Abstract
The current therapy of uveal melanoma (UM) metastases remains inefficient, which warrants the development of new treatment modalities. For the first time we investigated the effects of retinoic acid (RA) on a panel of UM cell lines and found that RA induces morphological changes compatible with differentiation, suppresses proliferation and causes apoptosis in these cells. RA treatment resulted in an increase of p21, p27 and p53 protein levels and G1 arrest in UM cells, which correlated with significant down-modulation of surface Her2/neu proto-oncogene expression. In addition, RA-treated UM cells exhibited increased sensitivity to both MHC class I-restricted killing by cytotoxic T lymphocytes and NK cell-mediated lysis that were accompanied by more efficient conjugate formation between UM cells and killer lymphocytes. Taken together, our results implicate UM as a new target for treatment with retinoids and suggest that retinoids and T- or NK-cell based immunotherapy can have mutually enhancing effects in UM patients.
Keywords: Retinoic acid, Uveal melanoma, Cell death, Cytotoxic T lymphocytes
Introduction
Uveal melanoma (UM), the most common primary intra-ocular tumour in adults [37], which accounts for about 13% of deaths in melanoma patients, is usually diagnosed after the establishment of micrometastases [35]. The current treatment modalities include enucleation of the eye, cryotherapy, radioactive therapy, transpupillary thermotherapy and photocoagulation, but currently no effective treatment prevents the development of metastases [1]. Average survival time after occurrence of liver metastases is less than 5 months [11]. Thus, development of novel therapeutic strategies aimed at prolonging survival of these patients is essential.
Retinoic acid (RA), the most potent natural form of vitamin A, plays an important role in mediating the growth and differentiation of both normal and transformed cells (reviewed in [3]). RA exerts its functions by activating the nuclear RA receptor (RAR) (α, β and γ) and rexinoid (RXR) (α, β and γ) receptors, which regulate transcription of multiple target genes essential for diverse biological functions including growth, vision, reproduction, embryonic development, differentiation of epithelial tissues and immune responses [18, 22]. Vitamin A deprivation in prenatal life results in a well-defined spectrum of symptoms during the postnatal period, demonstrating that retinoids play essential roles in differentiation and maintenance of differentiated tissue phenotypes in many organs including the eye (reviewed in [20]). Moreover, it has been demonstrated that RXRα-null animals display multiple ocular abnormalities indicating that RXRα plays a major role in eye development [15].
RA has been shown to inhibit tumour growth both in vitro and in vivo. Synthetic derivatives of RA, retinoids have been approved as pharmacological agents in cancer therapy due to their multiple anti-tumour effects [3]. RA is a potent agent in the treatment of acute promyelocytic leukaemia (APL) where it can reverse the dominant-negative effects which the PML-RAR-α oncoprotein exhibits on the functions of the wild type PML and RAR-α proteins [14]. In neuroblastoma, RA treatment can induce differentiation of malignant cells, loss of proliferative capacity and apoptosis [25, 27]. RA derivatives are also beneficial in leukaemia, cervical cancer, thyroid cancer, breast cancer, squamous cell carcinoma, skin cancer and head and neck cancer when administered alone or in combination with other therapies [3, 13].
At present, the effects of RA on UM are unknown. We assessed the effect of all-trans RA on a panel of UM cells. We show that RA dramatically reduces cell growth and viability in a panel of UM cell lines and induces morphological changes compatible with differentiation. In addition, RA sensitizes UM cells to killing mediated by cytotoxic lymphocytes.
Our results implicate RA as a novel therapeutic agent for UM and point to the potential usefulness of retinoids in combination with T- or NK-cell based immunotherapy in treatment of UM metastases.
Materials and methods
Cell lines and retinoic acid treatment
Tumour cell lines
Cell lines 92-1, Mel-202, Mel-290, OCM-1, OCM-3 and OCM-8 were established from primary lesions. Cell lines OMM-1.3 and -1.5 were obtained from a liver metastasis. Cell line 92-1 was established from a primary UM in the laboratory of Dr. Martine J. Jager (Department of Ophthalmology, Leiden University Medical Centre, Leiden, Netherlands). Cell lines Mel-202, Mel-290, OMM-1.3 and OMM-1.5 were the generous gift of Dr. Bruce R. Ksander (Schepens Eye Institute, Harvard Medical School, Boston, MA, USA). Cell lines OCM-1, −3, and −8 were kindly provided by Dr. J. Kan-Mitchell (University of California, San Diego, CA, USA). The T-cell leukaemia cell line Jurkat TIB 152 was from the American Type Culture Collection (Manassas, VA, USA).
The NK cell lines NKL and Nishi were a kind gift of Dr. Jonas Sundbäck (Microbiology and Tumourbiology Centre, Karolinska Institutet, Stockholm, Sweden). All cell lines were maintained in IMDM supplemented with 10% heat-inactivated foetal calf serum (FCS) (GibcoBRL, Life Technologies, Grand Island, NY, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (complete medium). Nishi and NKL cells were cultured in complete medium containing 200 IU/ml interleukin (IL)-2.
Treatment with RA was performed at a final concentration of 10 μM in complete medium for 6 days if not indicated otherwise. Cells kept in complete medium containing the corresponding amount of DMSO as a vehicle are hereafter referred to as “control”. Cell recovery was calculated at indicated time points using trypan blue exclusion of non-viable cells.
Changes in cell morphology in response to RA were monitored using a phase-contrast microscope.
Effectors
The generation and characterisation of the CD8+ HLA-A11 restricted CTL clones BK289, CAR30 and BK210 specific for the EBV nuclear antigen-4 (EBNA4) derived peptide IVTDFSVIK (IVT), were previously described [17].
Purification of NK cells and generation of polyclonal NK cell cultures was performed from peripheral blood lymphocytes of healthy donors by density gradient centrifugation on Ficoll-Hypaque with subsequent removal of cells adherent to plastic. The remaining cell population was incubated on ice with the mixture of monoclonal antibodies including anti-CD3 (JT3A), anti-CD4 (HP2.6), and anti-HLA-DR (D1.12), followed by incubation with goat anti-mouse IgG-coated Dynabeads (Dynal, Oslo, Norway). After immunomagnetic depletion, fluorescence-activated cell sorter (FACS) analysis of the resulting cell pool was performed demonstrating that 90–95% of these cells were CD3−, CD4−, and HLA-DR−. Cells were cultured with irradiated allogeneic feeders in the presence of 200 U/ml of recombinant IL-2 and 1.5 ng/ml of phytohemagglutinin (Life Technologies Inc., Paisley, Scotland).
Antibodies and chemicals
The RA derivative all-trans RA was purchased from Sigma (St. Louis, MO, USA) dissolved in DMSO as 100 mM stocks and stored at −70°C in small aliquots.
Hybridoma cells producing the HLA-A11-specific antibody (clone HB-164) were purchased from ATCC. Total mouse serum was prepared by the animal facility at the Microbiology and Tumorbiology Centre, Karolinska Institute. Human ICAM-1/CD54-FITC MAb, sheep anti-human ICAM-1 antibody and mouse IgG1-FITC isotype control were obtained from R&D Systems (Minneapolis, MN, USA).
