Abstract
Background:
Venous thromboembolism (VTE) is a dangerous postoperative complication after abdominal wall reconstruction (AWR). Intraoperative core body temperature has been associated with thrombotic events in other surgical contexts. This study examines the effects of intraoperative temperature on VTE rate after AWR.
Methods:
A retrospective study was performed on AWR patients. Cohorts were defined by postoperative 30-day VTE. Intraoperative core body temperature was recorded as the minimum, maximum, and mean intraoperative temperatures. Study variables were analyzed with logistic regression and cutoff analysis to assess for association with VTE.
Results:
In total, 344 patients met inclusion criteria. Fourteen patients were diagnosed with 30-day VTE for an incidence of 4.1%. The VTE cohort had a longer median inpatient stay (8 days versus 5 days, P < 0.001) and greater intraoperative change in peak inspiratory pressure (3 mm H2O versus 1 mm H2O, P = 0.01) than the non-VTE cohort. Operative duration [odds ratio (OR) = 1.32, P = 0.01], length of stay (OR = 1.07, P = 0.001), and intraoperative PIP difference (OR = 1.18, P = 0.045) were significantly associated with 30-day VTE on univariable regression. Immunocompromised status (OR = 4.1, P = 0.023; OR = 4.0, P = 0.025) and length of stay (OR = 1.1, P < 0.001; OR = 1.1, P < 0.001) were significant predictors of 30-day VTE on two multivariable regression models. No significant associations were found between temperature metrics and 30-day VTE on cutoff point or regression analysis.
Conclusions:
Intraoperative core body temperature did not associate with 30-day VTE after AWR, though operative duration, length of stay, immunocompromised status, and intraoperative PIP difference did. Surgeons should remain mindful of VTE risk after AWR, and future research is warranted to elucidate all contributing factors.
Takeaways
Question: Does intraoperative core body temperature affect the risk of developing a 30-day venous thromboembolism (VTE) event after complex abdominal wall reconstruction (CAWR)?
Findings: Operative duration, length of stay, and intraoperative PIP difference were significantly associated with 30-day VTE on univariable regression. Immunocompromised status and length of stay were significant predictors of 30-day VTE on multivariable regression. No significant associations were found between temperature metrics and 30-day VTE.
Meaning: Intraoperative core body temperature did not associate with 30-day VTE after CAWR; however, surgeons should remain mindful of VTE risk after CAWR, and future research is warranted to elucidate all contributing factors.
INTRODUCTION
Complex abdominal wall reconstruction (CAWR) describes the range of procedures intended to restore structure and function to the abdomen after compromise by defects of varying etiologies. These often manifest as one large defect or sometimes multiple smaller defects comprising a “Swiss cheese” configuration that commonly necessitate longer procedures involving muscle flaps as well as the need for inpatient hospitalization with multidisciplinary care teams for appropriate management (versus noncomplex repairs). After surgery, patients are at a high risk for postoperative complications due to unique clinical and operative challenges.1,2 Of these, venous thromboembolism (VTE) is one of the complications most feared by surgeons due to its high morbidity and mortality to patients. The reported incidence of postoperative VTE, specifically within the AWR population, has ranged from 0.6% to 7.9%.3–5 Although this may be due, in part, to increased intraabdominal pressure resulting in increased venous stasis within the lower extremities, the complete etiology of postoperative VTE is not fully understood, especially when it occurs despite proper preoperative risk stratification and perioperative prophylactic risk mitigation strategies being undertaken.3,6 Because of the severe morbidity inflicted upon recovering patients, there is a significant impetus to identify unexplored risk factors that may contribute to VTE in this patient population with the hopes of mitigating this risk to improve outcomes.
Intraoperative core body temperature is a surgical factor that has been previously associated with thrombotic events after surgical procedures such as ovarian cytoreductive surgery, intracranial tumor resection, and various forms of microsurgical free tissue transfer.7–12 Although investigations from the basic science literature have suggested that mild hypothermia might protect against thrombosis due to inhibition of platelet function and the clotting cascade, results from these studies have been discordant with clinical studies.13–15 Thus, the question arises whether intraoperative core body temperature may be a predictive factor of VTE after CAWR.
In this study, we aimed to analyze the impact of intraoperative body temperature, as well as other clinical factors, on the rate of clinically significant VTE after CAWR. As a secondary objective, we intended to describe the 30-day postoperative VTE rate in our patient sample and compare it against incidence rates currently reported in the literature. We hypothesized that intraoperative core body temperature would have an association with 30-day VTE rate.
