Introduction
Despite aggressive multi-modality therapy including image-guided surgical resection, maximum external beam radiation therapy, and prolonged cycles of adjuvant chemotherapy, the prognosis for patients diagnosed with malignant brain tumors remains poor [1]. In patients with malignant brain tumors, the normal brain parenchyma is deeply infiltrated by invasive tumor cells. Therefore, treatments that specifically target cancer cells without harming the surrounding normal brain are critically needed. Immunotherapy targeting tumor-specific antigens is a modality that can meet this specificity by taking advantage of the exquisite precision of the human immune system in targeting cells for cytolytic destruction. Currently, studies have demonstrated the feasibility of activating the immune system to eliminate tumors [2], and future efforts are aimed at identifying potential target antigens and maximizing the effectiveness of immunotherapy. Survivin was identified over a decade ago as an inhibitor of apoptosis that promotes tumorigenesis [3], and has been used as an antigenic target for immunotherapy in a variety of cancers such as pancreatic, gastric, and lung cancer [4–6]. However, immunotherapy efforts targeting survivin in patients with malignant brain tumors are relatively devoid despite considerable rationale for exploitation of this frequently over-expressed tumor antigen. In this review, we have summarized the data supporting the potential of survivin as an immunotherapeutic target antigen for adult and pediatric brain tumors.
Characteristics of a desirable immunotherapeutic antigen
An ideal antigen for cancer immunotherapy should possess the following characteristics:
-
Expression of the antigen should be restricted to tumor cells and absent in normal tissues (tumor-specific antigen or TSA), sparing normal cells from being targeted by immunotherapeutic treatments. However, to date, the identification of consistently expressed TSAs in most cancers is rare. A few notable exceptions include tumor-specific mutations that are key players in cellular signal transduction and tumorigenesis, such as EGFR, BRAF, and Ras. The expression of the epidermal growth factor receptor variant 3 (EGFRvIII) mutation was found in approximately a third of malignant gliomas and not found in normal adult tissues [7]; BRAF somatic missense mutations in the kinase domain are found in 66% of malignant melanomas. A single amino acid substitution (V599E) is accountable for 80% of these mutations [8, 9]; Mutational changes in the Ras family, leading to constitutively active Ras, are responsible for more than 15% of all human cancers [8, 10]. Another example of relative TSAs are cancer/testis (CT) antigens (MAGE, BAGE, GAGE, SEREX, etc.) that may also serve as desirable TSAs since they are found in a variety of malignancies, but are silent in normal tissues, except the restricted expression in immune privileged testis [11, 12].
More commonly identified are tumor-associated antigens (TAAs) which represent over-expressed proteins found in tumors relative to expression in other normal tissues. TAAs have served as targets in the majority of antigen-specific immunotherapy trials and these proteins may serve as effective targets for immunotherapy as long as the threshold for killing tumor cells exceeds that required for recognition and killing of the normal tissues with shared antigen expression, or if the toxicity associated with such recognition is acceptable. For example, vitiligo has been associated with effective immunologic responses to therapy targeting melanocyte differentiation antigens expressed in metastatic melanoma [13]. While such toxicity may be reasonably acceptable compared to the morbidity of metastatic melanoma, immunotherapy for brain cancers presents a significant concern for limiting toxicity against shared normal brain antigens, as significant autoimmune toxicity within the central nervous system (CNS) is not tolerable.
The antigen must be immunogenic, or capable of eliciting a specific-immune response in immunized hosts. The effectiveness of eliciting an immune response against any particular antigen will obviously be affected by the vaccine strategy employed and optimization thereof; however, the ideal antigen would be shown to be immunogenic in human in vitro assays or suitable experimental models before use in clinical evaluations in humans.
An ideal candidate tumor antigen for clinical immunotherapy would be over-expressed in many different cancer types, and also expressed within a large proportion of tumors of a given type, to allow for efficient clinical evaluation of immunotherapy directed against a single antigenic target in a broad spectrum of patients.
Within the context of what are frequently heterogeneous tumors, the antigen is expressed in a high percentage of the tumor cells, increasing the likelihood that effective targeting will have a significant impact on modulating disease progression.
The targeted antigen ideally has a key role in tumor development and progression, decreasing the chance for immunologic escape through down regulation of antigen expression. If tumor cells without the antigen have a less malignant phenotype then effective targeting may not be curative but still provide a significant survival benefit.
Survivin’s role in apoptosis and mitosis in human cancers
Survivin protein is over-expressed in a variety of human cancers, including but not limited to carcinomas of pancreas, lung, breast, colon, ovaries, liver, malignant melanoma, leukemia, and many CNS tumors such as malignant gliomas, medulloblastoma, neuroblastoma, and neurofibromas [14–21]. The expression of survivin is found to be highly tumor specific, with only minimum expression detected in normal cells [3]. Survivin is a mammalian inhibitor of apoptosis protein (IAP) that prevents cells from undergoing apoptotic cascade. The precise mechanisms of how survivin inhibits apoptosis remain unknown, although it is suggested that survivin, like other members in the IAP family (XIAP, c-IAP1, c-IAP2, NAIP, Apollon, Ts-IAP, and livin), exerts its anti-apoptotic effect by binding and inactivating caspases, a family of cysteine proteases that induce programmed cell death [22]. In addition to inhibiting apoptosis, survivin, a chromosomal passenger protein, also plays a role in cell division. In the mitotic phase, survivin associates with segments of the mitotic apparatus such as the microtubule spindles and centrosomes to ensure proper chromosome alignment and proper segregation [23, 24].
The dual roles of survivin in inhibiting apoptosis and promoting cell division highlight its importance in tumorigenesis. Survivin expression is strongly associated with malignant phenotype (one of the top four genes in the human genome that is found to be over-expressed in cancer [25]), but not expressed in most normal cells, making it a potentially desirable target for immunotherapy.
