Abstract
Background:
Merkel cell carcinoma (MCC) is a rare cutaneous malignancy with high metastatic potential. Sentinel lymph node biopsy (SLNB) is used to assess locoregional spread, facilitate staging, and inform prognosis. Positive nodal status is associated with higher recurrence rates and reduced overall survival.
Methods:
A systematic search was conducted. Eligible articles included patients diagnosed with MCC, who would be candidates for or who had SLNB. The Covidence tool was used for screening and data extraction, including additional treatments, disease-free survival, overall survival, and recurrence. Methodological quality was assessed using the Newcastle-Ottowa Scale criteria.
Results:
SLNB was associated with increased likelihood of completion lymphadenectomy (223 versus 41), regional radiotherapy (2167 versus 808), and systemic chemotherapy (138 versus 31). Overall survival for patients undergoing SLNB was 81% at 2 years, 75% at 3 years, and 72% at 5 years (odds ratio: 0.79). Hazard ratio for positive SLNB versus negative was 3.36 (P < 0.001). Five-year disease recurrence was 23.3% in patients undergoing SLNB.
Conclusions:
Lymph node metastases are associated with reduced overall survival and increased recurrence of MCC. Determining nodal status early can inform prognosis, facilitate staging, and determine need for adjuvant treatment. Adjuvant treatments are associated with reduced mortality and improved overall survival; SLNB is an important influencer of their use. Early prophylactic intervention should be considered in MCC in both positive and negative nodal status to improve overall outcomes. Widespread use of SLNB will allow more accurate assessment of the role of nodal status on adjuvant treatment and long-term outcomes.
Takeaways
Question: How can SLNB be used to assess locoregional spread, facilitate staging, and inform prognosis in MCC?
Findings: A systematic review was performed. SLNB was associated with increased likelihood of completion lymphadenectomy, regional radiotherapy, and systemic chemotherapy. Overall survival for patients undergoing SLNB was 81% at 2 years, 75% at 3 years, and 72% at 5 years (OR 0.79).
Meaning: Determining nodal status early in MCC can inform prognosis, facilitate staging, and determine need for adjunctive treatment.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, aggressive neuroendocrine-derived cutaneous neoplasm with high metastatic potential to the regional lymph nodes. Even with timely diagnosis and treatment, a high risk of disease recurrence and distant metastasis results in untoward prognoses and high mortality rates.1 Although recent extensive study of MCC has improved awareness and resulted in increased incidence, it remains uncommon.2 As a result, MCC was insufficiently described, with limited large prospective studies published relating to effective management, with the majority defined by a limited sample size.3
As with all solid-organ malignancies, accurate staging is required for informed treatment planning, development of clinical trials, and improvement of patient outcomes. Staging in MCC remains diagnostically challenging.4 Sentinel lymph node biopsy (SLNB) is widely used in the assessment and treatment of malignancies, including breast cancer and melanoma.5 Spread to the sentinel lymph node has been shown to be a significant prognostic factor, with positive SLNB being the single best predictor of recurrence and mortality in clinically node-negative cutaneous melanoma.5 To facilitate accurate staging and tailored treatment, use of SNLB in MCC has gained support based on its proven success in diagnosis and staging of melanoma, considering the clinical and histologic similarities of these malignancies.1 Both have high rate of regional and distant metastasis, with positive nodal status at presentation an indicator of poor outcomes, with significantly reduced 5-year survival rates of less than 50%.6,7
By identifying the first lymph node in the nodal basin that receives afferent lymphatic drainage from the tumor area, SLNB allows for careful pathologic evaluation of one or a few sentinel lymph nodes that are most likely to harbor metastatic disease.5 Thus, patients with locoregional spread or micrometastases can receive adjuvant therapy including completion lymph node dissection, radiotherapy, or systemic immunotherapy or chemotherapy.8,9 The current American Joint Committee on Cancer classification system for MCC, which requires pathologic node evaluation for complete staging, is based on a National Cancer Database study that demonstrated improved overall survival for patients with pathologically node-negative (pN0) disease compared with those with clinically negative nodes who did not have nodes excised for evaluation (cN0/NX).10 Subsequently, some have recommended that SLNB is performed in all patients regardless of clinical nodal status.1,11,12 Although SLNB is recommended in many Western countries, the strength of evidence for its use is limited, and routine SLNB is not widespread.11–13
This study aimed to determine the role of sentinel lymph node status in the management of patients with MCC, assess its prognostic significance and impact on overall survival, and the role of SLNB in accurate staging and tailored adjuvant treatment, to contribute toward the development of treatment guidelines for the management of MCC.
METHODS
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Reviews14 and has been reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.15 The protocol for this review was prospectively registered with International Prospective Register of Systematic Reviews (PROSPERO)(ID: CRD42023366368).16
SEARCH STRATEGY AND SELECTION CRITERIA
Studies meeting the following criteria were considered for inclusion: (1) randomized controlled trials, systematic reviews, cohort studies, case-control studies, literature reviews case series that qualify after critical appraisal by two separate reviewers, (2) studies with patients diagnosed with MCC anywhere in the body, (3) studies involving patients who would be candidates for or have had SLNB, and their outcomes.
