Abstract
Background
Cancer immunotherapy with NKT cells is a potential new treatment strategy for advanced head and neck cancer. NKT cell therapy is promising due to its unique anti-tumor activity and higher degree of safety compared to current therapies. Radiotherapy is indispensable as a standard treatment for advanced head and neck cancer. To elucidate the possibility of using NKT cells as an adjuvant immunotherapy with radiotherapy, we examined the effect of radiotherapy on NKT cells in patients with head and neck cancer.
Methods
The number, IFN-γ production and proliferation capacity of NKT cells were analyzed before and after 50 Gy radiation therapy in 12 patients with stage IV head and neck squamous cell carcinoma. The cytotoxic activity of NKT cells was examined in vitro.
Results
The number of NKT cells in the blood varied widely between patients. After radiation therapy, the population of CD3 T cells decreased significantly, while the NKT cell population remained stable. The number of NKT cells was the same after radiation therapy as before. IFN-γ production from NKT cells collected just after radiotherapy was impaired after stimulation with exogenous ligand, but the proliferative responses of these NKT cells was enhanced in comparison to those collected before radiation therapy. Furthermore, the proliferated NKT cells displayed a significant level of anti-tumor activity.
Conclusion
NKT cells are relatively resistant to radiation and might therefore be suitable for adjuvant immunotherapy to eradicate remnant cancer cells in patients who have undergone radiation therapy.
Keywords: NKT cell, Radiotherapy, Immunotherapy, Head and neck cancer
Introduction
The management of head and neck cancer generally involves the combined modalities of chemotherapy, radiotherapy and surgery [1]. In recent years, cisplatin-based chemotherapy together with radiotherapy has been widely used for the treatment of advanced head and neck cancer to preserve speech and swallowing abilities. Moreover, this combination therapy is used in the postoperative setting for patients at high risk for recurrence [2–4]. However, the prognoses of these patients are still poor [5, 6]. The development of new treatment strategies is essential for improving the prognoses and the quality of life (QOL) of patients with head and neck cancer.
Immunotherapy has emerged as a safe and promising approach in cancer therapy and has been demonstrated to induce significant immunological responses and beneficial clinical effects in patients with a variety of cancers [7, 8]. Immunotherapy is expected to become a common adjuvant for cancer therapy; however, its efficacy still needs to be improved. Although cytotoxic T cells play an important role in conventional immunotherapy, they are very sensitive to radiotherapy [9, 10]. Radiotherapy is indispensable for advanced cancers, particularly head and neck squamous cell carcinoma (HNSCC).
Natural killer T (NKT) cells are an unique lymphocyte subpopulation characterized by the co-expression of T cell and natural killer (NK) receptors [11–13]. Human NKT cells express the invariant Vα24JαQ paired with the Vβ11 antigen receptor and are activated by the specific glycolipid antigen α-galactosylceramide (α-GalCer) in a CD1d-dependent manner [14]. CD1d is an HLA class 1b antigen-priming molecule that does not exhibit any allelic polymorphism. After activation, humanVα24NKT cells display a strong anti-tumor activity against various malignant tumors both in vivo and in vitro [15, 16] and produce high levels of cytokines such as IFN-γ, thereby activating other anti-tumor effector cells. Decreased numbers of Vα24NKT cells in human peripheral blood mononuclear cells (PBMCs) have been observed in patients with malignant diseases, including head and neck cancer [17].
To elucidate the effect of irradiation on NKT cells, we examined the number and function of NKT cells from head and neck cancer patients who received radiation therapy.
Patients and methods
Patients
Patients less than 80 years of age with a histological diagnosis of stage IV squamous cell carcinoma at the pharyngeal site without any previous treatments were enrolled from January to December 2005. Further inclusion criteria included: a performance status of 0 or 1 and a negative response to HIV, hepatitis C virus, hepatitis B surface antigen; normal renal, hepatic and hematopoietic functions; the absence of active inflammatory disease or active autoimmune disease; and concurrent corticosteroid therapy. A total of 12 patients and 14 healthy subjects were included in the study as age- and gender-matched control subjects. The study received prior approval from the Ethics Committee of Chiba University. Written informed consent was obtained from each participant.
Radiation therapy
The patients received equalized fractionated radiotherapy of 4 MV X-rays from a linear accelerator at a dose of 2 Gy once daily. The entire neck was irradiated with a minimal dose of 50 Gy. The irradiation field included the entire neck, and the area in each patient ranged from 306 to 409 cm2. Chemotherapy was not administered. Peripheral blood was obtained before and after radiation therapy, and the number and activity of both the T cells and NKT cells were evaluated.