Antibodies specific for p21 (Anti-Cip1, clone 70) and mouse anti p27-Kip1 (G173-524) were obtained from BD, monoclonal Ab DO7 specific to human p53 was from DAKO. Actin-specific antibody was purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). Annexin V-FITC Apoptosis Detection Kit I and FITC-conjugated monoclonal anti-active caspase-3 antibody apoptosis Kit I was obtained from BD Pharmingen™ (BD Biosciences Pharmingen, San Diego, CA, USA). Propidium Iodide (PI) was purchased from Sigma. The pan-caspase inhibitor Z-VAD-fmk was purchased by Alexis Biochemicals corp. (Lausen, Switzerland). The apoptosis-inducing mouse IgM antibody CH-11 was from MBL (Nagoya, Japan). The mouse monoclonal anti-human FasL antibody 5G51 and the MICA specific antibody were obtained from Alexis Corp. (Lausen, Switzerland). The mouse monoclonal anti Her-2/neu antibody conjugated to PE and the IgG1-PE conjugated isotype were purchased from BD Biosciences.
Western blot analysis
All procedures were performed using Multiphor II Electrophoresis System and ExelGel SDS homogeneous precast gels (Amersham Pharmacia Biotech AB, Uppsala, Sweden). The UM cells cultured in complete medium containing DMSO, or in the presence of all-trans RA (10 μM) were pelleted down and lysed in electrophoresis sample buffer. Aliquots of total cell lysates corresponding to 105 cells were separated by SDS-PAGE followed by transfer onto nitro-cellulose membrane (Millipore AB, Sweden). Membranes were blocked in PBS containing 5% milk and 0.1% Tween-20 and probed with the respective specific antibody at the dilution recommended by the manufacturer. Rabbit anti-mouse IgGs conjugated to horseradish peroxidase (Amersham Pharmacia Biotech AB) were used as secondary antibodies. The reaction was visualised by enhanced chemiluminescence according to the manufacturers protocol (Amersham Pharmacia Biotech AB).
Cytotoxicity assays
Standard 4 h 51Cr-release assays were performed as previously described [31]. Briefly, HLA-A11-positive UM cell lines were pre-incubated with the IVT-peptide at a range of peptide concentrations and labelled with Na51CrO4 (0.1 μCi/106 cells at 37°C for 1 h). After extensive washing, target cells were incubated with HLA-A11 restricted IVT-specific CTLs at the indicated effector-to-target (E : T) ratio in triplicates for 4 h at 37°C. When NK cells were used as effectors, targets were labelled as described above and co-incubated with NK cell cultures for 4 h. In bystander cytotoxicity assays [12] target cells non-pulsed with the peptide were labelled with Na51CrO4 (0.1 μCi/106 cells at 37°C for 1 h) and subsequently incubated with either CTLs at 5:1 E:T ratio in triplicates for 16 h at 37°C or incubated with 250 ng/ml of anti-Fas antibody CH-11. 51Cr-release in the supernatants was measured by a γ-counter (Wallac Sverige AB, Stockholm, Sweden).
Fluorescence-activated cell sorter analysis
The UM cells either untreated or treated with RA were incubated with an excess of RPE-labelled HLA-ABC specific antibody W6/32 or relevant isotype control (IgG2a) and expression of total HLA class I molecules was measured by flow cytometry (Becton Dickinson, Mountain View, CA). The surface expression of the HLA-A11 allele or ICAM-1 was assessed after incubation of UM cells with HLA-A11-specific or ICAM-1-specific antibodies, respectively. Cells incubated with total mouse serum were used as a control for HLA-A11 staining. FITC-conjugated rabbit anti-mouse F(ab’)2-fragments were used as secondary antibodies. To monitor the surface expression of FasL and Fas UM cells were incubated with either 1 μg/ml of the FasL specific antibody 5G51 and mouse IgG1 control or with 1 μg/ml of Fas-specific antibody CH-11 and mouse IgM control antibodies respectively, for 30 min on ice. RPE-conjugated rabbit anti-mouse IgGs were used as secondary antibodies. Surface MICA expression was assessed using 10 μg/ml of mouse monoclonal MICA-specific antibody (clone AMO1) and a relevant isotype control (mouse IgG1) followed by incubation with rabbit anti-mouse PE-conjugated IgGs. Surface Her-2/neu expression was detected by a Her-2/neu specific PE-conjugated antibody (clone Neu 24.7). Mouse IgG1-PE was used as an isotype control.
Assessment of cell proliferation and death
To assess cell cycle distribution, UM cells were fixed on ice for 15 min in 1 ml of 70% v/v ethanol followed by centrifugation and incubation in 1 ml of the solution containing 1 mg/ml sodium citrate, 50 μg/ml PI, 50 μg/ml RNase A, 0.3% NP40, for 1 h at 4°C. The 104 cells were collected and analysed using the cellquest Software (Becton Dickinson). To monitor apoptosis-related changes, UM cells were incubated in 100 μl of Annexin V-binding buffer (10 mM Hepes/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2) containing 5 μl of Annexin V-FITC for 15 min in the dark at room temperature. Double staining with PI and Annexin V was performed in Annexin V binding buffer containing 5 μl of Annexin V-APC and 5 μl of PI at room temperature in the dark for 15 min prior to addition of another 300 μl of binding buffer. To monitor active caspase 3 expression, cells were washed twice in ice cold PBS and kept in Cytofix/Cytoperm™ solution at a concentration of 1×106 cells/ml for 20 min on ice. After two washes with Perm/Wash™ buffer cells were incubated in 100 μl of Perm/Wash™ buffer containing 20 μl of FITC-conjugated polyclonal rabbit anti-active caspase 3 antibody for 30 min on ice and washed twice in Perm/Wash buffer prior to flow cytometry. For inhibition of caspase activity UM cells were pre-treated with 50 μM of the pan-caspase inhibitor Z-VAD-fmk for 1 h at 37°C and subsequently treated with RA as described above in the presence or absence of the inhibitor.
Conjugation assay
Both targets (UM cells) and effectors (Nishi) were fluorescently labelled with dyes TFL2 and TFL4 obtained from the kit CyToxiLux® PLUS (OncoImmunin Inc., Gaithersburg, MD, USA) according to the manufacture’s protocol, mixed at 1:1 ratio in complete medium and incubated at +37°C or on ice (+4°C) in a total volume of 100 μl containing 105 cells. After indicated periods of time cell suspension was gently added to 400 μl of pre-warmed PBS and analysed by flow cytometry. Double-dye positive cells were considered as conjugates. For blocking of ICAM-1, UMs were pre-incubated with 30 μg/ml of anti-ICAM blocking Abs for 30 min at room temperature.
Statistical analysis
In general, mean values of at least three experiments were used to calculate standard deviations. Statistical significance was analysed using bifactorial variance analysis for comparison of RA treated versus untreated cells.
