial was to assess the safety and immunogenicity of TroVax injected intramuscularly and given before, during and after treatment with 5-fluorouracil, leukovorin and irinotecan. Here, we report on the safety of the vaccine in combination with chemotherapy and provide a detailed description of the immune responses induced by TroVax when administered to colorectal cancer patients.
Materials and methods
Patient characteristics
This phase II trial was an open label study of TroVax administered by intramuscular injection to patients with advanced colorectal cancer receiving 5-FU, leukovorin and irinotecan as first line therapy. All patients had histologically proven colorectal cancer, a WHO performance status of 0, 1 or 2, a life expectancy of ≥3 months, were aged ≥18 years and had adequate haematological and liver function. The trial protocol was approved by the United Kingdom Gene Therapy Advisory Committee (GTAC) and the study conducted under a Clinical Trial Exemption (CTX) granted by the Medicines and Healthcare products Regulatory Agency (formerly the MCA). The trial was approved by the Local Research Ethics Committees and informed consent was obtained from each patient prior to enrolment.
Vaccine composition
TroVax was produced by the homologous recombination of human 5T4 cDNA into deletion region III of MVA and placed under the control of the modified H5 promoter [6]. Clinical grade material was manufactured and vialled under GMP conditions (IDT, Rosslau, Germany).
Clinical trial design
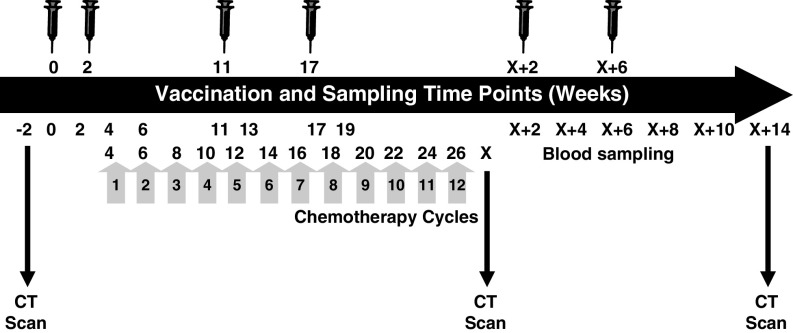
On entering the trial, each patient underwent chest, abdominal and pelvic CT scans to quantify tumor metastases. Further scans were taken at weeks 14 (non-protocol driven), X (completion of chemotherapy at approximately 26 weeks) and X + 14 (∼40 weeks) post primary TroVax immunization (Fig. 1). Patients received IrMdG [11; 180 mg m2 irinotecan combined with modified de Gramont FU/LV (bolus 5FU plus high dose 46 h 5FU infusion)] bi-monthly at 2 week intervals starting at week 4 with up to 12 cycles being administered, depending on clinical response and tolerance. Two TroVax immunizations were given before chemotherapy (weeks 0 and 2), 2 during chemotherapy (weeks 11 and 17) and 2 following completion of chemotherapy (weeks X + 2 and X + 6; completion of chemotherapy is indicated as week X). Patients received approximately 5 × 108 pfu TroVax via intramuscular injection in a volume of 1 ml into the deltoid muscle. Blood was taken at screening and before and after each immunization to assess the induction of immune responses to 5T4 and to the MVA vector. In addition, the plasma concentration of the surrogate marker, carcinoembryonic antigen (CEA), was measured throughout the trial. The primary objective was to assess the safety and immunogenicity of TroVax injected before, during and after treatment with 5-fluorouracil, leukovorin and irinotecan. Clinical efficacy was assessed by analysis of CT scan data according to RECIST criteria.
Fig. 1.
Vaccination, chemotherapy and blood sampling schedule. The schematic illustrates the timing of each vaccination (syringe) and the time points at which blood samples were taken for monitoring of immune responses and CT scans were performed to monitor disease progression
Antigens
Purified recombinant 5T4 protein [6] was used to monitor cellular and humoral responses by IFNγ ELISPOT and ELISA, respectively. In addition, overlapping 10mer peptides spanning the entire 5T4 sequence (Mimotopes, VIC, Australia), a pool of 23 known CTL epitopes, with multiple HLA restrictions, derived from CMV, EBV and Flu (CEF peptides; Mabtech, Sweden) and MVA were utilized to measure cellular responses. CEF peptides served as a positive control for the IFNγ ELISPOT and enabled any changes in immune responsiveness to be assessed before and after TroVax vaccination. 5T4 peptides were used in 20 sequential pools, each pool containing ten adjacent peptides. All 20 peptide pools were used in ELISPOT assays where PBMC availability allowed.
