Abstract
Background
We previously demonstrated that targeting lymphotoxin α (LTα) to the tumor evokes its immunological destruction in a syngeneic B16 melanoma model. Since treatment was associated with the induction of peritumoral tertiary lymphoid tissue, we speculated that the induced immune response was initiated at the tumor site.
Methods and results
In order to directly test this notion, we analyzed the efficacy of tumor targeted LTα in LTα knock-out (LTα−/−) mice which lack peripheral lymph nodes. To this end, we demonstrate that tumor-targeted LTα mediates the induction of specific T-cell responses even in the absence of secondary lymphoid organs. In addition, this effect is accompanied by the initiation of tertiary lymphoid tissue at the tumor site in which B and T lymphocytes are compartmentalized in defined areas and which harbor expanded numbers of tumor specific T cells as demonstrated by in situ TRP-2/Kb tetramer staining. Mechanistically, targeted LTα therapy seems to induce changes at the tumor site which allows a coordinated interaction of immune competent cells triggering the induction of tertiary lymphoid tissue.
Conclusion
Thus, our data demonstrate that targeted LTα promotes an accelerated immune response by enabling the priming of T cells at the tumor site.
Keywords: T cells, Cytokines, Tumor immunity, Lymphotoxin alpha knock-out mice, Antibody
Introduction
The adaptive immune system relies on the rapid induction of specific immune responses. In order to comply with this prerequisite, cellular responses are initiated by priming and activation of antigen specific T cells in specialized environments, i.e., lymphoid tissue in general and lymph nodes in particular. These organs accelerate the kinetics of immune responses by facilitating the interaction of the involved immune competent cells. After antigen capture in the periphery, Antigen presenting cells (APCs) migrate—attracted by a characteristic chemokine milieu—via afferent lymphatic vessels to secondary lymphoid tissues (reviewed in Ref. [3]). Likewise, naïve T cells enter the lymph node through specialized blood vessels such as high-endothelial venules. Importantly, within the lymph node the chemokines CCL19 (ELC) and CCL21 (SLC) guide mature, antigen presenting DC and T cells into the respective areas [7]. Thus, lymph nodes provide the suitable cytokine milieu and specialized structures to attract both APCs and naïve T cells, orchestrate their localization within the lymph node and thereby enable their interaction [5]. This contact ultimately leads to antigen specific activation, differentiation, and clonal proliferation of the T cells.
Several lines of evidence indicate that lymphotoxin α (LTα) is pivotal for lymph node organogenesis [12, 23, 36]. The lymphotoxins are a subfamily of the TNF ligand superfamily [4] and LTα exists either as a soluble or membrane bound molecule. The soluble form is a homotrimer which binds to the two TNF receptors (TNFRI/ CD120a and TNFRII/ CD120b) with similar affinities, whereas the heterotrimeric membrane form, LTα1β2, signals exclusively through the LTβ receptor (LTβR). The pathway triggered by LTα1β2/LTβR interaction seems to be responsible for lymph node genesis [13, 31]. Indeed, the formation of lymph nodes depends on cellular interactions between LTβR expressing organizing cells and LTα1β2 expressing inducer cells at the lymph node anlagen which leads to the initiation of the expression of adhesion molecules and the production of homeostatic chemokines (reviewed in Ref. [6]). Nevertheless, LTα seems to be able to partially rescue lymph node genesis in LTβ knock-out mice, either through LTα3/TNFRI or an yet unknown ligand-receptor interaction [23]. Accordingly, LTα knock-out (LTα−/−) mice are characterized by the absence of lymph nodes and Peyer’s patches. In addition, the mice have a disrupted spleenic architecture [30]. This phenotype is caused by a lack of LTα-dependent induction of chemokine expression by stromal cells such as CXCL13 and CCL21 [35]. As a consequence of these structural defects cellular immune responses are impaired, e.g., the initiation of cytotoxic T cells is delayed and the magnitude of the response reduced [28, 45].
On the other hand, ectopic expression of LTα induces formation of lymphoid tissue at the site of expression. For example, LTα expression under the control of a rat insulin promoter induced chronic inflammatory lesions in pancreas and kidneys resembling organized lymphoid tissue [24]. In addition, a LTα-transfected plasmacytoma cell line induced stromal characteristics typical for lymphoid tissue even in the absence of T and B cells [22]. Moreover, we previously demonstrated that targeting LTα to the tumor induced a lymphoid tissue at the tumor site [41]. From these experiments in wt mice we hypothesized that targeted LTα allows for priming of cellular anti-tumor immune responses directly at the tumor site. To proof this notion, we tested the functionality of targeted LTα-therapy in mice lacking secondary lymphoid tissues.
Methods
Animals
C57BL/6J wt mice and C57BL/6J LTα−/− mice were obtained from Jackson Laboratory (Bar Harbor, USA) at the age of 6 weeks. These animals were housed under specific pathogen-free conditions and all experiments were performed according to National Institute of Health guidelines for care and use of laboratory animals.
For splenectomy, mice were anesthetized using 15 μl/g Avertin [2.8 M 2,2,2-Tribromoethanol in 2-methyl-2-butanol (Sigma-Aldrich, Schnelldorf, Germany)]. After shaving, a small incision was made in the skin at the left flank and the peritoneum was opened with surgical scissors. After the splenic artery and vein were ligated with a synthetic absorbable suture (BIOSYN monofilament Glycomer 631, United States Surgical, Norwalk, CT, USA), the spleen was removed. The incisions were closed with surgical wound clips (#427631, BD Bioscience, Heidelberg, Germany). Splenectomy was performed 14 days prior to tumor induction.
Cell line, antibodies and fusion protein
The murine melanoma cell line B78-D14 has been described previously [18]. B78-D14 was derived from B16 melanoma by transfection with genes coding for β-1,4-N-acetylgalactosaminyltransferase and α-2,8-sialyltransferase inducing a constitutive expression of the disialogangliosides GD2 and GD3. B78-D14 melanoma cells were maintained as monolayers in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, 400 μg/ml G418 and 50 μg/ml Hygromycin B. Cells were passaged when sub-confluent.
The mouse/human chimeric antibody directed against GD2 (ch14.18) was constructed by joining the cDNA for the variable region of the murine 14.18 antibody with the constant regions of the human γ1 heavy chain and the κ light chain. From this, the ch14.18-LTα fusion protein was constructed by fusion of a synthetic sequence coding for human LTα—lacking the leader peptide—to the carboxyl end of the human Cγ1 gene [14]. The fused genes were inserted into the vector pdHL2 which encodes for the dihydrofolate reductase gene. The resulting expression plasmids were introduced into Sp2/0-Ag14 cells and selected in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 100 nM methotrexate. The fusion proteins were purified over a protein A-Sepharose affinity column.
