Abstract
Docetaxel has demonstrated therapeutic efficacy against breast, prostate, and ovarian cancer and other solid tumors. The tumoricidal activity of docetaxel is mainly attributed to its ability to block microtubule depolymerization, thus inducing G2-M arrest and apoptosis. Mounting evidence indicates that docetaxel also possesses immunomodulatory activity such as augmenting macrophage and lymphokine activated killer activity and inducing pro-inflammatory cytokines, suggesting that docetaxel may be a good chemotherapeutic agent to combine with cancer immunotherapies, assuming that it does not inhibit the vaccine-induced immune response. The anti-tumor activity of the combination of docetaxel and a GM-CSF-secreting B16F10 tumor cell vaccine (B16.GM) was evaluated in the murine B16 melanoma model. Dose levels of docetaxel and the B16.GM vaccine known to be ineffective when used as single agents were selected. Three iv treatments of 6 mg/kg docetaxel per injection given on days 5, 9, and 13 after tumor challenge or a single vaccination with 2–3×106 B16.GM cells on day 3 were ineffective at inhibiting tumor growth when used as single agents [median survival time (MST)=24 days in both treatment groups and in control animals]. However, combination of docetaxel and B16.GM vaccine significantly delayed tumor growth, increasing MST to 45 days. A similar improvement in anti-tumor efficacy was observed using multiple treatment cycles of the B16.GM vaccine/docetaxel combination. Administration of docetaxel every 4 days between bi-weekly B16.GM vaccinations increased the median survival of tumor-bearing mice from 31 to 52 days compared to multiple B16.GM vaccinations alone. In summary, these data demonstrate that rather than inhibiting the anti-tumor effects of a GM-CSF-secreting tumor cell vaccine, docetaxel combined with a whole cell vaccine significantly inhibits tumor growth, increases survival time and does not impede T-cell activation in the murine B16F10 melanoma tumor model. GM-secreting tumor cell vaccines in combination with docetaxel may represent a new strategy for combining chemo and immunotherapy for cancer.
Keywords: GM-CSF, Docetaxel, Cancer vaccine, Chemo-immunotherapy
Introduction
Conventional chemotherapy has demonstrated clinical benefit against a wide variety of human cancers. However, administration of many types of chemotherapeutic agents is often associated with significant toxicity in normal tissues. Docetaxel (Taxotere®, Bridgewater, NJ, USA), a semisynthetic analogue of paclitaxel, is used for the treatment of a number of human malignancies including ovarian, metastatic breast, prostate, and non-small cell lung cancer [1–5]. Its primary mechanism of action is via increasing tubulin polymerization and microtubule assembly and inhibiting tubulin depolymerization, resulting in cell cycle arrest at the G2/M interface [6–9]. Docetaxel also sensitizes tumors to radiation therapy [10–13], and recent evidence suggests that docetaxel possesses immunomodulatory properties, including the induction of macrophage-mediated tumor killing, stimulation of the production of pro-inflammatory cytokines (TNFα, IL-12, and IL-1), and increasing lymphokine activated killer (LAK) cell and NK cell anti-tumor activity [13–16]. However, the dose-limiting toxicity of docetaxel in humans is severe neutropenia following administration at a dose of 75 mg/m2 every 3 weeks.
Cytokine-secreting tumor cell vaccines are known to elicit systemic tumor-specific immune responses in mouse tumor models that protect against tumor challenge. In early studies, the capacity of different cytokines to enhance the immunogenicity of tumor cells was evaluated in the B16 melanoma model by transducing B16 cells with retroviral vectors expressing individual cytokines, including IL-2, IL-4, IL-5, IL-6, TNFα, and GM-CSF, irradiating the cells and using them as cellular vaccines [17]. These studies demonstrated that murine B16 melanoma tumor cells modified to secrete murine GM-CSF stimulated a potent, long-lasting and specific anti-tumor immune response that required both CD4+ and CD8+ T-cells. GM-CSF-secreting cellular vaccines (termed GVAX®, Cell Genesys Inc., CA, USA) have been evaluated in the clinic in melanoma, non-small cell lung carcinoma, pancreatic cancer, myeloma, leukemia and prostate cancer [18–24]. Currently, a randomized phase 3 clinical trial in metastatic hormone-refractory prostate cancer patients is comparing GVAX® prostate cancer vaccine to docetaxel chemotherapy. A second phase 3 trial compares the vaccine plus docetaxel to docetaxel alone.
The studies reported here were performed to evaluate whether administration of docetaxel at clinically relevant doses would negatively impact antigen-specific T cell activation and/or anti-tumor activity following immunization with a GM-CSF-secreting tumor cell vaccine. The results indicate that administration of docetaxel either prior to or after vaccination does not inhibit antigen-specific T cell responses, but enhances the anti-tumor efficacy of the vaccine resulting in prolonged survival even at docetaxel dose levels that induce neutropenia. These findings provide a rationale for testing docetaxel chemotherapy in combination with GM-CSF-secreting tumor cell vaccines in patients with cancer.
Materials and methods
Mice
Male C57Bl/6 mice and female OT-1 TCR transgenic (OT-1 TCR Tg) mice were purchased from Taconic (Oxnard, CA, USA) and The Jackson Laboratory (Bar Harbor, ME, USA), respectively, and maintained according to institutional and National Institute of Health guidelines. All mice were acclimated in the Cell Genesys Inc. (CGI, CA, USA) animal facility at least 1 week and used between 8 and 12 weeks of age. Water and food were provided ad libitum. Animal studies were approved by the CGI Animal Care and Use Committee and were conducted according to CGI Animal Care Guidelines and the Guide for the Care and Use of Laboratory Animals.
