Abstract
Gene MAGE-A3 encodes tumor-specific antigenic peptides recognized by T cells on many tumors. MAGE-A3 peptides presented by HLA class I molecules have been identified using CD8 lymphocytes stimulated with cells that either expressed gene MAGE-A3 or were pulsed with candidate peptides. One antigen identified with the latter method is peptide MAGE-A3195–203 IMPKAGLLI, presented by HLA-A24 molecules. It has been used to vaccinate advanced cancer patients. Here, we have used HLA/peptide tetramers to detect T cells recognizing this peptide. Their frequency was estimated to be 2 × 10−8 of the blood CD8 cells in non-cancerous HLA-A24+ individuals, which is tenfold lower than the reported frequencies of T cells against other MAGE peptides. In the blood of a patient vaccinated with MAGE-A3, the estimated frequency was 5 × 10−7. Anti-MAGE-3.A24 cytolytic T cell clones were derived, that lysed peptide-pulsed cells with half-maximal effect at the low concentration of 500 pM. However, these CTL did not recognize a panel of HLA-A24+ tumor cells that expressed MAGE-A3 at levels similar to those found in HLA-A1+ tumor cells recognized by anti-MAGE-3.A1 CTLs. Furthermore, 293-EBNA cells transfected with MAGE-A3 and HLA-A24 constructs were hardly recognized by the anti-MAGE-3.A24 CTL clones. These results suggest that peptide MAGE-A3195–203 is poorly processed and is not an appropriate target for cancer immunotherapy.
Keywords: CTL, Cancer, MAGE, HLA-A24, Antigen presentation
Introduction
The ‘cancer-germline’ genes are a small number of gene families that are expressed in male germline cells, not in other normal adult tissues, and in many tumors [5]. Male germline cells do not express HLA genes, and therefore cannot present antigenic peptides to T lymphocytes. Therefore, the cancer-germline genes encode antigenic peptides that are strictly tumor-specific and that can be recognized by CD4 or CD8 T cells on many tumors [20]. The prototype cancer-germline genes are the MAGE genes [21]. They are ranged in three families of genes located on chromosome X: MAGE-A, -B, and -C [5, 7]. The MAGE-A family comprises 12 genes, including genes MAGE-A1 and MAGE-A3, the first cancer-germline genes found to code for tumor-specific antigenic peptides recognized on human tumors by cytolytic T lymphocytes (CTL) [8, 21]. These antigenic peptides were identified by using tumor-specific CTL clones derived from blood lymphocytes restimulated in vitro with autologous tumor cells.
Many groups have contributed to the identification of more than 50 combinations of MAGE peptides and HLA class I or class II molecules, recognized by CD8 or CD4 T cells, respectively [20]. For most of these identifications, the T cells were obtained using one of the following two methods. The first is to use T cells, preferably T cell clones, derived after in vitro stimulation of blood or tumor-infiltrating lymphocytes with either tumor cells or antigen-presenting cells infected with a recombinant virus encoding a MAGE gene. This procedure normally ensures that the anti-MAGE T lymphocytes recognize cells that naturally process and present the antigenic peptide, which is then identified. The other strategy is to stimulate T cells with a candidate peptide selected because it contains an appropriate HLA-binding motif. Its binding to the HLA is then verified with cells or purified HLA molecules. Next, peptide-specific T cells are obtained, usually through an in vitro priming of blood lymphocytes with the peptide. The last and crucial step is then to examine whether the candidate antigenic peptide is naturally processed in tumor cells that express the appropriate HLA and MAGE genes, by testing their recognition by the peptide-specific lymphocytes.
Peptide MAGE-A3195–203, IMPKAGLLI, was identified using this peptide stimulation approach [17]. Peptide-specific CTL lines were reported to lyse HLA-A24+ MAGE-A3+ carcinoma cell lines but not cell lines that expressed only HLA-A24 or MAGE-A3 [17]. On this basis, several groups have vaccinated HLA-A24+ cancer patients with autologous dendritic cells (DC) pulsed with peptide MAGE-A3195–203. Six patients with advanced gastrointestinal carcinoma received i.v. injections of peptide-pulsed DC, and one patient displayed regression of a metastatic lymph node [16]. Four patients with advanced bladder carcinoma received s.c. injections of a similar vaccine, and objective tumor regressions were observed in three patients [15]. Blood lymphocytes from one regressor patient lysed autologous tumor cells after in vitro restimulation with peptide-pulsed DC. Finally, four HLA-A24+ patients with metastatic melanoma received DC pulsed with KLH and five antigenic peptides corresponding to defined melanoma antigens, including peptide MAGE-A3195–203 [1]. Regressions of metastases were observed in two patients, with T cell responses to the peptide mix detected using an ELISPOT assay performed after an in vitro restimulation over 2 weeks.
We undertook the present work to estimate the frequency of circulating anti-MAGE-A3195–203 CD8 T cells in normal HLA-A24+ individuals and in vaccinated patients. We used the method that we had set up for other tumor-specific antigens, namely restimulation of blood lymphocytes with the antigenic peptide followed by detection with the appropriate tetramer [6, 10]. The anti-MAGE-A3195–203 lymphocytes that we could detect were less frequent than the CTL against other MAGE peptides, but we discontinued these frequency analyses when we realized that the T cell clones derived from these experiments did not recognize a panel of HLA-A24+ melanoma lines expressing MAGE-A3.