With the exception of the anti-GD2 antibody (ch14.18), all other antibodies used [anti-CD4, clone RM4-5; anti-CD8, clone 53-6.7; anti-B220/CD45RB, clone RA3-6B2; anti-CD62L, clone MEL-14, anti-PNAd clone MECA-79 (all BD Bioscience); anti-TCA-4, clone AF457 (R&D Systems, Wiedbaden, Germany) and anti-CD11c antibody, clone N418 (LGC Promochem, Wesel, Germany)] are commercially available and have been described in detail by the manufacturer.
Experimental lung metastases and subcutaneous tumors
Single cell suspensions of 1.5 × 106 B78-D14 cells were injected into the lateral tail vein. To prevent pulmonary embolism caused by injection of tumor cells, mice were anesthetized by halothane inhalation; tumor cells were suspended in 500 μl PBS containing 0.1% BSA and administered i.v. over a period of 60 s.
Subcutaneous tumors were induced by s.c. injection of 1 × 106 B78-D14 melanoma cells in RPMI 1640, which resulted in tumors of ∼40 μl volume within 14 days.
Treatment schedule
Soluble LTα (Strathmann Biotec, Hamburg, Germany) or the ch14.18-LTα fusion protein were administered daily either by i.p. injections for pulmonary metastases or by i.v. injections for subcutaneous tumors. For pulmonary metastases therapy started on day 3, for subcutaneous tumor, dependent on the size of the tumor, at day 3 or day 7 after tumor cell inoculation and was maintained for 5 days or, in the case of splenectomized mice, for 7 days starting at day 11. The dose applied was 10 ng of sLTα or 32 μg ch14.18-LTα fusion protein as determined in previous experiments [41].
Immunohistochemistry
Frozen sections were fixed in cold acetone for 10 min followed by removal of endogenous peroxidase with 0.03% H2O2, and blocking of collagenous elements with 10% species-specific serum. All stainings were performed with the techmate automate (DAKO, Hamburg, Germany). For single staining, serial sections were incubated for 30 min with biotinylated antibodies at predetermined dilutions (usually 20 μg/ml). Subsequently, the streptavidin-peroxidase complex (DAKO) was applied for 30 min, followed by a 15 min incubation with the Chromogen AEC (DAKO). Finally, slides were counter stained. For double staining the last step was replaced by a 30 min incubation with an additional antibody, its detection by a species specific alkaline phosphatase-coupled secondary antibody during a 30 min incubation followed by the use of the Chromogen AP blue substrate kit (Zytomed, Berlin, Germany). Between each incubation step the slides were washed with tris buffer. After a final wash with water, the slides were mounted in aquatex (Merck Eurolab, Darmstadt, Germany). Alternatively, double staining was performed with two peroxidase reactions; the first antibody was detected by a species specific horseradish peroxidase (HRP) coupled antibody and Vector NovaRed (Linaris, Wertheim, Germany) for 15 min and the second, biotinylated antibody by straptavidin-HRP and Vector SG (gray; Linaris). After dehydration, slides were mounted in Shandon hypermount (Thermo Electron Corporation, Dreieich, Germany).
For detection of LTβR expression, acetone-fixed, air-dried sections were incubated for 60 min at room temperature with LTβR : Fc fusion protein (R&D Systems, Wiesbaden, Germany). The detection was carried out with a HRP-marked secondary antibody (anti-IgG human FC fragment; Dianova, Hamburg, Germany) and Vector NovaRed (Linaris) for 15 min. After a wash with aqua bidestillata, slides were counterstained, dehydrated and finally mounted.
Construction of multimeric TRP-2/Kb complexes and staining protocol
Tetramers of H-2Kb molecules complexed with TRP-2180–188 peptides were formed as described previously [16]. In brief, DNA encoding residues 1–280 of the extracellular domain of Kb was subcloned in the expression plasmid pET3a-pMbio which contains a peptidic biotinylation sequence for generation of C-terminally modified recombinant proteins. MHC class I heavy-biotag and light chain were expressed in E. coli strain BL21 (DE3) plysS, and were isolated as inclusion bodies. TRP-2 peptides were synthesized by conventional Fmoc chemistry. Folding of the H-2Kb peptide complexes was performed as described [50]. Purification of the folded complexes was performed by gel filtration chromatography on a Biosep SEC S 3000 column (Phenomenex, Aschaffenburg, Germany). Recombinant MHC class I complexes were biotinylated for 16 h at 20°C, using a recombinant His-tagged biotin ligase purified by native Ni2+ chromatography and biotinylated MHC monomers were purified by gel filtration chromatography. MHC class I tetramers were formed by the stepwise addition of streptavidin-APC (Molecular Probes, Leiden, The Netherlands).
The staining procedure for multimers has been described recently [43]. Briefly, sections of cryopreserved tumors were dried over-night and subsequently fixed in cold acetone for 5 min. All incubation steps were performed in the dark at room temperature as follows: (1) 45 min of the primary antibody (1:100 diluted), (2) streptavidin-FITC (1:100 diluted, BD Bioscience) for the biotinylated (ARK, DAKO) antibodies ch14.18, CD8, and CD62L for 45 min, biotinylated goat anti-hamster Ig antibody (1:100 diluted, code 107-066-142; Jackson Laboratory) for CD11c for 45 min followed by a 45 min incubation with streptavidin-FITC, and finally (3) the APC conjugated TRP-2/Kb tetramers for 75 min; for CD8, the unlabeled anti-CD8 antibody was reacted for 45 min and followed by an incubation with rabbit anti-rat Cy3 (1:100 diluted, code 312-165-004, Jackson Laboratory) for 45 min. Between each step the slides were washed twice for 10 min in PBS/BSA 0.1% and then mounted in vectashield and observed under a Leica Confocal Microscope (TCS 4D, Leica, Mannheim, Germany).
ELISPOT assay
The ELISPOT assay was performed as described before [48]. Briefly, splenocytes from experimental groups were isolated 11 days after therapy cessation and kept in culture for 4 days with Trp-2180–188 pulsed syngeneic LPS-blasts in complete medium supplemented with 200 U/ml of recombinant human IL-2 (Chiron, Marburg, Germany). Subsequently, 1 × 105 of these cells were added to wells coated with rat anti-mouse IFN-γ Ab (clone R4-6A2; BD Biosciences) in the presence of 1 × 105 B78-D14 tumor cells. After 24 h, the plates were washed, followed by incubation with biotinylated anti-mouse IFN-γ Ab (clone XMG 1.2; BD Biosciences). Spots were developed using freshly prepared substrate buffer.