Results
Retinoic acid induces cell loss in uveal melanoma cultures
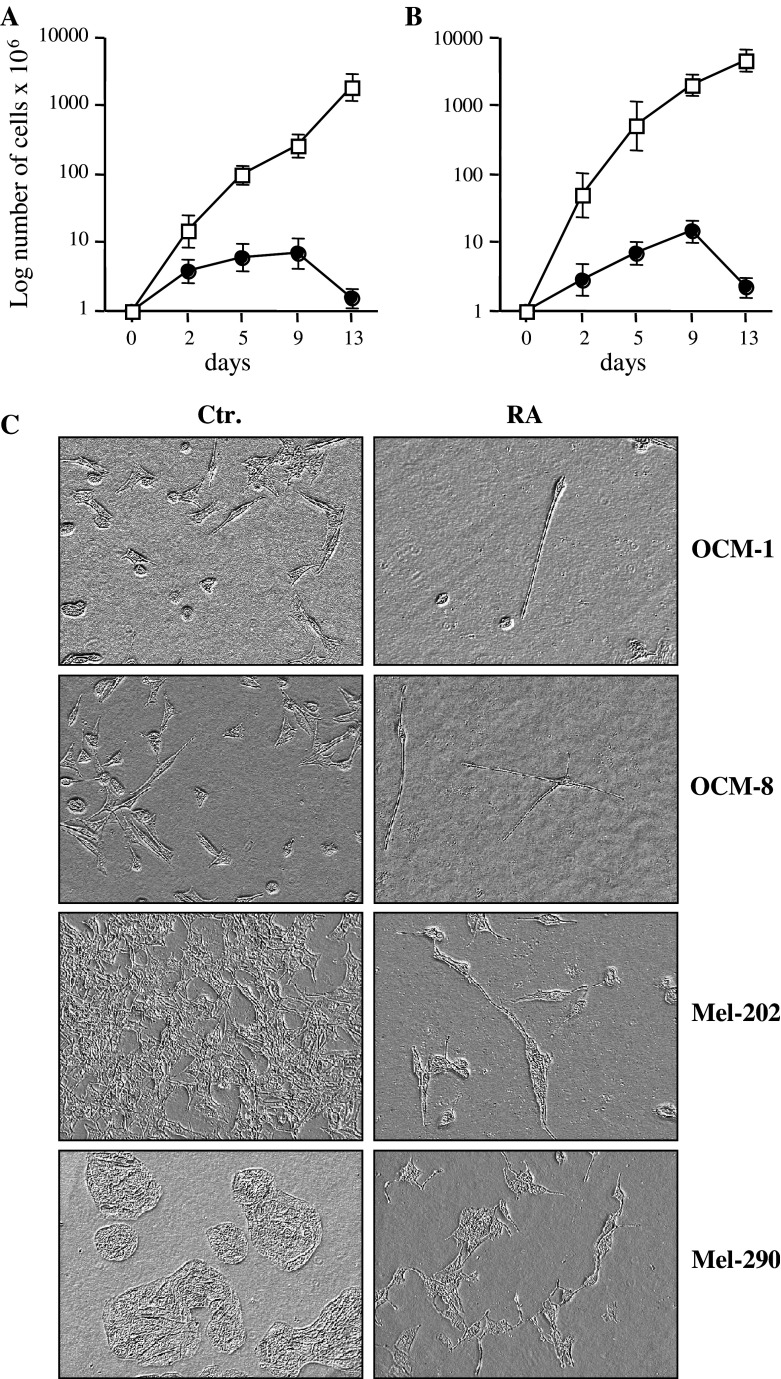
To investigate the effect of RA on UM, we exposed a panel of UM cell lines to 10 μM of all-trans RA, a concentration which has been previously used by others to analyse the effect of RA on tumours of different cellular origins [33, 36]. We found that RA decreases UM cell recovery in time-dependent manner, which could be already seen at day 2 of RA treatment in OCM-1 (Fig. 1a) and OCM-8 (Fig. 1b) cell lines. In addition, we also observed a dramatic change in cell morphology in response to RA treatment on a panel of UM cell lines (Fig. 1c and data not shown). When untreated UM cells were round or slightly elongated, had short, stunted branches emanating from the cell body, UM cells treated with RA appeared more neuronal, with small elongated cell bodies and long thin branches (Fig. 1c). These changes suggested that RA induces differentiation in UM cells.
Fig. 1.
Retinoic acid (RA) treatment blocks proliferation of uveal melanoma (UM) cell lines. Proliferation of OCM-1 (a) and OCM-8 (b) cells untreated (open squares) or treated with RA (closed circles). Cell recovery is expressed as numbers of trypan blue-negative cells counted at the respective day of treatment. The mean ± SD of three independent experiments is shown for each line. Changes in cell morphology in response to RA were monitored through a phase-contrast microscope and images were captured using digital camera (c)
Retinoic acid induces growth arrest in uveal melanoma cells
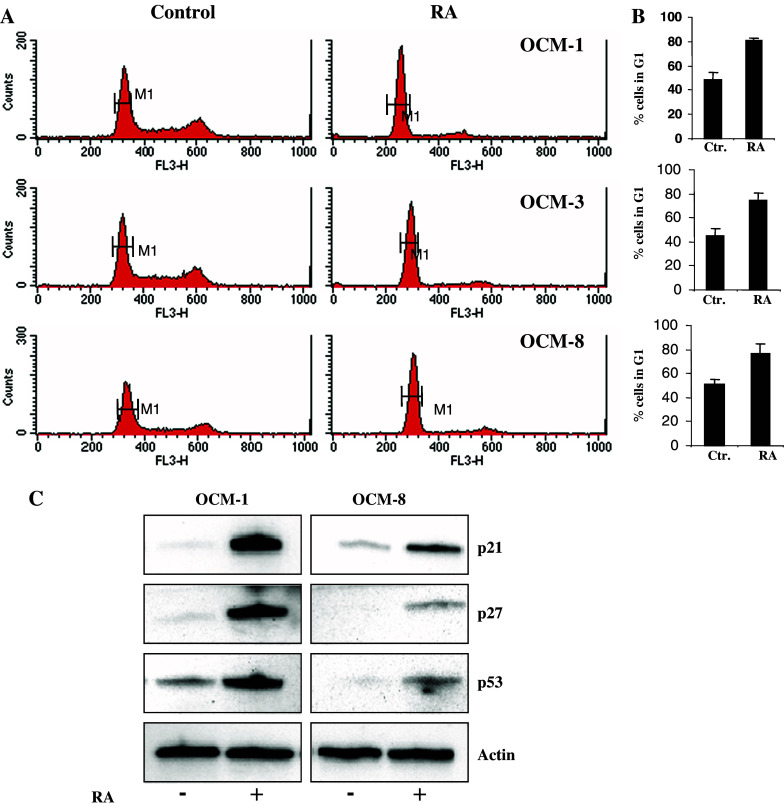
In order to understand the mechanisms of RA-triggered difference in UM cell recovery, we examined the cell cycle distribution in RA-treated and control UM cells (Fig. 2a). We found that RA induces accumulation of cells in G1/G0 phase of the cell cycle (Fig. 2a). The proportion of cells in G1 phase increased approximately 2-fold after 6 days of RA treatment (Fig. 2b). We have also detected accumulation of p21, p27 and p53 proteins in the total cell lysates of RA treated OCM-1 and OCM-8 cell lines (Fig. 2c). These observations together with the changes in cell cycle distribution led us to conclude that RA induces cell cycle arrest in G1 phase in UM cells.
Fig. 2.
