Abstract
Purpose: We conducted a phase I trial of interleukin 2 (IL-2) in combination with chimeric 14.18 (ch14.18) and murine R24 antibodies to determine the maximal tolerated dose (MTD), immunological effects, and toxicity of this treatment combination. Experimental Design: Twenty-seven patients with either melanoma (23 patients) or sarcoma (4 patients) were enrolled to receive a combination therapy with ch14.18 and R24 antibodies together with continuous infusion of Roche IL-2 (1.5×106 U/m2/day, 26 patients) or Chiron IL-2 (4.5×106 U/m2/day, 1 patient) given 4 days/week for 3 weeks. The antibodies ch14.18 (2–7.5 mg/m2/day) and R24 (1–10 mg/m2/day) were scheduled to be administered for 5 days during the second week of IL-2 therapy. Results: When given in combination in this study, the MTD for ch14.18 was 5 mg/m2/day and the MTD for R24 was 5 mg/m2/day. Dose-limiting toxicities were severe allergic reactions to both ch14.18 and R24 as well as pain related to ch14.18. This ch14.18 MTD was lower than the 7.5 mg/m2/day MTD previously determined for ch14.18 given alone with the same dose and schedule of IL-2. Immunological effects included the induction of lymphokine-activated killer (LAK) activity and antibody-dependent cell-mediated cytoxicity (ADCC). Anti-idiotype response to ch14.18 was seen in six patients, including two melanoma patients who had a partial response to treatment. In addition to two partial responses, four patients had a stable disease and one patient remained without any evidence of disease. Conclusions: Immunotherapy with IL-2 in combination with ch14.18 and R24 antibodies augments LAK function and ADCC measured in vitro in all patients. While there exist theoretical advantages of combining these two antibodies, the MTD of ch14.18 and of R24 were lower than the MTD of each antibody in prior studies evaluating single antibody therapy with IL-2. As such, the combination of these two antibodies together with IL-2 therapy appeared to influence the MTD and toxicity of each of the administered antibodies.
Keywords: Melanoma, Immunotherapy, Ganglioside GD2, Ganglioside GD3, Antibody-dependent cell cytotoxicity
Introduction
For over 2 decades, interleukin-2 has been evaluated for its potential antitumor effects. Pioneering studies in the early 1980s demonstrated that lymphocytes activated in vitro with IL-2 are able to lyse various solid tumors, and inhibition and regression of syngeneic melanoma and sarcoma were observed in mice treated with IL-2 [15, 25, 30]. These IL-2-activated effector cells have been termed lymphokine-activated killer (LAK) cells, which are thought to consist primarily of natural killer cells capable of non-MHC-restricted cytotoxicity [27]. Analysis of tumor-infiltrating lymphocytes and observation of tumor regression following T-cell adoptive immunotherapy suggest that antigen-specific T lymphocytes may also act as effectors in IL-2-potentiated tumor lysis [5, 12]. Therapy with recombinant IL-2 has had some measurable success in renal cell carcinoma and melanoma [31], but it has been limited by the short duration of effect for most patients as well as dose-limiting toxicities. A cumulative response rate of 16% in melanoma patients treated with high-dose IL-2 [6] indicates the need for improved therapies.
More recently, there has been interest in combining IL-2 therapy with monoclonal antibodies (mAbs) specific to the tumor cells. The rationale behind this approach is to increase LAK-mediated tumor lysis via antibody-dependent cell cytotoxicity (ADCC) and to possibly expand on the types of tumors that can be targeted by LAK cells. Indeed, early studies have demonstrated enhancement of ADCC when various types of tumors are exposed in vitro to IL-2-activated lymphocytes in the presence of the tumor-specific mAb [17]. Clinical trials of tumor-reactive mAbs together with IL-2 have been performed for several malignant diseases, including lymphoma, colon cancer, breast cancer, neuroblastoma, and melanoma [19, 20, 29, 33]. In melanoma patients, gangliosides GD2 and GD3 have been targets for mAbs. Both GD2 and GD3 are abundantly expressed on the surface of melanoma, neuroblastoma, and other tumor cells of neuroectodermal origin but on relatively few normal cell types [38]. Clinical trials using murine anti-GD3 R24 antibody or murine anti-GD2 14.G2a antibody in combination with IL-2 have provided evidence of enhanced ADCC and LAK activity as well as occasional antitumor responses [4, 8, 13, 34].
One important factor that may limit the clinical utility of mAbs is the development of human anti-mouse antibody (HAMA). Most patients with melanoma who have been treated with R24 and 14.G2a developed a significant HAMA response [21]. A strong HAMA response can cause rapid clearance of the murine mAb upon retreatment. In an effort to reduce the immunogenicity of mAb 14.G2a, the chimeric antibody ch14.18 consisting of murine variable regions and human constant regions of IgG1 and κ chains was constructed [9, 32]. While ch14.18 still generated an anti-idiotype (anti-Id) antibody response, this effect seems less pronounced compared to the HAMA response to 14.G2a [1, 2, 21]. In addition to being less immunogenic, ch14.18 is able to mediate ADCC with human effector cells that is 50–100 times greater than its murine counterpart [26].
Our previous phase I trial of ch14.18 plus IL-2 in patients with metastatic melanoma established the maximal tolerated dose (MTD) of antibody, demonstrated immune activation, and showed limited antitumor activity [2]. Similar phase I trials have been conducted by others using R24 plus IL-2 [8, 34, 37]. These antibodies each target different disialogangliosides expressed on melanoma and sarcoma. One potential method of improving the clinical response would be to administer ch14.18 and R24 concurrently, which may increase the total amount of mAb bound to melanoma cells and prevent escape of heterogeneous tumor cells expressing GD2 or GD3 but not both [16]. The purpose of this study was to investigate the combined effect of ch14.18 and R24 given together with IL-2 in patients with melanoma and sarcoma, characterizing the MTD, toxicity, immune response, and antitumor activity of this combination immunotherapy.
Materials and methods
Clinical Protocol
Patients
Over a 51-month period, 23 melanoma and 4 sarcoma patients were enrolled in this clinical trial (National Cancer Institute-Biological Response Modifiers Program Protocol B94-0002). These patients had either biopsy-proven refractory melanoma or soft tissue sarcoma that was surgically or medically incurable by standard clinical approaches. Patients could have either measurable or evaluable disease using standard Eastern Cooperative Oncology Group (ECOG) criteria, or they could be melanoma patients with no evidence of disease if they had prior surgical resection of distant or of multiple regional recurrences. All patients had an ECOG performance status of 0 or 1 and a life expectancy of at least 12 weeks. Eligibility criteria included normal hematological parameters (leukocyte count, ≥3,500/μl; hemoglobin, ≥10.0 g/dl; and platelet count, >100,000/μl), adequate liver function (total serum bilirubin, <2.0 mg/dl; and transaminases, <3× normal), and adequate renal function (serum creatinine, <2.0 mg/dl; or creatinine clearance, >60 ml/min/1.73 m2). Criteria for patient exclusion included treatment with cytotoxic chemotherapy within 3 weeks, radiation therapy within 2 weeks, or treatment with glucocorticoids within 2 weeks prior to entry into the study. Patients with central nervous system (CNS) disease, including intracerebral CNS metastases or a history of CNS metastases, were eligible for treatment if the CNS disease was previously treated and clinically stable for at least 4 weeks following radiotherapy. Patients who required continued therapy with corticosteroids, aspirin, or nonsteroidal anti-inflammatory agents were ineligible. Patients with significant cardiac disease or symptomatic respiratory disease were also not eligible for this study. Patients who had previously received murine mAb or human/mouse chimeric antibody for tumor therapy, imaging or for any other reason, were ineligible for this study. Written informed consent that was approved by the University of Wisconsin Health Sciences Institutional Review Board was obtained from all patients.
