Abstract
Melanoma chondroitin sulfate proteoglycan (MCSP; also called CSPG4, NG2, HMW-MAA, MSK16, MCSPG, MEL-CSPG, or gp240) is a surface antigen frequently expressed on human melanoma cells, which is involved in cell adhesion, invasion and spreading, angiogenesis, complement inhibition, and signaling. MCSP has therefore been frequently selected as target antigen for development of antibody- and vaccine-based therapeutic approaches. We have here used a large panel of monoclonal antibodies against human MCSP for generation of single-chain MCSP/CD3-bispecific antibodies of the BiTE (for bispecific T cell engager) class. Despite similar binding affinity to MCSP, respective BiTE antibodies greatly differed in their potency of redirected lysis of CHO cells stably transfected with full-length human MCSP, or with various MCSP deletion mutants and fusion proteins. BiTE antibodies binding to the membrane proximal domain D3 of MCSP were more potent than those binding to more distal domains. This epitope distance effect was corroborated with EpCAM/CD3-bispecific BiTE antibody MT110 by testing various fusion proteins between MCSP and EpCAM as surface antigens. CHO cells expressing small surface target antigens were generally better lysed than those expressing larger target antigens, indicating that antigen size was also an important determinant for the potency of BiTE antibody. The present study for the first time relates the positioning of binding domains and size of surface antigens to the potency of target cell lysis by BiTE-redirected cytotoxic T cells. In case of the MCSP antigen, this provides the basis for selection of a maximally potent BiTE antibody candidate for development of a novel melanoma therapy.
Keywords: BiTE antibody, MCSP, Melanoma, T cell, Redirected lysis, Epitope, Cytolytic synapse
Introduction
Numerous therapeutic approaches are currently testing the potential of T cells for the treatment of cancer. These include vaccination with tumor-derived proteins, RNA or peptide antigen, infusion of tumor-derived, ex-vivo expanded T cells (called adoptive transfer), T cell receptor gene transfer or direct engagement of T cells by bi- or trispecific antibodies. Likewise, many stimulants of T cell responses are clinically tested in combination or as monotherapy, such as ligands for Toll-like receptors, antibodies blocking CTLA-4 on T cells, immune stimulatory cytokines, or antibodies neutralizing molecules involved in immune escape of cancer cells such as TGF-beta or B7-H1 [1, 2]. The intense development of T cell-based therapies is spurred by the observation that patients appear to live significantly longer if their tumors are infiltrated by T cells. This has been observed, e.g., for patients with colorectal cancer [3], ovarian cancer [4], and non-Hodgkin lymphoma [5]. Moreover, numerous mouse models have shown that engagement of T cells by various means can eradicate even large tumors [6, 7].
A number of T cell therapies have recently made significant progress in treating various cancer indications. One is an EpCAM-specific trispecific antibody called catumaxomab, which has shown efficacy in prevention of malignant ascites in ovarian and other EpCAM-expressing cancer patients and just gained market authorization in Europe [8]. Antibodies blocking CTLA-4 on T cells [9, 10] or adoptive T cell transfer [11–13] showed stunning tumor regression in late-stage melanoma patients, albeit at low response rates. A vaccine based on prostate acidic phosphatase for mounting a prostate cell-specific T cell response apparently provided a significant survival advantage to prostate cancer patients [14]. Disease stabilization or tumor responses have been frequently reported in numerous trials with vaccines or adoptive transfer. However, response rates are typically low, which may relate to immune escape mechanisms accumulating in late-stage cancer cells by Darwinian selection [15].
Encouraging clinical results were also reported for bispecific antibody blinatumomab (MT103), which can engage T cells for redirected lysis of CD19-expressing lymphoma and leukemia cells [16]. It induced confirmed partial and complete regression of lymphoma lesions [16], and response rates of >80% were observed in early trials [17]. Blinatumomab belongs to the class of ‘bispecific T cell engager’ (BiTE) [18], which can transiently connect T cells and target cells via binding to CD3 and a selected surface target antigen. A number of additional BiTE antibodies with specificity for epithelial cell adhesion molecule [19], carcinoembryonic antigen [20], and tyrosine kinase EphA2 [21] have been characterized in vitro and in animal models. BiTE antibodies consistently show a high potency of redirected lysis in cell culture and animal models, serial lysis by BiTE-activated T cells, and a conditional activation of T cells requiring the presence of target cells [22, 23].
Here, we made a first attempt to generate novel BiTE antibodies targeting human melanoma-associated chondroitin sulfate proteoglycan (MCSP), also called high molecular weight melanoma-associated antigen (HMW-MAA), chondroitin sulfate proteoglycan 4 (CSPG4), or NG2 [24]. There is increasing evidence that MCSP is involved in tumorigenesis as a protein regulating cell adhesion, migration, and melanoma cell invasion through cytoskeletal rearrangement [25–27]. MCSP is very frequently and highly expressed on human melanoma cells [28], and has been selected for development of various vaccine- and antibody-based therapeutic approaches. These include antagonistic monoclonal antibodies [29], T cell-engaging trispecific antibodies [30], anti-idiotypic antibodies [31], immunotoxins [32], and bispecific antibodies [33]. So far, none of these candidates has advanced to later stages of clinical development.
A particular challenge of using MCSP as target antigen for bispecific antibodies is its large molecular weight and high degree of glycosylation. We have therefore created a large panel of MCSP-specific monoclonal antibodies in order to identify the most appropriate binding site for a BiTE antibody. Here, we report for the first time that BiTE antibodies are affected in their potency of redirected lysis by epitope distance to the target cell protein and by antigen size. This knowledge has then been used to generate a MCSP-specific BiTE antibody binding to the domain D3 of MCSP as basis for the development of a drug candidate to treat melanoma.