RA induces growth arrest in UM cell lines. a Cell cycle distribution in OCM-1, OCM-3 and OCM-8 cell lines prior and after RA treatment was assessed by labelling of cellular DNA with PI and subsequently analysed by flow cytometry. One representative experiment is shown for each line. b Percent of cells in G1 phase (M1 interval in panel “a”) prior and after RA treatment. Mean ± SD of three independent experiments is shown. c Expression of p21, p27 and p53 in total cell lysates of OCM-1 and OCM-8 cell lines was monitored by Western blot prior and after RA treatment. Expression of actin was used as a loading control
Retinoic acid down-regulates surface Her-2/neu expression on uveal melanoma cells
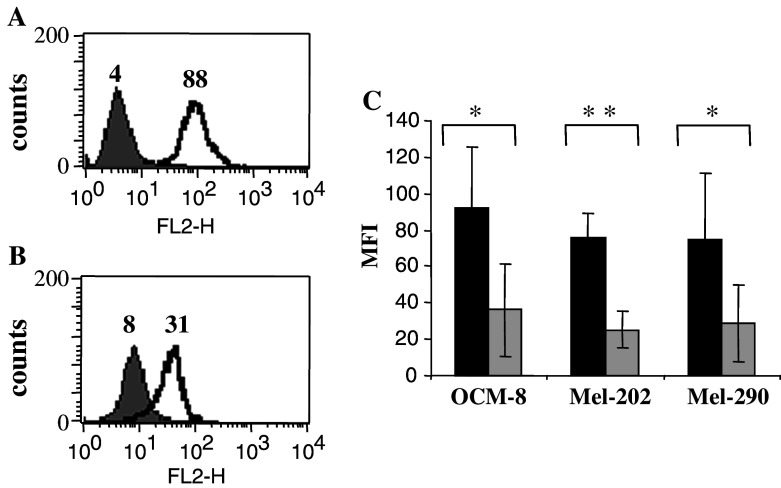
We previously found that most of UM cell lines described in our study express Her-2/neu at the cell surface (unpublished observation). This molecule, over-expressed by a variety of tumours, has been implicated in abnormal cell proliferation and often represents a marker of poor prognosis [16]. Since it has been shown earlier that RA down-regulates Her-2/neu [4], we monitored the expression of this protein on UM cells prior and after treatment with RA. We found that RA down-regulated surface Her-2/neu levels by more than 50% in all the Her-2/neu positive lines tested (Fig. 3). This suggests a possible mechanism for the suppressed proliferation in UMs in response to retinoids.
Fig. 3.
RA-treatment decreases Her2/neu expression at the surface of UM cells. Expression of Her-2/neu at the cell surface of 92-1 cell line, either untreated (a) or treated with RA (b) was assessed by flow cytometry using the Her-2/neu specific antibody. The grey histogram corresponds to the isotype control; the numbers indicate MFI (mean fluorescence intensity). c Expression of Her-2/neu at the cell surface of OCM-8, Mel-202 and Mel-290 either untreated (black bars) or treated with RA (grey bars) was determined by flow cytometry. Mean ± SD of three experiments is shown. Values *P<0.05 and **P 0.001 demonstrate significant differences in expression of Her2/neu in control versus RA-treated cells
Retinoic acid induces apoptotic changes in uveal melanoma cells
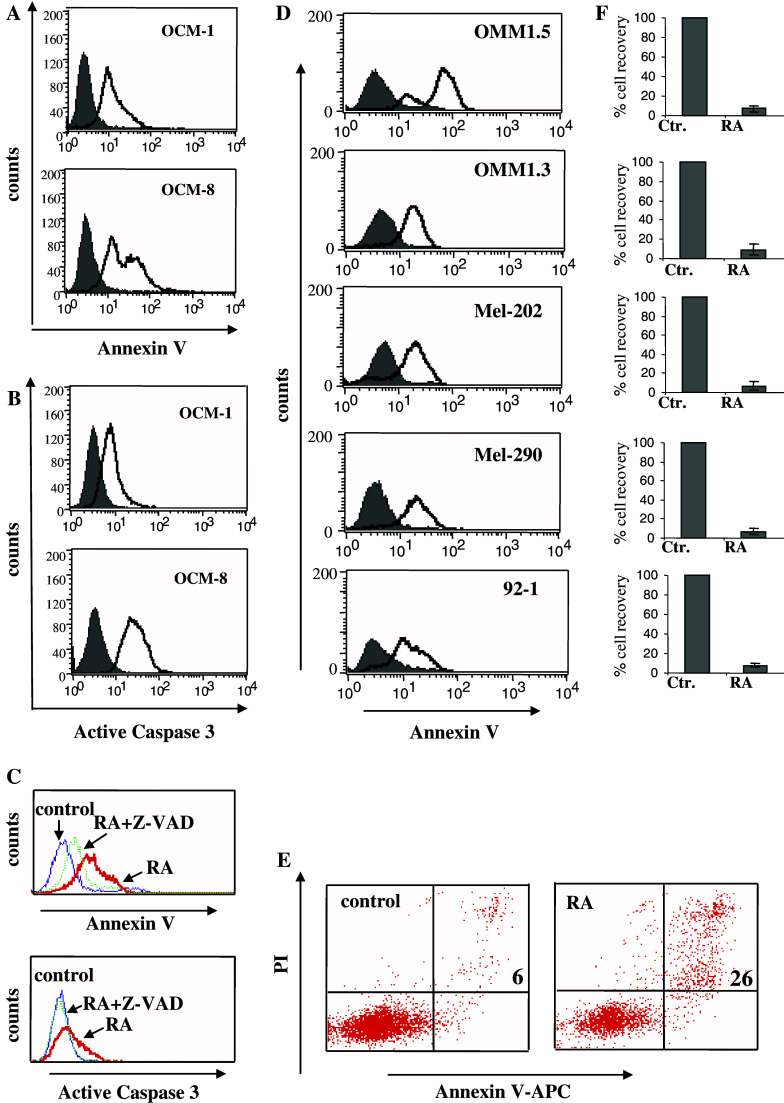
Besides differences in the rate of proliferation the difference in cell recovery may be explained by cell death induced by RA in UM lines. Indeed, RA treatment caused apoptosis-compatible alterations [9, 19] in OCM-1 and OCM-8 cell lines, such as externalization of phosphatidyl serine moieties (Fig. 4a) and increased expression of active caspase 3 (Fig. 4b) which could be significantly inhibited upon exposure of UM cells to pan-caspase inhibitor Z-VAD-fmk (Fig. 4c). The induction of apoptosis-related changes was also observed while measuring the externalization of phosphatidyl serine moieties on a panel of UM lines (Fig. 4d) as well as assessing an increase in numbers of Annexin V/PI double positive cells (Fig. 4e) in response to RA, which significantly reduced cell recovery in all lines tested (Fig. 4f).
Fig. 4.