METHODS
Patient Population and Study Design
After obtaining institutional review board approval (approval no.: 2023H0215), a retrospective chart review was conducted to identify patients between the ages of 18 and 89 who had undergone CAWR at the Ohio State University Wexner Medical Center performed by the senior author (J.E.J.) from February 2014 to December 2022. In total, 362 patients who underwent 388 CAWRs were identified for potential study inclusion. The first abdominal wall reconstruction procedure at the Ohio State University was considered for each patient in this study. To avoid repeat measures, repeat procedures were excluded from this analysis because only a few patients had more than one procedure. Patients who lacked intraoperative temperature data, who had an inpatient stay of fewer than two midnights, or who lacked at least 30 days of follow-up were also excluded.
Baseline patient characteristics were extracted, including age; gender; body mass index (BMI); smoking status; and patient comorbidities, including diabetes mellitus, hypertension, chronic kidney disease, chronic liver disease, immunocompromised status, chronic obstructive pulmonary disease, history of hypercoagulability, history of malignancy, and coronavirus disease 2019 (COVID-19) status. Smoking status was stratified into whether patients had active tobacco use between 30 days before surgery and 30 days postoperative versus a history of smoking outside that window. A history of hypercoagulability was defined as a predisposing condition (eg, factor V Leiden and lupus anticoagulant) or a previous VTE event. Patients who underwent surgery before the COVID-19 pandemic did not receive COVID-19 screening. Pandemic patients underwent preoperative COVID-19 screening and proceeded to surgery if results were negative. If admitted for an inpatient stay of at least two midnights, these patients underwent an additional admission screen. Further testing during the 30-day study period occurred if clinically indicated. Current use of anticoagulative or antiplatelet medications was also determined during surgical evaluation, and if so, whether the respective medication was held in the immediate preoperative period. Pertinent operative details were obtained, including use of muscle flaps (component separation); type of component separation; mesh use; mesh plane; defect location; defect size; defect width; fascial closure; concomitant plastic surgery procedure; operative duration; baseline intraoperative peak inspiratory pressure (PIP); PIP at the conclusion of the case; and the minimum, maximum, and mean intraoperative core body temperatures. The defect width was noted for patients with a single hernia, and in patients who had multiple “Swiss cheese” type hernias, these were consolidated intraoperatively into a single, larger defect and measured. Hospital length of stay was then documented along with the development of postoperative VTE within 30 days of surgery, and the postoperative day (POD) of VTE diagnosis. VTE events were defined as deep vein thrombosis (DVT) confirmed with vascular duplex ultrasonography or pulmonary embolism (PE) confirmed with computed tomography. Cohorts were defined by development of 30-day VTE.
Perioperative and Intraoperative Management
Perioperative management for all patients was conducted in accordance with the Enhanced Recovery After Surgery: Abdominal Wall Reconstruction/Complex Hernia Repair guidelines established by the Ohio State University Wexner Medical Center. Neuraxial analgesia was managed by the Acute Pain Service. For chemoprophylaxis, 5000 units of unfractionated heparin was given on the day of surgery 2 hours before the induction of general anesthesia. Sequential compression devices were placed and were functional before induction, as well as maintained throughout the procedure and entirety of the hospital stay. Intraoperative core body temperatures were monitored with a bladder temperature probe, with readings recorded every 15 minutes. Core body temperature was set at a goal of more than 36°C with the use of forced-air patient warming and intravenous fluid warming systems. If core body temperature dropped below 35.5°C, ambient room temperature was raised 2°C. Postoperatively, 40 mg of enoxaparin was injected daily in the subcutaneous thigh beginning on POD 1 and continued for the duration of the inpatient stay. In those with postoperative acute kidney injury (as defined by the Risk, Injury, Failure, Loss, and End-stage Kidney criteria), 5000 units of unfractionated heparin three times a day was used instead.16 Patients were required to ambulate with assistance at least once on POD 0 and then required to ambulate with assistance five or more times each day thereafter. Dedicated physical therapy was ordered only in cases of baseline mobility issues. Sequential compression devices remained on and functional throughout admission except during ambulation.