Results
Therapeutic effect of antibody-LTα fusion proteins on subcutaneous and pulmonary metastases
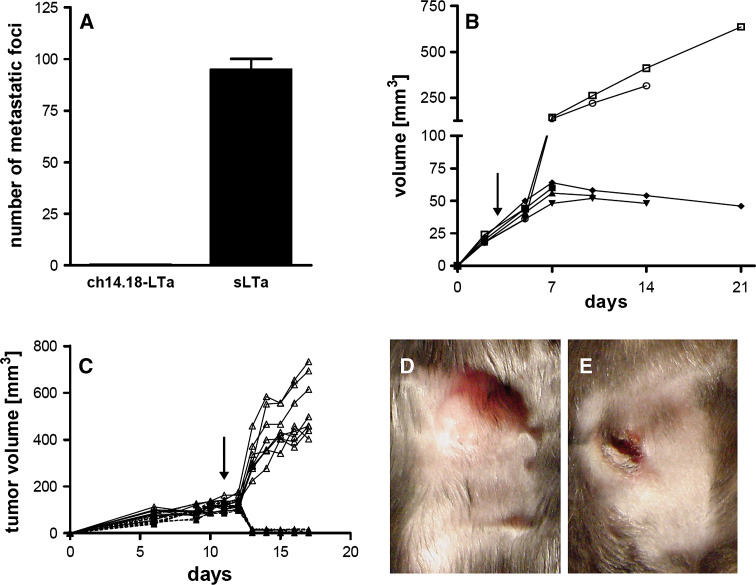
We demonstrated previously that treatment of melanoma bearing C57BL/6J wild type mice with ch14.18-LTα fusion proteins led to eradication of established tumor lesions by tumor specific T cells [41]. Notably, targeted LTα induced changes at the tumor site which are prerequisites for T-cell priming. In this regard, the therapeutic effect was associated with the induction of peritumoral lymphoid tissue. In order to determine whether the induction of the T-cell response is indeed initiated at the tumor site, we tested the effect of ch14.18-LTα in LTα−/− mice where spontaneous T-cell priming is hampered by lack of lymph nodes. The first series of experiments addressed the in vivo anti-tumor effect of the LTα fusion protein upon metastases of B78-D14 melanoma cells, i.e., a B16 melanoma sub-cell line, genetically engineered to express disialoganglioside GD2, the antigen recognized by the antibody portion of the ch14.18-LTα fusion protein, in syngeneic C57BL/6J LTα−/− mice. To this end, 3 days after induction of pulmonary metastases, one group of LTα−/− mice received 10 ng of recombinant soluble LTα (sLTα), and the other group 32 μg ch14.18-LTα fusion protein for five consecutive days. sLTα served as control, as we had previously demonstrated that neither systemic LTα administration, the ch14.18 antibody alone nor a fusion protein specific for an irrelevant antigen did possess any therapeutic effect in wt mice [41]. Importantly, the applied doses of sLTα and the fusion protein exhibited the same cytotoxic effect in the L929 assay [41]. On day 26, the metastatic state of the lungs was determined. Mice receiving ch14.18-LTα fusion protein revealed healthy lungs, completely free of metastatic foci. In contrast, lungs of control mice were covered with metastatic foci (Fig. 1a). Similar results were obtained for subcutaneous tumors, where the treatment for five consecutive days was the same as above, starting at a tumor volume of ∼50 μl. Measurement of the tumor volume over the course of the experiment indicated steady tumor growth in the control group receiving 10 ng sLTα. In contrast, administration of 32 μg ch14.18-LTα fusion protein not only delayed tumor growth but induced tumor regression (Fig. 1b).
Fig. 1.
Effect of ch14.18-LTα therapy on subcutaneous and pulmonary metastases. In C57BL/6J LTα−/− mice (a, b) or splenectomized LTα−/− mice (c, d, e) pulmonary metastases (a) were induced by i.v. injection of 1.5 × 106 and subcutaneous tumors (b–e) by s.c. injection of 1 × 106 B78-D14 melanoma cells. Therapy with 32 μg ch14.18-LTα for 5 or 7 (c–e) consecutive days was started on day 3 (a, b) or day 11 (c–e) after tumor cell inoculation. Control animals received 10 ng sLTα. Experiments depicted in a and b were each performed with seven animals (four fusion protein treated, three controls), in (c) with 17 animals (nine fusion protein treated, eight controls). On day 19 after therapy cessation the number of metastatic foci on the lung surface was determined (a). Individual subcutaneous tumor volumes of mice receiving 32 μg ch14.18-LTα (closed symbols) or 10 ng sLTα (open symbols) are plotted against time (b and c). Macroscopic appearance of tumors on day 7 after therapy cessation is depicted for animals treated with 10 ng sLTα (d) or 32 μg ch14.18-LTα (e)
In the first series of experiments mice still possessed their spleens as major secondary lymphoid organ. Consequently, we removed this last secondary lymphoid organ by splenectomy and tested the therapeutic effect of targeted LTα on subcutaneous tumors in these splenectomized LTα−/− mice. To this end, subcutaneous tumors were induced 14 days after splenectomy and treated from day 11 to day 18 with 32 μg ch14.18-LTα fusion protein or 10 ng sLTα, respectively. In accordance with the observations in the non-splenectomized mice, ch14.18-LTα fusion protein led to retardation of tumor growth even in splenectomized LTα−/− mice while the control mice treated with sLTα displayed uniform tumor growth (Fig. 1c). Accordingly, the regression was associated with phenotypic changes, i.e., the tumors became flat and necrotic, which was also evident histologically (Fig. 1e; data not shown). This observation was in striking contrast to tumors of sLTα treated mice (Fig. 1d). These experiments were terminated at a given time point in order to obtain samples for functional analysis not allowing to judge or compare survival benefit of the treatment between LTα−/− mice with or without splenectomy. It should be noted, however, that therapeutic efficacy in wild type mice also translated in prolonged survival [41].
Infiltration of lymphocytes into ch14.18-LTα treated tumors
Previous experiments demonstrated that LTα fusion protein treatment had no therapeutic effect on xenogenic tumors in immune deficient mice [38]. Nevertheless, since spontaneous T-cell responses to antigenic challenges are largely absent in LTα−/− mice and LTα is also known to exert direct cytotoxic effects on melanoma cells, tumors were analyzed for the presence of lymphocytes by immunohistochemistry.
