Abstract
A murine monoclonal anti-idiotype (Id) antibody, 3H1 has been developed and characterized previously. Anti-Id 3H1 mimics a specific epitope of carcinoembryonic antigen (CEA) and can be used as a surrogate antigen for CEA. 3H1 induced anti-CEA immunity in different species of animals as well as humans and showed promise as a potential vaccine candidate in phase I/II clinical trials for colon cancer patients. One area of interest to us has been the development of new immune adjuvants that may augment the potency of 3H1 as a tumor vaccine. Oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) are potent immunostimulatory agents capable of enhancing the Ag-specific Th1 response when used as immune adjuvants. In this study, we have evaluated the efficacy of 3H1 as a tumor vaccine when admixed with a select CpG ODN 1826 in transgenic mice that express human CEA. The vaccine potential of 3H1 was also assessed in the presence of another widely used adjuvant, QS-21. 3H1 coupled to keyhole limpet hemocyanin (KLH) and mixed with Freund’s adjuvant (FA) was used as a gold standard in this system. 3H1 vaccination with different adjuvants induced both humoral and cellular anti-3H1, as well as anti-CEA immunity in CEA transgenic mice. The immune sera could lyse CEA-transfected murine colon carcinoma cells, C15 effectively in an antibody-dependent cellular cytotoxicity assay. The anti-CEA antibody responses were somewhat comparable in each adjuvant-treated group of mice, whereas cellular immune responses were significantly greater when CpG was used as an adjuvant. Splenocytes obtained from 3H1–CpG-immunized mice showed an increased proliferative CD4+ Th1-type T-cell response when stimulated in vitro with 3H1 or CEA and secreted elevated levels of Th1 cytokines (IL-2, IFN-γ). This vaccine also induced MHC class I antigen-restricted CD8+ T-cell responses. In a solid tumor model, C15 tumor growth was significantly inhibited by 3H1 vaccinations. In 3H1–CpG-vaccinated mice, the duration of survival was, however, longer compared to the 3H1–QS21-vaccinated mice. These findings suggest that 3H1-CpG vaccinations can break peripheral tolerance to CEA and induce protective antitumor immunity in this murine model transgenic for human CEA.
Keywords: Anti-idiotype antibody, Cancer vaccine, Carcinoembryonic antigen, Transgenic mice, CpG ODN, Immunotherapy
Introduction
The specific activation of the immune system to control cancer growth in vivo has been a long- standing goal in cancer immunology. The identification of tumor-associated antigens (TAAs) has provided the basis for new concepts in antigen-specific immunotherapy. CEA is a glycoprotein over expressed in a high percentage of tumors of epithelial origin (colon, rectum, pancreas, gastric, breast, and so forth) and is an attractive target for immunotherapy [38]. Because tumor antigens such as CEA, is expressed at low levels in some normal cells, the induction of antitumor immune responses to CEA is difficult to achieve. An effective way of breaking tolerance is to present the required epitope in a different molecular environment to the tolerant host. A potential approach for the development of both cellular and humoral immune responses to known TAAs is the use of anti-Id antibodies. Anti-Id antibodies that share amino acid sequence homologies with nominal TAA could act as functional mimics of T-cell antigens and stimulate cellular immune responses. We have generated an anti-Id antibody, 3H1, that functionally mimics CEA. 3H1 was generated against an anti-CEA antibody, 8019, which reacts with a specific epitope of CEA that is highly restricted to tumor cells and not found on normal tissues [6]. We have shown previously that 3H1 can induce anti-CEA antibody in small animals [6, 31], non-human primates [7], and 3H1 was successfully tested earlier in several clinical trials [12–14].
