Abstract
Although animals can be immunized against the growth of some tumor implants, most of the attempts to use immunotherapy to cause the regression of animal and human tumors once they have become established have been disappointing even when strongly immunogenic tumors were used as target. In this paper, we demonstrate that the failure to achieve an efficient immunological treatment against an established strongly immunogenic murine fibrosarcoma was paralleled with the emergence of a state of immunological unresponsiveness (immunological eclipse) against tumor antigens observed when the tumor surpassed the critical size of 500 mm3. In turn, the onset of the immunological eclipse was coincidental with the onset of a systemic inflammatory condition characterized by a high number of circulating and splenic polymorphonucleated neutrophils (PMN) displaying activation and Gr1+Mac1+ phenotype and an increasing serum concentration of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 cytokines and C-reactive protein (CRP) and serum A amyloid (SAA) phase acute proteins. Treatment of tumor-bearing mice with a single low dose (0.75 mg/kg) of the synthetic corticoid dexamethasone (DX) significantly reduced all the systemic inflammatory parameters and simultaneously reversed the immunological eclipse, as evidenced by the restoration of specific T-cell-dependent concomitant immunity, ability of spleen cells to transfer anti-tumor activity and recovery of T-cell signal transduction molecules. Two other anti-inflammatory treatments by using indomethacin or dimeric TNF-α receptor, also partially reversed the immunological eclipse although the effect was not as striking as that observed with DX. The reversion of the immunological eclipse was not enough on its own to inhibit the primary growing tumor. However, when we used the two-step strategy of inoculating DX to reverse the eclipse and then dendritic cells loaded with tumor antigens (DC) as an immunization booster, a significant inhibition of the growth of both established tumors and remnant tumor cells after excision of large established tumors was observed, despite the fact that the vaccination alone (DC) had no effect or even enhanced tumor growth in certain circumstances. The two-step strategy of tumor immunotherapy that we present is based on the rationale that it is necessary to eliminate or ameliorate the immunological eclipse as a precondition to allow an otherwise ineffective anti-tumor immunological therapy to have a chance to be successful.
Keywords: Tumor, Immunotherapy, Dendritic cells, Systemic inflammation
Introduction
Many years ago, Foley [12] and Prehn and Main [42] demonstrated that the removal of a chemically induced murine tumor by ligation or surgical excision left the host immunized or protected against the subsequent injection of a normally lethal dose of viable cells of that tumor. In 1960, this finding was confirmed by Klein et al [22] who showed that immunization of mice against an implant of tumor cells could also be achieved by pretreatment with lethally X-irradiated tumor cells.
Since then, those pioneer discoveries were repeated and extended by numerous investigators who established, beyond all reasonable doubt, that some animal and human tumors express antigenic peptides which can elicit and become targets of an anti-tumor T cell immune response [20, 60].
On this basis, an immunological approach to clinical cancer emerged as an attractive method to efficiently and specifically kill the tumor cells, circumventing the limitations and the undesirable side effects of the conventional anti-tumor therapies. Thus, a high number of anticancer trials were designed over the past 40 years using different vaccination strategies. However, to date, most of the attempts to cause an immunologically mediated regression of animal or human tumors, once established, have been disappointing [9, 11, 58]. A few successful immunological therapies against experimental-growing tumors have been reported in the past few years by using dendritic cells or engineered tumor cells as new anti-tumor vaccines. However, in all cases, success was achieved only on incipient tumors; when tumors had grown beyond a minimal critical size, no effect was observed [7, 15, 28, 35, 41, 47, 66].
This means that the immunization procedures which are useful for preventing the growth of implants of viable tumor cells, are, in most cases, inefficient to eradicate and even slow down their growth once they become established, “established” meaning—according to the definition of Yu et al. [66]—the solid tumors that are at least 14 days old and have volumes of about 400–500 mm3. As a putative explanation of these conflicting observations, some authors have suggested that the immune system can efficiently deal with a limited number of tumor cells present in a small tumor fragment or in an incipient tumor but not with a larger number present in an established tumor [37]. If this was the case, specific immunotherapy should be more successful after surgically debulking the tumor. Indeed, a high percentage of the immunological therapies against human cancer have been attempted with the aid of debulking but, again, the results have, in general, been disappointing [9, 30, 32, 38].
The mechanisms, by which a host bearing an established tumor becomes refractory to immunotherapy, have been studied for decades; however, although several hypotheses have been raised, including tumor cell-associated and host immunoregulatory circuits [2, 19, 60], the underlying basis of this phenomenon has remained elusive.
In this paper, in an attempt to continue the line of thinking initiated by North some years before [36], we have studied the kinetics of the onset and decay of the immune response evoked by the progressive growth of an immunogenic murine tumor. We have assumed that a knowledge of the regulation of this concomitant immunity is a precondition to understand why antigenic tumors are not rejected by their immunocompetent hosts and why such tumors, once they become established, are refractory to any attempt of immunotherapy.
The decay of the antitumor immunity observed when a tumor exceeds a critical size—called by former authors as immunological eclipse [14, 36]—was correlated, in our model, with the emergence of a systemic inflammatory condition characterized by a sharp rise of polymorphonuclear neutrophils (PMN) in the blood and spleen and elevated serum concentrations of some pro-inflammatory cytokines and acute phase proteins. We have attempted to reverse this immunological eclipse using different anti-inflammatory treatments in order to test whether such reversion could enable an otherwise ineffective anti-tumor immunological therapy to become successful.
Materials and methods
Animals
BALB/c mice of both sexes, 3–5 months old were used throughout. They were raised in our own colony and maintained on Cooperation pellets (San Nicolás, Buenos Aires, Argentina) and water ad libitum. Parabiotic mice were prepared by joining pairs of mice in parabiotic union as previously described [50]; cross circulation was established around day 7 according to our previous experience. Animals were age and sex matched within each experiment. Care of animals was according to the policies of Academia Nacional de Medicina of Buenos Aires, Argentina (NIH Guide and Use of Laboratory Animals).
Tumor
MC-C: Fibrosarcoma induced in a 5-month-old BALB/c male, 3 months after the subcutaneous (s.c.) implantation of a methylcholanthrene pellet. It was used between s.c. passages 5–25. Tumor dose 50 (TD50) is defined as the number of tumor cells able to grow in 50% of mice. Tumor volume was calculated according to the formula of Attia and Weiss: volume = 0.4ab 2, where a and b are the larger and smaller diameters, respectively [13].
As control of specificity we have used two immunologically unrelated murine tumors: the fibrosarcoma MC-B and the lymphoma LB, which have been described previously [13].
Serum
Normal and tumor-bearing mice were bled through the retro-orbital plexus. The blood was kept at room temperature for 1 h for clotting. Serum obtained after centrifugation was stored at −20°C until used. For [3H] thymidine uptake assays, serum was decomplemented at 56°C for 30 min.
Histological studies
Skin with or without macroscopic tumor was removed and fixed in 15% formaldehyde, 5% acetic acid and 80% methanol. The tumor was sliced along the largest diameter and embedded with the overlying skin. Serial sections (3–5 μm) were obtained, stained with hematoxylin and eosin and studied.
Preparation of tumor lysates
Tumor tissue was removed and tumor cells were dispersed in phosphate-buffered saline (PBS) to create a single-cell suspension. Cells were lysed by four to five freeze cycles (on liquid nitrogen) and thaw cycles (room temperature). Larger particles were removed by centrifugation (10 min, 1,000 r.p.m.), supernatants were sonicated for 10 min in a Branson Digital Sonifier and then passed through a 0.2-μm filter, protein content determined and adjusted at 7.5 μg/ml and aliquots stored at −80°C until use.
Bone marrow-derived dendritic cells isolation
Femurs and tibiae of mice were removed and freed of muscles and tendons: The bones were placed in 70% ethanol for 2 min and subsequently washed in PBS. Both bone ends were cut off and the marrow was flushed out with RPMI 1640 medium. The red cells were lysed with ammonium chloride (0.45 M). The cells were centrifuged for 10 min at 1,500 rpm and 2 × 105 ml−1 cells were cultured in Petri dishes in 10 ml of RPMI medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum; 2 mM l-glutamine, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (complete medium) and supplemented with 5 × 10−5 M 2-mercaptoethanol and 15–30% of mouse granulocyte–macrophage colony-stimulating factor (m GM-CSF)-containing supernatant from a J558 cell line stably transfected with m GM-CSF. On day 8, dendritic cells were harvested by gentle pipetting.
Vaccination strategies
Tumor implantation and excision
Subcutaneous MC-C tumors were surgically excised when their volume had reached approximately 400 mm3; 2 weeks later, tumor challenge was carried out in the contra-lateral flank of the mice that had not relapsed.
Sublethal doses
Mice that had survived a first small MC-C tumor implant were re-inoculated with graded doses of the same tumor.
X-irradiated cells
Cell suspensions were irradiated with 90 Gy in a plastic irradiation chamber; X-rays were generated in a Philips 250/15 Radiotherapy apparatus at 220 kV, 14 mA and filtered with 1 mm Al. The dose rate was 3.15 Gy min−1 at a focus-target distance of 29 cm. Animals were pretreated with two s.c. doses of 4 × 106 irradiated MC-C tumor cells, 14 and 7 days before tumor challenge.
Heat-inactivated cells
Tumor cells (5 × 106) heated at 80–90°C for 1 min were inoculated s.c. 14 and 7 days before tumor challenge.
Dendritic cells pulsed with tumor lysate and X-irradiated cells
Bone marrow-derived day 8 dendritic cells (1 × 106) were incubated in 1 ml of complete medium with 0.25 ml of MC-C tumor-lysate or with 1 × 106 X-irradiated MC-C tumor cells for 2 days at 37°C. Cells were then washed and 3 × 105 dendritic cells pulsed with tumor lysate or X-irradiated MC-C tumor cells were injected s.c. in the foot pad of recipient mice 14 and 7 days before tumor challenge. Dendritic cells pulsed with LPS or not pulsed were injected as control.
Adoptive transference assay
Mice were inoculated by the intraperitoneal (i.p.) route with 1 × 108 spleen cells from normal, immunized and tumor-bearing mice. After 2 h, passively transferred mice were challenged with a s.c. tumor implant. The survival index (SVI) was calculated as the survival time in days divided by the ratio between the number of mice that died of tumor and the total number of mice inoculated. SVI is a measure of both the survival time and percentage of mortality [13].
