Abstract
Interleukin 10 (IL-10) is produced by various types of human cancer, including malignant melanoma, and plays an important role in negative regulation of cell-mediated immune responses against tumors. We have developed chimeric molecules (immunoadhesins), combining the extracellular domain of human interleukin 10 receptor 1 (IL-10R1) with the Fc regions of human IgG1 heavy chain and investigated their capability of blocking the biological activities of human IL-10. Monomeric and dimeric immunoadhesins (IL-10R1/IgG1) constructs were tested for capturing human IL-10 and blocking its biological activities. Plasmid vectors that contained the IL-10 immunoadhesin constructs were directly transfected into human melanoma cell lines. Transfection of plasmid vectors into melanoma cell lines resulted in capturing of exogenously added as well as endogeneously produced IL-10. The supernatants obtained from an IL-10 non-producing melanoma cell line transfected with monomeric IL-10 immunoadhesin plasmids most efficiently captured exogenously added IL-10, compared to those obtained with the dimeric IL-10R1/IgG1 plasmid vector. Transfection of IL-10-producing melanoma cells with the monomeric IL-10 immunoadhesin plasmids totally captured endogenously produced IL-10 and enhanced T cell responses against allogeneic melanoma cells. Furthermore, purified monomeric IL-10 immunoadhesin protein showed IL-10 capturing efficacy compatible with that of IL-10-specific monoclonal antibodies. Collectively, these studies indicate that IL-10 immunoadhesins, especially in monomeric form, are potent inhibitors of biological activities of IL-10 and suggest that these molecules, alone or in conjunctions with other immunotherapeutic approaches, can be utilized for the immuno-targeting of IL-10 producing tumors.
Keywords: Interleukin 10, Melanoma, Immunoadhesin, Cytokine receptors, Cytokines
Introduction
Interleukin-10 (IL-10) was first described as cytokine synthesis inhibitory factor (CSIF) that inhibits interferon (IFN)-gamma and GM-CSF production by Th1 cells [12, 14]. At present, IL-10 is considered an immunomodulatory cytokine that plays a major role in suppressing cellular immune and inflammatory responses [1, 9, 27]. Human IL-10 (hIL-10) consists of 178 amino acids (aa) including an 18 aa signal sequence and a 160 aa mature segment [10]. The concentration of hIL-10 in the blood is approximately 0.5 pg/ml in healthy donors [10]. Elevated serum levels of IL-10 have been reported in patients with various types of cancer, including malignant melanoma and non-Hodgkin’s lymphoma, and IL-10 levels appear to correlate with a poor survival in patients with these diseases [4, 35]. We have reported that more than 85% of single cell suspensions derived from human metastatic melanomas produced IL-10 and that the melanoma cells were the source of IL-10 production [35]. Furthermore, IL-10 production in melanoma tissues was negatively correlated to the survival of patients who received a hapten-modified autologous melanoma cell vaccine [31].
Evidence has accumulated that IL-10 plays an important regulatory role in anti-tumor cellular immune responses. For example, it was reported that IL-10 produced by melanoma cells suppresses the T cell response by inhibition of MHC class II expression and down-regulation of MHC class I [42]. Pretreatment with IL-10 resulted in a dose-dependent inhibition of autologous, cytotoxic T lymphocyte (CTL)-mediated, tumor specific lysis [24]. This effect is mediated by reduced expression of the transporter associated with antigen processing (TAP)-1 and -2 proteins, which in turn results in reduced translocation of peptides to the endoplasmic reticulum and impaired MHC class I peptide loading and diminished cell surface levels of target peptides [34]. It has also been reported that IL-10 down-regulates co-stimulatory molecules such as CD86 and ICAM-I on antigen-presenting cells including dendritic cells and induces immune tolerance [25]. Anergic T cells induced by IL-10 treated dendritic cells display increased extra-cellular and intra-cellular expressions of cytotoxic T-lymphocyte antigen (CTLA)-4 molecules [39]. The consequence of IL-10 presence and the resulting inhibition of the antitumor immune response might be critical in progression of cancers.
The important roles of IL-10 in cancer immunotherapy have been reemphasized as investigation of tumor-associated regulatory T cells has progressed. Reuben et al. [32] reported that patients who developed an antitumor response in conjunction with immune-related adverse events after CTLA-4 monoclonal antibody (mAb) treatment showed a significant decrease in constitutive production of IL-10 by peripheral blood mononuclear cells (PBMC). Since many tumors produce IL-10, the anti-CTLA4 mAb treatment might be restricted in inducing an anti-tumor immune response. In this regard, direct blocking of IL-10 in tumor sites in conjunction with anti-CTLA4 mAb might be a more potent approach to totally abrogate immunomodulatory effects of IL-10 in tumor microenvironment and facilitate the development of anti-tumor immune response. Furthermore, IL-10 is also considered an important factor in active specific immunotherapy. An IL-10 blockade in conjunction with a DNA vaccine, ineffective by itself, enhanced the efficacy of the vaccine and resulted in clearance of persistent chronic virus infection [5]. These investigations suggest that the antagonism of IL-10 would be an important and effective way to reverse the immune suppression in tumor microenvironment and might enhance the efficacy of therapeutic cancer vaccine.
Two types of cell surface receptors, IL-10 receptor 1 (IL10-R1) and receptor 2 (IL-10R2), are needed for transmission of IL-10 signal into cells [19, 20, 22, 36]. Human IL-10R1 (hIL-10R1) is a 90–110 kDa, type I transmembrane glycoprotein that encodes a 578 aa protein that contains a 21 aa signal sequence, a 215 aa extracellular domain, a 25 aa transmembrane segment, and a 317 aa cytoplasmic domain [22]. Human IL-10R2 (hIL-10R2) is a 60 kDa, type I transmembrane glycoprotein that encodes a 325 aa protein that contains a 19 aa signal sequence, a 201 aa extracellular domain, a 29 aa transmembrane segment, and a 76 aa cytoplasmic domain [23]. There are two fibronectin type III (FNIII) motifs within the extracellular domain and a STAT3 docking site plus a JAK1 association region within the cytoplasmic domain in hIL-10R1 [19, 20, 22]. hIL-10R2 has also two FNIII motifs within the extracellular domain and is associated with Tyk2 kinase via its cytoplasmic domain [19]. hIL-10 signaling requires the sequential assembly with the extracellular domains of hIL-10R1 and hIL-10R2 [22, 23]. At first, hIL-10 binds to hIL-10R1 with a high affinity interaction and forms an intermediate complex. Subsequently, the hIL-10/hIL-10R1 complex assembles with hIL-10R2 to form an active signaling complex through a low affinity interaction. Considering the hIL-10/hIL-10R1/hIL-10R2 complex formation, hIL-10R1 may be a potential target to block the effects of hIL-10.
To further investigate the above hypothesis, we developed molecules using a concept called “immunoadhesin”. An immunoadhesin is a chimeric, antibody-like molecule that combines the target-binding region of a receptor, a cell adhesion molecule, a ligand, or an enzyme, with the Fc region of an immunoglobulin [6]. Immunoadhesins gain the ability to prolong their half-lives in vitro and in vivo by being fused with the Fc portion of IgG [11, 28]. Plasmid DNAs that contain sequences of hIL-10R1/IgG1 were constructed and tested for their biological activities against hIL-10.
