Abstract
Drug dosing is commonly based on the dogma that, increasing the dose maximizes the therapeutic response until a dose level that is prohibitively toxic is reached. This doctrine also applies to antibody therapy, as several protocols have explored dose escalation. We have analyzed the effect of different amounts of a homophilic Herceptin targeting a human lung tumor cell line, and discovered that the normal dose-potency relationship does not apply. To study this paradoxical effect of antibody concentration on potency, we examined the molecular species of the homophilic Herceptin under different concentrations using size exclusion chromatography and gel electrophoresis. We also varied experimental conditions in FACS tumor targeting, such as concentration of antibody, membrane immobilization, temperature, and antibody homo/dimer immobilization. We observed that high concentrations of homophilic Herceptin reduce targeting, and also noted the tumor growth arrest in the xenograft mice after the tumor reached a critical size. The therapeutic window appears to be defined by tumor size and antibody concentration. Since the concentration of this homophilic antibody defines the optimum targeting window, our data suggest the therapeutic dose of antibody should be matched with the tumor burden.
Keywords: Immunotherapy, Homophilic antibody, Her2/neu, Lung tumor, Xenograft
Introduction
Our laboratory has developed a technology to enhance the binding of antibodies to tumors. The basis for this technology was adopted from a naturally occurring murine antibody that spontaneously homo-dimerizes [7, 11, 12]. The homophilic domain in the variable region of the so-called T15 monoclonal antibody comprises a 24mer peptide [10]. A similar homophilic antibody based on a different homophilic peptide has been described [9]. Recently, we developed methods to photochemically crosslink this homophilic peptide sequence to any antibody, thereby imparting the homodimerizing activity to the antibody of choice [17]. In addition, we have made homophilic antibodies that enhance both antibody targeting and effector functions (Kohler et al. Submitted) [20, 21, 23].
Antibody-based therapies, either alone or in combination with therapeutic agents, have demonstrated clinical success [1]. For example, Herceptin is an effective therapeutic antibody targeting the Her2/neu receptor. Currently, Herceptin is used to treat approximately 25% of breast cancer patients with tumors exhibiting high Her2/neu levels [14, 16]. Conversely, in lung cancer, which expresses low levels of Her2/neu, Herceptin failed [5].
Several mechanisms of how Herceptin kills tumors have been proposed, [8] including apoptosis. One way in which apoptosis can be induced is the crosslinking of specific cell surface receptors [2]. Antibodies can effectively crosslink receptors, provided the target receptor density is sufficiently high for bivalent antibodies to attach. Such antibody-mediated receptor crosslinking can be enhanced by the binding of a second antibody to the receptor bound antibody [6]. Based on these observations, low-target expression and unfavorable density of target distribution in tumors limit the efficacy of Herceptin.
Here we describe the generation of a homophilic Herceptin, and analyze the interaction of the homophilic Herceptin with a Her2/neu expressing NSCLC tumor. We found that, in comparison to naked Herceptin, homophilic Herceptin is more efficient in targeting and inducing apoptosis at a low concentration, and less efficient at a high concentration. The likely reason for this paradoxical dosing effect is a concentration monomer/dimer-equilibrium between the target and the solution phase.
Results
Optimization of conjugation and characterization of the homophilic Herceptin conjugate
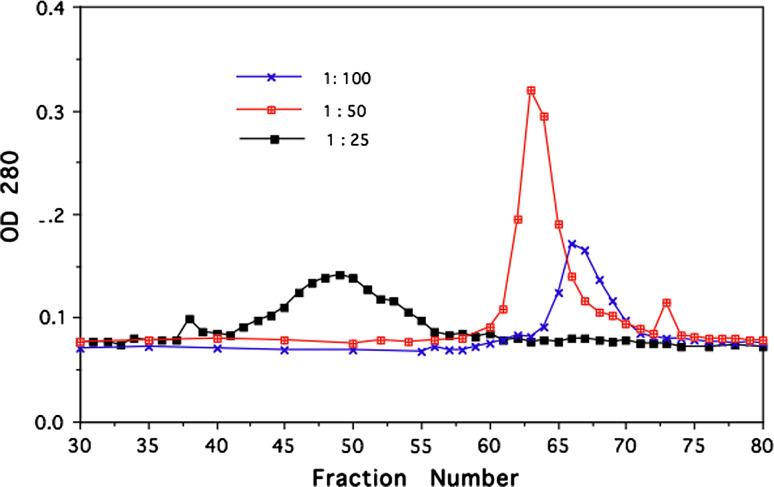
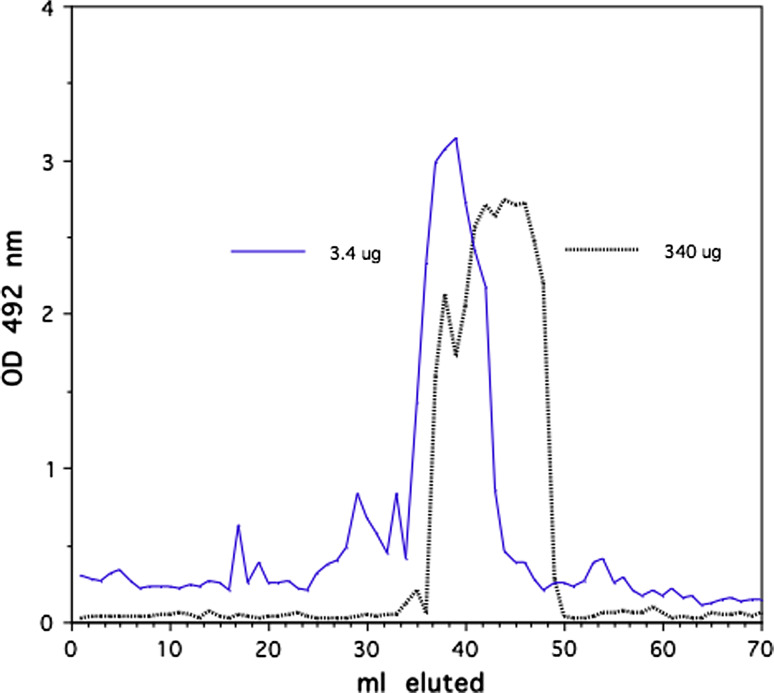
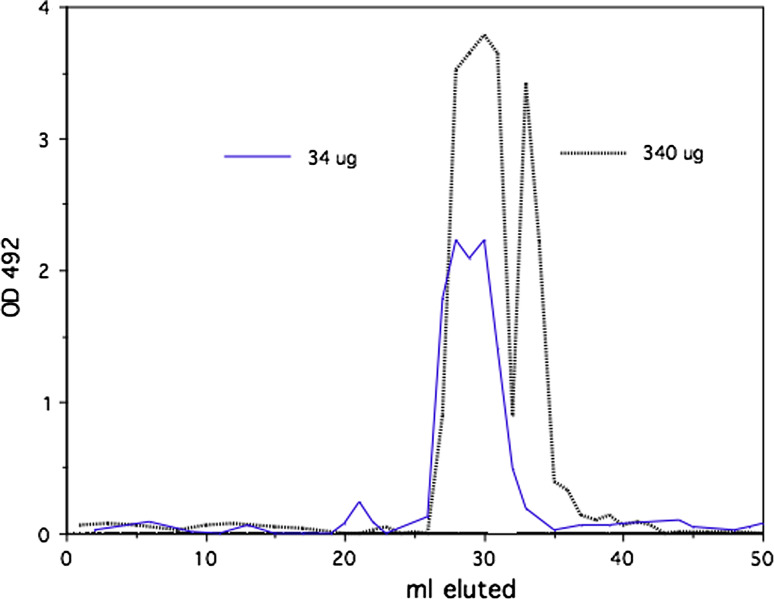