Cell lines, culture media, and reagents
The B16F10 melanoma and the retrovirally transduced GM-CSF-secreting B16.GM cell lines were previously described [17]. The latter generates 150 ng/106 cells/day of mouse GM-CSF. Cells were maintained in Dulbecco’s Minimum Essential Medium (DMEM; Hyclone, VT, USA) supplemented with 10% heat inactivated FBS (Hyclone), 2 mM L-glutamine, and 1X penicillin/streptomycin (JRH) in a humidified incubator with 5% CO2 at 37°C. The Kb binding peptide derived from ovalbumin (OVA) (SIINFEKL), Trp2 (SVYDFFVWL) or gp100 (TWGKYWQV) was purchased from Genmed Synthesis Inc. (South San Francisco, CA, USA). Cy-chrome anti-CD8, was obtained from BD Pharmingen (San Diego, CA, USA), and PE-conjugated Kb-ova tetramer was purchased from Immunomics (San Diego, CA, USA). Clinical-grade docetaxel (Taxotere; Aventis) was purchased from Cardinal Health (Dublin, OH, USA) as a single dose vial for injection. Docetaxel was freshly prepared on the day of injection according to the manufacturer’s instructions.
Generation of ovalbumin-expressing B16F10 tumor cells
B16F10 and the B16.GM tumor cells that express the CD16 OVA LAMP-1 fusion protein [25] were produced (B16-OVA, B16.GM-OVA). Using standard procedures, the OVA coding sequence was excised and cloned into a third generation lentiviral transfer vector. The chimeric OVA protein was expressed from an internal CAG promoter [26]. The third generation lentiviral vector system has previously been described [27]. Briefly, these vectors contain a 5′chimeric RSV/LTR promoter, cPPT [28], WPRE [29], and SIN LTR [30]. Vectors were generated by transient transfection into 293T cells as described previously [27]. B16F10 or B16.GM cells were transduced at an MOI of approximately 300. The resulting population was stained using an OVA-specific antibody conjugated to biotin (United States Biological, Swampscott, MA, USA), detected with a streptavidin-conjugated PE secondary antibody (BD Pharmingen), and sorted for OVA expression using a MoFlo (Cytomation Inc., Fort Collins, CO, USA).
In vivo B16F10 tumor model
Male C57Bl6 mice were inoculated (dorsal s.c.) with 1×105 B16F10 tumor cells. Three days later, mice were vaccinated either once (day 3) or three times (days 3, 17, 31) with 2–3×106 irradiated GM-CSF-secreting B16F10 (B16.GM) cells. Docetaxel was injected intravenously using several different dosing regimens. In a low-dose regimen, docetaxel was administered as three iv injections of 6 mg/kg each given at 3- or 4-day intervals beginning 2 days after the first vaccine only or after each vaccination. Alternatively, in a high-dose regimen, docetaxel was given as a single iv injection of 18 mg/kg 2 days prior or 2 days after the first vaccination and repeated either 2 days before or 2 days after each subsequent vaccination. Animals were monitored for the formation of palpable tumors twice weekly and euthanized if tumors became necrotic or exceeded 150 mm2 in size.
Hematologic and phenotypic analysis
Mice were injected intravenously with docetaxel using various dosing regimens as described above. Peripheral blood was collected by retro-orbital puncture into EDTA-coated capillary tubes on days 2, 4, 7, 10, and 14. Hematologic analysis was performed by IDEXX Preclinical Research Services (West Sacramento, CA, USA). Spleens were removed, processed into single cell suspensions, labeled with anti-CD4, anti-CD8, anti-B220, anti-NK1.1, anti-CD11b, and anti-CD11c (BD Pharmingen) and analyzed by flow cytometry.
OT-1 adoptive transfer tumor model
Spleen and lymph node cells (inguinal, axillary, lateral axillary, mesenteric, mandibular, and iliac) from OT-1 mice were homogenized, treated with ACK lysis buffer to remove RBC and washed three times in phenol red free HBSS. Lymphocytes, containing 1–2×106 CD8+/tetramer+ T cells, specific for the OVA peptide SIINFEKL, were transferred into C57Bl/6 mice by tail vein injection. Two days later, recipients were challenged s.c. with 1×105 B16-OVA cells (day 0). On day 3, mice were vaccinated s.c. with 1×106 irradiated cells. Docetaxel (6 mg/kg) was injected iv in a volume of 100 μl 5 and 9 days after tumor inoculation or a single iv injection of docetaxel (18 mg/kg) was administered 5 days after tumor inoculation. Spleen- and tumor-draining lymph nodes (axillary, lateral axillary) were isolated on the days indicated in the result section and the figure legends, stained with Kb-OVA tetramers and assayed via flow cytometry to determine the percentage and number of antigen-specific (CD8+OT-1+) T cells.