Results
Detecting anti-MAGE-3.A24 CD8 T cells in the blood of a melanoma patient
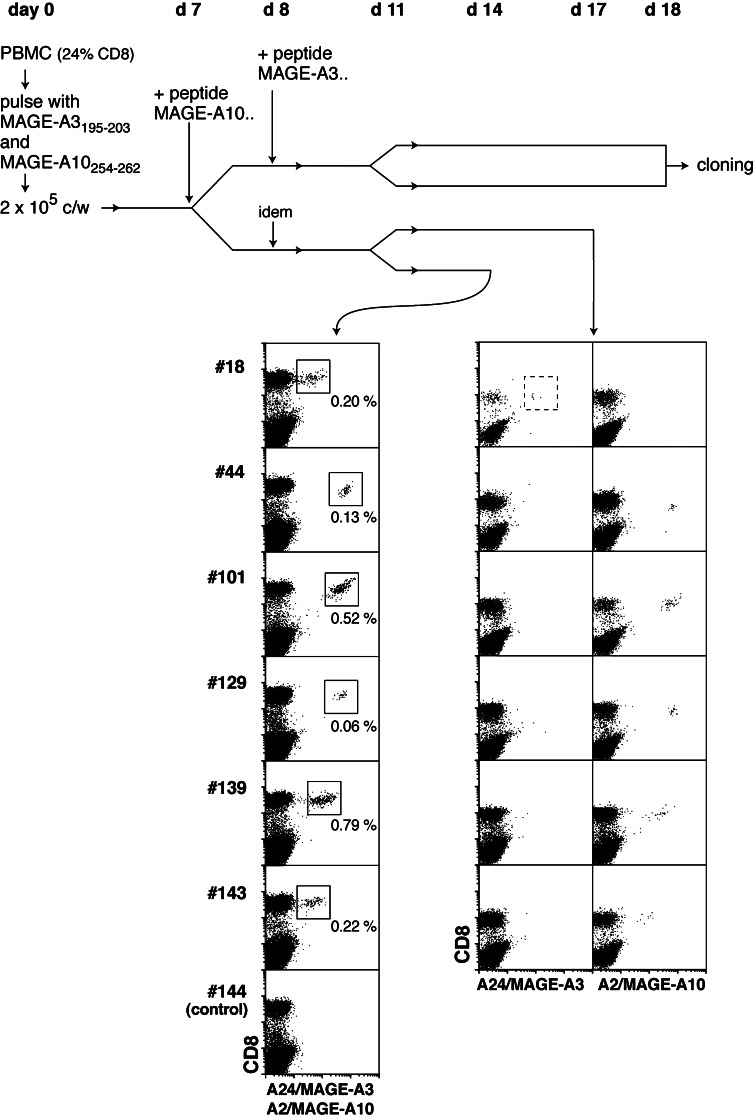
We first tried to detect anti-MAGE-3.A24 T cells in blood lymphocytes collected from a melanoma patient, LB2673, who was vaccinated with a recombinant MAGE-A3 protein and three peptides including peptide MAGE-A3195–203, referred to below as MAGE-3.A24, and peptide MAGE-A10254–262, which is presented by HLA-A2. Using the MAGE-A10 peptide as an internal control, we focused on these two peptides and used our mixed lymphocyte peptide culture (MLPC) approach involving the stimulation of lymphocytes with the antigenic peptides for 2 weeks under limiting dilution conditions, followed by labeling the responder cells with tetramers [6, 10]. An overview of the procedure is shown in Fig. 1. The tetramer screening was performed on day 14 with a mix of recombinant HLA-A24 molecules folded with peptide MAGE-A3195–203, and HLA-A2 folded with MAGE-A10254–262. Six out of the 156 microcultures contained CD8 cells stained by this mix of tetramers (Fig. 1). Three days later, cells of these six microcultures were labeled with each tetramer separately. One microculture contained anti-MAGE-3.A24 T cells, and the five others contained anti-MAGE-A10 lymphocytes (Fig. 1). Considering that the proportion of CD8 T cells in the peripheral blood mononuclear cells (PBMC) used in this experiment was 24%, the estimated frequency of precursors of anti-MAGE-3.A24 T cells is 1.3 × 10−7 of the blood CD8 T cells. In two other experiments, the estimated frequencies were 4.5 and 9.7 × 10−7 of the CD8 cells (Table 1).
Fig. 1.
Detection of anti-MAGE-3.A24 T cells using the MLPC/tetramer methodology. Blood lymphocytes from melanoma patient LB2673 were stimulated on days 0 and 7–8 with peptides and cytokines, and labeled on day 14 with anti-CD8 antibodies coupled to FITC, HLA-A24 tetramers containing peptide MAGE-A3195–203 and HLA-A2 tetramers containing peptide MAGE-A10254–262, all coupled to phycoerythrin. Microcultures that contained cells stained with tetramers were retested at day 17 with each tetramer separately. For the analysis of day 14, the proportions of CD8+ lymphocytes that were stained by the tetramer mix are indicated. The dotted line square in the plot of microculture #18 indicates the cells from which CTL clone 789 was derived
Table 1.