The exclusion criteria were (1) animal studies, experimental studies, in vitro studies, case reports, letters to editor, expert opinions, non-English language studies, (2) studies with no quantitative assessment of nodal status and patient outcomes (3) articles where no full text were available.
A preliminary search was conducted in Embase to identify seed articles. These articles were then used to develop comprehensive electronic search strategies for each database using a combination of relevant keywords and index headings. The search strategy was modified so that index headings relevant to each specific database were selected. The search strategy was peer reviewed by another information specialist using the Peer Review of Electronic Search Strategies checklist before execution.17
INFORMATION SOURCES
A systematic search of the literature was last conducted on October 15, 2022. Full search strategies and results are contained in Supplemental Digital Content 1. (See Supplemental Digital Content 1, which shows the search strategy. http://links.lww.com/PRSGO/D166.) A total of five bibliographic databases were searched. The interface, name, and coverage for each database are listed in Supplemental Digital Content 1 (http://links.lww.com/PRSGO/D166). Forward and backward citation searches were conducted on articles identified as eligible for full-text review. Forward citation searches were conducted using Web of Science Core Collection. Duplicate articles were identified and removed in Endnote 20.
DATA SCREENING AND EXTRACTION
Search results were uploaded to Covidence, a bespoke online tool for the conduct of systematic reviews.18 Two independent reviewers screened study titles and abstracts for potential inclusion, with consensus from a third, senior author. Potentially suitable articles were reviewed in full by two independent reviewers for final inclusion, with consensus from a third, senior author.
Two authors independently extracted data from each study with consensus from a third, senior author. Data on study characteristics (authors, publication year, country of study, design, start and end dates, funding sources, etc), participant characteristics (number, inclusion/exclusion, disease status), and outcomes (additional treatments, disease-free survival, overall survival) were extracted via a bespoke data collection tool built into Covidence, into a Microsoft Excel spreadsheet (Excel v16; Microsoft Corporation, Redmond, Wash.).
ASSESSMENT OF METHODOLOGICAL QUALITY
Risk of bias was assessed by two independent reviewers using the Newcastle-Ottowa Scale,19 with disagreements resolved by a third, senior reviewer. The Newcastle-Ottowa scale is a tool designed for assessing the quality of nonrandomised studies and consists of signaling questions relating to patient selection, comparability between groups, and outcome reporting. Methodological robustness in domains is awarded stars, with a study having a maximum of nine stars. The number of stars can then be converted to an overall assessment of “good,” “fair,” or “poor.”19 Results were presented using the tool robvis.20
STATISTICAL ANALYSIS
Data analysis was conducted using a combination of Microsoft Excel and RevMan 5 (RevMan 5.4; Cochrane Collaboration, 2020). Data items were compared; there was significant heterogeneity in study methodology, study populations, and outcome reporting, and so minimal meta-analyses of included data were undertaken. No subgroup analyses were undertaken due to a paucity of homogenous data suitable for synthesis. Results are presented narratively for the purposes of the review.
For overall survival, individual patient data presented at specific time points (2 years, 3 years, and 5 years) were pooled and presented as a proportion with 95% confidence interval (CI). For survival comparison between positive and negative SLNB, a random-effects meta-analysis using the inverse variance method was undertaken. For disease recurrence at 5 years, a random-effects meta-analysis was undertaken comparing patients undergoing SLNB and patients not undergoing SLNB. Both meta-analyses performed included an assessment of heterogeneity of included studies through calculation of I2.
RESULTS
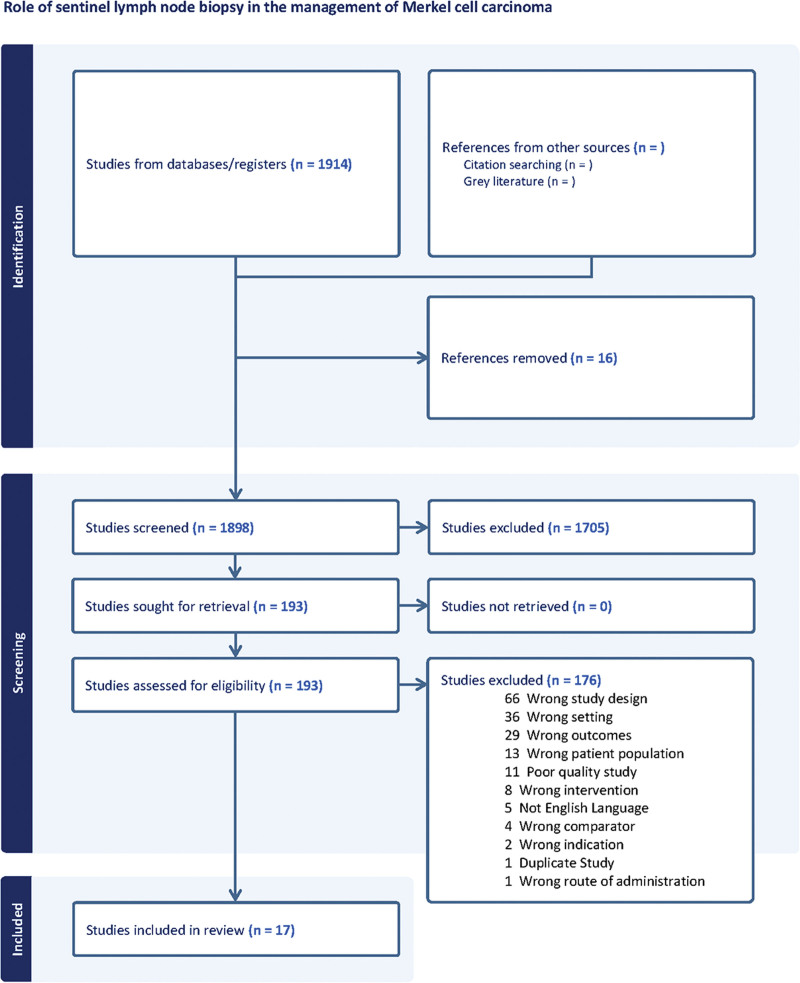
Figure 1 represents the full screening process. Searches identified 2913 studies, with 1898 available for screening after deduplication. There were 193 studies identified as potentially relevant that underwent full-text screening, and of these, 176 were excluded. “Wrong design” was the most common reason for exclusion, followed by “wrong setting.” In total, 17 studies were included in the final review.