Concomitant immunity assay
Mice received a s.c. tumor implant in the right flank followed, at different intervals, by a second s.c. implant of the same tumor in the left flank. Control mice were challenged in the left flank only. The titer of concomitant immunity was defined as the ratio between TD50 of the second challenge in tumor bearing mice and TD50 in control mice and expressed as a function of the primary tumor volume at the day of the second tumor challenge [13]. Strictly, the inhibition of a second tumor challenge in a tumor-bearing host is not itself an index of a concomitant immune response because mice bearing large tumors (in the case of MC-C when tumor size is >2,500 mm3) at the time of the second tumor challenge are capable of inhibiting it by an entirely different non-immunological mechanism, included, together with the immunological mechanism, in the broader concept of anti-tumor concomitant resistance [13, 44, 50, 51]. However, in this paper, we tested the inhibition of a second tumor challenge inoculated in mice bearing primary MC-C tumors ≤2,500 mm3 and in consequence, the inhibitory effect (when it were present) could only be associated with the immunological arm of this phenomenon, i.e., with concomitant immunity.
Winn test
The anti-tumor activity of spleen cells from normal, immunized and tumor-bearing mice was investigated with the in vivo Winn’s test [65] by mixing them with tumor target cells at an effector–target ratio of 100:1. The cells were then inoculated by the s.c. route and tumor growth evaluated.
Cell-mediated cytotoxicity assay
51Cr-labeled tumor cells in 0.1 ml of complete medium were incubated with the same volume of different spleen cell suspensions at an effector–target ratio of 100:1 for 4 h at 37°C in a 5% carbon dioxide humidified atmosphere. Afterward, cells were centrifuged and radioactivity in the supernatant was measured in a Gamma counter (Beckman). Percentage of specific lysis was calculated as (experimental cpm − normal cpm)/(maximal cpm with Triton − normal cpm) × 100. The assays were usually carried out in quadruplicate.
[3H]Thymidine uptake assay
Splenocytes from mice immunized against MC-C tumor (mice pretreated with lethally X-irradiated MC-C tumor cells) were prepared by mechanical disruption of spleens using standard protocols. Splenocytes (5 × 106 ml−1) were activated with tumor lysate (content of protein 7.5 μg/ml) for 72 h and then seeded, at a concentration of 2 × 106 ml−1, in 0.1 ml of medium, in 96-well microtiter plates (Corning, NY, USA) in the presence of 0.1 ml of several twofolded dilutions of serum from normal and tumor-bearing mice and 1μCi/ml of [3H] thymidine .The mixture was incubated at 37°C for 18 h in a 5% carbon dioxide humidified atmosphere and harvested with an automated cell harvester. The radioactivity incorporated into the cells was counted in a liquid scintillation Beta counter (Beckman). The assays were usually carried out in triplicate to sextuplicate. The titer of growth inhibitory activity was defined as the reciprocal of the serum dilution producing 50% inhibition of [3H] thymidine uptake by spleen cells as compared with medium only, and expressed as GIU50 ml−1.
Markers of systemic inflammation
Blood was collected at the indicated times from the retro-orbital plexus. Blood smears were prepared on microscope slides, fixed with methanol and stained with Giemsa. The number of polymorphonuclear neutrophils (PMN) was estimated by a light microscope and visual counting. Mouse serum amyloid A (SAA) and C-reactive protein (CRP) titration was on centrifuged serum using ELISA kits from BioSource International, Camarillo, CA, USA and Immunology Consultants Laboratory, Newberg, OR, USA, respectively, following manufacturer’s recommendations. Mouse TNF-α, IL-1β and IL-6 titration were on centrifuged serum using ELISA kits from RD Systems, Minneapolis, MN, USA, following manufacturer’s recommendations.
Anti-inflammatory treatments
An i.p. single dose of 0.75 mg/kg body weight (about 15 μg per mouse) of the synthetic corticoid dexamethasone (DX) (Decadron Shock, SIDUS, Argentina) was used.
Indomethacin (Sigma) was diluted in 0.015 M NaCl to obtain a dose of 0.5 mg/kg body weight (about 10 μg per mouse) and mice received two weekly injections of this preparation by the i.p. route.
Recombinant fusion protein composed of soluble dimeric human p80 tumor necrosis factor-α receptor (TNFR) linked to the Fc region of human IgG (TNFR:Fc, etanercept) was purchased from John Wyeth (Buenos Aires, Argentina). Each mouse received two weekly intravenous (i.v.) injections with 100 μg of TNFR/Fc in 0.1 ml of saline as previously described (4, 31).
In order to counteract the interaction of DX with the glucocorticoid (GC) receptor, a GC receptor antagonist, RU-486 (Sigma) was used. RU-486 was inoculated as a single dose (3 mg/ml en 1,2 propanediol) by the i.p. route 1 day and 5 h before inoculating DX. RU-486 competes with DX for rat kidney glucocorticoid receptor (GR) occupancy in vitro, exhibiting a higher association constant for binding to GR than DX [21].
Flow cytometry
For phenotypic analysis, spleen cells pooled from mice (five mice per group) were reacted with 100 ng of fluoresceinated anti-CD3, anti-CD4, anti-CD8, anti-CD25, anti-CD45 B220, anti-GR1 or anti-Mac1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Irrelevant isotype-matched Abs were used as controls. Cells were reacted with the desired Ab for 30 min at 4°C. Samples were washed in PBS with 1% FBS. Samples were analyzed for fluorescence using a Coulter Profile II flow cytometer (Palo Alto, CA, USA) following gating on the lymphocyte population for the analysis of CD3, CD4, CD8, CD25, CD45 B220 positive-cells and on the non-lymphocyte population for the analysis of Mac1 and GR1 positive cells.
For in vitro T cell enrichment, mononuclear spleen cells were obtained in a Ficoll–Hypaque density gradient as described [8] and then they were incubated at 37°C for 3 h in the presence of an anti-B220 antibody (20μg/1 × 106 cells) to eliminate B cells. Later, the suspension was incubated in a Petri dish at 37°C overnight to deplete the macrophages by adherence. After washing the suspension, an aliquot of cells was reacted with anti-CD3 mAb and analyzed via cytometry to assess the enrichment obtained. In all cases, more than 85% of the cells expressed the T cell marker.
For in vivo depletion studies, five mice per group were inoculated i.p. twice per week with 100 μl of anti-CD3 (clon 145-2C11, hamster IgG) or with an IgG isotype control. Selective depletion was tested in spleen preparations via FACS analysis and was always greater than 95% for T CD3+ cells; other subsets were unaffected.
Peripheral PMN from individual mice were obtained by layering on a Ficoll–Hypaque density gradient as previously described [8].
In order to measure their activation, 0.25 × 106 purified PMN were incubated with dihydrorhodamine 123 (DHR, Sigma) (1 mM) at 37°C in 5% CO2. DHR itself is non-fluorescent, but upon reactive oxygen species generation a brightly fluorescent FL-1 product is produced. Therefore, the fluorescent form of DHR was determined by flow cytometry.
Western blotting
Cells were washed and lysed in buffer composed of 20 mM Tris (pH 8), 150 mM NaCl, 1% Nonidet P-40, 200 μM EDTA, sodium pyro-phosphate, and 100 mM sodium fluoride containing protease inhibitors. Lysates corresponding to 4 × 106 enriched T cells/sample were separated on a 10% SDS-PAGE gel at 200 V for 45 min. Material was electroblotted to polyvinylidene difluoride membrane, blocked with 5% BSA, and immunoblotted with a rabbit anti-lck antiserum (Santa Cruz Biotechnology). To detect the level of the p56lcksignal transduction protein, the filter then was reacted with a secondary Ab conjugated with peroxidase and developed via enhanced chemiluminescence (DuPont-NEN, Boston, MA, USA); p56lck is a protein tyrosine kinase of the src family which is expressed in T cells and it has a significant role in signal transduction through the T cell receptor [52]. Calibrant molecular weight (Da) on tris-HCl gel: myosin 200,768, Β-galactosidase 115,281, bovine serum albumin 96,190, ovalbumin 51,783, carbonic anhydrase 37,659, soybean trypsin inhibitor 29,054, lysozyme 20,461, and aprotinin 7,100.
Statistical analysis
Student’s t test, Log-rank test and χ2 test were used. Values were expressed as mean ± standard error. Differences were considered to be significant whenever the P value was 0.05 or smaller.
Results
Immunization and immunotherapeutic assays against MC-C tumor
MC-C is a strongly immunogenic tumor. In effect, numerous active vaccination strategies—including tumor implantation and excision, pretreatment with sublethal tumor doses, X-irradiated or heat-inactivated tumor cells and dendritic cells pulsed with tumor lysates or X-irradiated tumor cells—and passive transference of immunity could successfully prevent the growth of implants of MC-C viable tumor cells (data not shown).
However, most of these procedures were unable to cause the regression or even slow down the kinetics of a growing MC-C tumor (data not shown). In our hands, the only exception was dendritic cells pulsed with MC-C-tumor lysate inoculated in mice bearing MC-C tumors ranging 10–70 mm3; in effect, this treatment caused the retardation of tumor growth in treated mice, prolonging their survival time (76.0 ± 8.4 days; n = 5) as compared with controls (60.1 ± 3.2 days; n = 11, P < 0.05).
However, even dendritic cells pulsed with MC-C tumor-lysate were unable to affect the growth of MC-C tumors larger than 100 mm3. In fact, as we will show below, in some cases, especially when tumors larger than 500 mm3 were used as target, tumor growth was accelerated upon this treatment.
Onset and decay of anti-tumor immunity during the progressive growth of MC-C tumor
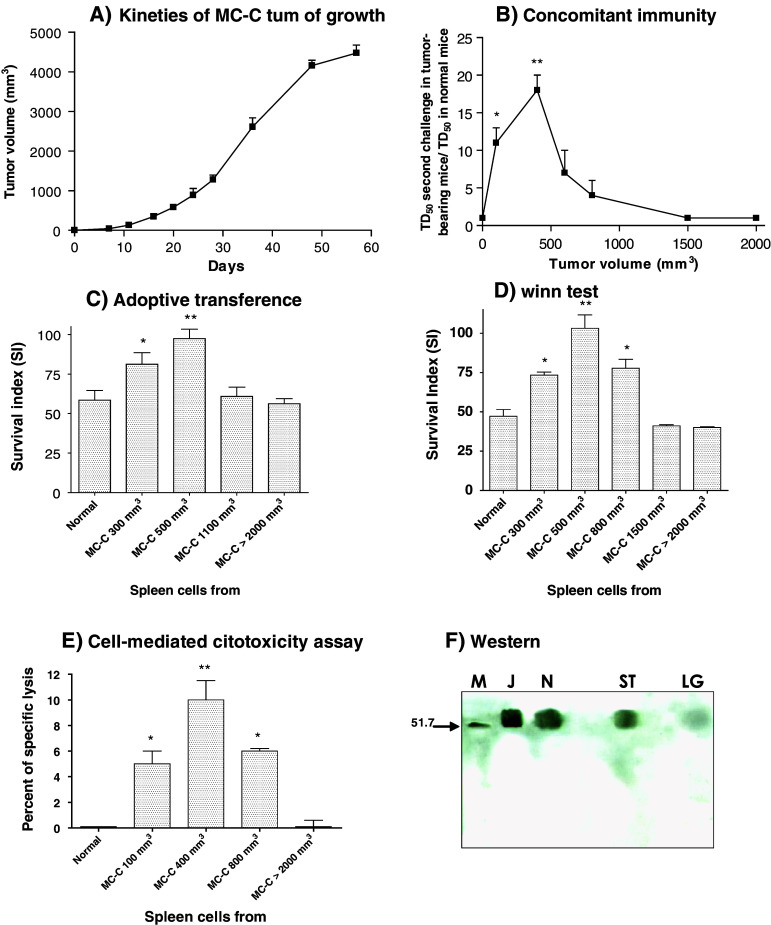
MC-C tumor growth initiated by a s.c. implant of 5 × 105 tumor cells is depicted in Fig. 1a.