Mixed lymphocyte-peptide cultures (MLPC) carried out with peptide MAGE-A3195–20 3
| Patient | Responding cells | Number of microcultures | Cells per well | Percentage of CD8 | Tet + | CTL clone | |
|---|---|---|---|---|---|---|---|
| LB2673 | Melanomaa | Post-vacc PBMC | 156 | 200,000 | 24 | 1 | ×b |
| Post-vacc PBMC | 67 | 134,000 | 23 | 2 | ×c | ||
| Post-vacc PBMC | 75 | 140,000 | 21 | 1 | |||
| HATT | Lung cancerd | Pre-vacc PBMC | 11 | 200,000 | 9 | 0 | |
| Post-vacc PBMC | 23 | 200,000 | 21 | 0 | |||
| LB2052 | Non-cancerouse | CD4− CD56−f | 528 | 200,000 | 21 | 1 | |
| CD8+g | 191 | 30,000 | 87 | 0 | |||
| LB2690 | Non-cancerouse | CD8+g | 718 | 200,000 | 45 | 1 | |
| LB2348 | Non-cancerouse | CD8+g | 93 | 28,000 | 93 | 1 | |
| LB2422 | Non-cancerouse | CD8+g | 297 | 30,000 | 87 | 2 |
aThis patient received four injections of a vaccine consisting of a recombinant MAGE-A3 protein [13] combined with adjuvant SBAS-15 (GlaxoSmithKline Biologicals), and peptides MAGE-A3195–203, MAGE-A3243–258, and MAGE-A10254–262 injected without adjuvant. PBMC were collected 3 weeks after the fourth vaccination
bCTL clone 789 was derived from this experiment
cThis CTL clone was named 806. It expressed another TCR than CTL clone 789. Its lytic activity is shown in Fig. 4
dThis patient had undergone surgery for stage IV (pT4N0M0) non-small cell lung cancer when he was vaccinated with 300 μg of peptide MAGE-A3195–203 combined with the streptococcal preparation OK-432 [14]. PBMC were collected before and 2 weeks after a course of six vaccinations, given over a period of 7 weeks
eNon-cancerous hemochromatosis patient
fPBMC, which contained 7% CD8 T cells, were enriched for CD8+ cells by depleting CD4+ and CD56+ cells with magnetic microbeads
gResponder cells were CD8+ cells enriched from PBMC with magnetic beads, stimulated by the CD8− cells incubated with the antigenic peptide
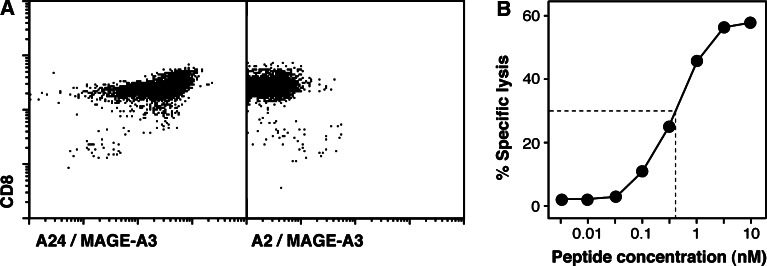
To confirm the specificity of the tetramer-stained cells, stable T cell clones were derived from tetramer-positive cells sorted at one cell per well and restimulated with HLA-A24+ Epstein-Barr virus (EBV)-transformed B cells pulsed with the antigenic peptide. Two independent clones were obtained, which expressed different T cell receptors (TCR) (Table 1). One clone, named CTL 789, is shown in Fig. 2. It was stained by the A24/MAGE-A3 tetramer but not by a control HLA-A2 tetramer folded with the same peptide MAGE-A3195–203. It lysed EBV-B cells incubated with peptide MAGE-3.A24. Half-maximal effect required only 500 pM of peptide, which is significantly less than what we usually observe for tumor-specific CTL clones, i.e., 1–30 nM. This result indicated that CTL clone 789 had a very good functional affinity for the peptide.
Fig. 2.
Characterization of anti-MAGE-A3.A24 CTL clone 789. a Cells were labeled with an anti-CD8 monoclonal antibody coupled to FITC, and with either an HLA-A24 tetramer containing peptide MAGE-A3195–203 or an HLA-A2 tetramer containing the same peptide, coupled to phycoerythrin. b Lytic activity of the CTL against HLA-A24+ EBV-transformed B cells incubated with the indicated concentrations of peptide MAGE-A3195–203. Chromium release was measured after 4 h of incubation at a CTL to target ratio of 10
As shown in Table 1, two MLPC/tetramer experiments were carried out with PBMC from a lung carcinoma patient vaccinated with peptide MAGE-3.A24, and no positive microculture was found.
Frequency of anti-MAGE-3.A24 CD8 T cells in the blood of non-cancerous individuals
MLPC/tetramer experiments were carried out with blood mononuclear cells from four donors, enriched for CD8+ cells by positive or negative selection with magnetic microbeads (Table 1). A total of 102 × 106 CD8 cells were used in five experiments, and only five microcultures were found to contain cells stained by the A24/MAGE-A3 tetramer. The resulting frequency of naive anti-MAGE-3.A24 lymphocytes was 5 × 10−8 of blood CD8 cells, which is tenfold lower than that observed in melanoma patient LB2673. It leaves open the possibility, but by no means proves, that this patient mounted a T cell response against peptide MAGE-3.A24. The frequency of 5 × 10−8 is also tenfold lower than that of naive T cells recognizing peptide MAGE-A3168–176 presented by HLA-A1, which we estimated to be around 4 × 10−7 of the CD8 cells in several donors [4, 12].
In view of these very low frequencies of anti-MAGE-3.A24 T cells detectable with our tetramer, we considered the possibility that the structure of the refolded antigen did not match exactly that of the natural HLA-A24/peptide complex, leading to false negatives. We therefore screened an MLPC experiment by measuring the lytic activity against HLA-A24+ EBV-B cells pulsed or not with the antigenic peptide, which is, in our hands, the most sensitive functional assay for human CD8 T cells. Reconstruction experiments for the detection assay were performed by spiking MLPC effector cells with a known CTL clone. They indicated a threshold of CTL detection of 1% among the effectors, i.e., about 30-fold higher than with a tetramer. We then used the lysis assay to screen an MLPC experiment, and found only one out of 96 microcultures with a low level of peptide-specific lytic activity. This microculture contained 0.9% of tetramer positive cells. Therefore, this experiment did not reveal the presence of anti-MAGE-3.A24 CTL that could not be stained by our tetramer preparation.