Data Analysis
Patient characteristics, clinical characteristics, and outcomes of interest were summarized using descriptive statistics overall and by VTE cohorts. Means with SDs or medians with interquartile ranges were used for continuous variables and frequencies, and proportions were used for categorical variables. Comparisons between study cohorts were analyzed using a chi-square test or Fisher exact test for categorical variables and a two-sample t test or Mann-Whitney U test for continuous variables. Univariable logistic regression analysis was performed to evaluate associations between clinical variables and development of 30-day VTE. Multivariable logistic regression analyses were also performed to evaluate the effect of minimum and mean intraoperative temperature on development of 30-day VTE, controlling for significant covariates. Stepwise multivariable logistic regression models were developed using forward selection. A P value of less than 0.05 was used for entry criterion into the model and for a covariate to remain in the model. Receiver operating characteristic (ROC) curves were created for the multivariable models to evaluate the diagnostic efficacy of core body temperature via area under curve (AUC) determinations. Cutoff point analysis was performed using sensitivity-specificity as the cutoff point criterion. Patients who were missing data were excluded from those respective analyses. All statistical analyses were conducted by a dedicated biostatistician using SAS 9.4 (SAS Institute, Inc.; Cary, N.C.) and R software 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient and Operative Characteristics
A total of 362 patients who underwent 388 CAWR procedures were evaluated for this study. Ten patients were excluded for lack of intraoperative temperature data, and eight patients were excluded for having an inpatient stay fewer than two midnights. In total, 26 procedures were excluded for being repeat AWR procedures. A final total of 344 AWR procedures between 2014 and 2022 were included. See Supplemental Digital Content 1 for a summary of patient characteristics. Of the 344 total patients, 14 developed VTE in the 30-day postoperative period for an incidence rate of 4.1%. (See table, Supplemental Digital Content 1, which shows patient characteristics. http://links.lww.com/PRSGO/D154.) There was no significant difference between age, gender, BMI, smoking status, comorbidity burden, or COVID-19 status between the VTE and non-VTE cohorts. One (0.3%) patient in the non-VTE group was positive for inpatient COVID-19 status; this patient was PCR negative for COVID-19 3 days before surgery but had a prolonged inpatient stay due to ileus and subsequently developed fever with a PCR-positive COVID-19 diagnosis on POD 14. He was discharged on POD 16. The proportion of patients using anticoagulation or antiplatelet medications was comparable across cohorts, and of these, the proportion of patients who had their medications held before surgery were also comparable. (See table, Supplemental Digital Content 1, http://links.lww.com/PRSGO/D154.)
Supplemental Digital Content 2 provides a summary of operative characteristics. All underwent a multidisciplinary approach. (See table, Supplemental Digital Content 2, which shows operative characteristics http://links.lww.com/PRSGO/D155.) The majority of patients in this study underwent component separation (n = 218, 63%), had mesh placed (n = 330, 96%), and had ventral hernia defects (n = 304, 88%); there was no significant difference between cohorts for these variables. In total, 35 (10%) patients were missing data on PIP. Although the median intraoperative baseline PIP [non-VTE: 19 (interquartile range: 17–22) versus VTE: 18 mm H2O (16–19); P = 0.256] and median end of case PIP [non-VTE: 21 (18–25) versus VTE: 22 mm H2O (21–26); P = 0.237] were not significantly different between groups, the VTE cohort had a significantly higher increase (variance) in PIP after AWR [3 (2–6) versus 1 mm H2O (0, 4); P = 0.0099]. When examining intraoperative core temperature measures, differences in the average minimum [non-VTE: 35.5°C (SD 0.8) versus VTE: 35.5°C (0.5); P = 0.487], maximum [non-VTE: 37.1°C (0.7) versus VTE: 37.3°C (1.1); P = 0.652], and mean [non-VTE: 36.4°C (0.6) versus VTE: 36.3°C (0.5); P = 0.602] temperatures were all found to be nonsignificant between groups. The median length of stay was longer for patients in the VTE cohort [8 days (7–12) versus 5 days (3–7); P < 0.001]. (See table, Supplemental Digital Content 2, http://links.lww.com/PRSGO/D155.) Of the 14 VTE patients, nine were diagnosed during their inpatient stay, and five were diagnosed after discharge (Table 1). For these five patients, VTE was diagnosed on average 9 days after discharge on POD 14 (range: PODs 7–26).
Table 1.
Time to VTE Diagnosis
| Patient | LOS | VTE POD Diagnosed |
|---|---|---|
| 1 | 33 | 25 |
| 2 | 33 | 19 |
| 3 | 4 | 0 |
| 4 | 8 | 13 |
| 5 | 10 | 5 |
| 6 | 7 | 26 |
| 7 | 10 | 3 |
| 8 | 7 | 22 |
| 9 | 8 | 1 |
| 10 | 7 | 2 |
| 11 | 12 | 2 |
| 12 | 6 | 10 |
| 13 | 76 | 18 |
| 14 | 5 | 7 |
Regression Analysis
Univariable logistic regression of study variables found operative duration (OR = 1.32, P = 0.012), length of stay (OR = 1.07, P = 0.001), and PIP change (OR = 1.18, P = 0.045) to be significantly associated with 30-day VTE, whereas minimum (P = 0.913), maximum (P = 0.282), and mean (P = 0.70) intraoperative core temperature were not found to be significantly associated (Table 2).