Measurement of antibody responses
The enzyme linked immunosorbent assay (ELISA) was used to measure 5T4 and MVA specific antibody titers as described previously [1]. Antibody titers were defined as the greatest dilution of plasma at which the mean optical density (OD) of the test plasma was ≥twofold the mean OD of the negative control (normal human plasma) at the same dilution. A positive response due to vaccination was reported if the post-injection antibody titer was ≥twofold the antibody titer determined prior to TroVax immunization.
Measurement of cellular responses
The IFNγ ELISPOT was used to monitor cellular responses throughout the trial as detailed previously [8]. A positive ELISPOT response induced by TroVax was reported if the mean spot forming units (SFU) per well in response to antigen was ≥threefold the mean SFU/well in wells containing medium alone and the mean SFU/well in response to antigen is ≥10 and the antigen specific precursor frequency (number of antigen specific cells per 106 total PBMCs), after immunization was ≥twofold the precursor frequency prior to TroVax vaccination.
In addition to analysis of cellular responses by IFNγ ELISPOT, MHC multimers (Pentamer; ProImmune, UK) were also used to quantify 5T4-specific CD8+ T cells. Two unlabelled MHC Pentamers specific for HLA-A2 restricted 5T4 CTL epitopes were synthesized (ProImmune, UK): A*0201/RLARLALVL (peptide #9) and A*0201/FLTGNQLAV (peptide #49). Following thawing of patients’ PBMCs, 2 × 106 cells were incubated with one test of unlabelled Pentamer for 10 min at room temperature. PBMCs were then washed with wash buffer (0.1% sodium azide, 2% fetal calf serum in PBS) and each sample incubated with Fluorotag (ProImmune, UK) and anti-CD8 FITC (PharMingen, UK) for 20 min on ice. Cells were again washed and incubated with anti-CD45RO APC (Caltag, UK) or anti-CD45RA APC (PharMingen) for 20 min on ice. Finally, cells were washed and resuspended in fixative (1% fetal calf serum, 2.5% formaldehyde in PBS) prior to flow cytometric analysis using a FacsCalibur (Becton Dickinson, UK).
Results
Patients
In total, 19 patients were enrolled into the trial and included in the intention-to-treat (ITT) population. Seven patients withdrew from the trial prior to becoming evaluable for assessment of immunological and clinical responses as follows; two patients were withdrawn due to progressive disease (010 at week 32 and 013 at week 19), one patient (001) withdrew consent and four patients (004, 006, 008 and 011) withdrew due to SAEs unrelated to TroVax. The SAEs leading to patient withdrawal were as follows: patient 004 had raised liver enzymes requiring insertion of a biliary stent; patient 006 presented with breathlessness and Hickman line infection; patients 008 and 011 had renal failure. The mean age (±SD) of the ITT population was 61.8 ± 6.4 years (range 46–73 years). The characteristics of the ITT patient group are detailed in Table 1. The median number of TroVax doses received in the ITT population was six (range 1–6 doses) and 12 cycles of chemotherapy (range 0–12 cycles). Twelve patients had blood samples taken following each of the six injections and were considered to be evaluable for assessment of immunological responses.
Table 1.
ITT patient characteristics
| Patient number | Age | Sex | Site of target metastatic lesion(s) | Number of TroVax vaccinations | No. of chemo cycles | Evaluable for immune response | Circulating CEA (μg/L) |
|---|---|---|---|---|---|---|---|
| Baseline | |||||||
| 001 | 60 | F | Presacral mass | 2 | 1 | No | 42 |
| 002 | 65 | M | Nodes | 6 | 12 | Yes | 3 |
| 003 | 66 | M | Lung, node | 6 | 12 | Yes | 15.4 |
| 004 | 55 | M | Nodes | 1 | 0 | No | 29.9 |
| 005 | 58 | M | Lung, liver, node | 6 | 12 | Yes | 476 |
| 006 | 66 | M | Lung, liver, adrenal | 2 | 0 | No | 369 |
| 007 | 62 | F | Lung, liver, node | 6 | 12 | Yes | 57 |
| 008 | 68 | F | Liver, lung | 1 | 0 | No | 363 |
| 009 | 64 | F | Liver | 6 | 12 | Yes | 361 |
| 010 | 49 | F | Liver | 4 | 12 | No | 4 |
| 011 | 66 | M | Aortocaval, para-aortic glands | 1 | 0 | No | 3 |
| 012 | 64 | M | Liver, lung, node | 6 | 12 | Yes | 9 |
| 013 | 65 | F | Lung | 3 | 6 | No | 8 |
| 014 | 73 | M | Lung | 6 | 12 | Yes | 3.8 |
| 015 | 46 | M | Node | 6 | 12 | Yes | 12 |
| 016 | 63 | M | Lung | 6 | 12 | Yes | 5 |
| 017 | 62 | M | Lung | 6 | 12 | Yes | 3.7 |
| 018 | 61 | M | Liver, node | 6 | 12 | Yes | 36 |
| 019 | 62 | M | Liver | 6 | 12 | Yes | 1,360 |
The table details the age, sex, main sites of metastatic disease and the total number of TroVax injections and chemotherapy cycles received. In addition, the level of circulating CEA (μg/L) detectable at baseline is tabulated. Key N/A = not available
Safety
No TroVax related SAEs were reported in the ITT population. The most frequent adverse event (probably or definitely) related to TroVax administration was soreness at the site of injection that occurred in nine (47%) patients, eight of which were CTC grade 1 and one which was grade 2. The other toxicities experienced by patients were in keeping with those expected from 5-fluoruracil/leukovorin/irinotecan given at these doses, e.g. high frequency of nausea, diarrhoea, lethargy, anemia and lymhpopenia.