We are interested to develop new immune adjuvants that will increase the potency of 3H1 as a tumor vaccine. Because oligodeoxynucleotides (ODN) containing CpG motifs have been extensively studied for their ability to stimulate activation of the innate and adaptive immune responses [22] and in the treatment of cancer [11, 35, 40], we have been interested in a select CpG ODN 1826 as a vaccine adjuvant. In a preclinical conventional murine tumor model, we have shown that naïve C57BL/6 mice immunized with 3H1 in combination with CpG ODN 1826 were able to induce both humoral and cellular anti-3H1 as well as anti-CEA immunity and were protected against challenge with lethal dose of CEA-transfected murine tumor cells [2]. Mice expressing human CEA as a transgene have been developed [9, 19] and used as experimental preclinical models to evaluate both the impact of immune tolerance to CEA as well as the effectiveness of vaccine strategies in overcoming tolerance and mediating tumor rejection [15, 17, 18, 20, 25, 27, 28, 42]. In these studies, a variety of protocols including DNA vaccine encoding CEA, DNA vaccine encoding both CEA and CD40 ligand, recombinant virus expressing CEA, or recombinant virus expressing CEA and different costimulatory molecules along with recombinant antibody-IL-2 fusion protein, recombinant GM-CSF protein and/or recombinant virus expressing GM-CSF were used as adjuvants to break the immune tolerance against the self-antigen CEA that leads to minor protection against implanted or spontaneous tumors in prophylactic settings [15, 17, 18, 20, 25, 27, 28, 42]. One study also used adoptive CTL transfer for inhibition of tumor growth where tumors were implanted two days prior to CTL transfer [27]. The immunization protocols used in these studies were complicated and yet less effective, and thus may or may not be suitable for clinical studies in human patients with CEA-positive tumors.
In the present study, using the CEA-transfected murine colon carcinoma model, we have investigated whether 3H1 along with CpG ODN 1826 could induce antitumor immunity in CEA transgenic (CEA.Tg) mice. Since CEA is a self-antigen in this transgenic murine model, we were interested to analyze whether the use of select CpG ODN as an adjuvant could enhance the potency of this TAA to break immune tolerance. Incomplete Freund’s adjuvant (IFA), the most commonly used so far in experimental models, has been included in this system. IFA has been successfully used in human immunotherapy against melanoma involving gp 100 peptide immunizations [33]. However, this adjuvant is not widely used in human vaccination protocols due to its undesirable side effects, such as erythema and induration at the injection site. Therefore, alternative potent and safe adjuvants need to be identified. A potent adjuvant, QS-21, which can enhance both B-cell [32] and T-cell responses [23] has been included in our study for comparison. Multiple clinical studies have shown QS-21 to be well tolerated by patients [13, 23, 32]. The primary end point was to determine whether by using CEA as a target antigen in a C57BL/6 murine model, 3H1 immunization could generate CEA-specific immune responses that can correlate with the host protection against challenge by a lethal dose of CEA-transfected murine colon carcinoma cells.
Materials and methods
Materials
The immunostimulatory synthetic ODN-1826 [41], containing two CpG motifs (5′-TCCATGA CGTTCCTGA CGTT-3′), was used throughout this work. QS-21, a purified saponin [21] was obtained from Aquila Biopharmaceuticals Inc. (Worcester, MA). Freund’s adjuvant, complete and incomplete, was obtained from Gibco Invitrogen Corp. (Grand Island, NY).
Mice and cell lines
CEA transgenic mice [C57BL/6J-TgN(CEAGe)18FJP], male and female, were obtained from Dr. F. James Primus. Expression of CEA in normal tissues of these CEA.Tg mice is restricted to the large intestine, and to a lesser extent in the stomach similar to the pattern of expression in humans [9]. The Institutional Animal Care and Use Committee approved all experimental protocols described here. MC-38 murine colon adenocarcinoma and human CEA-transfected MC-38 cells (clone C15-4.3) have been described previously [9]. YAC-1, a murine NK-sensitive cell line, was obtained from ATCC (Manassas, VA, USA).