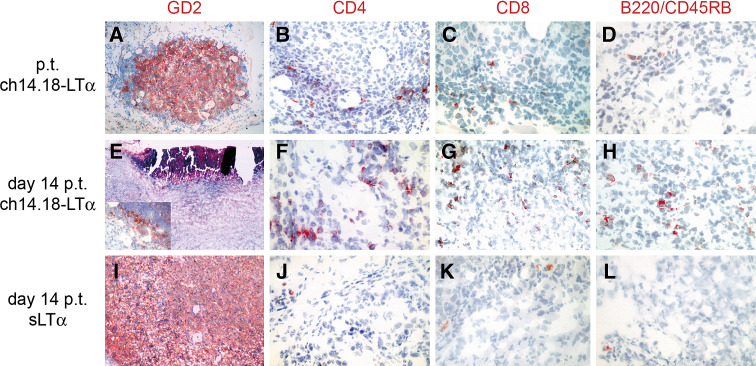
To this end, subcutaneous tumors were analyzed at the day of therapy cessation as well as one and 2 weeks thereafter. Treatment consisted of either 5 days with 10 ng sLTα or 32 μg ch14.18-LTα fusion protein starting at day 3 after tumor cell inoculation. Visualization of tumor cells by staining with an anti-GD2 antibody confirmed the anti-tumor effect of the LTα fusion protein therapy, i.e., the number of tumor cells decreased over time [Fig. 2a, e (insert)], and at day 21 tumors were almost completely necrotic (Fig. 2e) whereas sLTα treated mice were characterized by a heavy tumor load (Fig. 2i). Notably, in tumors of sLTα treated mice only few CD4+ (Fig. 2j), CD8+ cells (Fig. 2k) or B-lymphocytes (Fig. 2l) were detected even in the tumors excised 14 days after therapy cessation. In contrast, in ch14.18-LTα fusion protein treated mice, infiltrates of both CD4+ (Fig. 2b, f) and CD8+ cells (Fig. 2c, g) as well as B-lymphocytes (Fig. 2d, h) were already detectable immediately after cessation of therapy throughout the tumor. NK cells were virtually absent in the tumor of LTα−/− mice irrespective of the treatment (data not shown). This finding is in accordance with the observation that the number of NK cells is reduced in LTα−/− mice [21].
Fig. 2.
Immunohistological characterization of subcutaneous tumors. Tumors were induced by s.c. injection of 1 × 106 B78-D14 cells in LTα−/− mice. Treatment was administrated from days 3 through 7, consisting of 32 μg ch14.18-LTα (a–h) or 10 ng sLTα (i–l). Tumors were excised directly (a–d) or 14 days (e–l) after therapy was terminated (p.t.). Sections were subjected to staining with anti-GD2 [a, e (Insert), i), anti-CD4 (b, f, j), anti-CD8 (c, g, k) or anti-B220/CD45RB (d, h, l) antibodies. e Hematoxylin/eosin staining of a necrotic tumor
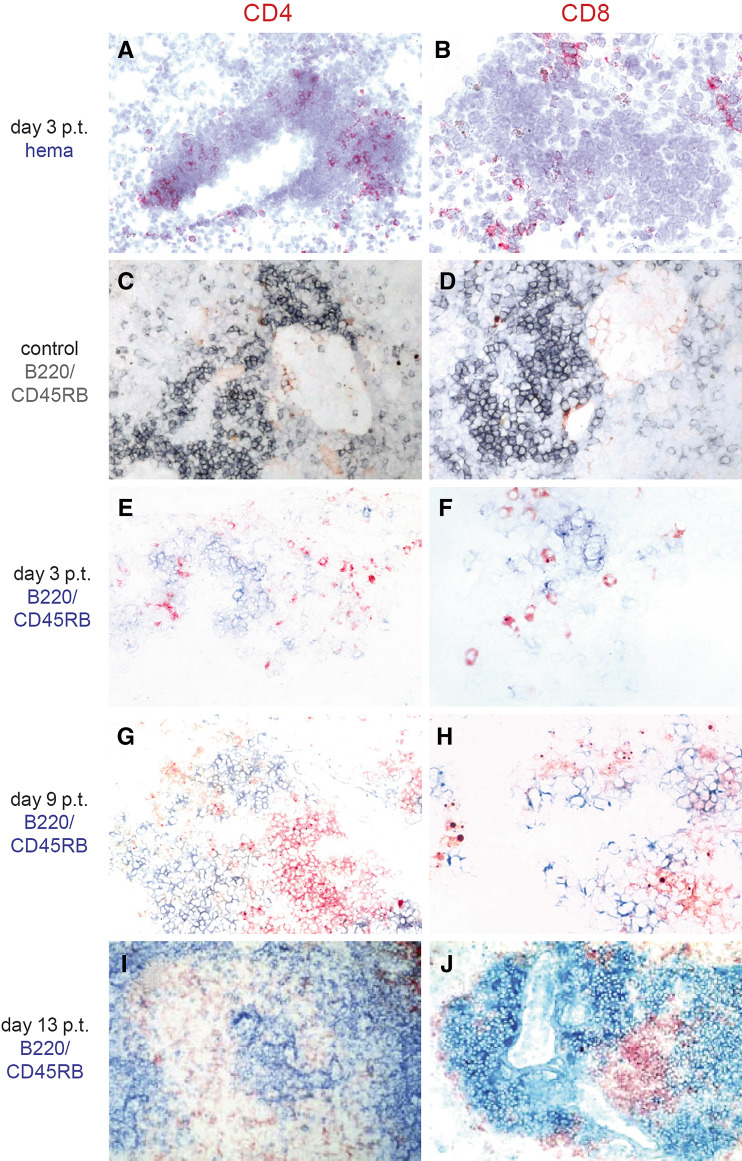
In pulmonary metastases, CD4+ (Fig. 3a) and CD8+ (Fig. 3b) cells were only present in the vicinity of tumors in LTα−/− mice treated with LTα fusion protein. As judged by hematoxylin and eosin staining, this lymphocytic infiltrate in close proximity of the pulmonary metastases resembled tertiary lymphoid tissue of chronic inflammatory processes rather than tumor infiltrating lymphocytes. Since lymphocytes in such lymphoid tissue are compartmentalized in defined T- and B-cell areas, we scrutinized the arrangement of T- and B-lymphocytes by double staining. It should be noted, however, that the occurrence of an infiltrate in lungs of naïve LTα−/− mice is a common finding (own personal experience). Consequently, lungs of LTα−/− mice not receiving any therapy served as controls. Indeed, these lungs contained an inflammatory infiltrate which consisted mainly of B cells with a few CD4+ cells and to an even lesser extend CD8+ cells (Fig. 3c, d). In contrast, in LTα−/− mice treated with LTα fusion protein, lungs removed 3 days after therapy cessation displayed B-cell aggregates surrounded by CD4+ (Fig. 3e) and CD8+ T cells (Fig. 3f) at the pulmonary metastases. Six days later, B cells were accumulated in follicle-like structures adjacent to the tumor, including enriched deposits of CD4+ and CD8+ T cells next to them (Fig. 3g, h) which were even more prominent after another 4 days (Fig. 3i, j). The observed compartmentalization of T- and B-cell areas was also present in subcutaneous tumors of ch14.18-LTα fusion protein treated LTα−/− mice (data not shown), similar to our previous results in targeted LTα treated wild type C57BL/6J mice [41]. Importantly, since the migration of naïve T cells in lymphoid tissue is mediated through high-endothelial venules (HEV), we stained tumors for PNAd and TCA-4, both markers for HEVs. To this end, some of the blood vessels in tumors of ch14.18-LTα treated LTα−/− mice expressed PNAd (Fig. 4c) or stained positive for TCA-4 (Fig. 4d), just as previous observed for wild type mice.