TroVax induced antibody responses
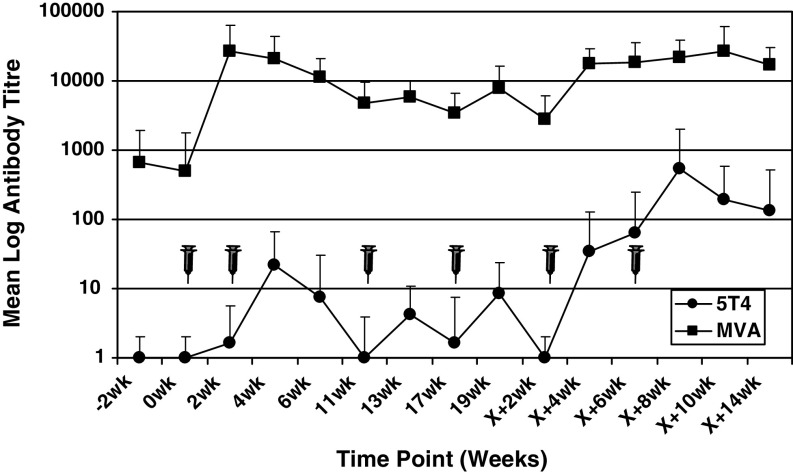
5T4 and MVA-specific antibodies were quantified by ELISA in the 12 patients who were evaluable for assessment of immune response. At each sampling time point, antibody levels were expressed as a titer compared to the negative control plasma. The kinetics of the mean MVA and 5T4 antibody titers for all 12 patients are plotted in Fig. 2 at each sampling time point throughout the clinical trial time course.
Fig. 2.
5T4 and MVA-specific antibody responses. The results are plotted as the mean log 5T4 (filled circle) or MVA (filled square) antibody titre at each sampling time point across the entire clinical trial time course. Standard deviations are plotted and vaccination time points illustrated with a syringe
No patient had a detectable 5T4 specific antibody titer prior to TroVax immunization and two patients (002 and 005) failed to show an increase in antibody titer following vaccination (data not shown). However, ten patients showed 5T4 specific antibody titers (ranging from 10 to 5, 120) which were detectable after two or more vaccinations in the majority of patients. During the period in which patients received chemotherapy (weeks 4–19), five patients had detectable antibody responses, while ten patients showed positive antibody responses following completion of chemotherapy and mean antibody titres increased significantly from 4.5 during chemotherapy to 163.3 following completion of chemotherapy (weeks X + 2 to X + 14; P < 0.01 signed rank test). It was encouraging to note that the mean antibody titer for all 12 evaluable patients was boosted after each TroVax immunization when compared to the titer measured on the day of vaccination.
As would be predicted, antibody responses to the viral vector (MVA) were significantly higher than to the 5T4 self-antigen. Three patients (003, 015 and 018) had detectable MVA-specific antibody titers (2,000 or 4,000) prior to TroVax immunization (data not shown). Following TroVax vaccination, all 12 evaluable patients showed either de novo MVA-specific antibody responses or an increase in antibody titer (patients 003, 015 and 018) with titers ranging from 4,000 to 128,000. Positive responses were detectable after a single vaccination in the majority of patients and remained elevated during the period in which patients received chemotherapy. However, patients 012 and 014 showed weak (patient 012) or no MVA specific antibody response (patient 014) before or during the period in which chemotherapy was administered. A similar pattern was seen for the 5T4 antibody response in these patients. The mean MVA antibody titer for all 12 evaluable patients during the period in which chemotherapy was administered (weeks 4–19) was 6,700 which increased significantly to 17,367 following completion of chemotherapy (weeks X + 2 to X + 14; P < 0.01 signed rank test).