Table 2.
Univariable Regression of Clinical Factors Associated with 30-day VTE
| Variable | Reference | Estimated OR | 95% CI | P |
|---|---|---|---|---|
| Age | Unit = 10 | 1.35 | (0.87, 2.18) | 0.184 |
| Sex | Female versus male | 1.77 | (0.58, 6.57) | 0.325 |
| Hypertension | Yes versus no | 1.20 | (0.41, 3.70) | 0.745 |
| Chronic obstructive pulmonary disease | Yes versus no | 1.08 | (0.06, 5.8) | 0.945 |
| Diabetes | Yes versus no | 0.93 | (0.21, 3.06) | 0.909 |
| Immunocompromised | Yes versus no | 3.04 | (0.90, 9.18) | 0.071 |
| Chronic kidney disease | Yes versus no | 0.45 | (0.02, 2.35) | 0.399 |
| Chronic liver disease | Yes versus no | 0 | (–, 6.66) | 0.412 |
| History of hypercoagulability | Yes versus no | 1.14 | (0.17, 4.38) | 0.866 |
| Inpatient COVID-19 status | Positive versus negative | 0 | (–, 141.41) | 0.773 |
| Smoking status | Yes versus no | 0 | (–, 22.11) | 0.617 |
| BMI (kg/m2) | Unit=5 | 0.80 | (0.50, 1.23) | 0.340 |
| Defect size (cm2) | Unit=10 | 1.03 | (0.99, 1.06) | 0.148 |
| Ventral hernia | Yes versus no | 0.78 | (0.20, 5.14) | 0.758 |
| Flank hernia | Yes versus no | 0 | (–, 1.36) | 0.089 |
| Umbilical hernia | Yes versus no | 0 | (–, 2.27) | 0.179 |
| Parastomal hernia | Yes versus no | 0.65 | (0.04, 3.41) | 0.663 |
| Component separation | Yes versus no | 3.61 | (0.96, 23.45) | 0.057 |
| Mesh use | Yes versus no | 0.53 | (0.09, 10.04) | 0.588 |
| Operative duration (min) | Unit=60 | 1.32 | (1.07, 1.63) | 0.012* |
| Length of stay (d) | Unit=1 | 1.07 | (1.03, 1.12) | 0.001* |
| Antiplatelet medications | Yes versus no | 1.20 | (0.27, 3.99) | 0.785 |
| Antiplatelet medications held | Yes versus no | 1.03 | (0.16, 3.93) | 0.971 |
| Anticoagulation medications | Yes versus no | 0.90 | (0.05, 4.8) | 0.919 |
| Anticoagulation medications held | Yes versus no | 0.98 | (0.05, 5.26) | 0.985 |
| Baseline PIP (mm H2O) | Unit = 5 | 0.60 | (0.22, 1.42) | 0.256 |
| Postoperative PIP (mm H2O) | Unit = 5 | 1.21 | (0.64, 2.12) | 0.541 |
| PIP change (mm H2O) | Unit = 1 | 1.18 | (1.00, 1.38) | 0.045* |
| Minimum intraoperative core temp (°C) | Unit = 1 | 0.96 | (0.53, 2.08) | 0.913 |
| Maximum intraoperative core temp (°C) | Unit = 1 | 1.48 | (0.71, 2.84) | 0.282 |
| Mean intraoperative core temp (°C) | Unit = 1 | 0.83 | (0.33, 2.15) | 0.700 |
Statistically significant (P < 0.05).