The number of CD3-positive T cells and NKT cells
From each subject, 50 ml of peripheral blood was collected before and after the radiation series. The lymphocytes were isolated with density-gradient centrifugation (Lympho separation medium; MP Biomedicals, OH, USA) and subsequently frozen in a Cell Banker (Nihon Zenyaku, Japan) at −80°C until the analysis was performed.
The numbers of CD3-positive T cells and NKT cells were assayed by a flow cytometer and at least 500,000 lymphocyte gate events were counted (FACSCalibur BD biosciences). NKT cells were defined as positive for Vα24 (C15; Immunotech, Marseilles, France), Vβ11 (C21; Immunotech) and CD3 (UCTH1; BD Biosciences PharMingen).
The following monoclonal antibodies (mAbs) were used: fluorescein isothiocyanate (FITC)-labeled anti-TCR Vα24 mAb (C15; Immunotech, Marseilles, France), phycoerythrin (PE)-labeled anti-TCR Vβ11 mAb (C21; Immunotech), anti-CD56 mAb (B159; BD Biosciences) and allophycocyanin (APC)-labeled anti-CD3 mAb (UCTH1; BD Biosciences). Isotype-matched control mAbs were used as negative controls in the flow cytometry analyses.
Proliferative activity of NKT cells
Fifty milliliters of peripheral blood was collected from each subject before and after the radiation series. The PBMCs were immediately separated by the Ficoll-Hypaque technique and stored in liquid nitrogen for later analyses. The number of NKT cells was calculated prior to use by flow cytometry as described above. The PBMCs were cultured in AIM-V medium containing 100 U IL-2 and 100 ng/ml α-GalCer, at 37°C in 5% CO2 for 7 days. This cell expansion method was identical to the method used in our clinical trial [18]. On the seventh day of culture, the number of NKT cells was assayed in the same manner. Thereafter, the degree of cell expansion was calculated.
IFN-γ production from NKT cells
Monocytes were isolated from the patient PBMCs and cultured with GM-CSF (1,600 U/ml) and IL-4 (1,000 U/ml) for 7 days to allow differentiation into immature dendritic cells. On the seventh day of culture, the cells were pulsed with α-GalCer (100 ng/ml) and then cultured for 6 h with PBMCs from the same cancer patients. A single-cell ELISPOT assay was used to detect IFN-γ-producing cells in PBMCs. Frozen PBMCs collected at each time point (0 and 50 Gy) were thawed and cultured for 6 h or 7 days. The cultured cells (5 × 105 per well) were washed and transferred to 96-well filtration plates (Millipore, Danvers, MA, USA) coated with an anti-IFN-γ capture antibody (Mabtech AB, Stockholm, Sweden) for 16 h. Stimulation was achieved by the addition of 100 ng/ml αGalCer or vehicle as described previously [18–20]. Concanavalin A was used as a positive control. A biotinylated antihuman IFN-γ antibody (Mabtech AB) was added. Two hours later, spots were detected with an avidin–biotin–peroxidase complex (Mabtech AB) and aminoethylcarbazole solution. Spot forming cells (SFCs) were objectively quantified using an ImmunoScan computer system and the ImmunoSpot software program (CTL, Cleveland, OH, USA).
Cytotoxic assay with NKT cells
K562 and U937 cells were used for a cytotoxicity assay and labeled overnight at 37°C in 5% CO2 with 300 µCi sodium 51Cr-chromate (Amersham).
PBMCs obtained before and after radiotherapy were cultured in AIM-V with 100 U IL-2 and 100 ng/ml α-GalCer for 7 days. Thereafter, NKT cells were purified with the MACS system (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) using a Vα24 antibody. The NKT cells were used as effector cells. Each well of the 96-well microtiter plate received target cells, the indicated proportions of effector cells and the appropriate medium at a total volume of 200 μl. The microplates were centrifuged for 1 min at 600×g and incubated for 4 h at 37°C. After additional centrifugation, 100 μl aliquots of the supernatants were assayed for radioactivity. The fraction of the total radioactivity released was calculated and the results were averaged from triplicate values.
The percentage of specific 51Cr release was calculated from the following formula: % of specific lysis = (sample cpm − spontaneous cpm) × 100/(maximum cpm−spontaneous cpm). Spontaneous cpm was calculated from the supernatant of the target cells alone, and the maximum release was obtained by adding 1 M HCl to the target cells. The data are expressed as the mean value of the triplicate cultures ± standard deviations.
Statistical analysis
The Mann–Whitney U test was performed to compare the NKT cell count between the control subjects and the patients. The Wilcoxon signed rank test was used for paired comparisons of each value before and after irradiation treatment. Statistical analyses were performed using the GraphPad Prism software program, Version 4. P values of less than 0.05 were considered to be statistically significant.