Recombinant IL-2, ch14.18, and R24
Recombinant IL-2 was provided through the National Cancer Institute-Biological Response Modifiers Program by Hoffmann-LaRoche, Inc. (Nutley, NJ, USA). The drug was lyophilized and reconstituted with sterile 0.9 N NaCl solution. Unitage corresponds to the initial Hoffmann–LaRoche IL-2 unit, which also corresponds to that of the National Cancer Institute-Biological Response Modifiers Program standard IL-2 unit, as used previously in our published IL-2 trials [1, 3, 18]. The current dosing conversion from Hoffmann–LaRoche IL-2 units to commercially available Chiron (Chiron Therapeutics, Emeryville, CA, USA) IL-2 units is: 1 Roche unit is approximately equivalent to 3– 6 international units of Chiron IL-2 [18]. One patient in this study received Chiron IL-2 because no additional Hoffman–LaRoche IL-2 was available at the time.
The ch14.18 antibody is a chimeric construct containing the same murine variable region as murine mAb 14.G2 a that is specific for the GD2 antigen, which is expressed on human neuroblastoma, melanoma, and certain sarcomas [9, 32]. The ch14.18 antibody was constructed by joining the cDNA for the variable region of the murine antibody with the cDNA for human constant regions of γ1 heavy chain and the κ light chain [32]. This antibody was developed by Stephen D. Gillies (Fuji Immunopharmaceutical Corporation, Lexington, MA, USA) and was provided through the National Cancer Institute-Biological Response Modifiers Program (Frederick, MD, USA).
The R24 antibody is an IgG3 murine mAb that is specific for the GD3 antigen, which is found in high levels on melanoma and sarcoma cells [38]. The R24 antibody is manufactured by CellTech Ltd, Slough, England, and was provided through the National Cancer Institute-Biological Response Modifiers Program. A chimeric or humanized version of the murine anti-GD3 R24 monoclonal antibody was not available at the time of this clinical trial.
Study design
The patients were to be accrued sequentially according to a dose-escalation scheme in which the dose of ch14.18 is escalated from 2 to 7.5 mg/m2/day and R24 from 1 to 50 mg/m2/day or until the MTD is reached. The plan was to demonstrate the safety of this combination therapy in the initial treatment group by using a dose of ch14.18 that was approximately one-third of the previously determined MTD of ch14.18 when given with IL-2 [2]. The second treatment group was scheduled to receive a dose of ch14.18 that was two-thirds of the previously determined MTD of ch14.18 when given with IL-2. Dose escalation of R24 was planned to take place in then subsequent treatment groups. The dose of R24 in the initial two treatment groups was 1 mg/m2/day, as this dose was well-tolerated in prior studies of R24 when given as single antibody therapy in combination with IL-2 [8]. Therefore, the first three patients received 2 mg/m2/day of ch14.18 and 1 mg/m2/day of R24. Subsequent groups of patients received 5 mg/m2/day of ch14.18 and escalating dose levels of R24. A final group of patients received 7.5 mg/m2/day of ch14.18 and the MTD of R24 as determined from the previous groups. When three patients at any dose level completed the treatment course without dose-limiting toxicity defined as grade 3 or 4 antibody-related toxicity, patients were entered onto the next dose level. When grade 3 or 4 toxicity was encountered in one or more patients, up to a total of six patients were treated at that dose level to define the nature and frequency of that toxicity. Escalations were terminated when grade 3 or 4 antibody-related toxicity occurred in two patients entered at a given dose level.
All patients were scheduled to initially receive IL-2 at a dose of 1.5×106 U/m2/day (Chiron IL-2 dose of 4.5×106 U/m2/day in one patient) that was given 4 days/ week for 3 weeks (days 1–4, 8–11, and 15–18 of each course). The IL-2 was administered as a continuous infusion throughout each 96-h period. The ch14.18 and R24 antibodies were given as daily 4-h and 18-h infusions, respectively, for 5 days in a row during week 2 of the treatment (days 9–13 of each course). The 4-h ch14.18 infusion duration was the same as previously utilized in our prior study of ch14.18 when given as single antibody therapy with IL-2 [2]. The 18-h R24 infusion duration was selected based on an earlier study suggesting that a slower infusion may allow for higher doses of the antibody [37]. Following completion of the 3 weeks of treatment, patients were observed without treatment for 2 weeks. At that time, patients who still met all eligibility criteria and had stable disease or a clinical antitumor response could receive an additional 3-week course of treatment. Patients without an ECOG grade 3 IL-2 toxicity during their first 3 weeks of IL-2 treatment were eligible for an IL-2 dose escalation to 2×106 U/m2/day of Hoffman–LaRoche IL-2 in the second course. Patients who received a second course of treatment were scheduled to receive an additional 5 days of ch14.18 and R24 antibodies at the same dose as given during course 1. These were also administered during the second week of this second course of treatment. Patients with a complete or partial clinical response after the second course were offered a third course of treatment.
All patients were treated in the inpatient or outpatient facility of the University of Wisconsin General Clinical Research Center. The IL-2-alone treatments were administered on an outpatient basis. All treatment involving ch14.18 and R24 antibodies were administered as inpatient therapy. On each day of antibody treatment, each patient received an initial test dose of 1/20 of his or her scheduled daily antibody dose over 10 min, followed by 20 min of observation for evidence of allergic reaction. The remaining antibody administration was then completed as a 3.5-h (ch14.18) or 17.5-h (R24) infusion. Premedications for the antibody infusions included 0.3 ml subcutaneous injection of Sus-phrine (1:200 aqueous suspension of epinephrine, Steris Laboratories, Inc., Phoenix, AZ, USA); 2 mg intravenous injection of morphine; and 650 mg of acetaminophen and 50 mg of diphenhydramine hydrochloride orally.
Toxicity grading and dose modifications
The ECOG common toxicity criteria were used for grading of toxicities. The University of Wisconsin Comprehensive Cancer Center clinical toxicity grading scale for IL-2 studies was used for weight gain, systolic blood pressure, temperature, and decline in performance status [36]. For these parameters, mild (grade 1) toxicity corresponded to a ≤20 mmHg decrease in systolic blood pressure, a 5–10% body weight gain, fever of ≥38°C, or a decline in performance status of one grade. Moderate toxicity corresponded to grade 2 toxicity of 20–40 mmHg decrease in systolic blood pressure, 11–14% body weight gain, fever of 38.1–39.9°C, or a decline of two grades in performance status. Severe toxicity corresponded to grade 3 with ≥40 mmHg decrease in systolic blood pressure, ≥15% body weight gain, fever of ≥40°C, or a decrease of 3 grades in performance status. When grade 3 toxicity occurred, treatment was withheld until the toxic reaction(s) improved to ≤grade 1 or symptoms returned to baseline. Treatment was then resumed at 50% dose.
Response criteria
A complete response was defined as complete disappearance of all evident tumor and return of tumor-related abnormal test values to normal levels. A partial response was defined as a decrease by at least 50% of the sum of the areas of all known lesions in the absence of progression of any lesion or the appearance of any new lesions. Progressive disease was defined as any of the following: development of any new area of malignant disease; increase by at least 25% in any pretreatment area of measurable malignant disease; or significant clinical deterioration related to malignant disease. Stable disease was defined as a change in measurable disease too small to meet the requirements for partial response or progression and appearance of no new lesions. For patients who had no evidence of disease at the time of enrollment, clinical response was either “no evidence of disease” or “progressive disease” if any new lesions developed.
Immunological monitoring
Cell lines
The Daudi Burkitt’s lymphoma cell line was obtained from the American Type Culture Collection (Rockville, MD, USA). The LA-N-5 human neuroblastoma cell line was kindly provided by R. Seeger (Children’s Hospital of Los Angeles, Los Angeles, CA, USA).
Surface marker analysis
The expression of the Fcγ receptor (CD16), α chain of the IL-2 receptor (CD25), NK marker (CD56), and MHC class II human leukocycle antigen-type DR (HLA-DR) was examined by flow cytometry on PBMCs gated for lymphocytes. All fluorescence labeling was performed at 4°C in the dark for 30 min. Patient PBMC populations were characterized by incubating 2×105 Ficoll-Hypaque-isolated PBMCs in 100 μl of PBS with FITC- or PE-conjugated antibodies (Becton Dickinson, San Jose, CA, USA) at the concentrations recommended by the manufacturer’s procedure. Propidium iodide, at a final concentration of 1 μg/ml, was added just prior to analysis to separate live from dead cell populations.