Herceptin was crosslinked with the homophilic 24mer peptide using photo-affinity protocol as described [17]. In initial experiments, we established an optimum peptide/antibody conjugation ratio of peptide over Herceptin (Fig. 1). Size-exclusion chromatography with 2% PEG homophilic antibodies appear as dimer and monomer species [13]. Under this condition Herceptin conjugated to the homophilic peptide at different molar ratios produces higher molecular species as the ratio peptide to antibody is varied. Herceptin conjugated with a 25 molar excess of peptide produced the highest amount of high molecular species in the chromatography. This conjugation ratio was used for all the experiments reported here. SDS PAGE of the conjugate Herceptin showed the typical banding of heavy and light chains (data not shown). The conjugate was analyzed by a series of size-exclusion chromatography runs on Sephadex and Sephacryl columns in comparison with native Herceptin under physiological condition. Different amounts of homophilic Herceptin were applied to the Sephacryl column and eluted material was monitored by OD 280 nm and with aliquots for Ig capture ELISA. Figure 2 shows a typical elution profile of homophilic conjugate using different amounts of protein; 3.4 and 340.0 μg were applied to Sephacryl S 300 columns. As shown, the apparent molecular weight of the homophilic Herceptin is higher with the low amount than with the high amount. Figure 3 depicts elution profiles from a G25 Sephadex column with 34 and 340 μg of homophilic Herceptin. The lower amount of antibody applied is eluted as a single peak with the higher amount in two peaks, the second peak at lower molecular weight position. Figure 4 shows the comparison of Herceptin and homophilic Herceptin applied to native gel electrophoresis using no detergent and stacking gel. The homophilic Herceptin indicates higher molecular protein aggregates than the native Herceptin. These data indicate that the apparent molecular weight of the homophilic Herceptin conjugate is greater at low-protein concentration than at highconcentration indicating that homophilic dimerization in solution is dependent on protein concentration. Control size exclusion runs with naked Herceptin at different concentrations did not produce any change in eluted peak volume (data not shown).
Fig. 1.
Photo-affinity conjugation of Herceptin with homophilic peptide at different ratios. Tryptophan containing homophilic peptide was added to Herceptin at different amounts and photo-crosslinked as described [17]. The conjugate was applied to Sephacryl S300 in PBS containing 2% PEG. Collected fractions were monitored at 280 nm
Fig. 2.
Conjugated Herceptin (homophilic Herceptin) at different amounts, 3.4 and 340 μg, was chromatographed on Sephacryl S300 in PBS. Overlay chromatograms with 3.4 and 340 μg, monitored with Ig-capture ELISA
Fig. 3.
Conjugated Herceptin, 340 and 34 μg, was chromatographed on Sephadex G25 in PBS. Eluted antibody was detected by IG-ELISA
Fig. 4.
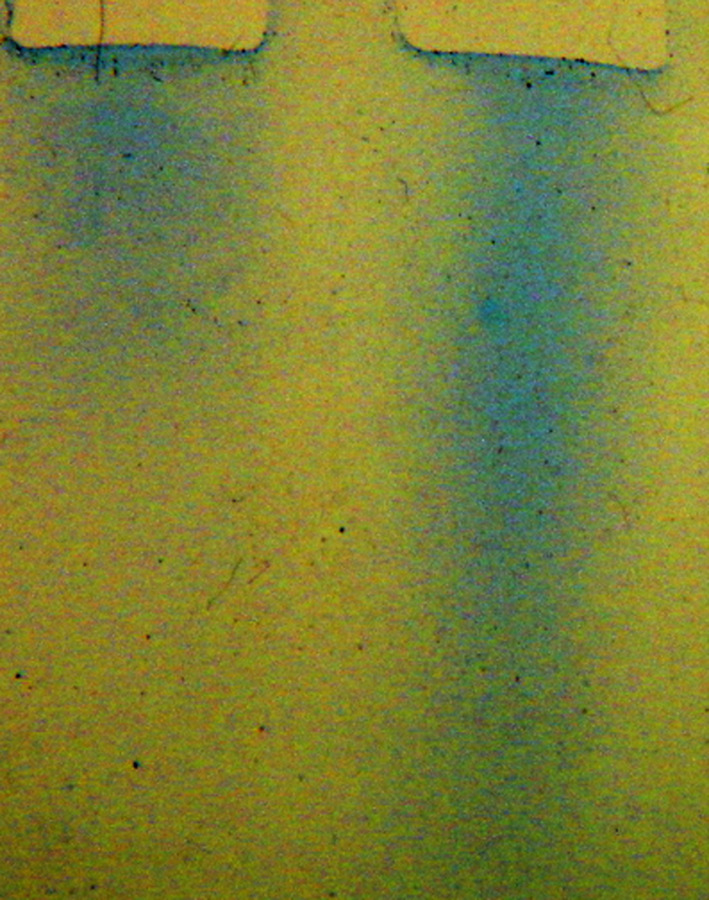
Native gel electrophoresis with a non-denaturing detergent as described. Left lane 3.4 μg Homophilic Herceptin, right lane 3.4 μg of Herceptin
Dynamics of homophilic targeting of the H1650 lung tumor cell line
Previous studies [13, 21] indicate that homophilic antibodies, natural occurring or chemically conjugated, exist in a dynamic equilibrium between monomers and dimers. This equilibrium heavily favors the monomeric form in physiological solutions, yet can be shifted toward the polymeric form by lowering the salt concentration. Homophilic antibodies dimerize upon attaching, either non-specifically (e.g., plastic) or specifically (e.g., cell surface antigen) [10, 12] to a solid surface. As cellular targets can move in the lipid bilayer, and are known to cluster upon binding to either ligands or antibodies, it was of interest to measure the binding of homophilic antibodies to cellular targets under different conditions. Parameters that influence homophilic antibody binding are (1) antibody concentration, (2) temperature, and (3) mobility of the cellular target.