Results
Effect of docetaxel treatment on hematology
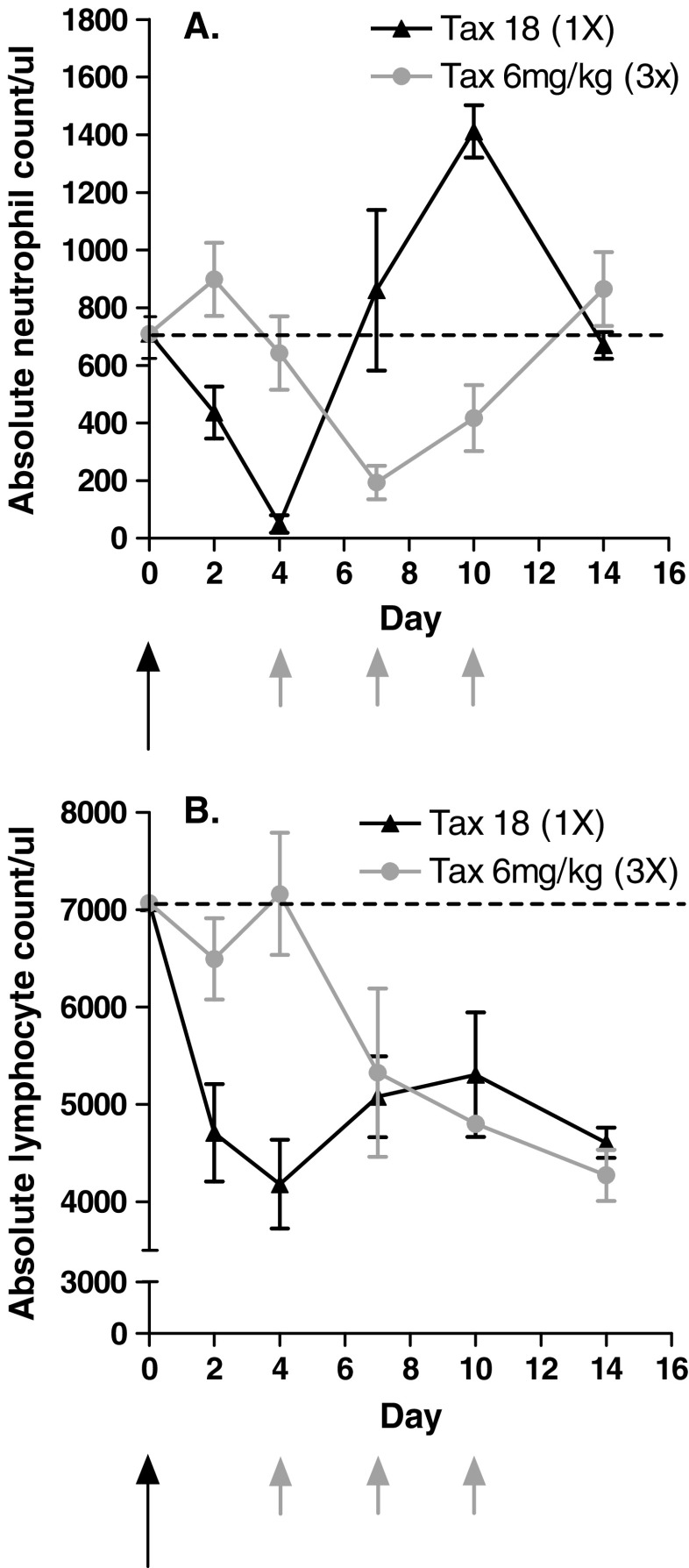
Prior to conducting the tumor model studies described below, the hematological toxicity of docetaxel at the selected dose levels was determined. Docetaxel-treated mice were monitored for neutropenia, the primary dose-limiting toxicity observed in patients, and lymphopenia. After a single injection of docetaxel (18 mg/kg), the absolute neutrophil count dropped to 50 neutrophils/μL by day 4, rebounded to control levels (710 neutrophils/μL) by day 7 and increased twofold (1,400 neutrophils/μL) on day 10. Neutrophil count returned to pretreatment levels by day 14 (Fig. 1a). Treatment with three doses of 6 mg/kg docetaxel given once every 3 days had a similar, but somewhat less marked effect on neutrophil counts (Fig. 1a, b). Interestingly, the absolute neutrophil count returned to pretreatment levels by day 14 despite the fact that two additional injections of docetaxel were given on day 7 and 10. Similar effects on lymphocyte count were seen, which dropped by greater than 40% 4 days after a single dose of 18 mg/kg docetaxel and remained significantly lower than in untreated animals up to day 14 (Fig. 1b). In animals that received three injections of 6 mg/kg docetaxel as described above, a significant drop in lymphocyte count was evident 3 days after the first treatment. The overall magnitude of lymphopenia observed on day 14 was similar for both dose regimens. These two dosing regimens were utilized in the tumor model studies described below to evaluate if docetaxel treatment would inhibit the anti-tumor efficacy of the B16.GM vaccine.
Fig. 1.
