Abstract
Carcinoembryonic antigen (CEACAM5) is commonly overexpressed in human colon cancer. Several antigenic peptides recognized by cytolytic CD8+ T-cells have been identified and used in colon cancer phase-I vaccination clinical trials. The HLA-A*0201-binding CEA694–702 peptide was recently isolated from acid eluted MHC-I associated peptides from a human colon tumor cell line. However, the immunogenicity of this peptide in humans remains unknown. We found that the peptide CEA694–702 binds weakly to HLA-A*0201 molecules and is ineffective at inducing specific CD8+ T-cell responses in healthy donors. Immunogenic-altered peptide ligands with increased affinity for HLA-A*0201 were identified. Importantly, the elicited cytolytic T lymphocyte (CTL) lines and clones cross-reacted with the wild-type CEA694–702 peptide. Tumor cells expressing CEA were recognized in a peptide and HLA-A*0201 restricted fashion, but high-CEA expression levels appear to be required for CTL recognition. Finally, CEA-specific T-cell precursors could be readily expanded by in vitro stimulation of peripheral blood mononuclear cell (PBMC) from colon cancer patients with altered CEA peptide. However, the CEA-specific CD8+ T-cell clones derived from cancer patients revealed low-functional avidity and impaired tumor-cell recognition. Together, using T-cells to demonstrate the processing and presentation of the peptide CEA694-702, we were able to corroborate its presentation by tumor cells. However, the low avidity of the specific CTLs generated from cancer patients as well as the high-antigen expression levels required for CTL recognition pose serious concerns for the use of CEA694-702 in cancer immunotherapy.
Keywords: CTL, Tumor immunology, Cancer vaccines, Tetramers
Introduction
Carcinoembryonic antigen (CEA), also designated CD66e or CEACAM5, belongs to a heterogeneous protein family that shares common immunoglobulin domains [13]. CEA confers homophilic and heterophilic cell adhesion and may contribute to the metastatic progression of tumors [16]. CEA has also been reported to block cell differentiation and to cooperate with other molecules in cellular transformation [8, 34].
The CEA has been proposed as a potential target antigen for immunotherapy because it is overexpressed by most colorectal, gastric, and pancreatic carcinomas, by 50% of breast cancers, and by 70% of non-small cell lung carcinomas [5, 38]. In contrast, CEA is expressed at lower levels in normal epithelial cells and fetal tissue [23]. Increased levels of soluble CEA can be detected in the serum from most patients with CEA-positive tumors, which allows the use of CEA as surrogate marker for disease progression and response to therapy [12, 20].
Numerous clinical studies have been performed in the last few decades targeting CEA for immunotherapy of colorectal carcinoma. CEA-derived antigens were delivered as recombinant vectors, peptides, or full-length protein (for review see [5, 6]). Overall, variable proportions of patients had positive DTH responses upon vaccination, and although the majority of vaccinated patients have been shown to mount CEA-specific T-cell and antibody responses, clinical results have been disappointing. Moreover, only rarely the CEA-specific cytolytic T lymphocyte (CTL) responses correlated with decreased CEA serum levels or clinical responses. Of note, one study testing immunization with autologous Flt3L-induced dissociation complex (DCs) pulsed with the HLA-A*0201-restricted peptide CEA-CAP1-6D, an analog of CEA605–613 peptide (YLSGANLNL), reported two complete clinical responses out of 12 metastatic colorectal cancer patients [10].
A novel HLA-A*0201-restricted peptide was recently identified in eluted peptides from MHC class-I molecules of a human colon cancer cell line (SW1116) and fresh human colon cancer tissue [33]. From a list of CEA-derived peptides predicted to be potential binders of HLA-A*0201, the authors could only identify one new single peptide derived from CEA, which was the peptide CEA694–702 (GVLVGVALI). Interestingly, both the cell line and the fresh tumor tissue presented the same peptide. This biochemical approach is independent of the preexistence of tumor-specific CD8+ T-cells, commonly used for the identification of novel processed and presented HLA-restricted epitopes [3]. Although the authors demonstrated the processing and presentation of the CEA694–702 peptide, no immunogenicity data have been reported [33].
In this manuscript, we evaluated the antigenicity and immunogenicity of the CEA694–702 peptide. We found that the native CEA694–702 peptide is poorly immunogenic in healthy donors. In addition, this peptide binds weakly to the HLA-*0201 (HLA-A2) molecule. In contrast, analogs with improved binding to HLA-A*0201 molecules were immunogenic. CD8+ T-cell clones cross-reactive with analog and native peptides were of low to intermediate avidity. Moreover, these clones recognized tumor cells with high-CEACAM5 expression, confirming that CEA694–702 peptide is processed by human tumor cell lines and recognized under certain circumstances. Finally, we found that CEA694–702-specific T-cell responses can be induced in peripheral blood mononuclear cell (PBMC) in colon cancer patients. However, in contrast to healthy donor-derived CTL, CD8+ T-cell clones generated from cancer patients had lower avidity and were unable to recognize CEA-expressing tumor cells.
Materials and methods
Synthesis of peptides
Peptides described in Table 2 as well as HIVpol589–597 (IVGAETFYV), FluMA58–66 (GILGFVFTL), and NY-ESO-1157–165 (SLLMWITQA) were synthesized in an automated peptide synthesizer 432A (Applied Biosystems, Weiterstadt, Germany) following the N-(9-fluorenyl)methoxycarbonyl/tert-butanol strategy. Synthesis products were analyzed by HPLC (Varian star; Zinsser, Munich, Germany) and MALDI-TOF mass spectrometry (G2025A; Hewlett-Packard, Waldbronn, Germany). Peptides were purified by preparative HPLC and dissolved in DMSO to a final concentration of 10 mM.
Table 2.
Predicted and experimental peptide binding to HLA-A*0201
| Peptide | BIMASa | SYFPEITHIa | MFI ratio Δ (n = 5)b | RA (n = 4)c | Stability DC50 (h)d |
|---|---|---|---|---|---|
| GVLVGVALI CEA694–702WT | 8 | 23 | 0.92 ± 0.37 | 23.74 ± 14.12 | <1 |
| G L LVGVALI CEA694–702L2 | 89 | 29 | 1.18 ± 0.39 | 3.15 ± 3.82 | 1–2 |
| GLLVGVAL V CEA694–702V9 | 52 | 25 | 1.87 ± 0.39 | 2.23 ± 0.89 | 2–4 |
| G L LVGVAL V CEA694–702L2V9 | 592 | 31 | 1.78 ± 0.19 | 0.98 ± 0.17 | 4–6 |
| GVL AR VALI CEA694AR4 | 8 | 21 | n.d. | n.d. | n.d. |
| GVL AGM ALI CEA694AGM4 | 8 | 19 | n.d. | n.d. | n.d. |
| GV VAL VALI CEA694VAL3 | 8 | 20 | n.d. | n.d. | n.d. |
| IVGAETFYV HIVpol589–597 | 330 | 18 | 3.28 ± 0.61 | – | n.d. |
aEach peptide sequence was entered into the BioInformatics and Molecular Analysis Section (BIMAS [26]) or the SYFPEITHI [30] algorithms. The HLA-A2 binding score output is annotated in the corresponding columns
bValues are the ratio between anti-HLA-A2 mean fluorescence intensity measured on T2-cells loaded with saturating concentrations of peptide in serum-free media and non-loaded T2-cells
cRA-relative HLA-A2 affinity values, which are calculated as described in the Materials and methods section
dStability DC50 is the number of hours required to attain half maximal anti-HLA-A2 fluorescence at the surface of T2-cells at 37°C after removal of peptide from media
Patients
Peripheral blood mononuclear cells of HLA-A*0201 colon cancer patients were obtained after informed consent. The study protocol was approved by the Ludwig Institute for Cancer Research protocol review committee, as well as by the medical and ethical committees of the University Hospital (Lausanne, Switzerland). All patients had primary colorectal adenocarcinomas and had not been enrolled in any adjuvant therapy at the time of blood sampling.