Fig. 1.
a Growth of s.c. MC-C tumor initiated (on day 0) with 5 × 105 MC-C tumor cells. Each point represents the mean ± standard error (SE) of 12 mice. b Concomitant immunity expressed, in the ordinate, as the ratio between TD50 of secondary MC-C tumor in MC-C tumor-bearing mice/TD50 in control mice. Abscissa indicates the primary tumor volume at the day of the second challenge. Each point represents the mean ± SE of five experiments. * P < 0.002 as compared with control mice and mice bearing MC-C tumors measuring 1,500 and 2,000 mm3; P < 0.05 as compared with mice bearing MC-C tumors measuring 800 mm3. ** P < 0.001 as compared with control mice and mice bearing MC-C tumors measuring 800, 1,500 and 2,000 mm3; P < 0.02 and P < 0.05 as compared with mice bearing MC-C tumors measuring 600 mm3 and 100 mm3, respectively. c In the adoptive transference assay, normal mice received 1 × 108 spleen cells by the i.p. route, from normal mice or mice bearing MC-C tumors with different sizes; 2 h later mice were challenged with 5 × 105 MC-C tumor cells by the s.c. route. Each bar represents the mean ± SE of six experiments. Ordinate: Survival index of recipient mice = survival time/t/n, where t = number of mice that died of tumor and n = number of mice inoculated. * P < 0.01 as compared with normal mice and mice bearing tumors measuring 1,100 and >2,000 mm3. ** P < 0.001 as compared with normal mice and mice bearing tumors measuring 1,100 and >2,000. d In the Winn test, 50 × 106 spleen cells from normal mice or mice bearing MC-C tumors with different sizes were mixed in vitro with 5 × 105 MC-C tumor cells and then the mixture was s.c. inoculated in naive mice. Each bar represents the mean ± SE of five experiments. Ordinate Survival index of mice inoculated. * P < 0.001 as compared with normal mice and mice bearing MC-C tumors measuring 1,500 and >2,000 mm3. ** P < 0.001 as compared with normal mice and mice bearing MC-C tumors measuring 1,500 and >2,000 mm3; P < 0.002 and P < 0.01 as compared with mice bearing tumors measuring 300 mm3 and 800 mm3, respectively. e Cytotoxic activity of 2 × 106 spleen cells from normal mice or mice bearing MC-C tumors with different sizes against 2 × 104 51Cr-labeled MC-C tumor cells. Each bar represents the mean ± SE of four experiments. * P < 0.01 as compared with normal mice and mice bearing MC-C tumors >2,000 mm3. ** P < 0.001 as compared with normal mice and mice bearing MC-C tumors >2,000 mm3 and P < 0.05 as compared with mouse bearing MC-C tumor measuring 100 and 800 mm3. f Levels of p56lck in T splenocytes throughout MC-C tumor growth. Cell lysates from enriched T cells (4 × 106 cell equivalent/lane) were separated on a 10% SDS-PAGE gel, transferred to a polyvinylidene difluoride membrane and immunoblotted with an anti-p56lck antibody, followed by a secondary antibody conjugated to peroxidase and developed via enhanced chemiluminescence. These results are representative of those obtained in two similar experiments. Lanes: M markers of molecular weight in Kilo Daltons, J Jurkat cells (positive control of p56lck expression), N, T splenocytes from normal mice, ST, T splenocytes from mice bearing small tumors (<500 mm3), LT T splenocytes from mice bearing large tumors (1,500–2,000 mm3)
Anti-tumor immunity during MC-C tumor growth was evaluated using five assays:
Capacity of tumor bearing mice to inhibit the growth of secondary tumor implants (concomitant immunity, Fig. 1b).
Ability of spleen cells to transfer anti-tumor immunity passively to naïve mice (adoptive transference of immunity, Fig. 1c).
Ability of spleen cells to counteract the tumorigenic capacity of tumor cells when spleen cells were mixed with tumor cells in vitro and then the mixture was inoculated into normal mice (the Winn test, Fig. 1d).
Ability of spleen cells to specifically kill 51Cr-labeled MC-C tumor cells in vitro (cell-mediated cytotoxicity, Fig. 1e).
Level of the tyrosine kinase p56lck, a signal transduction protein which is expressed in T cells and that it is activated following the binding of ligand with the TCR (Fig. 1f).
As shown in Fig. 1b–e, anti-tumor immunity against MC-C, as evidenced by the capacity of tumor bearing mice to inhibit the growth of a secondary tumor implant or by the ability of spleen cells to inhibit in vivo and in vitro tumor growth, peaked at the early stages of MC-C tumor growth but it was down regulated after MC-C tumor grew beyond 500 mm3, approximately. In the same way (Fig. 1f), by using Western blotting, we observed that while T cells from normal mice and mice bearing MC-C tumors <500 mm3 displayed a high level of p56lck, T cells from mice bearing MC-C tumors >500 mm3 exhibited a profound reduction of the expression of this protein.
The early and transient anti-tumor response proved to be tumor-specific and it was observed in euthymic but not in nude mice (data not shown) meaning that it was mainly a T-cell dependent mechanism.
Evidence that the down regulation of concomitant immunity is associated with the emergence of active suppressor mechanisms
The state of tolerance to MC-C tumor cells observed when MC-C tumor grows beyond 500 mm3 (immunological eclipse) could be due to clonal deletion or to active suppressor mechanisms. To distinguish between both possibilities, parabiosis between tolerant and immune mice to MC-C were carried out. Parabiosis between immune and normal mice and between normal and normal mice were also carried out as controls. As immune mice, we used mice pretreated with lethally X-irradiated MC-C tumor cells; as tolerant ones, we used mice bearing MC-C tumors larger than 500 mm3. If clonal deletion played a main role, transference of immune lymphocytes by parabiosis from the immune mouse would be expected to reverse the immunological eclipse in the tolerant mouse by replacing a cell type that had been eliminated from the latter. In contrast, such transference would probably not affect a state of tolerance if it were mainly due to an active suppressor mechanism. To test the state of tolerance or immunity, both partners of the parabiotic mice were challenged with an implant of 2 × 105 viable MC-C tumor cells, 9 days after parabiosis was carried out.
As can be seen in Table 1, in the pair tolerant-immune, tumor challenge grew in six of the six tolerant and in six of the six immune partners, indicating that parabiosis between immune and tolerant mice not only failed to abrogate tolerance to MC-C in the tolerant mice but, in fact, made the immune mice unresponsive. In contrast, in the pair immune-normal, the immune state could be easily transferred to normal mice. These results strongly suggest that the immunological eclipse would mainly be mediated by an active suppressor mechanism. If it were the case, this could explain the refractoriness of an established tumor to immunological therapies, because any mechanisms, which actively suppress the antitumor immune response mounted by the tumor-bearing host against its own tumor, would also be expected to suppress any attempt of active and passive antitumor immunotherapy.
Table 1.
Failure to abrogate tolerance to MC-C tumor in tolerant mice joined in parabiosis with immune mice
| Parabiotic miceb | Tumor takes of challenge implant of MC-C/totala | ||
|---|---|---|---|
| Tolerant | Immune | Normal | |
| Tolerant × immune | 6/6 | 6/6* | |
| Immune × normal | 0/4* | 0/4** | |
| Normal × normal | 9/9** | ||
aNumber of mice in which tumor challenge grew/total number of mice inoculated. Tumor challenge was 2 × 105 MC-C tumor cells implanted in the dorsum of both partners of parabiotic mice, 9 days after parabiosis
bParabiotoc mice: tolerant mice were mice bearing MC-C tumors growing s.c. in the opposite flank to the parabiotic junction and larger than 500 mm3 at the time when parabiosis was carried out. Immune mice were mice pretreated with lethally X-irradiated MC-C tumor cells, 14 and 7 days before parabiosis. Single tolerant and single normal mice failed to reject an implant of 2 × 105 MC-C tumor cells in five of the five and nine of the nine cases, respectively. Single immune mice rejected that tumor implant in six of the six cases
* P < 0.05 (6/6 vs 0/4); ** P < 0.01 (0/4 vs 9/9)
MC-C tumor growth and the emergence of a systemic inflammatory condition
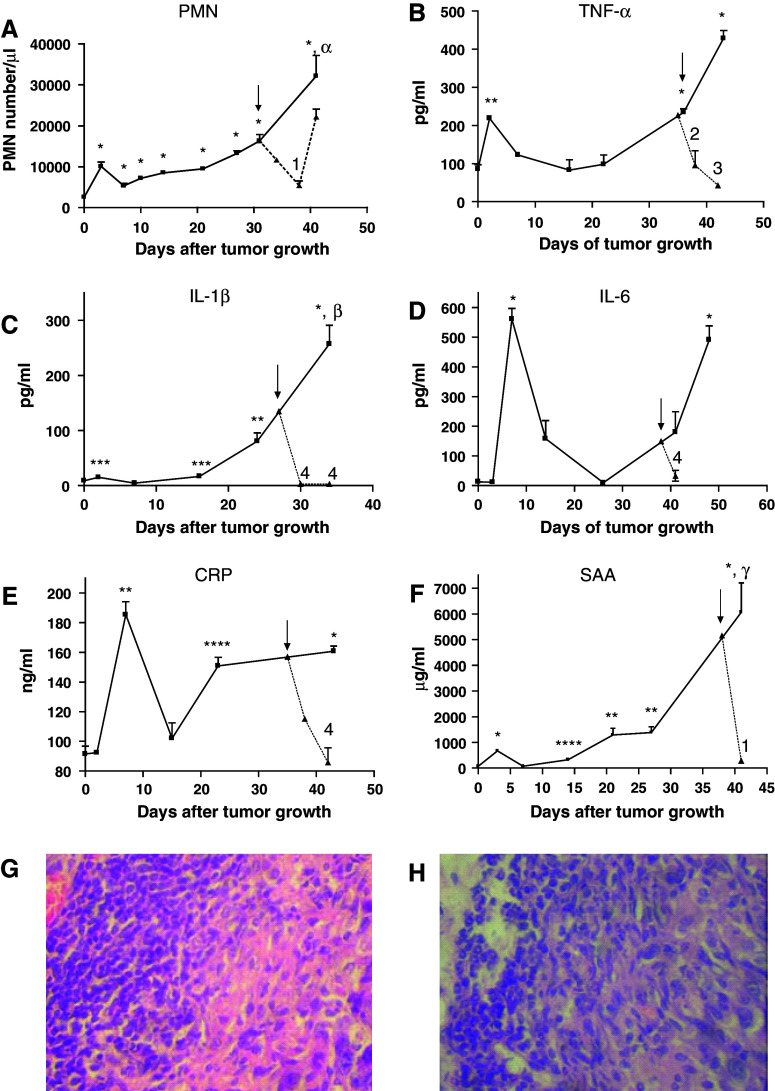
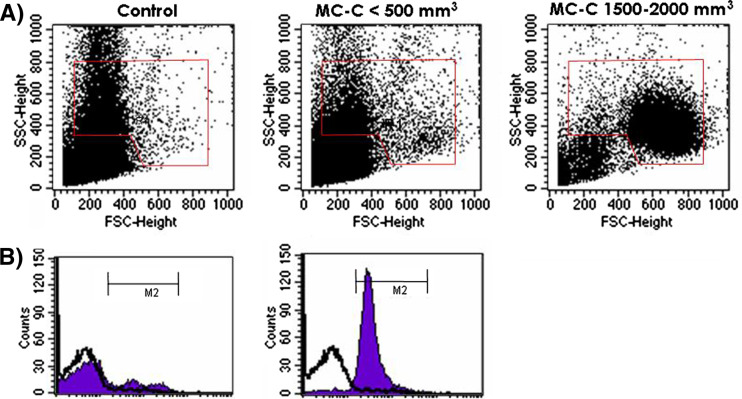
During the growth of MC-C tumor, two temporally separate peaks of systemic inflammation were detected as evidenced by an increase in both, the number of circulating PMN and the serum concentration of TNF-α, IL-1β and IL-6 cytokines and CRP and SAA phase acute proteins. The first peak was relatively small and transient since it was only detected during the first week after tumor inoculation and presumably it represented the response of the organism to the mechanical trauma of tumor inoculation. On the other hand, the second peak was observed starting after day 14, at the time when tumor began to grow exponentially and coincidental with the onset of the immunological eclipse. The second peak was, in general, more prominent than the first one and its intensity was proportional to tumor size (Fig. 2a–f). In mice bearing a large MC-C tumor, not only a high number of mature PMN but also immature PMN as well as myeloid precursors at different stages of differentiation were evident in circulation. Most of these cells were phenotypically larger than those observed in normal mice or mice bearing a small MC-C tumor and they exhibited a significant level of activation (Fig. 3). In contrast with the sharp increase of circulating PMN, circulating lymphocytes remained fairly constant throughout tumor growth, with counts not significantly different from normal values (6,955 ± 556 lymphocytes/μl; n = 6). Histological studies demonstrated that between 5 and 14 days after tumor inoculation, the incipient tumor was infiltrated by lymphocytes preferentially; afterward, as circulating PMN increased progressively, the growing tumor was mainly infiltrated by PMN (Fig. 2g, h).