Materials and methods
Molecular design and molecular modeling of hIL-10 immunoadhesins
The three-dimensional structures of immunoadhesins were constructed with MOE (version 2004.03, CCG Inc., Montreal, Canada). Sequences of IL-10R1 extracellular domain (ECD) and IgG1 (Hinges + CH2 + CH3) are obtained from the Brookhaven Protein Databank 1LQS and 1HZH, respectively. Then, these fragments were extended with joint amino acid sequences (Gly and Ala). The molecular mechanics calculations were performed using Amber94 force field. The energy minimization was performed using three methods: first the Steepest Descent, followed by the Conjugate Gradient, and finally the Truncated Newton. The resulting three-dimensional structures of immunoadhesins were displayed using MolFeat (Ver. 2, FiatLux, Tokyo, Japan).
Construction of recombinant transfer vectors and transfection
The ECD of human IL-10R1 [IL-10R1(ECD)] was cloned by RT-PCR from FirstChoice™ Human Cell Line Total RNA T-Cell Leukemia (Jurkat) (Ambion, Austin, TX). IL-10R1 (ECD) is the complementary DNA molecule encoding hIL-10R1 lacking the transmembrane and cytoplasmic domains, and the termination codon. The hinge and Fc regions of human immunoglobulin G1, [IgG1(Hinge + CH2 + CH3)] were cloned from  neo1-CD80/CD86/IgGFc (a gift from Dr. David B. Weiner, University of Pennsylvania, Philadelphia, PA) by PCR.
neo1-CD80/CD86/IgGFc (a gift from Dr. David B. Weiner, University of Pennsylvania, Philadelphia, PA) by PCR.
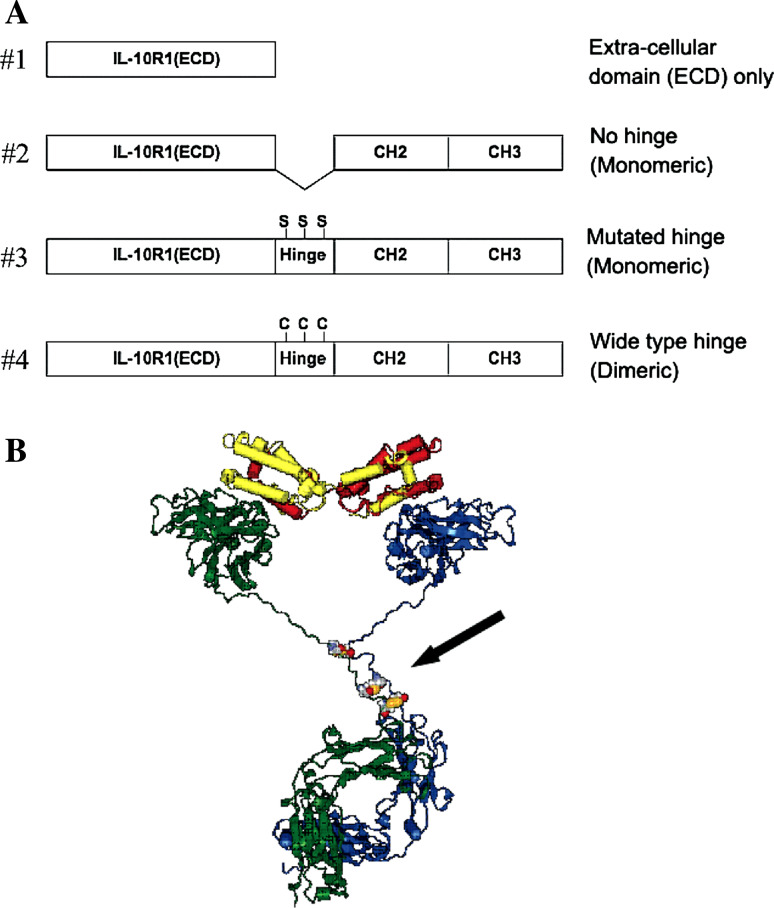
The resulting IL-10R1(ECD) and IgG1(CH2 + CH3) with or without the hinge were incorporated into the pVAX1 vector, which has a CMV promoter (Invitrogen, Carlsbad, CA). The schematic structures of the immunoadhesins used in this study are shown in Fig. 1a. The following plasmid vectors were constructed by using the corresponding primers:
#1 (ECD only): [pVAX1-IL-10R1(ECD)], IL-10R1_F_Hind3 and IL-10R1_1_R_EcoR1;
#2 (no hinge): [pVAX1-IL-10R1/IgG1(CH2 + CH3)], IL-10R1_F_Hind3 and IL-10R1_2_R_BamH1 for IL-10R1(ECD) and IgG1_1_F_BamH and IgG1_R_EcoR1 for IgG1(CH2 + CH3);
#3 (mutated hinge, cystines - > serines): [pVAX1-IL-10R1/IgG1 (mt-hinge + CH2 + CH3)], IL-10R1_F_Hind3 and IL-10R1_2_R_BamH1 for IL-10R1(ECD) and IgG1_2_F_BamH and IgG1_R_EcoR1 for IgG1(mt-hinge + CH2 + CH3);
#4 (wild type hinge): [pVAX1-IL-10R1/IgG1 (wt-hinge + CH2 + CH3)], IL-10R1_F_Hind3 and IL-10R1_2_R_BamH1 for IL-10R1(ECD) and IgG1_3_F_BamH and IgG1_R_EcoR1 for IgG1(wt-hinge + CH2 + CH3).
Fig. 1.
IL-10 Immunoadhesin constructs. a Schematic structures of IL10R1/IgG1Fc constructs, b Predicted three-dimensional molecular structure of human IL-10 homodimer with dimerized IL-10R1/IgG1Fc immunoadhesin (#4) Human IL-10, red and yellow; immunoadhesin (#4), blue and green. Three disulfide bonds of hinge region are presented with the CPK model (arrow)
Due to the lack of disulfide bond formation, plasmid vectors #2 (no hinge) and #3 (mutated hinge) produce monomeric immunoadhesin molecules. On the other hand, plasmid vector #4 (wild type hinge) produces a disulfide-bonded homodimeric IL-10 immunoadhesin via cystines on wild-type hinge. All of the inserted fragments were confirmed by DNA sequencing. The backbone pVAX1 vector was used as a control (#0, backbone).
The following primers were used for the plasmid DNA vector construction:
IL-10R1_F_Hind3, 5′-GCCCCCAAGCTTGCCGCCACCATGCTGCCGTGCCTCG-3′;
IL-10R1_1_R_EcoR1, 5′-ATCGGGGAATTCTCAGTTGGTCACGGTGAAATACTGC-3′;
IL-10R1_2_R_BamH1, 5′-ATCGGGGGATCCGTTGGTCACGGTGAAATACTGC-3′;
IgG1_1_F_BamH1, 5′-CGCGGATCCGCACCTGAACTCCTGGG-3′;
IgG1_2_F_BamH, 5′-CGCGGATCCGAGTCCAAATCTTCTGACAAAACTC-3′;
IgG1_3_F_BamH, 5′-CGCGGATCCGAGTCCAAATCTTGTGACAAAACTC-3′;
IgG1_R_EcoR1, 5′-ATCGGGGAATTCTCATTTACCCGGAGACAGGG-3′.
Cell lines and cell culture
Melanoma cell lines (H88, ZA, and JB) were established in our laboratory from metastatic melanoma tissues harvested from patients with stage III or IV cutaneous melanoma. The ZA cell line does not produce IL-10, whereas JB and H88 cell lines constitutively produce IL-10 (100–500 pg/106 cells per day). The melanoma cells were cultured in RPMI 1640 medium (Mediatech, Inc., Herndon, VA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 4 mM l-glutamine, 1% nonessential amino acids, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The COS-1 cell line (SV-40 transformed African green monkey kidney cells) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). COS-1 cells were cultured with Dulbecco’s Modified Eagle’s medium (DMEM; Mediatech, Inc., Herndon, VA) supplemented with 10% heat-inactivated FCS, 4 mM l-glutamine, 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Non-transfected COS-1 cells did not produce IL-10 (data not shown).