Major histocompatibility complex/peptide multimers and monoclonal antibodies
Phycoerythrin-labeled HLA-A2/peptide multimers [1] were synthesized around CEA694–702 peptide and designed peptide analogs. The cut-off value for detection of antigen-specific CD8+ T-cells with HLA-A2/peptide multimers was determined by flow cytometry analysis of CD8+ T-cells from HLA-A2+ and HLA-A2− human healthy donors stained with the multimers. Monoclonal antibodies (mAbs) were from Becton Dickinson, San Jose, CA, USA. Dead cells, in all situations, were electronically excluded by Propidium Iodine staining.
Cell lines
Human colon cell line SW1116 was the kind gift from H.G. Rammensee (Universitaet Tuebingen, Tuebingen, Germany). Human colon cell lines SW403, SW480, COLO201, SW620, LOVO, HCT-116, T84; gastric cancer cell line KATOIII; and NK-sensitive cell line K562 were bought from ATCC, Molsheim, France. Na8MEL was the kind gift from Francine Jotereau (INSERM, Nantes, France) and Me 290 melanoma cell line was generated from a melanoma patient (LAU 203) by Donata Rimoldi (Ludwig Institute for Cancer Research, Lausanne Branch, Switzerland—[40]). All tumor cell lines were used in CTL assays at exponential phase of cell culture growth and at ∼80% confluence.
Measurement of peptide relative affinity to HLA-A*0201
The protocol used was as previously described [39] and applied elsewhere [2, 3, 9]. Briefly, T2-cells were incubated at 37°C for 16 h with peptides at concentrations ranging from 100 to 0.1 μM, and then stained with BB7.2 mAb [25] to quantify the surface expression of HLA-A*0201. For each peptide concentration, HLA-A2-specific staining was calculated as the % of the staining obtained in the presence of 100 μM of the reference peptide (HIVpol589–597; IVGAETFYV). The RA score is the ratio of the concentration of each peptide to the concentration of the reference peptide that induces 20% of the HLA-A2 expression; the lower the RA is, the stronger the peptide binds to HLA-A2. The mean RA for each peptide was determined from at least three independent experiments. In all experiments, 20% of HLA-A2 expression using the reference peptide was obtained at concentrations of 1–3 μM.
Assessment of peptide/HLA-A*0201 complex stability
As previously described [39], T2-cells were incubated overnight with 100 μM of each peptide at 37°C in serum-free medium. The cells were then incubated with Brefeldin A (Sigma-Aldrich, St. Louis, MO, USA) at 10 μg/ml for 1 h, washed, incubated at 37°C during 0, 1, 2, 4, or 6 h in the presence of Brefeldin A (50 ng/ml) and finally stained with BB7.2 moAb. For each time point, peptide-induced HLA-A2 expression was calculated as: mean fluorescence of peptide-incubated T2-cells—mean fluorescence of T2-cells treated in similar conditions in the absence of peptide. DC50 DC was defined as the time required for the loss of 50% of the HLA-A*0201/peptide complexes stabilized at t = 0 h.
In vitro expansion of circulating antigen specific CD8+ T lymphocytes
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Beckman-Coulter, Fullerton, CA, USA) from the blood of human healthy donors. For generation of autologous dendritic (DC) cells, PBMCs were allowed to adhere into Petri-dishes during 2 h at 37°C. Adherent cells were incubated with 700 IU/ml of Granulocyte-Macrophage Colony Stimulating Factor (R&D Systems, Minneapolis, MN, USA) and 1,000 IU/ml of IL-4 (R&D Systems) in CTL culture medium [RPMI 1640 (Invitrogen, Basel, Switzerland ), 8% pooled human serum, Penicilin/Streptavidin (Invitrogen), 50 μM β-mercaptoethanol (Sigma)]. CD8+ T-cells were purified from non-adherent PBMC by negative selection using immunomagnetic beads (Miltenyi, Bergisch Gladbach, Germany) following the manufacturer’s instructions. For priming, DC were extensively washed and pulsed at least during 2 h with 10 μM of peptide in serum-free media. Pulsed DCs were later maturated with LPS (Sigma) at 5 μg/ml overnight. Co-culture of DCs with autologous CD8+ -purified T-cells was done at 1:5 ratio in CTL culture medium. IL-2 (Proleukin, Roche, Switzerland) at 20 IU/ml was added at day 2 and the culture medium was renewed every other day. At days 7 and 14, CTLs were restimulated with overnight peptide-pulsed (10 μM) T2-cells, which were lethally irradiated. The cultures were evaluated at day 21 for the presence of peptide-specific expanded CD8+ T-cells. In vitro stimulation of PBMC with CEA-derived peptides in microcultures was performed as previously described [21] using freshly isolated PBMCs at 0.2 × 106 cells/well (96 U-bottom plate) (FALCON, Franklin Lakes, NJ, USA) in CTL culture medium in the presence of 10 μM of peptide. IL-2 was added at day 2 at 1,000 IU/ml. The medium was changed every other day and the induction of specific CD8+ T-cells was evaluated at day 10.
Multimer-guided cell sorting and cloning
The expansion of CEA peptide-specific CD8+ T-cells stimulated by co-culture with autologous DC or in the peptide-stimulated PBMC microcultures was evaluated by multicolor flow cytometry of day 21 or 10, respectively, employing peptide-MHC multimers. The cultures were harvested and washed in PBS 0.2% BSA, 50 mM EDTA (Facs buffer). PE-conjugated multimers were incubated at room temperature during 1 h in FACS buffer. After washing, cells were incubated with FITC-conjugated anti-human CD8 mAb at 4°C during 20 min. Cells were immediately acquired in a FACsVantage cell sorter. Multimer+ CD8+ cells were electronically gated and sorted at 4°C. Sorted cells were promptly placed in culture in CTL culture medium with 150 IU/ml of rhIL-2 overnight. The next day, sorted cells were cloned by co-culture in limiting dilution conditions (0.3 cells/well) in NUNCLON plates (NUNC, Roskilde, Denmark) with 104 irradiated allogenic PBMC, 150 IU/ml of IL-2, and 1 μg/ml of phytohemagglutinin-leukoagglutinin (PHA-L) (REMEL, Dartford, UK). Proliferating wells were screened after 7–9 days of culture and expanded to 96 U-bottom plate wells. Cells were then restimulated with irradiated allogenic PBMCs, IL-2, and PHA-L as above. Cultures were replenished with media and split into new plates as required. CTL clones generated were evaluated with multimers for TCR specificity and later for functional antigen-triggered cytolitic activity.