Results
Patient profiles
The profiles of the patients are shown in Table 1. The patient ages ranged from 55 to 78 years of age, and the primary tumors were localized to mesopharyngeal and hypopharyngeal sites. Neck lymph node metastases were observed in all cases (Table 1). The pharyngeal tumors arose near the regional cervical lymph nodes, and the irradiation field and/or the area of the primary lesion and regional lymph nodes overlapped.
Table 1.
Patients’ profile
| Case | Age/gender | Primary lesion | Stage | Total dose (Gy) |
|---|---|---|---|---|
| Case001 | 59/male | Mesopharynx | T4N2b | 50 |
| Case002 | 67/male | Hypophrynx | T3N2b | 60 |
| Case003 | 72/male | Hypopharynx | T2N3 | 60 |
| Case004 | 66/male | Mesopharynx | T2N2b | 60 |
| Case005 | 58/male | Hypopharynx | T2N2b | 70 |
| Case006 | 56/male | Hypopharynx | T2N2b | 70 |
| Case007 | 73/male | Mesopharynx | T4N3 | 60 |
| Case008 | 66/male | Mesopharynx | T1N3 | 72 |
| Case009 | 76/male | Hypopharynx | T3N2b | 70 |
| Case010 | 58/male | Hypopharynx | T4N2b | 60 |
| Case011 | 69/male | Hypopharynx | T4N2b | 52 |
| Case012 | 55/male | Mesopharynx | T3N2b | 60 |
NKT cells in peripheral blood
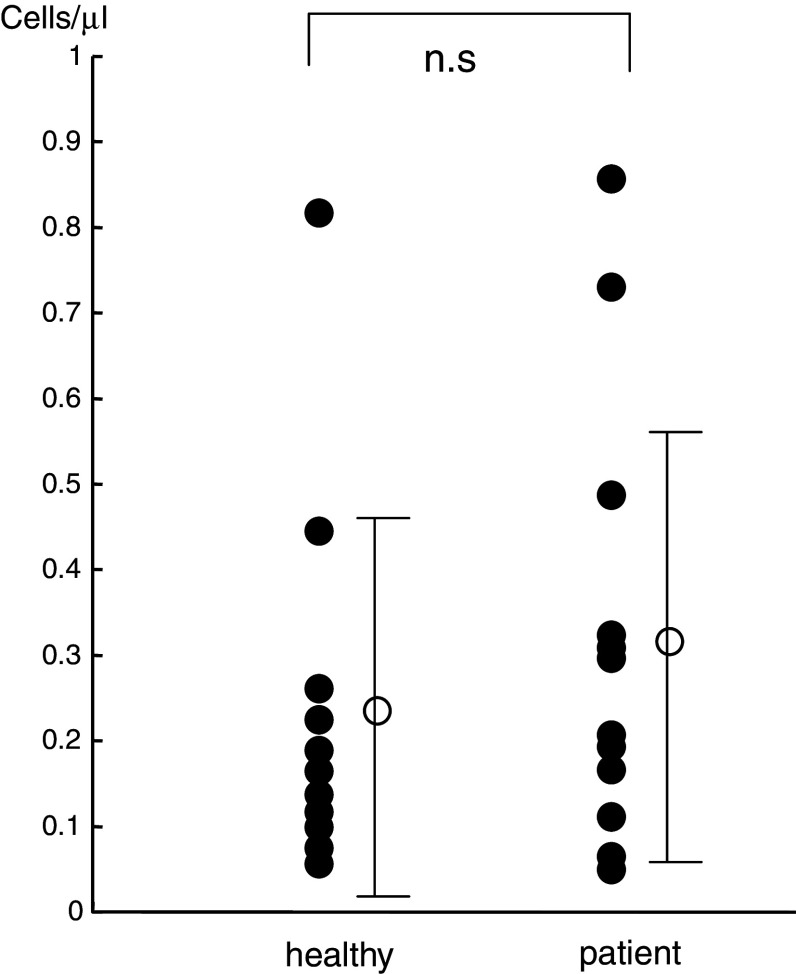
We also evaluated 14 age- and gender-matched healthy control subjects. The number of NKT cells in the circulating peripheral blood of non-treated SCC patients was not significantly different from that of the healthy control subjects (Fig. 1). NKT cells were detected in the peripheral blood from all examined cases, although the number represented less than 0.2% of all lymphocytes and varied widely between cases.
Fig. 1.