TroVax induced IFNγ ELISPOT and MHC multimer responses
IFNγ ELISPOT responses to a panel of antigens were monitored using thawed PBMCs which were allowed to recover overnight and then used directly without any in vitro restimulation steps. Responses to the CEF positive control peptide pool, MVA, 5T4 protein and 5T4 peptides are detailed in Table 2a and b. Positive responses to the control CEF peptide pool were detected in nine patients with frequencies reaching 1 in 548 PBMCs (0.18%) specific for one or more of the CEF peptides (Table 2a). Within the nine patients who responded to CEF, the frequencies of antigen-specific cells were highly consistent throughout the trial monitoring period both prior to, and post TroVax vaccination. The greatest difference in CEF responses detected prior to, versus post TroVax vaccination was 2.5, with a mean of 1.44 (range 0.92–2.5-fold).
Table 2.
Antigen-specific IFNγ ELISPOT responses
| a | ||||
|---|---|---|---|---|
| Patient number | Antigen | Maximum antigen specific T cell frequency | Fold increase post:pre TroVax immunization | |
| Pre-TroVax immunization | Post TroVax immunization | |||
| 002 | CEF | 1/885 | 1/936 | 0.95 |
| 5T4 Pep #77 | ≤1/200,000 | 1/4,717 | ≥42.4 | |
| MVA | 1/1,404 | 1/616 | 2.3 | |
| 003 | CEF | 1/588 | 1/639 | 0.92 |
| 5T4 Pep #8 | ≤1/200,000 | 1/27,027 | ≥7.4 | |
| MVA | 1/6,250 | 1/581 | 10.8 | |
| 005 | CEF | 1/1,029 | 1/688 | 1.5 |
| 5T4 Pep #41 | ≤1/200,000 | 1/2,096 | ≥95.4 | |
| MVA | 1/1,477 | 1/628 | 2.4 | |
| 007 | CEF | 1/10,526 | 1/4,292 | 2.5 |
| 5T4 Pep # | ≤1/200,000 | ≤1/200,000 | – | |
| MVA | 1/1,408 | 1/1,028 | 1.4 | |
| 009 | CEF | 1/665 | 1/566 | 1.2 |
| 5T4 Pep #8 | ≤1/200,000 | 1/726 | ≥275 | |
| MVA | N/A | 1/1,147 | N/A | |
| 012 | CEF | 1/625 | 1/548 | 1.1 |
| 5T4 Pep #5 | ≤1/200,000 | 1/2,033 | ≥98.4 | |
| MVA | N/A | 1/1718 | N/A | |
| 014 | CEF | N/A | 1/626 | N/A |
| 5T4 Pep | ≤1/200,000 | ≤1/200,000 | – | |
| MVA | N/A | 1/633 | N/A | |
| 015 | CEF | ≤1/200,000 | ≤1/200,000 | – |
| 5T4 Pep #41 | ≤1/200,000 | 1/23,256 | 8.6 | |
| MVA | 1/853 | 1/659 | 1.3 | |
| 016 | CEF | 1/8,547 | 1/4,587 | 1.9 |
| 5T4 Pep #41 | ≤1/200,000 | 1/3,597 | 55.6 | |
| MVA | 1/2421 | 1/792 | 3 | |
| 017 | CEF | ≤1/200,000 | ≤1/200,000 | – |
| 5T4 Pep #13 | ≤1/200,000 | 1/25,000 | ≥4 | |
| MVA | 1/730 | 1/762 | 0.96 | |
| 018 | CEF | 1/3,205 | 1/3,448 | 0.93 |
| 5T4 Pep #1 | ≤1/200,000 | 1/1,792 | ≥112 | |
| MVA | 1/919 | 1/832 | 1.1 | |
| 019 | CEF | 1/932 | 1/733 | 1.3 |
| 5T4 Pep #9 | ≤1/200,000 | 1/22,222 | 9 | |
| MVA | 1/1,770 | 1/1,429 | 1.2 | |
| b | ||||
|---|---|---|---|---|
| Patient number | Sum maximum 5T4 polyclonal precursor frequencies | |||
| Time point (week) | Peptides alone | Time point (week) | Protein ± peptides | |
| 002 | X + 10 | 1/4,717 | 13 | 1/2,208 |
| 003 | X + 8 | 1/27,027 | X + 8 | 1/9,372 |
| 005 | X + 6 | 1/868 | X + 6 | 1/706 |
| 007 | Any | ≤1/200,000 | X + 6 | 1/626 |
| 009 | 4 | 1/243 | 4 | 1/243 |
| 012 | X + 2 | 1/217 | X + 2 | 1/192 |
| 014 | Any | ≤1/200,000 | Any | ≤1/200,000 |
| 015 | 19 | 1/23,256 | 19 | 1/23,256 |
| 016 | X + 2 | 1/3,597 | X + 2 | 1/3,597 |
| 017 | X + 2 | 1/25,000 | X + 2 | 1/25,000 |
| 018 | X + 10 | 1/1050 | X + 6 | 1/625 |
| 019 | 19 | 1/22,222 | 19 | 1/22,222 |
aDetails the maximum antigen-specific T cell frequencies detected at baseline (pre-TroVax) and post-TroVax immunization following stimulation of patients’ PBMCs with the CEF positive control peptide pool, 5T4 peptide pools and MVA. The fold increase in antigen-specific T cell frequency following TroVax immunization is tabulated
bIllustrates the maximum 5T4 specific responses detected at any time point to ≥1 5T4 peptide pool or 5T4 peptide(s) plus protein (i.e. the sum of individual responses at one time point)
Positive ELISPOT responses to MVA were detected in nine patients prior to TroVax immunization (results from three patients were not available prior to TroVax administration) of which four showed a >twofold increase in MVA response following vaccination. The frequencies of MVA-specific T cells attained a similar magnitude as CEF with a maximum of one in 581 PBMCs (0.17%) specific for MVA. The mean fold increase in MVA-specific T cells was 2.7 in the nine patients where pre and post-vaccination results were available.