Antibody-dependent cell cytotoxicity
All ADCC assays were performed in RPMI 1640 medium supplemented with 10% human serum (HS) (Pel-Freez, Rogers, AR, USA), 25 m M HEPES buffer, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate (RPMI–HS) and in RPMI–HS supplemented with IL-2 at a final concentration of 100 U/ml. Effector cells in RPMI–HS, in a total volume of 50 μl, were plated in quadruplicate into 96-well U-bottom microtiter plates at E:T ratios of 50:1, 16.7:1, and 5.6:1. Fifty microliter of medium or IL-2 at a final concentration of 100 U/ml was added to the effector cells and incubated for 30–45 min at 37°C in an incubator with 5% CO2. Immediately prior to the addition of target cells, 50 μl of the medium containing ch14.18 was added to the effectors. The final concentration per well was 0.25 μg/ml for ch14.18. Effector cells in medium, IL-2, or ch14.18 were tested for their ability to mediate lysis of target cells, which were LA-N-5 human neuroblastoma cells labeled with 51 Cr as previously described [2, 28]. Percentage of cytotoxicity values were calculated for each E:T ratio as follows:
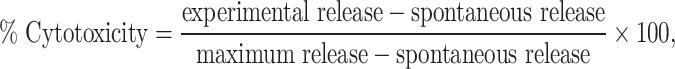 |
and results were expressed as lytic units, in which 1 lytic unit is the number of effector cells necessary to achieve 20% lysis of 5×103 targets [28].
LAK cell functional assays
PBMCs were assayed for their ability to kill the NK-resistant, LAK-sensitive Burkitt’s lymphoma cell line (Daudi), as previously described [2, 28].
R24 detection
A 96-well, C-8 immunomodule plate (Maxisorp, NUNC) was coated with 150 μl per well of goat anti-mouse IgG antibody at a concentration of 2 μg/ml in 0.05 M sodium carbonate buffer (pH 9.6) overnight at 4°C. The plate was washed three times with PBS, pH 7.4, containing 0.05% Tween 20 (Sigma). The plate was blocked with 200 μl per well of 5% milk in PBS + azide for three hours at room temperature in a moist chamber, then washed three times with Tris/Tween. Dilutions of R24 standards and serum test samples were made in 2.5% milk sample buffer (1:2 dilution in Tris/Tween of 5% milk in PBS + azide), and 100 μl of these samples were then added to the wells and incubated overnight at 4°C. After being washed five times with Tris/Tween, the plate was incubated with 100 μl of biotin-labeled goat anti-mouse IgG3 antibody (Sigma) at a 1:14,000 dilution in Tris/Tween for 3 h at room temperature before washing five times with Tris/Tween. The bound biotinylated antibody was detected by adding ExtrAvidin conjugated to alkaline phosphatase (Sigma) in Tris/Tween. After a 1-h incubation at room temperature and washing as above, staining was performed with p-nitrophenylphosphate (Sigma) in a concentration of 1 mg/ml in diethanolamine buffer. After 1-h incubation at room temperature in the dark, substrate conversion to colored product was determined at 405 nm with an ELISA reader (model EAR 400 AT; SLT Lab Instruments, Groeding/Salzburg, Austria).
Ch14.18 detection
ELISA detection of ch14.18 was performed using a modification of the method described previously [2, 14].
Evaluation of anti-idiotype antibody levels
Anti-Id antibody generated against the ch14.18 mAb was measured by ELISA as previously described [1, 2]. Briefly, the concept of this assay is based on how well patients’ serum specimens (obtained before and during the course of treatment) could “bridge” biotinylated ch14.18 antibody to ch14.18 antibody captured on the ELISA plate. Presence of these complexes is further detected by ExtrAvidin-alkaline phosphatase, and colorimetric readout >0.1 optical density (OD) over the background was considered as positive.
Statistical analysis
Treatment effects were assessed as the change in parameter values from baseline to various time points during treatment, and means are reported with standard errors (SEs). Wilcoxon signed rank test was used to test for treatment effects between baseline and follow-up times. Linear regression analysis was used to test for the time effect alone (in Fig. 1), and generalized least squares analysis was used to test for the time effect and the interaction terms between time and lymphocyte preparation (media alone or supplemented with IL-2 in Fig. 2 and media alone, supplemented with IL-2 or ch14.18 or both in a 2×2 factorial analysis in Fig. 3). The dose effect on the serum R24 mAb levels was assessed based on a piecewise linear regression (Fig. 4). The association between the presence of anti-Id antibody and antitumor effect was assessed using Fisher’s exact test. The data are presented in a simplified box plot in Figs. 1, 2, and 3 where the boxes represent the interquartile range from 25 to 75% percentile of lymphocyte count or lytic units per 107 effector cells and the line in the middle represents the median. The width of each box is proportional to the square root of the number of patients at each sample time. The dashed lines represent the full range, excluding the outliers (open circles) which fall outside of 1.5 times the length of the interquartile range.
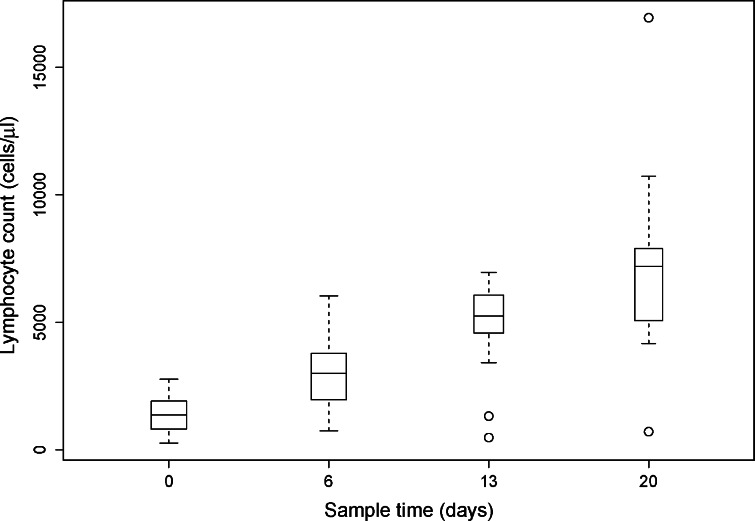
Fig. 1.
Lymphocyte counts were determined for patients at baseline before beginning protocol therapy (n = 27) and on protocol day 6 (following 1 week of IL-2; n = 27), day 13 (following the IL-2 plus ch14.18 and R24 administration; n = 19), and day 20 (following the last week of IL-2; n = 17). The change from baseline was significant for the lymphocyte counts on days 6, 13, and 20 (P < 0.001). Details of data presentation for Figs. 1, 2, 3 are provided in Statistical Analysis section
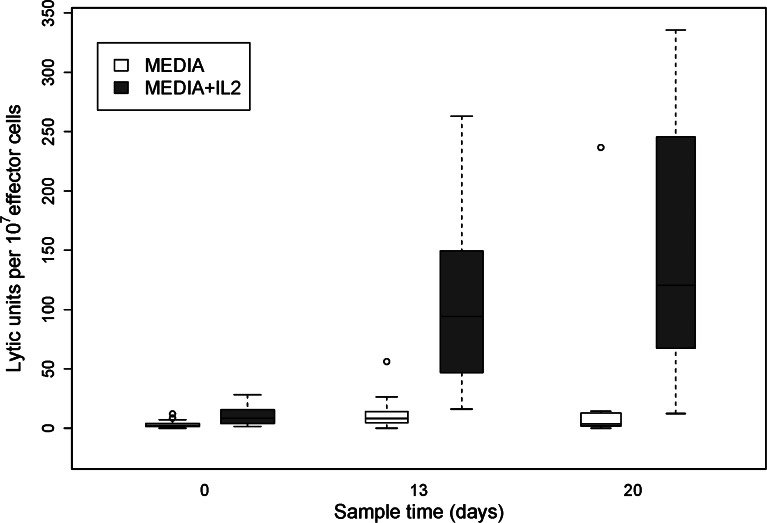
Fig. 2.