Docetaxel induces changes in CBC counts following two different treatment regimens. Mice were treated with low-dose docetaxel (three single injections of 6 mg/kg given once every 4 days; grey arrows) or high-dose docetaxel (a single injection of 18 mg/kg; black arrow). Peripheral blood was collected on the indicated days by retro-orbital puncture into EDTA-coated capillary tubes and analyzed for the presence of a neutrophils and b lymphocytes. Data are expressed as mean ± SD of four mice per group per timepoint. The horizontal line represents the absolute count/μl in age-matched naïve mice
Docetaxel does not impair the efficacy of a GM-CSF-secreting tumor cell vaccine
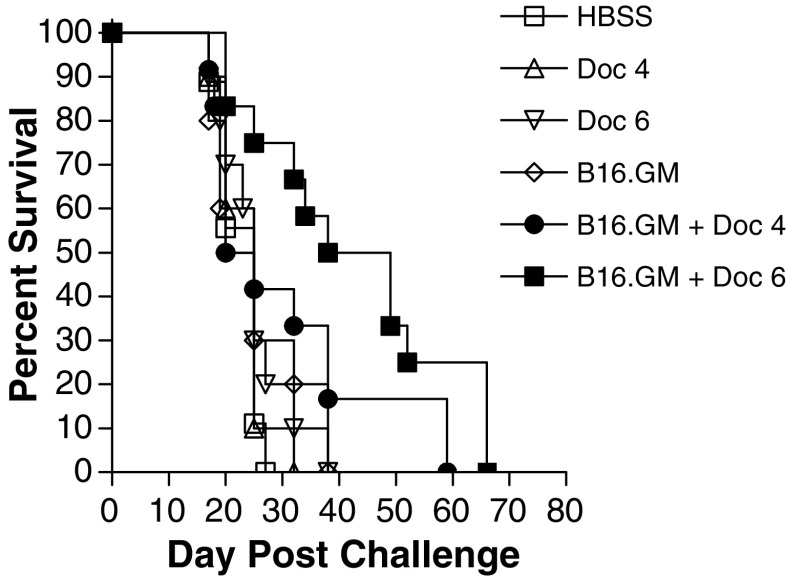
Mice bearing subcutaneous B16F10 tumors implanted on day 0 were treated with the B16.GM vaccine on day 3 and then received intravenous docetaxel on days 5, 9, and 13 to determine the effect of combination with docetaxel on the anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine. The dose levels of the GM-CSF secreting tumor cell vaccine (2×106 B16.GM cells) and docetaxel (4 or 6 mg/kg/treatment) were selected to provide minimal, if any, anti-tumor activity in the B16F10 tumor model when used as monotherapies to permit unambiguous detection of potential additive and/or synergistic effects of the two therapies. Both dose levels of docetaxel were ineffective when used alone (Fig. 2), with a MST of 25 days in both groups, identical to the MST in HBSS-treated control animals (Table 1). Similarly, a single vaccination with B16.GM tumor cells alone conferred no survival benefit, nor did the combination of the vaccine and 4 mg/kg docetaxel (MST=22.5 days in both treatment groups). In contrast, when B16.GM vaccination was followed by treatment with 6 mg/kg docetaxel, MST was significantly prolonged to 43.5 days demonstrating that docetaxel combination therapy enhances rather than inhibits the anti-tumor activity of the vaccine.
Fig. 2.
Docetaxel enhances the therapeutic efficacy following a single injection of a GM-CSF-secreting tumor cell vaccine. C57Bl/6 mice (n=10/group) were challenged with 1×105 B16F10 tumor cells, vaccinated with 2×106 irradiated B16.GM cells on day 3 and injected (iv) with 4 or 6 mg/kg of docetaxel on day 5, 9, and 13 relative to tumor challenge. Mice were monitored twice weekly for the development of subcutaneous tumors. Mice were sacrificed if tumors became necrotic or once tumor size exceeded 150 mm2. Data are presented as Kaplan–Meier survival curves indicating the percentage of surviving mice as a function of time after tumor challenge. The survival of mice treated with the combination of B16.GM and docetaxel (6 mg/kg) is significantly different when compared to B16.GM only (p<0.004) and docetaxel only (p<0.003) Results are comparable to three independent experiments
Table 1.
Median survival time (MST) of mice treated with a single administration regimena
| Treatment | MST (days) |
|---|---|
| HBSS | 25 |
| Docetaxel (4 mg/kg) | 25 |
| Docetaxel (6 mg/kg) | 25 |
| B16.GM | 22.5 |
| B16.GM + Doc 4 | 22.5 |
| B16.GM + Doc 6 | 43.5 |
aMale C57Bl6 mice were inoculated with 1×105 B16F10 tumor cells on day 0. Three days later, mice were vaccinated with 2×106 irradiated GM-CSF-secreting B16F10 (B16.GM) cells. Docetaxel (4 or 6 mg/kg) was injected iv on days 5, 9, and 13. Tumor growth was monitored twice weekly
Multiple treatments with docetaxel do not impair the efficacy of a GM-CSF-secreting tumor cell vaccine
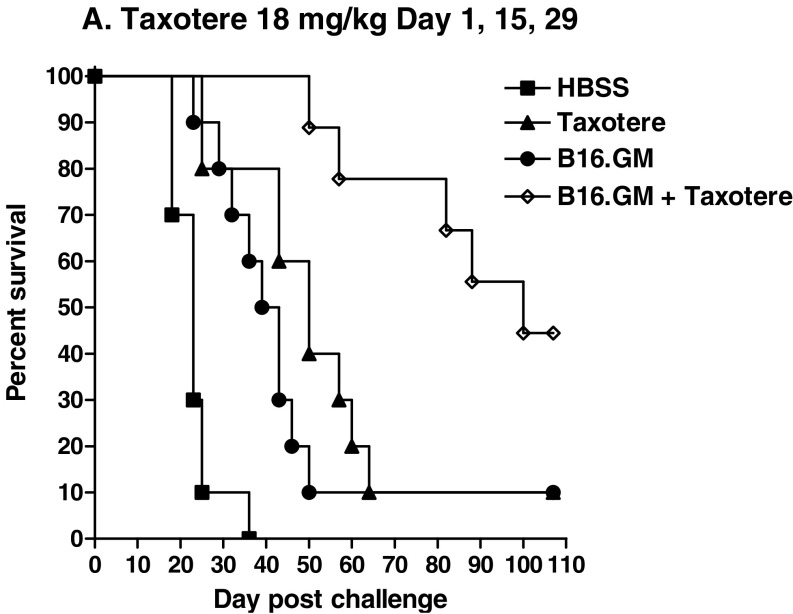