Positive ELISPOT responses to single 5T4 peptide pools occurred in patients with similar frequency to those detected for CEF and MVA. Ten patients demonstrated increases in 5T4 peptide specific T cells following TroVax vaccination with frequencies reaching 1 in 726 PBMCs (0.14%). Following vaccination, the mean fold increase in the frequency of 5T4-specific cells was ≥70.8 (range 1–≥275). If the responses to multiple peptides (i.e. 5T4-specific polyclonal responses) are considered at any one-time point, frequencies in excess of one in 1,000 PBMCs were detected in three patients (Table 2b). If responses to 5T4 protein are also included, five patients showed frequencies of 5T4-specific cells exceeding one in 1,000 with the maximum level detected being 1 in 192 PBMCs in patient 012.
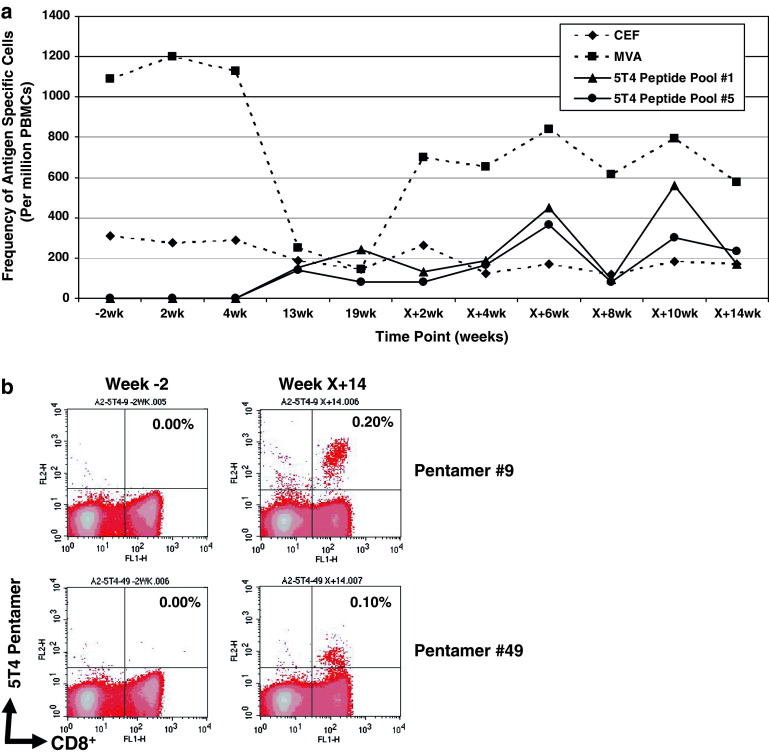
Unfortunately, the limited availability of PBMCs from all patients prevented a complete analysis of the kinetics of cellular responses at every time point and the dissection of positive responses detected to pools of peptides in some responding patients. However, Fig. 3a illustrates the kinetics of IFNγ ELISPOT responses in a responding patient (018) where PBMC yields enabled analysis at most sampling time points. Responses to the CEF peptide pool remained relatively consistent throughout the time course. MVA responses were strongly positive prior to TroVax vaccination and did not increase following vaccination. Indeed, during chemotherapy (weeks 13 and 19), the response to MVA decreased dramatically but recovered following completion of chemotherapy and remained relatively consistent until the end of the study. In contrast ELISPOT responses to 5T4 peptide pools 1 and 5 were negative prior to TroVax vaccination (week-2) but positive at weeks 13 and 19 (during the period in which the patient received chemotherapy). These responses increased further when additional TroVax vaccinations were administered following the completion of chemotherapy and were still strongly positive at the end of the trial (approximately 40 weeks after the first TroVax vaccination). Subsequently, the positive ELISPOT responses to peptide pools 1 and 5 were dissected by analysing the responses to the constituent peptides. Positive ELISPOT responses were detected against a single peptide in each pool, peptide #9 in pool 1 and peptide #49 in pool 5. Further analysis showed these peptides to be restricted through HLA-A2 (manuscript submitted). FACS analysis of PBMCs from patient 018 using HLA-A2 pentamers specific for peptides #9 and #49 demonstrated a negative response at baseline but positive responses at week X + 14 following TroVax vaccination (Fig. 3b) with 0.2 and 0.1% of CD8+ T cells being specific for peptide #9 (RLARLALVL) and #49 (FLTGNQLAV) respectively. Subsequent analysis demonstrated that these pentamer positive cells were CD45RO+ and CD45RA− indicative of an effector memory phenotype (data not shown). The use of a negative control pentamer gave no positive staining at either time point (data not shown).
