Abstract
Tumors often target dendritic cells (DCs) to evade host immune surveillance. DC injury is reported in many rodent and human tumors but seldom in tumors of other mammals. Canine transmissible venereal tumor (CTVT), a unique and spontaneous cancer transmitted by means of viable tumor cells. CTVT causes manifold damage to monocyte-derived DCs. This cancer provides an in vivo model of cancer to study the role of monocyte-derived DCs during spontaneous regression. Using flow cytometry and real-time reverse-transcription polymerase chain reactions, we compared the expression of surface molecules on monocyte-derived DCs between normal dogs and dogs with CTVT. These markers were CD1a, CD83, costimulatory factors (CD40, CD80, and CD86), and major histocompatability complex classes I and II. In immature DCs (iDCs) and lipopolysaccharide-treated mature DCs (mDCs), the surface markers were mostly downregulated during tumoral progression and regression. The tumor lowered endocytic activity of iDCs, as reflected in dextran uptake, and decreased allogeneic mixed lymphocyte reactions of mDCs. In addition, it decreased the number of monocytes in the peripheral blood by 40%. The tumor substantially impaired the efficiency with which DCs were generated from monocytes and with which mDCs were generated from iDCs. We also found that progression-phase CTVT supernatants that were cultured for 48 h and that contained protein components killed both monocytes and DCs. Additionally, DC numbers were significantly lower in the draining lymph nodes in CTVT dogs than in normal dogs. In conclusion, CTVT caused devastating damage to monocyte-derived DCs; this might be one of its mechanisms for evading host immunity. Reestablishment of monocyte-derived DC activity by the host potentially might contribute to spontaneous tumoral regression. These findings provide insight into the extent of tumoral effects on host immune systems and responses. This information is useful for developing cancer immunotherapies.
Keywords: Tumor Infiltrate Lymphocyte, Major Histocompatability Complex, Mixed Leukocyte Reaction, Canine Transmissible Venereal Tumor, Endocytic Activity
Introduction
Dendritic cells (DCs) are the most efficient antigen-presenting cells for initiating and maintaining immune responses [1]. They are present in almost all sites of antigen entry, and they can process tumoral antigens to present them on major histocompatability complex (MHC) class I and class II antigens [2, 3]. DCs express a high level of costimulatory molecules and produce a large array of regulatory cytokines [4] and chemokines [5]. These roles make them powerful components in initiating and promoting innate and adoptive immunity [4]. DCs are also important in modulating the activation of nature killer cells [6]. A recent study showed that a subset of mouse DCs that induced interferon-γ and that expressed nature killer cells surface molecules mediated the lysis of tumor cells dependant on tumor necrosis factor (TNF)-related apoptosis-inducing ligand; this process was believed to involve tumor immunosurveillance [7]. Taken together, DCs are crucial for inducing and maintaining antitumoral immunity.
Since the generation of DCs from CD14+ monocytes was established in 1994 [8], many researchers have used monocyte-derived DCs to study damage to DCs during tumoral growth [9–12]. Some have used these DCs as an option for cancer immunotherapy [13].
Tumors impair DC activities in a variety ways. Human renal carcinoma cells release soluble factors that prevent CD34 hematopoietic progenitor cells from differentiating into functional DCs [14]. Alpha-fetoprotein impairs APC function and induces their apoptosis [15]. Tumors also affect the ability of hematopoietic progenitor cells to differentiate into functional DCs during the early stage of maturation [16]. Tumors produce various immunosuppressive factors, such as cyclooxygenase-induced prostaglandin E2 [17], vascular endothelial growth factor [18], tumor growth factor (TGF)-β [19] and granulocyte-macrophage colony-stimulating factor [20] to block or impair the functions of DCs. Tumors also impair these functions by inducing CD4+CD25+ regulatory T cells [21]. Many studies have demonstrated alternations of DCs during tumor progression. However, because a suitable in vivo model is lacking, data about host immune responses, including changes in DCs during spontaneous tumor regression, are limited.
First described in 1820 [22], canine transmissible venereal tumor (CTVT) is a unique tumor that is transmitted by viable tumor cells [23] through injured mucosa and skin. CTVT cells have a stabilized genome with almost identical dog leukocyte antigen class II loci, which may have originated in wolves [23]. The tumor cells effectively evade the host’s histocompatibility barrier for long periods, and transplanted cells grow liberally in the progression (P) phase for a few months or over 1 year [24, 25]. This immunoevasion was found partly because of the high concentration of tumor-secreted TGF-β, which inhibits tumoral MHC antigen expression and the activity of nature killer cells [26]. However, the immune systems of most hosts eventually reject the transplanted cells in the regression (R) phase [27]. One mechanism for this rejection is related to interleukin (IL)-6 produced by tumor-infiltrating lymphocytes that counteract the activities of TGF-β [26]. CTVT is one of the few tumors that allow us to study detailed dynamic changes in host–cancer interactions during spontaneous regression of a tumor.
Canine DCs can be generated from monocytes and bone marrow [28–30], and some of their phenotypic characteristics have been described. The purpose of our study was to investigate the effects of CTVTs on CD14+ monocyte-derived canine DCs.
Materials and methods
Experimental animals, tumor inoculation, and determination of growth stage
The institutional animal care and use committee at the National Taiwan University approved this study before its start. Spontaneous CTVT on the external genitals of a male dog was used for the original transplantation. Fourteen beagles aged 1–1.5 years were used for the experiments. Each beagle was subcutaneously injected with a freshly prepared suspension containing 7.5 × 107 viable tumor cells in eight sites on their backs.
The tumors were allowed to grow. Their dimensions were measured with calipers once a week, and the volume was estimated as (π × length × width × thickness)/4 in cm3 [27].
A tumor increasing in volume was classified as a P-phase tumor, and one decreasing in volume was an R-phase tumor. MHC expression and tumor-infiltrating lymphocyte subpopulations of the tumor from the P and R phases were analyzed to confirm the growth phases.
Generation of peripheral blood mononuclear cells, monocytes, and monocyte-derived DCs
Peripheral venous blood samples were obtained from the dogs 1 and 2 weeks before CTVT inoculation, during the P phase 4 and 6 weeks after inoculation, and during the R phase 14 and 16 weeks after inoculation. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood, as previously described [31].
In brief, heparinized blood was overlayered on Ficoll-Hypaque medium (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and centrifuged at 800g for 20 min. PBMCs were harvested from the interface and washed three times in 1× phosphate-buffered saline (PBS; Amersco, Solon, OH, USA). They were resuspended in RPMI-1640 medium (Life Technologies, Gaithersburg, MD, USA) supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, and 5% canine autologous plasma that was collected before tumor inoculation. The PBMCs were allowed to adhere to a 25-cm2 flask (1 × 107 cells/ml) at 37°C in a 5% CO2 humidified atmosphere overnight. With gentle pipetting, nonadherent cells were collected as a population of peripheral blood lymphocytes (PBLs) in which the CD14+ cells were <5% (3.35 ± 0.98). Adherent cells were collected by scraping them down with small cell scrapers (Corning Glass, Corning, NY, USA) from the flasks and washed three times with PBS. The purity of the adherent cells was determined by staining them with antibodies against CD3, CD14, and CD21 and by passing them through a flow cytometer (Becton Dickinson, Mountain View, CA, USA).
Monocyte-derived DCs were generated by following procedures described previously [8]. To generate iDCs, adherent cells that were predominantly CD14+ monocytes were cultured in complete medium supplemented with 800 U/ml of human granulocyte-macrophage colony-stimulating factor (Leucomax; Schering-Plough, Kenilworth, NJ, USA), 500 U/ml of canine IL-4 (R&D Systems, Minneapolis, MN, USA) and 200 ng/ml of human flt3 ligand (R&D Systems) for 6 days. To produce activated mDCs, iDCs cultured for 6 days were incubated with 10 ng/ml lipopolysaccharide (Escherichia coli serotype 0128:B12; Sigma, St Louis, MO, USA) for 48 h. The iDCs and mDCs were harvested for further experiments.