Chromium release-based cytolytic assay
Cytolytic activity was assessed using a 4 h chromium release assay. To evaluate the relative avidity for the antigen ligand of the CTL clones generated upon multimer-guided cell sorting, radiolabeled T2-cells were plated in 96 V-bottom plates and pulsed with a range of peptide concentrations (10−5 to 10−14 M). Cells were incubated with peptide at least 1 h before addition of cloned CTL at an effector to target cell ratio of 10:1. In tumor recognition assays, “hot” targets were co-cultured with T-cell clones at different effector to tumor cell ratios. About 50 IU/ml of IFN-γ (R&D Systems) was added to tumor cells culture medium during 72 h prior to some tumor recognition assays. Na51Cr released in the media was quantified using LumaPlate-96 plates (PerkinElmer, Wellesley, MA, USA) and a TopCount-γ-counter (PerkinElmer).
Reverse-transcription and semi-quantitative PCR
Total RNA was extracted with RNeasy Mini Kit (Qiagen, Hilden, Germany). About 1 μg RNA was then reverse-transcribed to cDNA using M-MLV reverse transcriptase (Invitrogen). The following primers were used for semi-quantitative polymerase chain reaction (PCR): β-actin forward 5′-GGC ATC GTG ATG GAC TCC G-3′ reverse 5′-GCT GGA AGG TGG ACA GCG A-3′; CEACAM3 forward 5′-TTG CCA AAA CTG GAA GAA CC-3′ reverse 5′-AGC CAC TTC TGC TTT GTG GT-3′; CEACAM4 forward 5′CGC ATA CAG TGG TCG AGA GA-3′ reverse 5′-TGG GAC GTT GTT TTG GTG TA-3′; CEACAM5 forward 5′-AAC CCA GAA CCC AGT GAG TG-3′ reverse 5′-ATT GCT GGA AAG TCC CAT TG-3′. Thermocycling conditions for CEACAM3, CEACAM4, and CEACAM5 were the following: 94°C, 15″; 25 × (94°C 15″, 60°C 30″, 72°C 60″), 72°C 10′, 4°C pause. All reactions were performed in a T3 thermocycler (Biometra, Göttingen, Germany). PCR products were visualized by electrophoresis on agarose gel stained with ethidium bromide.
Results
Peptide CEA694–702 is poorly immunogenic and displays low-binding activity to HLA-A*0201 molecules
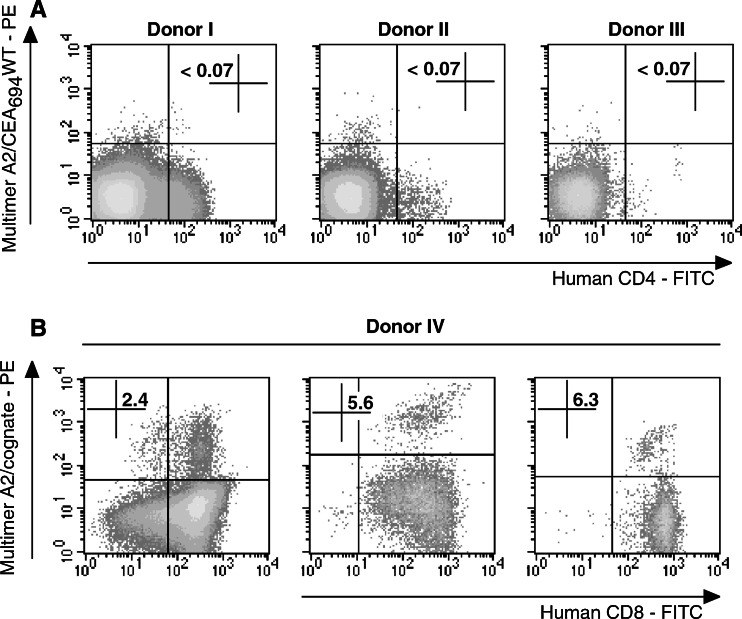
Labeling of PBMC samples from five HLA-A2+ healthy donors with A2/CEA694–702 multimers in conjunction with anti-hCD8 antibody did not detect CEA694–702-specific T-cells (not shown). The threshold of detection could be fixed at 0.07 ± 0.02%—a value comparable to the values determined for other HLA-A2/peptide multimers used in our laboratory [37]. To probe the potential immunogenicity of peptide CEA694–702, we performed multiple in vitro stimulations of PBMCs from HLA-A*0201+ healthy donors and evaluated the presence of expanded peptide-specific CD8+ T-cells by labeling with A2/CEA694–702 multimers and flow cytometry. As demonstrated in Fig. 1a and summarized in Table 1, the peptide CEA694–702 did not generate detectable peptide-specific responses either after stimulation of PBMC with peptide and IL-2, or after multiple stimulations of purified CD8+ T-cells with autologous peptide-pulsed dendritic cells.
Fig. 1.
Immunogenicity of CEA694–702WT and analog peptide in HLA-A*0201 healthy donors. a PBMCs from three HLA-A*0201 healthy donors were stimulated first with peptide-pulsed autologous matured dendritic cells and two consecutive times with irradiated T2-cells pulsed with peptide CEA694–702WT. The presence of peptide-specific CD8+ T-cells was assessed by multiparameter flow cytometry analysis 1 week after the last restimulation on cultured cells labeled with anti-CD4 mAb and A2/CEA694–702 multimers. b Representative results from PBMC of one HLA-A*0201 healthy donor stimulated with peptide CEA694–702L2 (left), CEA694–702V9 (center), and CEA694–702L2V9 (right) independently. Flow cytometry analysis was performed after three rounds of in vitro stimulation with anti-CD8 mAb and the corresponding A2/cognate peptide multimers
Table 1.
Immunogenicity of CEA694–702 and altered peptide ligands
| Peptide | In vitro expansion method | Number of significant expansionsc | Number of cultures tested |
|---|---|---|---|
| CEA694–702WT | Microculturea | 0 | 4 |
| DC basedb | 0 | 5 | |
| CEA694–702L2 | Microculture | 3 | 4 |
| DC based | 2 | 5 | |
| CEA694–702V9 | Microculture | 0 | 4 |
| DC based | 1 | 5 | |
| CEA694–702L2V9 | Microculture | 3 | 4 |
| DC based | 2 | 5 |
All experiments were performed by in vitro stimulation of PBMCs from nine HLA-A*0201 positive healthy donors with the indicated peptides
aIn vitro stimulation was carried out in multiple parallel PBMC microcultures as described in Materials and methods
bIn vitro stimulation was carried out by co-culture of purified CD8+ T-cells and autologous peptide-pulsed DCs and peptide-pulsed T2-cells at a ratio of 5:1
cAfter 10 days of microculture or three rounds of in vitro stimulation (DC based), cells were labeled with anti-CD8 and A2/CEA peptide multimers. A culture was considered as positive when the number of CD8 A2/CEA peptide multimer cells was higher than 0.1% of CD8+ T-cells
A major determinant of peptide immunogenicity is its ability to stably bind to the presenting MHC molecule. Therefore, we determined CEA694–702 peptide’s affinity and stability to HLA-A*0201 molecules using in silico and in vitro methods. As shown in Table 2, the native CEA694–702 peptide was predicted to have relatively low-affinity scores when compared with a known viral epitope (HIVpol589–597, a good binder to HLA-A*0201) [39]. These predictions were confirmed experimentally (Table 2). CEA694–702 presents low capacity to stabilize cell surface HLA-A*0201 on T2-cells: it presented bad relative affinity scores (RA > 10) and formed short lived complexes with HLA-A2 (t < 1 h).