Survivin and its spliced variants: their unique cellular functions and implications in human cancers
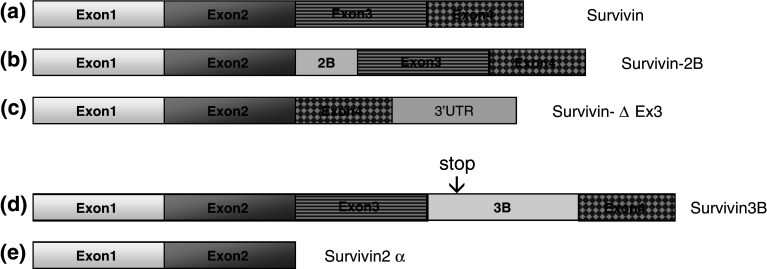
To date, five variants of survivin—survivin, survivin-2B, survivin-ΔEx3 [26], survivin 3B [27], and survivin 2-alpha [28]—have been identified (Fig. 1). Survivin-ΔEx3 and survivin-2B were first characterized in renal cell carcinomas. Unlike other IAPs, which have conserved caspase-recruitment domain and RING domain, survivin contains only a single baculovirus IAP repeat (BIR) domain. Exon 3 deletion and intron 2 insertion into the BIR domains of survivin results in survivin-ΔEx3 and survivin-2B, respectively, which greatly alters the structure of this anti-apoptotic protein and leads to unique cellular functions. Although the BIR domain in survivin-ΔEx3 is truncated, this variant still preserves its functional role as an anti-apoptotic protein. On the other hand, the ability of survivin-2B to inhibit apoptosis is highly reduced. Despite the different functional roles, all three survivin isoforms (survivin, survivin-ΔEx3, and survivin-2B) are expressed in renal cell carcinomas [26].
Fig. 1.
Survivin and its spliced variants. a Wild-type Survivin is consisted of four exons: exon 1 (37 aa), exon 2 (36 aa), exon 3 (40 aa), and exon 4 (29 aa). b An insertion of exon 2B in between exon 2 and exon 3 results in survivin-2B isoform. c Exon 3 is deleted in Survivin-∆ Ex3, and a frame shift leads to an extended open reading frame in the 3′ UTR. d Exon Survivin 3B (55 aa) is derived from intron 3. Exon 3B includes a stop codon which results in a truncated survivin with a length of 120 aa. e Survivin 2-alpha is consisted of exons 1 and 2, along with a fragment of 3′ intron 2. The stop codon in the intron 2 immediately truncates the survivin right after exon 2, resulting in a 74-aa spliced variants
Caldas et al. identified survivin 2-alpha and characterized its functional role through co-transfection experiments [28]. The co-expression of survivin 2-alpha with survivin increases early apoptosis and enhances the activation of caspase-3 when compared to cells without survivin 2-alpha. This particular variant seems to lessen the anti-apoptotic effects of survivin [28]. The presence of several survivin variants and their different functions adds yet another layer of complexity to the role of survivin in malignant diseases, and understanding the mechanisms of how each variant contributes to tumorigenesis becomes crucial for use of survivin expression as a prognostic or diagnostic biomarker in oncology.
The role of survivin in angiogenesis
Angiogenesis is a complicated process of vascular remodeling that involves endothelial cell migration, proliferation, sprouting, and regression, and the balance of these events is governed by various angiogenic growth factors and angiogenesis inhibitors [29]. Vascular endothelial cell growth factor (VEGF) [30], angiopoietin-1 [31], and basic fibroblast growth factor (bFGF) [32] are several important angiogenic growth factors that promote the formation of new blood vessels. VEGF binds to its receptor, VEGFR2, on the endothelial cells, which triggers the phosphatidylinositol 3-kinase (PI3K) pathway, leading to the activation of protein kinase B (PKB/Akt) [33, 34] and ultimately the up-regulation of inhibitors of apoptosis proteins such as Bcl-XL, XIAP, and survivin [35]. Survivin is induced by other angiogenic growth factors such as angiopoietin-1 and bFGF, and co-expression of multiple factors, such as VEGF and bFGF together, further enhances the up-regulation of survivin [34].
Survivin plays an important role in angiogenesis during embryonic development, as the deletion of survivin in early embryonic stage is lethal in murine models [36]. In a zebrafish model, inhibition of survivin expression significantly disrupted angiogenesis but not vasculogenesis during embryonic development [37]. In humans, survivin is highly expressed in proliferating endothelial cells while is minimally detectable in non-proliferating cells [38]. Recombinant protein survivin expression in endothelial cell culture diminishes the activities of caspase-3 and inhibits TNF-alpha and cycloheximide-induced apoptosis [38].
Targeting survivin suppresses tumor angiogenesis
Since tumor blood vessels provide nutrient supplies that are essential for the viability of growing tumors, reduction of tumor angiogenesis becomes a possible means to control tumor growth. The high expression of survivin in cancers and its role in promoting angiogenesis renders it a potential therapeutic target to suppress angiogenesis within solid tumors. Studies have confirmed that the ablation of survivin in endothelial cells of several cancers leads to a decrease of tumor-associated angiogenesis both in vitro and in vivo [6, 39, 40].
In a study conducted by Mesri et al., an antisense oligonucleotide targeting survivin reduced anti-apoptotic events mediated by VEGF and enhanced caspase-3 activities. In addition, antisense inhibition of survivin also repressed the formation of three-dimensional vasculature structures. The abolishment of survivin in endothelial cells promoted EC cell death and diminished the newly formed vascular network, providing evidence to support survivin as a potential target for reduction of tumor-associated angiogenesis [40].
Blanc-Brude et al. showed that the ablation of survivin reduced tumor angiogenesis in vivo in a xenograft model of breast cancer [39]. Adenoviruses encoding survivin (pAd-survivin) and a phosphorylation-defective survivin (pAd-T34A) dominant-negative mutants were transfected into endothelial cells. In endothelial cells transfected with adenoviruses encoding survivin, stable growth of vasculature was observed. In contrast, when pAd-T34A-transfected endothelial cells were injected into a breast cancer xenograft, a remarkable reduction (approximately 60%) of tumor-associated capillary network was observed. Similar experiments have confirmed that survivin antisense and a dominant-negative mutant in gastric cancer leads to decreased angiogenesis [6].
Expression of survivin in malignant brain tumors
Numerous studies have confirmed the presence of survivin in malignant brain tumors including astrocytomas, meningiomas, oligodendrogliomas, neurofibromas, and other histologic tumor types [18, 41–45]. In addition, the expression of survivin is found to correlate with pathologic grade of many tumors in the nervous system [43–46].