Anti-Id vaccine and purified CEA
Generation, purification, and characterization of anti-Id mAb 3H1 (Ab2), designated as CeaVac, have been described previously [6]. Isotype-matched control anti-Id mAb, 1A7 [36], which mimics a melanoma-associated antigen, ganglioside GD2, was also used in this study. Purified CEA was obtained commercially from Fitzgerald (Concord, MA). CEA was isolated from human liver metastasis of colonic tumors and purity amounted to >98% by SDS-PAGE. Murine anti-CEA mAb, 8019 (Ab1) reacted strongly with this purified preparation of CEA.
Immunization of mice
In most experiments, mice were immunized with 3H1 or CEA in the presence of CpG ODN 1826 or QS-21 as adjuvant. In some experiments, 3H1 was used along with a carrier and a potent adjuvant (KLH conjugated 3H1 + FA). All experiments were routinely performed in groups of 8–12 mice each. For mice receiving QS-21 adjuvant, 10–50 μg of protein in 100 μl of PBS was mixed with 10 μg of QS-21 and administered s.c. 2 weeks apart and boosted five times. For the mice receiving Freund’s adjuvant, the first immunization with protein was performed i.p. after emulsification with Freund’s complete adjuvant in a ratio of 1:1. Subsequent immunizations were performed s.c. 2 weeks apart using the same dose, after emulsification with Freund’s incomplete adjuvant. A total of six immunizations were performed. For mice included in the CpG arm, desired amount of protein was mixed with 50 μg of CpG ODN in a final volume of 100 μl and given s.c. 2 weeks apart and boosted three times. Mock vaccination with PBS was also performed in one group of mice for comparison. Mice were bled prevaccination and 8–10 days after each booster injection.
Serological analyses
Idiotype and epitope analysis of anti–anti-idiotype antibody (Ab3). Experiments were performed as described previously [2]. Serum from each mouse was checked for the ability to inhibit the binding of 125I-labeled 8019 to 3H1 bound to microtiter plates. Also, the inhibition of 8019 binding to CEA by murine sera were tested by RIA. Assays were performed in triplicate, and samples with a SD less than 10% were used to calculate the mean.
Enzyme-linked immunosorbent assay (ELISA). Anti-CEA antibodies were measured using an ELISA method as described previously [2]. Assays were performed in triplicate for each sample using a goat anti-mouse IgG antibody conjugated to alkaline phosphatase.
Cell surface reactivity determined by flow cytometry. The cell surface reactivity of immune sera were tested on human CEA-transfected murine colon carcinoma cells, C15 and nontransfected parental MC-38 cells as described previously [2]. Results are presented as percentage of positive cells.
ADCC assay. The ability of Ab3 to lyse CEA-positive tumor cells in conjunction with effector cells was tested by standard antibody-dependent cellular cytotoxicity (ADCC) as described previously [2]. Briefly, spleens were isolated from three mice per group, pooled, and a single-cell suspension was prepared by mechanical dissociation. These cells were used as effector cells. MC-38 and C15 tumor cells were labeled with [51Cr] and used as targets. Assays were performed in triplicate. Spontaneous release was always <25% of the total release. Percentage of cellular cytotoxicity was calculated using the formula: 100× [(experimental release − spontaneous release)/(total release − spontaneous release)].
T-cell proliferation assay
Spleens were harvested and pooled from three mice per group 10–14 days after the final immunization, and T-cell proliferation was measured by [3H] thymidine incorporation [2]. Concanavalin A (1.0 μg) was used as a positive control in these experiments. Each assay was performed at least in triplicate. T-cell proliferation was also assessed in the presence of antibodies (10 μg/ml) against the following antigens: CD4, CD8, MHC class I, and MHC class II. All antibodies used in blocking experiments were obtained from BD Pharmingen (San Diego, CA, USA).
Cytokine analysis
Supernatant from T-cell cultures were harvested at different time points and were analyzed for the presence of IL-2, IFN-γ, IL-4, and IL-10 by standard techniques with Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA) as described previously [34]. All samples were tested in triplicate. Results are expressed in pg/ml.