Transfection of cells with plasmid vectors and collection of supernatants
Twenty-four hours before transfection, either melanoma cells or COS-1 cells were seeded into a 6-well plate in 1 ml of culture medium at a concentration of 2 × 105 cells/ml. Two μg of backbone plasmid vector pVAX1 (#0, backbone), pVAX1-IL-10R1 (ECD) (#1, ECD only), pVAX1-IL-10R1/IgG1-Fc (#2, no hinge; #3, mutated hinge; #4, wild-type hinge) were separately transfected into the cells with FuGENE6 (Roche, Indianapolis, IN). Twenty hours after transfection, cells were washed and then fresh culture medium was added. Three days after transfection, the supernatants were collected, centrifuged (800×g for 10 min) and filtered through 0.22 μm filter (Millipore Corporation, Bedford, MA). The supernatants were stored at −30°C until use. The efficiency of plasmid DNA delivery into COS-1 cells was approximately 60%, as estimated from the transfection of the vector encoding green fluorescent protein, pMaxGFP (Amaxa Inc., Koeln, Germany) (data not shown).
Detection of immunoadhesin mRNA in transfected cells
One-step RT-PCRs were performed to detect mRNA derived from transfected DNA plasmid vectors according to the manufacturers’ instructions (Invitrogen, Carlsbad, CA). For the synthesis of cDNA, specimens were first treated at 50°C for 30 minutes and 94°C for 2 min. This was followed by 25 cycles of PCR amplification at 94°C for 40 s, 62°C for 45 s, 72°C for 2 min, with a final extension of 72°C for 10 min.
The following primer pairs were used:
IL-10 R1_F, 5′-AAGTACCTGCTATGAAGTGGCGCT-3′;
IL-10 R1_R, 5′-TCTTCCCGAGGATGAAGCCATTGT-3′;
IgG1_R, 5′-TCATTTACCCGGAGACAGGG-3′;
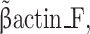 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′;
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′;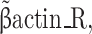 5′-CTAGAAGCATTGCGGTGGACGATGGAGGG-3′.
5′-CTAGAAGCATTGCGGTGGACGATGGAGGG-3′.
The PCR products were separated on a 1.5% agarose gel and stained with ethidium bromide. The sizes of final PCR products for individual plasmid vectors are as follow: #1 (ECD only), 700 base pairs (bps); #2 (no hinge), 1,360 bps; #3 (mutated hinge), 1,400 bps; #4 (wild-type hinge, dimeric), 1,410 bps. β-actin served as an internal control.
Detection of immunoadhesin proteins
Secretion of immunoadhesins from transfected COS-1 cells was confirmed by the Western blot analysis. Thirty-six hours after transfection of COS-1 cells with vectors, culture media were replaced with serum-free DMEM, and COS-1 cells were cultured for additional 36 h. The culture media were then collected and concentrated five times on Microcon YM50 filtration devise (Millipore, Bedford, MA). For the Western blotting, samples were prepared in reducing (denaturing) and non-reducing (native) conditions. Two μg of total protein per lane were separated on a 4–20% SDS PAGE (Invitrogen) followed by a transfer onto PVDF membranes. Membranes were probed with either goat anti-human IL-10R1 antibody (1:5,000; R&D SYSTEMS, Minneapolis, MN) for the detection of hIL-10R1, or with mouse anti-human IgG (Fc specific) antibody (1:10,000; SIGMA, St. Louis, MO) for the detection of IgG Fc. Immune complexes were then detected by horseradish peroxidase (HRP)-labeled anti-goat (Jackson ImmunoResearch, West Grove, PA) or anti-mouse (Promega, Madison, WI) secondary antibodies at a dilution of 1:200,000 with subsequent visualization using Supersignal WestFemto detection kit (Pierce).
Capturing assay for human IL-10
For the capturing assay for exogenously added IL-10, IL-10 immunoadhesin proteins or supernatants from transfected cells were mixed with recombinant human IL-10 (rhIL-10, NCI Biological Resources Branch, Rockville, MD). The mixtures were placed into a 96-well round plate (Corning Incorporated, Corning, NY) and incubated at 37°C for 30 min. After incubation, the levels of unbound human IL-10 in the supernatants were measured by a commercially available ELISA kit (BioSource International, Inc., Camarillo, CA).
In terms of culture supernatants obtained from transfected cells, the plasmid vectors were transfected into an IL-10 non-producing melanoma cell line (ZA), COS-1, and IL-10 producing melanoma cell lines (JB, H88), respectively. The 3-day-culture supernatants from the non-IL-10 producers (ZA, COS-1) were tested with exogenously added IL-10 with the method described above. As for the capturing assay for endogenously produced IL-10, the supernatants from the JB and H88 cells transfected with the plasmid vectors were assayed for the unbound IL-10 using the same ELISA kit. The minimum detection level of the human IL-10 ELISA kit is 1 pg/ml.
Neutralizing assay for human IL-10
The effectiveness of IL-10 immunoadhesins in blocking the biological activities of human IL-10 produced by tumor cells was tested in the co-culture of tumor cells with PBMC. Since autologous PBMC were not available for the established tumor cell lines, we tested an allogeneic response against the IL-10 producing tumor cell line JB, using PBMC obtained from an HLA-mismatched healthy donor. Twenty-four hours prior to pVAX1 transfection, IL-10 producing melanoma cells (JB) were seeded at 2 × 105 per well into a 12-well plate (Corning Incorporated) with 1 ml of culture medium. Two μg of plasmid vectors were separately transfected into the melanoma cells with FuGENE6 (Roche). After washing the plate, allogeneic PBMC from a healthy donor were added at 2 × 106/well and then incubated at 37°C in 5% CO2. After 3 days of incubation, the supernatants were collected. IFN-gamma production in the supernatants was measured by the above-mentioned ELISA kit.
Production and purification of IL-10 immunoadhesin
Based on the results of plasmid vector transfection to COS-1 cells as well as melanoma cells, we chose immunoadhesins construct #3 (mutated hinge) for the large-scale protein production and purification. Production of human IL-10 immunoadhesins was done in suspension CHO-S cells (Invitrogen, Carlsbad, CA) adapted to ProCHO5 media (Lonza, Walkersville, MD). To increase protein expression, cDNAs coding for immunoadhesins were re-cloned into pUB mammalian expression vector under the control on human ubiquitin promoter (pUbC) which provide elevated levels of protein expression [29]. The resultant plasmids were stably transfected into CHO-S cells. Protein-expressing cells were selected with blasticidin (Invitrogen), cultured and propagated to obtain 400 ml of culture containing 3 × 106 cells per ml. Then, culture media were collected, clarified by consecutive filtration through 9 and 0.45 μm and concentrated on Amicon Ultrafiltration YM50 membrane (Millipore). Immunoadhesins were purified from the media on Protein G column using FPLC system (GE Healthcare/AKTA) and dialyzed against PBS. The capturing capability of purified IL-10 immunoadhesin (#3, mutated hinge) was tested by adding recombinant human IL-10. Mouse anti-human IL-10 antibody (MAB 217, purchased from R&D systems Minneapolis, MN) was also included in individual experiments to compare the binding capacity.