In a study conducted by Katoh et al., the expression of survivin in gliomas, meningiomas, and schwannomas was assessed at the mRNA and protein level using RT-PCR, Western blot, and immunohistochemistry [47]. While survivin was highly expressed in the tumor cells, there was no expression detected in the surrounding normal brain [47].
The role of survivin in cell proliferation, apoptosis, and angiogenesis in brain gliomas was further investigated by measuring PI (proliferative index), AI (apoptotic index), MVD (microvessel density), and ODG (overall daily growth) in brain gliomas [14]. The level of survivin expression, measured by IRS (immunoreactivity score), was shown to positively correlate with pathologic grade in brain glioma (F = 7.578, P < 0.001). The rate of cell proliferation also correlated with pathologic grade of tumor. There is a sharp increase in PI observed as tumors progress from grade I to grade IV (F = 16.416, P < 0.001). However, there is also an increase in apoptotic activity in higher grade tumors as well. Some groups have reported that there is no clear association between AI and pathologic grade [16], while some other sources suggested that AI associates with tumor progression either positively or negatively [14, 48–51]. The measurement of MVD indicates the degree of angiogenesis in tumors. MVD is markedly higher in survivin-positive tumors in contrast to survivin-negative tumors, and there is a statistically significant association between increase in MVD and increase in pathologic grade [14].
Xie et al. described the differential expression of cytoplasmic survivin in primary and secondary human glioblastoma multiforme (GBM). Primary and secondary GBMs are derived from two different pathogenic pathways: primary GBMs form de novo, while secondary GBMs often develop from a less malignant grade glioma. Among 30 primary and 26 secondary GBMs, Xie et al. found that the expression of cytoplasmic survivin is significantly higher in the primary GBMs (83%) than in secondary GBMs (46%) (P < 0.001). The expression of survivin in the nucleus, however, was similar for both primary (73%) and secondary (81%) GBMs. Furthermore, 15 secondary GBM samples were compared to their corresponding low-grade precursors. Seventy-one percent of the secondary GBMs exhibit more intense expression level of cytoplasmic survivin when compared to the low-grade precursors, while there was no observable difference for expression of nucleus-localized survivin in secondary GBMs and their precursors. Cytoplasmic survivin thus appears to play a role in the development of primary GBMs and tumor progression [15].
In addition, survivin expression has been shown to be up-regulated in CD133+ glioma cancer stem-like tumor cells (CSCs) [52]. CSCs are a small population of cells in tumors that retain stem cell-like characteristics, and CSCs are thought to be tumorigenic due to their self-renewal properties. CSCs may contribute to tumor progression, angiogenesis, relapse, and metastasis, making therapies targeting cancer stem cell-specific antigens particularly desirable [53]. Preliminary evidence suggests that higher levels of survivin expression in these cells may contribute to their prolonged lifespan and treatment resistance.
Expression of survivin in pediatric brain tumors
Medulloblastoma
Survivin and its splice variants (survivin-2B, survivin-ΔEx3) are found to be expressed at an unusually high level in medulloblastoma, the most common type of pediatric brain tumor [20, 54]. Survivin and its splice isoforms seem to have unique cellular functions and localizations, and the statistically significant association between specific isoforms with histologic subtypes (classic, desmoplastic, nodular, and large-cell anaplastic) of medulloblastoma renders the possibility that the expression level of survivin and its isoforms can be predictors of clinical outcome.
Fangusaro et al. collected tumor samples from 19 pediatric patients with medulloblastomas of different grades and quantified the expression of survivin and its isoforms using PCR and Western blots. Survivin, survivin 2B, and survivin-ΔEx3 are over-expressed in pediatric medulloblastomas compared to the expression in normal brain. To rank the level of expression for each variant, survivin has the highest expression in most of the tumor samples (278-fold increase compared to normal cerebellum) relative to survivin-2B and survivin-ΔEx3, with survivin-2B (43-fold increase compared to normal cerebellum) and survivin-ΔEx3 (20-fold increase compared to normal cerebellum) being the second highest and lowest, respectively. Conducted by the same group, 40 medulloblastoma tumor samples were evaluated using immunohistochemistry. The higher level of survivin-positive cells was associated with large-cell anaplastic medulloblastoma, a highly malignant pathologic grade of medulloblastoma [54]. Immunohistochemical staining revealed that survivin is especially highly expressed in the internodular regions of the nodular and desmoplastic subtypes [20, 54].
However, when Li et al. investigated the differential expression of survivin splice isoforms in pediatric medulloblastoma, they confirmed a negative association between survivin expression and clinical outcome, and they did not find survivin isoforms associated with particular histologic subtypes of medulloblastoma. In this study, Li et al. quantified the mRNA level of three spliced variants of survivin (survivin, survivin-ΔEx3, and survivin-2B) in 20 pediatric medulloblastoma samples using quantitative RT-PCR and compared it to that of the normal brain. A low level of survivin-ΔEx3 is detected in normal brain while all three isoforms (survivin, survivin-ΔEx3, and surviving-2B) are over-expressed in medulloblastoma, with wild-type survivin being dominant [20]. Li’s findings further showed that isoform survivin-ΔEx3 is especially highly up-regulated in recurrent tumors, which seems to predict clinical outcome. Overall, survivin level serves as an indicator for prognostic tumor grades not only in medulloblastoma but in many other human cancers [48, 55–57], however, the role of each survivin variant in contributing to tumor progression needs further investigation.
Neuroblastoma
Neuroblastoma is ranked the most common solid extracranial tumor in infancy and childhood and accounts for 15% of death in children due to cancer [21]. The level of survivin expression is highly correlated with prognosis in these tumors.
Azuhata et al. showed that seven primary tumors that later developed recurrent tumors demonstrated high level of survivin expression, while eight tumors that went into long-term remission had undetectable survivin levels. In addition, a high level of survivin expression in tumors is associated with high cell proliferation rate, and among these cells, a high percentage of cells are in G2/M mitotic phase of the cell cycle. This is in contrast to the low proliferation rate of the tumors with low survivin level, where majority of cells are in the G0/G1 phase [55]. The eight tumors that went into remission also expressed a higher level of pro-apoptotic receptors such as Fas, TRAIL-R1, and (TNF)-R1 when compared to the seven tumors that recurred.