Assessment of in vitro cytolytic activity
Cytotoxicity assays were performed according to the standard protocols [2, 27]. Lymphocytes were isolated from harvested spleen of three mice per group 10–14 days after final immunization and these cells (2×106/ml) were stimulated by coculture with 3H1 or CEA (10–25 μg/ml) along with 20 units/ml rhIL-2 (Sigma-Aldrich, St. Louis, MO, USA). On day 5, these in vitro stimulated splenocytes were harvested and tested in a standard 6-h [51Cr]-release assay for their ability to lyse variety of target cell lines. All experimental determinations were performed in triplicate wells at different effector to target cell ratios as indicated. Spontaneous release was always <25% of the total release. Specific lysis was calculated by using the formula as described above.
Antibody-blocking experiments with anti-CD8 (53−6.7) or anti-CD4 (GK1.5) mAb were carried out by preincubating effector cells for 60 min at 37°C as described previously [34]. Antibody-blocking experiments with anti-H-2Kb/H-2Db or anti-I-Ab mAb were carried out by preincubating labeled target cells for 30 min at 37°C. Isotype-matched mAbs were used as control. Effector or labeled target cells were preincubated with 5 μg/ml antibody before the addition of untreated labeled target or effector cells, respectively [34]. Antibodies that were used in blocking experiments were obtained from BD Pharmingen.
Murine model for determining the efficacy of 3H1 vaccine
The human CEA-transfected murine colon carcinoma cells, C15, constitutively express CEA in culture, and routine flow cytometry analysis revealed cell surface expression of CEA at high intensity (>90%) by the cells. When the CEA-transfected C15 cells (1×106 cells/mouse) or nontransfected parental MC-38 cells (5×105 cells/mouse) were injected s.c. into syngeneic C57BL/6J (H-2b) mice transgenic for CEA, tumor developed in 100% of the mice within 10–15 days. Experiments included ten mice per group. Differences in the in vivo growth rates of the parental MC-38 cells and the CEA-transfected C15 cells necessitated injecting a different number of cells into mice.
Tumor protection in immunized mice
The effect of vaccination with different immunogenic adjuvants on tumor growth and survival was evaluated in prophylactic model. Each group of immunized mice was divided into two subgroups for tumor challenge. Two weeks after the final immunization, tumor challenge was performed s.c. with 1×106 of C15 cells or 5×105 of MC-38 cells. Tumor growth and survival were monitored daily and tumor size was determined weekly. Tumor size was recorded as a tumor area (in mm2) and mice were sacrificed when tumors became ulcerated or when they reached a size >250 mm2 and survival was recorded as the percentage of surviving animals of total animals on a given day [34].
Statistical analysis
Statistical analysis was performed by two-tailed Student’s t test or nonparametric Mann-Whitney rank-sum test using SigmaStat software (Jandel, San Rafael, CA, USA). The data are presented as mean ± SE. P<0.05 was considered to indicate statistical significance.
Results
Development of humoral immune responses
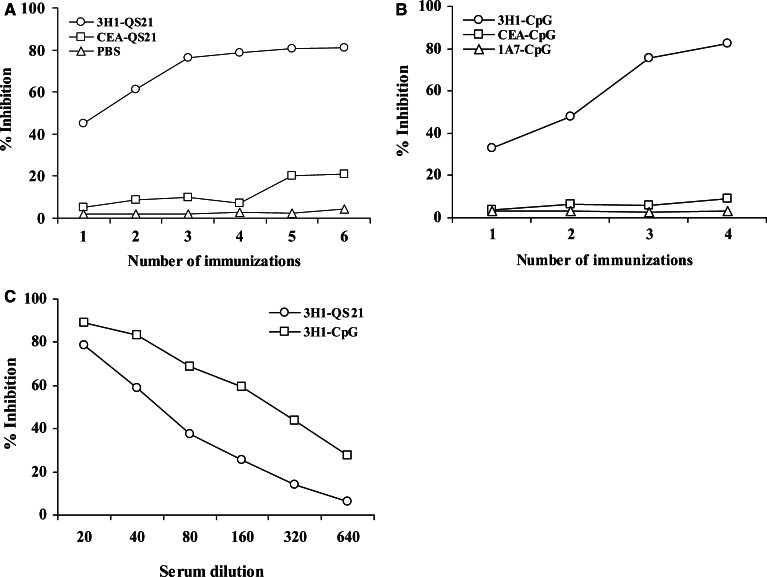