Fig. 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
INCLUDED STUDIES
Seventeen studies, published between 2006 and 2022, were included. Table 1 shows the characteristics, including country of origin and study design, of included studies. All studies were either case series10,23–25,27,30,32–36 or cohort studies.21,22,26,28,29,31
Table 1.
Characteristics of Included Studies
| Authors | Country | Study Type | Participants (n) | Follow-up (mo) |
|---|---|---|---|---|
| Conic et al21 | USA | Cohort study | 3048 | NS |
| Delisle et al22 | Canada | Cohort study | 750 | 32.4 |
| Fields et al23 | USA | Case series | 153 | 41 |
| Gunaratne et al24 | Australia | Case series | 737 | 22 |
| Gupta et al25 | USA | Case series | 61 | 17 |
| Harounian et al26 | USA | Cohort study | 122 | 64 |
| Jenkins et al27 | Australia | Case series | 41 | 27 |
| Kachare et al28 | USA | Cohort study | 1193 | 21 |
| Kouzmina et al29 | Finland | Cohort study | 28 | 42 |
| Lemos et al10 | USA | Case series | 10,020 | 64 |
| Maza et al30 | Germany | Case series | 23 | 36.1 |
| Sattler et al31 | Germany | Cohort study | 47 | 20 |
| Servy et al32 | France | Case series | 87 | 39.3 |
| Sims et al33 | USA | Case series | 150 | 45.6 |
| Song et al34 | USA | Case series | 133 | 36 |
| Straker et al35 | USA | Case series | 3643 | 60 |
| Xia et al36 | China | Case series | 1822 | NS |
NS, not stated.
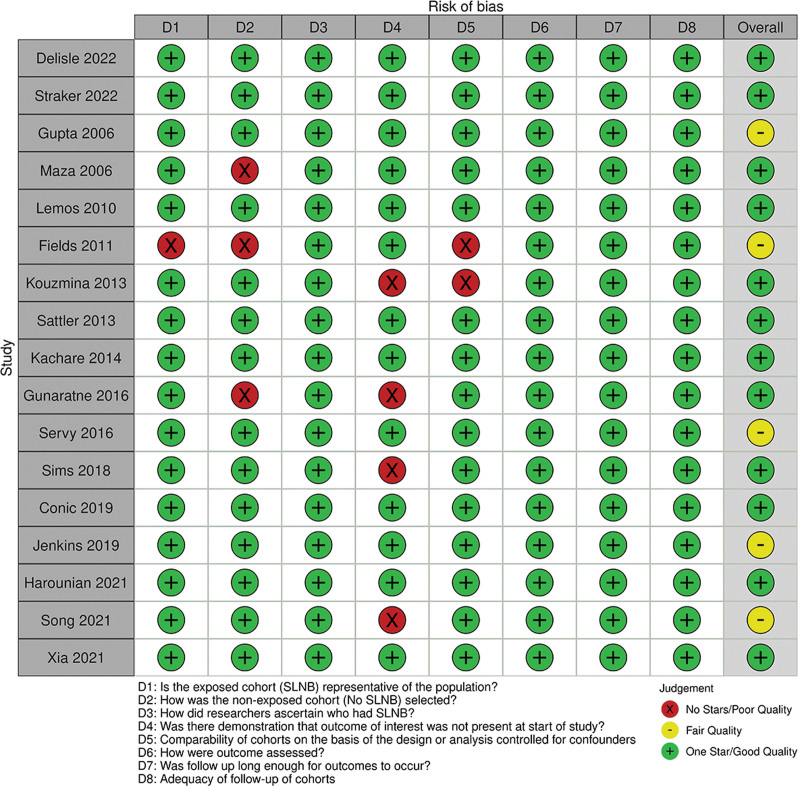
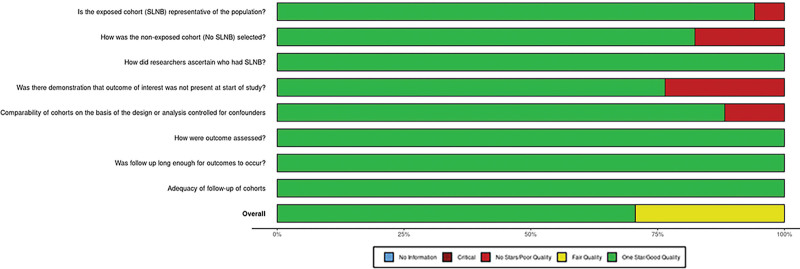
The overall risk of bias assessment for included studies is represented in Figure 2 and Figure 3.