Studies have shown that the ratios of survivin and these pro-apoptotic receptors present in tumors serve as predictors of the likelihood of developing recurrent neuroblastoma. For instance, it is known that survivin down-regulates the apoptotic-inducing effect exerted by TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand). The sensitivity to TRAIL varies according to tumor types, and NB cell lines with high level of survivin are TRAIL resistant, while NB cells lines with low level of survivin expression are more TRAIL sensitive [58]. When the TRAIL-resistant cell lines are treated with etoposide, the expression of survivin decreases greatly, and the presence of survivin is undetectable after 48 h of incubation. These originally TRAIL-resistant cells became TRAIL sensitive once the level of survivin subsided [58]. In another experiment, when high level of survivin was transfected into a TRAIL-sensitive cell line, the transfected cell line became TRAIL resistant, and conversely, when survivin is inhibited in the TRAIL-resistant cells, it renders the cells prone to apoptosis when given TRAIL treatment [58]. These transfection experiments demonstrated the link between survivin expression and tumor sensitivity to TRAIL-mediated apoptosis. Additional data demonstrated that a higher ratio of survivin to TRAIL expression predicts the likelihood of recurrent tumor growth. Eighty percent of the tumors that later recurred had a survivin:TRAIL ratio of 1.2 and greater, whereas only 17% of the cured tumors had a survivin:TRAIL ratio that exceeded this value [58]. Very similar observations have been made when investigating the survivin to Fas ratio as an indicator for the likelihood of recurrent disease. Tumors that went on to recur have a significantly higher value of survivin:Fas ratio (3.3) when compared to that of the tumors which went into remission (0.75) [59].
The frequency of survivin expression in various CNS tumors is summarized in Table 1.
Table 1.
Frequency of survivin expression in various tumor types
| Tumor type | Survivin expression (% positive) | Reference |
|---|---|---|
| Diffuse astrocytoma (grade II) | 37.5–50 | [18, 43] |
| Anaplastic astrocytoma (grade III) | 86.7–100 | [43, 45] |
| Glioblastoma multiforme | 80–100 | [18, 42, 45] |
| Meningiomas | 65–100 | [18, 41, 44] |
| Schwannomas | 100 | [18] |
| Oligodendroglioma | 100 | [18] |
| Medulloblastoma | 80–100 | [18, 20] |
| Oligoastrocytoma | 93 | [18] |
| Ependymoma | 100 | [18] |
| Nuerofibroma | 100 | [18] |
| Retinoblastoma | 25 | [18] |
Immunotherapy targeting survivin
Numerous published studies have demonstrated survivin as a potential candidate for immunotherapy [5, 60–66], and using survivin as a cancer target still remains an active area of research. Studies have used DNA-transfected dendritic cells (DCs) and peptide vaccines to target survivin in murine tumor models and a variety studies have shown immunologic targeting of survivin in human cancers such as carcinomas of prostate, breast, lung, and gliomas.
Vaccines targeting survivin in murine models
DNA- RNA- or peptide-pulsed DCs are an effective vehicle for induction of antitumor immune responses, and DC vaccines targeting survivin have been shown to generate immune responses in many studies [5, 61–63, 66]. Ciesielski et al. transfected bone marrow-derived DCs with pVAX vectors containing full-length human survivin (pVAX.hSVN I-V), full-length murine survivin (pVAX,mSVN I-V), and truncated human survivin containing survivin cDNA fragments II–V (pVAX.hSVN II-V). Immunized mice were subsequently challenged by intracranial injection of GL261 glioma cells. Vaccinated mice with pVAX.hSVN II-V-pulsed bone marrow DCs exhibited cytotoxic T cell responses and persisted a longer period without tumor growth (12 of 15 mice remained tumor free for 200 days) relative to the control mice (tumors grew in all mice within 50 days) [63]. Six of the 12 cured mice were re-challenged with intracerebral injection of GL261 cells to test the potency of immune memory response. Compared to the control group, pVAX.hSVN II-V-vaccinated mice survived the re-challenge with immunologic memory protection through entire period of observation (60 days). Furthermore, Ciesielski et al. showed that DC vaccines loaded with human survivin demonstrated better immunogenic protection against GL261 in mice than that of murine survivin-pulsed DCs. Full-length survivin was also shown to be more effective in prolonging survival in DC vaccines than using DCs pulsed with fragmented survivin epitopes (I–V) [63].
Cho et al. conducted protein-pulsed DC vaccination, with an additional recombinant tatPTD fused with the survivin protein. This approach took advantage of the ability of tatPTD to enhance the vaccine loading into the DCs through translocation into the cytosol. Mice vaccinated with tatPTD–survivin fusion demonstrated significantly higher CTL cytotoxicity compared to mice that received unmodified survivin-pulsed DC vaccination [62].
A study conducted by Kim et al. demonstrated that a combination of survivin vaccine with TMZ chemotherapy further enhances T cell cytotoxicity in murine glioma, providing better results than administering immunotherapy or chemotherapy alone [67].
DNA vaccines targeting survivin have also been shown to be effective in inducing survivin-specific CTL cytotoxicity in murine models for various cancer types [4, 5]. For instance, DNA vaccines encoding human and mouse survivin cDNAs in pCMVE mammalian expression vectors have been shown to slow the tumorigenesis and lengthen the survival time for mice with experimental pancreatic cancer and lymphoma [4].
In a study conducted by Xiang et al., a DNA vaccine encoding both survivin and chemokine CC21 was delivered orally to mice, and was shown to induce CTL. The investigators demonstrated that the co-expression of both survivin and CC21 vaccine is more effective than that of the survivin or CC21 alone. This survivin/CC21 DNA vaccine was shown not only to reduce lung metastases and enhance CLT-induced apoptosis but to also suppress angiogenesis in the tumor vascular bed [5].