Lack of tumor recognition by anti-MAGE-3.A24 CTL clones
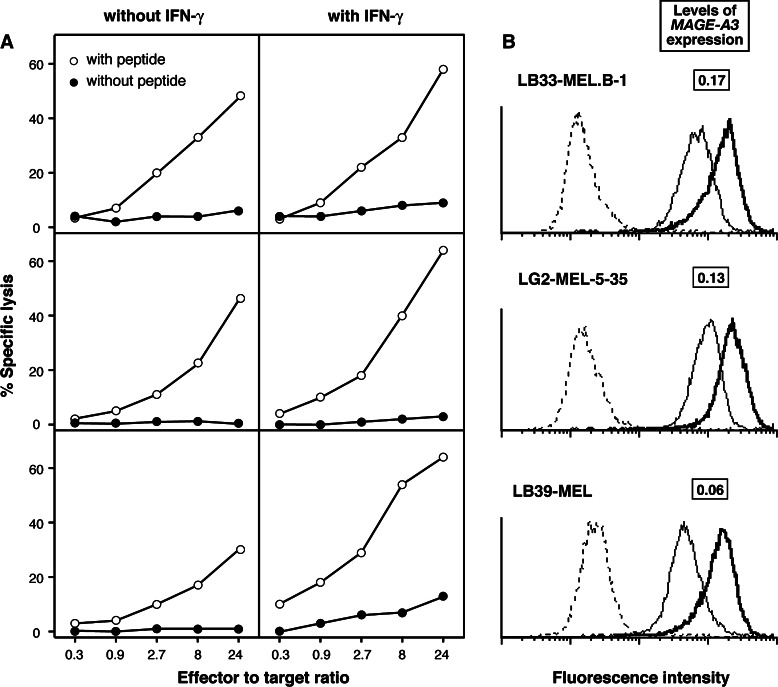
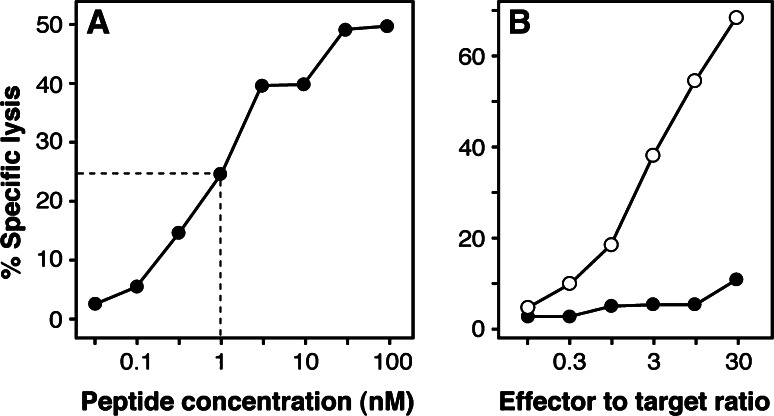
Even though CTL clone 789 recognized peptide MAGE-3.A24 presented by EBV-B cells (Fig. 2), it failed to significantly lyse any of three HLA-A24+ melanoma cell lines expressing MAGE-A3 (Fig. 3). This absence of lysis was not due to a low level of expression of HLA-A24 molecules, because the tumor cells were lysed after incubation with the antigenic peptide (Fig. 3). Furthermore, the melanoma cells were still not recognized after treatment with IFN-γ over 2 days, even though the surface expression of HLA-A24 molecules had increased, together with the sensitivity to lysis after peptide pulse (Fig. 3). Similar results were obtained with another CTL clone, named 806, derived from the same melanoma patient as CTL clone 789. As shown in Fig. 4, this CTL recognized peptide MAGE-A3195–203 very well, with half-maximal lysis of peptide-pulsed EBV-B cells obtained at 1 nM, and failed to significantly recognize melanoma cells LB39-MEL, unless these cells were pulsed with peptide.
Fig. 3.
Lytic activity of anti-MAGE-A3.A24 CTL clone 789 against HLA-A24+ melanoma cells expressing gene MAGE-A3. a Levels of lysis of three melanoma lines, treated or not over 2 days with 100 U/ml of IFN-γ, and incubated or not with peptide MAGE-A3195–203 at 100 nM. b Expression of HLA-A24 molecules at the surface of the melanoma cells pretreated (thick lines) or not (thin lines) with IFN-γ. Cells were labeled with a monoclonal anti-HLA-A24 antibody followed by goat anti-mouse Ig antibodies coupled to FITC. Dotted lines represent the staining of cells by the secondary antibodies alone. The levels of expression of gene MAGE-A3 by the melanoma lines are expressed as (copy number of MAGE-A3/copy number of ß-actin)
Fig. 4.
Characterization of anti-MAGE-A3.A24 CTL clone 806. a Lytic activity against HLA-A24+ EBV-transformed B cells incubated with the indicated concentrations of peptide MAGE-A3195–203. Chromium release was measured after 4 h of incubation at a CTL to target ratio of 5. b Lytic activity on melanoma cell line LB39-MEL, treated over 2 days with 100 U/ml of IFN-γ, and incubated (open symbols) or not (closed symbols) with peptide MAGE-A3195–203 at 1 μM
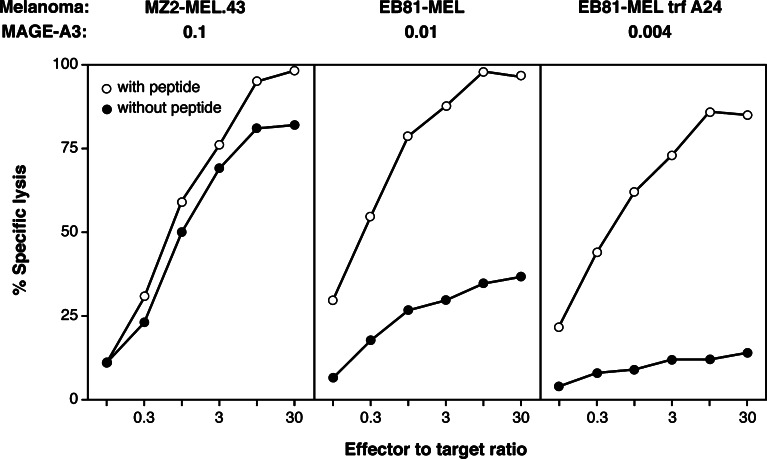
MAGE-A3 expression by the melanoma lines was quantified with real-time PCR. The ratios between the numbers of MAGE-A3 and ß-actin transcripts were 0.17, 0.13, and 0.06 (Fig. 3). To verify that these levels of expression were sufficient for recognition by anti-MAGE-A3 CTLs, we compared them with those of three other melanoma lines, which carried HLA-A1 molecules and which were tested with a CTL clone recognizing peptide MAGE-A3168–176 presented by HLA-A1. The MAGE-A3/ß-actin ratios were 0.1, 0.01, and 0.004 in these lines. The first two were lysed by the anti-MAGE-3.A1 CTL clone (Fig. 5). The third line was hardly lysed, but was recognized after peptide pulse, indicating that a MAGE-A3 expression level of 0.004 was too low for CTL recognition (Fig. 5). It is important to note that the functional affinity of the HLA-A1-restricted anti-MAGE-A3 CTL clone was significantly lower than that of CTL clone 789, as half-maximal lysis of EBV-B cells required 100 nM of the antigenic peptide (data not shown), i.e. 200 times more than what is required for CTL 789. We conclude that the three HLA-A24+ melanoma lines that were not recognized by CTL 789 expressed MAGE-A3 at levels that were sufficient for CTL recognition. Therefore, it had to be the amount of processed antigenic peptide that limited CTL recognition.