Experiments were performed according to the guidelines of the Biosafety Committee of Thomas Jefferson University.
Statistical analysis
Individual experiments were repeated at least three times to confirm the consistency of the data. Experiments were performed in triplicate to obtain the mean, standard deviation (SD), and standard errors (SE). The results were considered as statistically significant if P values were less than 0.05 with the two-tailed student t test.
Results
Molecular design of immunoadhesins
The crystal structure of IL-10/IL-10R1 complex revealed that IL-10 formed a homodimer comprised of two intertwining peptide chains and bound to two IL-10R1 molecules. [15, 16]. Computer modeling was used to predict the location and length of a hinge region of IgG1 Fc that would allow the formation of a stable immunoadhesin homodimer. The results of our molecular modeling suggested that the immunoadhesin can form a complex with IL-10 (Fig. 1b). However, the disulfide bond close to IL-10 (ECD) region in the immunoadhesin hinge is normally used for binding to the IgG1 light chain and thus, its state (i.e., free cystein or disulfide bond) in the immunoadhesin is not certain. It is also speculated that monomeric immunoadhesin might be sufficient to bind the circulating IL-10 homodimer and prevent its interaction with membrane-bound IL-10 receptors. Considering these possibilities, three different variants of the fusion junction, as shown in Fig. 1a, were designed to carry out a systematic investigation on IL-10 per immunoadehisin interactions.
Construction of immunoadhesins
Immunoadhesins were created by an in-frame fusion of the IL-10R1 (ECD) with the constant regions (CH2 and CH3 domains) of the human IgG1 heavy chain with or without the hinge region (Fig. 1b). The vector #1 (ECD only) only contains the extra-cellular domain of human IL-10R1. The vectors #2 and #3 were designed to secrete a monomeric immunoadhesin by eliminating disulfide attachment of the heavy chains via hinge regions. The plasmid vector #4 (wild-type hinge, dimeric) was designed to secret a disulfide-bonded homodimeric immunoadhesin via disulfide bonds at two or three cystine sites. These constructed plasmid vectors were tested for production of targeted molecules. The biological activity of excreted molecules was also tested in IL-10 capturing and neutralizing assays to investigate the most suitable structures for future pre-clinical and clinical studies.
Expression of transfected constructs
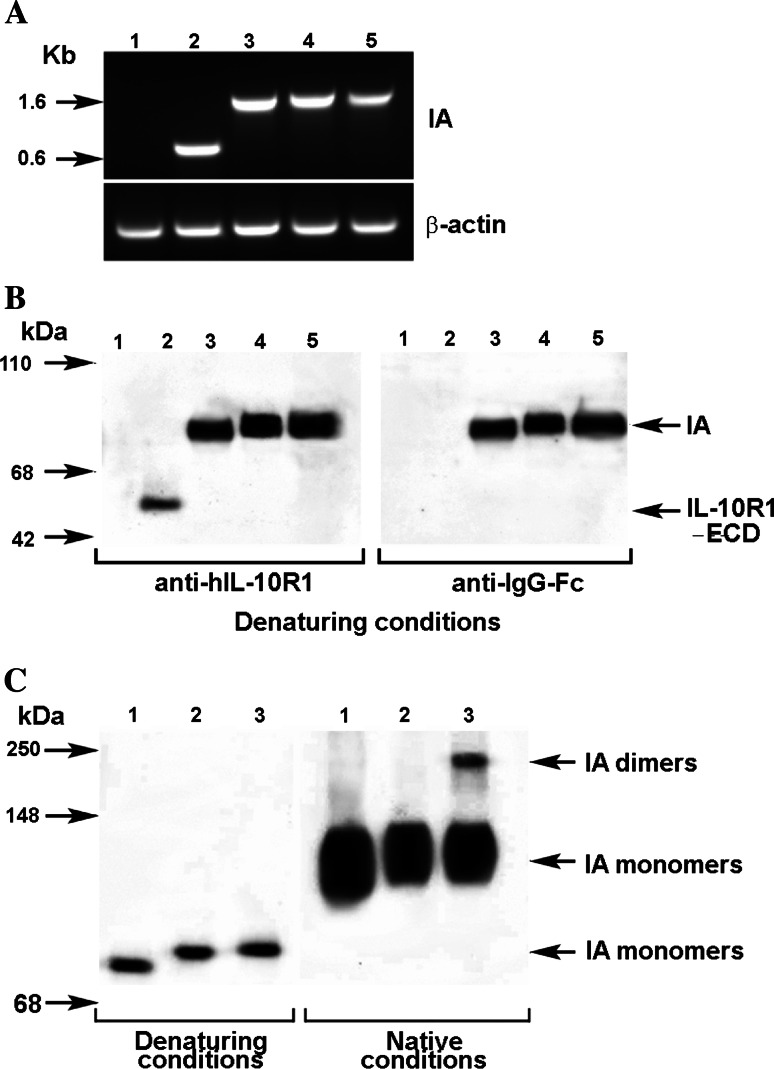
To confirm the transcription of transfected gene sequences, the expression of mRNA of immunoadhesins transcribed from plasmid DNAs #1 (ECD only), #2 (no hinge), #3 (mutated hinge) and #4 (wild-type hinge, dimeric), were assessed by RT-PCR analysis. As shown in Fig. 2a, the RT-PCR products obtained from transfected COS-1 cells matched the estimated sizes of transcribed products from individual pVAX1 vectors.
Fig. 2.
Detection of immunoadhesin. a mRNA derived from transfected pVAX1 vectors Total RNA was extracted from COS-1 cells that were transfected with immunoadhesins plasmid vectors. One-step RT-PCR was used to amplify the targeted mRNA. The PCR products were analyzed as described in Sect. Materials and methods. Immunoadhesins (IA): lane 1 #0 (wild type pVAX1), lane 2 #1 (IL10R1 extracellular domain only, 700 bps), lane 3 #2 (IL10R1/IgG1Fc with no hinge, 1,360 bps), lane 4 #3 (IL10R1/IgG1Fc with mutated hinge 1,400 bps), lane 5 #4 (IL10R1/IgG1Fc with wild type hinge 1,410 bps). β-actin (bottom) was served as an internal control to assure the integrity of mRNA. b Secretion of the immunoadhesin proteins. Serum-free culture media was collected 72 h after transfection of COS1 with different constructs. The culture media were then concentrated five times on Microcon YM50 filtration devise and separated on a 4–20% SDS PAGE followed by a transfer onto PVDF membranes in reducing (denaturing) conditions. Membranes were probed with either goat anti-human IL-10R1 antibody or with mouse anti-human IgG (Fc specific) antibody. Lane 1 #0 (backbone pVAX1), lane 2 #1 (IL10R1 ECD only), lane 3 #2 (IL10R1/IgG1Fc with no hinge), lane 4 #3 (IL10R1/IgG1Fc with mutated hinge), lane 5 #4 (IL10R1/IgG1Fc with wild type hinge). c Dimeralization of immunoadhesins. The concentrated culture supernatants obtained from transfected COS-1 cells were analyzed by the Western blotting method in reducing (denaturing) and non-reducing (native) conditions. Proteins were detected on blots by using anti-human IgG Fc specific antibodies. Dimerization of the immunoadhesin #4 (IA dimmers in lane 3) was confirmed in native conditions with the presence of higher molecular weight protein. Lane 1 #2 (IL10R1/IgG1Fc with no hinge), lane 2 #3 (IL10R1/IgG1Fc with mutated hinge), lane 3 #4 (IL10R1/IgG1Fc with wild type hinge)
Detection of Immunoadhesin proteins
The secretion of immunoadhesin proteins in supernatants obtained from transfected COS-1 cells was confirmed by Western blot analysis. As shown in Fig. 2b, IL-10 R1 protein was detectable in the supernatants obtained from COS-1 cells transfected with construct #1 (lane 2, ECD only), #2 (lane 3, no hinge), #3 (lane 4, mutated hinge) and #4 (lane 5, wild-type hinge, dimeric). The intensity of band #1 (ECD only) was weaker than those of immunoadhesin complexes (#2, #3, #4; IL-10 R1/IgG-Fc). Since the total protein concentration in individual supernatants was comparable and the amount of total protein that was used for the Western blotting analysis was adjusted, this would indicate the lower secretion of IL-10 R1 (ECD) protein into the supernatant #1 or instability of secreted ECD protein without IgG Fc. The intensity of bands #2 (no hinge), #3 (mutated hinge) and #4 (wild-type hinge, dimeric) are comparable. Furthermore, the comparison of the Western blotting results using reduced (denaturing) and non-reduced (native) conditions confirmed the monomeric nature of construct #2 (no hinge) and #3 (mutated hinge) (Fig. 2c, lanes 1 and 2). In contrast, the immunoadhesins with wild type hinge (construct #4, lane 3) showed dimerization of immunoadhesin molecules.