NKT cells in the peripheral blood of healthy control subjects and patients before radiotherapy. No significant differences were observed in the number of NKT cells between the patients and the control subjects
Influence of therapeutic irradiation on circulating T cells and NKT cells
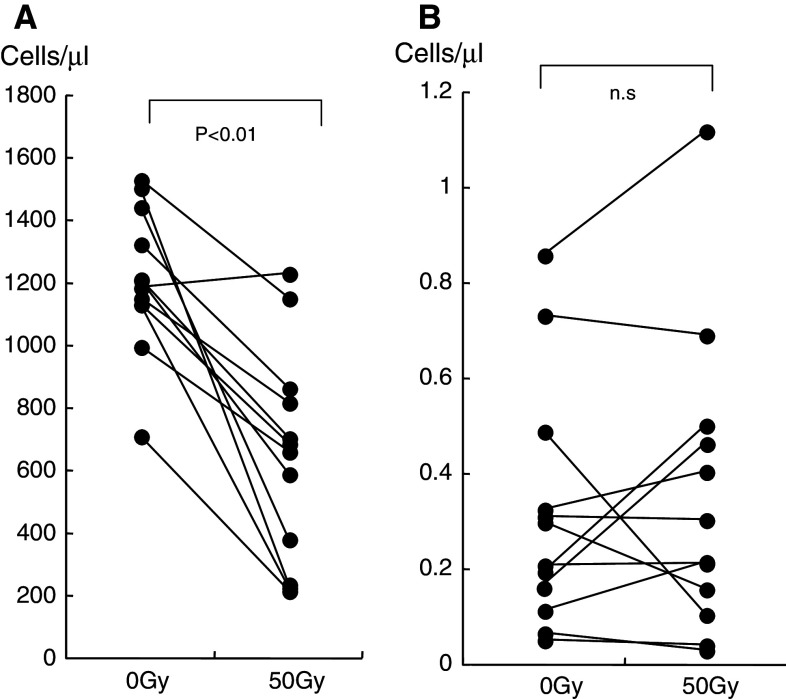
Consistent with other previous reports, the number of CD3-positive T cells was found to decrease as irradiation progressed [21]. At the time that the total dose of 50 Gy was attained, a remarkable decrease in the number of CD3-positive T cells was observed in each patient (Fig. 2a). However, the number of NKT cells (which were defined as Vα24- and Vβ11-positive cells) was not affected by radiation (Fig. 2b).
Fig. 2.
a The number of CD3-positive T cells is shown. The number of T cells was significantly decreased after irradiation. b The number of NKT cells is shown. NKT cells did not decrease significantly in number
Proliferation of NKT cells in response to α-GalCer and IL-2
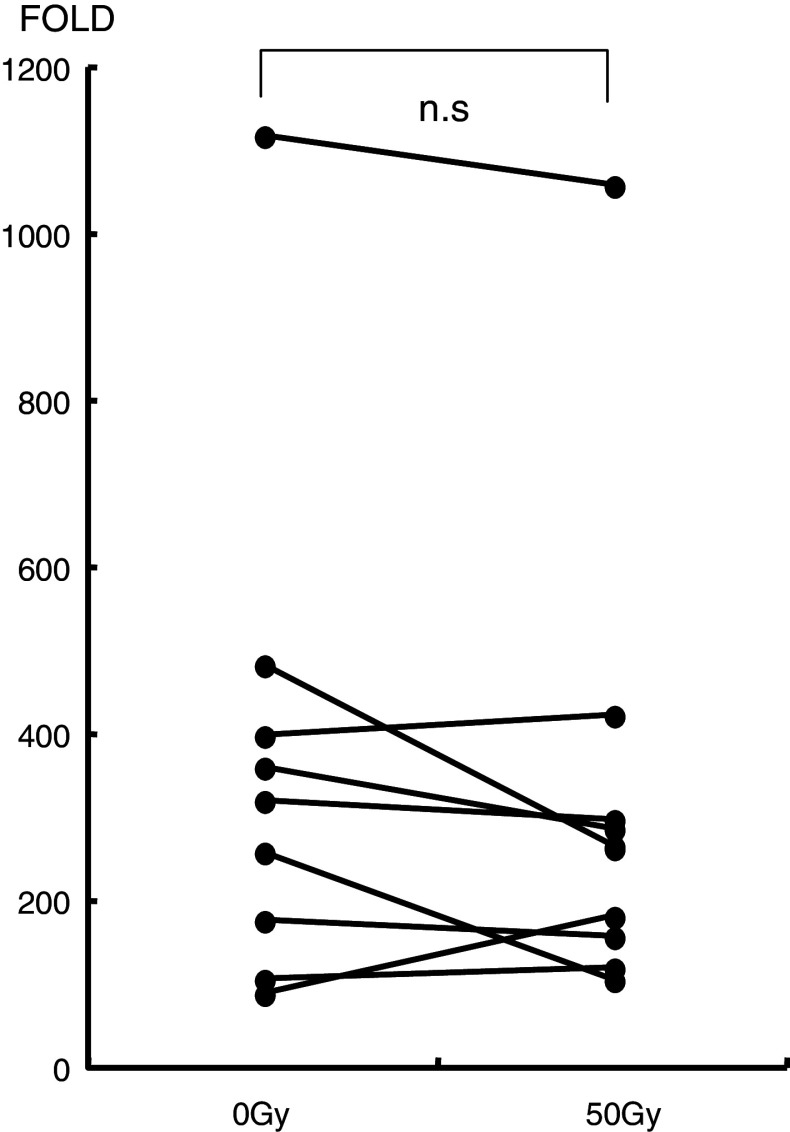
PBMCs (3 × 105/well) from cancer patients were cultured for 7 days with α-GalCer and IL-2. The expansion capacity of NKT cells in cancer patients in response to α-GalCer and IL-2 after irradiation was similar to that before irradiation (Fig. 3). The mean cell number of three wells is indicated.