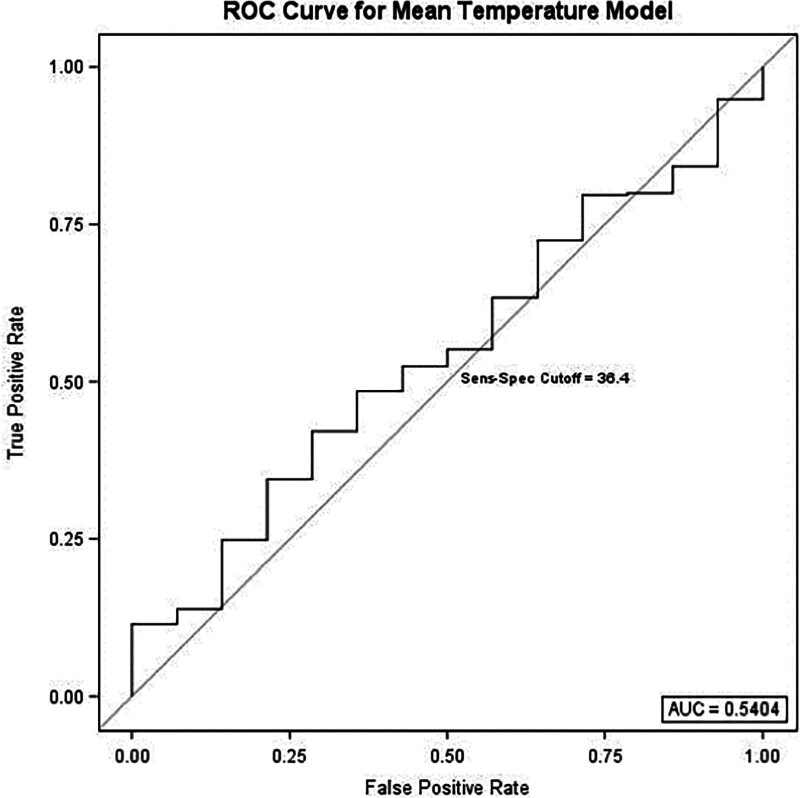
To further isolate the potential impact of intraoperative core temperature on the primary outcome, multivariable stepwise regression with forward selection was performed (Table 3). Using mean intraoperative temperature as the predefined predictor variable, the regression model selected immunocompromised status (OR = 4.1, P = 0.023) and length of stay (OR = 1.1, P < 0.001) as significant predictors of 30-day VTE. Component separation (P = 0.057) and mean temperature (P = 0.388) were nonsignificant as independent predictors. ROC analysis for diagnostic capability of the mean temperature regression model found an AUC = 0.54 (Fig. 1).
Table 3.
Multivariable Regression of Clinical Factors Associated with 30-day VTE
| Variable | Reference | Odds Ratio (95% CI) | P |
|---|---|---|---|
| Mean intraoperative core temperature | Unit = 1 | 0.7 (0.2, 1.7) | 0.388 |
| Immunocompromised | Yes versus No | 4.1 (1.2, 13.7) | 0.023 * |
| Length of stay | Unit = 1 | 1.1 (1.0, 1.1) | <0.001 * |
| Component separation | Yes versus No | 5.2 (1.0, 28.0) | 0.057 |
| Minimum intraoperative core temperature | Unit = 1 | 0.9 (0.4, 1.9) | 0.785 |
| Immunocompromised | Yes versus No | 4.0 (1.2, 13.4) | 0.025 * |
| Length of stay | Unit = 1 | 1.1 (1.0, 1.1) | <0.001 * |
| Component separation | Yes versus No | 4.5 (0.9, 22.9) | 0.070 |
CI: confidence interval.
Statistically significant (P < 0.05).
Fig. 1.
Receiver operating characteristic curve for the mean intraoperative temperature multivariable regression model with an AUC of 0.54. Sensitivity-specificity cutoff analysis determined 36.4°C to be optimal temperature cutoff.
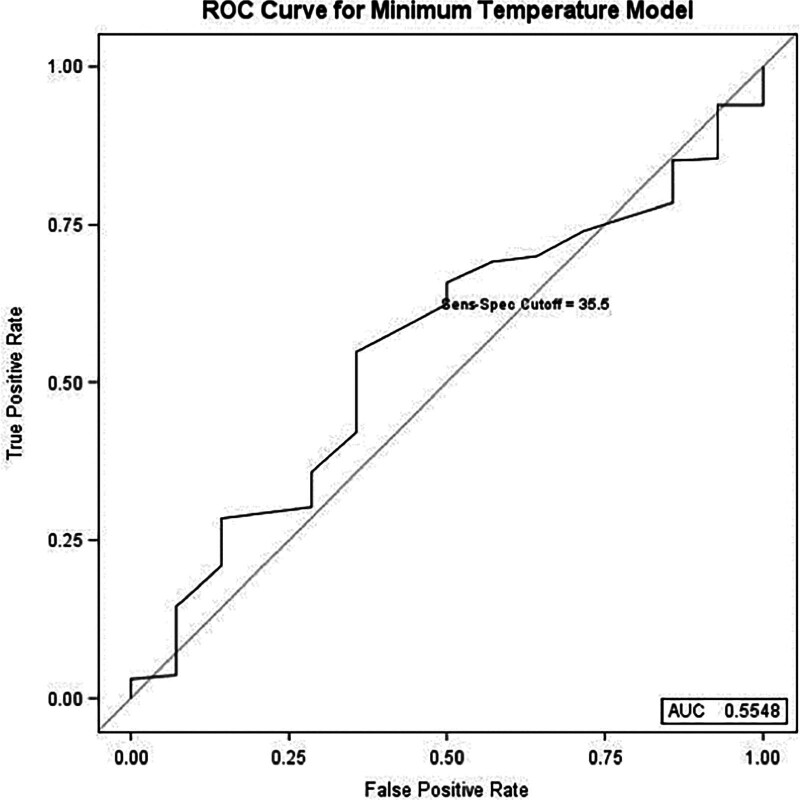
Additionally, when minimum intraoperative temperature was the predefined predictor variable, the regression model also selected immunocompromised status (OR = 4.0, P = 0.025) and length of stay (OR = 1.1, P < 0.001) as significant predictors of 30-day VTE. Component separation (P = 0.07) and minimum temperature (P = 0.785) were determined to be nonsignificant as independent predictors (Table 3). ROC analysis for diagnostic capability of the minimum temperature regression model found an AUC of 0.55 (Fig. 2).