Analogs of peptide CEA694–702 have higher HLA-A*0201-binding capacities
Because of the low affinity of peptide CEA694–702, we designed three peptide analogs incorporating canonical HLA-A*0201 anchor residues (leucine at position 2 and/or valine at position 9). These peptides with sequences GLLVGVALI, GVLVGVALV, and GLLVGVALV will be termed CEA694L2, CEA694V9, and CEA694L2V9, respectively. When the canonical HLA-A*0201 anchor motifs were introduced into the peptide sequence, their binding to HLA-A2 improved substantially as reflected by their enhanced ability to stabilize surface HLA-A2 expression in T2-cells (Table 2). Moreover, CEA694L2, CEA694V9, and CEA694L2V9 analog peptides showed relative affinity values 8–24 times higher than the wild-type peptide. The CEA694L2V9 variant presented the highest affinity. Moreover, the CEA694L2V9 and CEA694V9 and to a lesser degree CEA694L2 peptide analogs substantially increased the stability of surface HLA-A2 expression at 37°C. In summary, CEA694–702 peptide analogs have increased affinity to HLA-A*0201 MHC class-I allele as compared to the wild-type peptide.
CEA694–702 peptide analogs stimulate specific T-cells
A new series of in vitro stimulation with several human HLA-A*0201 healthy donors PBMCs were performed to assess the immunogenicity of the CEA694L2, CEA694V9, and CEA694L2V9 peptide analogs. As depicted in Fig. 1b and summarized in Table 1, the CEA694–702 peptide analogs lead to vigorous expansion of peptide analog-specific CD8+ T-cells, in clear contrast to the native CEA694–702. In particular, CEA694L2 and CEA694L2V9 were the analogs that elicited more frequently detectable responses and independent of the method of in vitro expansion used.
Cloned CD8+ T-cells specific for the CEA694L2 analog efficiently cross-recognize the native CEA694–702 peptide
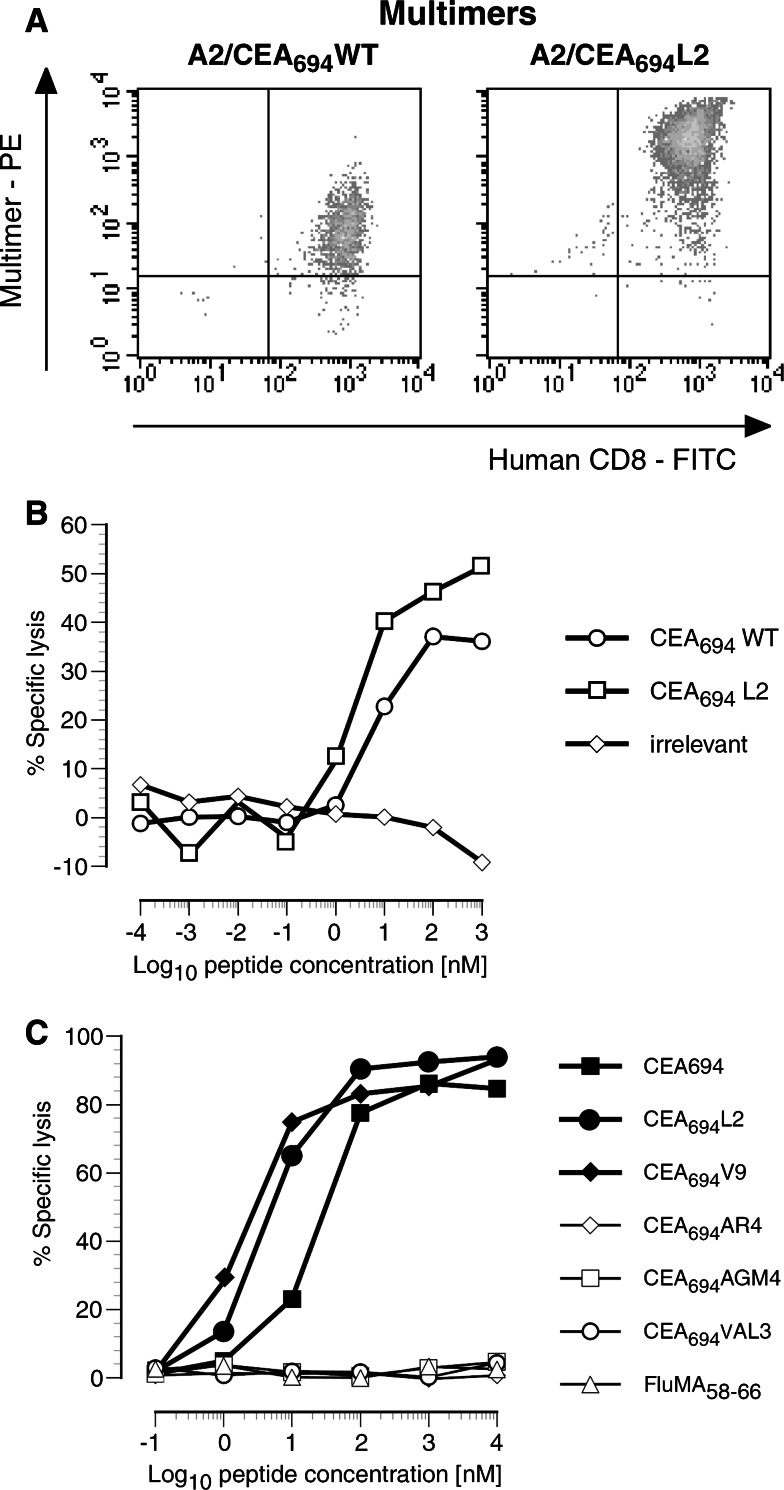
Using multimer-guided cell sorting and limiting dilution techniques, we isolated CD8+ T-cell clones specific for each of the CEA694–702 peptide analogs. Only stable CD8+ T-cell clones were obtained upon sorting with A2/CEA694L2 multimers (n = 11). Other clones isolated with A2/CEA694, A2/CEA694V9, or A2/CEA694L2V9 multimers were not specific or with very limited proliferative capacity and, therefore, detailed characterization was not possible. Thus, all functional assays were conducted using CTL clones obtained with CEA694L2-specific multimers. We first assessed the level of cross-reactivity between native (CEA694–702) and the analog (CEA694L2) peptides by staining the CEA694L2-specific CD8+ T-cell clones with HLA-A2 multimers incorporating either CEA694–702 or CEA694L2 peptides. As shown in Fig. 2a, the clone no. R1, representative of all clones analyzed, is efficiently labeled by the two multimers, albeit the intensity of the fluorescent signal was higher when stained with the A2/CEA694L2 specific multimer. This observation suggests the higher TCR avidity for the peptide analog-MHC complex [45].
Fig. 2.