Materials and methods
Generation of CHO cells expressing the MCSP model antigens and EpCAM/MCSP fusion proteins
The coding sequences of mutant MCSP constructs were obtained by cDNA gene synthesis and contained an immunoglobulin leader peptide, a FLAG epitope tag and the transmembrane and cytoplasmic domain of human MCSP [34]. Gene synthesis fragments were flanked by restriction sites for cloning into the mammalian cell expression vector pEF-DHFR [35] and transfection into DHFR deficient CHO cells.
The large extracellular domain of MCSP spanning >2,000 aa was divided into three structural domains according to Pluschke et al. [34], i.e., the N- and C-terminal cysteine-rich domains (called here domains D1 and D3), and a cysteine-free central domain (called here D2). MCSP domain D1 comprises amino acids 30–668 of the native human MCSP protein (NM_001897), D2 consists of amino acids 669–1,537, and D3 contains amino acids 1,538–2,221 adjacent to the transmembrane and cytoplasmic domain (amino acids 2,222–2,322). The coding sequences of the EpCAM/MCSP fusion proteins were obtained by gene synthesis as well and designed as described above. The leader is followed by the coding sequence of the extracellular domain of the mature human EpCAM protein (amino acids 17–265) followed by a FLAG epitope tag and the respective extracellular part of the human MCSP adjacent to the transmembrane and cytoplasmic domain of human MCSP.
Target cells stably expressing human MCSP, rat NG2, human EpCAM and the various mutant MCSP and EpCAM/MCSP fusion proteins were generated by transfecting CHO dhfr− (DSMZ, Braunschweig, Germany) with the pEF DHFR expression vector containing the respective cDNA sequences. Selection of clones and amplification of expression was performed in the presence of methotrexate.
Generation and characterization of anti-MCSP monoclonal antibodies
The extracellular domain of MCSP [34] was cloned into pEF DHFR and CHO dhfr− cells transfected with the cDNA expression vector encoding MCSP. By means of a hexahistidine tag, soluble MCSP was affinity purified from cell culture supernatant and used for immunization of CB6-F1 mice. Spleen cells of immunized mice were fused with myeloma cells and cell culture supernatants of hybridoma cells tested in an ELISA with bound soluble MCSP. Supernatants of MCSP-specific antibody expressing hybridoma cells were then tested for recognition of MCSP expressed on human melanoma cell lines. Thirty-two MCSP-specific monoclonal antibodies were isolated.
The MCSP-specific monoclonal antibodies were then tested by FACS analysis for binding to CHO cells expressing recombinant rat MCSP and, as background control, to dhfr− CHO cells. Seven antibodies cross-reacting with rat MCSP were discarded because they would not allow the use of chimeric MCSP/NG2 fusion proteins for domain mapping. Another six antibodies showed background binding to non-transfected CHO cells and were discarded as well.
The remaining antibodies were further tested by FACS analysis for binding to CHO transfectants expressing the isolated MCSP domains D1, D2 or D3, combinations of domains D1D2 and D1D3, and for binding to CHO transfectants expressing human/rat MCSP chimeric proteins combining human (h) and rat (r) D1, D2, and D3 domains in the following order: hhr, hrh, rhh, rrh, rhr, and hrr. This further eliminated two sets of monoclonal antibodies. One was binding to more than one MCSP domain, presumably, because of epitope redundancy. The other set did no longer bind to isolated MCSP domains, presumably, because binding epitopes were destroyed by the truncation.
A final selection of four monoclonal antibodies that showed strong and specific binding to either domain D1, D2 or D3 of MCSP in the FACS-based assay was based on their performance in the BiTE antibody format. As a structural element of BiTE antibodies, they had to be stable as a single-chain antibody in conjunction with an anti-CD3 single-chain antibody, be produced in sufficient amounts, and not shown the formation of aggregates.
For FACS analysis, CHO cells transfected with MCSP domain constructs (see Fig. 1b), human/rat MCSP chimeric molecules, and rat MCSP (called NG2) were incubated with cell culture supernatants of hybridoma cell lines. Bound MCSP antibodies were detected using a phycoerythrin (PE)-conjugated anti-mouse IgG antibody (Jackson Immuno Research). For generation of MCSP-specific BiTE antibodies, hybridoma lines 128, 113, 70, and 120 were selected.
Fig. 1.

Characterization of MCSP-specific BiTE antibodies. a Structures and binding affinities of MCSP-specific BiTE antibodies. All four BiTE antibodies share the same CD3-specific single-chain antibody L2K (open boxes), but differ in their MCSP-specific single-chain antibody (filled boxes). All had a C-terminal hexahistidine sequence for detection and affinity purification; N N-terminus; C C-terminus. b MCSP-derived constructs as expressed on transfected CHO cell lines. D1, D2, and D3 refer to the three domains of the dumbbell-shaped MCSP molecule. MCSP domain 1 corresponds to aa 30–668, D2 to aa 669–1,537, and D3 to aa 1,538–2,221 of human MCSP. All single and double domain constructs contain the transmembrane and cytoplasmic domain of human MCSP corresponding to aa 2,222–2,322; PM plasma membrane. c Surface expression of FLAG-tagged MCSP constructs on stably transfected CHO cells. d Number of binding sites for various anti-MCSP BiTE antibodies on stably transfected CHO lines. Binding sites were determined by the QIFIKIT kit. e Model of MCSP showing binding sites for four BiTE antibodies
Generation of MCSP-specific BiTE antibodies
Construction, expression in CHO cells, and purification of MCSP/CD3-bispecific BiTE antibodies MCSP120, MCSP128, MCSP113, and MCSP70 were essentially as described in detail earlier for BiTE antibody MT110 [19]. The MCSP-specific BiTE antibodies used in our study have been secreted by producer CHO cell clones into cell culture supernatants. Only the monomeric form of the BiTE antibodies has been characterized for potency of lysis in the shown experiments. MCSP-specific BiTE antibodies with a high tendency to aggregate have not been further investigated. High performance size exclusion chromatography has been used to test for the stability of monomeric BiTE antibodies upon storage.