Fig. 5.
Recognition by an anti-MAGE-3.A1 CTL clone of HLA-A1 melanoma lines with different levels of expression of gene MAGE-A3. Melanoma lines were treated over 2 days with 100 U/ml of IFN-γ and incubated or not with peptide MAGE-A3168–176, EVDPIGHLY, at 10 μM before addition of CTL clone LAU147–810A10. EB81-MEL trf A24 is a clonal line derived from the melanoma cells EB81-MEL, after transfection with an HLA-A24 construct. The levels of expression of gene MAGE-A3 are expressed as in Fig. 3
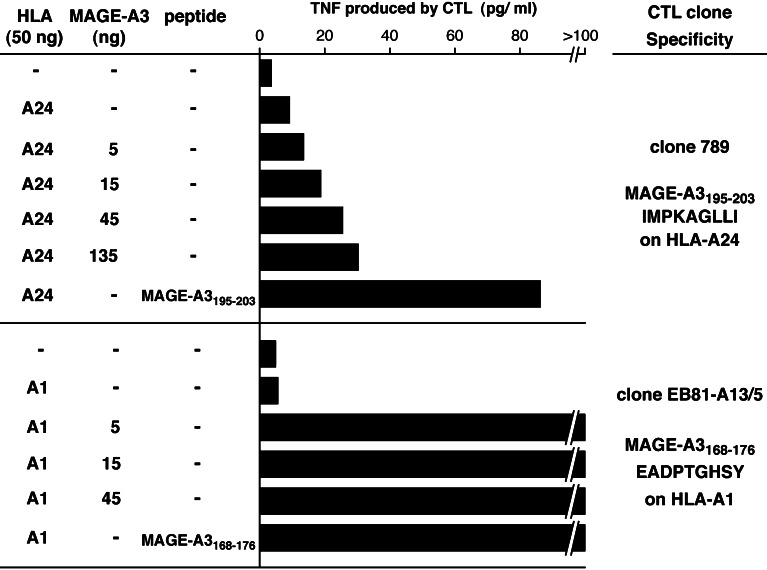
To confirm that peptide MAGE-3.A24 was poorly processed, we resorted to a transfection approach resulting in high levels of expression of the MAGE-A3 gene product. 293-EBNA cells, derived from the human embryonic kidney 293 cell line, constitutively express the EBNA-1 protein, which drives the amplification of transfected expression vectors containing the EBV origin of replication. These cells were cotransfected with EBV-ori(+) pCEP4 containing a MAGE-A3 cDNA and EBV-ori(−) pcDNA3 containing HLA-A24 or HLA-A1 constructs. This strategy results in a very high level of expression of protein MAGE-A3, and a lower but sufficient level of expression of the HLA presenting molecules. As shown in Fig. 6, CTL 789 produced TNF after stimulation with 293-EBNA cells transfected with HLA-A24 and pulsed with peptide MAGE-3.A24, but produced much less after stimulation with cells co-transfected with HLA-A24 and increasing doses of MAGE-A3. As a control, 293-EBNA cells transfected with HLA-A1 and low doses of MAGE-A3 stimulated the anti-MAGE-3.A1 CTL clone very well. Altogether, these results confirmed that peptide MAGE-3.A24 was poorly processed.
Fig. 6.
Recognition by CTL clones of 293-EBNA cells transfected with MAGE-A3 constructs. 293-EBNA cells were transfected with the indicated amounts of expression vectors pcDNA3 containing HLA-A1 or A24 cDNA clones, and pCEP4 containing a MAGE-A3 cDNA. After 24 h, untransfected cells were incubated with peptides MAGE-A3195–203 (30 nM) or MAGE-A3168–176 (1 μM) for 30 min. The indicated CTL clones were then added, and 18 h later the concentration of TNF released in the medium was estimated by measuring its cytotoxicity on the TNF-sensitive WEHI-164-clone 13 cells
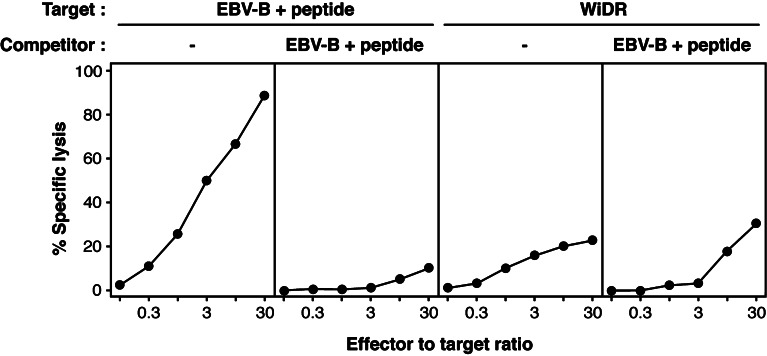
Finally, we tested the recognition of the colon carcinoma cell line WiDR, which expresses HLA-A24 and MAGE-A3, and was previously shown to be lysed by anti-MAGE-3.A24 T cells [17]. We also observed lysis by CTL clone 789, reaching about 25% at a CTL to tumor ratio of 30 (Fig. 7). However, this lysis could not be competed out by an excess of unlabeled peptide-pulsed EBV-B cells, whereas lysis of chromium-labeled peptide pulsed cells was completely abolished in the same assay. We provisionally conclude that this lytic activity is not a consequence of the recognition of peptide MAGE-3.A24 by the CTL, and did not explore its mechanism.
Fig. 7.