Fig. 3.
Immunhistological characterization of T-cell infiltrates. After i.v. (a, b, e–j) inoculation of 1.5 × 106 B78-D14 cells, LTα−/− mice received 32 μg ch14.18-LTα fusion protein (a, b, e–j) for five consecutive days. Lungs were removed of a naïve control LTα−/− mice not receiving any therapy (c, d) or 3 days (a, b, e, f), 9 days (g, h), and 13 days (I, J) after cessation of therapy (p.t.), respectively. Sections thereof were stained for CD4 (a, c, e, g, i) or CD8 (b, d, f, h, j). In C to J sections were subjected to double staining with an anti-B220/CD45RB antibody (gray; c, d or blue; e–j) instead of counter-staining with hematoxylin (hema)
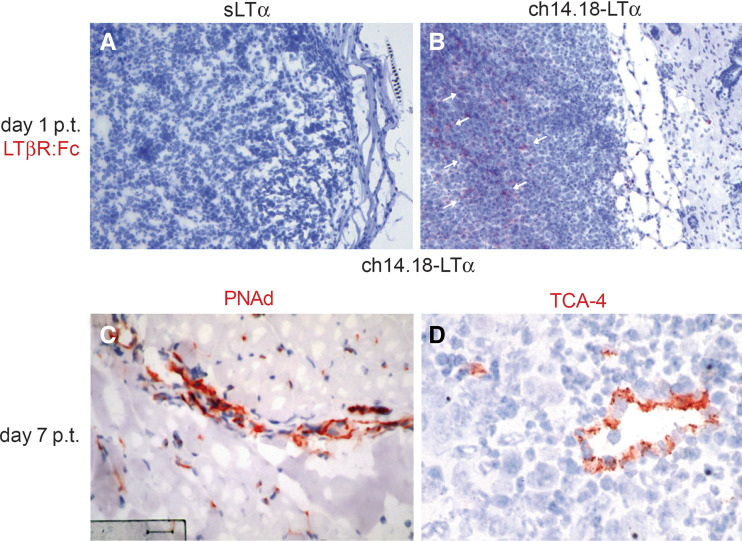
Fig. 4.
LTβR-ligand expressing cells and HEV characteristics in subcutaneous tumors. After s.c. inoculation of 1 × 106 B78-D14 cells LTα−/− mice received 32 μg ch14.18-LTα fusion protein (b–d) or 10 ng sLTα (a) for five consecutive days. Tumors were excised 1 day (a, b) or 7 days (c, d) after cessation of therapy (p.t.), respectively. Sections thereof were stained with an LTβR : Fc fusion protein which detects LTβR-ligand expressing cells (a, b), anti-PNAd (c) or anti-TCA-4 (d) antibodies. Sections were counter-stained with hematoxylin. LTβR-ligand expressing cells are indicated by arrows
The triggering of the LTβR mediated signaling is important for lymph node organogenesis. Therefore, we stained subcutaneous tumors obtained 1 day after therapy termination with a LTβR : Fc fusion protein. In mice treated with 32 μg ch14.18-LTα fusion protein, cells expressing LTβR ligand were present throughout the tumor (Fig. 4b), whereas in the tumors of sLTα treated mice none were detectable (Fig. 4a).
Tumor reactivity of ch14.18-LTα induced infiltrates
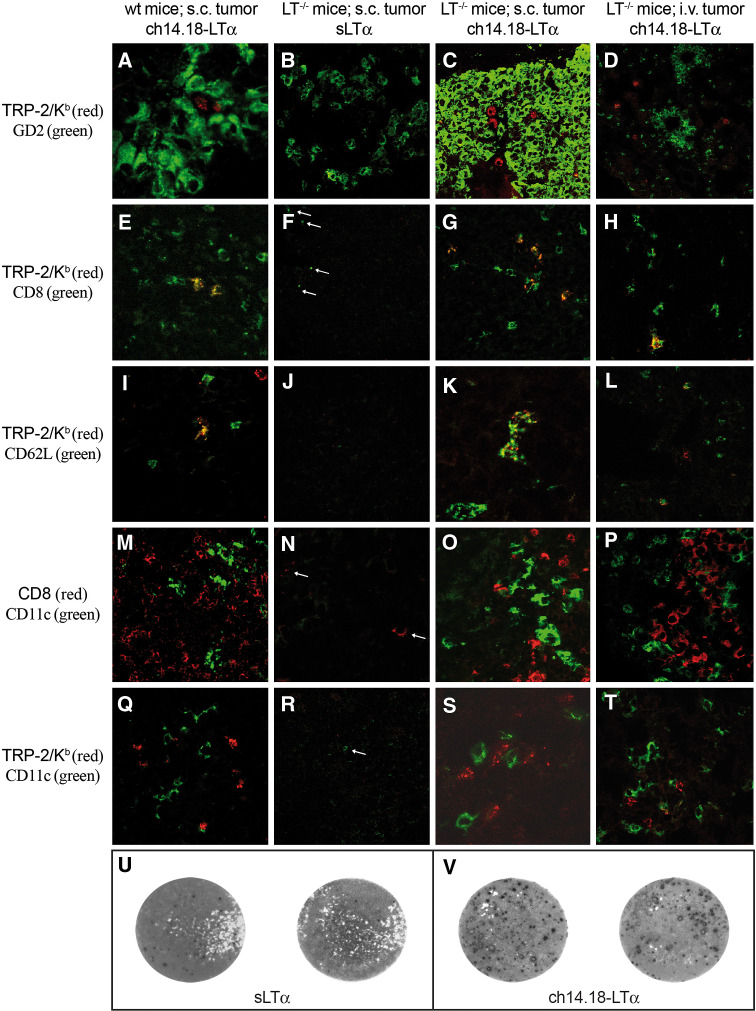
The mere presence of a T-cell infiltrate is not sufficient to conclude about its role in therapy efficacy. Thus, the inflammatory infiltrate observed in LTα−/− mice following administration of ch14.18-LTα fusion protein was tested for tumor-reactivity by in situ detection of specific T cell receptors by means of multimeric peptide/MHC class I complexes. The applicability and specificity of this method had been demonstrated both in humans and mice [17, 39, 43]. The immune dominant TRP-2180–188 epitope was used to analyze the specificity of T-cell infiltrates in subcutaneous and pulmonary tumors obtained from LTα−/− mice treated either with 10 ng sLTα or 32 μg ch14.18-LTα fusion protein since it is one of the best characterized Kb restricted melanoma-associated antigens. Subcutaneous tumors of C57BL/6J wt mice receiving LTα fusion proteins served as positive control, since we previously proved the presence of tumor reactive T cells in these animals by functional assays, i.e., ELISPOT and cytotoxicity assays [41]. Phenotypic characterization of TRP-2180−188/Kb reactive T cells within the infiltrate (Fig. 5a) demonstrated that TRP-2180–188/Kb tetramers only bind to CD8+ T cells (Fig. 5e). Notably, a fraction of TRP-2-specific T cells expressed L-Selectin (Fig. 5i), a marker for both naïve and lymphoid memory T cells. Moreover, CD8+ and TRP-2 specific T cells remained in direct contact with CD11c+ cells, e.g., DCs (Fig. 5m, q). In LTα−/− mice, which received only sLTα, this same analysis revealed that among the few CD8+ T cells present in and around tumor lesions (Fig. 5f, n), none were specific for TRP-2180–188/Kb tetramer (Fig. 5b, f, j, r). It should be further noted that CD11c-expressing cells were rarely observed in these sections and that the infiltrate was in general much weaker than in fusion protein treated mice (Fig. 5f, n, r). For LTα−/− mice with pulmonary metastases receiving sLTα the same observations were made (data not shown). In contrast, in LTα−/− mice treated with ch14.18-LTα, the staining patterns of sections from subcutaneous and pulmonary tumors were virtually identical with those of the positive control. In fact, TRP-2-specific T cells (Fig. 5c, d), positive for CD8+ (Fig. 5g, h) and partially expressing L-selectin (Fig. 5k, l) were found throughout the tumors and their microenvironment. These L-selectin+ TRP-2-specific T cells are likely to represent activated T cells after a few rounds of division [37]. Moreover, both CD8+ cells (Fig. 5o, p) and TRP-2-specific T cells (Fig. 5s, t) were in contact with CD11c+ cells.
Fig. 5.