Sample preparation of draining lymph nodes of CTVT dogs
Normal and CTVT dogs were surgery on 1, 2, 3, 4, 7, or 12 days after inoculation. Popliteal lymph nodes were taken out and mechanically disaggregated through a stainless-steel wire mesh. After washing, lymph node cells were suspended in complete medium. Cells were washed twice and suspended in complete medium. Cells were analyzed by flow cytometry to determine the number of CD1a and CD40 double positive cells.
Purification of tumor infiltrating lymphocytes (TIL)
The TIL were purified according to the procedures described previously [27]. Briefly, single cells suspension prepared from freshly collected CTVT mass were over-layered on 4 ml 42% PercollTM (Amersham pharmacia biotech, Uppsala, Sweden) gradient and centrifuged at 820×g and 18°C, for 25 min. The TIL in the bottom of the centrifugation tubes were collected.
Flow cytometry
To study the effect of CTVT on the differentiation of DCs, six monoclonal primary antibodies against antigens CD1a, CD11c, CD14, CD40, MHC class I, and MHC class II (Table 1) were used to stain the PBMCs, monocytes, iDCs, and mDCs that were isolated from the dogs before inoculation and in the P and R phases, as previously described [33]. CD3, CD4, CD8α, and CD21 were primarily used to stain the tumor-infiltrating lymphocytes. All of the monoclonal antibodies specifically reacted to canine species [29]. FITC-conjugated goat antimouse IgG antibody (Serotec) was used as the secondary antibody.
Table 1.
Monoclonal antibodies used in the phenotypic assays
| Specificity | Clone | Class |
|---|---|---|
| Canine CD3a | CA17.2A12 | Mouse IgG1 |
| Canine CD4a | CA13.1E4 | Mouse IgG1 |
| Canine CD8αa | CA9.JD3 | Mouse IgG2a |
| Canine CD21a | CA2.1D6 | Mouse IgG1 |
| Human CD1a-FITCb | NA1/34-HLK | Mouse IgG2a |
| Canine CD11cb | CA11.6A1 | Mouse IgG1 |
| Human CD14c | TÜK4 | Mouse IgG2a |
| Human CD40-FITCb | LOB7/6 | Mouse IgG2a |
| Canine MHC class Ib | 2G5 | Mouse IgG2b |
| Canine MHC class IIb | CA2.1C12 | Mouse IgG1 |
IgG immunoglobulin G, FITC fluorescein isothiocyanate
aFrom Dr. Peter F. Moor, University of California Davis
bFrom Serotec, Kidlington, UK
cFrom Dako, Glostrup, Denmark
For direct immunofluorescence analysis, cells were washed with fluorescence-activated cell sorter staining buffer (PBS supplemented with 1% bovine serum albumin and 0.02% sodium azide, pH 7.4). They were incubated for 30 min on ice in the dark with an isotype control or with specific monoclonal antibodies to detect CD1a and CD40.
For indirect immunofluorescence analysis, cells were incubated with an isotype control or with specific monoclonal antibodies against CD3, CD4, CD8α, CD11c, CD14, CD21, MHC class I, and MHC class II (Table 1). Cells were washed and further stained with FITC-conjugated goat antimouse IgG for 30 min on ice in the dark. Finally, the expression of surface antigens was analyzed with a flow cytometer (FACScaliber; Becton Dickinson). Propidium iodide staining was used to gate out dead cells. Fluorescence intensities were analyzed with software (Cell Quest; Becton Dickinson).
The differentiation rate of monocytes to iDCs (number of iDCs divided by number of monocytes) and the maturation rate of iDCs to mDCs were calculated.
Real-time RT-PCR analysis of surface markers CD80, CD83, and CD86 and cytokine IL-12 expression
Total RNA was extracted from iDCs and mDCs with Trizol reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s recommendations for cultured cells. The amount of total RNA was spectrophotometrically determined at 260 nm. A reverse transcriptase kit (SuperScript II; Invitrogen) and 2 mg mRNA was used to transcribe RNA into cDNA. We used a final concentration of 5.5 mM MgCl2, 0.5 mM of each dNTP, 2.5 mM random hexamers, 0.4 U/ml RNase inhibitor, and 1.25 U/ml multiscribe reverse transcriptase in a reaction volume of 10 ml. The samples were incubated at 80°C for 10 min, followed by transcription at 42°C for 60 min.
Primer sequences were designed to specifically bind to canine cytokine cDNA by using software (Primer Express; Applied Biosystems, Foster City, CA, USA) according to canine cytokine mRNA sequences published in GenBank and our previous work [29]. The designed primers were purchased (from Mission Biotech, Taipei, Taiwan). β-actin was chosen as the housekeeping gene. Table 2 shows the GenBank accession numbers of the primer sequences.
Table 2.
Primer sequences for canine β-actin, CD80, CD83, and CD86 genes
| Target gene, primer | Sequence (5′→3′) | GenBank accession number | Nucleotide position | Product length (bp) |
|---|---|---|---|---|
| β-actin | ||||
| Sense | CCG CGA GAA GAT GAC CCA GA | 281–300 | ||
| Antisense | GTG AGG ATC TTC ATG AGG TAG TCG G | Z70044 | 493–517 | 237 |
| CD80 | ||||
| Sense | ATG GAT TAC ACA GCG AAG TGG AGA A | 337–361 | ||
| Antisense | AGG CGC AGA GCC ATA ATC ACG AT | AF106824 | 637–659 | 323 |
| CD83 | ||||
| Sense | TCC CGG CCC ACT TTT TGT | XM847554 | 742–759 | |
| Antisense | AGG TGG CCC CAT GCT ACA | 791–808 | 67 | |
| CD86 | ||||
| Sense | ATG TAT CTC AGA TGC ACT ATG GAA C | 6–30 | ||
| Antisense | TTC TCT TTG CCT CTG TAT AGC TCG T | AF106827 | 202–226 | 221 |
| IL-12p40 | ||||
| Sense | CAGCAGAGAGGGTCAGAGTGG | 323–343 | ||
| Antisense | ACGACCTCGATGGGTAGGC | AF091134 | 413–432 | 109 |
Real-time reverse-transcription polymerase chain reaction (PCR) was performed by using a sequence-detection system with PCR master mix (ABI Prism 7000 and Sybr Green; Applied Biosystems) in accordance with the manufacturer’s instructions. PCR was conducted in 96-well optical reaction plates, as described elsewhere [32]. In brief, each well contained a 25 μl reaction mixture with 12.5 μl of the master mix, 1 μl of each forward and reverse primer, 9.5 μl of water, and 1 μl of the cDNA samples. The dye was measured at 530 nm during the extension phase.
The threshold cycle value reflects the cycle number at which the fluorescence generated in a reaction crosses a given threshold. Therefore, the value assigned to each well reflected the point in the reaction when a sufficient number of amplicons accumulated.
A single product at a specific melting temperature was found for each sample. The purity of the amplified product was determined as a single peak on the dissociation curve. Specificity of the PCR product, as based on the predicted size of the product, was confirmed by means of gel electrophoresis. All samples were tested in duplicate, and the mean was obtained for further calculations. Each run included a no-template control to test for contamination of the assay reagents.
The relative amount of mRNA in each sample was calculated where β-actin, and mRNA were ascribed a fold induction of 1. The results were determined as 2−(Ct of the target gene − Ct of the housekeeping gene)(2−ΔCt) in arbitrary units, where Ct is the threshold cycle, as described in Applied Biosystems’ user bulletin no. 2.