Fig. 2.
Number of circulating polymorphonuclear neutrophils (PMN) per μl (a) and serum concentration of the pro-inflammatory cytokines TNF-α (b), IL-1β (c), IL-6 (d) and the phase acute proteins CRP (e) and SAA (f) throughout MC-C tumor growth (solid lines). Dotted lines represent the values observed after treatment with a single dose (0.75 mg/Kg) of dexamethasone by the intraperitoneal route; day of treatment is indicated by arrow. Each point represents the mean ± SE of 2–4 determinations for the cytokines and phase acute proteins and the mean ± SE of 3–10 determinations for the PMN cell counts. Comparison with normal values: * P < 0.001, ** P < 0.01, *** P < 0.05, **** P < 0.02. Comparison between the second peak (days 34–48) and the first peak (days 2–7) of systemic inflammation: α P < 0.002, β P < 0.001; γ P < 0.01. Comparison between dexamethasone-treated and untreated mice: 1 P < 0,05, 2 P < 0.002, 3 P < 0.001, 4 P < 0.01. Peritumoral infiltrate preferentially composed by lymphocytes in a small (<500 mm3) tumor (g) or preferentially composed by PMN in a large (1,500–2,000 mm3) tumor (h) (×400)
Fig. 3.
a Change in phenotype of circulating PMN throughout MC-C tumor growth. b Pattern of activation of circulating PMN from mice bearing a large MC-C tumor (1,500–2,000 mm3 size; black area, graphic on the right) as compared with normal mice (white area) and mice bearing a small MC-C tumor (<500 mm3; black area, graphic on the left) as determined by a brightly fluorescent FL-1 product of dihydrorhodamine 123 upon reactive oxygen species generation. Similar results were obtained in three additional experiments
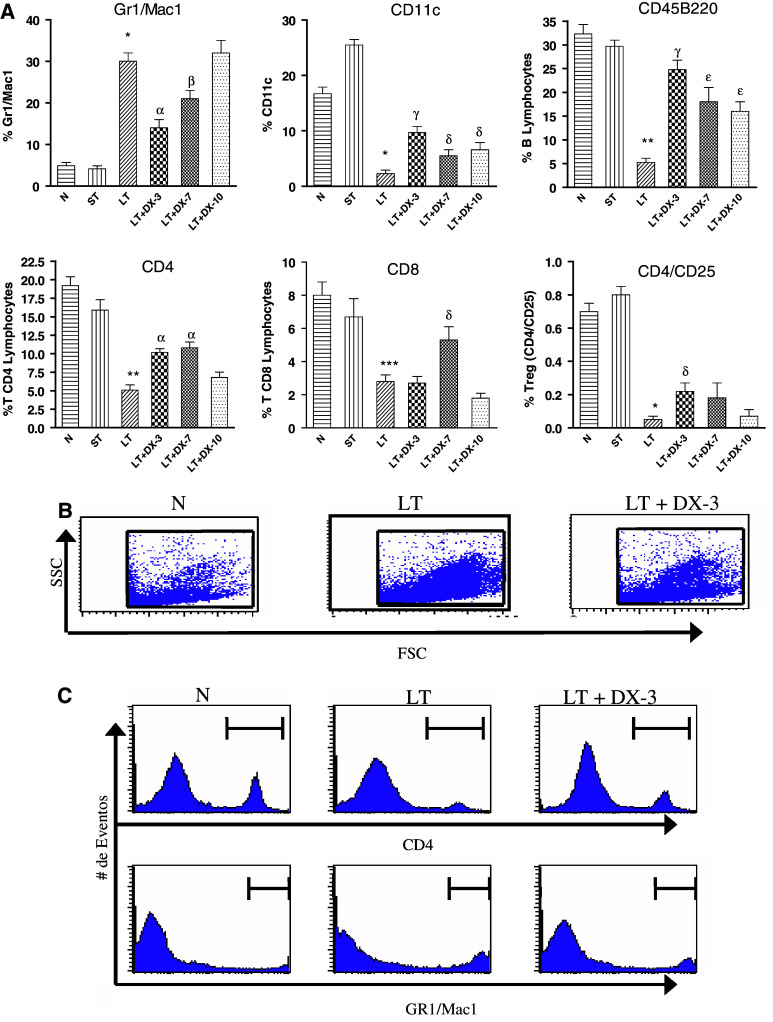
Similar to what occurred in circulation, a sharp increase of splenic PMN displaying high expression of Gr1+ Mac1+ markers was evident throughout tumor growth. In contrast, the percentage of splenic dendritic cells as well as splenic lymphocytes, including B cells, CD4+ and CD8+ T cells, CD4+CD25+ negative-regulatory T cells (T reg), etc., exhibited a significant reduction (Fig. 4).
Fig. 4.
a Change in phenotype of splenocytes throughout MC-C tumor growth and following DX treatment. Splenocytes from normal mice (N), mice bearing a small tumor (size < 500 mm3; ST) and mice bearing a large tumor (size 1,500–2,000 mm3) untreated (LT) or 3, 7 and 10 days after treatment with dexamethasone (LT + DX-3, LT + DX-7 and LT + DX-10) were studied. Splenocytes from all the groups were stained with 100 ng of fluoresceinated anti-Gr1 and anti-Mac1, anti-CD11c, anti-CD45 B220, anti-CD4, anti-CD8 and anti-CD 4/CD25. The percentage of cells staining with the irrelevant isotype-matched controls has been subtracted from the values obtained with the specific antibodies. Fluorescence was analyzed via flow cytometry. Bars represent the percentage of each cellular type as compared with the total number of splenic nucleated cells. Comparison between LT versus N: * P < 0.001; ** P < 0.01; *** P < 0.05. Comparison between LT + DX-3 or LT + DX-7 versus LT: α P < 0.002. β P < 0.02; γ P < 0.001; δ P < 0.05; ε P < 0.01. These values represent the mean ± ES of four similar experiments. Absolute number of splenocytes per spleen was similar in LT [(5.53 ± 0.80) × 108, n = 4 experiments] and LT + DX-3, LT + DX-7 and LT + DX-10 [(5.37 ± 0.82) × 108, n = 12]. On the other hand, a significantly lower number of splenocytes per spleen was seen in N [(1.33 ± 0.17) × 108, n = 4, P < 0.01 vs. LT] and ST [(2.05 ± 0.37) × 108, n = 4, P < 0.05 vs. LT ]. b Dot blot showing, in one representative experiment, the change in phenotype of splenocytes from LT as compared with LT + DX-3 and N mice. c Histograms from the representative experiment shown in b, showing splenic CD4+T lymphocytes and Gr1+/Mac1+ PMN from N, LT and LT + DX-3 mice
A closer association between the systemic inflammatory condition and the immunological eclipse was suggested by the following facts: first, Gr1+ Mac1+ cells, especially when present in a high number, have been reported to exert significant immunosuppressive effects by inhibiting CD8 T cells responses and by differentiating into suppressive tumor associated macrophages [25, 52]; second, proliferation of immune spleen cells (from mice immunized against MC-C) incubated with tumor antigens was inhibited by co-culturing with serum from mice bearing large MC-C tumors, which displayed a high concentration of some pro-inflammatory cytokines and phase acute proteins, but not with normal serum or serum from mice bearing small MC-C tumors (Table 2).
Table 2.