The magnitude and quality of humoral immune response induced by immunization with anti-Id mAb 3H1, in the presence of different adjuvants, was assessed by testing the sera obtained from mice after each immunization. Mice were immunized with 3H1 in PBS mixed in aqueous solution of QS-21. Anti-anti-Id antibody (Ab3) produced in the sera was determined by RIA. Preimmune sera were used as control. As illustrated in Fig. 1a, Ab3 that developed in mice after the first immunization moderately inhibited the binding of 125I-labeled 8019 (Ab1) to 3H1 (Ab2). However, 3–4 immunizations were required to obtain the peak value. Immunization of mice with 3H1 mixed with CpG ODN 1826 also developed detectable humoral response after the first immunization, although multiple booster immunizations were required to obtain and sustain peak immune reactivity (Fig. 1b). Similar results were obtained after immunization with 3H1-KLH mixed with FA (data not shown). Vaccination with control anti-Id mAb, 1A7 did not inhibit the binding of Ab1 to Ab2. Also, less than 20% inhibition was obtained with sera from CEA-vaccinated mice (Fig. 1a, b), irrespective of adjuvant used. The humoral immune response in the CpG group was higher than the response rate in the QS-21 group as determined by the inhibition of Ab1 binding to Ab2 by serial dilutions of immunized mice sera (Fig. 1c).
Fig. 1.
Ab1 (8019) binding inhibition to Ab2 (3H1) by immune sera. Plates were coated with 3H1 and the binding of 125I-labeled 8019 was tested for inhibition in the presence of immune sera at 1:20 dilution. a, b Sera were collected 8–10 days after each immunization and used for analysis. Preimmune sera at 1:20 dilution showed less than 10% binding inhibition. c Sera were collected from groups of mice after final immunization and were diluted with PBS before analysis. Results in a–c are representative of three independent experiments
The paratope of Ab3 developed in mice after 3H1 immunization was analyzed by inhibition of binding of 125I-labeled 8019 to CEA. As shown in Fig. 2, multiple immunizations were required to induce an Ab3 response that could significantly inhibit the binding of 8019 to CEA. The results suggest that Ab3 induced in 3H1-immunized mice share the same idiotype as Ab1 and may also contain Ab1′ antibodies. Ab1′ that developed in the Ab3 population is likely to have an antitumor property. Sera from CEA-immunized mice showed minor inhibition in these assays, indicating that the antibodies generated were directed against different epitopes of CEA or the titer and that the affinity of the antibodies were low.
Fig. 2.
Ab1 binding inhibition to CEA by immune sera. Sera were collected from groups of mice after each immunization. Plates were coated with CEA and the binding of 125I-labeled 8019 was tested for inhibition in the presence of immune sera at 1:20 dilution. Preimmune sera at 1:20 dilution were used as a control, which showed no binding inhibition. Results are representative of three independent experiments
Anti-CEA antibody developed in immunized mice was determined by ELISA. Immunization of mice with 3H1 in the presence of different adjuvants resulted in the induction of significant anti-CEA IgG antibody titers after multiple immunizations (Fig. 3a, b). We also analyzed whether immunization of mice with CEA could induce a humoral response to this self-Ag. Immunization of mice with CEA in the presence of different adjuvants also induced anti-CEA antibody responses (Fig. 3a, b). The high-titer anti-CEA antibody induced in CEA immunized mice may be explained by the fact that CEA is a heterogeneous molecule containing multiple epitopes and using intact whole CEA as a vaccine might have resulted in the generation of antibodies against different epitopes. Whereas 3H1 was developed against a specific epitope of CEA, using 3H1 as a vaccine, therefore resulted in the generation of anti-CEA antibody against that particular epitope.
Fig. 3.