Fine specificity of CEA analog peptide-specific T-cells. a A2/CEA694–702WT and A2/CEA694–702L2 multimer staining profile of CEA694–702L2 no. R1 CTL clone. b CEA694–702WT and CEA694–702L2 peptide recognition efficiency of the same CTL clone. c Efficiency of recognition of peptide analogs of CEA694–702 derived from CEACAM5 and other family members (CEACAM1—CEA694VAL3, CEACAM3—CEA694V9, CEACAM4—CEA694V9, CEACAM6 CEA694ARV4, CEACAM7—CEA694AGM4, and CEACAM8—CEA694ARV4) by CEA694–702L2 no. R1 CD8+ T-cell clone
We then assessed the level of functional cross-reactivity between the CEA694–702 and CEA694L2 peptides in a chromium release assay. As depicted in Fig. 2b, the representative T-cell clone no. R1 recognized and lysed peptide sensitized HLA-A2+ T2-cells efficiently. Half maximal lysis was observed at analog peptide concentrations ranging between 1 and 10 nM for all the clones tested (n = 11). Recognition of the native peptide was equally efficient. We also extended the analysis of CEA antigen cross-reactivity to naturally occurring CEA694–702 peptides present in other CEA family members. These include GVLVGVALV, which in fact is identical to one of the designed peptide analogs described above—CEA694V9 (CEACAM3 and CEACAM4), GVVALVALI (CEACAM1), GVLARVALI (CEACAM6 and CEACAM8), and GVLAGMALI (CEACAM7). As shown in Fig. 2c, only the GVLVGVALV analog was cross-recognized by the CEA694L2 peptide-specific no. R1 T-cell clone, thus suggesting the potential capacity to recognize CEACAM3-, CEACAM4-, and CEACAM5-derived antigens.
These results show that the clone CEA694–702L2 no. R1 is cross-reactive to native and high HLA-A2 affinity peptide analogs with intermediate to low-functional avidity.
Tumor-cell recognition by CEA-specific CTL clones
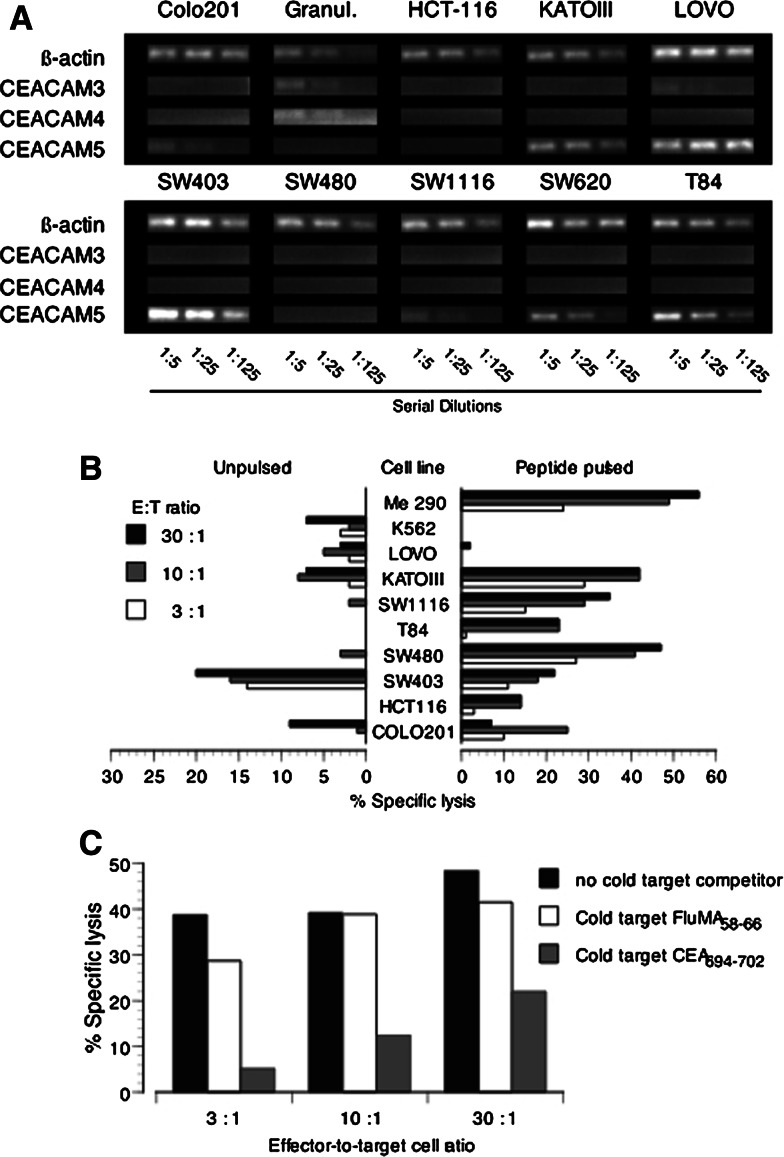
We next assessed the ability of peptide analog-specific cloned CD8+ T-cells to recognize CEA-expressing human tumor cell lines. We first evaluated the expression levels of CEACAM3, CEACAM4, and CEACAM5 in the tumor cells by semi-quantitative RT-PCR. As shown in Fig. 3a, CEACAM3 and CEACAM4 were found to be selectively expressed by granulocytes isolated from human PBMC [18, 22]. None of the epithelial cell lines tested detectably expressed those transcripts by semi-quantitative PCR. In contrast, several epithelial tumor cell lines expressed CEACAM5 mRNA especially SW403, KATOIII, and LOVO. The expression in those cells was higher or equal to β-actin expression levels. CEACAM5 was undetectable in HCT-116 and SW480. Other cell lines such as SW620, COLO201, SW1116, and T84 expressed relatively low levels. Similar results were found when the levels of protein expression were evaluated by flow cytometry with a pan-CEA-specific antibody (data not shown).
Fig. 3.
Naturally processed antigen presentation by CEA positive colorectal carcinoma cell lines. a Semi-quantitative mRNA expression analysis of human tumor cell lines. The transcripts for β-actin, CEACAM3, CEACAM4, and CEACAM5 were amplified by RT-PCR from serially diluted reverse-transcribed total RNA extracted from cell lines. b Recognition of human tumor cell lines by CEA694–702L2 no. R1 CD8+ T-cell clone in a chromium release assay. Pulsed targets were loaded with 1 μg/ml of CEA694–702 peptide. c Peptide-dependent recognition of SW403 colon cancer cell line assessed by cold-target inhibition. The ratio cold : hot was 10:1. Cold targets were T2-cells pulsed with either 1 μg/ml of CEA694–702 peptide or 1 μg/ml of FluMA58–66 peptide
We then assessed the ability of no. R1 T CTL clone to recognize and lyse tumor cell lines. As shown in Fig. 3b, the clone was able to recognize and efficiently kill all HLA-A*0201 tumor cell lines pulsed with the native CEA694–702 peptide. However, in the absence of exogenously added peptide, it only killed the SW403 colon cancer cell line. Close to background lysis levels were observed for KATO III (HLA-A2+), COLO201 (HLA-A2+), and SW620 (HLA-A2+). Importantly, SW1116 (HLA-A2+), the cell line from which the peptide CEA694–702 was eluted, was not recognized. A similar deficiency of recognition and lysis was observed with the T84 and SW480 tumor cell lines. As expected, background levels of lysis were present with the cell lines LOVO (HLA-A2−), Me 290 (HLA-A2 melanoma cell line, CEA−), and K562 (NK sensitive). We further evaluated the specificity of recognition of the SW403 cell line using a cold-target inhibition of cell lysis assay. As depicted in Fig. 3c, the recognition of SW403 by the clone CEA694L2 no. R1 was significantly reduced by the presence of excess cold-targets (T2-cells) pulsed with CEA694–702 native peptide. In contrast, cold-targets pulsed with an irrelevant peptide did not inhibit lysis of SW403, thus confirming the peptide specificity of the observed tumor cell lysis. We also tested the recognition of HLA-A*0201 positive granulocytes by both the T-cell clone and polyclonal CEA694L2-specific CTLs in cytolytic assays. However, no evidence of T-cell recognition could be detected even in the presence of exogenously added native CEA694–702 peptide (data not shown).