A novel cancer therapy called mimovirus has recently been described. Mimovirus is a virus-sized particle that contains a cell-penetrating peptide (CPP) Tat49–57, a survivin epitope, and a plasmid containing adjuvant IL-15. CPP Tat49–57 is shown to facilitate peptide transfection and MHC class I presentation, and encodes the same domain as the Tat47–57 PTD used in the study conducted by Cho et al. described above. The IL-15 cytokine was included to enhance the expansion of memory T cells. It was shown that the genes in the mimovirus were expressed in vivo and immunogenic survivin peptides were presented by antigen presenting cells. The mimovirus incorporating CPP and IL-15 with survivin peptides was effective in triggering cytotoxic CD8+ T cells response with long-term immunity involving CD8+ memory T cells [68]. No comparative efficacy of the mimovirus to other vaccination techniques against survivin was described in this study.
Survivin immunogenicity in human studies
Andersen et al. identified two potential survivin epitopes that are recognized by CTLs in patients with leukemia and melanoma. They tested nine epitopes (9–10 amino acids in length) that were predicted to have reasonable binding affinity to the HLA-A2 molecules. Among the tested epitopes, survivin95–104 (ELTLGEFLKL) and survivin96–104 (LTLGEFLKL) demonstrated higher affinity, and a single modification of the threonine at position 97 to leucine or methionine further enhanced the binding affinity of survivin96–104. survivin95–104 and survivin96–104 were subsequently shown to be capable of triggering CTL response in patients with CLL and melanoma [60].
In a study, Pisarev et al. transduced full-length dominant-negative survivin gene in human DCs in an attempt to stimulate T cell response against three algorithm-predicted survivin peptide T cell epitopes. Based on published literature and predictions from PaProC algorithm, Pisarev et al. determined three survivin peptides of 9–10 amino acids in length that were predicted to have optimal affinity to bind HLA-A2. These three peptides were ELTLGEFLKL (ELT), LDRERAKNKI (LDR), and FKELEGWEP (FKE). DCs were collected from four HLA-A2-positive health individuals and from four patients with prostate cancer. DCs were then transduced with full-length dominant-negative survivin gene by using an adenoviral vector. To reduce the risk of introducing a full-length survivin gene that encodes for an anti-apoptotic protein, the Thr 34 was altered to make a dominant-negative survivin mutant that still retains the coding regions for the predicted survivin T cell epitopes. After in vitro stimulation with full-length dominant-negative survivin-loaded DCs, the activity of CTL response against the three survivin peptide epitopes was assayed. All eight individuals in the study showed significant response against ELT peptide, while all health individuals and two of the four cancer patients showed immune response against survivin peptide LDR. The response against the third peptide FKE was not detected [69]. One potential limitation of this study was that it did not determine whether or not the simulated CTL could effectively exert its cytotoxic effect on tumor cells or other targets that expressed endogenous survivin antigen, since only peptide-pulsed cells were used as targets [65].
Idenoue et al. later showed that an HLA-A24-restricted antigenic peptide, surviving-2B80-88 (AYACNTSTL), is capable of inducing survivin-specific CTL, and furthermore, these CTLs exert potent cytotoxicity against tumor cells that express endogenous survivin. These investigators showed that an elevated level of CTL precursors exist in the PBMC of HLA-A24-positive patients of breast, colorectal, and gastric cancers (>0.1%) compared to that of the health individuals (<0.1%). The group subsequently demonstrated that peptide stimulation can induce survivin-specific CTL activity in vitro from PBMCs of patients with cancer. PBMCs were stimulated in vitro by survivin-2B80-88, and through HLA-A24/surviving-2B80-88 tetramer analysis, survivin-specific CTLs were found to increase in number from 0.07 to 0.89%. The cytotoxicity of the survivin-specific CTL cells was shown by 51Cr release assay, demonstrating higher cytotoxicity against survivin-2B80-88 pulsed C1R-A*2402 cells than nonpulsed target cells and negative control peptide-pulsed target cells. Furthermore, these induced CTL cells did not show cytotoxicity against cells that were not HLA-A24-restricted, such as survivin-2B80-88-pulsed C1R-*A31012 cells and nonpulsed K562 cells demonstrating HLA-A*2402-restricted recognition. The authors also demonstrated cytolytic killing of an adenocarcinoma breast cancer line, LNY-1-A*2402, that expressed endogenous survivin [64].
Clinical trials targeting survivin
Although the immunogenicity of survivin has been confirmed in vivo and in vitro in many studies, there are relatively few published clinical trials evaluating immunotherapy directed against survivin in humans.
Tsuruma et al. conducted two separate phase I clinical trials using HLA-A24-restricted survivin-2B epitope vaccines in patients with colorectal and breast cancers [70, 71]. Safety of survivin-2B peptide vaccines was demonstrated in both clinical trials. In the first clinical trial for advanced and recurrent colorectal cancer, subcutaneous vaccination resulted in slight toxicity in three patients which included anemia (grade 2), malaise (grade 1), and fever (grade 1). No severe adverse events were observed. There was a transient decrease of tumor marker levels (CEA and CA19-9) in six patients, and a slight reduction of tumor volume in one patient. HLA-A24/peptide tetramer showed an increase in CTL response against survivin-2B epitope in one patient.
The second clinical trial for patients with advanced or recurrent breast cancer involved the vaccination of HLA-A24-restricted survivin-2B epitope alone (n = 10) or with addition of Incomplete Freund’s adjuvant, IFA (n = 4). In the peptide alone group, no adverse events were observed. In the peptide plus IFA group, two patients showed indurations at the injection site. One patient had malaise (grade 1) and another had malaise (grade 1) and fever (grade 1). In the peptide-alone group, there were increases in tumor marker levels observed in eight patients, and a slight decrease in one patient. Two patients had stable disease. In 3 of 10 patients, there was an increase of the peptide-specific CTL frequency. In the peptide plus IFA group, an increase of CTL response was observed in all patients (n = 4), although the enhanced immunologic response did not lead to significant clinical responses. These two clinical trials are summarized in the Tables 2 and 3. A list of current ongoing survivin vaccine clinical trials (queried on http://www.clinicaltrials.gov on 4/08/09, search term: survivin vaccine) is shown in Table 4.
Table 2.