Lytic activity against Daudi cells using lymphocytes from patients obtained at baseline before beginning protocol therapy (n = 27) and on protocol day 13 (n = 17) and day 20 (n = 15). Lytic activity both with and without the addition of IL-2 to the assay is shown. The change from baseline was significant for values on day 13 (P <0.01) but only marginally on day 20 (P =0.083) for the assay in medium and was significantly increased over baseline on days 13 and 20 (P <0.001) for the assay in IL-2
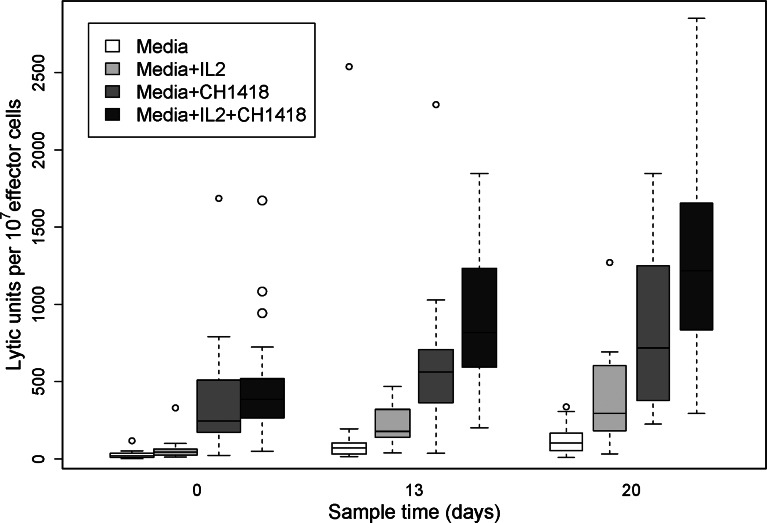
Fig. 3.
Lytic activity against the LA-N-5 neuroblastoma cell line using lymphocytes from patients obtained at baseline before beginning protocol therapy (n = 27) and on protocol day 13 (n = 17), and day 20 (n = 15). Lytic assays were performed in media alone, media supplemented with IL-2, media supplemented with ch14.18, and media containing both IL-2 and ch14.18. The change from baseline was significant for increased lysis of the LA-N-5 cell line in the assays in medium and containing IL-2, ch14.18, and both IL-2 and ch14.18 on day 13 (P <0.02) and day 20 (P <0.001)
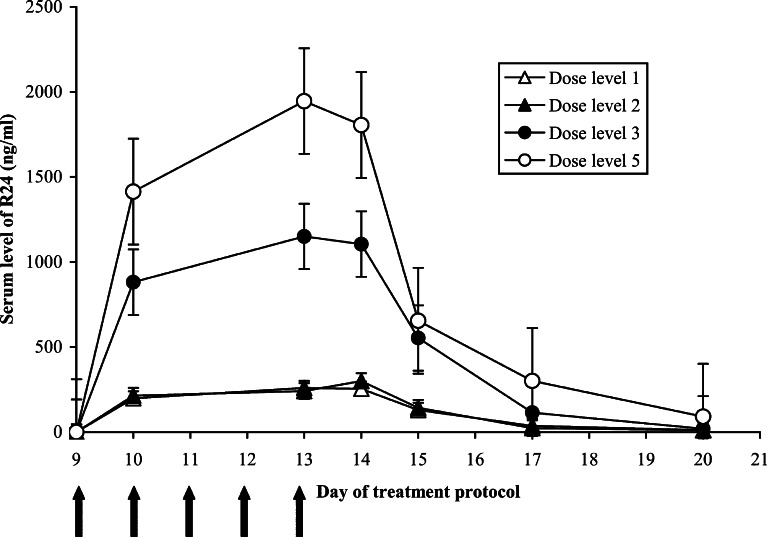
Fig. 4.
Serum R24 concentrations were measured at days 9, 10, 13, 14, 15, 17, and 20. The mean R24 concentration for each dose level group is shown with SE. R24 treatment was administered as an 18-h infusion on days 9, 10, 11, 12, and 13 as indicated by the arrows. Dose level 4 was not included as all but one patient in this group had an early termination or substantial dose reduction of R24
Results
Patient characteristics
Twenty-seven patients were entered into this study, and their pretreatment characteristics are outlined in Table 1. Twenty-three of the patients had melanoma and four patients had sarcoma. The four sarcoma patients had leiomyosarcoma of the uterus, leiomyosarcoma of the small bowel, gastrointestinal stromal sarcoma, and hemangiopericytoma. Twenty patients were men, the median age was 44 y, and all patients were of ECOG performance status 0 (14 patients) or 1 (13 patients). Six patients had received prior radiation therapy, 13 patients were treated with prior chemotherapy, and 6 patients had received prior immunotherapy. The most common sites of metastatic disease included nodal, skin, or subcutaneous disease (14 patients), pulmonary metastatic disease (10 patients), and metastatic disease to the liver (8 patients). One patient had a prior metastatic disease to the CNS. Four patients had no clinical evidence of disease at the time of entry into this study.
Table 1.
Characteristics of patients receiving immunotherapy with ch14.18 and R24 antibodies plus IL-2
| Characteristic | No. of patientsa |
|---|---|
| Total | 27 |
| Sex | |
| Male | 20 |
| Female | 7 |
| Prior therapy | |
| Surgery | 27 |
| Radiation therapy | 6 |
| Chemotherapy | 13 |
| Hormonal therapy | 7 |
| Immunotherapy | 6 |
| Hyperthermia | 3 |
| Sites of disease | |
| Nodal, skin, subcutaneous | 14 |
| Lung | 10 |
| Liver | 8 |
| Central nervous system | 1 |
| Bone | 2 |
| Intra-abdominal | 4 |
| Spleen | 5 |
| Other (adrenal, pleural, pelvis, neck mass) | 4 |
| Breast | 1 |
| No evidence of disease | 3 |
| Disease Status | |
| Melanoma | |
| M1ab | 2 |
| M1bc | 7 |
| M1cd | 14 |
| Sarcomae | 4 |
| Performance status (ECOG) | |
| 0 | 14 |
| 1 | 13 |
a Median age, 44; range, 24–62
b Metastatic disease to distant nodes, skin, or subcutaneous lesions
c Metastatic disease to lung
d Metastatic disease to other visceral sites or M1a, M1b disease with elevated LDH
e One patient each with leiomyosarcoma of the uterus, leiomyosarcoma of the small bowel, gastrointestinal stromal sarcoma, and hemangiopericytoma
Treatment summary
Twenty patients were evaluable to determine the MTD of R24 and ch14.18 when given together with IL-2. The remaining seven patients did not receive any antibody treatment due to IL-2-related toxicity requiring cessation of treatment prior to mAb treatment (patients 3, 4, 6, 8, 19, and 23) or patient decision to stop treatment (patient 1). The actual dose escalation scheme is shown in Table 2. Dose levels 1, 2, and 3 were well tolerated with no more than one antibody-related grade 3 toxicity in each group. Dose-limiting toxicity was seen in dose level 4 (ch14.18 at 5 mg/m2/day + R24 at 10 mg/m2/day), which consisted of severe allergic reactions characterized by angioedema causing partial upper airway obstruction in two patients. Dose escalation was therefore terminated at the R24 dose of 10 mg/m2/day. Dose-limiting toxicities were again encountered in dose level 5 (ch14.18 at 7.5 mg/m2/day + R24 at 5 mg/m2/day), which included severe allergic reaction causing partial upper airway obstruction in one patient, pain related to ch14.18 in one patient, and elevation of prothrombin time to greater than twice the normal value in one patient. The MTD for both ch14.18 and R24 when each was administered together in combination with IL-2 was thus determined to be 5 mg/m2/day.
Table 2.