Lytic activity of CTL clone 789 against colon carcinoma cells WiDR. 51Cr-labeled target cells were either HLA-A24+ EBV-transformed B cells incubated for 20 min with peptide MAGE-A3195–203 at 300 nM and washed, or human colon carcinoma cells WiDR. CTLs were added to 1,000 labeled target cells at the indicated CTL to target ratios, in the absence or in the presence of 50,000 unlabeled peptide-pulsed EBV-B cells. Chromium release was measured after 4 h. The results are representative of three independent experiments
Discussion
The main result of this report is that peptide MAGE-A3195–203 seems to be poorly if at all processed. None of three HLA-A24+ MAGE-A3+ melanoma lines could stimulate the anti-MAGE-3.A24 CTL clone 789, which recognizes the peptide pulsed onto HLA-A24+ cells. Similar results were obtained with another anti-MAGE-3.A24 CTL clone from patient LB2673. The problem is not limited to these three melanoma cell lines: transfected 293 cells were also hardly recognized. It is only when a high amount of MAGE-A3 cDNA was transfected into 293-EBNA cells, with an expression vector that is amplified in these cells, that stimulation of the CTL was observed. Similarly, the CTL clone recognized COS-7 cells transfected with a MAGE-A3 cDNA cloned into vector pcDNA3, which contains the SV40 origin of replication and is amplified through the SV40 T protein that is present in COS-7 (data not shown). The levels of protein expression that are obtained in these transfectants are very high, and recognition by CTL under these conditions gives no indication as to the level of antigen processing in physiological conditions.
Even though we have no indication that the low level of processing of peptide MAGE-A3195–203 involves the proteasome, we performed exploratory experiments with a synthetic precursor peptide digested in vitro by purified proteasomes (data not shown). Mass spectrometry analysis of the digests suggested the absence of the cleavage after isoleucine 203, which generates the C-terminus of the peptide. This C-terminal cleavage is considered to be an important step in the processing of antigenic peptides by proteasomes [11]. In addition, several fragments in the digests appeared to result from destructive cleavages within the antigenic peptide, after the glycine residue at P6 and the leucine at P7. If confirmed in a more detailed analysis, these results could help to explain why peptide MAGE-3.A24 is poorly processed.
This peptide is not the first reported candidate tumor antigen eventually found to be poorly or not processed in tumor cells. Other clear examples include peptide MAGE-A3271–279 and a telomerase peptide, both presented by HLA-A2 molecules, which were also identified with a peptide prediction approach followed by in vitro priming of PBMC [2, 19, 22, 23]. It is probably worth raising a cautionary flag about the validation of tumor antigens identified with this strategy. It is essential to verify that the peptide-specific T cells recognize tumor cells, and this should be performed either with a CTL clone or with a T cell population that is highly enriched for the peptide-specific cells. With less pure populations, there can be a considerable background of cytokine secretion or of target cell lysis, even more so when allogenic tumor cells are used as stimulators or targets.
The analysis of the blood frequency of HLA-A24-restricted T cells recognizing peptide MAGE-3.A24 is, in retrospect, of little interest for cancer immunotherapy as this antigen is not present on tumor cells. However, an interesting observation was the frequency estimate for precursor T cells against this antigen in non-cancerous individuals: about 5 × 10−8 of the blood CD8 cells. This is tenfold lower than our frequency estimates for naive CD8 T cells against peptide MAGE-A3168–176 presented by HLA-A1, and for T cells against other tumor antigens such as LAGE-1/NY-ESO-1157–165, for which we recently estimated that in one donor the frequency of naive CD8 T cells was 3.3 × 10−7 of the blood CD8 cells [4, 12, Coulie, unpublished observations]. On the basis of these results, we consider that the mean frequency of naive human CD8 T cells recognizing a given HLA-peptide combination is 4 × 10−7 of the CD8, the highest measured frequency being 5 × 10−4 of the CD8, for the Melan-A/MART-1 peptide EAAGIGILTV [24], and the lowest being 5 × 10−8, as shown in this report.
As mentioned in the introduction, peptide MAGE-3.A24 has been used to vaccinate cancer patients, and a few tumor regressions have been reported [1, 15, 16]. Our results suggest that anti-MAGE-3.A24 CD8 T cells played no role in these regressions. In one clinical trial, DC were pulsed with several different peptides including MAGE-3.A24, leaving open the possibility that T cells directed against the other antigens of the vaccine initiated the tumor regression process [1]. In the two other trials, patients received DC pulsed with peptide MAGE-3.A24 alone [15, 16]. Assuming that the vaccines caused the regressions of lymph node or liver metastases that were observed in three out of the four vaccinated bladder cancer patients of one study [15], and of lymph node metastases in one out of the six vaccinated patients with gastrointestinal carcinoma in the other [16], it is difficult at this stage to explain the mechanism of these regressions. One possibility is the non-antigen-specific immunostimulatory effect of the DC themselves [18], a possible effect which has not yet been tested in human clinical trials. Another possibility is that peptide MAGE-3.A24, IMPKAGLLI, is taken up by presenting cells and processed into a shorter antigenic peptide presented by HLA-A24 or other HLA molecules. Along this line, we tested whether the octamer IMPKAGLL, which also contains the HLA-A24 binding motif, could bind to HLA-A24 molecules, with negative results.
Materials and methods
Reagents and cell lines
Melanoma cell lines EB81-MEL, LB33-MEL.B-1, LB39-MEL, LG2-MEL-5-35 and MZ2-MEL.43, and the colon carcinoma cell line WiDR (ATCC, Lot No 3000366, Date Frozen: 05/18/05) were grown in Iscove’s modified Dulbecco’s medium (Gibco, Grand Island, NY) with 10% FCS (HyClone, Logan, UT). WiDr cells were used as targets after less than ten passages of the cells obtained from the ATCC. Lymphocyte stimulation experiments were carried out in Iscove’s medium supplemented with 10% human serum (HS), 100 μM 1-methyl-tryptophan, and standard amino acids. This medium will be referred to below as complete medium (CM). Human rIL-2 was from Eurocetus (Amsterdam, the Netherlands), and human rIL-7 and rIFN-γ from PeproTech (Rocky Hill, NJ). Monoclonal antibody C7709A2 is a murine IgG2a reagent produced in our laboratory and which recognizes HLA-A24 molecules.