Flow cytometry
The presence of MCSP or rat NG2 surface antigens on transfected CHO cells was examined by flow cytometry with a FLAG-specific antibody (Sigma) and a phycoerythrin-labeled goat anti-mouse IgG. The expression of EpCAM on CHO cells transfected with human EpCAM or EpCAM/MCSP fusion proteins was verified by flow cytometry with the murine anti-human EpCAM IgG1 antibody 5–10 [36]. As negative control, cells were incubated with PBS/2% FCS instead of the primary antibody. The relative fluorescence of cells was measured using a FACSCalibur flow cytometer; CellQuest software was used to acquire and analyze the data (Becton Dickinson Biosciences, Heidelberg, Germany).
Determination of dissociation constants
The dissociation constants were determined by Scatchard analysis. A dilution series with a starting concentration of 150 μg/ml (MT110) or 50 μg/ml (MCSP-BiTE antibodies) was made. EpCAM-CHO and MCSP-CHO cells were stained with MT110 or MCSP-BiTE antibodies (MCSP128, MCSP113, MCSP70, and MCSP120), respectively. Bound BiTE antibodies were detected with the afore determined saturated concentration of an anti-pentahistidine IgG (Qiagen) and a FITC-conjugated anti-mouse IgG antibody (DAKO). The relative fluorescence of cells was measured by a FACSCalibur flow cytometer and analyzed by the CellQuest software. By relating the mean values of the fluorescence intensity of stained cells to the BiTE molar concentrations, dissociation constants were calculated by the software Graphpad PRISM 4.0.
Quantification of cell surface antigen molecules
The number of surface antigen binding sites on transfected CHO dhfr− cells was determined by the DAKO QIFIKIT kit (DakoCytomation), according to the manufacturer’s instructions. Cells were incubated with the afore determined saturated concentration of BiTE antibodies and bound bispecific antibodies detected with the afore determined saturated concentration of an anti-pentahistidine IgG (Qiagen) and 20 μg/ml FITC-labeled goat-anti-mouse IgG antibody (DakoCytomation). Samples were analyzed by flow cytometry. Using a bead calibration curve, the number of surface binding sites was calculated by Microsoft Excel.
PBMC stimulation and T cell isolation
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density centrifugation and stimulated with 20 U/ml IL-2 (Proleukin, Chiron, Ratingen, Germany) and OKT3 and an CD28 antibody (BD Biosciences) at a final concentration of 1 μg/ml for 2 days. On the third day, cells were washed and IL-2 added to a final concentration of 20 U/ml. The following day cytotoxic CD8+ T lymphocytes (CTLs) were enriched by depleting CD4+ T cells, CD56+ NK and NKT cells according to standard protocols.
Cytotoxicity assay
Melanoma cells or stably transfected CHO cells expressing various MCSP or EpCAM/MCSP fusion proteins (see Fig. 1b or 4a) were loaded for 1 h at 37°C with 51Cr and then washed twice. Target cells were co-cultured for 18 h with stimulated T cells at an effector-to-target (E:T) ratio of 10:1 in the presence of the indicated BiTE concentrations. The starting concentration of dilution series for bispecific antibodies was 1 μg/ml. Cytotoxicity was measured as relative values of released chromium in the supernatant related to the difference of maximum lysis as achieved by addition of the non-ionic detergent Triton-X and spontaneous lysis in the absence of effector cells. Radioactive chromium released in the supernatants was quantified with a Wizard 3″ gamma counter (Perkin Elmer Life Sciences GmbH, Köln, Germany). For comparison of bioactivity, data were analyzed with GraphPad Prism 4 software. The assays were performed in triplicates.
Fig. 4.

Characterization of CHO cell lines stably expressing fusions between EpCAM and MCSP domains. a Schematic depiction of fusion proteins between EpCAM and MCSP. All fusion proteins use the transmembrane and cytoplasmic domain of human MCSP; PM plasma membrane. b Structure of EpCAM/CD3-bispecific BiTE antibody MT110. The overall structure is similar to that of MCSP-specific BiTE antibodies (see Fig. 1a). For purification and detection MT110 also contains a hexahistidine tag. c Expression of EpCAM/MCSP fusion proteins on the surface of stably transfected CHO cell lines. Cells were stained for FACS analysis with the parental anti-EpCAM antibody of MT110 and a PE-conjugated monoclonal anti-mouse-IgG antibody. d Quantitation of EpCAM-specific binding sites on the surface of transfected CHO cell lines
Results
BiTE antibodies binding to different domains of MCSP
A panel of 32 murine monoclonal antibodies (mAbs) was raised to human MCSP from which mAbs with specificity for different subdomains of MCSP were selected for generation of BiTE antibodies. Variable domains of mAbs were converted into single-chain antibodies, which were N-terminally fused by recombinant technology to a murine anti-human CD3 single-chain antibody, called L2K (Fig. 1a). Four selected BiTE antibodies were stably expressed in CHO cells and purified to homogeneity from cell culture supernatants as previously described for BiTE antibody MT110 [19]. Equilibrium dissociation constants of the four BiTE antibodies for human MCSP, as determined by Scatchard analysis, were similar with K D values ranging from 0.9 to 6.5 nM (Fig. 1a). All MCSP BiTE constructs share the same anti-CD3 scFv, which is flexibly linked to the targeting scFv. We therefore assume that the affinity for CD3 is comparable in all constructs. The equilibrium dissociation constant of the anti-CD3 scFv used in MCSP-specific BiTE antibodies has been determined for two other BiTE antibodies (anti-EpCAM and anti-CD19) and found to be approximately 1 × 10−7 M.