Biological activity of immunoadhesins in capturing exogenous and endogenous IL-10
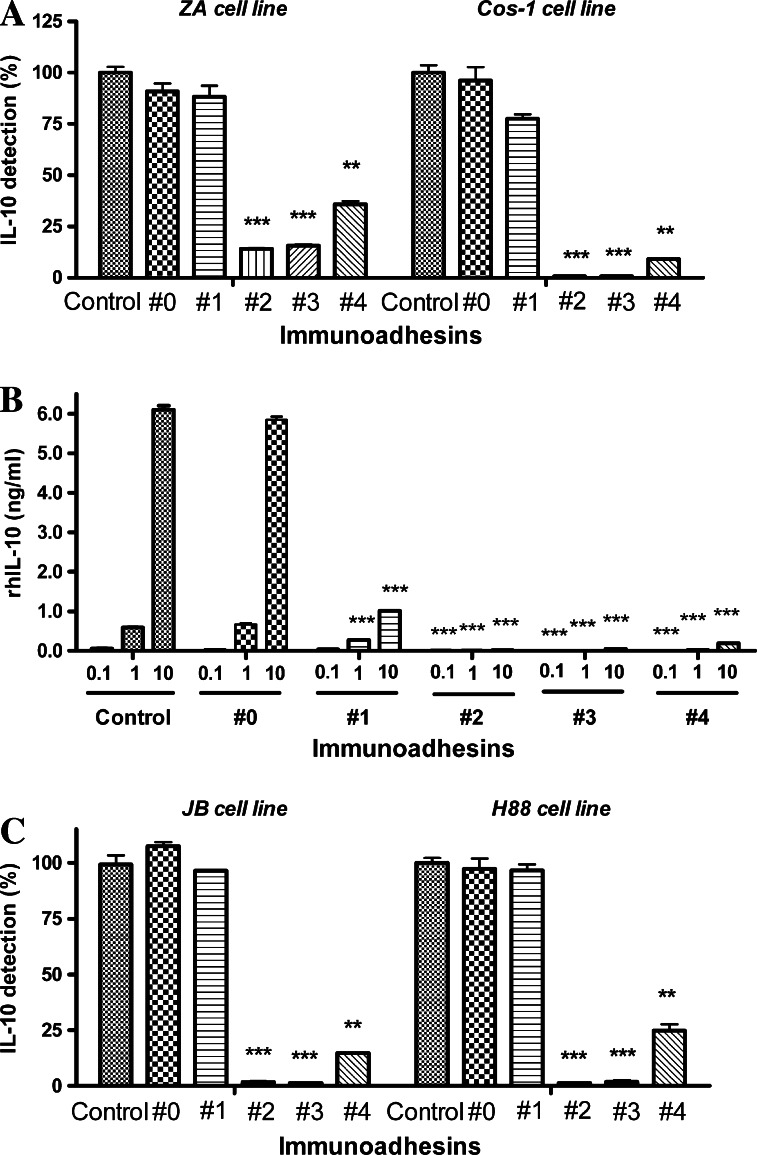
To investigate whether immunoadhesins that were secreted into the cell culture supernatants retained their biological activity in vitro, we performed the capturing assay for exogenously added and endogenously produced IL-10. In preclinical and clinical settings, we might consider a gene therapy using a viral vector containing IL-10 immunoadhesins. Therefore, we first investigated whether supernatants from IL-10 non-producing cell lines transfected with the plasmid vectors could capture exogenously added rhIL-10. As shown in Fig. 3a, the supernatants obtained after transfection with IL-10 immunoadhesin plasmid vectors were able to capture exogenously added rhIL-10. The capturing was much more efficient with monomeric immunoadhesins (#2, no hinge; #3, mutated hinge). The monomeric immunoadhesins from plasmid vectors #2 and #3 demonstrated almost complete blocking of exogenously added rhIL-10 up to 10 ng/ml (Fig. 3b). In contrast, the supernatant from plasmid vectors #1 (ECD only) was much less effective in capturing exogenous-added IL-10. Furthermore, the supernatant from plasmid vectors #4 (wild-type hinge, dimeric) showed the inferior activity compared to monomeric immunoadhesins. These results confirmed that transfection of IL-10 immunoadhesin plasmid vectors into cells resulted in the production and secretion of IL-10 immunoadhesins into supernatants, where they bound IL-10. Moreover, the results indicated that the plasmid vectors encoding monomeric immunoadhesins might be superior to those encoding dimeric immunoadhesin or IL-10 R1 ECD itself in capturing rhIL-10 after being transfected to cells.
Fig. 3.
Capturing of human IL-10 by immunoadhesins. a IL-10 non-producing human melanoma cell line ZA and COS-1 cells were transfected with plasmid vectors and culture supernatants from transfected cells were collected. The culture supernatants were incubated with 1 ng/ml of rhIL-10 for 30 min and unbound rhIL-10 was measured by an IL-10 ELISA kit. The data were shown as recovery rates (%) of rhIL-10. The recovery rates were calculated using the rhIL-10 levels detected in the supernatants obtained from untransfected cells as 100%. Control: supernatant from untransfected cells, #0 backbone pVAX1, #1 IL10R1 extracellular domain only, #2 IL10R1/IgG1Fc with no hinge, monomer, #3 IL10R1/IgG1Fc with mutated hinge, monomer, #4 IL10R1/IgG1Fc with wild type hinge, dimer. **P < 0.01, ***P < 0.001, compared to control. b Different concentrations of rhIL-10 (0.1, 1, 10 ng/ml) were incubated with COS-1 culture supernatants that contained different types of IL-10 immunoadhesin. After 30 min of incubation, unbound rhIL-10 was measured by an IL-10 ELISA kit. Control: supernatant from untransfected cells, #0 backbone pVAX1, #1 IL10R1 extracellular domain only, #2 IL10R1/IgG1Fc with no hinge, monomer, #3 IL10R1/IgG1Fc with mutated hinge, monomer, #4 IL10R1/IgG1Fc with wild type hinge, dimer. ***P < 0.001, compared to control. c IL-10 producing human melanoma cell lines JB and H88 were transfected with IL-10 immunoadhesin plasmid vectors and then incubated for 3 days. Endogenously produced human IL-10 in the culture supernatants were measured by an ELISA kit. The data were shown as IL-10 detection rates (%). The IL-10 detection rates were calculated using the IL-10 levels in the supernatants obtained from untransfected cells (control: JB 90 pg/ml, H88 620 pg/ml) as 100%. Control supernatant from untransfected cells, #0 backbone pVAX1, #1 IL10R1 extracellular domain only, #2 IL10R1/IgG1Fc with no hinge, monomer, #3 IL10R1/IgG1Fc with mutated hinge, monomer, #4 IL10R1/IgG1Fc with wild type hinge, dimer. **P < 0.01, ***P < 0.001, compared to control
We further investigated whether the immunoadhesins could capture endogenously produced IL-10. JB and H88 melanoma cell lines that constitutively produce human IL-10 (90 and 620 pg/ml, respectively) were transfected with the immunoadhesin plasmid vectors and detectable IL-10 levels in the supernatants were compared to that of backbone PVAX-1 (#0). As shown in Fig. 3c, the transfection with monomeric immunoadhesins plasmid vectors #2 (no hinge) and #3 (mutated hinge) demonstrated almost 100% capturing of endogenously produced IL-10. As was the case with the experiments using exogenously added IL-10, the dimeric immunoadhesin plasmid vector #4 (wild-type hinge) was less effective in capturing endogenously produced human IL-10. Transfection with plasmid vector #1 (ECD only) had only minimum effect on capture of endogenously produced IL-10.