Effect of serum from mice bearing large MC-C tumor on in vitro proliferation of immune spleen cells activated with tumor lysates
| 3[H] thymydine uptake by spleen cells (c.p.m. ± s.e.)a | |||||
|---|---|---|---|---|---|
| Final serum dilution | MC-C 2,000 mm3 | Normal | MC-C 2,000 mm3 + DX-3 | MC-C 2,000 mm3 + DX-7 | Medium only |
| – | – | – | – | – | 22,117 ± 981 |
| 1:2 | 3,757 + 749a | 8,507 ± 730 | 10,389 ± 213 | 3,107 ± 217e | |
| 1:4 | 6,072 ± 691b | 13,688 ± 1,267 | 14,543 ± 1,324 | 4,567 ± 234f | |
| 1:8 | 9,707 ± 1014c | 15,048 ± 1,219 | 14,340 ± 677 | 11,245 ± 1,005g | |
| 1:16 | 10,184 ± 784d | 15,367 ± 533 | 15,485 ± 1,248 | 13,034 ± 1,369 | |
| GIU50/ml | >160 | 28.1 | 22.9 | 78.5 | |
aEach value is the mean of triplicate to sextuplicate measurements except for medium only (n = 12) and for dilution 1:2 of MC-C 2,000 mm3 and MC-C 2,000 mm3 + DX-3 (n = 2)
a P < 0.05 versus normal (same dilution) and P < 0.02 versus MC-C 2,000 mm3 + DX-3 (same dilution)
b P < 0.001 versus normal and MC-C 2,000 mm3 + DX-3
c P < 0.05 versus normal and P < 0.01 versus MC-C 2,000 mm3 + DX-3
d P < 0.001 versus normal and P < 0.05 versus MC-C 2,000 mm3 + DX-3
e P < 0.001 versus normal and MC-C 2,000 mm3 + DX-3
f P < 0.01 versus normal and MC-C 2,000 mm3 + DX-3
g P < 0.05 versus MC-C 2,000 mm3 + DX-3
Treatment of mice bearing a large MC-C tumor, with a single dose of a synthetic corticosteroid, dexamethasone (DX, 0.75 mg/kg), by the i.p. route, significantly reduced the above-mentioned parameters of systemic inflammation, especially at days 3 and 7 after DX treatment (see Fig. 2). Treatment with DX also reversed the inhibitory effect of serum from tumor bearing mice on spleen cell proliferation; maximal effects were observed at day 3 after DX treatment (Table 2). In the same way, the number of splenic Gr1+ Mac1+ PMN was markedly reduced upon DX treatment while in contrast, the number of both, splenic dendritic cells and B and T lymphocytes, was enhanced (Fig. 4). A similar enhancement of lymphocytes was seen in lymph nodes upon DX treatment (not shown).
Treatment with dexamethasone can reverse the immunological eclipse associated with the progressive growth of MC-C tumor
Some workers have shown that, besides or associated with their anti-inflammatory properties, corticosteroids can prevent tolerance induction to protein antigens and may enhance antitumor cell mediated immune responses [17, 27]. These effects were claimed to be related to a depletion of precursors of suppressor cells although the nature of these cells has not yet been elucidated.
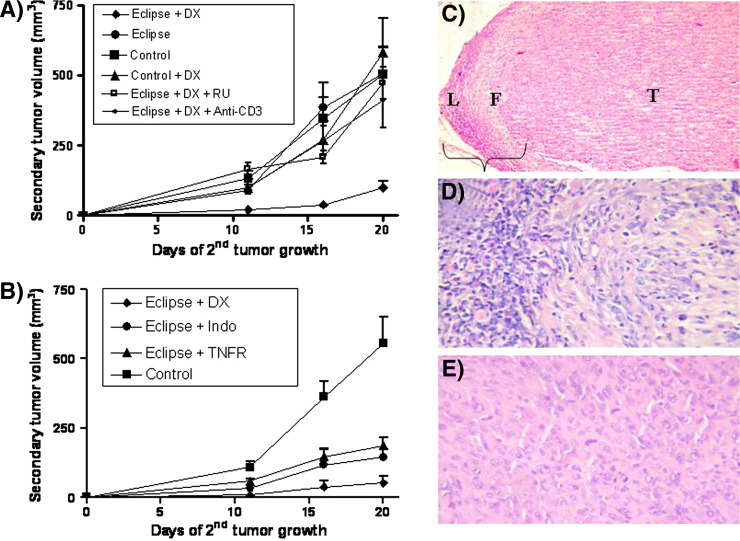
In order to determine whether corticosteroid treatment would reverse the immunological eclipse associated with the progressive growth of MC-C tumor, mice bearing a s.c. MC-C tumor 1,500–2,000 mm3 tumor size, which had received DX 3 days earlier, were challenged with a secondary s.c. implant of 5 × 105 MC-C tumor cells in the contralateral flank. Untreated tumor bearing mice as well as untreated and DX-treated normal mice challenged with 5 × 105 MC-C tumor cells served as controls. As shown in Fig. 5a, a significant inhibition of the secondary tumor challenge was observed in DX-treated tumor bearers as compared with control mice. The reversion of the immunological eclipse mediated by DX was dependent on the interaction of DX with its glucocorticoid receptor since tumor-bearing mice receiving both DX and a glucocorticoid receptor antagonist (RU-486) did not exhibit such reversion (see also Fig. 5a).
Fig. 5.
a Reversion of the immunological eclipse mediated by dexamethasone (DX) as evidenced by the restoration of the capacity of mice bearing a large MC-C tumor to restrain the growth of a secondary MC-C tumor implant (concomitant immunity). The figure shows the volume of the secondary tumor (ordinate) as a function of the days after the secondary tumor implant (abscissa). Mice bearing a s.c. MC-C tumor 1,500–2,000 mm3 tumor size, which had received DX 3 days earlier, were challenged with a secondary s.c. implant of 5 × 105 MC-C tumor cells in the contralateral flank (eclipse + DX, n = 14). Untreated tumor bearing mice (eclipse, n = 16) as well as untreated (control, n = 16) and DX-treated normal mice (control + DX, n = 10) challenged with 5 × 105 MC-C tumor cells served as controls. Growth of secondary tumor implant was also determined in tumor-bearing mice which received DX plus the glucocorticoid antagonist RU-486 (eclipse + DX + RU; n = 3) or DX plus anti-CD3 antibody (eclipse + DX + anti-CD3, n = 6). Comparison of Eclipse + DX with the other groups: Day 11: P < 0.01 versus eclipse and control + DX; P < 0.001 versus control and eclipse + DX + RU; P < 0.02 versus eclipse + DX + anti-CD3. Day 16: P < 0.001 versus all the other groups. Day 20: P < 0.01 versus control; P < 0.002 versus eclipse and control + DX; P < 0.001 versus eclipse + DX + anti-CD3 and eclipse + DX + RU. b Reversion of the immunological eclipse mediated by dexamethasone (eclipse + DX), indomethacin (eclipse + indo) and tumor necrosis factor receptor (eclipse + TNFR) as evidenced by the restoration of the capacity of mice bearing a large MC-C tumor (1,500–2,000 mm3) to restrain the growth of a secondary tumor s.c. implant of 5 × 105 MC-C tumor cells in the contralateral flank (concomitant immunity). Control group was represented by normal mice challenged with a s.c. implant of 5 × 105 MC-C tumor cells. The figure shows the volume of the secondary tumor (ordinate) as a function of the days after the secondary tumor implant (abscissa). Comparison between eclipse + DX versus control: day 11: P < 0.01; day 16: P < 0.001; day 20: P < 0.002. Comparison between eclipse + DX versus eclipse + TNFR: days 11, 16 and 20: P < 0.01. Comparison between Eclipse + DX versus eclipse + indo; day 20: P < 0.05. Comparison between eclipse + TNFR and eclipse + indo versus control: days 16 and 20: P < 0.05. c Secondary MC-C tumor challenge prevented to grow in a dexamethasone-treated mice bearing a large primary MC-C tumor (day 25 after secondary tumor implant). Note a dense lymphocyte infiltration (L) followed by a dense fibrotic area (F) surrounding a tumor core (T) (H–E, ×100). d Magnification of c showing an immunological reaction with abundant mononuclear host cells infiltrating the tumor (HE, ×400). e Secondary MC-C tumor challenge growing in untreated mice bearing a large primary MC-C tumor (day 25 after secondary tumor implant). See viable tumor cells displaying mitosis and without lymphocyte infiltration (H–E, ×400)
Two other anti-inflammatory treatments using two weekly injections of indomethacin or a dimeric TNF-α receptor (TNFR), starting 1 or 3 days, respectively, before the secondary tumor implant, also reversed partially the immunological eclipse associated with a large MC-C tumor, although the inhibition of the secondary tumor implant was not as striking as that observed by using DX (Fig. 5b).
The immunological nature of the inhibition of the secondary MC-C tumor challenge in DX-treated MC-C tumor-bearing mice, was indicated by the following facts:
Histological studies showed an immunological reaction with abundant mononuclear cells infiltrating the MC-C tumor challenge (Fig. 5c, d) while, on the other hand, in untreated MC-C tumor-bearing mice, the secondary tumor challenge grew undisturbed without lymphocyte infiltration (Fig. 5e).
The inhibition was specific because these mice failed to reject the challenge of two immunologically unrelated tumors (the fibrosarcoma MC-B and the lymphoma LB, data not shown).
Spleen cells of these mice recovered the ability to transfer antitumor immunity to naive mice. In effect, survival index of mice receiving s.c. MC-C tumor cells plus i.p. spleen cells from DX-treated MC-C tumor-bearing mice was significantly higher (97.8 ± 6.0 days; n = 3 experiments) than that observed in mice receiving MC-C plus spleen cells from non-treated MC-C tumor-bearing mice (62.5 ± 4.0 days; n = 3 experiments; P < 0.01).
The inhibition was dependent upon the presence of T cells. In effect, DX-treated MC-C tumor-bearing mice receiving 100 μl of anti-CD3 mAb starting 2 days after DX inoculation and then biweekly for the length of the experiment did not inhibit the growth of the secondary challenge of MC-C tumor (see also Fig. 5a). Selective depletion of T cells was confirmed in spleen preparations of those mice by flow cytometry.
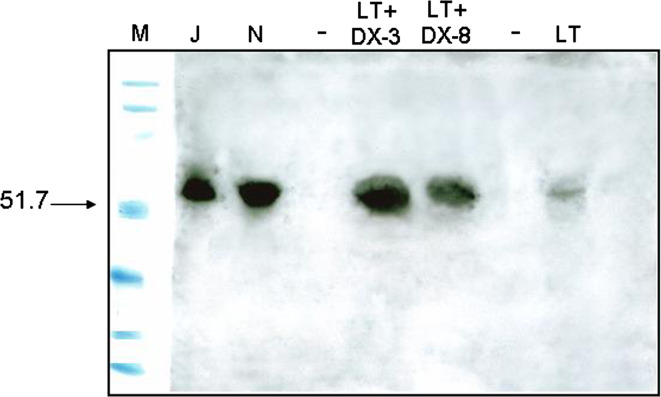
Levels of p56lck were normalized or almost normalized in spleen T cells. In effect, using Western blotting, we observed that while T cells from mice bearing large tumors displayed decreased levels of p56lck compared with control mice, levels of this protein were increased up to near control values, 3 and 8 days following treatment with DX (Fig. 6).