Actual treatment groups for IL-2 combined with ch14.18 and R24 as delivered in this study
| Dose levels | No. of patients | ch14.18 (mg/m2/day) | R24 (mg/m2/day) | IL-2 (U/m2/day) |
|---|---|---|---|---|
| 1 | 3 | 2 | 1 | 1.5×106 |
| 2 | 3 | 5 | 1 | 1.5×106 |
| 3 | 6 | 5 | 5 | 1.5×106 |
| 4 | 4 | 5 | 10 | 1.5×106 |
| 5a | 4 | 7.5 | 5 | 1.5×106 |
a The last patient in this treatment group received Chiron IL-2 dose of 4.5×106 U/m2/day
Table 3 summarizes the antibody dose level, number of antibody courses, presence of anti-Id antibodies, clinical response, and duration of response for each patient treated on this study. Among the patients who completed at least one course of antibody treatment, those who required IL-2 dose reductions or modifications (skipped doses or dose reductions) in planned ch14.18 or R24 infusions are identified. The IL-2 schedule was interrupted or delayed in four patients (patients 7, 9, 13, and 14) due to pump error or infection, but the change in the total IL-2 dose administered to these four patients was minor to none. Eight patients had more significant dose reduction or early termination of IL-2 (patients 9, 10, 18, 21, 22, 25, 26, and 27). The reasons included severe toxicity related to antibody treatment in three patients, grade 3 hypotension, depression, infection, new metastasis to the brain, and pump error. Modifications in the planned ch14.18 or R24 infusions occurred in nine patients. Patients 9, 13, 15, 21, 22, 24, and 27 received a lower dose or had an early termination of R24 infusion secondary to significant allergic reactions (grade 3 except patient 15, who had grade 2 hives). Patient 9 had both antibodies held during course 3 due to IL-2-related grade 3 hypotension. Patient 24 developed angioedema following a ch14.18 infusion during course 1 and thus did not receive any ch14.18 during course 2. Patients 18 and 25 were taken off the study during antibody treatment due to severe infection and grade 4 elevation in prothrombin time, respectively. Patient 27 required a 50% dose reduction of ch14.18 due to neuropathic pain.
Table 3.
Treatment summary
| Patient no.a | Dose levelb | No. of antibody Coursesc | Anti-Id antibodyd | Clinical responsee | Duration of response or stable disease (months) |
|---|---|---|---|---|---|
| 1 | 1 | 0 | N/Ag | N/Ah | |
| 2l | 1 | 1 | − | Progression | |
| 3 | 1 | 0 | N/A | N/A | |
| 4l | 1 | 0 | N/A | N/A | |
| 5 | 1 | 1 | − | Progression | |
| 6 | 1 | 0 | N/A | N/A | |
| 7 | 1f | 1 | − | Progression | |
| 8 | 2 | 0 | N/A | N/A | |
| 9k | 2f | 3 | + | Partial | 18 |
| 10 | 2f | 1 | + | Stable | 2 |
| 11 | 2 | 1 | − | Stable | 3 |
| 12k, l | 3 | 2 | − | Progression | |
| 13k | 3f | 2 | + | Stable | 1 |
| 14l | 3f | 1 | − | Stable | 1 |
| 15 | 3 | 1 | + | Progression | |
| 16k | 3 | 2 | + | Progression | |
| 17 | 3 | 2 | + | Partial | 1 |
| 18 | 4f | 1 | N/Ai | No evidence of disease | 5 |
| 19 | 4 | 0 | N/A | N/A | |
| 20 | 4 | 1 | − | Progression | |
| 21 | 4f | 2 | − | Progression | |
| 22 | 4f | 1 | − | Progression | |
| 23 | 5 | 0 | N/A | N/A | |
| 24 | 5 | 2 | − | Progression | |
| 25 | 5f | 1 | − | N/Aj | |
| 26 | 5f | 1 | − | Progression | |
| 27 | 5f | 2 | − | Progression |
a Each patient entered was given a sequential patient number
b Modifications in planned ch14.18 or R24 infusions were required for patients 9, 13,15, 18, 21, 22, 24, 25, and 27
c Patients 1, 3, 4, 6, 8, 19, and 23 were taken off the study before any antibody was given
d Patients who had anti-Id antibody against ch14.18 detected at any time during protocol treatment are identified with “+”, and patients who did not are identified with “−”
e Clinical response was assessed following completion of all protocol therapy. The best clinical response for each patient is indicated. Patients 3, 18, 21 and 23 had no measurable disease at the time of starting protocol therapy
f IL-2 dose reductions were required for these patients
g N/A = not assessed
h Patients not receiving mAb were not assessed for clinical response to mAb
i Anti-Id level was not measured because the patient received only one dose of mAb
j The patient was taken off study due to a grade 4 toxicity and was not assessed for clinical response to mAb. The toxicity occurred after one dose of mAb
k These patients had dose escalation of IL-2 per protocol during the second course of therapy
l Patients 2, 4, 12, and 14 had sarcoma
Toxicity
Most patients treated on this clinical study had well-described IL-2 constitutional symptoms, including fever, chills, hypotension, anorexia, and decrease in performance status. Additional clinical toxicities that were grade 3 or greater, by either the common toxicity criteria or the University of Wisconsin Comprehensive Cancer Center clinical toxicity grading scale for IL-2 studies, are shown in Table 4. Patients subjected to this study did not receive prophylactic antibiotics, and all patients were treated with 1 mg of daily warfarin as prophylaxis for catheter-related thrombosis. Grade 3 allergic reactions to ch14.18 or R24 occurred in five patients—one each in dose levels 2 and 3, two in dose level 4, and one in dose level 5. The patient in dose level 2 developed wheezing and fever while receiving R24. The patient in dose level 3 developed hives with throat fullness. The allergic reactions in dose levels 4 and 5 involved tongue swelling leading to partial upper airway obstruction and were dose-limiting. The reactions in dose levels 2 and 3 were clearly related to R24, while the allergic reactions in patients at dose levels 4 and 5 seemed to be related to both ch14.18 and R24. Symptoms generally resolved after stopping the infusion and treating with diphenhydramine. One patient in dose level 5 developed upper airway edema and responded to symptomatic treatment with epinephrine and inhalers. Neuropathic pain in association with infusion of the ch14.18 antibody occurred in some patients receiving 5 mg/m2/day and the majority of patients receiving 7.5 mg/m2/day. The pain was controlled with IV morphine in all but one patient. The uncontrolled pain was dose-limiting for this patient. None of the patients in this study had any objective evidence of peripheral neuropathy. Patient 27 had grade 3 confusion but was subsequently found to have new CNS metastasis.
Table 4.
Significant clinical toxicities during IL-2 and ch14.18/R24 therapy
| Clinical toxicitya | No. of patients (total = 27) | No. of treatment courses (total = 36) |
|---|---|---|
| Fever ≥40°C | 4 | 4 |
| Hypotension (>40 mmHg reduction in systolic blood pressure or blood pressure <85) | 11 | 12 |
| Decline in ECOG performance status of 3 (0→3) | 3 | 3 |
| Weight gain ≥15% of total body weight | 0 | 0 |
| Central venous catheter thrombosis | 2 | 2 |
| Infection requiring intravenous antibiotics | 10 | 12 |
| Transient dyspnea or hypoxemia requiring oxygen | 3 | 3 |
| Severe allergic reaction | 5 | 6 |
| Severe depression/confusion | 2 | 2 |
| Objective peripheral neuropathy | 0 | 0 |
| Pain uncontrolled with intravenous morphine | 1 | 1 |
Most patients had some constitutional toxicities including fever, malaise, anorexia, and a decline in performance status
a The clinical toxicities that were ≥ grade 3 by either the common toxicity criteria or the University of Wisconsin Comprehensive Cancer Center clinical toxicity grading scale for IL-2 studies are indicated
Significant laboratory changes noted during this IL-2 and ch14.18/R24 antibody treatment are indicated in Table 5. One patient developed grade 4 thrombocytopenia after 1 week of IL-2 treatment and was taken off the study. Another patient developed grade 4 elevation of prothrombin time during week 2 after 1 day of antibody treatment. Other than the above two cases, toxicities were related to IL-2 and improved following the completion of protocol treatment. These laboratory changes were similar to those seen in our prior IL-2 protocols [2, 26] and represent toxicities attributable to IL-2.