RA induces caspase activation and affects viability of multiple UM cell lines. OCM-1 and OCM-8 cell lines were stained for Annexin V (a) or for active caspase 3 expression (b), prior (grey histogram) and after (black line) RA treatment and analysed by flow cytometry. One representative experiment of three is shown in the figure. c RA treated and control OCM-8 cells were monitored for the expression of Annexin V and active caspase 3 in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk. One representative experiment of three is shown. d UM cell lines either untreated (grey histogram) or treated with RA (black line) were stained for Annexin V and analysed by flow cytometry. e Control and RA treated OCM-8 cells were double stained with Annexin-V and PI. Numbers indicate percentage of double positive cells. One representative of three experiments is shown. f The same cultures as in panel “d” were stained with trypan blue and viability was expressed as percentage of cell recovery relative to that in control cultures
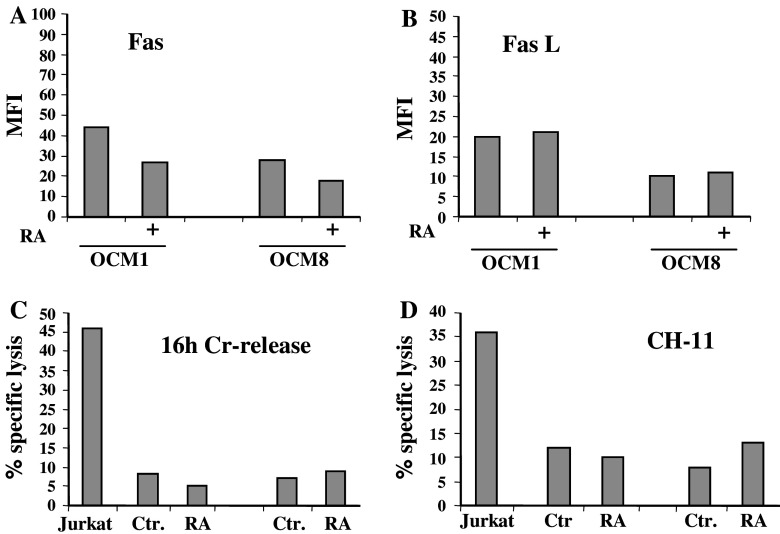
Retinoic acid treatment does not interfere with the death receptor-mediated killing of uveal melanomas
We have previously shown that UM cells are protected from Fas-mediated death due to the autocrine secretion of soluble FasL [12]. It has been reported that RA can increase surface expression of both Fas and FasL in tumour cells of different origins [30]. We speculated that RA may have similar effects on UM cells which may lead to restoration of susceptibility to Fas-mediated killing. We measured the expression of Fas (Fig. 5a) and FasL (Fig. 5b) at the surface of UM cells but failed to detect any up-regulation of these molecules. On the contrary, we observed a slight down-regulation of surface Fas expression in OCM-1 and OCM-8 cell lines. In agreement with these data we did not observe any significant changes in either CTL- or CH11-induced UM cell death as monitored by a 16 h 51Cr-release assay (Fig. 5c,d). We also found that RA did not sensitize UMs to killing by the recombinant killer TRAIL or/and cytokines such as INF-γ, INF-α and TNF-α, all used as sole agents or in combinations (data not shown). Our data suggest that an extrinsic pathway of cell death does not play a major role in the RA-induced death of UM cell lines.
Fig. 5.
RA does not affect Fas mediated killing of UM cells. Surface Fas (a) and FasL (b) levels were detected in control or RA-treated UM cells by specific relevant antibodies and analysed by FACS. The same cell lines, either untreated or treated with RA, were used as targets for either CTLs (c) or agonistic Fas-specific antibody CH-11 (d) in a 16 h 51Cr-release assay. Jurkat cells were used as a control for Fas-mediated killing. One representative of 3–4 reproducible experiments is shown
Retinoic acid sensitizes uveal melanoma cells to killing by cytotoxic lymphocytes
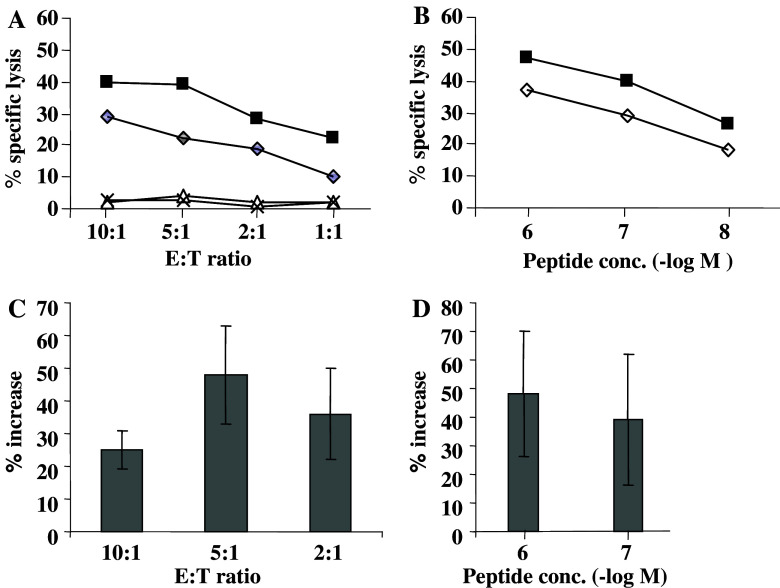
Since we have previously shown that RA sensitizes neuroblastoma cells to CTL killing [33], we speculated that RA may have similar effects on UM cells. We tested the HLA-A11 positive OCM-1 cell line for the sensitivity to killing by an HLA-A11 restricted CD8+ EBV-specific CTL clones in a 4 h 51Cr-release assay (Fig. 6).
Fig. 6.
RA treatment sensitizes UM cells to MHC class I-restricted peptide-specific lysis. a The HLA A11+ UM cell line OCM-1, either untreated (open diamonds) or treated with RA for 6 days (closed squares) was pre-pulsed with 0.1 μg/ml of the IVT peptide and tested for sensitivity to lysis by the peptide specific HLA-A11-restricted CD8+ CTL clone BK289 in a standard 4 h 51Cr-release assay at the indicated effector-to-target ratio. Cells non-pulsed with peptide (triangle for untreated and black cross for RA treated) were used as controls. b OCM-1 cell line either untreated or treated with RA was pre-pulsed with the indicated concentrations of IVT peptide and tested in a 4 h 51Cr-release assay against the CD8+ CTL clone BK289 at a 5:1 effector-to-target ratio. One representative of three performed experiments is shown. c Percent increase in cytotoxicity at the indicated E:T ratio achieved after RA treatment is expressed as the mean ± SD of three independent experiments. d Percent increase in cytotoxicity at the indicated peptide concentrations achieved after RA treatment is expressed as the mean ± SD of three independent experiments. CTL clones CAR30 and BK210 were also used as effectors in experiments shown in panels “c” and “d”
We found an increase in an MHC class I-restricted peptide-specific cytotoxicity that was observed at different effector-to-target ratios (Fig. 6a) and different peptide concentrations (Fig. 6b). These data were reproduced in multiple experiments using different CTLs as effectors (Fig. 6c, d). In order to understand the mechanism of this phenomenon we determined the total MHC class I and HLA-A11 expression at the cell surface of UM cells, but failed to detect any significant changes in response to RA treatment (data not shown).