Fig. 3.
The expansion capacity of NKT cells in cancer patients in response to α-GalCer and IL-2 is shown. NKT cells collected after and before radiation therapy proliferated
Cytokine activity of NKT cells
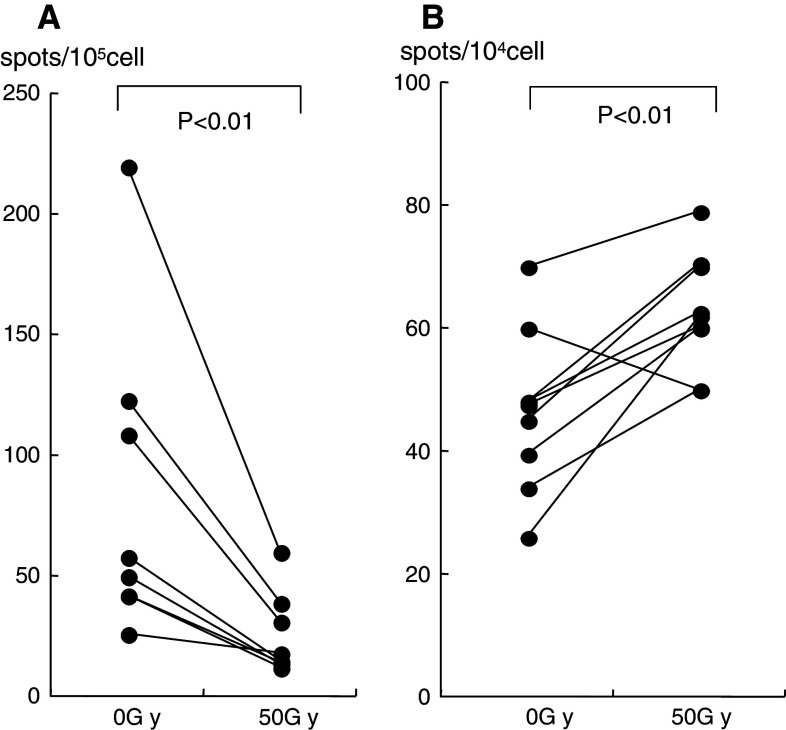
Although the number of NKT cells did not decrease after irradiation, the IFN-γ-producing cells reflecting α-GalCer-pulsed DCs decreased significantly (Fig. 4a). However, after culturing for 7 days in AIM-V medium containing 100 U IL-2 and 100 ng/ml α-GalCer, which was the same technique as in our clinical setting [19], a significant number of IFNγ-producing cells were observed in the cultured cells collected after radiation therapy. The IFN-γ activity in the cells collected after radiation therapy was higher than in those before radiation therapy (Fig. 4b).
Fig. 4.