Fig. 3.
IFNγ ELISPOT and MHC multimer (pentamer) responses in patient 018. a Illustrates the kinetics of IFNγ ELISPOT responses following incubation of patients’ PBMCs with MVA, CEF peptides and two 5T4 peptide pools (1 and 5). Responses are plotted as the number of antigen-specific T cells per million PBMCs. b Illustrates HLA-A2 pentamer responses specific for peptides #9 and #49 detected by flow cytometry prior to TroVax vaccination (week-2) and following six TroVax vaccinations (week X + 14). The percentage of CD8+, pentamer positive cells is detailed in the top right quadrant of each plot
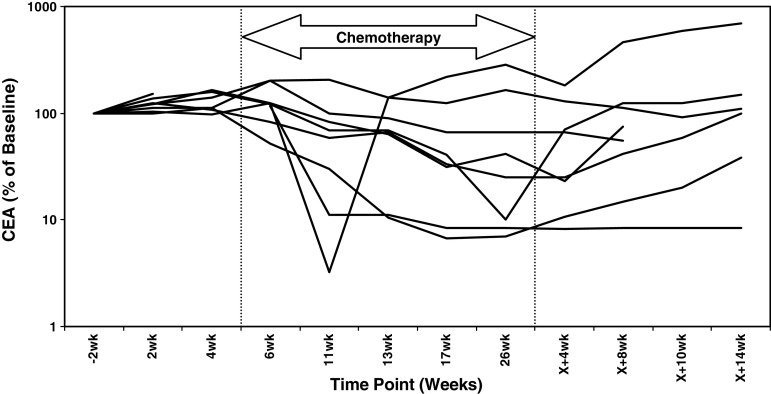
CEA responses
Circulating CEA levels were determined at baseline for all ITT patients (Table 1). Furthermore, CEA levels were quantified before, during and after completion of chemotherapy in the 12 patients who were evaluable for assessment of immune responses. Of these 12 patients, eight had elevated CEA levels (>5 μg/L) at baseline and six showed a >50% reduction at ≥1 time point throughout the trial (Fig. 4). In the majority of patients, the nadir in CEA levels occurred during the period in which both chemotherapy and TroVax were administered. However, CEA levels remained stable in four patients (003, 012, 018 and 019) for ≥1 month following completion of chemotherapy when TroVax was administered alone. These four patients mounted both antibody and cellular responses specific for 5T4 in the period when TroVax was administered alone following completion of chemotherapy (weeks X + 2 to X + 14).
Fig. 4.
Normalized CEA levels. The figure plots the magnitude of circulating CEA levels as a percentage relative to baseline concentrations in eight patients over the clinical trial time course. The period in which chemotherapy was administered is illustrated
Clinical responses
CT scans were mandated at weeks X (approximately 26-week-post primary immunization and following completion of chemotherapy) and X + 14 for all patients. However, it should be noted that an analysis of clinical responses was not possible on the ITT population as seven patients withdrew from the study prior to receiving the first protocol mandated CT scan at week X. CT scan data were also available for nine patients at week 14 (six cycles of chemotherapy and three TroVax administrations) and a partial response was observed in one patient (Table 3). Upon completion of chemotherapy (week X, approximately 26 weeks following primary immunization), seven patients (58.3%) showed radiological responses which consisted of one CR (8.3%) and six PRs (50%). As clinical efficacy was not a primary endpoint in this study, confirmatory CT scans ≥4 weeks later were not mandated. However, a CT scan performed at week X + 14 (40 weeks post primary immunization) showed a continued response (CR) in patient 002. that for all but two patients the response observed at week 14 was maintained. The median survival was 15.4 months in the 19 ITT patients and 18.6 months in the 12 evaluable patients. Three patients (002, 015 and 017) remain alive with a minimum follow up of 33.6 months. Patient 015 had positive 5T4-specific immune response detectable at week X + 10 (but was negative at week X + 14) while patients 002 and 017 had robust 5T4-specific immune responses at the end of the trial time course (week X + 14).