For mapping of binding domains, the dumbbell-shaped MCSP protein [34, 37] was divided into the globular domains D1 and D3 and the rod-like domain D2, as shown in Fig. 1b. Calculated molecular sizes of domains not considering glycosylation are 69 kDa (638 aa) for D1, 95 kDa (870 aa) for D2, and 73 kDa (684 aa) for domain D3. The three domains and various combinations thereof were stably expressed as FLAG-tagged proteins in CHO cells, which then served as target cells for studying redirected lysis by BiTE antibodies. For testing species crossreactivity of antibodies, also rat NG2 was expressed. Transfected CHO cells showed robust surface binding signals by FACS analysis with an anti-FLAG antibody, indicating the proper and comparable cell surface expression of constructs (Fig. 1c).
Saturation binding analysis showed that BiTE antibody MCSP128 only reacted with constructs containing MCSP domain D1, BiTE MCSP113 only with constructs containing domain D2, and BiTE antibodies MCSP120 and MCSP70 only with constructs containing domain D3 of human MCSP (Fig. 1d). None of the BiTE antibodies bound to CHO cells expressing rat NG2 (data not shown). The number of surface antibody binding sites of the various MCSP construct-expressing CHO cell lines ranged from 33,000 (D1) to 754,000 (D1D2) with a mean of 303,600 (Fig. 1d). With CHO cell lines to which two or three different BiTE antibodies could bind to, the number of binding sites determined was very similar. As illustrated in Fig. 1e, we obtained BiTE antibodies specifically binding to domains D1, D2, and D3 of human MCSP.
BiTE antibodies binding to the membrane proximal domain D3 of MCSP are most potent
We compared the BiTE antibodies for their potency of redirected lysis of a CHO line and human melanoma Mel888 cells both expressing full-length human MCSP in a standardized 18-h 51Cr release assay.
For the 51Cr release assay, pre-stimulated CD8+ human T cells were used as effectors at an E:T ratio of 10:1 (Fig. 2). For each experimental series, T cells from the same human donor were used. With both target cell lines, BiTE MCSP70, which is binding the most membrane-proximal domain D3 of MCSP, showed the highest potency of redirected lysis. EC50 values of 1.9 ng/ml for CHO-MCSP (Fig. 2a, c) and 1.2 ng/ml for Mel888 cells (Fig. 2b, c) were determined. This corresponds to 34 and 22 pM BiTE antibody. BiTE MCSP70 reached near complete target cell lysis during the 18-h assay period. The BiTE antibody MCSP113, which is binding the rod-like domain D2, showed the lowest activity. The domain D1-binding BiTE MCSP128 was more potent than the D2-binding BiTE, but likewise did not reach complete lysis. MCSP128 showed 35% specific lysis versus 87% by MCSP70 (p = 0.001) for Mel888 and 46 versus 76% (p = 0.009) for MCSP-CHO, respectively. This indicated that targeting the most membrane-proximal domain D3 of MCSP may be suited best for generating a potent MCSP-specific BiTE antibody.
Fig. 2.
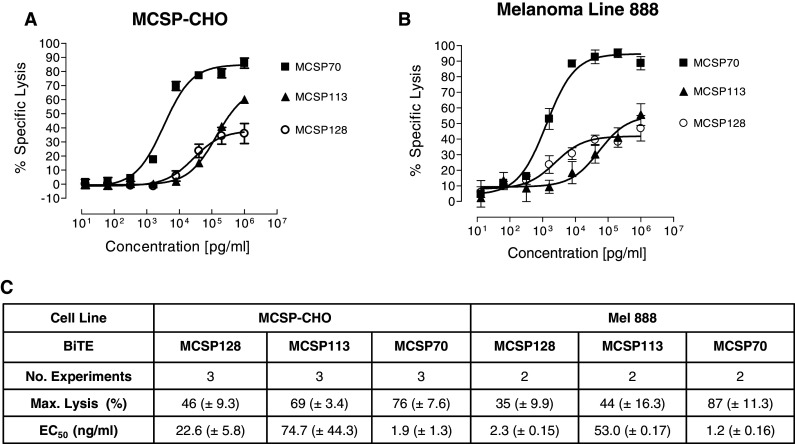
Redirected lysis of human MCSP-expressing CHO line and human melanoma cells by MCSP domain-specific BiTE antibodies. Dose–response analysis for redirected lysis of a human MCSP-expressing CHO line and b human melanoma line 888 by three BiTE antibodies. 51Cr-labeled target cells were co-cultured for 18 h with stimulated human CD8+ T cells at an E:T ratio of 10:1 with the indicated BiTE concentrations. c Values for half maximal lysis (EC50) and overall lysis during the 18-h assay period are shown. Standard deviations of the mean are shown from three independent experiments for MCSP-CHO and two for Mel888
Short distance of binding domains to the target cell membrane improves BiTE potency
We next analyzed the various BiTE antibodies for redirected lysis of CHO cells expressing isolated MCSP domains or combinations of domains. As reference, lysis of CHO cells expressing full length MCSP and, as negative control, of CHO cells expressing rat NG2 was determined under the same assay conditions. The domain D1-binding BiTE MCSP128 lysed all CHO cells expressing a MCSP construct containing domain D1 (Fig. 3a, b). CHO cells solely expressing the domain D1 were most potently killed by BiTE MCSP128 with an EC50 value of 0.9 ng/ml, and with close to complete lysis. Increasing the distance of the D1 domain to the target cell membrane by fusion with either domain D3 or D2 had a negative impact in both cases on the potency of redirected lysis, resulting in EC50 values of 13.2 and 42 ng/ml, respectively. Fusion of domain D1 to the rod-like domain D2 showed a stronger impairment of lysis than fusion to the globular domain D3. Specific lysis of D1-MCSP-CHO by MCSP128 was 76% compared to 63% lysis of D1D2-MCSP-CHO (p = 0.39).
Fig. 3.