Fig. 6.
Normalization or cuasi-normalization of the levels of p56lck in splenic T cells from mice bearing a large MC-C tumor (1,500–2,000 mm3), 3 and 8 days after DX treatment. For more technical details see Fig. 1f. Lanes: M markers of molecular weight in Kilo Daltons; J Jurkat cells (positive control of p56lck expression), N T splenocytes from normal mice, LT + DX-3 splenocytes from mice bearing large tumors pretreated with DX 3 days earlier, LT + DX-8 splenocytes from mice bearing large tumors pretreated with DX 8 days earlier, LT T splenocytes from mice bearing large tumors non-pretreated with DX
Combined therapy with dexamethasone and dendritic cells pulsed with MC-C tumor lysate
In order to test whether the reversion of the immunological eclipse by DX would allow an otherwise ineffective immunological therapy against large established tumors to be successful, two experiments simulating two putative clinical settings were carried out.
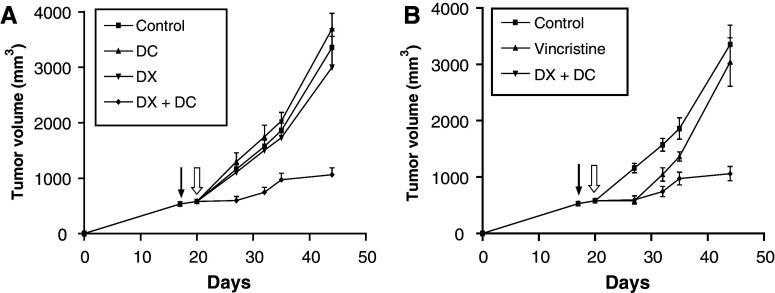
In the first experiment, MC-C tumor-bearing mice after the onset of the immunological eclipse (about 550 mm3 tumor size) received DX and 3 days later (about 600 mm3 tumor size), they were inoculated intra foot pad (i.f.p.) with dendritic cells pulsed with MC-C tumor lysate (DX + DC). As shown in Fig. 7a, a profound inhibition of tumor growth was observed in DX + DC-treated mice as compared with mice receiving dexamethasone only (DX) or dendritic cells only (DC) or none. This inhibition was maximal during the first 7–10 days after DX + DC treatment. Afterward, tumors began to grow again but with a significantly lower kinetics than those in the other three groups. This was reflected in the survival of mice treated with DX + DC: 90.5 ± 3.0 days (n = 6) which was significantly greater (P < 0.001) than that observed in mice receiving DC only (50.0 ± 2.8 days, n = 8), DX only (57.2 ± 5.2 days, n = 6) or none (50.5 ± 0.6 days, n = 11). Incidentally, it is worth noting that, on treatment with DC alone, growth of these large MC-C tumors was even faster (P < 0.05) than that observed in untreated and DX-treated mice, at least for the first 7 days following treatment (see also Fig. 7a).
Fig. 7.
a Two-step immunotherapy against an established MC-C tumor using DX treatment followed by dendritic cell vaccination. Mice bearing an MC-C tumor (about 550 mm3 of tumor size) received DX and 3 days later (when tumor size was about 600 mm3) dendritic cells (DC) pulsed with MC-C tumor lysate (DX + DC, n = 6). Tumor growth was evaluated in this group as well as in tumor bearing mice receiving DX only (DX, n = 6), DC only (DC, n = 8) or none (control, n = 11). Comparison of DX + DC with the others groups: day 27: P < 0.001 versus control, P < 0.01 versus DC; and P < 0.02 versus DX; day 32: P < 0.001 versus control, P < 0.01 versus DC and DX; day 35: P < 0.01 versus control, P < 0.001 versus DC and P < 0.05 versus DX; day 44: P < 0.001 versus control and DC and P < 0.01 versus DX. Comparison between DC versus control and DX: day 27: P < 0.05. Arrow: day of DX inoculation, open arrow day of DC inoculation. b Comparison between the therapeutic effects of the two-step immunotherapy (DX + DC) and conventional chemotherapy by using vincristine against an established MC-C tumor. Although vincristine-treated mice (vincristine, n = 10) as well as DX, DC and DX + DC-treated and control mice (control) were studied simultaneously, for clarity, data from vincristine were shown in b instead of a. For comparative purposes, data from DX + DC-treated and control mice shown in a were reproduced in b. Comparison between vincristine versus control: P < 0.001, P < 0.01 and P < 0.05 at days 27, 32, and 35, respectively. Comparison between vincristine versus DX + DC: P < 0.02 and P < 0.01 at days 35 and 44, respectively. Arrow Day of DX inoculation. Open arrow Day of DC or vincristine inoculation
To compare the efficiency of our two-step immunotherapeutic protocol to a standard chemotherapeutic treatment, an additional group of mice bearing MC-C tumor measuring 600 mm3 was treated with a single dose of vincristine (1 mg/kg body weight) by the i.p. route. Vincristine is a standard treatment of murine fibrosarcomas [29, 57] and the dose used was determined to be the highest dose that is tolerated by the mice without major weight loss. As shown in Fig. 7b, chemotherapy was able to retard tumor growth during the next 7 days after treatment in a similar fashion to our immunotherapeutic protocol. However, in chemotherapy-treated mice, tumor growth was reinitiated after day 7 with a kinetic rather similar to that observed in controls while, in contrast, in mice treated with the two-step immunotherapeutic protocol, tumor growth remained sharply retarded, meaning that in our model, this procedure was more effective than chemotherapy. In effect, although vincristine-treated mice displayed a survival time (65.6 ± 6.1 days, n = 10) greater than untreated mice (50.5 ± 0.6 days, n = 11, P < 0.02) and DC-treated mice (50.0 ± 2.8, n = 8, P < 0.05), that survival time was, in turn, significantly lower than that observed in DX + DC-treated mice: 65.6 ± 6.1 days, n = 10 versus 90.5 ± 3.0 days, n = 6; P < 0.01.
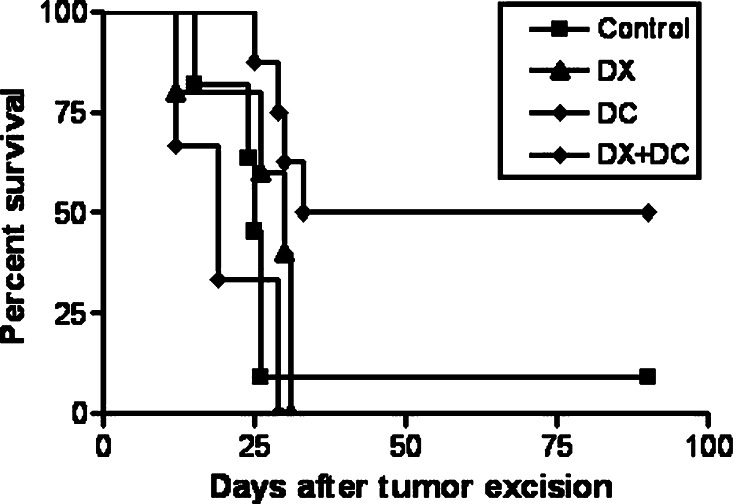
In the second experiment, MC-C tumors measuring 2,500 mm3 were surgically excised. As expected by previous experience of our laboratory, when such large tumors were excised, local tumor recurrence was observed in more than 90% of mice (Fig. 8). Tumor recurrences were, in most cases, more aggressive than the original tumors and killed the hosts rapidly. Similar results were observed when DX alone or DC alone was inoculated 3 days before or 2 days after surgery, respectively. On the other hand, when mice received both, DX (3 days before surgery) and DC (2 days after surgery), a significant reduction in local tumor recurrence was observed (see also Fig. 8) meaning that the immunological eclipse needed to be ameliorated with DX before surgical excision of the tumor, to allow a vaccination booster (DC) to have a chance to eliminate the remnant tumor cells.
Fig. 8.
Percent survival of mice after surgical excision of MC-C tumors measuring 2,500 mm3. Death of mice (if occurred) was associated with local tumor recurrences. The figure shows the percentage of the survivors of the different groups (ordinate) as a function of the days after surgery (abscissa). Control (n = 11): tumor-excised mice without additional treatment. DX (n = 10): mice receiving dexamethasone (DX) 3 days before surgery. DC (n = 6): mice receiving dendritic cells loaded with MC-C tumor lysate (DC) 2 days after surgery. DX + DC (n = 8): mice receiving DX and DC, 3 days before and 2 days after surgery, respectively. Comparison of DX + DC versus control and DC, P < 0.01; versus DX, P < 0.05. The mice displaying no-recurrence which had received the two-step immunotherapeutic protocol (DX + DC, n = 4 of 8) or none (control, n = 1 of 11) remained disease-free until day 180 after surgery, when the study was terminated
Discussion
In 1957, Prehn and Main began their classical paper in the Journal of the National Cancer Institute [42], with the following sentence: “The history of attempts to immunize against cancer is one of long frustration”. However, despite the discouragements accumulated up to that time, these authors were able to demonstrate, by using methylcholanthrene-induced fibrosarcomas and implantation and excision of tumor as the immunization procedure, that vaccination against at least some types of malignant tumors, was feasible. Since then, numerous immunization trials aimed to prevent the growth of implants of experimental tumors or virus-associated human cancer as well as in vitro assays to test the antigenic properties of some animal and human malignant cells proved to be successful [3, 20, 54]. These promissory results prompted researchers and physicians to apply immunologic schedules to treat animal as well as human established tumors. However, 50 years after that pioneer paper we can paraphrase its first sentence saying that up to date “the history of attempts to treat established tumors using immunological therapies is one of long frustration”. Failures of anticancer immunotherapeutic schedules observed in clinical settings might, at first sight, be attributed to the weak antigenicity of many clinical cancers on the basis that spontaneous tumors of mice, rats and presumably humans, display, in general, a significantly lower immunogenicity than experimental ones induced by massive doses of chemicals and viruses [13, 16, 49, 55].
However, although the weak antigenicity can be an explanation for those failures, other causes can play a main role [64] as evidenced by the fact that despite their enormous promise, therapeutic vaccine strategies have shown only very limited success even in the case of strongly immunogenic chemically induced tumors in mice as well as in renal cell carcinoma in human beings, two prototypical malignancies for the application of immunotherapy [7, 9, 30, 58].
In this paper, by studying a methylcholanthrene-induced fibrosarcoma similar to those used by Prehn and Main many years before, we were able to demonstrate that the same immunological procedures which successfully prevented the growth of implants of cells of that tumor were, in contrast, largely inefficient to eradicate, inhibit or even slow down the growth of the same tumor cells when they were growing as a tumor larger than a minimal critical size.