Table 5.
Significant laboratory changes during IL-2 and ch14.18/R24 therapy
| Laboratory abnormality | No. of patients (total =27) | No. of treatment courses (total =36) |
|---|---|---|
| Hematological | ||
| Hemoglobin <10 g/dl | 12 | 15 |
| Neutrophil count <1000/μl | 5 | 6 |
| Eosinophil count >10,000/μl | 9 | 11 |
| Platelet count <50,000/μl | 1 | 1 |
| Prothrombin time | ||
| >2 times normal | 1 | 1 |
| Hepatic | ||
| Aspartate aminotransferase | ||
| 2.5–5 times normal | 9 | 10 |
| >5 times normal | 2 | 2 |
| Total bilirubin | ||
| 1.5–3 times normal | 1 | 1 |
| Renal (creatinine) | ||
| 2.0–2.9 mg/dl | 1 | 1 |
Antitumor effects
The best clinical response and duration of that response is shown for all patients in Table 3. Of the 20 patients who received antibody treatment, only 19 could be evaluated for response. Of the seven patients who had partial response, stable disease, or no evidence of disease, six had malignant melanoma and one (patient 14) had gastrointestinal stromal sarcoma. Patient 9 achieved a partial response after two courses of dose level 2 (ch14.18 at 5 mg/m2/day and R24 at 1 mg/m2/day), which consisted of >50% decrease in splenic metastasis and stable right axillary disease. Because this partial response was noted after course 2, this patient received a third course of immunotherapy, and the partial response was maintained for 18 months prior to disease progression. Patient 17 had a partial response after two courses of dose level 3 (ch14.18 at 5 mg/m2/day and R24 at 5 mg/m2/day) but developed new CNS metastasis before the third course could be started. Four patients had stable disease—two patients in dose level 2 and two in dose level 3. One patient who had no evidence of disease at the beginning of the treatment protocol remained without any evidence of disease for 5 months after receiving only 1 day combined antibody treatment (treatment was stopped due to sepsis). The patient with gastrointestinal stromal sarcoma did have stable disease after one course of therapy but elected not to proceed with a second course due to declining performance status.
Immunological results
Lymphocyte number and phenotype
Peripheral blood samples were obtained from patients to determine the treatment-associated changes in lymphocyte count and phenotype. As shown in Fig. 1, there was a progressive increase in lymphocyte counts after each 96-h IL-2 infusion. Lymphocyte counts obtained from patients on protocol treatment days 6, 13, and 20 reflect significant increases over baseline (P<0.005).
Lymphocyte cell surface phenotype was also evaluated. As presented in Table 6, the lymphocyte cell surface phenotype showed a therapy-induced increase in the percentage of both CD16+ Fc-receptor-expressing cells and CD56+ NK cells (P <0.05). These changes were initially seen following the first week of treatment with IL-2 alone and were maintained during each of the subsequent 2 weeks of treatment with antibodies. There was an increase in the percentage of PBMCs positive for CD25 on day 13 (P <0.05), but this was not maintained on day 20 of protocol treatment. There was also an increase in the percentage of PBMC positive for HLA-DR on days 13 and 20.
Table 6.
Changes in lymphocyte phenotype following IL-2 therapy
| CD16+ (%) | CD56+ (%) | CD25+ (%) | HLA-DR (%) | |
|---|---|---|---|---|
| Baseline | 22±2 | 21±2 | 25±2 | 22±3 |
| Day 13 | 28±3a | 33±4a | 39±5a | 43±3a |
| Day 20 | 33±4a | 39±5a | 28±4 | 41±5a |
Values are Mean±SE
a The change from baseline was significant on days 13 and 20 for all four markers (P <0.05) except for the percent of CD25+ lymphocytes at day 20
LAK and ADCC activity
Fresh PBMCs were evaluated for LAK activity at baseline and treatment days 13 and 20. As shown in Fig. 2, significant LAK activity against the Daudi cell line was induced in vivo with this combined IL-2 plus ch14.18 and R24 therapy and was best detected when IL-2 was included in the in vitro assay (P <0.001). This LAK activity was seen starting at second week of treatment and increased further after completion of the first course of therapy. In addition to the LAK activity against the Daudi target, ADCC was induced against the LA-N-5 neuroblastoma cell line known to express GD2 and to bind the ch14.18 antibody. Fresh PBMCs were evaluated at baseline and on protocol days 13 and 20 either in the presence or absence of 0.25 μg/ml of the ch14.18 antibody. As shown in Fig. 3, ADCC against the LA-N-5 cell line is seen on day 0 when media + ch14.18 and media + IL-2 + ch14.18 are compared with media alone or media + IL-2 (P<<0.001). Enhanced ADCC with ch14.18 antibody against this cell line is seen with PBMCs collected on protocol day 13 (P <0.02) and day 20 (P <0.001) when compared with results with PBMCs collected on day 0. The observed differences between days 13 and 20 is statistically significant with media + IL-2 (P=0.013), but only marginally so with media + ch14.18 (P = 0.104) and with media + ch14.18 + IL-2 (P = 0.068).
Serum R24, ch14.18, and Anti-idiotype antibody levels
Serum R24, ch14.18, and anti-idiotype antibody levels were measured before, during, and after the second week of treatment when ch14.18 and R24 were administered. Figure 4 illustrates the mean serum R24 concentration in each dose level group. Patients receiving R24 at 5 mg/m2/day achieved higher serum concentrations than those receiving 1 mg/m2/day, although the R24 levels declined rapidly by day 15 (3 days after the last dose) regardless of the dose received.
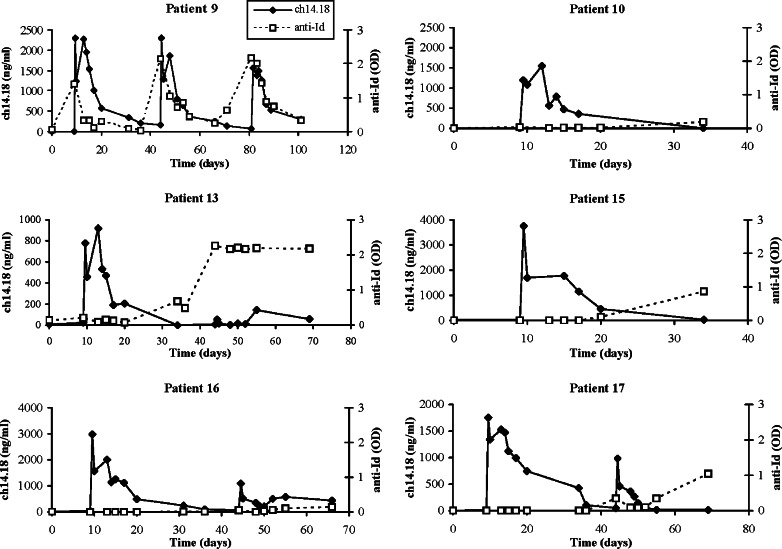
Six of nineteen patients who received antibody therapy and had anti-Id antibody levels measured (two patients at dose level 2 and four patients at dose level 3) developed significant levels (given as OD >0.1 at 1 : 5 serum dilution) of anti-Id antibody against ch14.18 (Table 4). Patient 18 was taken off the study after only one day of antibody therapy and therefore was not evaluated for an anti-Id response. Excluding patient 18, anti-idiotype antibody was present in 4 out of 6 patients who had a partial response or stable disease, compared to only 2 out of 13 patients who did not (P =0.046). The serum ch14.18 concentration and anti-Id level for each of the six patients showing anti-Id activity are shown in Fig. 5. Patients 10, 13, and 15 developed an anti-Id response near day 20, while patients 16 and 17 did not have a detectable response until the second course of therapy. In patient 13, the anti-Id response detected early in course 2 was associated with lower serum ch14.18 concentrations during the second course of therapy than during the first course. In contrast to the other patients, patient 9 had an anti-idiotypic response detected following the first week of IL-2 but prior to receiving antibody therapy, with no clear prior treatment that would be expected to induce this response. Moreover, the anti-Id response was not associated with lower concentrations of ch14.18 during the second and third courses (this patient only received 3 days of antibody therapy during course 3 due to a severe allergic reaction).