Table 3.
Summary tumor responses (RECIST) detected in all evaluable patients
| Patient number | Tumor response (week) | ||
|---|---|---|---|
| 14 | X (26 weeks) | X + 14 | |
| 002 | SD | CR | CR (40) |
| 003 | N/A | PR | PD (39) |
| 005 | SD | PR | PD (40) |
| 007 | SD | SD | PD (40) |
| 009 | SD | SD | PD (38) |
| 012 | PR | PR | PD (47) |
| 014 | N/A | SD | N/A |
| 015 | SD | PR | PD (40) |
| 016 | SD | PR | PD (40) |
| 017 | N/A | PR | PD (42) |
| 018 | SD | SD | PD (40) |
| 019 | SD | SD | PD (40) |
The table details the reported tumor responses at weeks 14, X (week 26) and X + 14 (the actual week post-primary immunization is indicated in parentheses)
Discussion
Currently, few clinical studies have investigated the use of an immunotherapy alongside chemotherapy due to the perceived negative impact of cytotoxic agents on cells of the immune system. However, this may not be true for all chemotherapy regimens and a number of publications have demonstrated that certain chemotherapeutic agents could have a positive impact on the induction of immune responses [3, 21]. Despite this fact, few studies have undertaken a systematic analysis of vaccine-induced immune responses occurring in cancer patients before, during and after treatment with a standard of care chemotherapy regimen.
We have demonstrated that the administration of TroVax in combination with 5FU/leukovorin and irinotecan is both safe and capable of inducing potent 5T4-specific immune responses. Indeed, all 12 evaluable patients mounted 5T4-specific cellular and/or humoral immune responses at one or more time point(s) throughout the trial. 5T4-specific antibody responses were, in general, of greater magnitude and longevity than those detected in a phase I/II study in which TroVax was administered as a monotherapy to stage IV colorectal cancer patients [7]. There was some evidence that immune responses (primarily antibody) were diminished during the period in which patients received chemotherapy and this was more evident in the context of 5FU/leukovorin and irinotecan than we reported previously using a different chemotherapy regimen [8].
The magnitude of IFNγ ELISPOT responses specific for 5T4 was very high and in some patients, was comparable to those detected to MVA and CEF. Since MVA and CEF contain multiple antigens derived from viruses, the similarity in the magnitude of the responses to a self-antigen (5T4) was surprising. Using PBMCs from patients enrolled in this study, we have identified two HLA-A2 restricted epitopes and confirmed the magnitude of the 5T4-specific responses using MHC multimers. These responses were detectable at the end of the study (week X + 14), which was approximately 10 months following the first TroVax vaccination and 2 months following the final immunization, suggesting good longevity of 5T4-specific responses.
Few, if any, cancer vaccines have demonstrated the induction of cellular responses specific for a tumor antigen in such a large proportion of patients and at such a high magnitude [5]. Data from a similar trial in which patients received TroVax in combination with 5FU/leukovorin and oxaliplatin also demonstrated the induction of potent 5T4-specific cellular and humoral immune responses in the majority of patients [8]. Furthermore, a significant correlation was detected between 5T4, but not MVA-specific ELISPOT responses and clinical benefit (tumor response). No similar correlation was evident in the study reported here despite the magnitude of 5T4-specific ELISPOT responses being similar in both studies. Differences in the kinetics of the 5T4-specific immune responses detected in these two TroVax plus chemotherapy studies may potentially explain the lack of correlation. For example, 5T4 antibody responses were significantly higher at earlier time points in the vaccination schedule in patients receiving 5FU/leukovorin and oxaliplatin.
Since this trial was a small, open-label single arm study, in which tumor response was not a primary end-point, it is not appropriate to comment extensively on the clinical responses detected in this patient cohort. However, the frequency of radiological responses were of a similar order to those seen in studies using comparable chemotherapy regimens alone [12, 20] indicating no negative impact of TroVax on delivery or activity of the chemotherapy. Levels of the surrogate marker CEA decreased during chemotherapy in most patients and it was encouraging that four patients showed stable CEA concentrations for more than 1 month following completion of chemotherapy. Since these four patients mounted 5T4-specific antibody and/or humoral responses in this post-chemotherapy period, it is possible that such immune responses could be responsible for the stabilization of CEA levels.