The failure to achieve an efficient immunological treatment against established tumors was paralleled in our model with the emergence of a state of specific immunological unresponsiveness against tumor antigens, historically known as “immunological eclipse”, observed when the tumor surpassed the critical size of 500 mm3. Actually, the refractoriness to any attempt of immunotherapy was observed when treatment was initiated even before the onset of the eclipse (about 100 mm3). This discrepancy may be more apparent than real. In effect, tumor-bearing hosts may not be in the state of eclipse when a therapy is initiated, but this state could be reached before the immunological treatment had had a chance to generate a strong antitumor immune response. Alternatively, immunosuppressive conditions operating at the site of tumor growth could precede the establishment of a systemic antitumor unresponsiveness [2].
During the state of eclipse, all immunological markers of anti-tumor response, which had been raised at the very early stages of tumor growth, were down regulated. However, the mere absence of immune competence was not the only or even the main problem because if it were the case, immunological unresponsiveness could be solved by passive transfer of immunity. A main problem, suggested by experiments of parabiosis between immune and tumor bearing mice in the state of eclipse, was the emergence of a suppressor mechanism which actively suppressed the anti-tumor immune response mounted in the organism against the tumor. This could explain the refractoriness of established animal and human tumors to immunological therapies because any mechanism present in a tumor-bearing host, which is actively suppressing its own anti-tumor response, would also be expected to sterilize any attempt of active or passive anti-tumor immunotherapy.
Different mechanisms have been reported to be associated etiologically with the immunological eclipse which might limit or impede successful immunotherapy of cancer. Some of these impediments might be tumor cell-associated, including the loss of class I MHC expression, tumor-expression of the vascular cell adhesion molecule-1 (VCAM-1), enhanced expression by tumor cells and by the surrounding stromal cells of the tolerogenic enzyme indoleamine 2,3-dioxygenase (IDO) or the production by tumor cells of factors such as IL-10, TGF-β or galectin-1 that could inhibit effective immune responses [26, 48, 60, 67]. Other impediments have been associated with a series of negative immunoregulatory mechanisms including the anomalous expression of the negative co-stimulatory molecule CTLA-4 in cytotoxic T-lymphocytes or the increased expression of CD4+ CD25+ T reg or CD4+ NK T cells [59, 60]. However, although reduction of these putative immunosuppressive mechanisms was effective for preventing the growth of tumor implants or for the treatment of incipient tumors, up to date, to our knowledge, it has been largely inefficient against tumors once they grow beyond a minimal critical size [39, 66]. It is worth noting that, in our model, although a slight and transient increase of T reg cells and foxp3 expression was seen in the draining lymph nodes two days after tumor implantation (G. Dran, unpublished results), their number was progressively reduced in both, spleen and lymph nodes throughout tumor growth, suggesting that this subset of T suppressor cells could play a significant role during tumor implantation but presumably not (or at least not a main role) in the development of the immunological eclipse associated with MC-C tumor growth. However, more experiments are necessary to address this issue.
In our model, the onset of the immunological eclipse was coincident with the onset of a systemic inflammatory condition the intensity of which was proportional to tumor size and characterized by a high number of circulating and splenic PMN displaying activation and Gr1+Mac1+ phenotype and an increasing serum concentration of the pro-inflammatory cytokines TNFα, IL-1β and IL-6 and the CRP and serum A amyloid phase acute proteins. A quantum of inflammation is a prerequisite for the development of an efficient immune response [34]. However, under certain conditions, for example, when inflammation becomes systemic and/or chronic, adverse effects can be induced, directly, by the “wrong” inflammatory condition itself or indirectly, by a compensatory anti-inflammatory reaction [33–35, 61]. In effect, during progression of human and animal tumors, an accumulation of immature myeloid precursors and PMN Gr1+Mac1+ cells in the blood and infiltrating the spleen has been associated with a decreased number of dendritic cells and the absence of antitumor responsiveness by T cells. These effects have been claimed to be associated with: (i) tumor-derived factors that can activate Jak2/Stat3 signaling in myeloid cells and prevent dendritic cells differentiation; (ii) migration of Gr1+Mac1+ cells into peripheral lymphoid organs where they can inhibit CD8 T cell immune response; (iii) migration of Gr1+Mac1+ cells into tumor site where they can differentiate into highly immune suppressive tumor associated macrophages (TAM) [5, 6, 10, 24, 25, 40, 52]. In the same way, overproduction of some pro-inflammatory cytokines has been implicated in a wide range of pathological conditions [62]. In our model, in vitro proliferation of immune spleen cells incubated with tumor antigens was inhibited by co-culturing with serum from mice bearing large MC-C tumors, which displayed elevated concentration of cytokines and phase acute proteins, but not with normal serum or serum from mice bearing small tumors. The above considerations suggest that different cellular and humoral factors, directly or indirectly associated with the systemic inflammatory condition, could contribute to the establishment and the maintenance of the immunological eclipse.
Treatment of tumor-bearing mice with a relatively low single dose of the synthetic corticoid dexamethasone, significantly reduced the number of circulating and splenic Gr1+Mac1+ cells as well as the concentrations of circulating pro-inflammatory cytokines and acute phase proteins. This dexamethasone-mediated anti-inflammatory effect, which has recently been claimed to be dependent on induction of dual specificity phosphatase I [1], was paralleled with an increase in the number of both dendritic cells and lymphocytes, in lymph nodes and spleen, together with a loss of the serum inhibitory activity on the in vitro proliferation of immune spleen cells incubated with tumor antigens.
In turn, these dexamethasone-mediated effects were associated with the reversion of the immunological eclipse as evidenced by the restoration of specific T-cell dependent concomitant immunity, ability of spleen cells to transfer anti-tumor activity to naive mice and recovery of immune response-associated transducing signals by spleen T cells.
At first glance, the restoration of the anti-tumor immune competence by dexamethasone seems paradoxical since corticoids have usually been associated with immunosuppressive properties. However, the latter have generally been observed when using pharmacological doses of corticosteroids while stimulating effects on the immune system have been reported by using lower doses [63].
Recent evidence suggests that the immune-stimulating effect of dexamethasone could be produced, at least in part, through the up-regulation of GITR (glucocorticoid-induced tumor-necrosis-factor-receptor-related protein) expression and the consequent activation of the GITR pathway in T cells. In effect, activation of this pathway upon treatment with agonistic GITR-specific antibodies has been shown to promote T-cell responses to tumor antigens or viral pathogens that otherwise fail to elicit significant responses, suggesting that GITR–GITR ligand interactions co-stimulate responder T cells and allow their escape from immunosuppressive mechanisms [23, 53]. Others have suggested that glucocorticoids could stimulate the immune response by enhancing the expression of toll-like receptor 2 [18].
Two other anti-inflammatory treatments, by using non-steroid anti-inflammatory molecules, such as a dimeric TNF-α receptor or indomethacin, also reversed partially the immunological eclipse, although the results were not as striking as those observed with dexamethasone, suggesting that the latter might have the potential to affect more broadly the network of tumoral immune tolerance.
Taken together, the above mentioned results suggest that the loss of anti-tumor immunity displayed after the tumor grows beyond 500 mm3 is not an irreversible event and that the systemic inflammation associated with tumor progression would mask the existence of T cells bearing anti-tumor reactivity. In the same way, although it is possible that the increase in p56lck upon dexamethasone treatment was due to the death of abnormal T cells followed by an influx of normal T cells, the rapidity with which the response was observed suggests that levels of p56lck might have been restored in those cells in which they had been decreased.
Although the reversion of the immunological eclipse by an anti-inflammatory treatment was a promissory achievement, it was not enough on its own to inhibit the primary growing tumor in the same way that, in the pre-eclipse stage, the existence of an anti-tumor response which is sufficient to prevent a secondary tumor implant to grow, is at the same time ineffective to inhibit the growth of the established primary tumor. In order to attack a primary large tumor during the immunological eclipse, we used the two-step strategy of inoculating dexamethasone to reverse the eclipse and three days later, dendritic cells loaded with tumor antigens as an immunization booster. When mice bearing tumors measuring 600 mm3 approximately, received this combined treatment, a striking effect on the growth of the tumors was achieved. For the first 7–10 days after dendritic cell inoculation, tumors stopped their growth and thereafter, when growth was reinitiated, it was markedly slower than that of the controls inoculated with dexamethasone alone, dendritic cells loaded with tumor antigens alone or none. This was reflected in the survival time of the former which almost duplicated that of the controls. In the same way, the combined treatment of dexamethasone plus dendritic cells was able to partially prevent the local tumor recurrences observed when tumors measuring 2,500 mm3 were excised.
The two-step strategy of tumor immunotherapy presented herein demonstrates that it is necessary to eliminate or ameliorate the immunological eclipse as a precondition to allow any immunization procedure to have a chance to be successful. This might explain why immunotherapy alone is not usually effective in clinical settings even against a relatively small number of local and distant residual tumor cells after debulking the primary tumor by surgery, radiation or chemotherapy. Moreover, the application of immunological therapies without withdrawing that eclipse could accelerate rather than inhibit tumor growth according to the predictions of the immunostimulatory theory proposed by Prehn [43, 45, 46]. In effect, in our hands, when an immunological strategy of vaccination, on the basis of dendritic cells only, was applied to treat established tumors, a slight but significant stimulation of tumor growth was observed, despite the fact that the same vaccination strategy was very efficient to prevent the growth of tumor implants or to slow down the growth of incipient tumors.
While having the theoretical advantage of specificity and lower toxicity, immunotherapy is usually thought to be less effective than chemotherapy for treating established tumors. However, this comparison has usually been made between chemotherapy and immunological procedures applied individually, i.e., without previously eliminating the immunological eclipse. In this paper, we have compared the standard vincristine-based chemotherapeutic treatment of murine fibrosarcomas [29, 57] to the two-step immunotherapeutic protocol presented herein. Our results show that even though chemotherapy slowed down the growth of MC-C murine fibrosarcoma and prolonged the survival time of tumor-bearing mice as compared with untreated mice or mice treated with dendritic cells alone, these inhibitory effects were significantly less important than those achieved by the two-step immunotherapeutic protocol, meaning that in our model, this procedure was more efficient than conventional chemotherapy.
In conclusion, and even admitting the limitations of mouse tumor models to predict the efficacy of any approach for the therapy of human tumors [56], some interesting conclusions can be drawn:
The immune system would be capable of inhibiting the growth of both, established tumors and residual tumor cells after excision of large established tumors, but only when the immunological eclipse had previously been eliminated.
In our model, the immunological eclipse seemed to be associated, directly or indirectly, with some cellular and humoral components of the systemic inflammatory condition exhibited in mice bearing large tumors.
The two-step immunotherapeutic protocol was applied systemically, circumventing the limitations of an intra-tumor therapy that might be impractical in many clinical settings.
In some cases, immune therapy of a solid tumor can be more effective than conventional chemotherapy.