Overall, these results show that peptide CEA694–702 can be processed and presented by tumor cells lines expressing HLA-A*0201 and high levels of CEACAM5.
Analysis of CEA694–702-specific CD8+ T-cell responses in colon cancer patients
We next addressed the existence of CEA694–702-specific CD8+ T-cell responses in a group of HLA-A2+ colon cancer patients. To assess the possible existence of high frequencies of CEA694–702-specific CD8+ T–cells, we labeled PBMCs from three HLA-A*0201 colon cancer patients with A2/CEA694–702L2 multimers. As summarized in Table 3, none of the three cancer patients had detectable CEA694L2-specific CD8+ T-cells ex vivo. However, after a single stimulation of PBMCs with peptide CEA694L2, CEA694L2-specific CD8+ T-cells significantly expanded in two of three patients to numbers as high as 3% of the total CD8+ T-cell population. The patient that failed to respond to the stimulation protocol also showed no detectable expansion of FluMA58–66 peptide-specific CD8+ T-cells, suggesting low responsiveness of the T-cells. PBMCs stimulated with the immunogenic HLA-A*0201-restricted peptide NY-ESO-1157–165 did not generate CEA694L2-specific CD8+ T-cells, demonstrating the antigen specificity of the induced T-cells (Fig. 4a).
Table 3.
Immunogenicity of CEA694–702L2 peptide in HLA-A*0201 colon cancer patients
| Peptide | Ex vivoa | In vitrob | ||||
|---|---|---|---|---|---|---|
| LAU 892c | LAU 896d | LAU 919e | LAU 892 | LAU 896 | LAU 919 | |
| Irrelevant | n.d. | n.d. | n.d. | 0.4 | 0.1 | 0.6 |
| FluMA58–66 | <0.07 | <0.07 | n.d. | 0.6 | 6.4 | 76.6 |
| CEA694L2 | <0.07 | <0.07 | <0.07 | 0.2 | 3.2 | 2.4 |
n.d. not done
aEx vivo flow cytometry analysis of CD8+ A2/CEA694L2 multimer+ T-cells in PBMC analysis
bPBMCs from HLA-A*0201 colon cancer patients were stimulated with peptide CEA694–702L2 at 10 μg/ml. rIL-2 at 1,000 IU/ml was added 48 h after stimulation and every other day after that. Anti-CD8 and A2/CEA694L2 multimer analyses were performed at day 10. The figures represent the percentage of CD3+ CD8+ multimer+ events
c57 years old, TMN: pT4 pN1 Mx
d56 years old, TMN: pT4 pN2 pMx
e57 years old, TMN pT4 pN2 pMx
Fig. 4.
Immunogenicity of the CEA694–702L2 peptide analog in HLA-A*0201 colon cancer patients and tumor reactivity of CD8+ T-cell clones. a PBMCs from three HLA-A2 colon cancer patients were stimulated with 10 μg/ml of peptide and cultured for 10 days in 1,000 IU/ml IL-2. FluMA58–66 and NY-ESO-1157–165 peptides were used as controls of peptide-specific CTL induction. Cells were labeled with the indicated A2 multimer and anti-CD8 mAb. Data is summarized in Table 3. b CEA694–702WT and CEA694–702L2 peptide recognition efficiency of the 18 patient-derived CTL clones generated from A2/CEA694–702 multimers-guided cell sorting and limiting dilution. c Tumor-cell recognition of tumor cell lines expressing CEA antigen. Left panel shows CD8+ T-cell functional avidity of polyclonal and clonal populations. Center panel presents histograms of tumor recognition in chromium release assay unpulsed or pulsed with CEA694–702 peptide. Right panel shows CTL recognition of tumor cells unpulsed or pulsed with CEA694–702 peptide but pre-treated for 72 h with IFN-γ
These results reveal the presence of CEA694–702-specific T-cells in the peripheral blood of cancer patients that can be mobilized and expanded using peptide analogs of higher HLA-A*0201 affinity.
We further extended the study of these in vitro responses by CEA694L2 multimer-specific guided cell sorting and establishment of CD8+ T-cell clones by limiting dilution. These clones were then characterized for their cross-recognition of the native peptide. As shown in Fig. 4b, a surprisingly low efficiency of antigen recognition was recorded for the great majority of the T-cell clones evaluated in native peptide titration assays. They attained half maximal lysis with concentrations of native CEA694–702 peptide that were approximately one log higher than those required by CTL clones isolated from PBMC of healthy donors (Fig. 2b). At the same time, these series of T-cell clones obtained from cancer patients displayed a marked high level of recognition efficiency of the CEA694L2 analog peptide. The reduced cross-recognition activity of these CTL clones and their lower lytic efficiency forecasted a reduced or complete abrogation of tumor-cell recognition. Cytolytic assays were performed with multimer-sorted bulk T-cell populations and several T-cell clones of different avidities (Fig. 4c). The results obtained confirmed our expectations on the potential failure of the CTL clones generated from cancer patients, as these CTL were unable to recognize tumor cells expressing CEA antigen even when treated with IFN-γ for 72 h, a treatment commonly used to increase HLA class-I expression [35].
Discussion
We have addressed the potential immunogenicity of a CEA-derived non-apeptide identified 6 years ago as an HLA-A2 binding peptide [33]. The promise of this peptide as a bona fide tumor-associated antigen stemmed from the evidence that the peptide is naturally processed. Indeed, the peptide was found via the biochemical analysis of the pool of peptides eluted from MHC class-I molecules that had been purified from an HLA-A2+ colorectal carcinoma cell line. Our experimental analysis lead to the following conclusions: (1) the native peptide binds weakly to the HLA-A2 molecule and is poorly immunogenic in an in vitro culture system, (2) introduction of the two major anchors for HLA-A2 substantially improves both binding to HLA-A2 and in vitro immunogenicity, (3) peptide analog-specific CTL clones generated from healthy donor lymphocytes can be tumor reactive and display intermediate functional TCR avidity, and (4) peptide analog-specific CTL generated from colorectal carcinoma patients have a significantly lower TCR avidity for cross-recognition of the native CEA694–702 peptide and fail to recognize tumor cells expressing both HLA-A2 and CEA.