Fig. 2.
Methodological quality assessment of included studies.
Fig. 3.
Risk of bias of included studies.
PARTICIPANT DEMOGRAPHICS
Some 22,042 patients were described across all studies; almost half of these were described in a single registry-based cohort study.10 Men made up 59.5% of the cohort; two studies (4393 patients) did not declare what proportion of the cohort were men.22,35 The weighted mean age was 75.1 ± 2.12 years; four studies (7496 patients) did not describe the age of their cohort.21,22,29,35 The disease stages of included patients within each study are outlined in Table 2.
Table 2.
Disease Stage (T Stage) of Participants in Individual Studies
| Study | Patients Undergoing SLNB | Observation Only | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 (n) | T2 (n) | T3 (n) | T4 (n) | T Stage Unknown (n) |
T1 (n) | T2 (n) | T3 (n) | T4 (n) | T Stage Unknown (n) |
|
| Conic et al21 | 541 | 37 | 11 | 585 | 672 | 40 | 15 | 1110 | ||
| Delisle et al22 | 84 | 45 | 66 | 183 | 100 | 7 | ||||
| Fields et al23 | 122 | 31 | ||||||||
| Gunaratne et al24 | 737 | |||||||||
| Gupta et al25 | 33 | 19 | 24 | 5 | ||||||
| Harounian et al2 | 73 | 29 | 2 | 9 | ||||||
| Jenkins et al27 | 41 | |||||||||
| Kachare et al28 | 330 | 114 | 17 | 13 | 449 | 198 | 38 | 34 | ||
| Kouzmina et al4 | 25 | 7 | 1 | |||||||
| Lemos et al10 | 937 | 277 | 8806 | |||||||
| Maza et al30 | 23 | |||||||||
| Sattler et al31 | 10 | 6 | 3 | 10 | 12 | 6 | ||||
| Servy et al32 | 25 | 28 | 34 | |||||||
| Sims et al33 | 150 | |||||||||
| Song et al34 | 64 | 13 | 48 | 8 | ||||||
| Straker et al35 | 2442* | 124* | 989* | 94* | ||||||
| Xia et al36 | 905 | 408 | 81 | |||||||
Indicates multiple stages combined within study.
DISEASE LOCATION
It is without doubt that SLNB in the head and neck is technically more challenging and that there may be a difference in outcomes for tumors of the head and neck.21,22 However, no articles presented data in a way that was amenable to meta-analysis. Several articles presented survival data for MCC, where head and neck tumors are compared with trunk or limb tumors; however, this was independent of SLNB and nodal status and therefore outside the scope of our review.10,22–25,27,29,31–33,35,36 Conic et al highlighted that location of lesion had no bearing on overall survival, or node positivity, and Harounian et al presented comparative statistic demonstrating that head and neck location had no correlation with survival.21,26 No articles presented granular outcome data for SLNB and no SLNB in head and neck tumors only; they were pooled in the results, with no extractable statistics.36 Location of lesion has been associated with likelihood of undergoing SLNB, with the technical challenge of head and neck SLNB a limiting factor of use in these cases.31,32
ADDITIONAL TREATMENTS
SLNB appeared to be a gateway to further treatment of the nodal basin, or for systemic treatment. One study outlined patients with MCC who went on to have subsequent treatments but did not relate these to SLNB.34 Across four studies,23,24,34,35 138 patients had systemic chemotherapy following SLNB, compared with 31 after no SLNB in one study.35 Regional radiotherapy was given to 2167 patients after SLNB in 11 studies.22–28,30,32,33,35 Only a single study24 described which patients undergoing radiotherapy had positive SLNB. In those without SLNB, 808 patients from four studies22,26,28,35 underwent regional radiotherapy without a prior SLNB. Finally, 225 patients across 11 studies22–24,26–30,32–34 underwent completion lymphadenectomy, with 41 patients in two studies26,34 undergoing lymphadenectomy without prior SLNB.
OVERALL SURVIVAL
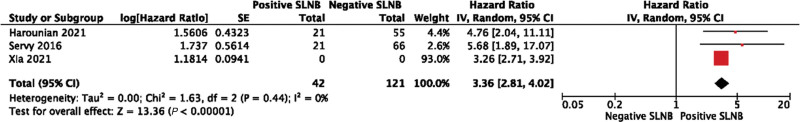
Overall survival was presented in a variety of forms. Studies described overall survival at 1 year,33 2 years,21,24,27 3 years,29 5 years,33,36 and up to 14 years (with variable follow-up within the cohort).23 Studies compared survival between SLNB and observation only but presented data in an unextractable format;21,28,31 between positive and negative SLNB presenting a hazard ratio;26,32,36 between positive and negative SLNB but with data in an unextractable format;30,33,35 and between clinically positive and pathologically positive nodes.10 Due to this clinical and methodological heterogeneity relating to this outcome in included studies, meta-analysis was limited. The pooled overall survival for patients having SLNB was 81% (95% CI, 79%–83%) at 2 years, 76% (95% CI, 68%–83%) at 3 years, and 72% (95% CI, 70%–74%) at 5 years. The pooled hazard ratio for positive SLNB compared with negative was 3.36 (95% CI, 2.81–4.02; P < 0.001) (Fig. 4). The heterogeneity of studies included in meta-analysis was I2 = 0, suggesting the effect size of these studies was comparable.