Fig. 2.
Receiver operating characteristic curve for the minimum intraoperative temperature multivariable regression model with an AUC of 0.55. Sensitivity-specificity cutoff analysis determined 35.5°C to be optimal temperature cutoff.
Sensitivity-specificity Cutoff Analysis
Subsequent temperature cutoff point analysis using the sensitivity-specificity criterion identified an optimal mean temperature cutoff of 36.4°C and an optimal minimum temperature cutoff of 35.5°C. Neither the mean temperature cutoff (P = 0.59) nor the minimum temperature cutoff (P = 0.404) was found to be significant in their diagnostic efficacy for 30-day VTE (Table 4).
Table 4.
Sensitivity-specificity Cutoff Analysis
| Variable | Level | Total | No 30-day DVT/PE | DVT/PE | P |
|---|---|---|---|---|---|
| Optimal mean cutoff temperature 36.4°C | Above | 178 (52%) | 172 (52%) | 6 (43%) | 0.590 |
| Below | 166 (48%) | 158 (48%) | 8 (57%) | ||
| Optimal min. cutoff temperature 35.5°C | Above | 213 (62%) | 206 (62%) | 7 (50%) | 0.404 |
| Below | 131 (38%) | 124 (38%) | 7 (50%) |
DISCUSSION
The effects of intraoperative core body temperature on operative morbidity have previously been the subject of robust investigation in multiple surgical fields, with relative hypothermia associated with increased risk for prolonged length of stay, ischemic cardiac events, and blood loss.17–21 Although studies have also evaluated temperature effect on the risk of thrombotic events, none have done so specifically in the setting of CAWR. We found that intraoperative core body temperature was not associated with VTE development as a standalone variable using univariable regression analysis or predictive of VTE development when controlling for covariates with multivariable regression. Further, ROC curve analysis of the multivariable regression models determined the AUC to be 0.54 and 0.56 for mean and minimum intraoperative core body temperature, respectively; this is only slightly superior to a random classifier AUC of 0.50. These results were confirmed by cutoff point analysis, which also did not find a temperature cutoff point with significant diagnostic efficacy.
Other studies in varying surgical specialties with heterogenous cohorts have reported discordant results regarding the association of intraoperative core body temperature on thrombosis and VTE incidence. Moslemi-Kebria et al found that intraoperative hypothermia increased the risk of postoperative DVT after cytoreductive surgery in ovarian cancer patients.8 A similar study evaluating patients who underwent intracranial tumor resection reported a significant association between hypothermia and DVT incidence.7 Both studies defined hypothermia as less than 36.0°C, but they differed in that the former considered the intraoperative nadir, whereas the latter examined only the endoperative temperature. Within plastic and reconstructive surgery, examinations of this topic have generally focused on vascular thrombosis after microvascular free tissue transfer. While pedicle thrombosis may be affected by a different host of clinical considerations compared with coagulopathy, research teams have also reported conflicting results when evaluating its relationship to intraoperative temperature.9–11,22 Our current study is the first to investigate the relationship between intraoperative temperature and VTE specifically in CAWR, and we found no meaningful association between the two.