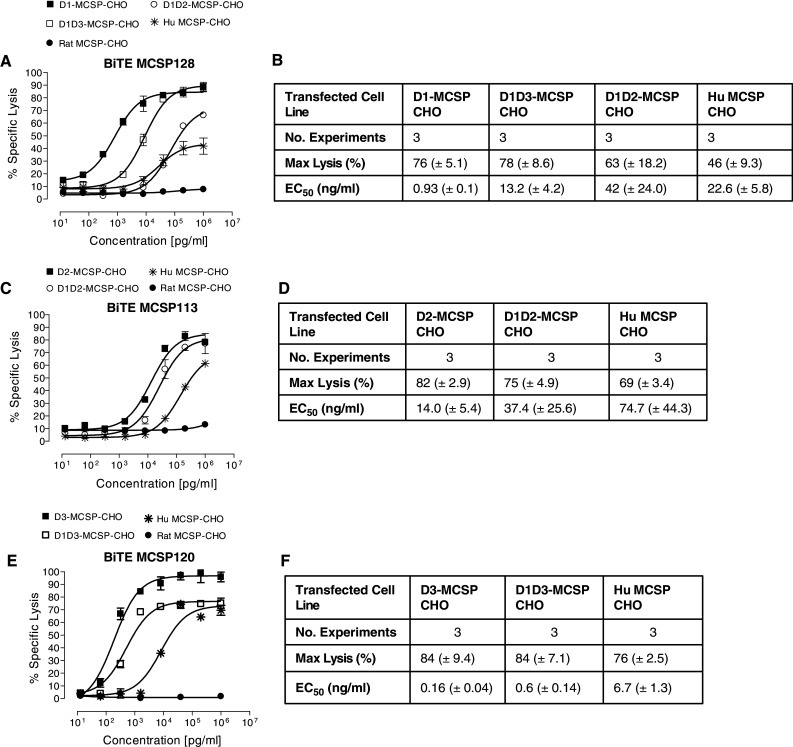
Redirected lysis of CHO lines expressing MCSP subdomains by MCSP-specific BiTE antibodies. Dose–response analyses of redirected lysis were performed for a, b domain D1-specific BiTE antibody MCSP128, c, d domain D2-specific BiTE antibody MCSP113, and e, f for domain D3-specific BiTE antibody MCSP120 by a 51Cr release assay. Standard deviations of the mean are shown from three independent experiments
BiTE MCSP113, which is binding the rod-like domain 2 of MCSP, was similarly potent in mediating lysis of CHO cells expressing solely domain D2, and CHO cells expressing domain D2 fused with domain D1 (Fig. 3c, d). EC50 values were 14.0 and 37.4 ng/ml, and also the degree of lysis was similar.
BiTE MCSP120, which is binding the most membrane proximal domain D3 of MCSP (see Fig. 1b, e), has shown very similar activity as MCSP70 (data not shown), which is why we interchanged the two BiTE antibodies in some experiments. BiTE MCSP 120 mediated a high degree of redirected lysis of CHO cells solely expressing domain D3, and of CHO cells expressing domain D3 fused with domain D1 (Fig. 3e, f). EC50 values were lowest among all experiments in this series being 0.16 ng/ml for lysis of D3-expressing CHO cells and 0.6 ng/ml for lysis of D1D3-expressing CHO cells.
These results corroborate that a BiTE antibody specific for the membrane proximal domain D3 is most potent. Moving the most distant domain D1 close to the target cell membrane by omission of domains D2 and D3 (Fig. 3a, b) considerably increased the potency of D1-specific BiTE antibody, indicating that BiTE potency is positively affected by a more membrane proximal binding on the target cell membrane. In all cytotoxicity experiments of this series, CHO cells expressing full-length MCSP were lysed with lower efficacy than CHO cells expressing individual MCSP domains. Specific lysis of D1-MCSP-CHO was on average 76% compared to 46% for MCSP-CHO (p = 0.0082). This indicates that antigen size was also a key determinant for BiTE activity. CHO cells expressing NG2 were not lysed (Fig. 3a, c, e), showing that CHO cell lysis was in each case specific for the expressed human MCSP sequences.
Potency of EpCAM-specific BiTE antibody MT110 decreased with increasing distance of EpCAM to target cell membrane
In order to show that epitope distance effects are not restricted to the MCSP antigen or MCSP-specific BiTE antibodies, we engineered fusion proteins between the entire extracellular domain of EpCAM and various domains of MCSP, or full-length MCSP (Fig. 4a). We used the CD3/EpCAM-bispecific antibody MT110, which has the same domain arrangement as the four MCSP-specific BiTE antibodies (Fig. 4b), for lysis of transfected CHO cells by human T cells. MT110 is binding to EpCAM with an equilibrium dissociation constant of approximately 10−8 M [36].
As shown by FACS analysis with the parental anti-EpCAM mAb, all CHO transfectants showed reactivity for human EpCAM on their cell surface (Fig. 4c). EpCAM expression levels on stably transfected CHO cells ranged from 236,000 to 1,478,000 antibody binding sites per cell with a mean of 661,570 (Fig. 4d).
BiTE antibody MT110 mediated the redirected lysis of human EpCAM-transfected CHO cells with very high potency (Fig. 5a). The EC50 value for redirected lysis was 14 pg/ml (Fig. 5b). All fusions of EpCAM with a domain of MCSP, which in each case increased the distance of EpCAM to the target cell membrane, dramatically reduced the potency of redirected lysis by MT110. It appeared that the loss in potency directly correlated with membrane distance and molecular mass introduced by the respective MCSP domains (compare Fig. 4a with Fig. 5). Attachment of EpCAM to the rod-like domain D2, to a fusion of domains D1 and D2, or to full-length MCSP did not show detectable lysis of transfected CHO cells under the assay conditions and broad concentration range tested.
Fig. 5.