Acknowledgments
This work was supported by grants from CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Fundación Roemmers and Agencia Nacional de Promoción Científica y Tecnológica (PICT 05-38197/2005), Argentina. One of the authors (M. Vulcano) was a recipient of an International Cancer Technology Transfer Fellowship (ICRETT 2005) from the International Union Against Cancer (UICC). The authors are grateful to Dr. Richmond T. Prehn and Dra. Christiane D. Pasqualini for critical discussion of this article.
References
- 1.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase I. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler AJ. Mechanisms of T cell tolerance and suppression in cancer mediated by tumor-associated antigens and hormones. Curr Cancer Drug Target. 2007;7:3–14. doi: 10.2174/156800907780006931. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Dillner J. Review of current knowledge on HPV vaccination: an appendix to the European guidelines for quality assurance in cervical cancer screening. J Clin Virol. 2007;38:189–197. doi: 10.1016/j.jcv.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Bustuoabad OD, Ruggiero RA, di Gianni P, Lombardi MG, Belli C, Camerano GV, Dran G, Schere-Levy C, Costa H, Isturiz MA, Narvaitz M, van Rooijen N, Bustuoabad VA, Meiss RP. Tumor transition zone: a putative new morphological and functional hallmark of tumor aggressiveness. Oncol Res. 2005;15:169–182. doi: 10.3727/096504005776367933. [DOI] [PubMed] [Google Scholar]
- 5.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac1+/Gr1+ cells. J Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 6.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte–macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 7.Chiarella P, Vulcano M, Laborde E, Vermeulen M, Bruzzo J, Rearte B, Bustuoabad OD, Ruggiero RA. Reversion of the immunological eclipse and therapeutic vaccination against cancer in an experimental model [Article in Spanish] Medicina (Bs. As.) 2007;67:44–48. [PubMed] [Google Scholar]
- 8.Coligan J, Knisblek A, Margulies D, Shevach E, Warren S, et al. In vitro assays for mouse lymphocyte function. In: Coligan J, Knisblek A, Margulies D, et al., editors. Current protocols in immunology, National Institute of Health, USA. New York: Wiley; 1994. pp. 320:3–320:4. [Google Scholar]
- 9.Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immmunol Immunother. 2004;53:275–306. doi: 10.1007/s00262-003-0432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.V97.2.339. [DOI] [PubMed] [Google Scholar]
- 11.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 12.Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953;13:835–837. [PubMed] [Google Scholar]
- 13.Franco M, Bustuoabad OD, di Gianni PD, Goldman A, Pasqualini CD, Ruggiero RA. A serum-mediated mechanism for concomitant resistance shared by immunogenic and non-immunogenic murine tumours. Br J Cancer. 1996;74:178–186. doi: 10.1038/bjc.1996.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelik E. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv Cancer Res. 1983;39:71–120. doi: 10.1016/s0065-230x(08)61033-7. [DOI] [PubMed] [Google Scholar]
- 15.Heckelsmiller K, Beck S, Rall K, Sipos B, Schlamp A, Tuma E, Rothenfusser S, Endres S, Hartmann G. Combined dendritic cell-and CpG oligonucleotide- based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol. 2002;32:3235–3245. doi: 10.1002/1521-4141(200211)32:11<3235::AID-IMMU3235>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976;33:241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiramoto Y, Sugimachi K. Effect of glucocorticoid deficiency after adrenalectomy on antitumor immunity. Cancer Immunol Immunother. 1987;25:157–160. doi: 10.1007/BF00199141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imasato A, Desbois-Mouthon C, Han J, Kai H, Cato AC, Akira S, Li JD. Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae induced expression of toll-like receptor 2. J Biol Chem. 2002;277:47444–47450. doi: 10.1074/jbc.M208140200. [DOI] [PubMed] [Google Scholar]
- 19.Inman BA, Frigola X, Donq H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 20.Janeway CA, Travers P, Walport M, Shlomckik MJ (2001) Manipulation of the immune response. In: Immunobiology. Garland, New York, pp 566–577
- 21.Kalimi MY, Agarwal MK. Interaction of antiglucocorticoid RU-486 with rat kidney glucocorticoid receptor. Biochem Biophys Res Commun. 1988;153:365–371. doi: 10.1016/S0006-291X(88)81232-4. [DOI] [PubMed] [Google Scholar]
- 22.Klein G, Sjögren HO, Klein E, Hellström KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561–1572. [PubMed] [Google Scholar]
- 23.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+ CD4+CD25+ T regulatory cells. J Exp Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L (2007) Tumor-derived hyaluronan induces formation of immunosuppressive macrophages. Blood. doi:10.1182/blood-2007-01-068031 [DOI] [PubMed]
- 25.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin KY, Lu D, Huang CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, Kim DJ, Jaffee E, Pardoll D, Wu TC. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res. 2007;67:1832–1841. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maehara Y, Hiramoto Y, Akazawa K, Sakaguchi Y, Tamada R, Sugimachi K. Effect of glucocorticoid replacement on tumor growth alter adrenalectomy in mice. Cancer Res. 1989;49:2048–2051. [PubMed] [Google Scholar]
- 28.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumor immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 29.Meyvisch C, Storme GA, Bruyneel E, Mareel MM. Invasiveness and tumorigenicity of MO4 mouse fibrosarcoma cells pretreated with microtubule inhibitors. Clin Exp Metastasis. 1983;1:17–28. doi: 10.1007/BF00118469. [DOI] [PubMed] [Google Scholar]
- 30.Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part 1: Vaccines for solid tumors. Lancet Oncol. 2004;5:681–689. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 31.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 32.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Roger-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nature Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 34.Nathan C. Points of Control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 35.Nelson D, Ganss R. Tumor growth or regression: powered by inflammation. J Leukoc Biol. 2006;80:685–690. doi: 10.1189/jlb.1105646. [DOI] [PubMed] [Google Scholar]
- 36.North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984;35:89–155. doi: 10.1016/S0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- 37.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 38.Offringa R. Cancer: enhanced: cancer immunotherapy is more than a number game. Science. 2006;314:68–69. doi: 10.1126/science.1133893. [DOI] [PubMed] [Google Scholar]
- 39.Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol. 2006;16:137–139. doi: 10.1016/j.semcancer.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Pekarek LA, Starr B, Toledano A, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrotta C, Falcone S, Capobianco A, Camporeale A, Sciorati C, De Palma C, Pisconti A, Rovere-Querini P, Bellone M, Manfredi AA, Clementi E. Nitric oxide confers therapeutic activity to dendritic cells in a mouse model of melanoma. Cancer Res. 2004;64:3767–3771. doi: 10.1158/0008-5472.CAN-04-0668. [DOI] [PubMed] [Google Scholar]
- 42.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 43.Prehn RT. The immune reaction as a stimulator of tumor growth. Science. 1972;176:170–171. doi: 10.1126/science.176.4031.170. [DOI] [PubMed] [Google Scholar]
- 44.Prehn RT. Two competing influences that may explain concomitant tumor resistance. Cancer Res. 1993;53:3266–3269. [PubMed] [Google Scholar]
- 45.Prehn RT. An adaptive immune reaction may be necessary for cancer development. Theor Biol Med Model. 2006;3:6. doi: 10.1186/1742-4682-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prehn RT. Immunostimulation and immunonoinhibition of premalignant lesions. Theor Biol Med Model. 2007;4:6. doi: 10.1186/1742-4682-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossi GR, Mautino MR, Unfer RC, Seregina TM, Vahanian N, Link CJ. Effective treatment of preexisting melanoma with whole cell vaccines expressing α (1,3)-galactosyl epitopes. Cancer Res. 2005;65:10555–10561. doi: 10.1158/0008-5472.CAN-05-0627. [DOI] [PubMed] [Google Scholar]
- 48.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboin L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection: a potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–251. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 49.Ruggiero RA, Bustuoabad OD, Bonfil RD, Meiss RP, Pasqualini CD. “Concomitant immunity” in murine tumors of non-detectable immunogenicity. Br J Cancer. 1985;51:37–48. doi: 10.1038/bjc.1985.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggiero RA, Bustuoabad OD, Cramer P, Bonfil RD, Pasqualini CD. Correlation between seric antitumor activity and concomitant resistance in mice bearing nonimmunogenic tumors. Cancer Res. 1990;50:7159–7165. [PubMed] [Google Scholar]
- 51.Ruggiero RA, Bustuoabad OD. The biological sense of cancer: a hypothesis. Theor Biol Med Model. 2006;3:43. doi: 10.1186/1742-4682-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salvadori S, Martinelli G, Zier K. Resection of solid tumors reverses T cell defects and restores protective immunity. J Immunol. 2000;164:2214–2220. doi: 10.4049/jimmunol.164.4.2214. [DOI] [PubMed] [Google Scholar]
- 53.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 54.Sioud M. An overview of the immune system and technical advances in tumor antigen discovery and validation. Methods Mol Biol. 2007;360:277–318. doi: 10.1385/1-59745-165-7:277. [DOI] [PubMed] [Google Scholar]
- 55.Speiser DE, Miranda R, Zakarian A, Bachmann MF, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel RM, Ohashi PS. Self antigens expressed by solid tumors do not efficiently stimulate naïve or activated T cells: implications for immunotherapy. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teicher BA, Holden SA, Al-Achi A, Herman TS. Classification of antineoplastic treatments by their differential toxicity toward putative oxygenated and hypoxic tumor subpopulations in vivo in the FSaIIC murine fibrosarcoma. Cancer Res. 1990;50:3339–3344. [PubMed] [Google Scholar]
- 58.Vieweg J, Su Z, Dahm P, Kusmartsev S. Reversal of tumor-mediated immunosuppression. Clin Cancer Res. 2007;13:727–732. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 59.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;4:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 60.Waldman TA. Immunotherapy: past, present and future. Nat Med. 2003;3:269–277. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]
- 61.Ward PA. Inflammation. In: LaVia MF, Hill RB Jr, editors. Principles of Pathology. 2. New York: Oxford University Press; 1975. pp. 97–140. [Google Scholar]
- 62.Watson B. TNF inhibitors: a review of the recent patent literature. I Drugs. 2002;5:1151–1161. [PubMed] [Google Scholar]
- 63.Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today. 1997;18:418–424. doi: 10.1016/S0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 64.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 65.Winn HJ. Immune mechanisms in homotransplantations: II. Quantitative assay of the immunological activity of lymphoid cells stimulated by tumor homograft. J Immunol. 1961;86:228–239. [PubMed] [Google Scholar]
- 66.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Zheng X, Koropatnick J, Li M, Zhang X, Ling F, Ren X, Hao X, Sun H, Vladau C, Franek JA, Feng B, Urquhart BL, Zhong R, Freeman DJ, García B, Min WP. Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. J Immunol. 2006;177:5639–5646. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]