The understanding of risk factors for developing VTE after CAWR has been a great area of interest, with many studies looking outside intraoperative temperatures. Kim et al used the American College of Surgeons National Surgical Quality Improvement Program database and found an overall VTE rate of 0.5%. Component separation was not found to be associated with VTE in this study.23 Kraft et al found a 2.3% VTE rate in CAWR patients that all manifested as isolated PEs without DVT.3 Nelson et al specifically evaluated patients who underwent component separation using the American College of Surgeons National Surgical Quality Improvement Program database and reported a 2.1% VTE rate with obesity as a significant risk factor for VTE development.5 Finally, Andriyashkin et al reported component separation, BMI, and operative duration as significant predictors of VTE in a cohort that reflected a 7.9% incidence rate.4 Our findings of a 4.1% VTE rate are within range of these previous reports, albeit higher than most. This may be explained by the nature of the operations included in this study; all patients included in this study underwent a multidisciplinary approach (at least two services, including plastic surgery, and sometimes more), the majority of which required components separation, which reflected the complexity of the case and often required longer reconstructive procedures. Indeed, we found that operative duration (P = 0.012) was significantly associated with 30-day VTE on univariable analysis, a finding supported by Andriyashkin et al4 and Kraft et al, who noted that all four VTE patients in their study had a procedural duration of more than 240 minutes.3 One noteworthy result that has not been previously reported was the significant association between variance of baseline intraoperative to end of case PIP with 30-day VTE on univariable analysis (P = 0.045). This is a sensible result; intrathoracic and intraabdominal pressures can be affected by factors such as closures with higher tension as well as the use of postoperative compressive garments.24–27 High compartmental pressures may subsequently impair ventilatory function and compress the iliac vessels, reducing venous return and promoting stasis.28–30 However, given that a minority of patients in our cohort were missing this variable and were therefore excluded from this specific analysis, further in-depth evaluation may be necessary to better elucidate the impact of PIP on VTE incidence. Finally, we found that length of stay (P = 0.001) was significantly associated with 30-day VTE on univariable analysis; however, it is important to consider causal inference when evaluating the relationship between length of stay and VTE. It was noted that nine of 14 VTE patients were diagnosed during their inpatient stay; thus, VTE diagnosis was an influential factor on length of stay in this cohort.
This study is not without limitations. These data were collected from a single surgeon’s experience at a single institution in a retrospective manner. Any shortcomings inherent to such a design thus apply to this study, including potential limitations in patient diversity, external validity, and data fidelity. Secondly, the cohort of VTE patients was relatively small at 14, which left any multivariable regression analyses prone to overfitting. Indeed, this was reflected by the wide confidence intervals for covariates selected in the models presented herein. Stepwise regression with forward selection was used to further isolate temperature effect on 30-day VTE while controlling for variables that may have influenced the primary outcome. As such, although we can state that these multivariable regression models confirm a nonsignificant temperature effect, we could not make any valid conclusions on the effects of these covariates.
CONCLUSIONS
Intraoperative core body temperature was not associated with or predictive of 30-day VTE after abdominal wall reconstruction. Despite using an evidence-based protocol for VTE prophylaxis, the incidence rate remains nonzero, and surgeons caring for abdominal wall reconstruction patients must be aware of the risks inherent to this patient population. Future research is warranted to evaluate clinical factors that may contribute to VTE in an effort to further reduce this morbidity.
DISCLOSURES
Dr. Janis receives royalties from Thieme and Springer Publishing. The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 19 April 2024.
Presented in part at Plastic Surgery The Meeting 2023, Austin, Tex., October 27-29, 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Seaman AP, Sarac BA, ElHawary H, et al. The effect of negative pressure wound therapy on surgical site occurrences in closed incision abdominal wall reconstructions: a retrospective single surgeon and institution study. Hernia. 2021;25:1549–1555. [DOI] [PubMed] [Google Scholar]
- 2.Janis JE, Khansa I. Evidence-based abdominal wall reconstruction. Plast Reconstr Surg. 2015;136:1312–1323. [DOI] [PubMed] [Google Scholar]
- 3.Kraft CT, Janis JE. Venous thromboembolism after abdominal wall reconstruction: a prospective analysis and review of the literature. Plast Reconstr Surg. 2019;143:1513–1520. [DOI] [PubMed] [Google Scholar]