Redirected lysis of CHO lines expressing EpCAM/MCSP fusion proteins by BiTE antibody MT110. a MT110 dose–response analysis of redirected lysis of CHO lines stably expressing fusion proteins between EpCAM and MCSP. b Quantitation of assay results for maximal lysis and half maximum lysis (EC50). Standard deviations of the mean are shown from three independent experiments
BiTE potency is influenced by antigen size
Using the constructs depicted in Fig. 6a, we next tested how the size of the antigen impacted redirected lysis of CHO transfectants by domain D3-binding BiTE MCSP120. Attachment of EpCAM to domain D3 barely affected redirected lysis (Fig. 6b, c). An EC50 value of 0.1 ng/ml was observed for lysis of D3/EpCAM expressing CHO cells, which is similar to the EC50 value of 0.19 ng/ml observed for lysis of domain D3-expressing CHO cells in a previous experiment (see Fig. 3e, f). A 20-fold drop in potency of BiTE MCSP120 was observed if EpCAM plus the domain D1 was added on top of domain D3; the EC50 value for redirected lysis was reduced to 1.95 ng/ml (Fig. 6b, c). If domain D2 was added on top, a further but small decrease in potency to 4.39 ng/ml was observed. As observed before (see Fig. 5), the potency of BiTE MT110 severely dropped with increasing the distance of EpCAM to the target cell membrane.
Fig. 6.
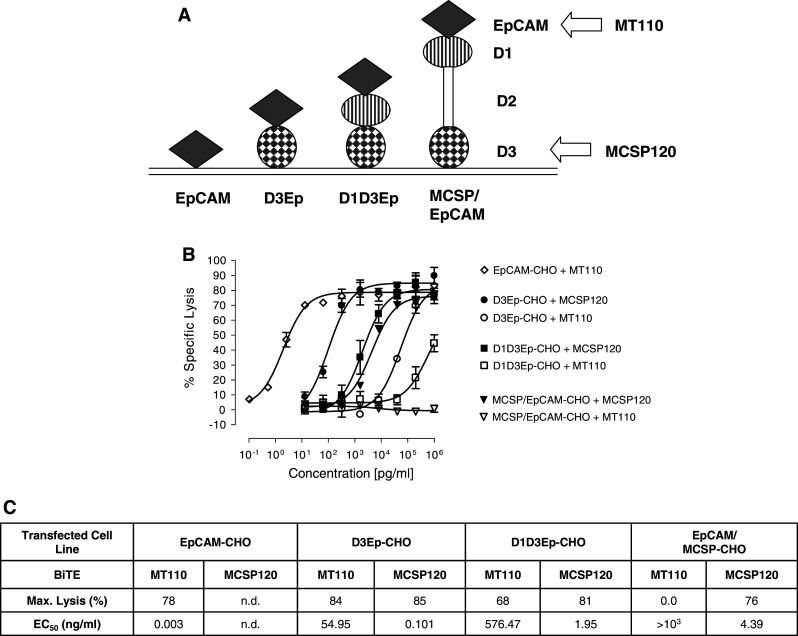
The effect of antigen size on redirected lysis of transfected CHO cells by domain D3-specific BiTE antibody MCSP120. a CHO cell lines expressing EpCAM/MCSP fusion proteins or EpCAM alone were used as targets. b Dose–response analysis of redirected lysis for D3-specific BiTE antibody MCSP120 or EpCAM-specific BiTE antibody MT110 of CHO lines stably expressing fusion EpCAM/MCSP proteins or EpCAM. c Quantitation of assay results for maximal lysis and half maximum lysis (EC50)
Discussion
We have explored in this study the impact of epitope distance and antigen size on the potency of target cells lysis by human cytotoxic T cells that were redirected by bispecific antibodies of the BiTE class. In order to obtain comparable data for the various target antigens, we tried to minimize experimental variables to a minimum within each experimental series. All full-length antigens, fusion proteins, and deletion mutant proteins were expressed in hamster CHO cells, providing for the same target cell background. Expression levels of target antigens were quantified and, where possible, cell clones selected with similar expression levels. Likewise, target-binding affinity of BiTE antibodies were determined and found to differ by less than tenfold among the four domain-specific BiTE antibodies tested. Cytotoxicity conditions in a 51Cr release assay were standardized by using an incubation period of 18 h, an E:T ratio of 10:1, and pre-stimulated, CD8+ enriched T cells as effectors. Pre-stimulation of T cells was found to reduce the large donor variation typically seen with non-stimulated human PBMC [23]. PBMC donor variation was further minimized by using the same donor for each experimental series. By combining all these measures, differences of BiTE antibodies in the potency of redirected lysis of CHO transfectants were largely due to the nature of the target antigen and not to variations of the assay.
We found that both the distance of the binding domain to the target cell membrane and the overall size of the antigen had a substantial impact on the potency of T cell-engaging BiTE antibodies. The following conclusions are derived from testing a total of five BiTE antibodies and using a total of 14 different CHO target cell lines expressing various recombinant versions of MCSP and EpCAM antigens: (1) MCSP-specific BiTE antibodies binding the most membrane proximal domain D3 of MCSP were most potent; (2) direct attachment of MCSP domains to the plasma membrane of target cells in isolation in each case increased the potency of BiTE antibodies; (3) stepwise increasing the distance of domains to the plasma membrane of target cells decreased BiTE potency; (4) stepwise increasing the size of the target antigen while keeping the epitope distance constant also decreased the potency of BiTE antibodies binding to a membrane proximal domain; (5) CHO cells expressing full-length MCSP consistently required the highest BiTE concentrations for redirected lysis. In several experiments, both epitope distance and antigen size may have determined BiTE potency, since the two parameters were changed at the same time.