Since the clinical treatment regimen of GM-CSF-secreting tumor cell vaccines involves multiple vaccine administrations, typically given bi-weekly, the effect on survival of repeated treatments with docetaxel interspersed in a bi-weekly vaccination regimen was evaluated. Mice were vaccinated three times (days 3, 17, 31 after tumor inoculation) with irradiated B16.GM cells, and cycles of treatment with 6 mg/kg docetaxel were initiated 2 days after each vaccination; a “cycle” of docetaxel treatment was defined as treatment 2, 6, and 10 days after each vaccination. Although multiple vaccinations and multiple treatments with docetaxel both significantly increased MST compared to HBSS-treated control mice (MST=41, 37.5, and 23 days, respectively), the combination of the B16.GM vaccine and docetaxel, each given multiple times, increased MST still further, to 60 days (Table 2), and 30% of the mice treated with the combination remained tumor-free for more than 100 days. No tumor-free mice were seen in the other treatment groups. Combination of repeated B16.GM vaccinations with a more clinically relevant dose level of docetaxel (18 mg/kg, equivalent to approximately 60 mg/m2), given as a single dose 2 days after each vaccination with B16.GM cells, was significantly more effective than either the vaccine or docetaxel given as single agents (combination versus B16.GM alone, p=0.04; combination versus docetaxel alone, p=0.0005). MST in the combination therapy group was 65.5 days, compared to 34 and 41 days in single agent docetaxel and vaccine groups, respectively; MST in the control groups was 23 days (Fig. 3a, b).
Table 2.
MST of mice treated with multiple administration regimena
| Treatment | MST (days) |
|---|---|
| HBSS | 23 |
| Docetaxel (6 mg/kg)×9 | 37.5 |
| Docetaxel (18 mg/kg)×3 | 34 |
| B16.GM | 41 |
| B16.GM + Doc 6×9 | 60 |
| B16.GM + Doc 18×3 | 65.5 |
aMale C57Bl6 mice were inoculated with 1×105 B16F10 tumor cells on day 0. Mice were vaccinated three times (day 3, 17, 31) with 2×106 irradiated B16.GM cells. Docetaxel was injected intravenously using various dosing regimens. Low-dose docetaxel, consisting of three injections of 6 mg/kg. Every 4 days, was given after each vaccination. Alternatively, high dose docetaxel, consisting of a single injection of 18 mg/kg was initiated 2 days after the first vaccine and repeated for each subsequent vaccine
Fig. 3.
Docetaxel enhances the efficacy of a GM-CSF-secreting tumor cell vaccine in a multiple administration treatment regimen. C57Bl/6 mice (n=10/group) were challenged with 1×105 B16F10 tumor cells, vaccinated with 2×106 irradiated B16.GM cells on days 3, 17, and 31. Mice were then treated with a low-dose docetaxel (6 mg/kg) 2, 6, and 10 days after each vaccine or b high-dose docetaxel (18 mg/kg) 2 days after each vaccine. Mice were monitored twice weekly for the development of subcutaneous tumors. Mice were sacrificed if tumors became necrotic or once tumor size exceeded 150 mm2. Data are presented as Kaplan–Meier survival curves indicating the percentage of surviving mice as a function of time after tumor challenge. The survival of mice treated with the combination of a low dose docetaxel and B16.GM is significantly improved when compared to each monotherapy (B16.GM versus combination p=0.016; docetaxel versus combination p=0.004). The survival is also significantly different in the high dose docetaxel (B16.GM versus combination p=0.04; docetaxel versus combination p=0.0005)
Order of administration does not alter the potency of a GM-CSF-secreting tumor cell vaccine
The effect of order of administration of the vaccine and docetaxel at the beginning of therapy was evaluated. In the study described above, 18 mg/kg docetaxel administered 2 days after each of three B16.GM vaccinations resulted in an MST of 65.5 days. In this study, 18 mg/kg docetaxel was administered 2 days prior to the first vaccination and repeated 2 days prior to each of two additional vaccinations. With this treatment regimen, docetaxel alone significantly delayed tumor growth compared to control animals (p<0.0001; MST=50 vs. 23 days, respectively; Fig. 4), and tumor-free survival was 10% by 80 days after tumor inoculation. Vaccine treatment alone had a similar effect on MST (41 days), and overall tumor-free survival at days 80 was also 10%. Combination therapy (docetaxel given 2 days prior to each vaccination) significantly (p<0.006) increased both MST (100 days) and overall tumor-free survival (50% at day 80) compared to either agent as a monotherapy.
Fig. 4.