Phase I clinical trial targeting survivin for advanced and recurrent colorectal cancer (15 patients)
| Vaccination | Survivin2B 80-88 (AYACNTSTL) peptide alone is given every 14 days for a total of six times |
| Toxicities | Three patients demonstrated toxicities: anemia (grade 2), general malaise (grade 1), and fever (grade 1) |
| Surrogate marker response | Six patients showed temporary decreased in CEA and CA 19-9 levels during the time of vaccination |
| Tumor volume | One patient demonstrated minor tumor reduction. |
| Immunologic response | In one patient, HLA-A24/peptide tetramer demonstrated increased CTL response against survivin 2B peptide from 0.09 to 0.35% of CD8+ T cells after four vaccinations |
Table 3.
Phase I clinical trial targeting survivin for advanced and recurrent breast cancer (15 patients)
| Survivin 2B 80-88 (AYACNTSTL) peptide vaccine alone (ten patients) | |
|---|---|
| Vaccination | 0.1–1.0 mg of survivin-2B peptide every 2 weeks for a total of four times |
| Toxicities | No adverse response |
| Surrogate marker response | Eight patients showed increased level of tumor markers (CEA, CA15-3, NCC-ST-439, and ICTP are examined); level decreased slightly in one patient |
| Immunologic response | Three patients (30%) showed increased peptide-specific CTL |
| Survivin 2B 80-88 (AYACNTSTL) peptide + IFA (four patients) | |
| Vaccination | 1.0 mg of survivin 2B peptide mixed with IFA every 2 weeks for a total of four times |
| Toxicities | Two patients had induration; one patient had general malaise (grade 1); one patient had general malaise and fever |
| Surrogate marker response | All patients showed increased level of tumor markers (CEA, CA15-3, NCC-ST-439, and ICTP are examined) |
| Immunologic response | Four patients (100%) demonstrated increased peptide-specific CTL |
Table 4.
Current phase I/II survivin vaccine clinical trials
| Conditions | Intervention | PI/sponsor/NCT identification | Phase |
|---|---|---|---|
| Malignant melanoma, pancreatic cancer, colon cancer, cervical cancer | Survivin peptide vaccine | J. C Becker, MD, PhD/Julius-Maximilians University/NCT00108875 | I/II |
| Breast neoplasm, breast cancer, cancer of the breast, carcinoma, ductal | hTERT/Survivin multi-peptide vaccine | S. Domchek, MD & K. Fox, MD/ University of Pennsylvania/ NCT00573495 | I |
| Melanoma (skin) | Melan-A, MAGE-3, and Survivin RNA-transfected DC vaccine | G. Schuler, MD/Dermatologische Klinik MIT Poliklinik-Universitaetsklinikum Erlangen/NCT00074230 | I/II |
| Multiple myeloma and plasma cell neoplasm | Survivin peptide vaccine | A. Rapoport, MD/University of Maryland Greenebaum Cancer Center; National Cancer Institute (NCI)/NCT00499577 | I/II |
| Advanced renal cell carcinoma | Survivin and telomerase peptide-pulsed dendritic cells | I. Svane, MD, PhD/Herlev Hospital/NCT00197860 | I/II |
| Advanced melanoma | p53, survivin, and telomerase peptide-pulsed dendritic cells | I. Svane, MD, PhD/Herlev Hospital/NCT00197912 | I/II |
Information collected from http://www.clinicaltrials.gov; search term: survivin vaccine
Concerns of targeting survivin: the minimum expression of survivin in normal cells
One characteristic that makes survivin a desirable cancer target is its tumor specificity, with an almost undetectable level in differentiated normal cells. However, survivin, being a chromosomal passenger protein that regulates cell proliferation, is expressed at low level during the cell cycle in hematopoietic cells, T lymphocytes, basal colonic epithelial cell [72], and vascular endothelial cells [73]. The regulation of the proliferation and apoptosis of endothelial cells is dependent on survivin expression, which is manipulated by a variety of factors through the Akt signaling pathway. The level of survivin also seems to play a role in T cell development in the thymus, and survivin is found to be expressed in both thymocytes and matured T cells. Survivin is fundamental for embryonic development, and knocking out survivin during fetal development is lethal.
Being an important protein during development, survivin is also expressed in human stem cells populations, which may be a crucial determinant in assessing the safety of using survivin as an immunotherapeutic target. In humans, CD34+ cells express survivin [73]. Hematopoietic growth factors regulate the cell cycle of CD34+ stem cells by manipulating survivin expression level.
One study addressed the potential for targeting CD34+ stem cells with survivin-specific T cells. The full-length dominant-negative survivin peptide-induced CTL generated by Pisarev et al. was tested for cytotoxicity against CD34+ cells [69]. Although CD34+ cells express a higher level of survivin compared to normal cells, the peptide-induced CTL did not exhibit cytotoxicity against these stem cells. This may be due to the reported absence of MHC class I and class II molecules on the surface of stem cell populations for recognition by activated T lymphocytes [74, 75]. Although more than one study has established the safety of using survivin as vaccine target, as methods for generating increasingly potent immune responses against this protein are developed, concern for potential toxicity in critical tissues is warranted.
Survivin as an immunotherapeutic target for malignant brain tumors
To date, there has not been a published clinical trial of immunotherapy targeting survivin in patients with brain cancers. Survivin appears to be a potential ideal candidate antigen for evaluation in immunotherapy trials in brain cancer due to several factors outlined in this review.
In summary, survivin is over-expressed in most common adult and pediatric brain tumors but minimally expressed in normal brain, a critical issue for the safety of targeting CNS tumors. Numerous studies have shown that cytotoxic immune responses can be induced in humans, and survivin-targeted vaccines are capable of reducing tumor growth in experimental brain tumor models. Additionally, survivin is demonstrated to play a critical role in tumor progression and angiogenesis, so the effective targeting of survivin could attenuate the malignant phenotype of brain tumor cells. Finally, since survivin over-expression is a phenotype shared by a high percentage of patients with various CNS and systemic cancers, immunotherapy targeting survivin has the potential to be a widely applicable therapeutic platform with relevance to several different malignant diseases.