Although no firm conclusions can be drawn from this study about possible synergy between TroVax and cytotoxic chemotherapy, it was encouraging that 5T4-specific immune responses were induced in all evaluable patients and the primary objective was met.
With the possible exception of surgery, it is unlikely that a single treatment modality will deliver a cure for malignancies, especially in the context of metastatic disease. For this reason, it is essential that combination therapy approaches are explored. However, many cytotoxic or cytokine therapies have significant toxicity profiles, therefore great care is needed in combining two different treatment modalities. Previously, we have demonstrated that TroVax has an excellent safety profile and causes no or very limited side effects when used as a monotherapy in colorectal cancer patients [7]. We have now extended this observation and demonstrated that the safety profile and immunological efficacy of the vaccine has been maintained in the context of two widely used chemotherapy regimens (this publication and 8). Combining a cancer vaccine such as TroVax with chemotherapy may be beneficial for a number of reasons. The use of a cytotoxic agent will result in some tumor cell death which could provide an ideal inflammatory environment for the migration of TAA specific cells to the tumor site. In addition, the release of TAAs such as 5T4 from dying tumor cells could further boost immune responses. For these reasons, the priming of an immune response prior to administration of chemotherapy may be beneficial. Furthermore, the fact that chemotherapy results in a reduced tumor load in most patients may provide the ideal setting of minimal residual disease for a cancer vaccine approach to demonstrate efficacy. We believe that these observations provide a sound justification for the continued development of TroVax alongside other standard of care therapies.
References
- 1.Braybrooke JP, Slade A, Deplanque G, et al. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin Cancer Res. 2005;11:1512. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- 2.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632. [PubMed] [Google Scholar]
- 3.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths RW, Gilham DE, Dangoor A, et al. Expression of the 5T4 oncofoetal antigen in renal cell carcinoma: a potential target for T-cell-based immunotherapy. Br J Cancer. 2005;93:670. doi: 10.1038/sj.bjc.6602776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv Drug Deliv Rev. 2006;58:931. doi: 10.1016/j.addr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Harrop R, Ryan MG, Myers KA, et al. Active treatment of murine tumors with a highly attenuated vaccinia virus expressing the tumor associated antigen 5T4 (TroVax) is CD4+ T cell dependent and antibody mediated. Cancer Immunol Immunother. 2006;55:1081. doi: 10.1007/s00262-005-0096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrop R, Connolly N, Redchenko I, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 8.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 9.Hermans IF, Chong TW, Palmowski MJ, et al. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408. [PubMed] [Google Scholar]
- 10.Hole N, Stern PL. A 72 kD trophoblast glycoprotein defined by a monoclonal antibody. Br J Cancer. 1988;57:239. doi: 10.1038/bjc.1988.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake RA, Robinson BWS. Immunotherapy and chemotherapy—practical partnership. Nature Rev Cancer. 2005;5:397. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 12.Leonard P, Seymour MT, James R, et al. Phase II study of irinotecan with bolus and high dose infusional 5-FU and folinic acid (modified de Gramont) for first or second line treatment of advanced or metastatic colorectal cancer. Br J Cancer. 2002;87:1216. doi: 10.1038/sj.bjc.6600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 15.Rochlitz C, Figlin R, Squiban P, et al. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5:690. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- 16.Southall PJ, Boxer GM, Bagshawe KD, et al. Immunohistological distribution of 5T4 antigen in normal and malignant tissues. Br J Cancer. 1990;61:89. doi: 10.1038/bjc.1990.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starzynska T, Rahi V, Stern PL. The expression of 5T4 antigen in colorectal and gastric carcinoma. Br J Cancer. 1992;66:867. doi: 10.1038/bjc.1992.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzynska T, Marsh PJ, Schofield PF, et al. Prognostic significance of 5T4 oncofetal antigen expression in colorectal carcinoma. Br J Cancer. 1994;69:899. doi: 10.1038/bjc.1994.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutter G, Moss B. Non-replicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 21.Weihrauch MR, Ansen S, Jurkiewicz E, et al. Phase I/II combined chemoimmunotherapy with carcinoembryonic antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer. Clin Can Res. 2005;11:5993. doi: 10.1158/1078-0432.CCR-05-0018. [DOI] [PubMed] [Google Scholar]
- 22.Wrigley E, McGowan AT, Rennison J, et al. 5T4 oncofetal antigen expression in ovarian carcinoma. Int J Gynecol Cancer. 1995;5:269. doi: 10.1046/j.1525-1438.1995.05040269.x. [DOI] [PubMed] [Google Scholar]