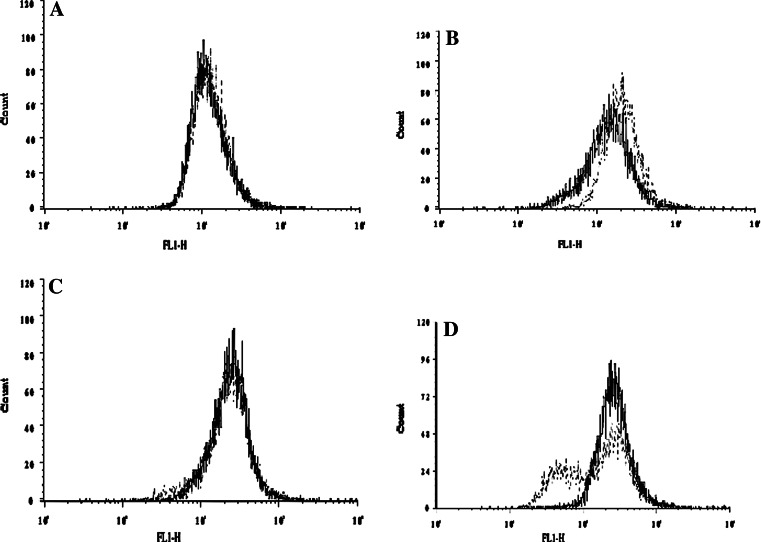
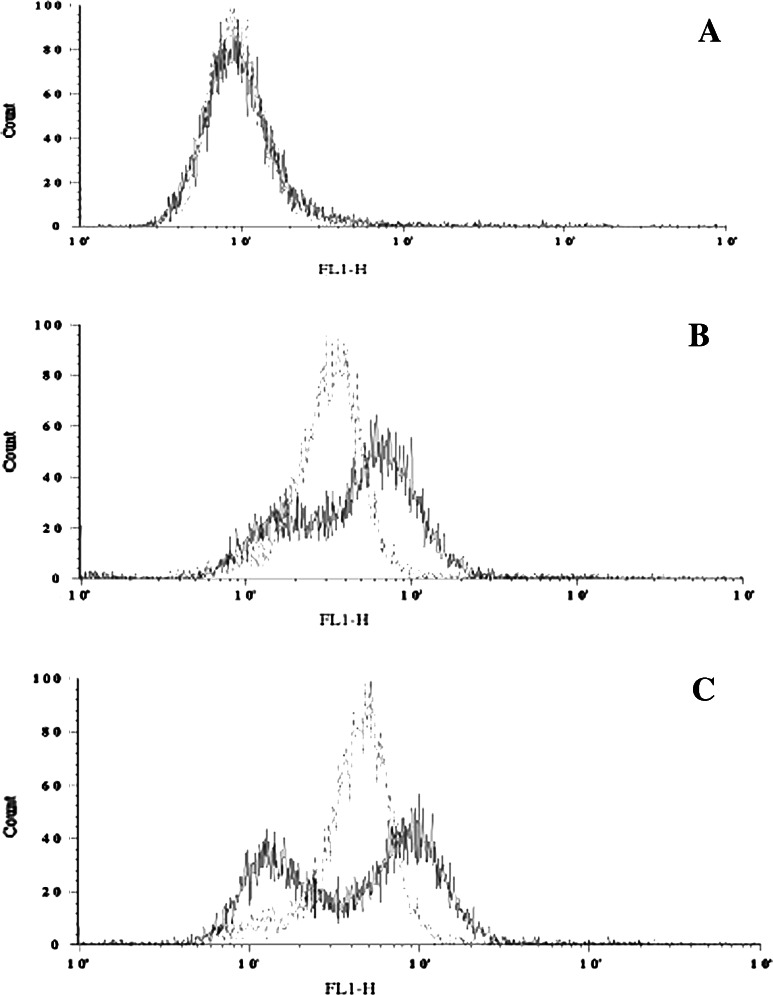
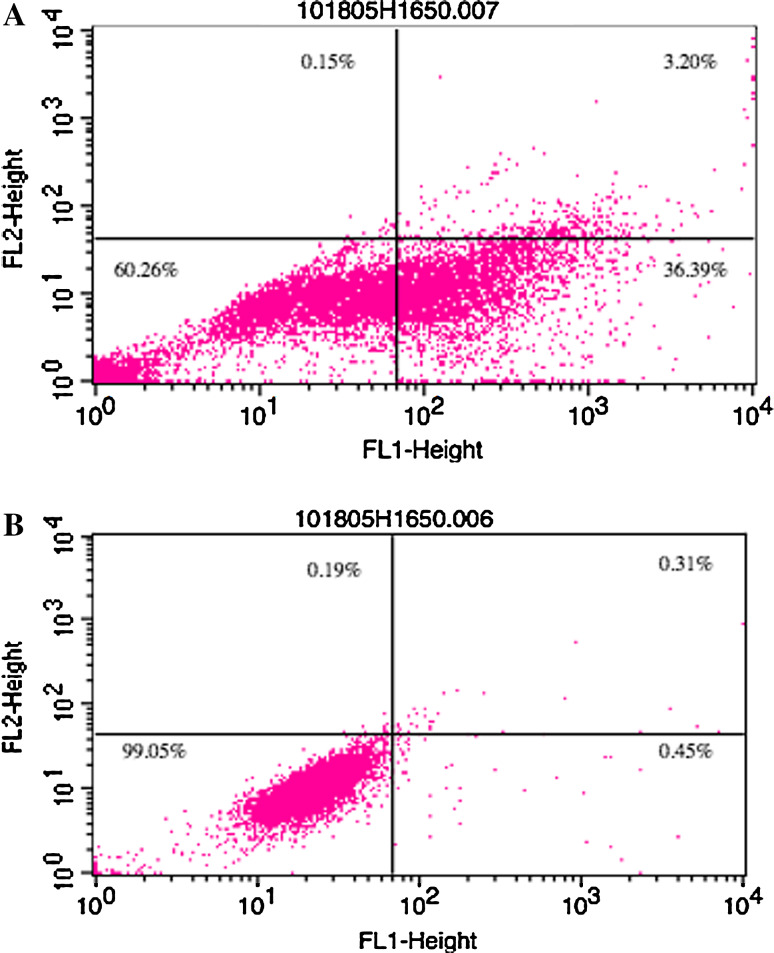
We compared the ability of naked Herceptin and homophilic Herceptin to bind to the NSCLC H1650 cells using fluorescence activated cell sorting (FACS). In our FACS analysis, the concentration of Herceptin and homophilic Herceptin ranged from 0.05 to 5.0 μg/ml. As seen in Fig. 5, the fluorescent intensity is weak for both antibodies at the lowest concentrations (Fig. 5a); as the concentration is increased, the fluorescent signal for the homophilic antibody is higher than that for the naked antibody (Fig. 5b). Upon increasing the concentration, both the naked and homophilic antibody show equal fluorescence intensity (Fig. 5c). However, at the highest concentration, the homophilic Herceptin reveals two populations, of which one has a fluorescence intensity that is higher than that of the naked Herceptin (Fig. 5d) and the intensity of the other is lower than that of the naked Herceptin.