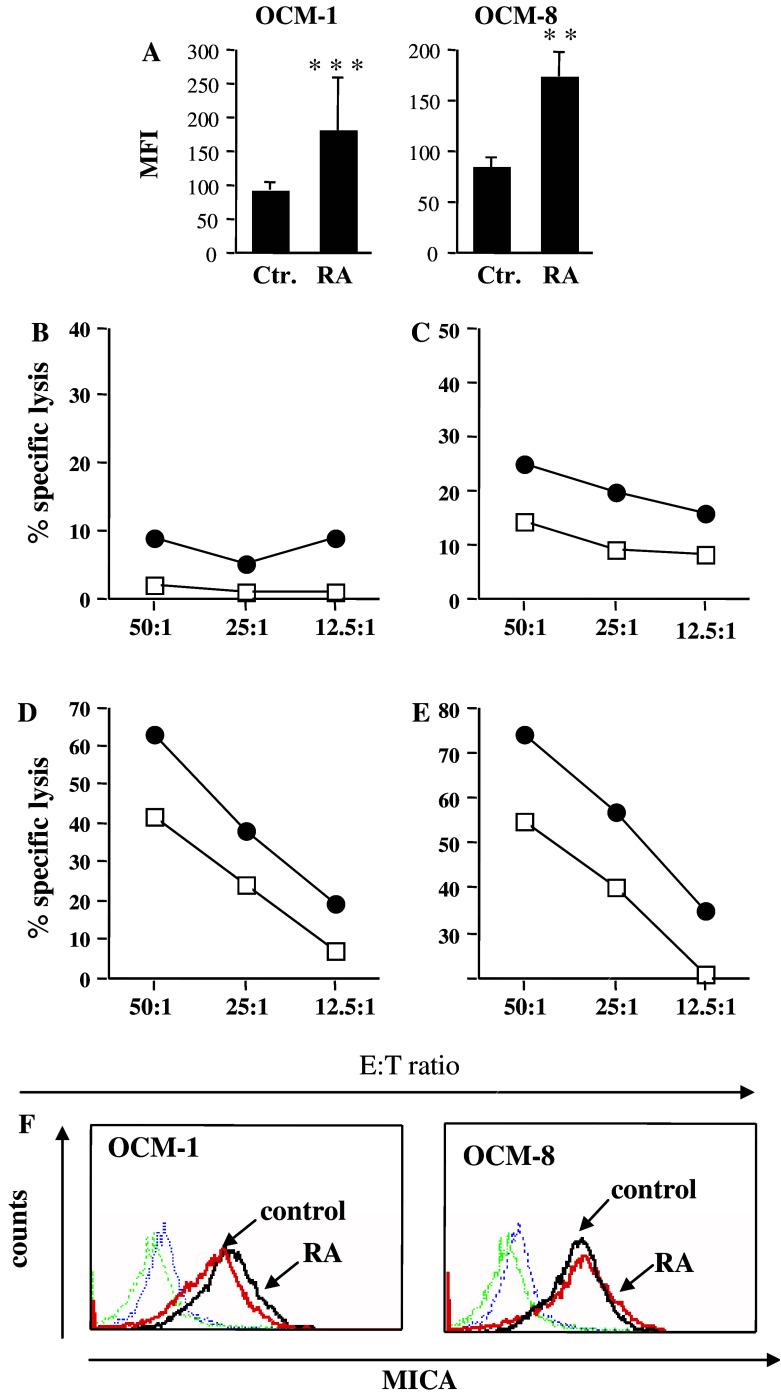
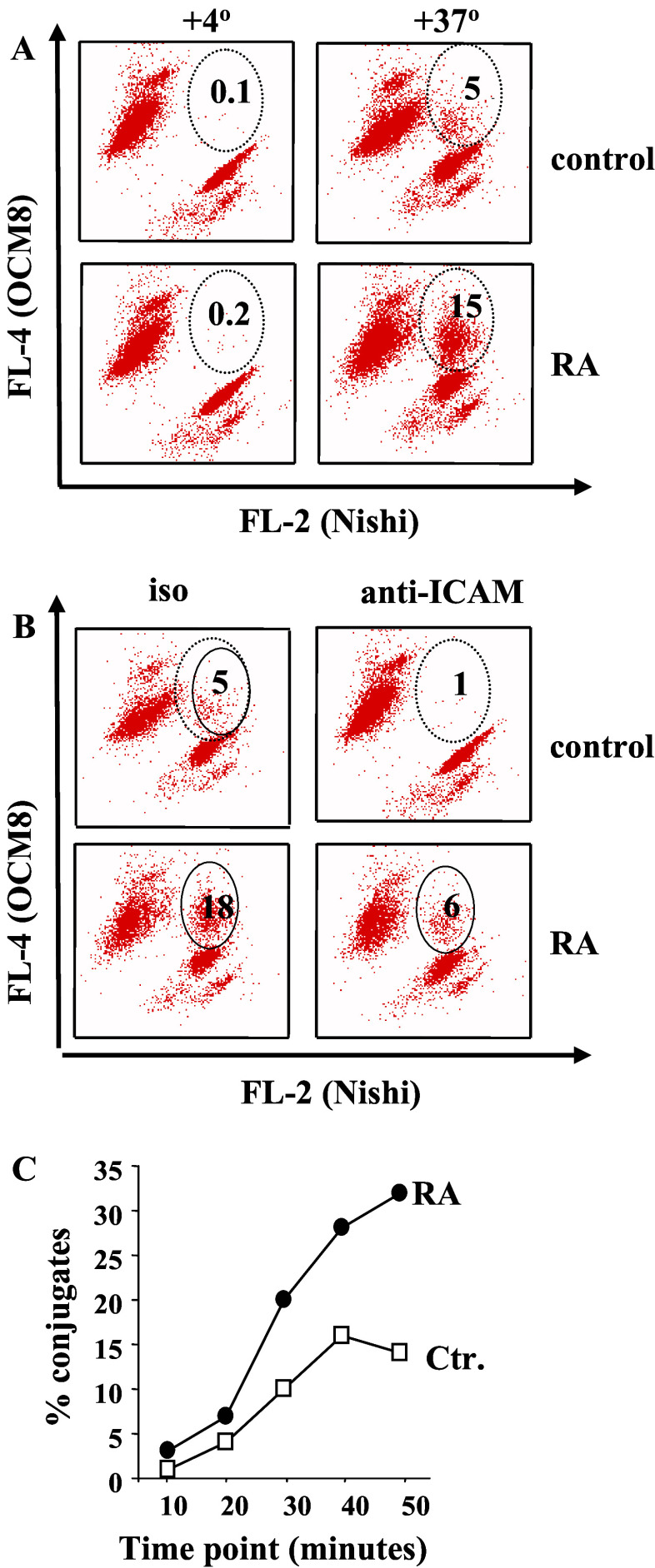
Because it has been previously reported that RA can modulate the expression of the adhesion molecule ICAM-1 [34] and that increased expression of ICAM-1 can affect killing by cytotoxic lymphocytes [5], we measured the surface levels of ICAM-1 and found a significant up-regulation of this molecule by RA in UMs (Fig. 7a). Up-regulation of adhesion molecules can lead to an increase in NK-mediated cytotoxicity, even in the absence of alterations in surface MHC class I expression [2]. Therefore, we tested UM cells prior to and after RA treatment for the sensitivity to NK cell-mediated killing using a panel of effectors (Fig. 7b–e). No significant difference in 51Cr-release from RA-treated versus control UM cell line was seen when NKL cell line was used as effectors (Fig. 7b), whereas Nishi NK cell line killed RA-treated UM cells with higher efficacy at different effector-to-target ratios (Fig. 7c). A similar pattern was observed when freshly isolated NK cultures from two different donors were used as effectors (Fig. 7d, e). Since MICA, one of the ligands for NK activating receptors can be induced by RA [23], we measured its expression at the cell surface of UM cells. Control UM cells express MICA at the cell surface but it is not up regulated upon RA treatment (Fig. 7f). We then speculated that the increase in killing by cytotoxic lymphocytes may result from the enhanced adhesion of RA-treated UM targets to effector cells, which is one of the major processes in the formation of immunological synapse and initiation of killer lymphocytes degranulation (reviewed in [7]). Indeed, RA-treated OCM-8 cells formed more conjugates with Nishi as compared to untreated cells after 20 min of co-incubation (Fig. 8a) and the conjugate formation was significantly reduced in the presence of ICAM-1 blocking antibodies (Fig. 8b). We have also observed an accelerated time kinetic of conjugate formation when UM cells were pre-treated with RA (Fig. 8c).