Enzyme-linked immunosorbent assay of TNF-α
Cell-free supernatants from the DC culture were collected and analyzed for canine TNF-α by performing enzyme-linked immunosorbent assay (ELISA) with a kit (R&D Systems) according to the manufacturer’s instructions. In brief, anti-canine TNF-α monoclonal antibody and biotinylated goat anti-canine TNF-polyclonal antibody (R&D Systems) were used on microtiter plates (Costar, Cambridge, MA, USA). Recombinant canine TNF-α was used to generate a standard curve. The test was developed with 3,3′,5,5′-tetramethylbenzidine in accordance with the manufacturer’s instructions. Plates were read on a plate reader (MRX; Dynex Technologies, Denkendorf, Germany) at 450 nm. Minimal sensitivity was 150 pg/ml.
FITC-dextran uptake
Endocytic activity was assessed by incubating 105 DCs in 200 μl PBS with FITC-dextran 1 mg/ml for 2.5 h at 4 or 37°C. The cells were then washed with PBS 3 times. FITC-dextran uptake was quantified as the mean fluorescence intensity [30]. Expression of fluorescence intensity was analyzed with the flow cytometer, and the data were analyzed with software (Cell Quest; Becton Dickinson).
Mixed leukocyte reaction
A mixed leukocyte reaction (MLR) was performed according to the manufacturer’s instructions (CellTrace CFSE cell proliferation kit; Invitrogen). In brief, 5 mM carboxyfluorescein diacetate succinimidyl ester stock solution was prepared immediately before use by dissolving the contents of a vial of component A in 18 μl of dimethylsulfoxide (component B).
PBL samples isolated from PBMCs of normal dogs and dogs with P- or R-phase tumors were suspended in prewarmed PBS and 0.1% bovine serum albumin at a final concentration of 1 × 106 cells/ml. We added 1 μL of 5-mM stock CFSE solution per milliliter of 1 × 106 cells and incubated them at 37°C for 10 min in PBS. The solution was quenched by adding five volumes of ice-cold culture media, and it was further incubated for 5 min on ice. The cells were washed 3 times by resuspending the pellet in fresh medium and centrifuged at 820g for 10 min. The washed PBLs were used as responder and co-cultured with allogeneic DCs samples (stimulator) at a PBL-to-DC ratio of 10:1 for 4 days.
Decay of CFSE fluorescence due to the proliferation of T cells was measured by using the flow cytometer with 488-nm excitation. MLR results were calculated as (experimental data − negative control)/negative control.
Effect of CTVT cell-culture supernatants on monocyte-derived DCs
For 3 days we incubated 1 × 106 CTVT cells in 1 ml of RPMI-1640 medium at containing 10% fetal calf serum supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine. To ensure that the effect of CTVT supernatant on iDCs or mDCs was specific, we also cultured tumor cells isolated from two simple carcinomas of the canine mammary gland (tubulopapillary carcinoma) that were diagnosed according to the histologic classification of the World Health Organization for mammary tumors of the dog and the cat.
The cells were cultured for 3 days in a medium similar to that previously described. Cell-free supernatants were collected and preserved in a refrigerator at −80°C for further experiments. Thawed tumor-cell supernatants were forced through a 0.2-μm filter. Part of the tumor supernatants were treated at 56°C for 30 min. We co-cultured 500 μl of each of the tumor supernatants with and without heat treatment plus 5 × 105 iDCs or mDCs in l ml RPMI-1640 supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, and 10% fetal bovine serum. The cultures were kept 37°C for 24 h with 5% CO2.
To test if the protein component was involved, 1 mg/ml of the CTVT supernatant was also treated with protease K (Promega, Madison, WI, USA) for 16 h under 5% CO2 at 37°C. We co-cultured 500 μl of the protease K-treated CTVT supernatant with 5 × 105 iDCs or mDCs in l ml RPMI-1640 supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, and 10% fetal bovine serum in 5% CO2 at 37°C for another 24 h. The cells were washed 3 times and stained with propidium iodide and an annexin-V-FITC apoptosis kit (Strong Biotech Corp., Taipei, Taiwan). They were analyzed with flow cytometry.
Effect of CTVT on monocytes
CTVT supernatants and monocytes collected as described were co-cultured for 48 h. Apoptosis of the monocytes was assessed by staining the cells with propidium iodide and annexin-V and then analyzed with the flow cytometer.
Statistical analysis
Samples were compared for significant differences by using a two-tailed Student t test for independent samples. Results were statistically significant at P < 0.05.
Results
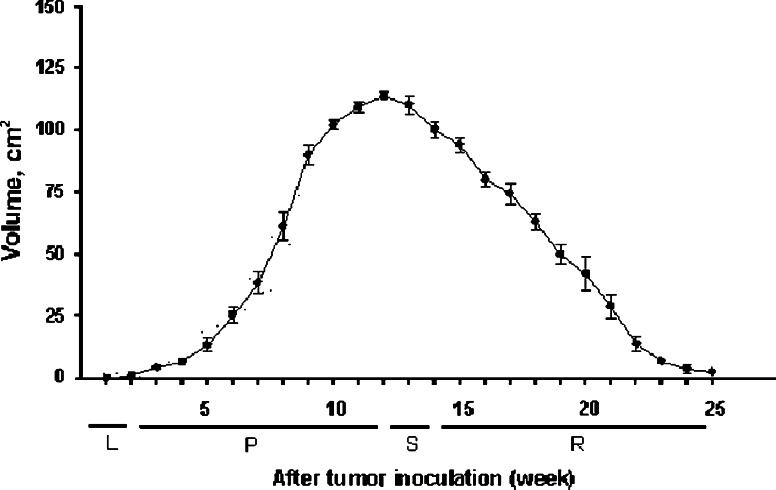
Growth phases
Figure 1 shows the CTVT growth stages classified according to growth patterns. Tumoral growth progressed for about 11–13 weeks and regressed thereafter.
Fig. 1.
Tumoral growth patterns after CTVT cells were inoculated into eight sites on the backs of four beagles. Phases are latency (L), stable (S), progression (P), and regression (R)
Changes in numbers of PBMCs, monocytes, and DCs during tumoral growth
The number of PBMCs significantly decreased (P < 0.05) in the R phase compared with the number before inoculation, but the number of PBMCs in the P phase did not (Table 3). During both phases, CTVT decreased monocytes by about 40%. In the P and R phases, the numbers of iDCs dramatically decreased to about 22% and about 40% the number before inoculation, respectively. A similar but more severe situation was seen with mDCs. The number of mDCs generated during the P phase were only about 12% that observed before inoculation.
Table 3.
Effects of CTVT on numbers of monocytes, iDCs, and mDCs
| Cell or ratio | Number (1 × 106 per 10 ml of blood) | ||
|---|---|---|---|
| Before inoculation | P phase | R phase | |
| PBMCs | 93.40 ± 2.61 | 94.63 ± 5.56 | 76.58 ± 6.98a |
| Monocytes | 12.00 ± 2.00 | 7.33 ± 1.53a | 7.10 ± 1.15a |
| iDCs | 4.34 ± 1.24 | 0.95 ± 0.51a | 1.73 ± 0.86a,b |
| mDCs | 2.33 ± 0.50 | 0.27 ± 0.15a | 0.57 ± 0.12a,b |
| Monocytes/PBMCs | 0.13 ± 0.02 | 0.08 ± 0.01a | 0.09 ± 0.02a |
| iDCs/monocytes | 0.38 ± 0.17 | 0.12 ± 0.04a | 0.24 ± 0.1a,b |
| mDCs/iDCs | 0.54 ± 0.05 | 0.27 ± 0.08a | 0.40 ± 0.2b |
a P < 0.05 versus the number before inoculation
b P < 0.05 versus the number from the P phase
CTVT greatly hampered the differentiation rate of monocytes to iDCs from 0.38 per monocyte in normal dogs to 0.12–0.24 in dogs with the tumor. It also reduced the maturation rate of mDCs to iDCs from 0.54 per iDC in normal dogs to 0.27–0.40 in dogs with CTVT. However, during the R phase, the iDC–mDC differentiation and maturation rate was restored, and it was not statistically different from preinoculation rates (Table 3).