Pre-vaccine administration of docetaxel increases the efficacy of a GM-CSF-secreting tumor cell vaccine in a multiple administration treatment regimen. C57Bl/6 mice (n=10/group) were challenged with 1×105 B16F10 tumor cells. Docetaxel treatment (18 mg/kg) was initiated 2 days prior to each vaccination (days 3, 17, 31). Mice were monitored twice weekly for the development of subcutaneous tumors. Mice were sacrificed if tumors became necrotic or once tumor size exceeded 150 mm2. Data are presented as Kaplan–Meier survival curves indicating the percentage of surviving mice as a function of time after tumor challenge. Survival of mice treated with the combination is significantly increased when compared to each monotherapy (p<0.006)
Docetaxel does not inhibit the expansion and/or survival of antigen-specific T cells
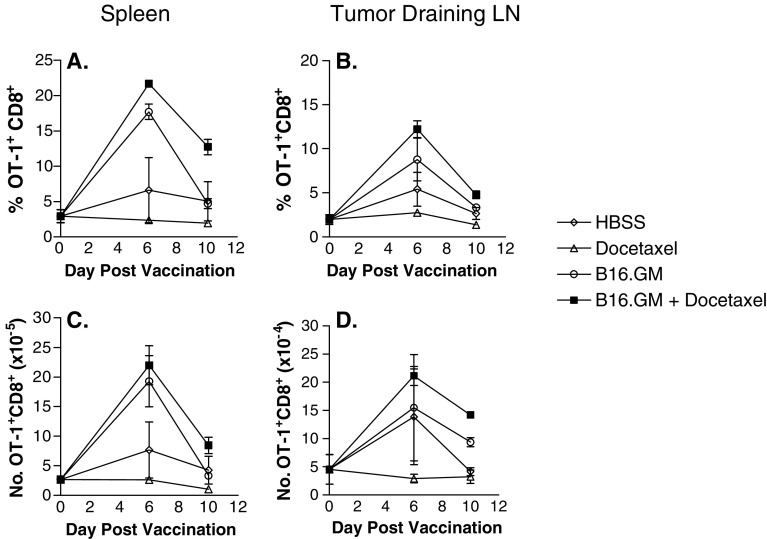
The combination of a chemotherapeutic agent such as docetaxel with a vaccine like B16.GM could result in chemotherapy-related inhibition of the activation or premature death of immune cells that are stimulated to divide rapidly by the vaccine. A series of studies were conducted to determine whether docetaxel could inhibit antigen (Ag)-specific T cell activation after vaccination with a GM-CSF-secreting tumor cell vaccine. An adoptive T cell transfer system was developed in which OVA is expressed on the surface of the tumor cells (B16.OVA) to serve as a surrogate tumor-associated antigen. Matching, i.e., OVA-expressing, B16.GM vaccine cells (B16.GM-OVA) were also developed. Ova-specific T-cell receptor transgenic (TCR) precursor frequencies of 2–3% were detected in the spleen and lymph nodes of mice that were adoptively transferred with approximately 1.5×106 OVA-specific T cells only at day 0, and their frequency was unchanged in mice bearing B16-OVA tumors that were treated with either HBSS or docetaxel (Fig. 5a, c). In contrast, a large increase in the fraction of OT-1-specific T cells was seen in the spleens of mice 6 days after vaccination with B16.GM-OVA cells compared to control mice (18% vs. 6% of CD8+ T cells) (Fig. 5a), and a slightly greater, but not significant, expansion was detected in mice treated with the combination of B16.GM-OVA vaccine and docetaxel (22%). By day 10 post-vaccination, the percentage of Ova-specific T cells in mice vaccinated with B16.GM-OVA returned to control levels, whereas the percentage of ova-specific T cells remained slightly elevated in mice treated with the vaccine plus docetaxel (13% vs. 5% in the vaccine-only treatment group). Similar effects on the expansion and survival of ova-specific T cells were also observed in tumor-draining lymph nodes (Fig. 5b, d). Furthermore, treatment with a single dose of 18 mg/kg docetaxel 2 days after the B16.GM-OVA vaccination did not alter the expansion and survival of ova-specific T-cells when compared to B16.GM-OVA alone (data not shown). Thus, a dose regimen of docetaxel that induces moderate levels of lymphopenia does not inhibit the activation or survival of antigen-specific T cells (Table 3).
Fig. 5.
Docetaxel does not inhibit the expansion and/or survival of antigen-specific T cells. Mice were adoptively transferred with lymphocytes from OT-1 TCR transgenic mice containing 1–2×106 CD8+/tetramer+ T cells. Two days later recipients were challenged s.c. with 1×105 B16-OVA cells (day 0). On day 3, mice were vaccinated s.c. with 1×106 irradiated B16.GM-OVA cells. Docetaxel (6 mg/kg) was injected iv in a volume of 100 μl 5 and 9 days after tumor inoculation. The percentage (a) and (b) and total number (c) and (d) of spleen (a) and (c) and tumor draining lymph nodes cells (b) and (d) that express CD8+/tetramer+ phenotype were analyzed by flow cytometry. Data represent mean ± SD of three mice per group. Results are comparable to three independent experiments
Table 3.