Neutralizing activity of immunoadhesins for IL-10 produced by melanoma cells
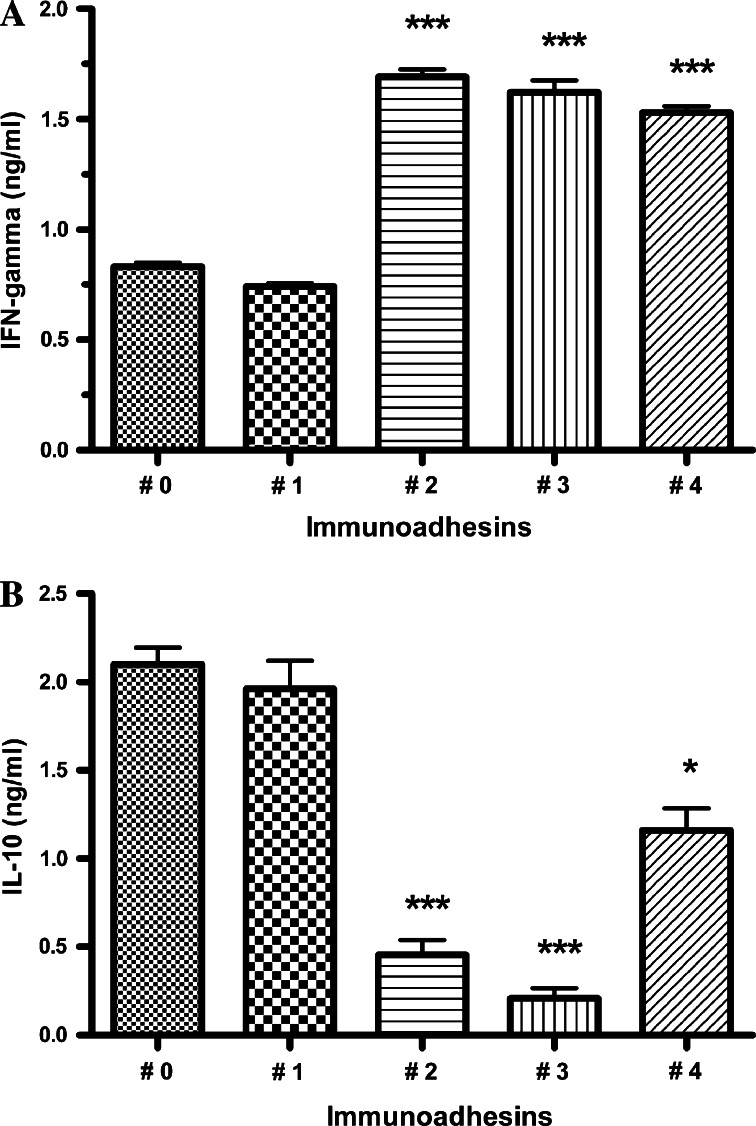
As D’Andrea et al. [7] reported, human IL-10 inhibits the production of IFN-gamma from PBMC. To ascertain whether IL-10 immunoadhesins could neutralize this biological activity of human IL-10 produced by melanoma cells, the production of IFN-gamma from PBMC in response to allogeneic tumor cells in the presence of immunoadhesins was measured. As shown in Fig. 4a, the IL-10 producing melanoma cells (JB) transfected with plasmid vectors #2 (no hinge), #3 (mutated hinge), and #4 (wild-type hinge, dimeric) induced significant production of IFN-gamma by allogeneic PBMC in comparison with melanoma cells transfected with backbone vector (#0). The increase in IFN-gamma production by PBMC was correlated to the decrease in IL-10 levels in the supernatants (Fig. 4b).
Fig. 4.
IL-10 immunoadhesins enhance IFN-γ productions by PBMC against allogeneic IL-10-producing melanoma cells by capturing secreted IL-10. PBMC from a healthy donor were cultured with allogeneic IL-10-producing melanoma cells (JB) transfected with plasmid vectors. After 3 days of co-culture, the culture supernatants were collected and productions of IFN-gamma (a) and IL-10 (b) were measured by ELISA kits. #0 backbone pVAX1, #1 IL10R1 extracellular domain only, #2 IL10R1/IgG1Fc with no hinge, monomer, #3 IL10R1/IgG1Fc with mutated hinge, monomer, #4 IL10R1/IgG1Fc with wild type hinge, dimer. *P < 0.05, ***P < 0.001, compared to #0
Potency of IL-10 immunoadhesin proteins with mutated hinge
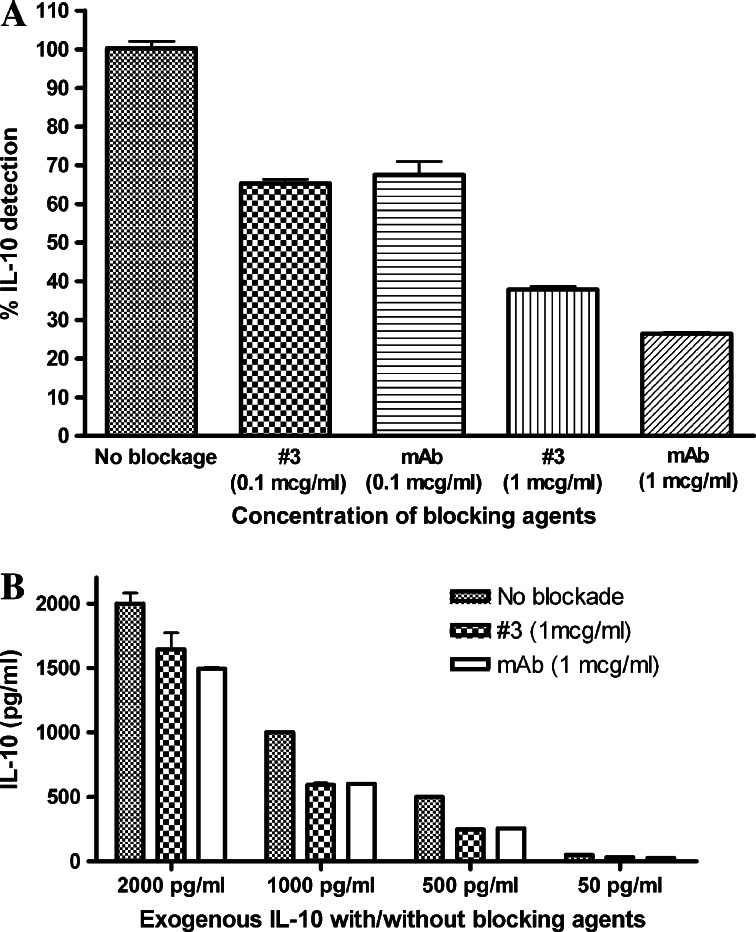
Monomeric IL-10 immunoadhesin (#3, mutated hinge) was selected for the large-scale protein production based on the results obtained from the plasmid vector transfection to melanoma cells. As seen in Fig. 5a, purified human IL-10 immunoadhesin #3 captured recombinant human IL-10 (500 pg/ml) in a dose-dependent manner. The binding efficacy of IL-10 immunoadhesin #3 is comparable to that of anti-human IL-10 monoclonal antibody. This was further confirmed by adding different amounts of IL-10 to the fixed amount of IL-10 immunoadhesin or anti-IL-10 antibody (1 μg/ml) (Fig. 5b).