Fig. 4.
Pooled hazard ratios for positive SLNB compared with negative.
DISEASE RECURRENCE
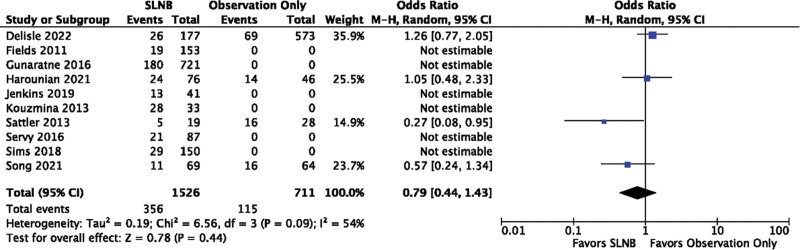
Ten studies gave details of recurrence of MCC within 5 years; Four of these compared patients who had had SLNB with those who had not,22,26,31,34 with the remaining six only describing patients who underwent SLNB.23–25,27,29,32,33 Recurrence within 5 years occurred in 23.3% (95% CI, 21.2%–25.5%) of patients undergoing SLNB. There was no significant difference in recurrence between those undergoing SLNB and those not [OR = 0.79 (95% CI, 0.44–1.43); P = 0.44]. The I2 value was 54%, suggesting moderate to substantial heterogeneity according to the Cochrane Handbook37 (Fig. 5).
Fig. 5.
Recurrence rates for SLNB compared with observation.
METHODOLOGICAL QUALITY ASSESSMENT
Studies were rated either good10,21,22,24,26,28–31,33,35,36 or fair23,27,30,32,34 overall (Fig. 2). All studies received one star for patient categorization, outcome assessment and follow-up length, and completeness. The worst performing measure was for demonstration of the outcome of interest at the start of the study, with four studies receiving no stars in this domain (Fig. 3). Due to the overall good quality of included studies, and the heterogeneity of clinical and methodological differences in included studies and outcome reporting, no subgroup analysis based on study quality was undertaken.
DISCUSSION
SLNB is increasingly used in the management of patients with MCC for prognostic assessment, to facilitate accurate staging, and influence proposed surgical and adjuvant treatment strategies.1,38 Positive lymph node status in MCC, particularly when detected at initial presentation or early in the disease stage, is associated with poorer patient outcomes and 5-year survival.39 MCC is associated with high rates of morbidity and mortality; SLNB remains the most reliable predictor of overall survival in patients with MCC.40 Determining the accuracy of SLNB in assessing the likelihood of regional and distant metastasis, the influence nodal status should have on initial management and subsequent adjuvant treatment, and its effects on overall survival and recurrence remains of significant importance.41
Presence of lymph node metastasis is one of the most influential predictors of overall survival in MCC.42 An awareness of nodal status is important in informing and influencing postoperative and adjuvant management strategies. Our study highlighted that SLNB was associated with an increased likelihood of adjuvant postoperative treatments in MCC, including chemotherapy, radiotherapy, and completion lymph node dissection. Detection of a positive node may prompt earlier intervention with completion lymph node dissection and locoregional radiotherapy to the lymph node basin; a negative nodal status allows informed decision-making relation to prophylactic adjuvant treatments that may be beneficial in reducing the risk of disease recurrence.23
Both completion lymph node dissection and radiotherapy have been associated with significantly reduced rates of regional and distant metastasis and improved long-term outcomes in MCC.43,44 Although MCC remains highly sensitive to chemotherapy, it is commonly used in advanced cases, with associated increased rates of toxicity and subsequent relapse.45 Early and aggressive use of adjuvant treatments in patients with positive nodes has been associated with significantly improved overall survival and may reduce the likelihood of metastasis, recurrence, and further adjuvant treatment requirement.46–48 Multimodal treatment has been associated with improved outcomes compared with those treated with surgery alone, highlighting the relevance of adjuvant treatment measures in improving prognosis in MCC.49
Prophylactic use of adjuvant measures, regardless of initial nodal status, may have an important role in reducing locoregional recurrence and improving long-term outcomes.50 Prophylactic regional lymph node dissection in node-negative disease has been associated with significantly reduced rates of recurrence at the lymph node basin compared with later therapeutic nodal dissection; suggesting early nodal resection may be an important prognostic influencer.51 In addition, irradiation of the nodal basin in confirmed nodal negative disease has been associated with reduced recurrence rates.48 Prophylactic adjuvant treatment, informed by nodal status, may therefore have an important role in reducing rates of regional and distant metastasis and improve associated long-term outcomes.52 Moreover, there remains a risk of false-negative SLNB resulting from aberrant lymphatic drainage or multiple sentinel nodes, particularly in the head and neck region; therefore, prophylactic treatments should be considered in all cases to reduce the rates of disease recurrence and improved overall survival.48,52
Positive nodal status was associated with significantly reduced overall survival. These findings are congruent with the available literature that highlights both the prognostic value of SLNB, and the significance of morbidity and mortality associated with regional metastasis in MCC.53 Our study demonstrates SLNB to be associated with 72% survival at 5 years; overall survival for MCC is suggested at approximately 30% at 5 years, though this is strongly stage specific.54,55 While there was limited quantitative data available in this study to assess the relationship between nodal status and disease recurrence, the literature suggests positive nodal status has been associated with significantly higher rates of both occult micrometastases and systemic disease recurrence.25,56 This highlights the importance of early SLNB in both prognostic assessment and informed use of adjuvant treatment strategies to improve long-term outcomes in MCC.