It is noteworthy that in a study with an EphA2-specific BiTE antibody an inverse linear relationship between EC50 values for redirected lysis and target density on tumor cell lines has been observed [21]. However, our present data suggest that epitope and antigen size are much more critical determinants for potency than the target expression level. For instance, the expression level of D1D2-MCSP on CHO cells is more than 20-fold higher than that of D1-MCSP (Fig. 1d), but the EC50 value of BiTE MCSP128 is 45-fold lower for CHO target cells expressing D1-MCSP compared to CHO cells expressing D1D2-MCSP (Fig. 3b).
It is possible that the inhibitory effects of epitope distance and antigen size on BiTE potency had the same structural basis. A prerequisite for lysis by cytotoxic T cells is the formation of a cytolytic synapse [38]. This structure enables T cells to tightly adhere to target cells for a highly controlled delivery of perforin and granzymes, the proteins responsible for target cell lysis. We have previously shown that BiTE antibodies induce cytolytic synapses that are indistinguishable in structure and composition from regular cytolytic synapses induced by matching T cell receptor, peptide antigen, and MHC class I molecules [39]. Our present results indicate that synapse formation or function may be more efficient, the closer T cell and CHO target cell membranes can adhere to each other. Closest approximation would be possible by BiTE antibodies binding to the most proximal domain D3 of MCSP. More distant domains, such as D1, only show high BiTE activity when they are directly attached to the target cell membrane by omission of other domains. A second determinant for effective synapse formation or function is the size of the target antigen. The target antigen serves to transiently connect the BiTE antibody to CD3 on T cells and, therefore, has to be accommodated within the forming synapse. It seems obvious that it is more difficult to force a 450-kDa polypeptide like MCSP into the forming synaptic cleft than a 30–40-kDa polypeptide such as EpCAM.
EpCAM-mediated homotypic adhesion induces a distance of opposing intercellular membranes of approximately 10–14 nm [40]. This suggests that the length of an EpCAM molecule from its tip to the plasma membrane is in the range of 5–7 nm. Electron microscopy of rat MCSP showed that its two globular domains are separated by a distance ranging from 30 to 110 nm [37], suggesting that MCSP is extending much further from the plasma membrane than EpCAM. The length of the TCR/peptide-MHC complex is approximately 14 nm [41, 42]. A typical BiTE antibody has an estimated distance between its two tandemly arranged binding domains of approximately 3.3 nm. The CD3ε subunit of the TCR extrudes from the cell surface by 4 nm [43]. These numbers predict that an intercellular bridge formed by EpCAM on the target cell, a BiTE antibody, and the CD3ε subunit on the T cell would add up to a length of 12–14 nm. This comes rather close to the length of a natural TCR/peptide/MHC complex of 14 nm, which is naturally bridging target and cytotoxic T cells. To get to a similar distance with MCSP, the epitope recognized by the BiTE antibody on MCSP is crucial, which would argue for selection of the membrane-proximal globular domain D3 of MCSP.
Due to space limitation within the synaptic cleft, lower copy numbers of a large antigen may fit into the forming synapse than of a smaller antigen. As indicated by our experiments with cell lines expressing full-length MCSP, increasing the BiTE concentration may help in reaching the critical antigen density needed for effective synapse formation and function. We have observed that not only pre-activated PBMC but also unstimulated PBMC can mediate redirected lysis of target cells with a D3 domain-specific MCSP BiTE antibody [44]. Depending on PBMC donor, melanoma cell line, and E:T ratio, unstimulated PBMC required between 1 and 100 ng/ml MCSP BiTE for half-maximal lysis of human melanoma lines. This is the same range observed in our present study for CHO cells expressing full-length MCSP and using pre-stimulated CD8+ T cells as effectors.
Our data suggest that functional cytolytic synapses can form between target cells expressing full-length MCSP and T cells albeit this required higher BiTE antibody concentrations than seen with smaller target antigens. This observation is corroborated by BiTE antibodies targeting CEA (CD66e; CEACAM5), a surface protein with a size of 180 kDa [20]. Redirected lysis of CEA-expressing target cells likewise required higher BiTE concentrations than seen with EpCAM-specific BiTE antibody MT110 or CD19-specific BiTE antibody MT103. Despite the need of relatively high in vitro concentrations for redirected lysis, CEA-specific BiTE antibodies showed a very significant anti-tumor activity in two different mouse models [20].
In conclusion, our data suggest that development of a MCSP-specific BiTE antibody for treatment of human melanoma will profit from targeting the membrane proximal domain D3 and from using higher BiTE antibody concentrations for redirected lysis than seen with EpCAM- and CD19-specific BiTE antibodies. Effective cytolytic synapses can be induced by BiTE antibodies between T cells and target cells expressing a wide variety of target antigens, including the very large proteoglycan MCSP.