Anti-CEA IgG serum titers developed in immunized mice was detected by ELISA as outlined in “Materials and methods.” Plates were coated with CEA and sera collected after each immunization were tested at 1:20 dilution. 1A7-immunized mice sera were used as a control. Data represent mean ± SE of six individual mice per group. One of three experiments is shown
The cell-surface reactivity of anti-CEA antibodies induced by 3H1 immunization was tested on CEA-transfected C15 cells and on nontransfected parental MC-38 cells by flow cytometry. Sera collected from three mice per group were pooled and used for analysis. Results in Table 1 demonstrate that sera from 3H1-immunized mice could bind to the C15 cell surface and binding was optimum when immunizations were performed with QS-21. Of interest, sera from CEA-immunized mice also showed reactivity with C15 cells, whereas binding with preimmune sera, or sera from PBS-vaccinated mice was negligible. Anti-CEA mAb 8019 was used as a positive control in this assay. Immunized mice sera did not bind to MC-38 cells and sera from 1A7-immunized mice also did not bind with either C15 or MC-38 cells (data not shown). These results confirmed that the relevant Ab1′ had been induced by 3H1 immunization.
Table 1.
Immunofluorescence analysis of CEA-positive tumor cells after reaction with immune seraa
| Immunizations with | C15 cells | MC-38 cells |
|---|---|---|
| 3H1-QS21 | 30.1 | 4.6 |
| CEA-QS21 | 31.9 | 3.9 |
| 3H1-CpG | 26.2 | 3.1 |
| CEA-CpG | 27.0 | 2.8 |
| PBS | 4.0 | 2.3 |
| Preimmune sera | 2.2 | 2.1 |
| 8019 | 97.4 | 5.7 |
a Pre- and postimmune sera at 1:10 dilution were tested for binding on CEA-transfected C15 tumor cells or nontransfected parental MC-38 cells. Anti-CEA mAb 8019 at 1.0 μg was used as a positive control for C15 cells. Results are presented as percentage of positive cells. Data are a representation of two independent experiments
Tumor cell lysis in vitro in the presence of immune sera
Next, we tested whether anti-CEA antibody generated by 3H1 immunization was cytolytic for CEA-positive tumor cells. As shown in Fig. 4, sera obtained from 3H1-immunized mice could lyse C15 cells effectively, but not MC-38 cells, suggesting that tumor cell lysis was dependent on antigen expression by target cells. The lysis of C15 cells in the presence of immune sera in 3H1-QS21 and 3H1-KLH-FA groups were higher than the response in the 3H1-CpG group, although the difference in lysis was not significant (P>0.07). Of interest, ADCC with sera from CEA-immunized mice were lower than those measured for the corresponding 3H1-immunized littermates and the difference in lysis was significant (Fig. 4). Anti-CEA antibodies generated against multiple epitopes of CEA in CEA-immunized mice might have resulted in a mixture of counterproductive immune responses. Of note, sera obtained from control groups of mice could not lyse the C15 target cells effectively.
Fig. 4.