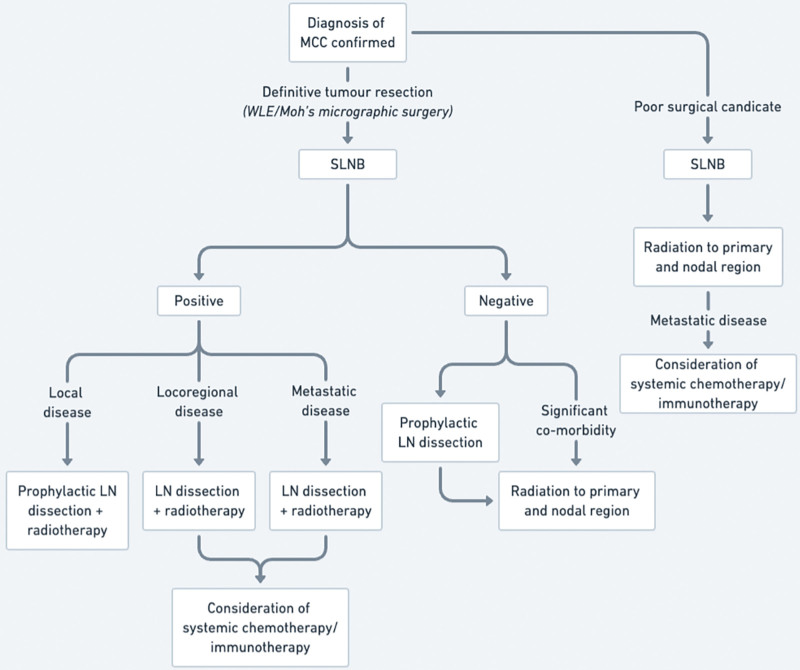
Both positive and negative nodal status have been associated with a high incidence of regional and distant metastasis in MCC, with associated effects on overall survival.41 While the association of T stage and utility of SLNB could not be determined in this study, early aggressive treatment for MCC has been associated with improved long-term outcomes.48 A negative node can inform a decision for prophylactic treatment strategies, to reduce rates of disease recurrence and overall mortality, particularly in patients with otherwise minimal comorbidities or good performance status.57 Early detection of a positive node provides valuable prognostic and diagnostic information, allowing informed management planning and early adjuvant systemic and locoregional treatment, to improve overall survival.41,58 As a result, the following treatment algorithm is recommended, to utilize the prognostic benefit of SLNB, prompting earlier adjuvant treatment in nodal positive disease, and essential prophylactic measures in node-negative disease, to improve overall patient survival and reduce likelihood of disease recurrence (Fig. 6).
Fig. 6.
Proposed treatment algorithm for MCC.
Limitations of this review include limited availability of studies with prospective design and robust methodology comparing the benefits of SLNB to control in improving overall survival and reducing recurrence in MCC. This relates to several factors. MCC is a rare neurocutaneous malignancy. Prospective recruitment of a meaningful sample allowing randomization and accurately matching across trial arms remains challenging. As a result, much of the available data is of retrospective and observational nature, with small sample sizes or from national databases. This leads to heterogeneity of data between available studies, missing data, and incomplete follow-up and assessment of overall survival rates and disease recurrence. Therefore, complete and valid data synthesis and statistical assessment are challenging, limiting the reliability of inferences possible from the available literature and true assessment of the role of SLNB in MCC. However, the results of this study, within the wider available evidence base, suggest a promising role for SLNB in MCC as both a prognostic marker and influencing ongoing treatment strategies. The evidence suggests that early adjuvant treatment can significantly influence overall survival and recurrence rates even in node-negative disease; this study demonstrates that SLNB is an important factor in influencing the likelihood of their use, highlighting its role in improving long-term patient outcomes. With increasing use of SLNB and availability of robust evidence, determination of the long-term effects of early assessment of nodal status will be possible.