MLPC/tetramer
MLPC were set up as described [6, 10], with as responder cells either total PBMC or cells enriched for CD8+ lymphocytes using the MACS microbeads technology (Miltenyi Biotec, Bergisch Gladbach, Germany). In several experiments, CD8+ cells were enriched with beads coated with anti-CD8 antibodies, and in one experiment they were enriched by removing CD4+ and CD56+ cells with beads coated with the corresponding antibodies. The proportion of CD8+ T cells in the resulting populations was analyzed by flow cytometry with anti-CD3 and anti-CD8 antibodies. For stimulation at day 0, responder cells were incubated with peptide at 10 μM for 1 h at room temperature in Iscove’s medium containing 1% HS, washed, and distributed in microwells in CM supplemented with IL-2 (20 U/ml) and IL-7 (10 ng/ml). Cell numbers are indicated in Table 1. At day 7, all microcultures were restimulated by the addition of peptide at 5 μM. Between days 7 and 14, according to proliferation, half of the medium was replaced by fresh CM containing IL-2, or cultures were split. On day 14, an aliquot of cells from each microculture was labeled with an HLA-A24 tetramer containing peptide MAGE-A3195–203 and coupled to phycoerythin, a control A24 tetramer containing PRAME peptide LYVDSLFFL [9] and coupled to allophycocyanin, and anti-CD8 antibodies coupled to FITC. The procedure used to distinguish low numbers of tetramer-positive cells from background has been described [10]. In the experiment shown in Fig. 1, MLPC were stimulated simultaneously with two peptides, MAGE-A3195–203 and MAGE-A10254–262. For restimulation, one peptide was added at day 7 while another was added at day 8. As shown in Fig. 1, the tetramer screening was performed at day 14 with two tetramers coupled to phycoerythin: A24 with the MAGE-A3 peptide, and A2 with the MAGE-A10 peptide.
Derivation of CTL clones
Two CTL clones were derived that recognized peptide MAGE-A3165–203 presented by HLA-A24 (Table 1). Briefly, tetramer-positive cells were sorted at one cell/microwell and stimulated with 5,000 irradiated (100 Gy) HLA-A24 EBV-transformed B cells LG2-EBV-B pulsed for 1 h with the antigenic peptide at 10 μM and washed, and 50,000 irradiated allogenic PBMC as feeder cells, in CM containing IL-2 (50 U/ml) and IL-7 (10 ng/ml). Clones could be transferred into M24 wells after 3–4 weeks, and were maintained in culture by weekly restimulation with peptide-pulsed EBV-B cells and feeder PBMC as above, using 20 times more cells than in microwells. Overall, the cloning efficiency and the proliferation rate of these anti-MAGE-A3195–203 T cells were however lower than those we observed for T cells against other MAGE peptides. We have no satisfactory explanation for this difference.
Functional assays
Lytic activities were assessed with conventional chromium release assays, after a 4 h incubation of CTLs and targets. For the peptide titration experiment shown in Fig. 2, target cells (1,000 cells/well) were HLA24+ LB2348-EBV-B cells incubated with various concentrations of peptide for 30 min at room temperature in 100 μl of Iscove’s medium + 1% HS, before addition of the CTL clone (10,000 cells) in 50 μl of CM. For the recognition of transfected 293-EBNA cells shown in Fig. 6, confluent cultures of 293-EBNA cells in microwells were cotransfected, using 1 μl of the Lipofectamine reagent (Invitrogen) and following the manufacturer’s recommendations, with 50 ng of plasmid pcDNA3 containing an HLA-A24 or HLA-A1 cDNA sequence, and 5–135 ng of plasmid pCEP4 containing a MAGE-A3 sequence encoding the full-length gene product. After 24 h, control untransfected cells were pulsed for 30 min with peptides MAGE-A3195–203 at 30 nM or MAGE-A3168–176 at 1 μM, before addition of 10,000 anti-MAGE-3.A24 CTL clone 789 or 5,000 anti-MAGE-3.A1 CTL clone EB81-CTL-35 [10]. After 18 h of coculture, the supernatant was collected and its TNF content determined by testing its cytotoxic effect on WEHI-164 clone 13 cells as described [8].
Quantitative analysis of MAGE-A3 expression
RNA extracted with the QIAgen RNeasy kit (Qiagen, Hilden, Germany) was treated with DNAse using the DNA-free kit (Ambion, Austin, TX). Aliquots of 2 μg were converted to cDNA using an oligo(dT) primer and 200 units of the M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA), and 1/40th of the products were used as templates in real-time PCR amplifications carried out with the ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Warrington, UK) using the TaqMan methodology as described [3].
The following primers (sense and antisense) and probes were used. For MAGE-A3: M3T634 5′-GTCGTCGGAAATTGGCAGTAT, M3T742 5′-GCAGGTGGCAAAGATGTACAA, pMAGA3 (Applied Biosystems) 6FAM-5′-AAAGCTTCCAGTTCCTT-MGBNFQ. For ß-actin: OPC1652 5′-ATTGCCGACAGGATGCAGAA, OPC1653 5′-GTCATACTCCTGCTTGCTGA, ACTp2 (Eurogentec, Seraing, Belgium) 6FAM-5′-TCAAGATCATTGCTCCTCCTGAGCGC-TAMRA (kindly provided by Dr B. Lethé).
Acknowledgments
We thank Therèse Aerts for expert technical assistance, André Tonon for cell sorting experiments, and Suzanne Depelchin for editorial help. This work was supported by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programing, and by grants from the Fonds J. Maisin (Belgium), the Fondation contre le Cancer (Belgium), the Fondation Salus Sanguinis (Belgium), the Fonds National de la Recherche Scientifique (Belgium) and the FB Assurances and VIVA (Belgium).