Fig. 5.
Comparison of FACS intensity using Herceptin and homophilic Herceptin on live NSCLC H1650 cells. Solid and the dashed lines show Herceptin- and homophilic Herceptin-treated cells, respectively. a 0.05 μg/ml, b 0.1 μg/ml, c 0.5 μg/ml, d 1 μg/ml antibody
The decreased fluorescence of the homophilic Herceptin at the highest concentration could be caused by one of two mechanisms: (1) the amount of target bound dimer could be affected by the solution dimer/monomer equilibrium, or (2) a high homophilic antibody concentration could induce Her2/neu target clustering, which would in turn be followed by endocytosis. To address the role of internalization, cells were fixed prior to adding the primary homophilic Herceptin antibody to inhibit/reduce membrane mobility. Figure 6a shows that there is no difference in fluorescence intensity between the fixed and live cells exposed to secondary fluorescent antibody only. Using 1 μg/ml of homophilic Herceptin on fixed H1650 NSCLC cells (Fig. 6b) there is a significant increase in fluorescence over the control in Fig. 6a, and with 5 μg/ml (Fig. 6c) there is a further increase in fluorescence intensity. In contrast, using live H1650 NSCLC cells, homophilic Herceptin reveals two populations of fluorescent cells (Fig. 6b), with an increase in the lower fluorescent population at a concentration of 5 μg/ml (Fig. 6c).
Fig. 6.
Comparison of FACS staining with homophilic Herceptin on fixed and live H1650 cells (dashed line and solid line, respectively). a FACS using secondary FITC antibody only. b Using 1 μg/ml of homophilic antibody. c Using 5 μg/ml of homophilic antibody
These data suggest that the binding of homophilic Herceptin to live Her2/neu positive cells induces reduction of the Her2/neu target by one, or a combination, of the following mechanisms: (1) internalization of receptor antibody complexes (2) shedding of receptor antibody complexes or (3) redistribution of receptors, thereby reducing the amount of secondary fluorescent antibody binding. Of these three mechanisms, we can rule out the induction of apoptosis due to the short incubation time with homophilic Herceptin H1650 (data not shown). Homophilic Herceptin stains fixed cells in a dose dependant manner similar to naked Herceptin, however, it does not reach the intensity seen with live cell (Fig. 6b, c).
The effect of temperature on naked and homophilic Herceptin using FACS at low and high fluorescence intensity gates
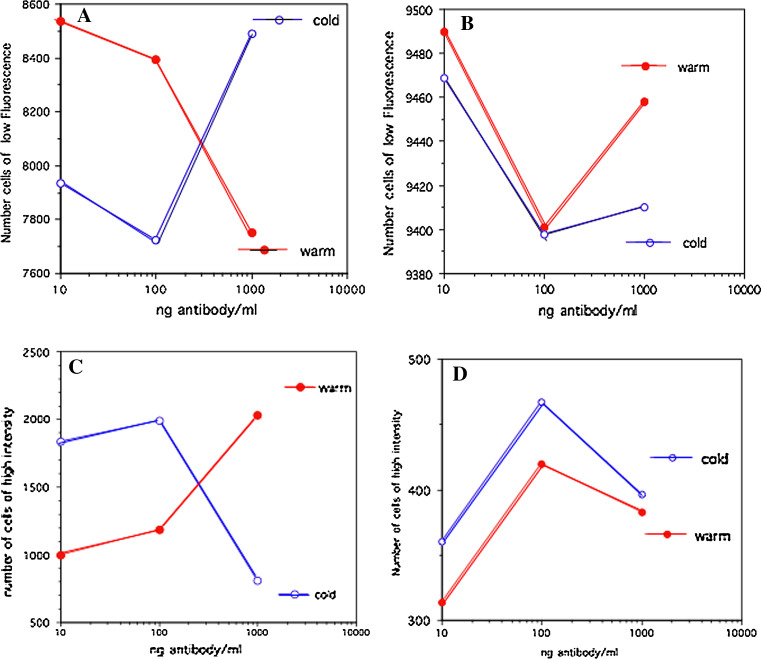
Live H1650 cells were stained with antibody at either 37°C or at 4°C, and FACS analyses were performed gating populations with high- or low-fluorescence intensity. In Fig. 7, the number of fluorescent cells is plotted against increasing amounts of antibody: The antibody concentration ranged from 10 to 1,000 ng/ml; The 1,000 ng/ml concentration of homophilic antibody produces the highest intensity (Fig. 5). Figure 5a, c shows data with homophilic Herceptin, and Fig. 5b, d exhibits FACS data for naked Herceptin. Using homophilic Herceptin staining at 37°C (Fig. 5a, c; closed circles) the number of cells in the low-intensity gate decreases (Fig. 5a), while the number of cells in the high-intensity gate increases (Fig. 5c). We observed the reverse pattern for homophilic Herceptin when staining was performed at 4°C (Fig. 5a, c; open circles). The cells incubated with naked Herceptin at 37 and 4°C showed a biphasic pattern, with the highest concentration yielding a shift to lower fluorescence intensity (Fig. 5b, d). Interestingly, the biphasic patterns for cold and warm antibody labeling are similar indicating that the mechanism producing this pattern is relatively independent of temperature. At lower temperatures, the cell membrane loses its fluidity, becoming rigid and inhibiting receptor movement within the bilayer. This decreased fluidity may affect the binding of dimerized homophilic antibody, and also the monomer/dimer equilibrium, thereby resulting in reduced binding of the homophilic Herceptin (Fig. 7c; open circles).