References
- 1.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 2.King J, Waxman J, Stauss H. Advances in tumour immunotherapy. QJM. 2008;101:675–683. doi: 10.1093/qjmed/hcn050. [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predicts clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Wahlin BE, Sander B, Christensson B, Kimby E. CD8 + T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 6.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, Carroll RG, Riley JL, Pastan I, June CH. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebastian M, Kiewe P, Schuette W, Brust D, Peschel C, Schneller F, Rühle KH, Nilius G, Ewert R, Lodziewski S, Passlick B, Sienel W, Wiewrodt R, Jäger M, Lindhofer H, Friccius-Quecke H, Schmittel A. Treatment of malignant pleural effusion with the trifunctional antibody catumaxomab (Removab) (anti-EpCAM × Anti-CD3): results of a phase 1/2 study. J Immunother. 2009;32:195–202. doi: 10.1097/CJI.0b013e318195b5bb. [DOI] [PubMed] [Google Scholar]
- 9.Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675, 206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 10.Langer LF, Clay TM, Morse MA. Update on CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7:1245–1256. doi: 10.1517/14712598.7.8.1245. [DOI] [PubMed] [Google Scholar]
- 11.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harzstark AL, Small EJ. Immunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC8015 (Provenge) Expert Opin Biol Ther. 2007;7:1275–1280. doi: 10.1517/14712598.7.8.1275. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmüller G, Reinhardt C, Baeuerle PA, Kufer P. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 17.Topp M, Goekbuget N, Kufer P, et al. Treatment with anti-CD19 BiTE antibody blinatumomab (MT103/MEDI-538) is able to eliminate minimal residual disease (MRD) in patients with B-precursor acute lmphoblastic leukemia (ALL): first results of ongoing phase 2 study (ASH Annual Meeting Abstract) Blood. 2008;112:1926. [Google Scholar]
- 18.Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 19.Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, Offner S, Locher M, Urbig T, Raum T, Kleindienst P, Wimberger P, Kimmig R, Fichtner I, Kufer P, Hofmeister R, da Silva AJ, Baeuerle PA. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43:1129–1143. doi: 10.1016/j.molimm.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Lutterbuese R, Raum T, Kischel R, Lutterbuese P, Schlereth B, Schaller E, Mangold S, Rau D, Meier P, Kiener PA, Mulgrew K, Oberst MD, Hammond SA, Baeuerle PA, Kufer P. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother. 2009;32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 21.Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E, Kinch MS, Coats S, Baeuerle PA, Kufer P, Kiener PA. Selective targeting and potent control of tumor growth using an EphA2/CD3-bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, Itin C, Prang N, Baeuerle PA. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 23.Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbuese R, Schlereth B, Kufer P, Baeuerle PA. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology. 2009;214:441–453. doi: 10.1016/j.imbio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama A, Dahlin KJ, Prince JT, Johnstone SR, Stallcup WB. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burg MA, Grako KA, Stallcup WB. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J Cell Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Eisenmann KM, McCarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, Lim L, Manser E, Furcht LT, Iida J. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- 27.Fang X, Burg MA, Barritt D, Dahlin-Huppe K, Nishiyama A, Stallcup WB. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natali PG, Bigotti A, Cavaliere R, Nicotra MR, Ferrone S. Phenotyping of lesions of melanocyte origin with monoclonal antibodies to melanoma-associated antigens and to HLA antigens. J Natl Cancer Inst. 1984;73:13–24. [PubMed] [Google Scholar]
- 29.Hafner C, Breiteneder H, Ferrone S, Thallinger C, Wagner S, Schmidt WM, Jasinska J, Kundi M, Wolff K, Zielinski CC, Scheiner O, Wiedermann U, Pehamberger H. Suppression of human melanoma tumor growth in SCID mice by a human high molecular weight-melanoma associated antigen (HMW-MAA) specific monoclonal antibody. Int J Cancer. 2005;114:426–432. doi: 10.1002/ijc.20769. [DOI] [PubMed] [Google Scholar]
- 30.Ruf P, Jäger M, Ellwart J, Wosch S, Kusterer E, Lindhofer H. Two new trifunctional antibodies for the therapy of human malignant melanoma. Int J Cancer. 2004;108:725–732. doi: 10.1002/ijc.11630. [DOI] [PubMed] [Google Scholar]
- 31.Mittelman A, Chen ZJ, Yang H, Wong GY, Ferrone S. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci USA. 1992;89:466–470. doi: 10.1073/pnas.89.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitler LE, del Rio M, Khentigan A, et al. Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res. 1987;47:1717–1723. [PubMed] [Google Scholar]
- 33.Pfosser A, Brandl M, Salih H, Grosse-Hovest L, Jung G. Role of target antigen in bispecific-antibody-mediated killing of human glioblastoma cells: a pre-clinical study. Int J Cancer. 1999;80:612–616. doi: 10.1002/(SICI)1097-0215(19990209)80:4<612::AID-IJC21>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.Pluschke G, Vanek M, Evans A, Dittmar T, Schmid P, Itin P, Filardo EJ, Reisfeld RA. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raum T, Gruber R, Riethmüller G, Kufer P. Anti-self antibodies selected from a human IgD heavy chain repertoire: a novel approach to generate therapeutic human antibodies against tumor-associated differentiation antigens. Cancer Immunol Immunother. 2001;50:141–150. doi: 10.1007/PL00006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brischwein K, Parr L, Pflanz S, Volkland J, Lumsden J, Klinger M, Locher M, Hammond SA, Kiener P, Kufer P, Schlereth B, Baeuerle PA. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother. 2007;30:798–807. doi: 10.1097/CJI.0b013e318156750c. [DOI] [PubMed] [Google Scholar]
- 37.Tillet E, Ruggiero F, Nishiyama A, Stallcup WB. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central nonglobular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- 38.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/S1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 39.Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–771. doi: 10.1016/j.molimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Balzar M, Prins FA, Bakker HA, Fleuren GJ, Warnaar SO, Litvinov SV. The structural analysis of adhesions mediated by Ep-CAM. Exp Cell Res. 1999;246:108–121. doi: 10.1006/excr.1998.4263. [DOI] [PubMed] [Google Scholar]
- 41.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 42.Garcia KC, Degano M, Pease LR, Huang M, Peterson PA, Teyton L, Wilson IA. Structural basis of plasticity in T cell receptor recognition of a self peptide-MHC antigen. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 43.Sun ZJ, Kim KS, Wagner G, Reinherz EL. Mechanisms contributing to T cell receptor signaling and assembly revealed by the solution structure of an ectodomain fragment of the CD3 epsilon gamma heterodimer. Cell. 2001;105:913–923. doi: 10.1016/S0092-8674(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 44.Torisu-Itakura H, Schoellhammer H, Huynh Y, Hausmann S, Lutterbuese R, Kufer P, Baeuerle PA, Morton DL (2009) Anti-tumor activity of a T cell-engaging MCSP-specific BiTE antibody at very low effector to target ratios: a new approach to treat metastatic melanoma. Am Assoc Cancer Res Abstract 3248