Abbreviations
- CTL
Cytolytic T lymphocytes
- DC
Dendritic cells
- EBV
Epstein-Barr virus
- MLPC
Mixed lymphocyte peptide culture
- PBMC
Peripheral blood mononuclear cells
- TCR
T cell receptor
References
- 1.Akiyama Y, Tanosaki R, Inoue N, Shimada M, Hotate Y, Yamamoto A, Yamazaki N, Kawashima I, Nukaya I, Takesako K, Maruyama K, Takaue Y, Yamaguchi K. Clinical response in Japanese metastatic melanoma patients treated with peptide cocktail-pulsed dendritic cells. J Transl Med. 2005;3:4. doi: 10.1186/1479-5876-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyoub M, Migliaccio M, Guillaume P, Lienard D, Cerottini JC, Romero P, Levy F, Speiser DE, Valmori D. Lack of tumor recognition by hTERT peptide 540–548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol. 2001;31:2642–2651. doi: 10.1002/1521-4141(200109)31:9<2642::AID-IMMU2642>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Bonehill A, Heirman C, Tuyaerts S, Michiels A, Breckpot K, Brasseur F, Zhang Y, Van Der Bruggen P, Thielemans K. Messenger RNA-electroporated dendritic cells presenting MAGE-A3 simultaneously in HLA class I and class II molecules. J Immunol. 2004;172:6649–6657. doi: 10.4049/jimmunol.172.11.6649. [DOI] [PubMed] [Google Scholar]
- 4.Chaux P, Vantomme V, Coulie P, Boon T, van der Bruggen P. Estimation of the frequencies of anti-MAGE-3 cytolytic T lymphocyte precursors in blood from individuals without cancer. Int J Cancer. 1998;77:538–542. doi: 10.1002/(SICI)1097-0215(19980812)77:4<538::AID-IJC11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–5551. [PubMed] [Google Scholar]
- 6.Coulie PG, Karanikas V, Colau D, Lurquin C, Landry C, Marchand M, Dorval T, Brichard V, Boon T. A monoclonal cytolytic T-lymphocyte response observed in a melanoma patient vaccinated with a tumor-specific antigenic peptide encoded by gene MAGE-3. Proc Natl Acad Sci USA. 2001;98:10290–10295. doi: 10.1073/pnas.161260098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora J-P, De Smet C, Brasseur F, van der Bruggen P, Lethé B, Lurquin C, Brasseur R, Chomez P, De Backer O, Cavenee W, Boon T. Structure, chromosomal localization and expression of twelve genes of the MAGE family. Immunogenetics. 1994;40:360–369. doi: 10.1007/BF01246677. [DOI] [PubMed] [Google Scholar]
- 8.Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E, Lethé B, Brasseur F, Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda H, Lethé B, Lehmann F, Van Baren N, Baurain J-F, De Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 10.Karanikas V, Lurquin C, Colau D, van Baren N, De Smet C, Lethé B, Connerotte T, Corbière V, Demoitié M-A, Liénard D, Dréno B, Velu T, Boon T, Coulie PG. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J Immunol. 2003;171:4898–4904. doi: 10.4049/jimmunol.171.9.4898. [DOI] [PubMed] [Google Scholar]
- 11.Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2:179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- 12.Lonchay C, van der Bruggen P, Connerotte T, Hanagiri T, Coulie P, Colau D, Lucas S, Van Pel A, Thielemans K, van Baren N, Boon T. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14631–14638. doi: 10.1073/pnas.0405743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchand M, Punt CJA, Aamdal S, Escudier B, Kruit WHJ, Keilholz U, Håkansson L, van Baren N, Humblet Y, Mulders P, Avril M-F, Eggermont AMM, Scheibenbogen C, Uiters J, Wanders J, Delire M, Boon T, Stoter G. Immunization of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: clinical report. Eur J Cancer. 2003;39:70–77. doi: 10.1016/S0959-8049(02)00479-3. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara S, Tsunoda T, Baba T, Asabe S, Tahara H. Dendritic cells stimulated with a bacterial product, OK-432, efficiently induce cytotoxic T lymphocytes specific to tumor rejection peptide. Cancer Res. 2003;63:4112–4118. [PubMed] [Google Scholar]
- 15.Nishiyama T, Tachibana M, Horiguchi Y, Nakamura K, Ikeda Y, Takesako K, Murai M. Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res. 2001;7:23–31. [PubMed] [Google Scholar]
- 16.Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, Fujie T, Tanaka F, Inoue H, Takesako K, Akiyoshi T, Mori M. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res. 2001;7:2277–2284. [PubMed] [Google Scholar]
- 17.Tanaka F, Fujie T, Tahara K, Mori M, Takesako K, Sette A, Celis E, Akiyoshi T. Induction of antitumor cytotoxic T lymphocytes with a MAGE-3-encoded synthetic peptide presented by human leukocytes antigen-A24. Cancer Res. 1997;57:4465–4468. [PubMed] [Google Scholar]
- 18.Triozzi PL, Kim J, Aldrich W. Infusion of unpulsed dendritic cells derived from granulocyte/macrophage colony-stimulating factor-mobilized peripheral blood CD34+ cells and monocytes in patients with advanced carcinoma. J Hematother Stem Cell Res. 2003;12:279–287. doi: 10.1089/152581603322023016. [DOI] [PubMed] [Google Scholar]
- 19.Valmori D, Lienard D, Waanders G, Rimoldi D, Cerottini JC, Romero P. Analysis of MAGE-3-specific cytolytic T lymphocytes in human leukocyte antigen-A2 melanoma patients. Cancer Res. 1997;57:735–741. [PubMed] [Google Scholar]
- 20.Van den Eynde B, van der Bruggen P Peptide database of T-cell defined tumor antigens. URL: http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm [DOI] [PubMed]
- 21.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 22.van der Bruggen P, Bastin J, Gajewski T, Coulie PG, Boel P, De Smet C, Traversari C, Townsend A, Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994;24:3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- 23.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytolytic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 24.Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, Ellefsen K, Valmori D, Liénard D, Plum J, MacDonald HR, Speiser DE, Cerottini J-C, Romero P. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]