While survivin alone is an attractive immunotherapeutic target, evoking immune responses against multiple antigens provides the possibility to enhance the efficacy of immunotherapeutic approaches. Targeting multiple cancer antigens may render heterogeneous populations of tumor cells more susceptible to immunologic attack and prevent immune evasion due to selective loss of a single targeted antigen. Combining survivin targeting strategies with other known or newly identified cancer antigens may be a promising therapeutic strategy [76]. Furthermore, administering standard therapy, such as chemotherapy, along with immunotherapy targeting survivin is likely to enhance the efficacy of treatment, since the expression of apoptosis regulators such as survivin correlate with resistance to chemotherapy [76].
In our opinion, the assessment of the safety and potential efficacy of targeting survivin in malignant brain tumors with immunotherapeutic approaches deserves continued and thorough evaluation in preclinical and clinical experimental systems, and holds significant potential as a novel therapeutic target for the treatment of adult and pediatric malignant brain tumors.
References
- 1.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 4.Zhu K, et al. Survivin DNA vaccine generated specific antitumor effects in pancreatic carcinoma and lymphoma mouse models. Vaccine. 2007;25(46):7955–7961. doi: 10.1016/j.vaccine.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Xiang R, et al. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65(2):553–561. [PubMed] [Google Scholar]
- 6.Tu SP, et al. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63(22):7724–7732. [PubMed] [Google Scholar]
- 7.Wikstrand CJ, et al. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57(18):4130–4140. [PubMed] [Google Scholar]
- 8.Davies H, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Andersen MH, et al. Immunogenicity of constitutively active V599EBRaf. Cancer Res. 2004;64(15):5456–5460. doi: 10.1158/0008-5472.CAN-04-0937. [DOI] [PubMed] [Google Scholar]
- 10.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 11.Simpson AJ, et al. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 12.Yokoe T, et al. Efficient identification of a novel cancer/testis antigen for immunotherapy using three-step microarray analysis. Cancer Res. 2008;68(4):1074–1082. doi: 10.1158/0008-5472.CAN-07-0964. [DOI] [PubMed] [Google Scholar]
- 13.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhen HN, et al. Survivin expression and its relation with proliferation, apoptosis, and angiogenesis in brain gliomas. Cancer. 2005;104(12):2775–2783. doi: 10.1002/cncr.21490. [DOI] [PubMed] [Google Scholar]
- 15.Xie D, et al. Expression of cytoplasmic and nuclear Survivin in primary and secondary human glioblastoma. Br J Cancer. 2006;94(1):108–114. doi: 10.1038/sj.bjc.6602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takekawa Y, Sawada T, Sakurai I. Expression of apoptosis and its related protein in astrocytic tumors. Brain Tumor Pathol. 1999;16(1):11–16. doi: 10.1007/BF02478896. [DOI] [PubMed] [Google Scholar]
- 17.Satoh K, et al. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271–278. doi: 10.1002/1097-0142(20010715)92:2<271::AID-CNCR1319>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, et al. Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol. 2002;104(1):105–109. doi: 10.1007/s00401-002-0532-x. [DOI] [PubMed] [Google Scholar]
- 19.Sarela AI, et al. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86(6):886–892. doi: 10.1038/sj.bjc.6600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li XN, et al. Differential expression of survivin splice isoforms in medulloblastomas. Neuropathol Appl Neurobiol. 2007;33(1):67–76. doi: 10.1111/j.1365-2990.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 21.Borriello A, et al. Proliferate and survive: cell division cycle and apoptosis in human neuroblastoma. Haematologica. 2002;87(2):196–214. [PubMed] [Google Scholar]
- 22.Tamm I, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–5320. [PubMed] [Google Scholar]
- 23.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 24.Li F, et al. Pleiotropic cell-division defects and apoptosis induced by interference with survivin function. Nat Cell Biol. 1999;1(8):461–466. doi: 10.1038/70242. [DOI] [PubMed] [Google Scholar]
- 25.Velculescu VE, et al. Analysis of human transcriptomes. Nat Genet. 1999;23(4):387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 26.Mahotka C, et al. Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59(24):6097–6102. [PubMed] [Google Scholar]
- 27.Badran A, et al. Identification of a novel splice variant of the human anti-apoptosis gene survivin. Biochem Biophys Res Commun. 2004;314(3):902–907. doi: 10.1016/j.bbrc.2003.12.178. [DOI] [PubMed] [Google Scholar]
- 28.Caldas H, Honsey LE, Altura RA. Survivin 2alpha: a novel Survivin splice variant expressed in human malignancies. Mol Cancer. 2005;4(1):11. doi: 10.1186/1476-4598-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- 31.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Circ Res. 2008;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 32.Karsan A, et al. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2-dependent and independent mechanisms. Am J Pathol. 1997;151(6):1775–1784. [PMC free article] [PubMed] [Google Scholar]
- 33.Gerber HP, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 34.Tran J, et al. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA. 2002;99(7):4349–4354. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem. 1998;273(21):13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 36.Zwerts F, et al. Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood. 2007;109(11):4742–4752. doi: 10.1182/blood-2006-06-028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma A, et al. The role of survivin in angiogenesis during zebrafish embryonic development. BMC Dev Biol. 2007;7:50. doi: 10.1186/1471-213X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor DS, et al. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156(2):393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanc-Brude OP, et al. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9(7):2683–2692. [PubMed] [Google Scholar]
- 40.Mesri M, et al. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001;158(5):1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das A, Tan WL, Smith DR. Expression of the inhibitor of apoptosis protein survivin in benign meningiomas. Cancer Lett. 2003;193(2):217–223. doi: 10.1016/S0304-3835(02)00741-3. [DOI] [PubMed] [Google Scholar]