Antibody-dependent cellular cytotoxicity by sera from immunized mice. Sera were collected from groups of mice after final immunization and were diluted 1:5 with PBS before analysis. Specific cell lysis was determined by 6-h [51Cr]-release assay using C15 and MC-38 as target cells. Preimmune sera were used as a control. Data represent mean ± SE of three mice per group. Results are a representation of two independent experiments
T-cell response in immunized mice
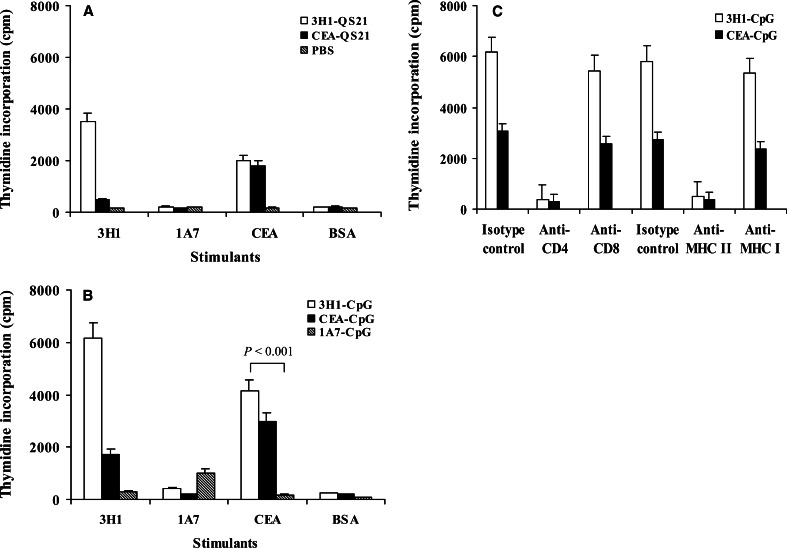
Next, we assessed whether 3H1 immunization in CEA.Tg mice were able to induce antigen-specific T-cell responses in vitro. 3H1-immunized mice splenocytes proliferated significantly in the presence of 3H1 or CEA and the response rate in the CpG group (Fig. 5b) was significantly higher than the response rate in the QS-21 group (Fig. 5a) and FA group (data not shown). The proliferation of T cells in the presence of 1A7 as a stimulant in the assay was significantly lower than the value obtained with 3H1 or CEA (P<0.002). CEA-immunized mice splenocytes could proliferate in the presence of CEA. However, the CEA-specific response was stronger in 3H1-immunized littermates (Fig. 5a, b). Spleen cells harvested from 1A7 or PBS-vaccinated mice showed negligible stimulation with either 3H1 or CEA.
Fig. 5.
Proliferation of immunized spleen cells in response to antigens in vitro. T-cell proliferation was determined by [3H]thymidine incorporation, and proliferation in the presence of media alone has been deducted from values obtained in the presence of different stimulants. a, b Splenocytes were obtained from groups of mice as indicated and cells were cultured in the presence of different stimulants. c Analysis of subsets of T cells in splenocytes obtained from mice immunized with 3H1-CpG or CEA-CpG. 3H1–CpG-immunized mice splenocytes were cultured in the presence of 3H1 and CEA–CpG-immunized mice splenocytes were cultured in the presence of CEA. Antibodies (10 μg/ml) against the indicated antigens were added at the beginning of culture. Results in a–c are the mean ± SE of one representative experiment of three performed experiments
We analyzed the subsets of T-cell populations that become responsive by CpG vaccinations. Spleen cells from 3H1–CpG-immunized mice and CEA–CpG-immunized mice were assayed for proliferation in the presence of antibodies to block specific accessory interactions. The proliferation of splenocytes in the presence of 3H1 or CEA was significantly inhibited by the antibodies directed against CD4 and MHC class II Ags (P<0.005), whereas, antibodies against CD8 and MHC class I Ags were relatively less effective (P>0.07) compared to the isotype-matched control antibodies (Fig. 5c). These results suggested that in vitro T-cell proliferation was primarily mediated by CD4+ T cells.
Secretion of cytokines by stimulated T cells
Since cellular immunity is an important effector arm in mediating antitumor effects, we analyzed the pattern of cytokines released in vitro by stimulated T cells of immunized mice. Immune splenocytes were cultured in vitro in the presence of 3H1 or CEA, and culture supernatants were then analyzed for the detection of IL-2, IFN-γ, IL-4, and IL-10 by ELISA. A Th1-associated immune response was observed, with a moderate enhancement of IL-2 and IFN-γ production in the group of mice immunized with 3H1-CpG (Table 2). The levels of the Th2-associated cytokines, IL-4 and IL-10, obtained from the culture supernatants were much lower than that of the Th1-associated cytokines (P<0.005). The secretion of IL-2 and IFN-γ were 47–67% lower in mice immunized with 3H1-QS21 or 3H1-KLH-FA (data not shown) over the levels observed in mice immunized with 3H1-CpG. Of interest, the secretion of IL-2 and IFN-γ were 38–56% lower in mice immunized with CEA-CpG compared to 3H1–CpG-immunized littermates.