Fig. 5.
Potency of purified monomeric IL-10 immunoadhesin protein. a Purified human IL-10 immunoadhesin #3 proteins (0.1 and 1 μg/ml) were incubated with recombinant human IL-10 (500 pg/ml). After 30 min of incubation, unbound IL-10 was measured by an ELISA assay. Mouse anti-human IL-10 mAb was used with the same concentration. The results are shown as percentage of detectable IL-10 compared to IL-10 detectable in control wells that did not contain either IL-10 immunoadhesins or anti-IL-10 mAb. b Various amounts of IL-10 (2,000, 1,000, 500, and 100 pg/ml, respectively) were added to the wells that contained 1 μg/ml of immunoadhesins #3. The same amounts of IL-10 were also added to the wells that contained 1 μg/ml of mouse anti-human IL-10 mAb. After 30 min of incubation, unbound IL-10 was measured by the ELISA assay
Discussion
Our results confirmed that transfection of human melanoma cells with plasmid vectors containing DNA constructs for IL-10R1/IgG1-Fc induces production of fusion proteins (IL-10 immunoadhesins) into culture supernatants and capture endogenously produced as well as exogenously added human IL-10. Furthermore, the transfection of IL-10-producing melanoma cells with IL-10 immunoadhesin vectors enhances IFN-gamma production by allogeneic PBMC.
As to the biological activity of immunoadhesins in capturing exogenous and endogenous IL-10 after being transfected to cells, the monomeric immunoadhesins (#2, no hinge; #3, mutated hinge) were much more effective than the dimeric configuration (#4, wild-type hinge). This result is rather surprising since we added several amino acids to the traditional binding portion of the hinge region to increase the flexibility of IL-10R1 portion when we designed the fusion molecules. Although the Western blotting data indicate comparable amounts of dimeric and monomeric immunoadhesin production in the supernatants (Fig. 2b, c), this could still be related to the lower concentrations of dimeric immunoadhesin that was released to the culture supernatants by transfected cells. Nevertheless, the result of this experiment indicates the inferiority of IL-10 immunoadhesin with wild type hinge when used for a gene therapy.
For more than a decade, humanized antibodies and immunoadhesins have been developed and investigated for their potential use in human therapy [21]. Since the first attempt by Gascoigne et al. [13], more than 50 immunoadhesins have been developed and investigated for their biological functions. An immunoadhesin for TNF-alpha is currently in clinical use [2] and clinical trials using immunoadhesin for vascular endothelial growth factor (VEGF trap) has been conducted [33]. Additionally, a gene therapy using soluble CD4-IgG immunoadhesin has shown its efficacy in treatment of HIV in preclinical models [40]. According to Chamow et al. [6], the majority of immunoadhesins combine the hinge and Fc regions of an IgG1 heavy chain with the ECD of a type I transmembrane protein that recognizes a specific ligand. Immunoadhesin would play a key role in situations in which human monoclonal antibodies for specific molecules are not available or difficult to develop. They are considered to be less immunogenic, compared to monoclonal antibodies obtained from other species or chimeric antibodies. Furthermore, the increased size conferred by the Fc region of immunoadhesin prolongs its half-life in vitro and in vivo by evading renal clearance and by the stability of fusion protein [11, 28].
In this regard, extensive comparison has been made of various TNF-alpha blocking agents and it has been reported that TNF-alpha receptor-Fc fusion protein (TNFR-Fc) has distinct characteristics compared to anti-TNF alpha mAbs. Kohno et at. [18] compared binding characteristics of TNFR-Fc (etanercept) with those of two different types of anti-TNF mAbs (adalimumab and infliximab). It was found that one or two TNFR-Fc molecules bind to one TNF trimer, while both mAbs form a variety of complexes with a wide range of sizes and stoichiometries. Formation of large complexes with antigens via cross-linking is common phenomenon in antibodies and this could trigger subsequent immune responses such as complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity. This is a significant concern for the application of IL-10 mAb in patients since there might be significant numbers of IL-10 producing cells such as B cells or monocytes circulating in the blood. Significant systemic side effects such as anaphylactic shock might develop if IL-10 mAb triggers antibody-dependent or antibody-induced immune responses. In fact, steroid pretreatment has been used prior to infusion of commercially available anti-cancer mAbs to avoid anaphylactic reactions. Usage of steroids is a concern when mAbs are to be used to enhance a T cell immune response against cancer. In this regard, monomeric IL-10 immunoadhesins are considered to be ideal compounds since cross-linking is not a major concern.
Despite the conflicting data published in animal systems [26, 37, 38], it is clearly the consensus of opinion that human IL-10 has significant suppressive effects on anti-tumor T cell immune response based on various clinical observations in cancer patients, [3, 4, 8, 17, 30, 41]. The results presented here suggest that immunoadhesins for IL-10 may be useful as therapeutic agents for cancer and could be a useful strategy to reverse or neutralize the effects of IL-10 produced in tumor microenvironment. There are several therapeutic applications of IL-10 immunoadhesins in cancer patients. For example, intra-tumoral injection of either IL-10 immunoadhesin vectors or proteins could block the local effect of IL-10 that is produced or induced by tumor cells and might induce an anti-tumor response in injected tumor site. This might result in subsequent development of a systemic anti-tumor immune response. Alternatively, the systemic infusion of IL-10 immunoadhesin proteins could capture the circulating IL-10 and shift the Th2-dominant immune environment to the Th1 type environment in cancer patients. Furthermore, the IL-10 immunoadhesins could be used as an immuno-adjuvant for cancer vaccine. Co-administration of IL-10 immunoadhesins with cancer vaccine could enhances a Th-1 type immune response in the vaccine injection site and facilitates the establishment of systemic anti-tumor immunity. We are in the process of developing human IL-10 immunoadhesin products suitable for human use for the purpose of initiating clinical trials to define pharmacokinetics, immunopharmacology, toxicity and utility in cancer patients.
Acknowledgments
This study was supported by the Bonnie Kroll Research Fund, the Siobhan McDonald Research Fund, and the Eye Melanoma Research Fund.
Footnotes
M. Terai and Y. Tamura contributed equally to this work. Yutaka Tamura and Eiko Otsuka are listed as inventors in the pending patent application for IL-10 Immunoadhesins (PCT/JP2004/013090).