Specificity and reactivity characterization of T cells. Pulmonary metastases were induced by i.v. injection of 1.5 × 106 and subcutaneous tumors by s.c. injection of 1 × 106 B78-D14 melanoma cells in C57BL/6J wt mice (a, e, i, m, q) or C57BL/6J LTα−/− mice (all others). Animals received either 10 ng sLTα (b, f, j, n, r, u) or 32 μg ch14.18-LTα fusion protein (all others) for five consecutive days starting on day 3 after tumor induction. Tumor specimens were obtained 7 days after therapy was terminated. Sections of pulmonary metastases (d, h, l, p, t) or subcutaneous tumors (all others) were stained with TRP-2180–188/Kb tetramers (red) and a series of antibodies (green). The antibodies used for staining were anti-GD2 (a–d), anti-CD8 (e–h), anti-CD62L (i–l), and anti-CD11c (m–t). In one series of double stained tissue (m–p) an anti-CD8 antibody (red) was used instead of the tetramer. Double positive cells appear yellow. Since in the controls only few cells are present throughout a section, overviews are given and positive cells indicated by arrows. Representative examples of ELISPOT measuring the reactivity of splenocytes against B78-D14 tumor cells (u, v). About 11 days after therapy cessation splenocytes were generated from sLTα (u) or ch14.18-LTα (v) treated mice, cultured for 4 days with TRP-2180–188 pulsed syngeneic LPS-blasts and finally cocultured with tumor cells to measure the secretion of IFN-γ by ELISPOT. Each spot represents an IFN-γ secreting cell
To confer that targeted LTα can induce tumor reactive T cells in LTα−/− mice, we analyzed the functionality of spleen cells in an ELISPOT assay. To this end, spleen cells obtained from mice treated with ch14.18-LTα secreted IFN-γ in the presence of B78-D14 tumor cells (Fig. 5v), while those from control animals, i.e., sLTα treated mice, did not (Fig. 5u). Thus, similar to our observation in wild type mice, therapy with ch14.18-LTα fusion protein induces tumor reactive T cells in LTα−/− mice.
Discussion
The specificity of the adaptive immune system is maintained by a complex interaction of different immune competent cells. It has been proposed that one of the major functions of lymphoid tissues is to ensure that these interactions occur in a timely manner. In the present study of a murine tumor model, we confirmed previous observations that LTα−/− mice, devoid of lymph nodes and Peyer’s patches, exhibit a delayed development of specific immune responses [8, 28]. Indeed, experiments with an antibody-IL2 fusion protein demonstrated the virtual absence of spontaneous anti-tumor immune responses in these mice [42]. Transient reconstitution of LTα, however, allows the rapid induction of cellular anti-tumor immune responses.
Antibody-cytokine fusion proteins provide the means to target cytokines to a specific site while they still sustain effective cytokine function [40]. We previously demonstrated that an antibody-LTα fusion protein elicited a specific immune response which resulted in the eradication of tumors in immune competent animals. The importance of lymphocytes for this therapeutic effect of targeted LTα is evident from experiments in C57Bl/6J scid/scid mice where this treatment did not demonstrate any therapeutic effect (data not shown). Furthermore, since treatment of wild type mice with the parental antibody alone is also not effective, complement activation seems not to contribute efficiently to the anti-tumor effect of targeted LTα. It should be noted, that treatment was accompanied by the induction of a tertiary lymphoid tissue. However, no conclusive evidence directly demonstrated that naïve T-cell priming indeed occurs in this local tertiary lymphoid organ microenvironment [9]. Thus, to be able to distinguish between T-cell priming in draining lymph nodes versus in loco priming, we scrutinized the anti-tumor effect of an antibody-LTα fusion protein targeted to the tumor microenvironment in LTα−/− mice. To this end, antibody-LTα fusion protein therapy induced an inflammatory infiltrate consisting of tumor specific T cells as demonstrated in situ by peptide-epitope/Kb multimer staining in LTα−/− mice. Moreover, tumor eradication induced by tumor targeted LTα−/− as well as tumor reactivity in the ELISPOT assay implies the functionality of these cells. To even further impoverish the LTα−/− mice of possible spontaneous T-cell responses to the tumor we removed the spleen of the animals prior to tumor induction. Therapeutic efficacy of ch14.18-LTα was still present in these splenectomized LTα−/− mice devoid off all major secondary lymphoid tissues. This is of importance since LTα−/− mice are still able to induce effective T- and B-cell responses, e.g., against respiratory viruses, albeit with delayed kinetics [25, 28]. In this regard, such immune responses directed against influenza seem to be initiated at sites of induced bronchus-associated lymphoid tissue [32]. In accordance, the observations that (1) even 14 days after tumor cell inoculation, no tumor specific T cells could be detected in tumors of LTα−/− mice not treated with ch14.18-LTα, (2) therapy with targeted IL-2 proved to be ineffective even though it efficiently enhances preexisting T-cell responses in wt mice [42, 47], and (3) targeted LTα mediates an anti-tumor effect in splenectomized LTα−/− mice with tumor reactive T cells in close proximity to APCs argue for a priming of tumor-specific T cells at the tumor site, rather than a boost of a preexisting T-cell response. Importantly, it was recently elegantly demonstrated that transferred skin allografts harboring tertiary lymphoid tissue were able to activate naïve T cells in mice devoid of all secondary lymphoid tissue. In fact, these intra-graft tertiary lymphoid tissues did generate both effector and memory T cells responses [34].