Fig. 7.
RA increases ICAM-1 expression and sensitizes UM cells to NK–mediated killing. a Surface levels of ICAM-1 prior and after RA treatment were determined by immunostaining with relevant antibodies and FACS analysis. Mean ± SD of mean fluorescence intensity (MFI) obtained in three independent experiments. Values **P=0.007673 and ***P=0.000443 demonstrate significant differences in ICAM-1 expression observed in RA-treated versus untreated cells. OCM-1 cells either untreated (open squares) or treated with RA (black circles) were used as targets for the NK lymphoma cell line NKL (b), the NK cell line Nishi (c) or polyclonal NK cultures established from two different healthy donors (d, e) in a standard 4 h 51Cr-release assay. One representative experiment for each effector is shown. f Control or RA-treated OCM-1 and OCM-8 cell lines were stained with MICA-specific antibody and analysed by flow cytometry. One representative of three reproducible experiments is shown for each cell line
Fig. 8.
RA increases immunological conjugate formation between UM cells and NK cells. a Conjugate formation between OCM-8 cells, either untreated or RA-treated, and Nishi cells (all labelled with fluorescent dyes as described in materials and methods) after 20 min of incubation on ice (+4°C) and at +37°C. Numbers indicate percent of double-dye positive cells (conjugates). Data from one out of four performed experiments are shown. b Conjugate formation between OCM-8 cells, either untreated or RA-treated, and Nishi cells after 20 min of incubation at +37°C in the presence of ICAM-blocking antibodies. c Time kinetic of conjugate formation between OCM-1 cells, either untreated or treated with RA, and Nishi cells were monitored by flow cytometry. Data from one out of three performed experiments are shown
In addition, in our experiments, ICAM-1 blocking on UMs completely abolished 51Cr-release induced by the NK cells and CTLs in either RA-treated or untreated target cells (data not shown). Therefore, the extent to which RA-induced ICAM-1 up-regulation contributes to the observed level of cytotoxicity is difficult to assess. However, this indirectly supports the importance of ICAM-1 modulation in the increased sensitivity of UM cells to CTL- and NK-mediated cytotoxicity.
Discussion
In the present study we identified retinoids as agents causing a significant reduction in UM cell recovery in response to treatment in vitro (Fig. 1), which results from inhibition of the proliferative capacity of tumour cells (Fig. 2) as well as cell death (Fig. 4).
It is well documented that down-regulation of surface proto-oncogene Her2/neu reverses transformed phenotypes and leads to a reduction in proliferation of tumour cells (reviewed in [6]). We found that all UM lines used in our study were Her2/neu positive (Fig. 3 and our unpublished results). In agreement with previous observations [24, 29], we demonstrate that the RA-induced decrease of surface Her2/neu expression is accompanied by accumulation of UM cells in the G0/G1 phase of the cell cycle, a decrease in the percentage of cells in the S-phase (Fig. 2a, b) and increased expression of the cell cycle inhibitors p21, p27 as well as the p53 protein (Fig. 2c). These findings provide a plausible molecular explanation for the RA-mediated anti-proliferative effects in UMs observed in our experiments. It has been recently demonstrated, both in vitro and in vivo, that retinoids can promote apoptosis in cancer cells by activating the intrinsic and/or extrinsic apoptosis pathway; the latter mediated by the Fas–Fas ligand interactions and associated with the up-regulation of both proteins [8, 28, 30]. We also observed that RA induced changes compatible with apoptosis, such as externalization of phosphatidyl serine moieties at the outer cell membranes (Fig. 4a–d) and as activation of caspase 3 (Fig. 4b); both alterations were diminished by the pan-caspase inhibitor Z-VAD-fmk. However, in contrast to previous observations [8], UM cell lines failed to up-regulate surface Fas and Fas ligand in response to RA (Fig. 5a, b) and, more importantly, remained resistant to Fas-mediated killing by CTLs and Fas agonistic antibody (Fig. 5c, d) as well as to TRAIL-mediated killing (data not shown) ruling out the involvement of the surface death receptors in RA-induced death of UM cells.
RA can modify the immunogenicity of tumour cells both in vitro and in vivo [10, 32, 33], through up-regulation of MHC class I and ICAM-1 molecules [34]. We did not detect significant up-regulation of either total surface MHC class I or HLA-A11 in UM cells upon RA-treatment (data not shown), whereas ICAM-1 was always significantly induced by RA in all UM lines tested (Fig. 7a and data not shown). This may explain the enhanced susceptibility of UM cells to lysis by MHC class I-restricted peptide specific CTLs (Fig. 6) without detectable induction of the relevant restriction element. The up-regulation of the adhesion molecule ICAM-1 has been previously reported to increase the sensitivity of targets to killing by NK cells [5, 21, 33]. In agreement with these observations, UM cells treated with RA were more readily killed by NK line Nishi as well as polyclonal NK cultures obtained from healthy donors (Fig. 7b–e). In search for other potential molecular changes leading to the increased sensitivity of UM cells to lysis by cytotoxic lymphocytes, we analysed the expression of the RA-inducible molecule MICA known to be a ligand for NK activating receptors [23, 26], but failed to detect accumulation of this molecule at the surface of UM cells in response to RA treatment (Fig. 7f). Therefore, one of the plausible explanations for the enhanced sensitivity of RA-treated UM cells to killing by cytotoxic lymphocytes may be more efficient adhesion between effectors and targets, leading to an increased rate of conjugate formation (Fig. 8).
Collectively, our study identifies UM as a target for treatment by retinoids and suggests a potential synergistic cytotoxic effect of RA and immunotherapy based on cytotoxic lymphocytes. Our findings may be of special clinical importance due to the fact that a number of retinoids have been already approved as therapeutic reagents. This significantly simplifies clinical logistics associated with application of these substances for treatment of UM metastases.
Acknowledgments
This work has been supported by the Swedish Cancer Foundation, Swedish Research Council, the Cancer Society in Stockholm and King Gustav the Fifth Jubilee Fund. We would like to thank Mikael Hanson for his help in the preparation of the manuscript and Dr. Ashley Miller for the critical review of this manuscript.