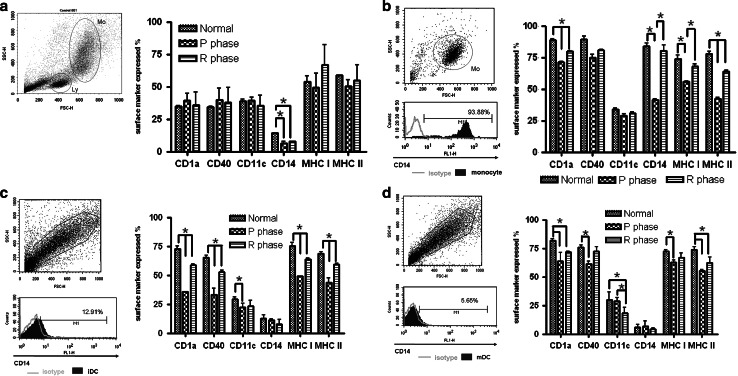
Expression of surface markers on PBMCs, monocytes, iDCs, and mDCs
For PBMCs, the only statistically significant change in the expression of surface markers was observed with CD14, which was downregulated after CTVT inoculation. As for monocytes, CD1a, CD14, MHC I, and MHC II were significantly downregulated during the P phase, especially CD14, which was suppressed the most. However, expression partially recovered during the R phase. Adherent cells in PBMCs were predominantly CD14 positive (94.6% ± 3.82). For monocyte-derived iDCs in CTVT dogs, all markers except CD14 decreased during the P phase; expression of CD14 was already low before inoculation. Similar suppression was found for lipopolysaccharide-stimulated mDCs. An exception was CD11c, which was unchanged during the P phase but which decreased in expression in the R phase. During the R phase, all suppressed markers were significantly restored but not to normal levels (Fig. 2).
Fig. 2.
Six monoclonal antibodies were used to study expression of the corresponding antigen on PBMCs (a), monocytes (b), iDCs (c), and mDCs (d) with flow cytometry. Images show cells are identified on forward scatter (FSC-H)-side scatter (SSC-H) dot plots and results of representative antibody staining. Data are means and standard deviations. Ly lymphocyte, Mo monocyte. *P < 0.05
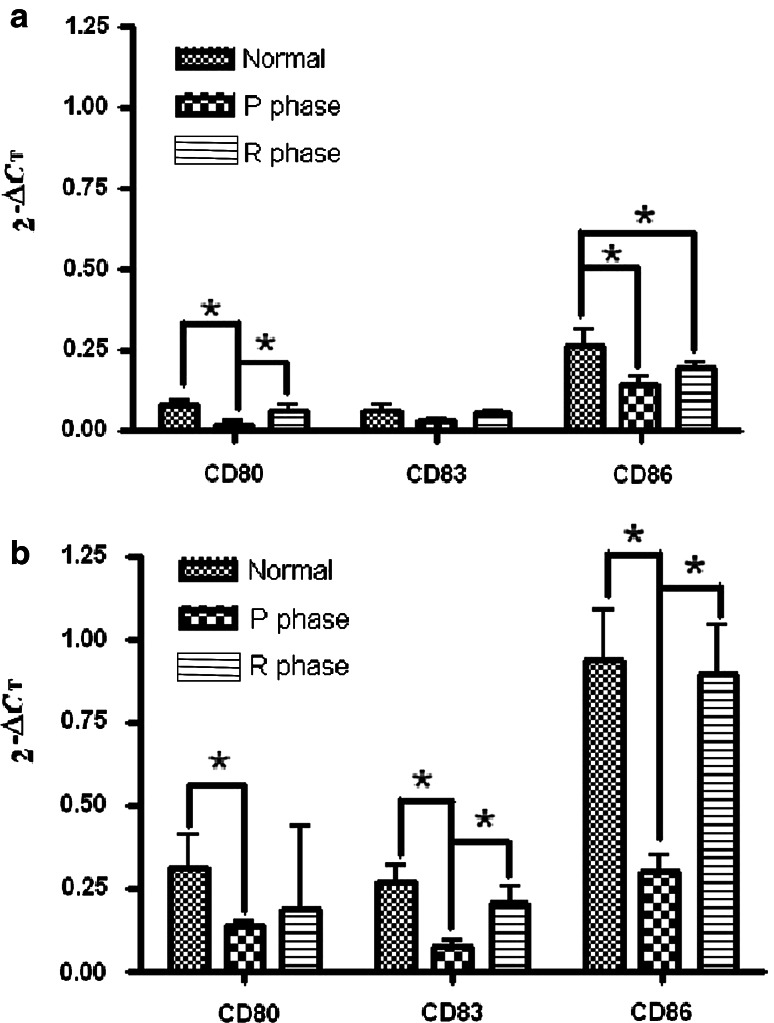
Figure 3 shows the results of real-time RT-PCR to determine the expression of DCs-associated molecules including CD80, CD83, and CD86. In general, mRNA expression of the three molecules tested in iDCs or mDCs was suppressed during the P phase. Nonsignificant changes were observed for CD83 in iDCs. In both DCs, mRNA expression of these markers was significantly restored during the R phase.
Fig. 3.
Effect of CTVT growth on the expression of CD80, CD83, and CD86 mRNA of DCs. Results for iDCs (a) and mDCs (b) are presented as 2−(Ct of the target gene − Ct of the housekeeping gene)(2−ΔCt), where Ct is the threshold cycle. *P < 0.05
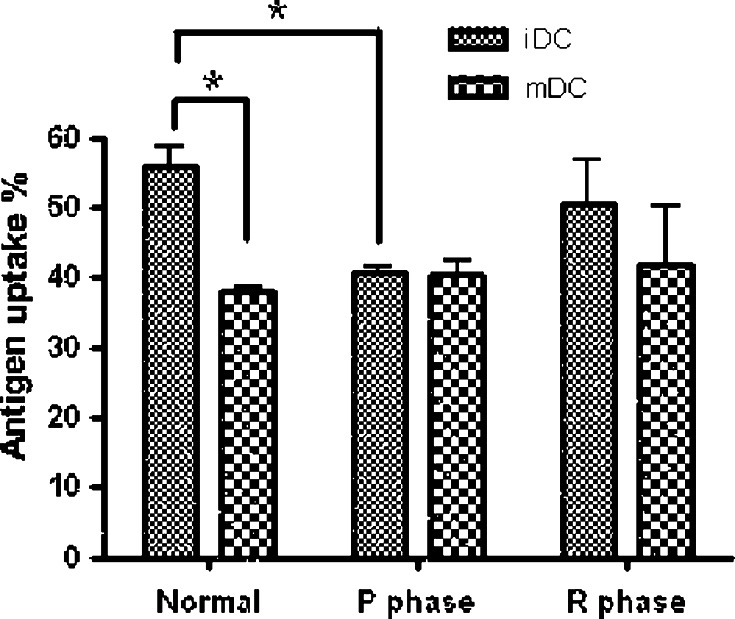
DC endocytic activity and allogeneic MLR results
In our antigen-uptake experiments, iDCs had normal endocytic activity at 37°C, whereas treatment at 4°C inhibited iDC activity. During the P phase, endocytic activity of the iDCs was significantly inhibited, but it was restored during the R phase (Fig. 4).
Fig. 4.