Median survival of mice treated with docetaxel 2 days prior to each B16.GM vaccine
| Treatment | Median survival (days) |
|---|---|
| HBSS | 23 |
| Docetaxel 18 mg/kg | 50 |
| B16.GM | 41 |
| B16.GM + Doc 18 mg/kg | 100 |
aMale C57Bl6 mice were challenged with 1×105 B16F10 tumor cells. Mice were vaccinated three times (day 3, 17, 31) with 2×106 irradiated B16.GM cells. Docetaxel, consisting of a single injection of 18 mg/kg was initiated 2 days prior to the first vaccine and repeated for each subsequent vaccine
Discussion
The studies described here were designed to address the question of whether combination therapy with docetaxel had the potential to inhibit the anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine via impairment of the vaccine-induced immune response. Combination therapy of chemotherapy and a cancer vaccine has the potential to induce the anti-tumor immune response in a setting of minimal residual disease resulting from chemotherapy-related cytotoxicity, but only if the chemotherapy does not inhibit tumor-specific immune activation elicited by the vaccine. Docetaxel has direct cytotoxic effects, preventing microtubule disassembly [6–9], inhibiting tumor angiogenesis, increasing expression of Fas and increasing sensitivity to Fas- and radiation-induced apoptosis [10, 11, 31, 32]. It also has multiple immunomodulatory functions, including induction of macrophage-mediated tumor killing, stimulation of pro-inflammatory cytokines (TNFα, IL-12, and IL-1) and increasing lymphokine-activated killer (LAK) and NK cell anti-tumor activity [12, 14–16]. These attributes suggest that docetaxel may be a good candidate for combination with tumor-specific vaccines such as GM-CSF-secreting tumor cell vaccines.
The current study showed that the combination of a GM-CSF-secreting tumor cell vaccine and docetaxel significantly inhibits tumor growth in a murine tumor model compared to either agent as a monotherapy. Improved anti-tumor activity of the combination therapy was evident in several different dosing regimens, ranging from a single injection of 18 mg/kg docetaxel given 2 days before or 2 days after vaccination to a multi-treatment regimen of 6 mg/kg docetaxel administered 2 days after vaccination and then every 3–4 days thereafter for up to three cycles of treatment. Docetaxel given 2 days prior to vaccination had improved anti-tumor activity compared to the same dose administered 2 days after vaccination, which is consistent with published reports demonstrating that pre-vaccination treatment with paclitaxel in a murine model of Her2-expressing breast cancer improved vaccine potency [33]. The better pre-vaccination outcome in our study may reflect the direct anti-proliferative actions of docetaxel on the tumor cells since pre-vaccination administration was given 1 day after tumor challenge whereas post-vaccination docetaxel was given 5 days post-tumor challenge. Tumor burden would therefore be lower and the cytotoxic effect of docetaxel stronger at the time of vaccination in the former case. Reduced tumor burden, and the increased antigen availability resulting from dying tumor cells, would potentially result in a more effective anti-tumor response and consequent improved survival.
Pre-vaccination administration of docetaxel, which leads to moderate lymphopenia, might also result in the induction of a stronger anti-tumor T cell response after vaccination. Several studies have shown that a lymphopenic environment, as a result of chemotherapy, radiation therapy or RAG deficiency, is conducive to the expansion and survival of antigen-specific T cells, presumably by eliminating the inhibitory effects of the CD4+CD25+ T regulatory cells [34–37]. However, whether docetaxel increases the potency of GM-CSF-secreting tumor cell vaccines by removal of CD4+CD25+ regulatory T cells or simply reducing tumor burden and increasing antigen accessibility through direct tumor cytolytic effects or by both mechanisms remains to be investigated.
In contrast to the observation of Machiels et al. [33], in which post-vaccination administration of paclitaxel did not enhance anti-tumor activity of a vaccine, post-vaccination administration of docetaxel in this study significantly increased the survival of mice. The dissimilar outcome may reflect the different timing of chemotherapy treatment. Machiels et al. administered paclitaxel 7 days after vaccination, whereas in our studies docetaxel was administered 2 days after vaccine [33]. It is possible that administration of docetaxel 7 days after vaccination eradicates the rapidly dividing T cells stimulated by the vaccination. However, since docetaxel has a short half-life in vivo [38, 39], residual drug levels will be very low when given 2 days after vaccination, before T cells start their vaccine-induced proliferation.
Docetaxel is an approved therapy for prostate cancer, and a GM-CSF-secreting tumor cell vaccine (GVAX) is currently being evaluated in a phase 3 clinical trial in hormone-resistant prostate cancer patients. When undertaking combination therapy with these two treatment modalities for prostate cancer, particular attention should be given to the timing of administration of the two therapies. Docetaxel is typically given every 3 weeks in current treatment regimens, in part to allow for the recovery of drug-induced neutropenia. One could envisage a regimen in which a GM-CSF-secreting tumor cell vaccine such as the GVAX prostate cancer vaccine is administered 2–3 days after each docetaxel treatment. Using this treatment schedule, docetaxel would kill the proliferating tumor cells, increasing antigen accessibility, or remove CD4+CD25+ regulatory T cells or both, but be cleared from the circulation in time to have a minimal effect on the vaccine-induced anti-tumor immune response.
The data reported herein demonstrate that administration of docetaxel either prior to or after vaccination enhances the anti-tumor efficacy of the vaccine resulting in prolonged survival at docetaxel dose levels that induce neutropenia. Furthermore, vaccine-induced T cell responses are unaffected by docetaxel administration. These findings provide a rationale for testing docetaxel chemotherapy in combination with GM-CSF-secreting tumor cell vaccines in patients with cancer.
Acknowledgements
The authors would like to thank Drs. Peter Working and Natalie Sacks for their critical review of the manuscript.