References
- 1.Albert DM, Niffenegger AS, Willson JK. Treatment of metastatic uveal melanoma: review and recommendations. Surv Ophthalmol. 1992;36(6):429–438. doi: 10.1016/S0039-6257(05)80024-4. [DOI] [PubMed] [Google Scholar]
- 2.Alexander CL, Edward M, MacKie RM. The role of human melanoma cell ICAM-1 expression on lymphokine activated killer cell-mediated lysis, and the effect of retinoic acid. Br J Cancer. 1999;80(10):1494–1500. doi: 10.1038/sj.bjc.6690551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 4.Bacus SS, Kiguchi K, Chin D. Differentiation of cultured human breast cancer cells (AU-565 and MCF-7) associated with loss of cell surface HER-2/neu antigen. Mol Carcinog. 1990;3(6):350–362. doi: 10.1002/mc.2940030607. [DOI] [PubMed] [Google Scholar]
- 5.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173(6):3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J. Monoclonal antibodies directed at growth factor receptors. Ann Oncol. 2000;11(Suppl 3):187–190. doi: 10.1023/A:1011144502974. [DOI] [PubMed] [Google Scholar]
- 7.Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 8.Chun KH, Pfahl M, Lotan R. Induction of apoptosis by the synthetic retinoid MX3350-1 through extrinsic and intrinsic pathways in head and neck squamous carcinoma cells. Oncogene. 2005;24(22):3669–3677. doi: 10.1038/sj.onc.1208339. [DOI] [PubMed] [Google Scholar]
- 9.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 10.Eccles SA, Barnett SC, Alexander P. Inhibition of growth and spontaneous metastasis of syngeneic transplantable tumors by an aromatic retinoic acid analogue. 1. Relationship between tumour immunogenicity and responsiveness. Cancer Immunol Immunother. 1985;19(2):109–114. doi: 10.1007/BF00199717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskelin S, Pyrhonen S, Summanen P, et al. Screening for metastatic malignant melanoma of the uvea revisited. Cancer. 1999;85(5):1151–1159. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1151::AID-CNCR20>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Hallermalm K, De Geer A, Kiessling R, et al. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. 2004;64(18):6775–6782. doi: 10.1158/0008-5472.CAN-04-0508. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Lara AM, Clarke N, Altucci L, et al. Retinoic-acid-induced apoptosis in leukemia cells. Trends Mol Med. 2004;10(10):508–515. doi: 10.1016/j.molmed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kambhampati S, Verma A, Li Y, et al. Signalling pathways activated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Leuk Lymphoma. 2004;45(11):2175–2185. doi: 10.1080/10428190410001722053. [DOI] [PubMed] [Google Scholar]
- 15.Kastner P, Grondona JM, Mark M, et al. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 16.Kiessling R, Wei WZ, Herrmann F, et al. Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res. 2002;85:101–144. doi: 10.1016/S0065-230X(02)85004-7. [DOI] [PubMed] [Google Scholar]
- 17.Levitsky V, de Campos-Lima PO, Frisan T, et al. The clonal composition of a peptide-specific oligoclonal CTL repertoire selected in response to persistent EBV infection is stable over time. J Immunol. 1998;161(2):594–601. [PubMed] [Google Scholar]
- 18.Marill J, Idres N, Capron CC, et al. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab. 2003;4(1):1–10. doi: 10.2174/1389200033336900. [DOI] [PubMed] [Google Scholar]
- 19.Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morriss-Kay GM, Sokolova N. Embryonic development and pattern formation. FASEB J. 1996;10(9):961–968. doi: 10.1096/fasebj.10.9.8801178. [DOI] [PubMed] [Google Scholar]
- 21.Naganuma H, Kiessling R, Patarroyo M, et al. Increased susceptibility of IFN-gamma-treated neuroblastoma cells to lysis by lymphokine-activated killer cells: participation of ICAM-1 induction on target cells. Int J Cancer. 1991;47(4):527–532. doi: 10.1002/ijc.2910470410. [DOI] [PubMed] [Google Scholar]
- 22.Nagy L, Thomazy VA, Heyman RA, et al. Retinoid-induced apoptosis in normal and neoplastic tissues. Cell Death Differ. 1998;5(1):11–19. doi: 10.1038/sj.cdd.4400337. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K, Dimasi N, Wang J, et al. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 24.Offterdinger M, Schneider SM, Huber H, et al. Retinoids control the expression of c-erbB receptors in breast cancer cells. Biochem Biophys Res Commun. 1998;251(3):907–913. doi: 10.1006/bbrc.1998.9570. [DOI] [PubMed] [Google Scholar]
- 25.Pahlman S, Ruusala AI, Abrahamsson L, et al. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14(2):135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 26.Poggi A, Venturino C, Catellani S, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64(24):9172–9179. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds CP. Differentiating agents in pediatric malignancies: retinoids in neuroblastoma. Curr Oncol Rep. 2000;2(6):511–518. doi: 10.1007/s11912-000-0104-y. [DOI] [PubMed] [Google Scholar]
- 28.Sako T, Nakayama Y, Minagawa N, et al. 4-[3,5-Bis(trimethylsilyl)benzamido] benzoic acid (TAC-101) induces apoptosis in colon cancer partially through the induction of Fas expression. In Vivo. 2005;19(1):125–132. [PubMed] [Google Scholar]
- 29.Schneider SM, Offterdinger M, Huber H, et al. Involvement of nuclear steroid/thyroid/retinoid receptors and of protein kinases in the regulation of growth and of c-erbB and retinoic acid receptor expression in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1999;58(2):171–181. doi: 10.1023/A:1006377006816. [DOI] [PubMed] [Google Scholar]
- 30.Sun SY, Yue P, Hong WK, et al. Induction of Fas expression and augmentation of Fas/Fas ligand-mediated apoptosis by the synthetic retinoid CD437 in human lung cancer cells. Cancer Res. 2000;60(22):6537–6543. [PubMed] [Google Scholar]
- 31.Torsteinsdottir S, Masucci MG, Ehlin-Henriksson B, et al. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci USA. 1986;83(15):5620–5624. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toulouse A, Loubeau M, Morin J, et al. RAR beta involvement in enhancement of lung tumor cell immunogenicity revealed by array analysis. FASEB J. 2000;14(9):1224–1232. doi: 10.1096/fasebj.14.9.1224. [DOI] [PubMed] [Google Scholar]
- 33.Vertuani S, De Geer A, Levitsky V, et al. Retinoids act as multistep modulators of the major histocompatibility class I presentation pathway and sensitize neuroblastomas to cytotoxic lymphocytes. Cancer Res. 2003;63(22):8006–8013. [PubMed] [Google Scholar]
- 34.Wang Z, Cao Y, D’Urso CM, et al. Differential susceptibility of cultured human melanoma cell lines to enhancement by retinoic acid of intercellular adhesion molecule 1 expression. Cancer Res. 1992;52(17):4766–4772. [PubMed] [Google Scholar]
- 35.Wang MX, Shields JA, Donoso LA. Subclinical metastasis of uveal melanoma. Int Ophthalmol Clin. 1993;33(3):119–127. doi: 10.1097/00004397-199303330-00017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Rosdahl I. Expression profiles of p53, p21, bax and bcl-2 proteins in all-trans-retinoic acid treated primary and metastatic melanoma cells. Int J Oncol. 2004;25(2):303–308. [PubMed] [Google Scholar]
- 37.Zierhut M, Streilein JW, Schreiber H, et al. Immunology of ocular tumours. Immunol Today. 1999;20(11):482–485. doi: 10.1016/S0167-5699(99)01513-3. [DOI] [PubMed] [Google Scholar]