Fig. 7.
Comparison of FACS staining of live H1650 cells at 4 and 37°C [open circle (cold) and solid circle (warm), respectively]. a, b FACS gated at low-fluorescence intensity a using dilutions of homophilic Herceptin, and b dilutions of Herceptin. c, d FACS gated at high-fluorescence intensity c using dilutions of homophilic Herceptin, and d dilutions of Herceptin
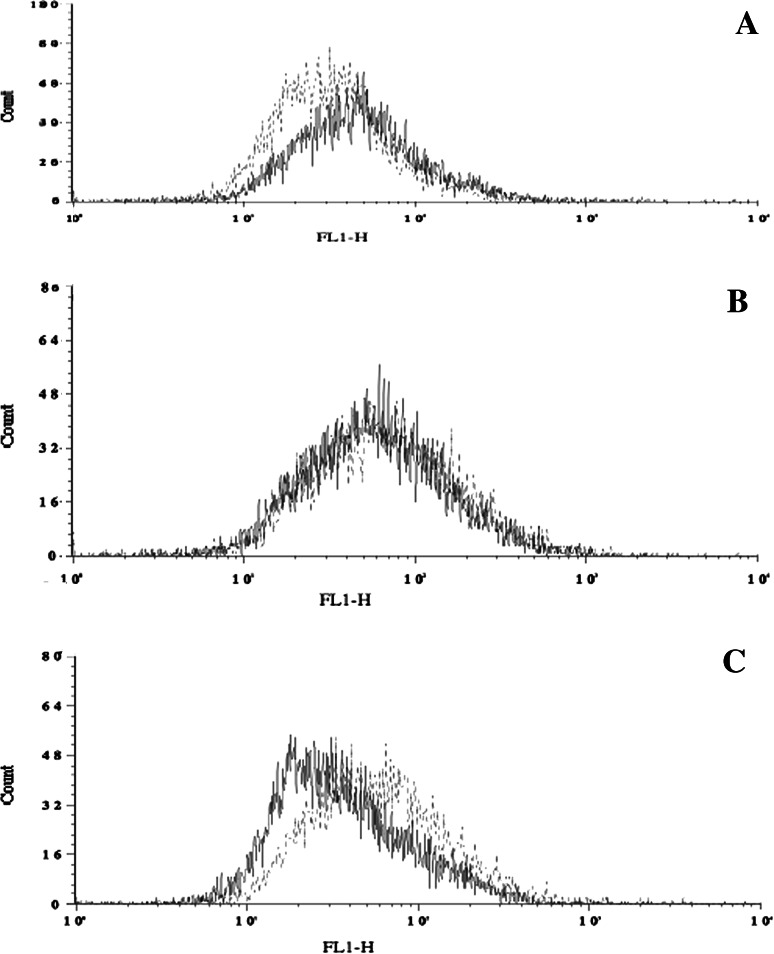
To further investigate the temperature- and concentration-induced shifts in the monomer/dimer equilibrium of homophilic Herceptin, we stabilized the concentration-dependent equilibrium of the monomeric/dimeric forms. This was done by treating the homophilic antibody stock solution with a protein crosslinker, gluteraldehyde, at a low concentration that did not cause aggregation or precipitation (“Materials and methods”). Different concentrations (1, 5, 10 μg/ml) of homophilic Herceptin, gluteraldehyde-treated and untreated, were used to stain live H1650 cells for FACS. In Fig. 8, the FACS histograms of cells exposed to gluteraldehyde-treated and untreated homophilic Herceptin are overlaid. With increasing amounts of gluteraldehyde-treated homophilic Herceptin (dashed line), the fluorescence intensity increased, while with untreated homophilic Herceptin (solid line) the fluorescence decreased. Fixing the homophilic Herceptin in solution abolishes the inverse dose effect relationship of binding to target cells. Based on this data, we hypothesize that the inverse relationship between concentration and fluorescence intensity for homophilic Herceptin is based on equilibrium shifts in solution, and not on the changes in the membrane bilayer of the target cell.
Fig. 8.
FACS of live H1650 cells using untreated homophilic Herceptin or treated with gluteraldehyde (solid line and dotted line, respectively). a 1 μg/ml, b 5 μg/ml, and c 10 μg/ml
Titrating homophilic Herceptin in the induction of apoptosis of H1650 cells
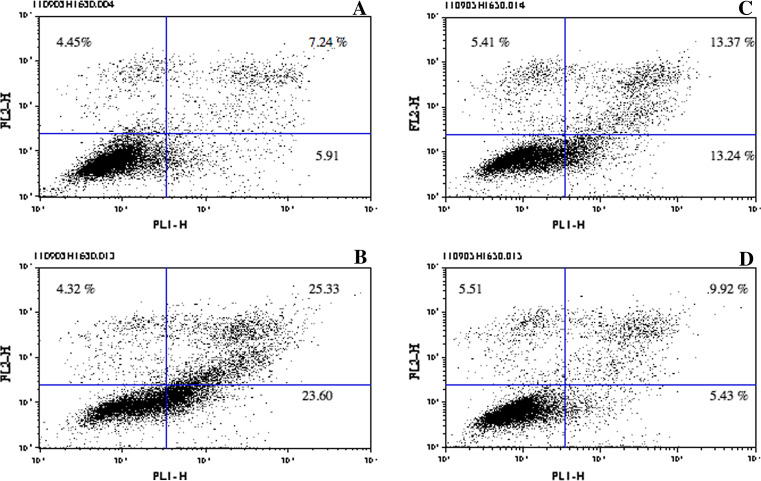
One of the implied therapeutic effects of Herceptin is the induction of apoptosis [4]. H1650 NSCLC cells were incubated with increasing concentrations of homophilic Herceptin as described in “Materials and methods”. Apoptotic and pre-apoptotic cells were measured in FACS using Annexin V and PI. Figure 9 shows the number of apoptotic and pre-apoptotic cells induced by increasing antibody concentration. With 5 μg/ml, 32.02% of cells are pre-apoptotic/apoptotic (sum of upper left, upper right and lower right panels), with 10 μg/ml, 53.25% and with 20 μg/ml 20.83%. To show the greatest difference between homophilic and naked Herceptin, we used 2 μg/ml. Figure 10 compares apoptosis induced by homophilic Herceptin (Fig. 10a) and Herceptin (Fig. 10b): homophilic Herceptin yields 39.74% pre-apoptotic and apoptotic cells, while the naked Herceptin generates only 0.95% pre-apoptotic and apoptotic cells.