Effect of CTVT growth on DC endocytic activity. Monocyte-derived DCs were generated from PBMCs collected from dogs before CTVT inoculation (normal) and in the P and R phases. Data are the percentage of FITC-dextran uptake at 37°C minus the percentage of uptake at 4°C to exclude background contamination. *P < 0.05
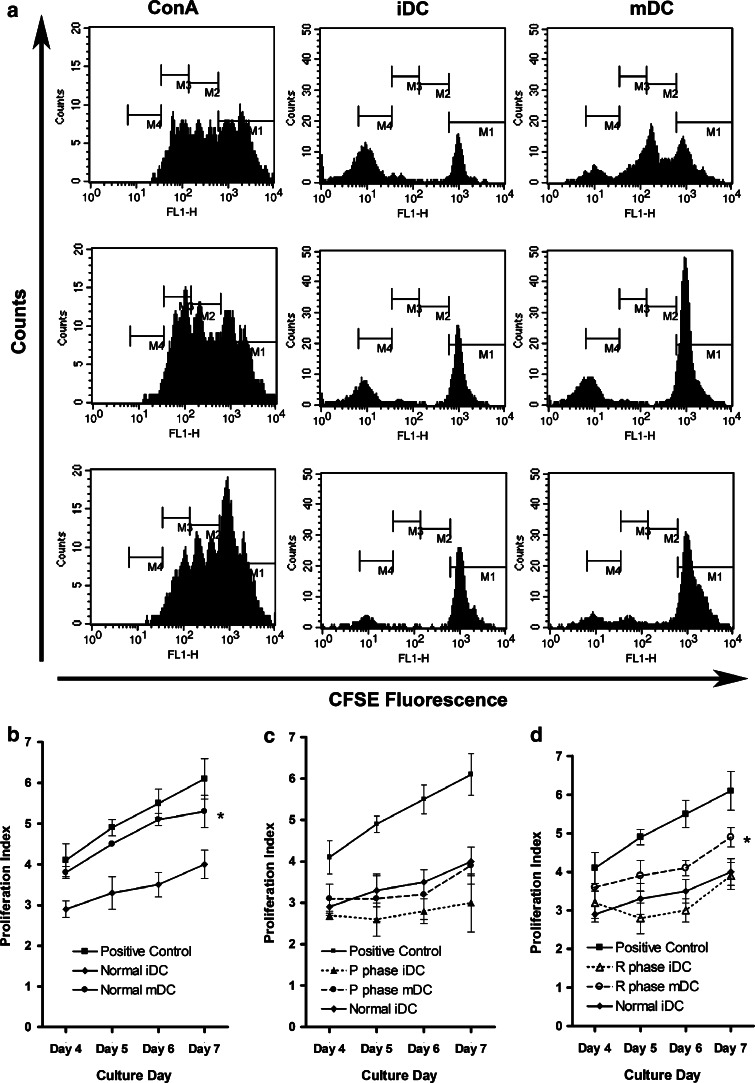
Decay of CFSE intensity was observed as PBLs proliferated. As expected, MLR values were higher in mDCs compared with iDCs. However, the MLR of mDCs from the P phase was significantly impeded (P < 0.05) to the level of normal iDCs. During the R phase, MLR activity of mDCs increased significantly (P < 0.05) (Fig. 5).
Fig. 5.
Effects of CTVT growth on allogeneic MLR activity of DCs. Representative results from a triplicate experiment are shown (a). The stimulator is DC and responder is PBLs. Histograms were used to define generations originating from the progenitor cells. Data include the percentage of cells that underwent at least one division step; this was detected as a reduced CFSE signal when compared with that of undivided cells. Numbers on the graphs are the sum of M2, M3, and M4 values in a ± the standard deviation of triplicate wells of three independent experiments. PBLs were co-cultured with allogeneic DCs, including DCs from normal (b), P-phase (c), and R-phase (d) dogs for 4 days. ConA concanavalin A. *P < 0.05
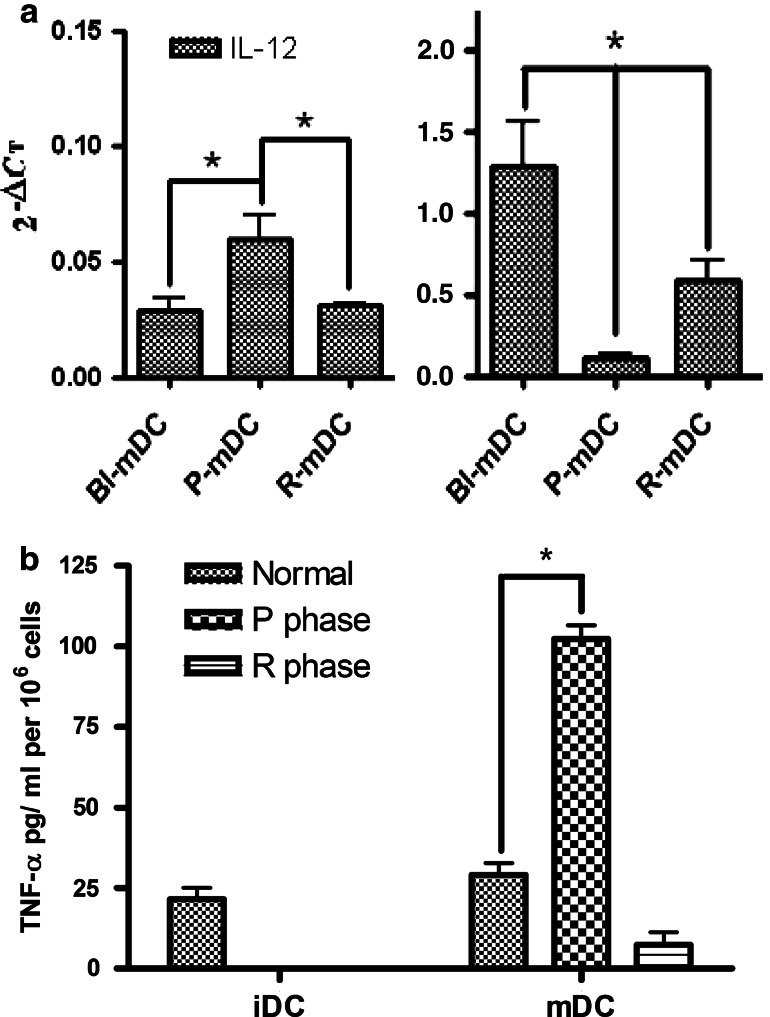
IL-12 gene expression and TNF-α secretion by normal, P-phase, and R-phase DCs under lipopolysaccharide stimulation
Gene expression for IL-12 was significantly reduced in P-phase mDCs compared with DCs before inoculation but reverted in R-phase mDCs (Fig. 6a). Regarding TNF-α expression, iDCs and mDCs did not significantly change in the normal condition, whereas P-phase mDCs expressed high levels of TNF-α after stimulation with lipopolysaccharide. By comparison, R-phase mDCs only slightly expressed TNF-α (Fig. 6b). The normal function of DCs to release IL-12 was prohibited in P-phase mDCs, but the effect could revert in the R phase. The inflammatory response to release TNF-α was induced in P-phase mDCs.
Fig. 6.