Tertiary lymphoid tissue, i.e., ectopic lymphoid aggregates exhibiting characteristics usually associated with secondary lymphoid organs, can also be observed at sites of transgenic expression of LTα and various other homeostatic chemokines [10, 24, 29]. In addition, by embedding LTα producing stromal cells in biocompatible scaffold, a functional synthetic lymphoid tissue-like organoid can be generated [44]. In contrast to these models, where LTα is constantly expressed, LTα levels in our model were only transiently elevated. Nevertheless, even this short period of ectopic expression of LTα appears sufficient to initiate the formation of tertiary lymphoid tissue. This formation continues and leads to large T- and B-cell clusters after the cessation of therapy. Thus, the high-local concentrations of LTα generated by in vivo targeted therapy might trigger a positive feedback loop involving other cytokines. In normal lympho-organogenesis, such a positive feedback loop has been suggested for the role of progenitor cells in developing lymphoid organs [33]. CD3−CD4+ cells developing from fetal liver precursors express IL7Rα and interact with stromal cells through LTα1β2/LTβR activating NFκB. This leads to the induction of homeostatic and inflammatory chemokines and adhesion molecules which, in turn, attract more CD3−CD4+ cells to the developing lymph node. It seems that the number of CD3−CD4+ inducer cells and stromal organizer cells has to exceed a threshold in order that they can stimulate each other and thereby form a cluster of activated cells (reviewed from [11]). In accordance, for tertiary lymphoid tissue the state of activation of both innate and adaptive immune cells recruited to inflammatory sites may be crucial for increasing the local concentrations of key mediators above a threshold required for the initiation of lymphoid tissue organization [2]. Notably, in our model the threshold level needed for induction of tertiary lymphoid tissue might be decreased through the expression of chemokines like CCL19, CXCL10, CXCL12, and CCL21 by the tumor per se (data not shown).
Since the molecular mechanisms involved in secondary lymphoid ontogeny and tertiary lymphoid tissue formation are similar, TNF family members are likely to be involved in development and organization of tertiary lymphoid tissue [2]. In this regard, sustained T-cell activation seems to induce LTα3 and LTα1β2 production which, in turn, results in the secretion of chemokines able to attract lymphocytes and APCs, as well as expression of membrane-bound molecules participating in their compartmentalization [1, 46]. For example, LTα3 induces chemokines like CCL21 and CXCL13 in stromal and endothelial cells which are potent chemoattractants for lymphocytes, DCs and CD45+CD4+CD3− lymphoid tissue inducer cells [19, 20]. LTα1β2, in its heterotrimeric form, is also important for compartmentalization of leucocytes within lymphoid tissues by induction of ICAM-1 and VCAM-1 on stromal cells [27]. In our current model, LTα3 is formed after cleavage of LTα from the fusion protein at its plasmin cleavable site [15]. Signaling through LTβR, i.e., the only known receptor for LTα1β2, can be triggered by LIGHT which is for example expressed on activated lymphocytes [49]. Indeed, in tumors treated with the LTα fusion protein, cells expressing a LTβR ligand were present, which actually due to the absence of endogeneous LTα is likely to be LIGHT. Interestingly, tumors expressing LIGHT induced a massive infiltration of naïve T cells that correlated with an upregulation of chemokines and adhesion molecules [51]. In addition, the important role of LIGHT for the organization of tertiary lymphoid organ preceding the development of spontaneous insulin-dependent diabetes mellitus has been recently demonstrated [26].
Thus, it is conceivable that in tissues where the threshold is already decreased, i.e., in our model the tumormicroenvironment, a transient high concentration of LTα is able to trigger a cascade or positive feedback loop, respectively, which results in the formation of a tertiary lymphoid tissue. Importantly, this induced tissue allows for T-cell priming directly at the tumor site which may ease the initiation of specific T-cell responses.
Acknowledgments
We thank Carrie Dolman, Eva Baumann, Katrin Mueller-Blech and Claudia Siedel for excellent technical assistance; Susan Schellworth for animal care. This work was supported by Deutsche Forschungsgemeinschaft grant Be 1394/5-2 and grant SFP1330 from EMD-Lexigen Research Center, Bedford, MA. (R.X., R.A.R.). D. S. was supported by the Deutsche Krebshilfe.