CONCLUSIONS
SLNB is an important early prognostic marker in MCC to allow accurate disease staging; reduce likelihood of recurrence following initial surgical intervention; and aid decision-making regarding adjuvant perioperative treatment measures including radiotherapy, chemotherapy, and completion lymphadenectomy. Early prophylactic intervention at the lymph node basin guided by sentinel lymph node status may provide significant overall morbidity and mortality benefits given the high metastatic potential of MCC, reducing risk of future recurrence and overall mortality. Presence of lymph node metastasis remains one of the most important predictors of overall survival in MCC, and early assessment of nodal status with SLNB can aid informed decision-making relating to early adjuvant treatment, improving overall patient outcomes. Increased widespread use of SLNB will allow for ongoing quantitative assessment of the relationship between nodal status, recurrence, and overall mortality, and the effects of prophylactic or therapeutic adjuvant treatment strategies on these outcomes.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENT
Search strategy peer reviewed by Ms Jennie Roe, Clinical Librarian, Morriston Hospital, Swansea Bay University Health Board.
Supplementary Material
Footnotes
Published online 19 April 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Dimitrios Kanakopoulos and Hester Lacey share first authorship.
REFERENCES
- 1.Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. [DOI] [PubMed] [Google Scholar]
- 3.Angeles CV, Wong SL. Importance of sentinel lymph node biopsy in Merkel cell carcinoma. J Oncol Pract. 2016;12:647–648. [DOI] [PubMed] [Google Scholar]
- 4.Schmalbach CE, Lowe L, Teknos TN, et al. Reliability of sentinel lymph node biopsy for regional staging of head and neck Merkel cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:610–614. [DOI] [PubMed] [Google Scholar]
- 5.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. [DOI] [PubMed] [Google Scholar]
- 6.Pitale M, Sessions RB, Husain S. An analysis of prognostic factors in cutaneous neuroendocrine carcinoma. Laryngoscope. 1992;102:244–249. [DOI] [PubMed] [Google Scholar]
- 7.Mehrany K, Otley CC, Weenig RH, et al. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28:113–117; discussion 117. [DOI] [PubMed] [Google Scholar]
- 8.Gessner K, Wichmann G, Boehm A, et al. Therapeutic options for treatment of Merkel cell carcinoma. Eur Arch Otorhinolaryngol. 2011;268:443–448. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock CL, Bland KI, Laney RG, III, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988;207:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemos BD, Storer BE, Iyer JG, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol. 2010;63:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellitteri PK, Takes RP, Lewis JS, Jr, et al. Merkel cell carcinoma of the head and neck. Head Neck. 2012;34:1346–1354. [DOI] [PubMed] [Google Scholar]
- 12.The National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Merkel Cell Carcinoma. Version 2. 2011. Plymouth Meeting, Pa.: NCCN. 2023:11–18.
- 13.Warner RE, Quinn MJ, Hruby G, et al. Management of Merkel cell carcinoma: the roles of lymphoscintigraphy, sentinel lymph node biopsy and adjuvant radiotherapy. Ann Surg Oncol. 2008;15:2509–2518. [DOI] [PubMed] [Google Scholar]
- 14.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74:790–799. [DOI] [PubMed] [Google Scholar]
- 16.National institute for health and care research—international prospective register of systematic reviews. Available at https://www.crd.york.ac.uk/prospero/. Published 2011. Accessed December 10, 2022.
- 17.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
- 18.Veritas Health Innovation. Covidence systematic review software. 2022.
- 19.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford. 2000:1–4. [Google Scholar]
- 20.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods. 2020;12:55–61. [DOI] [PubMed] [Google Scholar]
- 21.Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: Predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364–372. [DOI] [PubMed] [Google Scholar]
- 22.Delisle ME, Dingley B, Apte S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: a multi-institutional study from the Pan-Canadian Merkel Cell Collaborative. J Clini Oncol. 2022;40(16 Supplement 1):9583–9583. [Google Scholar]
- 23.Fields RC, Busam KJ, Chou JF, et al. Recurrence and survival in patients undergoing sentinel lymph node biopsy for Merkel cell carcinoma: analysis of 153 patients from a single institution. Ann Surg Oncol. 2011;18:2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunaratne DA, Howle JR, Veness MJ. Sentinel lymph node biopsy in Merkel cell carcinoma: a 15-year institutional experience and statistical analysis of 721 reported cases. Br J Dermatol. 2016;174:273–281. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SG, Wang LC, Penas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma—the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. [DOI] [PubMed] [Google Scholar]
- 26.Harounian JA, Molin N, Galloway TJ, et al. Effect of sentinel lymph node biopsy and LVI on Merkel cell carcinoma prognosis and treatment. Laryngoscope. 2021;131:E828–E835. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins LN, Howle JR, Veness MJ. Sentinel lymph node biopsy in clinically node-negative Merkel cell carcinoma: the Westmead Hospital experience. ANZ J Surg. 2019;89:520–523. [DOI] [PubMed] [Google Scholar]
- 28.Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624–1630. [DOI] [PubMed] [Google Scholar]