IL-12 gene expression and TNF-α secretion by DCs during stimulation of CTVT supernatants. a P-phase mDCs have impaired IL-12 secretion compared with mDCs before inoculation. Monocyte-derived DCs were generated from PBMCs collected from dogs before CTVT inoculation and in the P and R phases. After 6 days of culture, iDCs matured for 12 h with 10 μg/ml of lipopolysaccharide to become mDCs for real-time RT-PCR. b After 6 days of culture, DCs matured for 48 h with 10 μg/ml of lipopolysaccharide. Supernatants were harvested and stored at -20°C. TNF-α in the culture supernatants was analyzed by using commercially available ELISA kits. *P < 0.05 (n = 4)
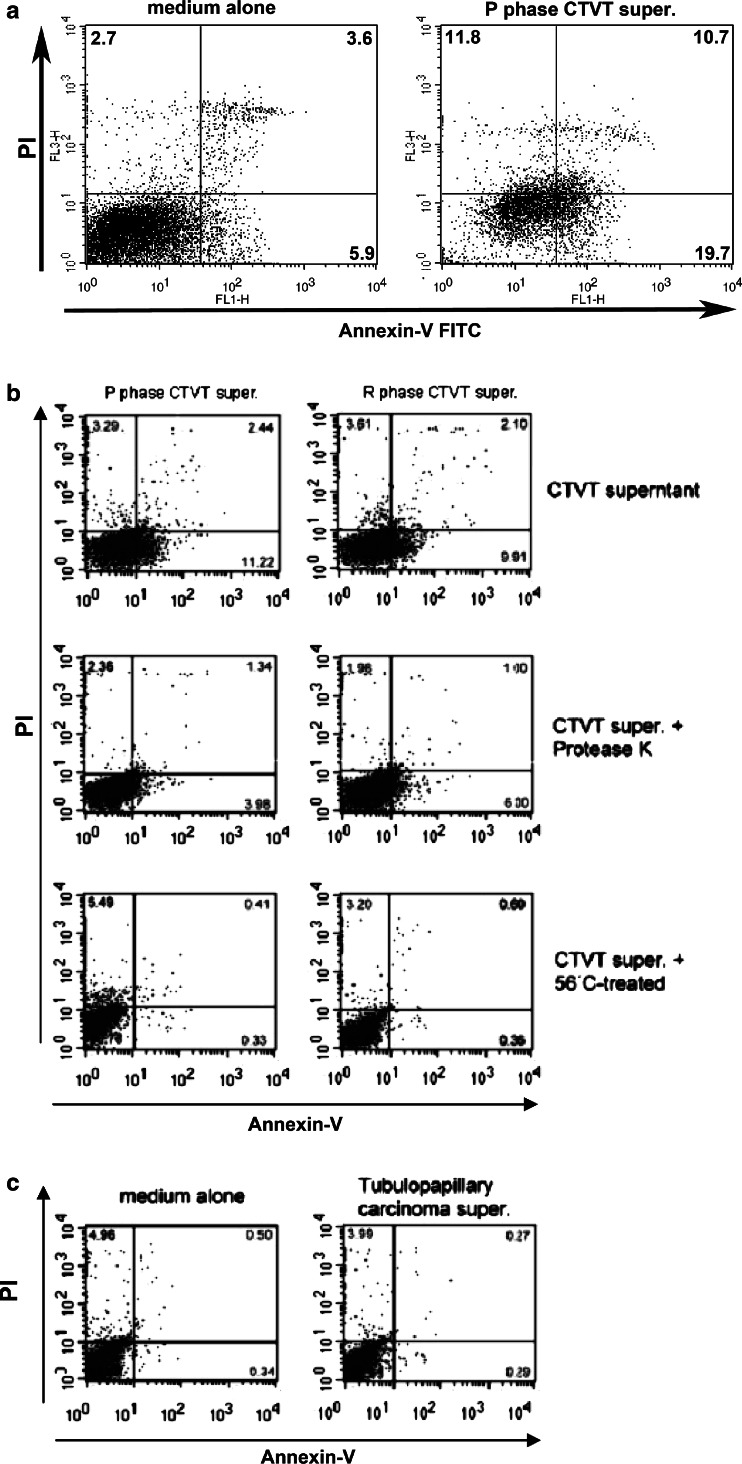
DC killing by CTVT cell-culture supernatants
When co-cultured with monocytes or DCs, CTVT supernatants caused serious apoptosis of monocytes (31% ± 5.5) and DCs (15% ± 3.2 and 12% ± 1.2 for P- and R-phase supernatants, respectively, Fig. 7a). When CTVT supernatants from the P and R phases were heated at 56°C for 30 min, both lost their apoptotic activity. Protease K-treated supernatants from P- and R-phase tumor cells also lost their apoptotic effect on DCs. The medium-only group and supernatants from the simple carcinoma of the canine mammary gland did not cause apoptosis of DCs (Fig. 7a, b).
Fig. 7.
Apoptotic activity of CTVT supernatants (super.) on normal monocytes (a) and monocyte-derived DCs (b). Supernatants from P- and R-phase tumor cells were heated at 56°C for 30 min or treated with protease K before co-culturing with monocyte-derived DCs (b). Medium alone and supernatants from canine mammary gland tumor (tubulopapillary carcinoma) were used as controls (c). Apoptosis was determined by measuring the percentage of annexin-V-positive cells with flow cytometry. Images show individual death rates. Total apoptosis rates were calculated as the late-phase rate of apoptosis (numbers in upper right corners) plus the early-phase rate (numbers in lower right corners). PI propidium iodide uptake. P progression, R regression
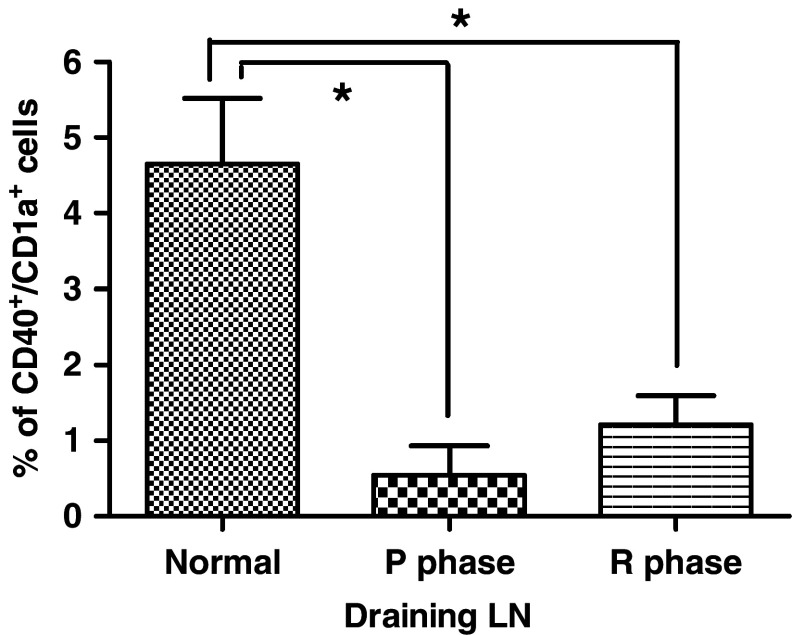
Decreased number of DCs in the draining LN of CTVT dogs
Dual-color flow cytometry estimates of the percentages of DC in LN revealed significantly less DCs in the draining LNs (P < 0.05) from P phase (0.54 ± 0.68%) and R phase (1.21 ± 0.66%) in CTVT dogs as compare with normal dogs (4.65 ± 1.50%) (Fig. 8). This result further confirmed that DCs were impaired during CTVT invasion. Tumor infiltrated lymphocytes were also detected for CD1a and CD40 double positive cells. However, no cells positive for CD1a and CD40 were evident.
Fig. 8.
Component of CD40+/CD1a+ cells in draining lymph nodes (LN). Staining for CD40 and CD1a in draining LN cells using dual-color flow cytometry, compare to the normal, P and R phase CTVT LN. Data are means and standard deviations. *P < 0.05 (n = 3)
Discussion
DCs are important in the development of antitumor immunity [7, 13]. Therefore, they are also susceptible to tumor-mediated immunosuppression [33, 34]. However, few investigators have reported tumor-caused DC damage in mammals other than humans and rodents. CTVT induced damage to monocytes and DCs in several ways and caused serous differentiation and functional destruction. The tumor also significantly suppressed most of the tested surface molecule expressions on DCs. A similar situation of diminished surface molecules was reported in cancer cells of humans and mice [35, 36]. The lowered levels of surface markers and the impaired differentiation efficiency of DCs seen in colon and prostate cancers could cause functional damage [9, 37]. Confirming these functional disturbances are our findings that dextran uptake to reflects antigen uptake [38] and MLR to determine antigen-presentation capability [8] were substantially retarded. In addition, IL-12 expression was significantly reduced in P-phase mDCs. These results indicated that even the surviving DCs did not carry out the functions as efficiently as normal DCs. Of interest, most of the damage to the DCs was significantly restored during spontaneous regression.