Fig. 5.
Serum ch14.18 concentrations and anti-idiotype levels for six patients who developed an anti-Id response. The anti-Id levels are presented as optical densities (OD), and only OD >0.1 was considered significant. Patient 9 had three courses of therapy and patients 13, 16, and 17 had two courses
Discussion
This study evaluated combined immunotherapy with IL-2 and the ch14.18 and R24 mAbs in patients with melanoma or sarcoma. When the antibodies were given together as a concurrent therapy with this IL-2 regimen; the MTD for both ch14.18 and R24 was 5 mg/m2/day. As expected, most patients experienced constitutional side effects such as fever, chills, and hypotension while receiving IL-2. Allergic reactions to R24 and neuropathic pain associated with ch14.18 were also common, both of which have been described in other studies using these antibodies [2, 23]. Dose-limiting toxicities were severe allergic reaction to either antibody in three patients, uncontrolled neuropathic pain in one patient, and elevation of prothrombin time to greater than twice the normal value in one patient. It is worth noting that the MTD of R24 in this study was significantly lower than in previously reported trials, although a direct comparison is not possible since none of them used the same IL-2 dose or treatment schedule. A total dose as high as 600 mg/m2 over 6–8 days has been tolerated when R24 was given alone [7], and 12 mg/m2/day has been given concurrently with a similar IL-2 regimen without dose-limiting toxicity [8]. Moreover, the MTD of ch14.18 in this study was lower than the 7.5 mg/m2/day determined in our previous phase I trial using the identical IL-2 regimen [2]. The lower-than-expected MTD for both ch14.18 and R24 suggests the possibility that some of the toxicities, particularly the allergic reactions, overlap when these antibodies are administered together.
There were several immunological changes associated with this combined IL-2 plus ch14.18 and R24 therapy. There was a treatment-related increase in lymphocyte count and LAK activity, both of which were the greatest at the end of the 3-week course. There was an increase in the percentage of CD16+ and of CD56+ lymphocytes, suggesting an increased percentage of Fc-receptor-bearing NK cells. The peripheral blood lymphocytes demonstrated significantly greater ability to mediate ADCC following treatment with IL-2. All of the above changes are similar to those seen in our previous studies using IL-2 alone and IL-2 plus ch14.18 [2, 3].
In this study, anti-Id response to ch14.18 was seen in 6 of 20 patients receiving the antibody. Two of these six patients had only a weakly positive anti-Id response, measuring just above 0.1 OD. The clinical significance of the anti-Id levels near the lower limit of detection is unclear, and there are too few patients to make a meaningful generalization about the pattern of the anti-Id response or any dose-related effects. The possible role of anti-Id and human anti-mouse antibodies against R24, neither of which was measured in this study (because of lack of appropriate reagents); also warrants further investigation. It is interesting to note that one patient who had a partial antitumor response (patient 9) had a detectable level of anti-Id antibody prior to receiving any ch14.18. Moreover, anti-Id response seemed to be more frequent in patients who had partial response or stable disease (4 of 6 evaluated patients) compared with those who did not (2 of 13 patients), a statistically significant difference. This finding is similar to the anti-Id responses observed in our IL-2 plus ch14.18 study [1, 2], and it raises the possibility that antitumor response may be associated with activation of the Id network. A relationship between antitumor effects and development of an anti-Id response has also been reported for children with neuroblastoma [11]. Vaccination against melanoma and other tumors using anti-Id antibodies is currently an area of intensive research [10].
Antitumor activity of the combined IL-2, ch14.18, and R24 therapy was rather limited despite evidence of immune activation in all of the evaluable patients. None of the patients had a complete response. A partial response was seen in one melanoma patient that received three courses of treatment, which was maintained for 18 months. One other patient with melanoma had a partial response after two courses, but a new CNS metastasis was discovered before the third course could be started. Five patients had either a stable disease or no evidence of disease after treatment, but all showed progressive disease within 5 months. While it is anticipated that the optimal clinical setting for in vivo ADCC will be for patients with micrometastatic disease, additional modifications of this combined immunotherapy are needed prior to proceeding to clinical testing in the adjuvant setting.
In conclusion, immunotherapy with IL-2 in conjunction with ch14.18 and R24 antibodies augments LAK and ADCC in all patients but is associated with significant toxicity requiring dose reduction of IL-2 or the antibodies in a substantial number of patients. We have previously reported a phase I trial of IL-2 plus ch14.18 [2], and this study explored the approach of targeting more than one tumor antigen with the rationale of further enhancing ADCC. Because the MTD of ch14.18 and of R24 when given in combination with IL-2 is lower than when given as a single mAb therapy with IL-2, it remains unclear whether the theoretical advantage of combining these two mAbs are outweighed by the need to decrease the dose when used in combination. Furthermore, since allergic reactions to the murine R24 mAb were significant toxicities, these might be avoided by using a chimeric or humanized R24 mAb, potentially allowing an increase in the R24 dose. This may be clinically important, especially when treating patients with bulky tumors, as higher antibody doses may be needed to penetrate into tumors and trigger ADCC. Thus, this regimen using ch14.18 and murine R24 plus IL-2 at the MTD shown here is not being recommended for phase II testing. If a chimeric or humanized R24 mAb becomes more widely available, exploration of combining it with ch14.18 and IL-2 may be warranted. Another approach to improving the antitumor activity using anti-GD2 mAb and IL-2 has been the development of a hu14.18-IL-2 recombinant fusion protein. This immunocytokine (IC) is comprised of the humanized form of the 14.18 mAb linked to IL-2 [35]. Preclinical studies demonstrate enhanced antitumor activity with this IC compared to separate administration of 14.18 mAb and IL-2 [24]. We have recently completed a phase I trial using the humanized IC hu14.18-IL-2 and have demonstrated in vivo immune activation and reversible clinical toxicities [22]. Phase II testing of hu14.18-IL-2 is currently in progress and will evaluate potential antitumor activity of this antibody-based therapy aimed at gangliosides on melanoma.
Acknowledgements
The authors thank the University of Wisconsin General Clinical Research Center (M01 RR03186), the Steve Leuthold Family Foundation (Jay Van Sloan Memorial) and Kathy Eagle (Tim Eagle Memorial) for gifts to the University of Wisconsin Comprehensive Cancer Center supporting our research on melanoma immunotherapy. The authors thank Brad Javorsky and Jean Surfus for technical assistance and Gloria St. Cyr for assistance with manuscript preparation.