a The IFN-γ spots induced by α-GalCer-pulsed DCs from fresh isolated PBMCs are shown. These spots decreased significantly after radiation therapy compared with those before therapy. b After culture for 7 days with α-GalCer and IL-2, the IFN-γ spots reflected by α-GalCer-pulsed DCs are shown. Those spots were significantly increased after radiation therapy
Cytotoxic activity of NKT cells
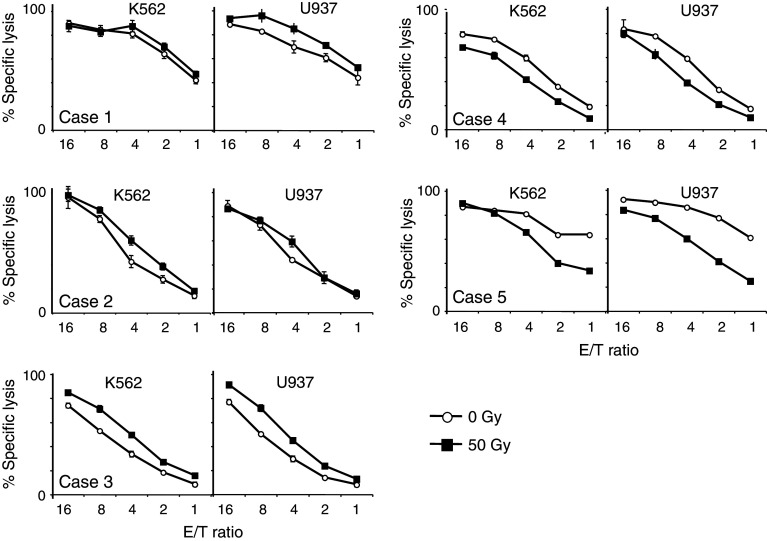
The cytotoxic activity of the NKT cells toward the target K562 and U937 cells is shown in Fig. 5. PBMCs were obtained before and after radiotherapy, and NKT cells were purified by magnetic bead selection. The cytotoxic activity of NKT cells collected after irradiation was equal to that prior to irradiation in the same patient.
Fig. 5.
The cytotoxic activity of NKT cells is shown. The target cells were K562 and U937. The E:T ratios were 16:1, 8:1, 4:1, 2:1 and 1:1. Effector cells from pre-radiation and post-radiation are represented by open circles and closed boxes, respectively
Discussion
In the present study, the number of circulating NKT cells did not decrease in patients with pharyngeal cancer in comparison to age- and gender-matched healthy controls. Other studies have reported a decrease in the number of NKT cells in patients with advanced cancer, including those with head and neck carcinoma [22]. The patients recruited in the present study were not at an advanced cancer stage, and the NKT cell number varied among individuals, thus suggesting a reason for the decrease in the NKT cell number.
The effect of the therapeutic irradiation on NKT cells and T cells was evaluated in patients with pharyngeal cancer, and the irradiation region in all the cases was similar. The number of CD3-positive T cells isolated from the patients was markedly decreased after radiation with a total dose of 50 Gy to the head and neck area; however, NKT cells were relatively resistant to radiation and did not decrease in number.
The radioresistant activity of NKT cells has been previously reported. In a mouse model, NKT cells were found to be more resistant to in vivo irradiation than other blood lymphocytes, including T cells and B cells [23]. Another study demonstrated that the number of NKT cells, but not the number of ordinary T cells, in patients with a prior irradiation history was similar to that of patients with no irradiation history in several types of cancer [24]. However, a detailed comparison in the same patients before and after irradiation has not yet been conducted.
The mechanisms involved in NKT cell radioresistance are poorly understood. It is well known that T cells are very sensitive to irradiation in the clinical setting. Irradiation induces cell death by causing direct damage to DNA and altering the intracellular signaling pathways. The high production of reactive oxygen species in mitochondrial DNA following irradiation might be the origin of the hyper-radiosensensitivity of T cells [21]. NKT cells may also respond differently to free radicals induced by irradiation. Further investigations are therefore necessary to elucidate the difference between the radioresistance of NKT cells and ordinary T cells.
NKT cells produce many cytokines, including IFN-γ and IL-4, as well as GM-CSF, TNFα and IL-2, -3, -5, -6, -9, -10, -13, -17 and -22 [25]. After co-culture with α-GalCer, NKT cells proliferate and differentiate into massive IFN-γ-producing NKT or massive IL-4 producing cells [26]. The former has anti-tumor properties, whereas the latter suppresses anti-tumor responses. It is very difficult to control such a large number of cytokines, but IFN-γ and IL-4 have been controlled to some extent in recent studies. The interaction of DC and NKT cells may influence IFN-γ or IL-4 production by NKT cells. Interleukin-12 production by DCs upregulates IFN-γ production by NKT cells [27, 28]. The CD40–CD40L interaction also regulates IFN-γ and IL-4 production of NKT cells [29]. These reports help explain tumor protection via NKT and urge the development of the DC–NKT axis to provide innate and adaptive immunity to human cancers.
Although the function of NKT cells, as measured by the capacity for IFN-γ production, was impaired after radiotherapy, the intact proliferative capacity of NKT cells was observed after stimulation with α-GalCer-pulsed DCs and was comparable to that of cells obtained before radiotherapy. These proliferated NKT cells exhibited significant cytotoxic activities toward the target tumor cells. These results suggest that even after radiotherapy, NKT cells are well-tolerated and demonstrate normal levels of proliferation by α-GalCer-pulsed DCs. Moreover, these cells display potent killing activity against remnant cancer tissue. The effective anti-tumor activity of activated NKT cells in vivo could therefore be expected with the administration of α-GalCer-pulsed DCs to head and neck cancer patients.