In contrast to the so-called direct immunology approach to identify CTL-defined tumor antigens [14, 42], where tumor cell lysis by CTLs already proves that naturally processed antigen is recognized and can trigger CTLs, both reverse immunology [43] and biochemical methods for identification of T-cell epitopes [29, 33] require proof that the hypothetical antigenic candidates are naturally processed and immunogenic. In this regard, for several tumor antigen candidates, contradictory data have been reported concerning peptide immunogenicity, presentation by tumor cells, and tumor cell peptide-specific CTL tumor recognition [2, 4, 7, 19, 27, 31, 32, 41, 44]. Therefore, it is critical for the appropriate validation of tumor-derived CD8+ T-cell epitopes that reproducible data on the antigen processing, immunogenicity, functional performance of induced CTLs, and tumor cell recognition are provided.
From the list of eluted peptides, reported by Shirle et al., the previously unknown peptide CEA694–702 was described [33]. Surprisingly, other previously known CEA HLA-A*0201 CD8+ peptides were not detected, raising doubts concerning the efficiency of their antigen processing. However, peptides may be present at too low numbers, or other reasons may account for lack of peptide identification by biochemical analysis of material obtained with acid elution.
Our findings pose a paradox: that a peptide with low affinity for HLA-A*0201 and forming unstable complexes could be successfully identified from the mixture of MHC class-I eluted peptides, in both a tumor cell line and a tumor tissue fragment [33]. Nevertheless, successful identification of naturally processed peptides with low affinity to HLA-A2 molecules has been reported [36]. It is conceivable that the original eluted fraction from which the peptide was identified was composed of a mix of closely related peptide analogs, which provided fragment signals leading to the CEA694–702 sequence prediction. However, screening for HLA-A*0201-binding natural CEA694–702 analogs in human protein databases consistently provided CEA family-related protein-derived peptides (CEACAM1436–444: GVVALVALI, CEACAM3159–167: GVLGVGALV, CEACAM4159–167: GVLGVGALV, CEACAM5694–702: GVLVGVALI, CEACAM6336–344: GVLARVALI, CEACAM7257–265: GVLAGMALI, and CEACAM8341–349: GVLARVALI). Among these, only the GVLVGVALI and GVLVGVALV peptides were cross-recognized by the CEA694L2 no. R1 clone (Fig. 2c). Other variants were not detectably cross-recognized and are predicted as very weak binders to HLA-A*0201 (Table 2).
Furthermore, the semi-quantitative PCR analysis suggests that CEACAM5 is the likely source of the peptide present in the peptide elution pool, as all colon epithelial tumor cells, including the SW1116 cell line, expressed detectable amounts of the CEACAM5 cDNA. Moreover, CEACAM3 and CEACAM4, which code for cross-recognized peptides with GVLVGVALI, are not expressed by tumor cell lines (Fig. 3a). Alternatively, it can be hypothesized that the off-rate of the low-affinity peptide CEA694 is compensated by a high rate of antigen synthesis, proteolysis, and derived peptide presentation at the cell surface [28, 46]. It is interesting to note that the CEA694-702 epitope is located at the carboxyl terminus of CEACAM5, which ensures its release in the cytoplasm by proteasomal degradation [11].
The expression of CEACAM5 in epithelial tissues may likely result in the induction of both central and peripheral tolerance [17], as has been previously described for other tumor-associated antigens, e.g., p53, gp100 [15, 24]. Our findings are in agreement with the hypothesis that tolerance to CEA antigen may exist in humans. First, the immunogenic CEA peptide analogs display differential CTL induction efficiency, as their relative immunogenicity does not strictly correlate with their relative HLA-A*0201-binding capacity. Second, the peptide-specific CD8+ T-cell clones all had low to intermediate TCR avidities irrespective of whether they had been induced by CEA694L2, CEA694V9, or CEA694L2V9. In addition, clones derived by stimulation with the CEA694V9 and CEA694L2V9 had a very limited proliferation capacity. Moreover, other independent studies with different CEACAM5-derived peptides have also reported low to intermediate TCR avidities [47]. Third, CD8+ T-cell clones generated from colon cancer patients had in general lower avidity toward the CEA694–702 peptide than CD8+ T-cell clones generated from human healthy donors and were unable to recognize CEACAM5 expressing tumor cells even when treated with IFN-γ. These results suggest that tumor-derived CEA may drive in parallel both the expansion of CEA694–702-specific CD8+ T-cells in vivo and the deletion of T-cells with relatively high-TCR avidity. The fact that these patients often present high levels of CEA antigen in their serum might contribute to these processes.
Altogether, the present findings raise serious concerns on the efficacy of immunological tumor surveillance provided by the CEA694–702-specific CD8+ T–cells; particularly because the native epitope is poorly immunogenic, the specific T-cell repertoire induced by peptide analogs shows low avidity in colon cancer patients and fails to recognize allogenic tumor cells expressing CEACAM5.
Acknowledgments
This study was supported in part by Swiss National Science Foundation special program NCCR Molecular Oncology. We would like to acknowledge the technical support of Estelle Devevre and Frederic Grosjean and Nicole Montandon.
Abbreviations
- h
Hour
- HLA-A2
HLA-*0201
- mAb
Monoclonal antibody
- MFI
Mean fluorescence intensity
- n.d.