Footnotes
This work is supported by NIH grants M01-RR03186, R01-CA32685, and P30-CA14520
References
- 1.Albertini MR, Gan J, Jaeger P, Hank JA, Storer B, Schell K, Rivest T, Surfus J, Reisfeld RA, Schiller JH, Sondel PM. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J immunother. 1996;19:278–295. doi: 10.1097/00002371-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Albertini MR, Hank JA, Schiller JH, Khorsand M, Borchert AA, Gan J, Behhofer R, Storer B, Reisfeld RA, Sondel PM. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–1288. [PubMed] [Google Scholar]
- 3.Albertini MR, Hank JA, Sondel PM. Strategies for improving antitumor activity utilizing IL-2: preclinical models and analysis of antitumor activity of lymphocytes from patients receiving IL-2. Biotherapy. 1992;4:189–198. doi: 10.1007/BF02174205. [DOI] [PubMed] [Google Scholar]
- 4.Alpaugh RK, von Mehren M, Palazzo I, Atkins MB, Sparano JA, Schuchter L, Weiner LM, Dutcher JP. Phase IB trial for malignant melanoma using R24 monoclonal antibody, interleukin-2/alpha-interferon. Med Onco1. 1998;15:191–198. doi: 10.1007/BF02821938. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH, Gehl J, Reker S, Pedersen LO, Becker JC, Geertsen P, thor Straten P. Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol. 2003;13:449–459. doi: 10.1016/j.semcancer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Onco1. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 7.Bajorin DF, Chapman PB, Wong GY, Cody BV, Cordon-Cardo C, Dantes L, Templeton MA, Scheinberg D, Oettgen HF, Houghton AN. Treatment with high dose mouse monoclonal (anti-GD3) antibody R24 in patients with metastatic melanoma. Melanoma Res. 1992;2:355–362. doi: 10.1097/00008390-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bajorin DF, Chapman PB, Wong G, Coit DG, Kunicka J, Dimaggio J, Cordon-Cardo C, Umacher C, Dantes L, Templeton MA. Phase I evaluation of a combination of monoclonal antibody R24 and interleukin-2 in patients with metastatic melanoma. Cancer Res. 1990;50:7490–7495. [PubMed] [Google Scholar]
- 9.Barker E, Mueller BM, Handgretinger R, Herter M, Yu AL, Reisfeld RA. Effect of a chimeric anti-ganglioside GD2 antibody on cell-mediated lysis of human neuroblastoma cells. Cancer Res. 1992;54:144–149. [PubMed] [Google Scholar]
- 10.Bhattacharya-Chatterjee M, Chatterjee SK, Foon KA. The anti-idiotype vaccines for immunotherapy. Curr Opin Mol Ther. 2001;3:63–69. [PubMed] [Google Scholar]
- 11.Cheung NV, Guo H, Heller G, Cheung IY. Induction of Ab3 and Ab3’ antibody was associated with long-term survival after anti-GD2 antibody therapy of stage 4 neuroblastoma. Clin Cancer Res. 2000;6:2653–2660. [PubMed] [Google Scholar]
- 12.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nature Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost JD, Hank JA, Reaman GH, Frierdich S, Seeger RC, Gan J, Anderson PM, Ettinger LJ, Cairo MS, Blazar BR, Krailo MD, Matthay KK, Reisfeld RA, Sondel PM. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer. 1997;80:317–333. doi: 10.1002/(SICI)1097-0142(19970715)80:2<317::AID-CNCR21>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Gan J, Kendra K, Ricci M, Hank JA, Gillies SD, Sondel PM. Specific enzyme-linked immunosorbent assays for quantitation of antibody-cytokine fusion proteins. Clin Diagn Lab Immunol. 1999;6:236–242. doi: 10.1128/cdli.6.2.236-242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. The lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hank JA, Albertini MR, Schiller J, Sondel PM. Activation of multiple effector mechanisms to enhance tumor immunotherapy. J immunother. 1993;14:329–335. doi: 10.1097/00002371-199311000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Hank JA, Robinson RR, Surfus J, Mueller BM, Reisfeld RA, Cheung NK, Sondel PM. Augmentation of antibody dependent cell mediated cytotoxicity following in vivo therapy with recombinant interleukin 2. Cancer Res. 1990;50:5234–5239. [PubMed] [Google Scholar]
- 18.Hank JA, Surfus J, Gan J, Albertini MR, Lindstrom M, Schiller JH, Hotton KM, Khorsand M, Sondel PM. Distinct clinical and laboratory activity of two recombinant interleukin-2 preparations. Clin Cancer Res. 1999;5:281–289. [PubMed] [Google Scholar]
- 19.Hotton KM, Khorsand M, Hank JA, Albertini M, Kim KM, Wilding G, Salamat MS, Larson M, Sondel P, Schiller JH. A phase Ib/II trial of granulocyte-macrophage-colony stimulating factor and interleukin-2 for renal cell carcinoma patients with pulmonary metastases: a case of fatal central nervous system thrombosis. Cancer. 2000;88:1892–1901. doi: 10.1002/(SICI)1097-0142(20000415)88:8<1892::AID-CNCR19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Kendra K, Gan J, Ricci M, Surfus J, Shaker A, Super M, Frost JD, Rakhmilevich A, Hank JA, Gillies SD, Sondel PM. Pharmacokinetics and stability of the ch14.18-IL-2 fusion protein in mice. Cancer Immunol Immunother. 1999;48:219–229. doi: 10.1007/s002620050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khazaeli MB, Conry RM, LoBuglio AF. Human immune response to monoclonal antibodies. J Immunother. 1994;15:42–52. doi: 10.1097/00002371-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 22.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. A phase I clinical trial of the immunocytokine EMD 273063 (hu14.18-IL2) in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood JM, Mascari RA, Edington HD, Rabkin MS, Day RS, Whiteside TL, Vlock DR, Shipe-Spotloe JM. Analysis of therapeutic and immunologic effects of R24 anti-GD3 monoclonal antibody in 37 patients with metastatic melanoma. Cancer. 2000;88:2693–2702. doi: 10.1002/1097-0142(20000615)88:12<2693::AID-CNCR7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;92:1706–1715. [PubMed] [Google Scholar]
- 25.Lotze MT, Grimm EA, Mazumder A, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumor by lymphocytes cultured in T cell growth factor. Cancer Res. 1981;41:4420–4425. [PubMed] [Google Scholar]
- 26.Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144:1382–1386. [PubMed] [Google Scholar]
- 27.Nirmala R, Narayanan PR. Flow cytometry—a rapid tool to correlate functional activities of human peripheral blood lymphocytes with their corresponding phenotypes after in vitro stimulation. BMC Immunol. 2002;3:p 9. doi: 10.1186/1471-2172-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pross HF, Maroun JA. The standardization of NK cell assays for use in studies of biological response modifiers. J Immunol Methods. 1984;68:235–249. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]
- 29.Rakhmilevich AL, Timmins JG, Janssen K, Pohlmann EL, Sheehy MJ, Yang NS. Gene gun-mediated IL-12 gene therapy induces antitumor effects in the absence of toxicity: a direct comparison with systemic IL-12 protein therapy. J Immunother. 1999;22:135–144. doi: 10.1097/00002371-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Mule JJ, Speiss PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. doi: 10.1001/jama.271.12.907. [DOI] [PubMed] [Google Scholar]
- 32.Saleh MN, Khazaeli MB, Wheeler RH, Allen L, Tilden AB, Grizzle W, Reisfeld RA, Yu AL, Gilles SD, LoBuglio AF. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14.18 in patients with malignant melanoma. Hum Antib Hybrid. 1992;3:19–24. [PubMed] [Google Scholar]
- 33.Shi FS, Weber S, Gan J, Rakhmilevich AL, Mahvi DM. GM-CSF secreted by cDNA-transfected tumor cells induces a more potent antitumor response than exogenous GM-CSF. Cancer Gene Ther. 1999;6:81–88. doi: 10.1038/sj.cgt.7700012. [DOI] [PubMed] [Google Scholar]
- 34.Soiffer RJ, Chapman PB, Murray C, Williams L, Unger P, Collins H, Houghton AN, Ritz J. Administration of R24 monoclonal antibody and low-dose interleukin 2 for malignant melanoma. Clin Cancer Res. 1997;3:17–24. [PubMed] [Google Scholar]
- 35.Sondel PM, Hank JA, Gan J, Neal Z, Albertini MR. Preclinical and clinical development of immunocytokines. Curr Opin Investig Drugs. 2003;4:696–700. [PubMed] [Google Scholar]
- 36.Sosman JA, Kohler PC, Hank JA, Moore KH, Bechhofer R, Storer B, Sondel PM. Repetitive weekly cycles of IL-2. II. Clinical and immunologic effects of dose, schedule, and addition of indomethacin. J Natl Cancer Inst. 1988;80:1451–1460. doi: 10.1093/jnci/80.18.1451. [DOI] [PubMed] [Google Scholar]
- 37.Vadhan-Raj S, Cordon-Cardo C, Carswell E, Mintzer D, Dantis L, Duteau C, Templeton MA, Oettgen HF, Old LJ, Houghton AN. Phase I trial of a mouse monoclonal antibody against GD3 ganglioside in patients with melanoma: induction of inflammatory responses at tumor sites. J Clin Oncol. 1988;6:1639–1648. doi: 10.1200/JCO.1988.6.10.1636. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73:42–49. doi: 10.1002/(SICI)1097-0215(19970926)73:1<42::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]