Head and neck cancer occurs in more than a half million patients worldwide each year [30]. The management of head and neck cancer generally involves the combined modalities of chemotherapy, radiotherapy and surgery. However, even after these combined therapies, relapses and distant metastasis occur at a high frequency, and the 5-year survival rate has been reported to be less than 40% [31–33]. Therefore, a new treatment strategy must be developed to improve the survival and QOL of patients with advanced head and neck cancer.
We recently conducted a phase-one study evaluating the safety and efficacy of the administration of α-GalCer-pulsed antigen presenting cells (APCs) to the nasal mucosa of nine patients with either unresectable or recurrent head and neck cancer [19].
In addition, we performed a phase-one study of the administration of in vitro-expanded natural killer T cells and α-GalCer-pulsed antigen presenting cells to the tumor-feeding arteries and the nasal submucosal of eight patients with either unresectable or recurrent head and neck cancer. We observed that there were beneficial clinical effects associated with the use of this treatment strategy for advanced HNSCC [20].
Although there were only limited effects with regard to the reduction of the unresectable tumors, no adverse events exceeding CTCAE grade 2 were observed and significant anti-tumor activity was detected. These results suggest that NKT immunotherapy for the treatment of head and neck cancer might be useful for destroying both small remnant cancer tissue and micro-metastases.
Radiotherapy is indispensable for many cases of advanced head and neck cancer. In this study, we demonstrated that NKT cells are relatively resistant to radiation therapy. Even after a total radiation dose of 50 Gy, a high degree of proliferation was induced in NKT cells following stimulation with α-GalCer-pulsed DCs. The beneficial cytotoxic effects of this type of therapy are therefore highly anticipated. NKT cells might be suitable as an effective adjuvant immunotherapy to eradicate remnant and micro-metastatic cancer cells just after radiotherapy. Further investigation of immunotherapy utilizing NKT cells should therefore be conducted to fully elucidate the clinical efficacy of this treatment modality.
Abbreviations
- α-GalCer
α-Galactosylceramide
- APCs
Antigen presenting cells
- CTCAE
Common terminology criteria for adverse events
- DCs
Dendritic cells
- E/T
Effector cell/target cell
- GM-CSF
Granulocyte macrophage colony stimulating factor
- mAbs
Monoclonal antibodies
- NK
Natural killer
- NKT
Natural killer T
Footnotes
K. Kobayashi and Y. Tanaka contributed equally to this work.
References
- 1.Hainsworth JD, Meluch AA, McClurkan S, Gray JR, Stroup SL, Burris HA, III, Yardley DA, Bradof JE, Yost K, Ellis JK, Greco FA. Induction paclitaxel, carboplatin, and infusional 5-FU followed by concurrent radiation therapy and weekly paclitaxel/carboplatin in the treatment of locally advanced head and neck cancer: a phase II trial of the Minnie Pearl Cancer Research Network. Cancer J. 2002;8:298–300. doi: 10.1097/00130404-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Harrison LB, Fass DE, Armstrong J, Spiro RH, Shah JP, Strong EW. Postoperative radiotherapy for oral cavity cancers: impact of anatomic subsite on treatment outcome. Head Neck. 1993;12:470–475. doi: 10.1002/hed.2880120604. [DOI] [PubMed] [Google Scholar]
- 3.Clark J, Li W, Smith G, Shannon K, Clifford A, McNeil E, Gao K, Jackson M, Mo Tin M, O’Brien C. Outcome of treatment for advanced cervical metastatic squamous cell carcinoma. Head Neck. 2005;27:87–94. doi: 10.1002/hed.20129. [DOI] [PubMed] [Google Scholar]
- 4.Hicks WL, Jr, Loree TR, Garcia RI, Maamoun S, Marshall D, Orner JB, Bakamjian VY, Shedd DP. Squamous cell carcinoma of the floor of mouth: a 20-year review. Head Neck. 1997;19:400–405. doi: 10.1002/(SICI)1097-0347(199708)19:5<400::AID-HED6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Metelmann HR. Chemotherapy and radiochemotherapy in tumors of the head–neck area. Mund Kiefer Gesichtschir. 2000;4(suppl 1):S155–S159. doi: 10.1007/PL00014536. [DOI] [PubMed] [Google Scholar]
- 6.Wanebo HJ, Glicksman AS, Landman C, Slotman G, Doolittle C, Clark J, Koness RJ. Preoperative cisplatin and accelerated hyperfractionated radiation induces high tumor response and control rates in patients with advanced head and neck cancer. Am J Surg. 1995;170:512–516. doi: 10.1016/S0002-9610(99)80342-0. [DOI] [PubMed] [Google Scholar]