Abbreviations
- APCs
Antigen presenting cells
- HEV
High endothelial venules
- LTα
Lymphotoxin α
- LTα−/−
LTα knock-out
- LTβR
LTβ receptor
- sLTα
Soluble lymphotoxin α
References
- 1.Agyekum S, Church A, Sohail M, Krausz T, Van Noorden S, Polak J, Cohen J. Expression of lymphotoxin-beta (LT-beta) in chronic inflammatory conditions. J Pathol. 2003;199:115–121. doi: 10.1002/path.1249. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 5.Caux C, Vanbervliet B, Massacrier C, Ait-Yahia S, Vaure C, Chemin K, Dieu-Nosjean M, Vicari A. Regulation of dendritic cell recruitment by chemokines. Transplantation. 2002;73:S7–S11. doi: 10.1097/00007890-200201151-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cupedo T, Mebius RE. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 7.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 8.Davis IA, Knight KA, Rouse BT. The spleen and organized lymph nodes are not essential for the development of gut-induced mucosal immune responses in lymphotoxin-alpha deficient mice. Clin Immunol Immunopathol. 1998;89:150–159. doi: 10.1006/clin.1998.4601. [DOI] [PubMed] [Google Scholar]
- 9.Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 10.Fan L, Reilly CR, Luo Y, Dorf ME, Lo D. Cutting edge: ectopic expression of the chemokine TCA4/SLC is sufficient to trigger lymphoid neogenesis. J Immunol. 2000;164:3955–3959. doi: 10.4049/jimmunol.164.8.3955. [DOI] [PubMed] [Google Scholar]
- 11.Finke D. Fate and function of lymphoid tissue inducer cells. Curr Opin Immunol. 2005;17:144–150. doi: 10.1016/j.coi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- 13.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/S1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 14.Gillies SD, Young D, Lo KM, Foley SF, Reisfeld RA. Expression of genetically engineered immunoconjugates of lymphotoxin and a chimeric anti-ganglioside GD2 antibody. Hybridoma. 1991;10:347–356. doi: 10.1089/hyb.1991.10.347. [DOI] [PubMed] [Google Scholar]
- 15.Gillies SD, Young D, Lo KM, Roberts S. Biological activity and in vivo clearance of antitumor antibody/cytokine fusion proteins. Bioconjug Chem. 1993;4:230–235. doi: 10.1021/bc00021a008. [DOI] [PubMed] [Google Scholar]
- 16.Haanen JB, Toebes M, Cordaro TA, Wolkers MC, Kruisbeek AM, Schumacher TN. Systemic T cell expansion during localized viral infection. Eur J Immunol. 1999;29:1168–1174. doi: 10.1002/(SICI)1521-4141(199904)29:04<1168::AID-IMMU1168>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Haanen JB, van Oijen MG, Tirion F, Oomen LC, Kruisbeek AM, Vyth-Dreese FA, Schumacher TN. In situ detection of virus- and tumor-specific T-cell immunity. Nat Med. 2000;6:1056–1060. doi: 10.1038/79573. [DOI] [PubMed] [Google Scholar]
- 18.Haraguchi M, Yamashiro S, Yamamoto A, Furukawa K, Takamiya K, Lloyd KO, Shiku H. Isolation of GD3 synthase gene by expression cloning of GM3 alpha-2,8- sialyltransferase cDNA using anti-GD2 monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:10455–10459. doi: 10.1073/pnas.91.22.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, Nishikawa SI. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer’s patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iizuka K, Chaplin DD, Wang Y, Wu Q, Pegg LE, Yokoyama WM, Fu YX. Requirement for membrane lymphotoxin in natural killer cell development. Proc Natl Acad Sci USA. 1999;96:6336–6340. doi: 10.1073/pnas.96.11.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Kammertoens T, Janke M, Schmetzer O, Qin Z, Berek C, Blankenstein T. Establishment of early lymphoid organ infrastructure in transplanted tumors mediated by local production of lymphotoxin alpha and in the combined absence of functional B and T cells. J Immunol. 2004;172:4037–4047. doi: 10.4049/jimmunol.172.7.4037. [DOI] [PubMed] [Google Scholar]
- 23.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/S1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 24.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. Lymphotoxin-alpha-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J Virol. 2000;74:2786–2792. doi: 10.1128/JVI.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Chin RK, Christiansen P, Sun Y, Tumanov AV, Wang J, Chervonsky AV, Fu YX. Recruitment and activation of naive T cells in the islets by lymphotoxin beta receptor-dependent tertiary lymphoid structure. Immunity. 2006;25:499–509. doi: 10.1016/j.immuni.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 28.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 29.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Iwamasa K, Rennert PD, Yamada T, Suzuki R, Matsushima A, Okabe M, Fujita S, Yokoyama M. Involvement of distinct cellular compartments in the abnormal lymphoid organogenesis in lymphotoxin-alpha-deficient mice and alymphoplasia (aly) mice defined by the chimeric analysis. J Immunol. 1999;163:1584–1591. [PubMed] [Google Scholar]
- 31.Matsushima A, Kaisho T, Rennert PD, Nakano H, Kurosawa K, Uchida D, Takeda K, Akira S, Matsumoto M. Essential role of nuclear factor (NF)-kappaB-inducing kinase and inhibitor of kappaB (IkappaB) kinase alpha in NF-kappaB activation through lymphotoxin beta receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 33.Muller G, Lipp M. Concerted action of the chemokine and lymphotoxin system in secondary lymphoid-organ development. Curr Opin Immunol. 2003;15:217–224. doi: 10.1016/S0952-7915(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 34.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, Lakkis FG. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transpl. 2007;7:1071–1079. doi: 10.1111/j.1600-6143.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 35.Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065X.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 37.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 38.Reisfeld RA, Gillies SD, Mendelsohn J, Varki NM, Becker JC. Involvement of B lymphocytes in the growth inhibition of human pulmonary melanoma metastases in athymic nu/nu mice by an antibody-lymphotoxin fusion protein. Cancer Res. 1996;56:1707–1712. [PubMed] [Google Scholar]
- 39.Schrama D, Pedersen LO, Keikavoussi P, Andersen MH, thor Straten P, Brocker EB, Kampgen E, Becker JC. Aggregation of antigen-specific T cells at the inoculation site of mature dendritic cells. J Invest Dermatol. 2002;119:1443–1448. doi: 10.1046/j.1523-1747.2002.19604.x. [DOI] [PubMed] [Google Scholar]
- 40.Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–159. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- 41.Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, Becker JC. Targeting of lymphotoxin-alpha to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14:111–121. doi: 10.1016/S1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 42.Schrama D, Voigt H, Eggert AO, Xiang R, Reisfeld RA, Becker JC. Therapeutic efficacy of tumor-targeted IL2 in LTalpha(-/-) mice depends on conditioned T cells. Cancer Immunol Immunother. 2005;55:861–866. doi: 10.1007/s00262-005-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schrama D, Xiang R, Eggert AO, Andersen MH, Pedersen Ls LO, Kampgen E, Schumacher TN, Reisfeld RR, Becker JC. Shift from systemic to site-specific memory by tumor-targeted IL-2. J Immunol. 2004;172:5843–5850. doi: 10.4049/jimmunol.172.10.5843. [DOI] [PubMed] [Google Scholar]
- 44.Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol. 2004;22:1539–1545. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- 45.Suresh M, Lanier G, Large MK, Whitmire JK, Altman JD, Ruddle NH, Ahmed R. Role of lymphotoxin alpha in T-cell responses during an acute viral infection. J Virol. 2002;76:3943–3951. doi: 10.1128/JVI.76.8.3943-3951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O’Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- 47.thor Straten P, Guldberg P, Seremet T, Reisfeld RA, Zeuthen J, Becker JC. Activation of preexisting T cell clones by targeted interleukin 2 therapy. Proc Natl Acad Sci USA. 1998;95:8785–8790. doi: 10.1073/pnas.95.15.8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voigt H, Schrama D, Eggert AO, Vetter CS, Muller-Blech K, Reichardt HM, Andersen MH, Becker JC, Luhder F. CD28-mediated costimulation impacts on the differentiation of DC vaccination-induced T cell responses. Clin Exp Immunol. 2006;143:93–102. doi: 10.1111/j.1365-2249.2005.02972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 50.Young AC, Zhang W, Sacchettini JC, Nathenson SG. The three-dimensional structure of H-2Db at 2.4 A resolution: implications for antigen-determinant selection. Cell. 1994;76:39–50. doi: 10.1016/0092-8674(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 51.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]