- 42.Das A, et al. Expression of survivin in primary glioblastomas. J Cancer Res Clin Oncol. 2002;28(6):302–306. doi: 10.1007/s00432-002-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajiwara Y, et al. Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer. 2003;97(4):1077–1083. doi: 10.1002/cncr.11122. [DOI] [PubMed] [Google Scholar]
- 44.Kayaselcuk F, et al. The expression of survivin and Ki-67 in meningiomas: correlation with grade and clinical outcome. J Neurooncol. 2004;67(1–2):209–214. doi: 10.1023/B:NEON.0000021823.05163.2c. [DOI] [PubMed] [Google Scholar]
- 45.Saito T, et al. Survivin subcellular localization in high-grade astrocytomas: simultaneous expression in both nucleus and cytoplasm is negative prognostic marker. J Neurooncol. 2007;82(2):193–198. doi: 10.1007/s11060-006-9267-1. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarti A, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–1068. doi: 10.1200/JCO.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 47.Katoh M, et al. Survivin in brain tumors: an attractive target for immunotherapy. J Neurooncol. 2003;64(1–2):71–76. doi: 10.1007/BF02700022. [DOI] [PubMed] [Google Scholar]
- 48.Heesters MA, et al. Analysis of proliferation and apoptosis in brain gliomas: prognostic and clinical value. J Neurooncol. 1999;44(3):255–266. doi: 10.1023/A:1006398613605. [DOI] [PubMed] [Google Scholar]
- 49.Ralte AM, et al. Clinicopathological features, MIB-1 labeling index and apoptotic index in recurrent astrocytic tumors. Pathol Oncol Res. 2001;7(4):267–278. doi: 10.1007/BF03032383. [DOI] [PubMed] [Google Scholar]
- 50.Yew DT, Wang HH, Zheng DR. Apoptosis in astrocytomas with different grades of malignancy. Acta Neurochir (Wien) 1998;140(4):341–347. doi: 10.1007/s007010050107. [DOI] [PubMed] [Google Scholar]
- 51.Nakamizo A, et al. Enhanced apoptosis in pilocytic astrocytoma: a comparative study of apoptosis and proliferation in astrocytic tumors. J Neurooncol. 2002;57(2):105–114. doi: 10.1023/A:1015705305540. [DOI] [PubMed] [Google Scholar]
- 52.Jin F, et al. Comparison between cells and cancer stem-like cells isolated from glioblastoma and astrocytoma on expression of anti-apoptotic and multidrug resistance-associated protein genes. Neuroscience. 2008;154(2):541–550. doi: 10.1016/j.neuroscience.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 53.Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 54.Fangusaro JR, et al. Survivin, Survivin-2B, and Survivin-deItaEx3 expression in medulloblastoma: biologic markers of tumour morphology and clinical outcome. Br J Cancer. 2005;92(2):359–365. doi: 10.1038/sj.bjc.6602317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azuhata T, et al. The inhibitor of apoptosis protein survivin is associated with high-risk behavior of neuroblastoma. J Pediatr Surg. 2001;36(12):1785–1791. doi: 10.1053/jpsu.2001.28839. [DOI] [PubMed] [Google Scholar]
- 56.Sohn DM, et al. Expression of survivin and clinical correlation in patients with breast cancer. Biomed Pharmacother. 2006;60(6):289–292. doi: 10.1016/j.biopha.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Ye CP, et al. Relationship between survivin expression and recurrence, and prognosis in hepatocellular carcinoma. World J Gastroenterol. 2007;13(46):6264–6268. doi: 10.3748/wjg.13.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azuhata T, et al. Survivin inhibits apoptosis induced by TRAIL, and the ratio between survivin and TRAIL receptors is predictive of recurrent disease in neuroblastoma. J Pediatr Surg. 2006;41(8):1431–1440. doi: 10.1016/j.jpedsurg.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Sandler A, et al. The survivin:Fas ratio is predictive of recurrent disease in neuroblastoma. J Pediatr Surg. 2002;37(3):507–511. doi: 10.1053/jpsu.2002.30857. [DOI] [PubMed] [Google Scholar]
- 60.Andersen MH, et al. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61(3):869–872. [PubMed] [Google Scholar]
- 61.Charalambous A, et al. Dendritic cell targeting of survivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin. J Immunol. 2006;177(12):8410–8421. doi: 10.4049/jimmunol.177.12.8410. [DOI] [PubMed] [Google Scholar]
- 62.Cho HI, et al. Enhanced induction of anti-tumor immunity in human and mouse by dendritic cells pulsed with recombinant TAT fused human survivin protein. Cancer Lett. 2007;258(2):189–198. doi: 10.1016/j.canlet.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 63.Ciesielski MJ, et al. Antitumor effects of a xenogeneic survivin bone marrow derived dendritic cell vaccine against murine GL261 gliomas. Cancer Immunol Immunother. 2006;55(12):1491–1503. doi: 10.1007/s00262-006-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Idenoue S, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11(4):1474–1482. doi: 10.1158/1078-0432.CCR-03-0817. [DOI] [PubMed] [Google Scholar]
- 65.Reed JC, Wilson DB, et al. Cancer immunotherapy targeting survivin: commentary re: V. Pisarev et al., full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9(17):6523–6533. [PubMed] [Google Scholar]
- 66.Wobser M, et al. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55(10):1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim CH, et al. Enhanced antitumour immunity by combined use of temozolomide and TAT-survivin pulsed dendritic cells in a murine glioma. Immunology. 2007;122(4):615–622. doi: 10.1111/j.1365-2567.2007.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, et al. A novel mimovirus vaccine containing survivin epitope with adjuvant IL-15 induces long-lasting cellular immunity and high antitumor efficiency. Mol Immunol. 2008;45(6):1674–1681. doi: 10.1016/j.molimm.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 69.Pisarev V, et al. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9(17):6523–6533. [PubMed] [Google Scholar]
- 70.Tsuruma T, et al. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2(1):19. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuruma T, et al. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang T, et al. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61(24):8664–8667. [PubMed] [Google Scholar]
- 73.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5(5):1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- 74.Deeg HJ, Huss R. Major histocompatibility complex class II molecules, hemopoiesis and the marrow microenvironment. Bone Marrow Transplant. 1993;12(5):425–430. [PubMed] [Google Scholar]
- 75.Serke S, Huhn D. Expression of class I, II and III epitopes of the CD34 antigen by normal and leukemic hemopoietic cells. Cytometry. 1996;26(2):154–160. doi: 10.1002/(SICI)1097-0320(19960615)26:2<154::AID-CYTO9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 76.Andersen MH, Becker JC, Straten P. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov. 2005;4(5):399–409. doi: 10.1038/nrd1717. [DOI] [PubMed] [Google Scholar]