- 7.Beatty JD. Immunotherapy of colorectal cancer. Cancer. 1992;70:1425–1433. doi: 10.1002/1097-0142(19920901)70:3+<1425::AID-CNCR2820701534>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Cortesina G, De Stefani A, Sacchi M, Rosso S, Galeazzi E. Immunomodulation therapy for squamous cell carcinoma of the head and neck. Head Neck. 1993;15:266–270. doi: 10.1002/hed.2880150318. [DOI] [PubMed] [Google Scholar]
- 9.Gualde N, Goodwin JS. Effect of irradiation on human T-cell proliferation: low dose irradiation stimulates mitogen-induced proliferation and function of the suppressor/cytotoxic T-cell subset. Cell Immunol. 1984;84:439–445. doi: 10.1016/0008-8749(84)90118-7. [DOI] [PubMed] [Google Scholar]
- 10.Rose H, Moldenhauer H, Kehrberg G. Lymphocyte damage caused by ionizing radiation. Radiobiol Radiother. 1985;26:289–297. [PubMed] [Google Scholar]
- 11.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi M, Koseki H, Tokuhisa T, Masuda K, Sato H, Kondo E, Kawano T, Cui J, Perkes A, Koyasu S, Makino Y. Essential requirement of an invariant Vα14 T cell antigen receptor expression in the development of natural killer T cells. Proc Natl Acad Sci USA. 1996;93:11025–11028. doi: 10.1073/pnas.93.20.11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 14.Hameg A, Apostolou I, Leite-De-Moraes M, Gombert JM, Garcia C, Koezuka Y, Bach JF, Herbelin A. A subset of NKT cells that lacks the NK1.1 marker, expresses CD1d molecules, and autopresents the alpha-galactosylceramide antigen. J Immunol. 2000;165:4917–4926. doi: 10.4049/jimmunol.165.9.4917. [DOI] [PubMed] [Google Scholar]
- 15.Nicol A, Nieda M, Koezuka Y, Porcelli S, Suzuki K, Tadokoro K, Durrant S, Juji T. Human invariant valpha24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumor activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99(2):229–234. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molling JW, Kölgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Peripheral blood IFN-gamma-secreting Valpha24 + Vbeta11 + NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 18.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 19.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, Taniguchi M, Nakayama T, Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Ohnishi T, Hamasato S, Seguchi H, Yoshida S. Reactive oxygen species-producing site in radiation-induced apoptosis of human peripheral T cells: involvement of lysosomal membrane destabilization. Int J Mol Med. 2004;13:69–73. [PubMed] [Google Scholar]
- 22.Molling JW, Langius JA, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJ, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–868. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 23.Kajioka EH, Andres ML, Li J, Mao XW, Moyers MF, Nelson GA, Slater JM, Gridley DS. Acute effects of whole-body proton irradiation on the immune system of the mouse. Radiat Res. 2000;153:587–594. doi: 10.1667/0033-7587(2000)153[0587:AEOWBP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Valpha24(+)Vbeta11(+)NKT cells by age, malignancy and conventional anticancer therapy. Br J Cancer. 2004;91:1880–1886. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1–NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 28.Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Nagahira K, Fukuda Y, Nishimura T. Murine NKT cells produce Th17 cytokine interleukin-22. Cell Immunol. 2009;254:81–84. doi: 10.1016/j.cellimm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M, Yahata T, Meng H, Koda T, Nishimura S, Kawano T, Taniguchi M, Nishimura T. Alpha-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 30.Mignogna MD, Fedele S, Lo Russo L. The world cancer report and the burden of oral cancer. Eur J Cancer Prev. 2004;13:139–142. doi: 10.1097/00008469-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Zhen W, Karnell LH, Hoffman HT, Funk GF, Buatti JM, Menck HR. The national cancer data base report on squamous cell carcinoma of the base of tongue. Head Neck. 2004;26:660–674. doi: 10.1002/hed.20064. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho AL, Ikeda MK, Magrin J, Kowalski LP. Trends of oral and oropharyngeal cancer survival over five decades in 3267 patients treated in a single institution. Oral Oncol. 2004;40:71–76. doi: 10.1016/S1368-8375(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 33.Sessions DG, Lenox J, Spector GJ, Chao C, Chaudry OA. Analysis of treatment results for base of tongue cancer. Laryngoscope. 2003;113:1252–1261. doi: 10.1097/00005537-200307000-00026. [DOI] [PubMed] [Google Scholar]