- 4.Andriyashkin AV, Loban KM, Kalinina AA, et al. Risk factors of venous thromboembolism after incisional ventral hernia repair. Hernia. 2022;27:895–899. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JA, Fischer JP, Cleveland EC, et al. Abdominal wall reconstruction in the obese: an assessment of complications from the National Surgical Quality Improvement Program datasets. Am J Surg. 2014;207:467–475. [DOI] [PubMed] [Google Scholar]
- 6.Pannucci CJ, Alderman AK, Brown SL, et al. The effect of abdominal wall plication on intra-abdominal pressure and lower extremity venous flow: a case report. J Plast Reconstr Aesthet Surg. 2012;65:392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Huang J, Chen X, et al. A Retrospective analysis of the postoperative effect of intraoperative hypothermia on deep vein thrombosis after intracranial tumor resection. World Neurosurg. 2022;167:e778–e783. [DOI] [PubMed] [Google Scholar]
- 8.Moslemi-Kebria M, El-Nashar SA, Aletti GD, et al. Intraoperative hypothermia during cytoreductive surgery for ovarian cancer and perioperative morbidity. Obstet Gynecol. 2012;119:590–596. [DOI] [PubMed] [Google Scholar]
- 9.Laitman BM, Ma Y, Hill B, et al. Mild hypothermia is associated with improved outcomes in patients undergoing microvascular head and neck reconstruction. Am J Otolaryngol. 2019;40:418–422. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y-J, Hirsch B, Shah A, et al. Mild intraoperative hypothermia reduces free tissue transfer thrombosis. J Reconstr Microsurg. 2011;27:121–126. [DOI] [PubMed] [Google Scholar]
- 11.Moellhoff N, Broer PN, Heidekrueger PI, et al. Impact of intraoperative hypothermia on microsurgical free flap reconstructions. J Reconstr Microsurg. 2021;37:174–180. [DOI] [PubMed] [Google Scholar]
- 12.Zhang KK, Reddy N, Janis JE. Office-based plastic surgery—evidence-based clinical and administrative guidelines. Plast Reconstr Surg Global Open. 2022;10:e4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kermode JC, Zheng Q, Milner EP. Marked temperature dependence of the platelet calcium signal induced by human Von Willebrand factor. Blood. 1999;94:199–207. [PubMed] [Google Scholar]
- 14.Valeri CR, MacGregor H, Cassidy G, et al. Effects of temperature on bleeding time and clotting time in normal male and female volunteers. Crit Care Med. 1995;23:698–704. [DOI] [PubMed] [Google Scholar]
- 15.Reed RL, Bracey AW, Hudson JD, et al. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141–152. [PubMed] [Google Scholar]
- 16.Shelby RD, Eiferman DS, Janis JE. Optimizing perioperative fluid management in complex abdominal wall reconstruction to prevent postoperative acute kidney injury. Am Surg. 2023;89:1879–1886. [DOI] [PubMed] [Google Scholar]
- 17.Frank SM, Beattie C, Christopherson R, et al. Unintentional hypothermia is associated with postoperative myocardial ischemia. The perioperative ischemia randomized anesthesia trial study group. Anesthesiology. 1993;78:468–476. [DOI] [PubMed] [Google Scholar]
- 18.Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 19.Schmied H, Kurz A, Sessler DI, et al. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet (London, England). 1996;347:289–292. [DOI] [PubMed] [Google Scholar]
- 20.Aitken LM, Hendrikz JK, Dulhunty JM, et al. Hypothermia and associated outcomes in seriously injured trauma patients in a predominantly sub-tropical climate. Resuscitation. 2009;80:217–223. [DOI] [PubMed] [Google Scholar]
- 21.Cohen B, Meilik B, Weiss-Meilik A, et al. Intraoperative factors associated with postoperative complications in body contouring surgery. J Surg Res. 2018;221:24–29. [DOI] [PubMed] [Google Scholar]
- 22.Hill JB, Sexton KW, Bartlett EL, et al. The clinical role of intraoperative core temperature in free tissue transfer. Ann Plast Surg. 2015;75:620–624. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Mella JR, Ibrahim AMS, et al. Is there an association between component separation and venous thromboembolism? Analysis of the NSQIP. Plast Reconstr Surg Glob Open. 2015;3:e429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosloski FR, Barbosa MVJ, Rodrigues MA, et al. Effect of compression garments on the ventilatory function after abdominoplasty. Aesthetic Surg J. 2023;44:174–182. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa MVJ, Nahas FX, Ferreira LM. A variation of the components’ separation technique that preserves the semilunaris for treatment of abdominal wall deformities. Indian J Plast Surg. 2022;55:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa MVJ, Ayaviri NAM, Nahas FX, et al. Improving tension decrease in components separation technique. Hernia. 2014;18:123–129. [DOI] [PubMed] [Google Scholar]
- 27.Fontes de Moraes BZ, Ferreira LM, Martins MRC, et al. Do compression garments prevent subcutaneous edema after abdominoplasty? Aesthetic Surg J. 2023;43:329–336. [DOI] [PubMed] [Google Scholar]
- 28.Corp A, Thomas C, Adlam M. The cardiovascular effects of positive pressure ventilation. BJA Educ. 2021;21:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukácsovits J, Nava S. Inspiratory pressure during noninvasive ventilation in stable COPD: help the lungs, but do not forget the heart. Eur Respir J. 2013;41:765–766. [DOI] [PubMed] [Google Scholar]
- 30.Torquato JA, Lucato JJJ, Antunes T, et al. Interaction between intra-abdominal pressure and positive-end expiratory pressure. Clinics (Sao Paulo). 2009;64:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.