Fig. 9.
Induction of apoptosis in H1650 cells using different concentrations of homophilic Herceptin. H1650 cells were incubated overnight at 37°C with either no antibody or homophilic Herceptin. Cells were stained with Annexin V and PI, and analyzed on FACS. a No antibody. b 5 μg/ml antibody. c 10 μg/ml antibody. d 20 μg/ml antibody
Fig. 10.
Induction of apoptosis in H1650 cells using 2 μg/ml of homophilic Herceptin (a) or Herceptin (b)
Comparison of the in vivo antitumor activity of homophilic and naked Herceptin in mouse xenografts
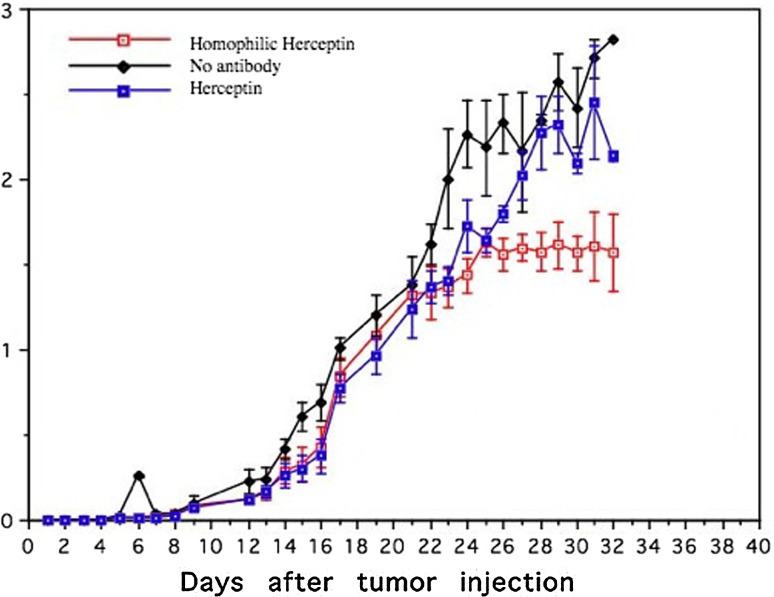
Groups of nude mice (12 per group) were injected with 5 × 106 H1650 cells, and treatment was initiated 24 h post-tumor inoculation. The mice were divided into three groups: (1) untreated control group (no antibody), (2) Herceptin treatment (110 μg/mouse) and (3) homophilic Herceptin treatment (110 μg/mouse). The tumor size was recorded daily. Figure 11 shows the mean tumor size with standard deviation of the three groups. Collectively, the error bars recorded throughout the daily observation of Herceptin treated and untreated groups do not indicate statistically significant difference in tumor growth. In contrast, beginning at day 24, the error bars in the homophilic Herceptin-treated group indicate statistical differences in growth rates versus the untreated and Herceptin-treated groups. It is interesting to note that tumor growth in all three groups is the same for the first 24 days; after day 24, the tumor growth of the homophilic Herceptin group was inhibited while the tumors in the untreated and Herceptin group continued to grow.
Fig. 11.
Xenograft using H1650 cells in nude mice. 5 × 106 H1650 cells were injected into the flank of each mouse. Groups (12 mice each) were untreated (diamond), treated with Herceptin (solid box), or were treated with homophilic Herceptin (open box). Tumor growth was monitored three times weekly using a caliper and is shown on the y axis as cm3. Mice were treated twice weekly with antibody (110°μg/mouse) starting 24 h post-tumor inoculation. All groups of mice were euthanized at day 32. Error bars indicate the standard deviation of each group of 12 mice
Discussion
The novel finding of this work is the paradoxical relationship between homophilic antibody concentration and targeting efficiency. Non-homophilic anti-Her2/neu (Herceptin) shows increased binding to a target cell when the antibody concentration is increased. In contrast, increasing the concentration of homophilic Herceptin reduces both targeting efficiency and induction of apoptosis. Indeed, at the low end of the antibody titration, targeting and apoptosis are higher with homophilic Herceptin than with naked Herceptin at the same concentration. One important property of the homophilic antibody is that its dimerizing potential increases its avidity for its target. Unlike conventional antibody dimers or pentamers, which are covalent structures, homophilic antibodies facultatively dimerize, a process that is controlled by the equilibrium between target-bound antibody and in-solution antibody. Since less homophilic antibody demonstrates better targeting than more, which contradicts the dosage dogma of the direct and proportional relationship between amount and binding, targeting with homophilic Herceptin was studied under different conditions in order to elucidate the unique mechanism of homophilic antibody binding.
The increased potency of a homophilic antibody was first discovered in an animal infection model [3]. In this study, antibody protection from a bacterial challenge (Pneumoccocus pneumoniae) was compared between a non-homophilic and a homophilic antibody (both against the same epitope and with identical affinity). The homophilic antibody T15 (TEPC15) delivered 10,000-fold greater protection than the non-homophilic antibody. Our laboratory has developed methods [17, 20–23] to endow antibodies with increased biological potency by transplanting the homophilic domain of the natural T15 antibody. Such man-made homophilic antibodies show increased CDC, ADDC activity, and greater induction of apoptosis than the non-homophilic counterpart ([20, 23] and unpublished).
The exact mechanism(s) that increases antibody potency are not known, but distinct biophysical properties may contribute to the functional differences between homophilic antibodies and “normal” antibodies. First, homophilic complexes or homophilic dimers can be induced by lowering the ionic strength [20] or adding PEG [13], suggesting a solution state equilibrium. Secondly, ELISA measurements of the amount of homophilic antibody bound to a plastic surface or antigen are greater than that of non-homophilic antibodies [11, 22]. These two phenomena may be involved in the increased potency of homophilic antibodies.
In this study, we have further investigated the biophysical properties of the homophilic Herceptin. We have used size-excluding chromatography and electrophoresis in native gel and buffer. Using different concentrations of homophilic Herceptin, we detected equilibrium of high- and low-molecular species. At low-protein concentration of homophilic Herceptin, a significant amount of antibody is eluted at higher than at monomer position on gel filtration, while at higher concentration only monomeric antibody is detected. These findings demonstrate that homophilic Herceptin exhibits in physiological solution equilibrium of monomeric and dimeric species controlled by concentration of the antibody.