Functional damage to the DCs is frequently described in various tumors in humans and mice [9, 35, 39]. The DC system is believed to be the major contributor to immune surveillance against malignancy [34, 40], and damage to this system is important in protecting tumors from host immune surveillance. Monocytes are important precursors to DCs. A decreased number of monocytes influences the yield of DCs [41]. Therefore, in addition to the apoptosis of iDCs the CTVT supernatants caused, the 40% decrease in monocytes during tumoral progression should contribute to the reduction of monocyte-derived iDCs generated in this phase. The apoptotic effect of tumors on monocytes we observed is seldom described. However, the induction of DC apoptosis by tumor-derived soluble factors is frequently reported in humans and in murine systems [17, 19]. This activity is considered to be a key mechanism that enables tumor cells to escape immune recognition [42].
DCs and monocytes also produce an array of cytokines during the host-tumor interactions to moderate immune responses [43, 44]. Impairment of the monocytes exerts serious effects in terms of iDC dysfunction, including suppressed expression of surface markers, a low differentiation rate from monocytes to iDCs, and altered antigen-uptake capability [9]. As we previously reported, CTVT produced soluble protein containing substances that eliminated circulating B cells [31]. The substance that killed monocytes and monocyte-derived DCs in the present study had similar properties. Because human lung cancers produce TGF-β1 that causes apoptosis of monocyte-derived DCs [45] and CTVT produces high level of TGF-β1 [26], it would be interesting to investigate the relationship between this cytokine and the apoptosis of the DCs. However, the molecular weight of the B-cell killing substance from CTVT is within 30–100 kD and it is 25 kD for a mature active TGF-β1 dimer [46]. Further study to identify the killing substances is important before we can delineate the mechanism. DC defects are believed to be strongly associated with abnormal differentiation of myeloid cells [33]. [33]. Presently, we show that the percentage of CD40+/CD1a+ cells is significantly decreased in P and R phase draining LNs as compared to the normal situation. Although in vivo DC markers are not well defined currently, CD40+/CD1a+ cells should be close to the DC in vivo. In addition to CD1a+/CD40+ cells, we also coincidentally detected TUNEL and CD1a from draining LNs. However, cells positive for both CD1a and TUNEL were not detected in P and R phase draining LN. This may be due to the low percentage of CD1a+/CD40+ positive cells in CTVT draining LN.
Many reports describe DC damage [10, 12, 15], but few describe DC recovery in detail, especially during in vivo spontaneous regression. We demonstrated that, while CTVT spontaneously regressed, most of the indicators that we tested were significantly restored from suppression during the P phase, though the recovered levels were not as high as those seen in normal animals. These finding suggested that, with an unknown mechanism, the dogs developed an efficient way to overcome suppression after experiencing CTVT-induced immunologic suppression for a few months. Recovery of DC activities is likely an important factor that aid animals in fighting tumors and in forcing them toward to regression.
Tumor-derived TGF-β causes serious immunoinhibition in CTVT dogs. Because it inhibits TGF-β, IL-6 produced by tumor-infiltrating lymphocytes are responsible for the overexpression of CTVT MHC antigens and for partially recovery of nature killer cell activities [26]. As mentioned before, human lung cancer derived-TGF-β1 causes apoptosis of monocyte-derived DCs from healthy individuals, and the incidence of DC apoptosis is higher in sentinal lymph nodes than in nonsentinal lymph nodes [45]. On the other hand, Bacteroides vulgatus-induced IL-6 is associated with the maturation of bone marrow-derived DCs [47]. CTVT-derived TGF-β may play a role in damaging DCs during the P phase, but whether tumor-infiltrating lymphocyte-derived IL-6 is responsible for the recovery of DC activity during the R phase is an interesting issue to be explored. These host–cancer interactions and the mechanisms behind them deserve further study. Another valuable endeavor is research into the mechanisms by which hosts develop efficient ways to defend themselves against after tumors that grow over months.
CTVT supernatants hampered the differentiation of CD14-positive monocytes to DCs; therefore, more undifferentiated or partially differentiated CD14-positive cells were left in the culture. Because CD14 is a receptor for lipopolysaccharide (LPS) [48] and will response to the LPS that we used for the TNF-α secretion test. Thus the increased numbers of the undifferentiated or partially differentiated CD14-positive cells during the growth of CTVT might elevated the secretion of TNF-α as found in this experiment. In addition, monocyte-derived DCs also display a decrease of IL-12 and an increased level of TNF-α in humans [49, 50] in an immunosuppressive condition similar to CTVT [26, 27, 31]. Although these studies did not determine the number of mococytes, the expression of CD14 (a marker for monocytes) was observed to be significantly increased [49, 50]. Thus, the immunosuppressive status and the cytokine expressions in CTVT coincide with the results in humans. Taken together, CTVT damages DCs in several ways, which hampers the differentiation of monocytes to iDC, and of iDC to mDC. These damages were further confirmed by the decreased production of IL-12 by DCs. TNF-α did not display a corresponding decrease, probably partially due to the production of TNF-α by the undifferentiated monocytes during the growth of CTVT.
In conclusion, CTVT possesses several mechanisms of evading host immune surveillance. This tumor produces a high concentration of TGF-β to inhibit MHC expression and natural killing activity [25], and it secretes soluble substances to kill B cells [33]. In this study, we also demonstrated that CTVT impaired the differentiation of DCs, inhibited antigen uptake and presentation, and caused apoptosis of monocytes and DCs. Rarely are tumors in dogs or other species so powerful in escaping from host immune responses in such a variety of ways. During spontaneous regression, DC activity substantially recovered. Reestablished DCs are believed to be potentially important host factors that push the tumor toward to regression. These findings also suggest that CTVT is an excellent model to study the immunologic interactions between hosts and tumor cells.
Acknowledgments
This research was supported by the National Science Council, Taiwan, ROC (NSC 95-2323-B-002-025).