References
- 1.Katsumata N. Docetaxel: an alternative taxane in ovarian cancer. Br J Cancer. 2003;89(Suppl 3):S9–S15. doi: 10.1038/sj.bjc.6601495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghersi D, Wilcken N, Simes J, Donoghue E (2003) Taxane containing regimens for metastatic breast cancer. Cochrane Database Syst Rev CD003366 [DOI] [PubMed]
- 3.Davies AM, Lara PN, Jr, Mack PC, Gandara DR. Docetaxel in non-small cell lung cancer: a review. Expert Opin Pharmacother. 2003;4:553–565. doi: 10.1517/14656566.4.4.553. [DOI] [PubMed] [Google Scholar]
- 4.Trump D, Lau YK. Chemotherapy of prostate cancer: present and future. Curr Urol Rep. 2003;4:229–232. doi: 10.1007/s11934-003-0074-3. [DOI] [PubMed] [Google Scholar]
- 5.Obasaju C, Hudes GR. Paclitaxel and docetaxel in prostate cancer. Hematol Oncol Clin North Am. 2001;15:525–545. doi: 10.1016/S0889-8588(05)70230-6. [DOI] [PubMed] [Google Scholar]
- 6.Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- 7.Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Cancer Drug Targets. 2003;3:193–203. doi: 10.2174/1568009033481967. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Khuri FR. Mode of action of docetaxel—a basis for combination with novel anticancer agents. Cancer Treat Rev. 2003;29:407–415. doi: 10.1016/S0305-7372(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 9.Lin HL, Liu TY, Chau GY, Lui WY, Chi CW. Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer. 2000;89:983–994. doi: 10.1002/1097-0142(20000901)89:5<983::AID-CNCR7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Nabell L, Spencer S. Docetaxel with concurrent radiotherapy in head and neck cancer. Semin Oncol. 2003;30:89–93. doi: 10.1053/j.seminoncol.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Milas L. Docetaxel/radiation combinations: rationale and preclinical findings. Clin Lung Cancer. 2002;3(Suppl 2):S29–36. doi: 10.3816/CLC.2002.s.011. [DOI] [PubMed] [Google Scholar]
- 12.Mason K, Staab A, Hunter N, McBride W, Petersen S, Terry N, Milas L. Enhancement of tumor radioresponse by docetaxel: involvement of immune system. Int J Oncol. 2001;18:599–606. doi: 10.3892/ijo.18.3.599. [DOI] [PubMed] [Google Scholar]
- 13.Mason KA, Hunter NR, Milas M, Abbruzzese JL, Milas L. Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res. 1997;3:2431–2438. [PubMed] [Google Scholar]
- 14.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong AW, Seamour B, Lawson JM, Ordonez G, Vukelja S, Hyman W, Richards D, Stein L, Maples PB, Nemunaitis J. Cellular immune profile of patients with advanced cancer before and after taxane treatment. Am J Clin Oncol. 2000;23:463–472. doi: 10.1097/00000421-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrlinger U, Kramm CM, Johnston KM, Louis DN, Finkelstein D, Reznikoff G, Dranoff G, Breakefield XO, Yu JS. Vaccination for experimental gliomas using GM-CSF-transduced glioma cells. Cancer Gene Ther. 1997;4:345–352. [PubMed] [Google Scholar]
- 19.Hrouda D, Perry M, Dalgleish AG. Gene therapy for prostate cancer. Semin Oncol. 1999;26:455–471. [PubMed] [Google Scholar]
- 20.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, Abrams RA, Pardoll DM, Cameron JL, Yeo CJ. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 21.Jaffee EM, Thomas MC, Huang AY, Hauda KM, Levitsky HI, Pardoll DM. Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J Immunother Emphasis Tumor Immunol. 1996;19:176–183. doi: 10.1097/00002371-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kusumoto M, Umeda S, Ikubo A, Aoki Y, Tawfik O, Oben R, Williamson S, Jewell W, Suzuki T. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol Immunother. 2001;50:373–381. doi: 10.1007/s002620100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim M, Simons JW. Emerging concepts in GM-CSF gene-transduced tumor vaccines for human prostate cancer. Curr Opin Mol Ther. 1999;1:64–71. [PubMed] [Google Scholar]
- 24.Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Curr Opin Immunol. 2000;12:571–575. doi: 10.1016/S0952-7915(00)00144-8. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes DM, Vidard L, Rock KL. Characterization of MHC class II-presented peptides generated from an antigen targeted to different endocytic compartments. Eur J Immunol. 2000;30:2333–2343. doi: 10.1002/1521-4141(2000)30:8<2333::AID-IMMU2333>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K. Expression vector system based on the chicken beta-actin promoter directs efficient production of interleukin-5. Gene. 1989;79:269–277. doi: 10.1016/0378-1119(89)90209-6. [DOI] [PubMed] [Google Scholar]
- 27.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 29.Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW., Jr The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 32.Piechocki MP, Lonardo F, Ensley JF, Nguyen T, Kim H, Yoo GH. Anticancer activity of docetaxel in murine salivary gland carcinoma. Clin Cancer Res. 2002;8:870–877. [PubMed] [Google Scholar]
- 33.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 34.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 35.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI200215175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 37.Terando A, Mule JJ. On combining antineoplastic drugs with tumor vaccines. Cancer Immunol Immunother. 2003;52:680–685. doi: 10.1007/s00262-003-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruno R, Hille D, Riva A, Vivier N, Bokkel Huinnink WW X, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 39.van Tellingen O, Beijnen JH, Verweij J, Scherrenburg EJ, Nooijen WJ, Sparreboom A. Rapid esterase-sensitive breakdown of polysorbate 80 and its impact on the plasma pharmacokinetics of docetaxel and metabolites in mice. Clin Cancer Res. 1999;5:2918–2924. [PubMed] [Google Scholar]