To correlate these biophysical properties of homophilic Herceptin with the finding that low concentration of antibody is more effective in vitro than higher amounts led to a better understanding of the enhancing effects of a homophilic antibody. The dimerizing homophilic Herceptin at low concentration, as pre-formed complex, targets the tumor more effectively than monomeric homophilic Herceptin at higher concentration or as naked Herceptin at any concentration. Pre-formed homophilic complexes latch on to tumor target leading to antibody lattice formation. This in turn increases the biological anti-tumor effects of the homophilic antibody observed in this study. This model also posits a protein–protein complex dissociating force, which becomes effective as the antibody concentration increases. With increasing concentration, the in-solution homophilic complex reaches a critical size that reduces the stability of the complex and the complex dissolves.
We investigated the mechanism(s) of enhanced targeting and apoptosis in a NSCLC cell with the homophilic antibody Herceptin by altering (1) the membrane mobility, (2) inhibiting target movement and (3) freezing the in-solution monomer/dimer equilibrium. Collectively these experiments exclude membrane mobility, which is required for receptor clustering and internalization, as a mechanism of enhanced targeting and apoptosis for the homophilic antibody, and instead support a mechanism that involves concentration-induced changes in the monomer/dimer solution equilibrium.
A paradoxical observation in the tumor binding studies with homophilic Herceptin is the decrease in binding when antibody concentration is increased. This could be due to induction of receptor clustering and internalization, for which there is evidence [4, 15, 18]. However, the finding that both reducing membrane fluidity at low temperature and crosslinking the Her2/neu target did not reverse the loss of binding upon increasing homophilic antibody concentration excludes these mechanisms for homophilic Herceptin.
Immobilizing the in-solution equilibrium of homophilic Herceptin by mild gluteraldehyde fixation converts the binding activity of the homophilic antibody into that of a normal antibody showing concentration-dependant increase of binding. This observation suggests a critical factor that influences targeting of the homophilic antibody is the monomer/dimer in-solution equilibrium, which changes upon varying the antibody concentration. This equilibrium creates a concentration-dependent targeting window.
The xenograft tumor study shows that, with a constant level of circulating antibody maintained by twice weekly injections, the tumor growth ceases after reaching a critical size in homophilic Herceptin treated group. Tumors smaller than this critical size appear not to respond the homophilic antibody. As the tumor continues to increase in size, the antibody reaches a favorable antibody tumor ratio for homophilic lattice formation. This formation is influenced not only by tumor size and tumor target expression, but also by characteristics of antibody distribution, clearance, and, most importantly, dosing. The present observations and further work will lead to guidelines for adjusting the dose of homophilic antibody in relation to tumor burden. Experiments addressing the dosing regimen in xenograft models are subject to future studies.
Materials and methods
Cell lines and reagents
Herceptin was a generous gift from Genentech (San Francisco, CA). Goat α-Human IgG FITC (SB 2040-0) antibodies used for the FACS analyses were obtained from Southern Biotech (Birmingham, AL). NCI-H1650 NSCLC cells were obtained from American Type Culture Collection (Manassas, VA) (CRL 5883). Up to 95% of NCI-H1650 cells express Her-2/neu at moderate levels (Wroblewski and Bixby et al. [19]). AnnexinV (Alexa Fluor®488 conjugate Molecular Probes, Inc. Eugene, OR) and Propidium Iodide were obtained from Sigma–Aldrich (St. Louis, MO).
Photo-affinity conjugation of Herceptin
A 26 mer homophilic peptide [10], which included the C-terminal Ala-Trp homophilic domain, was made by AnaSpec, Inc (San Jose, CA). Conjugation was done as described [17], using a 25 molar excess of peptide over Herceptin.
Size-exclusion chromatography
Sephacryl S300 (Sigma–Aldrich, St. Louis, MO) (70 ml volume column) was equilibrated in PBS or PBS containing 2% PEG1500 (Bohringer Mannheim, Mannheim, Germany). Antibody samples of 1 ml were applied and 1 ml fractions were collected and monitored at 280 nm and/or with aliquots in Ig-capture ELISA.
Native gel electrophoresis
Herceptin and homophlic Herceptin (10 μg) were applied to Novex Tris-Glycine Gels (Invitrogen, Calsbad, CA) using non-denaturing buffers containing 0.2% Nonidet P40 (Boering Mannheim, Mannheim, Germany) run at 125 V constant for 65 min. Gels were stained with SeeBlue (Invitrogen, Carlsbad, CA) and de-stained in H2O.
Ig-capture ELISA
Microtiter plates (Greiner Bio-one, Monroe, NC) were coated with aliquots from chromatography runs, blocked with milk powder, washed and developed with goat anti-human Ig-HRP (Sigma, St Louis, MO). Plates were read on Multiscan MCC/340 (Thermolab System, Helsinki, Finland).
Cell culture
NCI-H1650 NSCLC cells [19] were maintained in RPMI + GlumaMAX medium 1640 (Gibco, Carlsbad, CA) growth media supplemented with 1% Penicillin–Streptomycin 10,000 units/ml (Gibco) and 10% serum HyClone Fetal Clone III (Fisher Sci., Pittsburg, PA). Cells were maintained at 37°C in 4.5% CO2. Cells were harvested using 0.5% Trypsin–EDTA (Gibco).
Measuring surface binding
H1650 cells were harvested, and washed with PBS and then FACS staining buffer (1% Bovine Serum Albumin in PBS). The cells were resuspended in Herceptin or homophilic Herceptin at varying concentrations for 30 min at room temperature or 4°C. The cells were washed two times to remove unbound antibody and reacted with a secondary antibody (Goat α-Human IgG FITC) for 30 min at room temperature or 4°C. The stained cells were fixed using 2% formaldehyde for a minimum of 30 min. They were then processed by Flow Cytometry using a Becton Dickinson (BD, Mountain View, CA) FACS Calibur system.
Measurement of apoptosis
H-1650 cells were plated into 6-well plates at a concentration of 1 × 106 cells per well and allowed to incubate at 37°C for 3 h to facilitate attachment to the plate surface. After 3 h, the media was removed and replaced with fresh media plus the desired concentration of primary antibody (either homophilic or normal Herceptin). Cells were incubated overnight, and, the following day, the cells were harvested, washed in cold (4°C) Annexin-binding buffer (10 mM HEPES, 140 mM NaCl and 2.5 mM CaCl2 pH 7.4), and stained with Annexin, AlexaFluor 433 Annexin, A13201 (Molecular Probes, Eugene, OR) at a concentration of 5 μl per 100 μl of buffer solution for 15 min and was diluted 1:4 using Annexin-binding buffer. Prior to sorting via a Becton Dickinson (BD, Mountain View, CA) FACS Calibur system flow Cytometry, 10 μl propidium iodide (1.5 mM) was added.