References
- 1.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Barrera MV, Habicheyn S, Mendiola MV, Herrera Ceballos E. Etanercept in the treatment and retreatment of psoriasis in daily clinical practice. Eur J Dermatol. 2008;18:683–687. doi: 10.1684/ejd.2008.0541. [DOI] [PubMed] [Google Scholar]
- 3.Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, Grosse-Wilde H, Broelsch CE, Gerken G, Cicinnati VR. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10:7260–7269. doi: 10.1158/1078-0432.CCR-04-0872. [DOI] [PubMed] [Google Scholar]
- 4.Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, Banchereau J, Favrot MC. Serum interleukin-10 in non-Hodgkin’s lymphoma: a prognostic factor. Blood. 1993;82:2169–2174. [PubMed] [Google Scholar]
- 5.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamow SM, Ashkenazi A. Immunoadhesins: principles and applications. Trends Biotechnol. 1996;14:52–60. doi: 10.1016/0167-7799(96)80921-8. [DOI] [PubMed] [Google Scholar]
- 7.D’Andrea A, Aste-Amezaga M, Valiante MN, Ma X, Kubin MGT. Interleukine 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killter cell stimulatory factor/IL-10 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vita F, Orditura M, Galizia G, Romano C, Roscigno A, Lieto E, Catalano G. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest. 2000;117:365–373. doi: 10.1378/chest.117.2.365. [DOI] [PubMed] [Google Scholar]
- 9.de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE (1992) Interleukin-10. Curr Opin Immunol 4:314–320 [DOI] [PubMed]
- 10.Denizot Y, Turlure P, Bordessoule D, Trimoreau F, Praloran V. Serum IL-10 and IL-13 concentrations in patients with haematological malignancies. Cytokine. 1999;11:634–635. doi: 10.1006/cyto.1998.0468. [DOI] [PubMed] [Google Scholar]
- 11.Donohue JH, Rosenberg SA. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130:2203–2208. [PubMed] [Google Scholar]
- 12.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gascoigne NR, Goodnow CC, Dudzik KI, Oi VT, Davis MM. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci USA. 1987;84:2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard M, O’Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 15.Jones BC, Logsdon NJ, Josephson K, Cook J, Barry PA, Walter MR. Crystal structure of human cytomegalovirus IL-10 bound to soluble human IL-10R1. Proc Natl Acad Sci USA. 2002;99:9404–9409. doi: 10.1073/pnas.152147499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josephson K, Logsdon NJ, Walter MR. Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site. Immunity. 2001;15:35–46. doi: 10.1016/S1074-7613(01)00169-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 18.Kohno T, Tam LT, Stevens SR, Louie JS. Binding characteristics of tumor necrosis factor receptor (TNFR)-Fc fusion proteins vs anti-tumor necrosis factor mAbs. J Investig Dermatol Symp Proc. 2007;12:5–8. doi: 10.1038/sj.jidsymp.5650034. [DOI] [PubMed] [Google Scholar]
- 19.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–5903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotenko SV, Pestka S. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene. 2000;19:2557–2565. doi: 10.1038/sj.onc.1203524. [DOI] [PubMed] [Google Scholar]
- 21.Liossis SN, Tsokos GC. Monoclonal antibodies and fusion proteins in medicine. J Allergy Clin Immunol. 2005;116:721–729. doi: 10.1016/j.jaci.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wei SH, Ho AS, l Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. [PubMed] [Google Scholar]
- 23.Lutfalla G, Gardiner K, Uze G. A new member of the cytokine receptor gene family maps on chromosome 21 at less than 35 kb from IFNAR. Genomics. 1993;16:366–373. doi: 10.1006/geno.1993.1199. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci G, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cell Immunol. 2002;215:162–172. doi: 10.1016/S0008-8749(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 26.Mocellin S, Panelli M, Wang E, Rossi CR, Pilati P, Nitti D, Lise M, Marincola FM. IL-10 stimulatory effects on human NK cells explored by gene profile analysis. Genes Immun. 2004;5:621–630. doi: 10.1038/sj.gene.6364135. [DOI] [PubMed] [Google Scholar]
- 27.Moore KW, O’Garra A, l Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau RW, Satoh H, Scheide S, Crowl R, Conroy R, Garland WA, Liberato DJ. A comparison of mass balance, pharmacokinetics and disposition of [14C(U)]- and [125I]recombinant human interleukin-2 in cynomolgus monkeys. Drug Metab Dispos. 1995;23:904–909. [PubMed] [Google Scholar]
- 29.Novak L, Igoucheva O, Cho S, Alexeev V. Characterization of the CCL21-mediated melanoma-specific immune responses and in situ melanoma eradication. Mol Cancer Ther. 2007;6:1755–1764. doi: 10.1158/1535-7163.MCT-06-0709. [DOI] [PubMed] [Google Scholar]
- 30.Ordemann J, Jacobi CA, Braumann C, Schwenk W, Volk HD, Muller JM. Immunomodulatory changes in patients with colorectal cancer. Int J Colorectal Dis. 2002;17:37–41. doi: 10.1007/s003840100338. [DOI] [PubMed] [Google Scholar]
- 31.Patel KB, Berd D, Mastrangelo MJ, Terai M, Sato T. Prognostic importance of interleukin-10 (IL-10) production by metastatic melanoma tissues from patients receiving autologous vaccine therapy. Proc of ASCO. 2002;21:432a. [Google Scholar]
- 32.Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, Hernandez IM, Gutierrez C, Lopez-Berestein G, Camacho LH. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–2444. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 33.Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ, Yancopoulos GD. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene-based discovery of the next generation of angiogenesis targets. Cold Spring Harb Symp Quant Biol. 2005;70:411–418. doi: 10.1101/sqb.2005.70.052. [DOI] [PubMed] [Google Scholar]
- 34.Salazar-Onfray F, Charo J, Petersson M, Freland S, Noffz G, Qin Z, Blankenstein T, Ljunggren HG, Kiessling R. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing IL-10. J Immunol. 1997;159:3195–3202. [PubMed] [Google Scholar]
- 35.Sato T, McCue P, Masuoka K, Salwen S, Lattime EC, Mastrangelo MJ, Berd D. Interleukin 10 production by human melanoma. Clin Cancer Res. 1996;2:1383–1390. [PubMed] [Google Scholar]
- 36.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stearns ME, Garcia FU, Fudge K, Rhim J, Wang M. Role of interleukin 10 and transforming growth factor beta 1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin Cancer Res. 1999;5:711–720. [PubMed] [Google Scholar]
- 38.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5:189–196. [PubMed] [Google Scholar]
- 39.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk A. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.V99.7.2468. [DOI] [PubMed] [Google Scholar]
- 40.Touraine JL, Sanhadji K, Sembeil R. Gene therapy for human immunodeficiency virus infection in the humanized SCID mouse model. Isr Med Assoc J. 2003;5:863–867. [PubMed] [Google Scholar]
- 41.Uwatoko N, Tokunaga T, Hatanaka H, Osada H, Kawakami T, Yamazaki H, Abe Y, Kijima H, Ueyama Y, Nakamura M. Expression of IL-10 is inversely correlated with distant metastasis of renal cell carcinoma. Int J Oncol. 2002;20:729–733. [PubMed] [Google Scholar]
- 42.Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S, Burg G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer. 1997;71:630–637. doi: 10.1002/(SICI)1097-0215(19970516)71:4<630::AID-IJC20>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]