Footnotes
C.-C. Liu and Y.-S. Wang contributed equally.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Onishi H, Kuroki H, Matsumoto K, Baba E, Sasaki N, Kuga H, Tanaka M, Katano M, Morisaki T. Monocyte-derived dendritic cells that capture dead tumor cells secrete IL-12 and TNF-alpha through IL-12/TNF-alpha/NF-kappaB autocrine loop. Cancer Immunol Immunother. 2004;53:1093–1100. doi: 10.1007/s00262-004-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tourkova IL, Shurin GV, Chatta GS, Perez L, Finke J, Whiteside TL, Ferrone S, Shurin MR. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J Immunol. 2005;175:3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 4.Rossi G, Heveker N, Thiele B, Gelderblom H, Steinbach F. Development of a Langerhans cell phenotype from peripheral blood monocytes. Immunol Lett. 1992;31:189–197. doi: 10.1016/0165-2478(92)90145-E. [DOI] [PubMed] [Google Scholar]
- 5.Nimura F, Zhang LF, Okuma K, Tanaka R, Sunakawa H, Yamamoto N, Tanaka Y. Cross-linking cell surface chemokine receptors leads to isolation, activation, and differentiation of monocytes into potent dendritic cells. Exp Biol Med (Maywood) 2006;231:431–443. doi: 10.1177/153537020623100409. [DOI] [PubMed] [Google Scholar]
- 6.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, Pequignot M, Casares N, Terme M, Flament C, Opolon P, Lecluse Y, Metivier D, Tomasello E, Vivier E, Ghiringhelli F, Martin F, Klatzmann D, Poynard T, Tursz T, Raposo G, Yagita H, Ryffel B, Kroemer G, Zitvogel L. A novel dendritic cell subset involved in tumor immunosurveillance. Nature Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/S0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 9.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46:68–75. doi: 10.1002/1097-0045(200101)46:1<68::AID-PROS1010>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 11.Mahanonda R, Sa-Ard-Iam N, Yongvanitchit K, Wisetchang M, Ishikawa I, Nagasawa T, Walsh DS, Pichyangkul S. Upregulation of co-stimulatory molecule expression and dendritic cell marker (CD83) on B cells in periodontal disease. J Periodontal Res. 2002;37:177–183. doi: 10.1034/j.1600-0765.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323–331. doi: 10.1016/S0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 13.Babatz J, Rollig C, Oelschlagel U, Zhao S, Ehninger G, Schmitz M, Bornhauser M. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: a phase I study. J Hematother Stem Cell Res. 2003;12:515–523. doi: 10.1089/152581603322448222. [DOI] [PubMed] [Google Scholar]
- 14.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 15.Um SH, Mulhall C, Alisa A, Ives AR, Karani J, Williams R, Bertoletti A, Behboudi S. {alpha}-Fetoprotein Impairs APC Function and Induces Their Apoptosis. J Immunol. 2004;173:1772–1778. doi: 10.4049/jimmunol.173.3.1772. [DOI] [PubMed] [Google Scholar]
- 16.Katsenelson NS, Shurin GV, Bykovskaia SN, Shogan J, Shurin MR. Human small cell lung carcinoma and carcinoid tumor regulate dendritic cell maturation and function. Mod Pathol. 2001;14:40–45. doi: 10.1038/modpathol.3880254. [DOI] [PubMed] [Google Scholar]
- 17.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–550. doi: 10.1007/s00262-003-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirano A, Brown WC, Estes DM. Cloning, expression and biological function of the bovine CD40 homologue: role in B-lymphocyte growth and differentiation in cattle. Immunology. 1997;90:294–300. doi: 10.1046/j.1365-2567.1997.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty A, Li L, Chakraborty NG, Mukherji B. Stimulatory and inhibitory maturation of human macrophage-derived dendritic cells. Pathobiology. 1999;67:282–286. doi: 10.1159/000028080. [DOI] [PubMed] [Google Scholar]
- 21.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF- and IL-10. Cancer Immunol Immunotherapy. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell JM, Ishmael J, Joshua JO. Transmissible venereal tumour of dogs. Vet Rec. 1969;84:418–419. doi: 10.1136/vr.84.16.418. [DOI] [PubMed] [Google Scholar]
- 23.Murgia C, Pritchard J, Kim SY, Fassati A, Weiss RA. Clonal Origin and Evolution of a Transmissible Cancer. Cell. 2006;126:477–487. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu RM, Lin CY, Liu CC, Yang SY, Hsiao YW, Hung SW, Pao HN, Liao KW. Proliferation characteristics of canine transmissible venereal tumor. Anticancer Res. 2001;21:4017–4024. [PubMed] [Google Scholar]
- 25.Cohen D. The canine transmissible venereal tumor: a unique result of tumor progression. Adv Cancer Res. 1985;43:75–112. doi: 10.1016/S0065-230X(08)60943-4. [DOI] [PubMed] [Google Scholar]
- 26.Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta1 and restores the lymphokine-activated killing activity. J Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao YW, Liao KW, Hung SW, Chu RM. Effect of tumor infiltrating lymphocytes on the expression of MHC molecules in canine transmissible venereal tumor cells. Vet Immunol Immunopathol. 2002;87:19–27. doi: 10.1016/S0165-2427(02)00026-0. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefont-Rebeix C, de Carvalho CM, Bernaud J, Chabanne L, Marchal T, Rigal D. CD86 molecule is a specific marker for canine monocyte-derived dendritic cells. Vet Immunol Immunopathol. 2006;109:167–176. doi: 10.1016/j.vetimm.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Wang YS, Chi KH, Liao KW, Liu CC, Cheng CL, Lin YC, Cheng CH, Chu RM. Characterization of canine monocyte-derived dendritic cells with phenotypic and functional differentiation. Can J Vet Res. 2007;71:165–174. [PMC free article] [PubMed] [Google Scholar]
- 30.Hagglund HG, McSweeney PA, Mathioudakis G, Bruno B, Georges GE, Gass MJ, Moore P, Sale GE, Storb R, Nash RA. Ex vivo expansion of canine dendritic cells from CD34+ bone marrow progenitor cells. Transplantation. 2000;70:1437–1442. doi: 10.1097/00007890-200011270-00007. [DOI] [PubMed] [Google Scholar]
- 31.Liao KW, Hung SW, Hsiao YW, Bennett M, Chu RM. Canine transmissible venereal tumor cell depletion of B lymphocytes: molecule(s) specifically toxic for B cells. Vet Immunol Immunopathol. 2003;92:149–162. doi: 10.1016/S0165-2427(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::AID-IJC1545>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 35.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esche C, Shurin MR, Lotze MT. B16 melanoma induces downregulation of beta2 integrin and ICAM-1 expression on murin dendritic cells. J Investig Dermatol. 1997;108:640. [Google Scholar]
- 37.Chaux P, Favre N, Bonnotte B, Moutet M, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression. A role in the immune tolerance to antigenic tumors. Adv Exp Med Biol. 1997;417:525–528. doi: 10.1007/978-1-4757-9966-8_86. [DOI] [PubMed] [Google Scholar]
- 38.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1111–1113. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 39.Mohty M, Jarrossay D, Lafage-Pochitaloff M, Zandotti C, Briere F, de Lamballeri XN, Isnardon D, Sainty D, Olive D, Gaugler B. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–3756. doi: 10.1182/blood.V98.13.3750. [DOI] [PubMed] [Google Scholar]
- 40.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 41.Bohnenkamp HR, Burchell JM, Taylor-Papadimitriou J, Noll T. Apoptosis of monocytes and the influence on yield of monocyte-derived dendritic cells. J Immunol Methods. 2004;294:67–80. doi: 10.1016/j.jim.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Muta M, Matsumoto G, Nakashima E, Toi M. Mechanical analysis of tumor growth regression by the cyclooxygenase-2 inhibitor, DFU, in a Walker256 rat tumor model: importance of monocyte chemoattractant protein-1 modulation. Clin Cancer Res. 2006;12:264–272. doi: 10.1158/1078-0432.CCR-05-1052. [DOI] [PubMed] [Google Scholar]
- 43.Lans TE, Van Horssen R, Eggermont AM, Ten Hagen TL. Involvement of endothelial monocyte activating polypeptide II in tumor necrosis factor-alpha-based anti-cancer therapy. Anticancer Res. 2004;24:2243–2248. [PubMed] [Google Scholar]
- 44.Micheva I, Thanopoulou E, Michalopoulou S, Karakantza M, Kouraklis-Symeonidis A, Mouzaki A, Zoumbos N. Defective tumor necrosis factor alpha-induced maturation of monocyte-derived dendritic cells in patients with myelodysplastic syndromes. Clin Immunol. 2004;113:310–317. doi: 10.1016/j.clim.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Ito M, Minamiya Y, Kawai H, Saito S, Saito H, Nakagawa T, Imai K, Hirokawa M, Ogawa J. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 46.Roberts AB, Anzano MA, Meyers CA, Wideman J, Blacher R, Pan YC, Stein S, Lehrman SR, Smith JM, Lamb LC, et al. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983;22:5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- 47.Frick JS, Zahir N, Muller M, Kahl F, Bechtold O, Lutz MB, Kirschning CJ, Reimann J, Jilge B, Bohn E, Autenrieth IB. Colitogenic and non-colitogenic commensal bacteria differentially trigger DC maturation and Th cell polarization: an important role for IL-6. Eur J Immunol. 2006;36:1537–1547. doi: 10.1002/eji.200635840. [DOI] [PubMed] [Google Scholar]
- 48.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 49.Duperrier K, Velten FW, Bohlender J, Demory A, Metharom P, Goerdt S. Immunosuppressive agents mediate reduced allostimulatory properties of myeloid-derived dendritic cells despite induction of divergent molecular phenotypes. Mol Immunol. 2005;42:1531–1540. doi: 10.1016/j.molimm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Velten FW, Duperrier K, Bohlender J, Metharom P, Goerdt S. A gene signature of inhibitory MHC receptors identifies a BDCA3(+) subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur J Immunol. 2004;34:2800–2811. doi: 10.1002/eji.200324732. [DOI] [PubMed] [Google Scholar]