Not done
- PCR
Polymerase chain reaction
References
- 1.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 2.Alves PM, Faure O, Graff-Dubois S, Cornet S, Bolonakis I, Gross DA, Miconnet I, Chouaib S, Fizazi K, Soria JC, Lemonnier FA, Kosmatopoulos K. Steap, a prostate tumor antigen, is a target of human CD8(+) T cells. Cancer Immunol Immunother. 2006;55:1504–1514. doi: 10.1007/s00262-006-0165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves PM, Faure O, Graff-Dubois S, Gross DA, Cornet S, Chouaib S, Miconnet I, Lemonnier FA, Kosmatopoulos K. Epha2 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Cancer Res. 2003;63:8476–8480. [PubMed] [Google Scholar]
- 4.Ayyoub M, Migliaccio M, Guillaume P, Lienard D, Cerottini JC, Romero P, Levy F, Speiser DE, Valmori D. Lack of tumor recognition by Htert peptide 540–548-specific CD8(+) T cells from melanoma patients reveals inefficient antigen processing. Eur J Immunol. 2001;31:2642–2651. doi: 10.1002/1521-4141(200109)31:9<2642::AID-IMMU2642>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46:33–57. doi: 10.1016/S1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 7.Dupont J, Latouche JB, Ma C, Sadelain M. Artificial antigen-presenting cells transduced with telomerase efficiently expand epitope-specific, human leukocyte antigen-restricted cytotoxic T cells. Cancer Res. 2005;65:5417–5427. doi: 10.1158/0008-5472.CAN-04-2991. [DOI] [PubMed] [Google Scholar]
- 8.Eidelman FJ, Fuks A, DeMarte L, Taheri M, Stanners CP. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differentiation of rat myoblasts. J Cell Biol. 1993;123:467–475. doi: 10.1083/jcb.123.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faure O, Graff-Dubois S, Bretaudeau L, Derre L, Gross DA, Alves PM, Cornet S, Duffour MT, Chouaib S, Miconnet I, Gregoire M, Jotereau F, Lemonnier FA, Abastado JP, Kosmatopoulos K. Inducible Hsp70 as target of anticancer immunotherapy: identification of HLA-A*0201-restricted epitopes. Int J Cancer. 2004;108:863–870. doi: 10.1002/ijc.11653. [DOI] [PubMed] [Google Scholar]
- 10.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/S0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 12.Graham RA, Wang S, Catalano PJ, Haller DG. Postsurgical surveillance of colon cancer: preliminary cost analysis of physician examination, carcinoembryonic antigen testing, chest X-ray, and colonoscopy. Ann Surg. 1998;228:59–63. doi: 10.1097/00000658-199807000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammarstrom S. The carcinoembryonic antigen (Cea) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 14.Herin M, Lemoine C, Weynants P, Vessiere F, Van Pel A, Knuth A, Devos R, Boon T. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer. 1987;39:390–396. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez J, Lee PP, Davis MM, Sherman LA. The use of HLA A2.1/P53 peptide tetramers to visualize the impact of self tolerance on the Tcr repertoire. J Immunol. 2000;164:596–602. doi: 10.4049/jimmunol.164.2.596. [DOI] [PubMed] [Google Scholar]
- 16.Jessup JM, Petrick AT, Toth CA, Ford R, Meterissian S, O’Hara CJ, Steele GJ, Thomas P. Carcinoembryonic antigen: eanhancement of liver colonisation through retention of human colorectal carcinoma cells. Br J Cancer. 1993;67:464–470. doi: 10.1038/bjc.1993.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreuwel HT, Sherman LA. The T-cell repertoire available for recognition of self-antigens. Curr Opin Immunol. 2001;13:639–643. doi: 10.1016/S0952-7915(01)00272-2. [DOI] [PubMed] [Google Scholar]
- 18.Kuroki M, Arakawa F, Matsuo Y, Oikawa S, Misumi Y, Nakazato H, Matsuoka Y. Molecular cloning of nonspecific cross-reacting antigens in human granulocytes. J Biol Chem. 1991;266:11810–11817. [PubMed] [Google Scholar]
- 19.Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G, Cytron S, Lemonnier F, Tzehoval E, Eisenbach L. Human Ctl epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. 2005;65:6435–6442. doi: 10.1158/0008-5472.CAN-05-0133. [DOI] [PubMed] [Google Scholar]
- 20.Marshall J. Carcinoembryonic antigen-based vaccines. Semin Oncol. 2003;30:30–36. doi: 10.1016/S0093-7754(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 21.Montes M, Rufer N, Appay V, Reynard S, Pittet MJ, Speiser DE, Guillaume P, Cerottini JC, Romero P, Leyvraz S. Optimum in vitro expansion of human antigen-specific CD8 T cells for adoptive transfer therapy. Clin Exp Immunol. 2005;142:292–302. doi: 10.1111/j.1365-2249.2005.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel G, Grunert F, Kuijpers TW, Watt SM, Thompson J, Zimmermann W. Genomic organization, splice variants and expression of Cgm1, a CD66-related member of the carcinoembryonic antigen gene family. Eur J Biochem. 1993;214:27–35. doi: 10.1111/j.1432-1033.1993.tb17892.x. [DOI] [PubMed] [Google Scholar]
- 23.Nap M, Mollgard K, Burtin P, Fleuren GJ. Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol. 1988;9:145–153. doi: 10.1159/000217555. [DOI] [PubMed] [Google Scholar]
- 24.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. Gp100/Pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parham P, Brodsky FM. Partial purification and some properties of Bb7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3:277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- 26.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 27.Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the Htert:540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/S1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishna V, Ross MM, Petersson M, Gatlin CC, Lyons CE, Miller CL, Myers HE, McDaniel M, Karns LR, Kiessling R, Parmiani G, Flyer DC. Naturally occurring peptides associated with HLA-A2 in ovarian cancer cell lines identified by mass spectrometry are targets of HLA-A2-restricted cytotoxic T cells. Int Immunol. 2003;15:751–763. doi: 10.1093/intimm/dxg074. [DOI] [PubMed] [Google Scholar]
- 30.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 31.Rodeberg DA, Nuss RA, Elsawa SF, Celis E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin Cancer Res. 2005;11:4545–4552. doi: 10.1158/1078-0432.CCR-04-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, Tourdot S, Chouaib S, Nadler LM, Lemonnier FA, Vonderheide RH, Cardoso AA, Kosmatopoulos K. Her-2/Neu and HTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900–5906. doi: 10.4049/jimmunol.168.11.5900. [DOI] [PubMed] [Google Scholar]
- 33.Schirle MKW, Weber B, Gouttefangeas C, Dumrese T, Becker HD, Stevanovic S, Rammensee HG. Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. Eur J Immunol. 2000;30:2216–2225. doi: 10.1002/1521-4141(2000)30:8<2216::AID-IMMU2216>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997;137:939–952. doi: 10.1083/jcb.137.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw AR, Chan JK, Reid S, Seehafer J. Hla-Dr synthesis induction and expression in HLA-Dr-negative carcinoma cell lines of diverse origins by interferon-gamma but not by interferon-beta. J Natl Cancer Inst. 1985;74:1261–1268. [PubMed] [Google Scholar]
- 36.Skipper JC, Gulden PH, Hendrickson RC, Harthun N, Caldwell JA, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CLJ. Mass-spectrometric evaluation of HLA-A*0201-associated peptides identifies dominant naturally processed forms of Ctl epitopes from Mart-1 and Gp100. Int J Cancer. 1999;82:669–677. doi: 10.1002/(SICI)1097-0215(19990827)82:5<669::AID-IJC9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Speiser DE, Pittet MJ, Guillaume P, Lubenow N, Hoffman E, Cerottini JC, Romero P. Ex vivo analysis of human antigen-specific CD8(+) T-cell responses: quality assessment of fluorescent HLA-A2 multimer and interferon-gamma elispot assays for patient immune monitoring. J Immunother. 2004;27:298–308. doi: 10.1097/00002371-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 39.Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, Lemonnier FA, Kosmatopoulos K. A general strategy to enhance immunogenicity of low-affinity HLA-A2. 1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411–3421. doi: 10.1002/1521-4141(2000012)30:12<3411::AID-IMMU3411>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/Mart-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 41.Valmori D, Gileadi U, Servis C, Dunbar PR, Cerottini JC, Romero P, Cerundolo V, Levy F. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen Mage-3. J Exp Med. 1999;189:895–906. doi: 10.1084/jem.189.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Eynde B, Hainaut P, Herin M, Knuth A, Lemoine C, Weynants P, van der Bruggen P, Fauchet R, Boon T. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int J Cancer. 1989;44:634–640. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- 43.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Van Den Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065X.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 44.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 45.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive Ctl from heterogeneous populations using peptide-Mhc tetramers. J Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 46.Yewdell JW. Not such a dismal science: the economics of protein synthesis, folding, degradation and antigen processing. Trends Cell Biol. 2001;11:294–297. doi: 10.1016/S0962-8924(01)02030-X. [DOI] [PubMed] [Google Scholar]
- 47.Zhu MZ, Marshall J, Cole D, Schlom J, Tsang KY. Specific cytolytic T-cell responses to human Cea from patients immunized with recombinant Avipox-Cea vaccine. Clin Cancer Res. 2000;6:24–33. [PubMed] [Google Scholar]