Gluteraldehyde treatment of antibodies
Herceptin or homophilic Herceptin (1 mg/ml) was combined with 10 μl fresh 5% gluteraldehyde (S-A G6257). The mixture was allowed to react for 10 min at room temperature with agitation. The reaction was stopped using 10 μl of 2 M l lysine (S-A L1884), and dialyzed overnight against H2O at 4°C to remove unreacted gluteraldehyde.
Xenograft studies
Mouse studies were performed under the University of Kentucky Department of Lab Animal Resources (DLAR) Protocol #929M2005. Daily care was provided by the UK DLAR. Hsd Athymic Nude-Foxn1™/ƒoxn1+ strain mice, 6–7 weeks old, were obtained from Harlan Labs (Indianapolis, IN). Mice were injected subcutaneously on the upper back with 7 × 106 H1650 cells, which had been harvested from tissue culture, washed two times in PBS, and resuspended in 500 μl sterile PBS. Twenty-four hours after inoculation, the mice were divided into three groups of 12 mice each. The first group received no further treatment. The second group was treated with 100 μl of PBS containing 110 μg of naked Herceptin, and the third group with PBS containing 110°μg of homophilic Herceptin. The antibody treatments were administered twice weekly. Measurements of the tumors were taken three times weekly. When the tumors of the control group reached 1.5 cm2, all mice were humanely killed.
Acknowledgments
We wish to thank Mr. Fred Orego, Mr. Mike Russ and Benno Kohler of the Coldstream Innexus facility for performing the FACS analysis and conjugation of Herceptin. We wish to thank Genentech Inc. for the generous gift of Herceptin. Work was supported by Commonwealth of Kentucky Lung Cancer Research Program and The Lucille Parker Markey Cancer Center of University of Kentucky.
References
- 1.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23(9):1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 2.Bazil V, Brandt J, et al. Apoptosis of human hematopoietic progenitor cells induced by crosslinking of surface CD43, the major sialoglycoprotein of leukocytes. Blood. 1995;86(2):502–511. [PubMed] [Google Scholar]
- 3.Briles DE, Forman C, et al. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae . J Exp Med. 1982;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuello M, Ettenberg SA, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61(12):4892–4900. [PubMed] [Google Scholar]
- 5.Gatzemeier U, Groth G, et al. Randomized phase II trial of gemcitabine–cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15(1):19–27. doi: 10.1093/annonc/mdh031. [DOI] [PubMed] [Google Scholar]
- 6.Ghetie MA, Podar EM, et al. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci USA. 1997;94(14):7509–7514. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halpern R, Kaveri SV, et al. Human anti-phosphorylcholine antibodies share idiotopes and are self-binding. J Clin Invest. 1991;88(2):476–482. doi: 10.1172/JCI115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson ES, Hu X, et al. Herceptin sensitizes ErbB2-overexpressing cells to apoptosis by reducing antiapoptotic Mcl-1 expression. Clin Cancer Res. 2006;12(3 Pt 1):845–853. doi: 10.1158/1078-0432.CCR-05-0754. [DOI] [PubMed] [Google Scholar]
- 9.Kaminski MJ, MacKenzie CR, et al. The role of homophilic binding in anti-tumor antibody R24 recognition of molecular surfaces. Demonstration of an intermolecular beta-sheet interaction between vh domains. J Biol Chem. 1999;274(9):5597–5604. doi: 10.1074/jbc.274.9.5597. [DOI] [PubMed] [Google Scholar]
- 10.Kang CY, Brunck TK, et al. Inhibition of self-binding antibodies (autobodies) by a VH-derived peptide. Science. 1988;240(4855):1034–1036. doi: 10.1126/science.3368787. [DOI] [PubMed] [Google Scholar]
- 11.Kang CY, Kohler H. A novel chimeric antibody with circular network characteristics: autobody. Ann NY Acad Sci. 1986;475:114–122. doi: 10.1111/j.1749-6632.1986.tb20861.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang CY, Kohler H. Immunoglobulin with complementary paratope and idiotope. J Exp Med. 1986;163(4):787–796. doi: 10.1084/jem.163.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaveri SV, Halpern R, et al. Self-binding antibodies (autobodies) form specific complexes in solution. J Immunol. 1990;145(8):2533–2538. [PubMed] [Google Scholar]
- 14.Kern JA, Schwartz DA, et al. p185neu expression in human lung adenocarcinomas predicts shortened survival. Cancer Res. 1990;50(16):5184–5187. [PubMed] [Google Scholar]
- 15.Kobayashi H, Shirakawa K, et al. Rapid accumulation and internalization of radiolabeled herceptin in an inflammatory breast cancer xenograft with vasculogenic mimicry predicted by the contrast-enhanced dynamic MRI with the macromolecular contrast agent G6-(1B4 M-Gd)(256) Cancer Res. 2002;62(3):860–866. [PubMed] [Google Scholar]
- 16.Micke P, Hengstler JG, et al. c-erbB-2 expression in small-cell lung cancer is associated with poor prognosis. Int J Cancer. 2001;92(4):474–479. doi: 10.1002/ijc.1229. [DOI] [PubMed] [Google Scholar]
- 17.Russ M, Lou D, et al. Photo-activated affinity-site cross-linking of antibodies using tryptophan containing peptides. J Immunol Methods. 2005;304(1–2):100–106. doi: 10.1016/j.jim.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Wehrman TS, Raab WJ, et al. A system for quantifying dynamic protein interactions defines a role for Herceptin in modulating ErbB2 interactions. Proc Natl Acad Sci USA. 2006;103(50):19063–19068. doi: 10.1073/pnas.0605218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wroblewski JM, Bixby DL, et al. Characterization of human non-small cell lung cancer (NSCLC) cell lines for expression of MHC, co-stimulatory molecules and tumor-associated antigens. Lung Cancer. 2001;33(2–3):181–194. doi: 10.1016/S0169-5002(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Kohler H. Enhancing tumor targeting and apoptosis using noncovalent antibody homodimers. J Immunother. 2002;25(5):396–404. doi: 10.1097/00002371-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Lou D, et al. Enhanced anti-B-cell tumor effects with anti-CD20 superantibody. J Immunother. 2002;25(1):57–62. doi: 10.1097/00002371-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Russ M, et al. Therapeutic applications of superantibodies. Drug Discov Today. 2005;10(18):1231–1236. doi: 10.1016/S1359-6446(05)03530-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Russ M, et al. Endowing self-binding feature restores the activities of a loss-of-function chimerized anti-GM2 antibody. Cancer Immunol Immunother. 2007;56(2):147–154. doi: 10.1007/s00262-006-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]