- 29.Kouzmina M, Leikola J, Bohling T, et al. Positive sentinel lymph node biopsy predicts local metastases during the course of disease in Merkel cell carcinoma. J Plast Surg Hand Surg. 2013;47:139–143. [DOI] [PubMed] [Google Scholar]
- 30.Maza S, Trefzer U, Hofmann M, et al. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: results of a prospective study and review of the literature. Euro J Nuclear Med Molecular Imag. 2006;33:433–440. [DOI] [PubMed] [Google Scholar]
- 31.Sattler E, Geimer T, Sick I, et al. Sentinel lymph node in Merkel cell carcinoma: to biopsy or not to biopsy? J Dermatol. 2013;40:374–379. [DOI] [PubMed] [Google Scholar]
- 32.Servy A, Maubec E, Sugier PE, et al. Merkel cell carcinoma: value of sentinel lymph-node status and adjuvant radiation therapy. Ann oncol. 2016;27:914–919. [DOI] [PubMed] [Google Scholar]
- 33.Sims JR, Grotz TE, Pockaj BA, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: the Mayo Clinic experience of 150 patients. Surg Oncol. 2018;27:11–17. [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Azari FS, Tang R, et al. Patterns of metastasis in Merkel cell carcinoma. Ann Surg Oncol. 2021;28:519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straker RJ, Sharon CE, Fraker DL, et al. Contemporary analysis of sentinel lymph node biopsy performance among patients with clinically localized Merkel cell carcinoma. Ann Surg Oncol. 2022;29:7261–7264. [DOI] [PubMed] [Google Scholar]
- 36.Xia Y, Cao D, Zhao J, et al. Does regional lymph node status have a predictive effect on the prognosis of Merkel cell carcinoma? J Plast Reconstr Aesthet Surg. 2021;74:845–856. [DOI] [PubMed] [Google Scholar]
- 37.The Cochrane Collaboration. Identifying and measuring heterogeneity. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. www.handbook.cochrane.org. Published 2011. Accessed December 10, 2022. [Google Scholar]
- 38.Arruda EP, Higgins KM. Role of sentinel lymph node biopsy in the management of Merkel cell carcinoma. J Skin Cancer. 2012;2012:176173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messina JL, Reintgen DS, Cruse CW, et al. Selective lymphadenectomy in patients with Merkel cell (cutaneous neuroendocrine) carcinoma. Ann Surg Oncol. 1997;4:389–395. [DOI] [PubMed] [Google Scholar]
- 40.Wang TS, Byrne PJ, Jacobs LK, et al. Merkel cell carcinoma: update and review. Semin Cutan Med Surg. 2011;30:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maher NG, Murrell DF. A systematic review of patients with Merkel cell carcinoma of the head and neck and a negative sentinel lymph node biopsy. Int J Womens Dermatol. 2015;1:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez MC, Oliver DE, Weitman ES, et al. Management of sentinel lymph node metastasis in Merkel cell carcinoma: completion lymphadenectomy, radiation, or both? Ann Surg Oncol. 2019;26:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jouary T, Leyral C, Dreno B, et al. ; Groupe de Cancérologie Cutanée of the Société Française de Dermatologie. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study. Ann Oncol. 2012;23:1074–1080. [DOI] [PubMed] [Google Scholar]
- 44.Ott MJ, Tanabe KK, Gadd MA, et al. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999;134:388–392; discussion 392. [DOI] [PubMed] [Google Scholar]
- 45.Villani A, Fabbrocini G, Costa C, et al. Merkel cell carcinoma: therapeutic update and emerging therapies. Dermatol Ther (Heidelb). 2019;9:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubois M, Abi Rached H, Escande A, et al. Outcome of early-stage Merkel carcinoma treated by exclusive radiation: a study of 53 patients. Radiat Oncol. 2021;16:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang SH, Haydu LE, Goh RYH, et al. Radiotherapy is associated with significant improvement in local and regional control in Merkel cell carcinoma. Radiat Oncol. 2012;7:4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayak AL, Pickett AT, Delisle M, et al. Survival of patients with head and neck Merkel cell cancer: findings from the pan-Canadian Merkel cell cancer collaborative. JAMA Netw Open. 2023;6:e2344127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronan Tanguy SO, Laetitia R, Christian C, et al. Role of prophylactic regional lymph node radiation therapy on locoregional recurrence in Merkel cell carcinomas. J Clin Oncol. 2015;33(15_suppl):e20034–e20034. [Google Scholar]
- 51.Victor NS, Morton B, Smith JW. Merkel cell cancer: is prophylactic lymph node dissection indicated? Am Surg. 1996;62:879–882. [PubMed] [Google Scholar]
- 52.Cramer JD, Suresh K, Sridharan S. Completion lymph node dissection for Merkel cell carcinoma. Am J Surg. 2020;220:982–986. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang E, Brower JV, Rice SR, et al. Merkel cell carcinoma analysis of outcomes: a 30-year experience. PLoS One. 2015;10:e0129476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEvoy AM, Lachance K, Hippe DS, et al. Recurrence and mortality risk of Merkel cell carcinoma by cancer stage and time from diagnosis. JAMA Dermatol. 2022;158:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howle JR, Hughes TM, Gebski V, et al. Merkel cell carcinoma: an Australian perspective and the importance of addressing the regional lymph nodes in clinically node-negative patients. J Am Acad Dermatol. 2012;67:33–40. [DOI] [PubMed] [Google Scholar]
- 57.Kokoska ER, Kokoska MS, Collins BT, et al. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997;174:688–693. [DOI] [PubMed] [Google Scholar]
- 58.Senchenkov A, Barnes SA, Moran SL. Predictors of survival and recurrence in the surgical treatment of Merkel cell carcinoma of the extremities. J Surg Oncol. 2007;95:229–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.