Table 2.
Th1-associated immune response in splenocytes from immunized micea
| Immunizations with | mIL-2 | mIFN-γ | mIL-4 | mIL-10 |
|---|---|---|---|---|
| 3H1-QS21 | 112.0±16 | 430.0±20 | 10.0±2 | 16.0±8 |
| CEA-QS21 | 64.0±8 | 180.0±10 | 10.0±2 | 8.0±8 |
| 3H1-CpG | 336.0±24* | 925.0±30** | 36.0±6 | 56.0±16 |
| CEA-CpG | 208.0±16 | 410.0±15 | 24.0±4 | 40.0±8 |
| 1A7-CpG | 40.0±8 | 145.0±10 | 16.0±2 | 24.0±8 |
| PBS | 16.0±8 | 50.0±5 | 6.0±2 | 8.0±8 |
Results are expressed in pg/ml
aSplenocytes (2×105 /well) obtained from groups of immunized mice were cocultured in the presence of 1.0 μg of 3H1 or CEA in 96-well flat-bottomed microtiter plates in a final volume of 200 μl. Cell-free supernatants were harvested after 48 h (for quantitation of IL-2, IL-4, and IL-10) and 72 h (for quantitation of IFN-γ) of culture and cytokine levels were measured by standard ELISA kits. One representative experiment of two is shown
* P < 0.001 with respect to other experimental groups for IL-2 production
** P < 0.005 with respect to other experimental groups for IFN-γ production
CpG ODN 1826 is an efficient adjuvant for Ag-specific CTL response
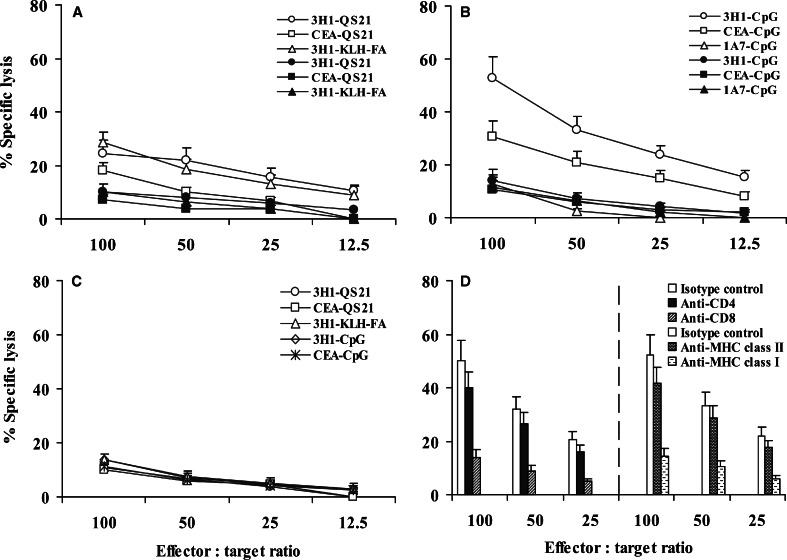
Cytotoxic T lymphocytes (CTLs) are a critical component of the immune response to tumors. To determine the ability of different adjuvants for the induction of Ag-specific CTL response following vaccination, splenic lymphocytes were isolated from different groups of immunized mice and stimulated in vitro in the presence of 3H1 or CEA along with rhIL-2 for 5 days, and these cells were used as effector cells. CTLs obtained from mice immunized with 3H1 mixed with QS-21 or FA could lyse the CEA-transfected C15 cells but not parental MC-38 cells (Fig. 6a), and the difference in lysis was modest (P<0.05). Immunization with CEA-QS21 resulted in negligible Ag-specific CTL response in vitro (Fig. 6a). CTLs obtained from mice immunized with 3H1-CpG proved to be most effective, inducing up to 60% lysis of C15 cells compared with 30–35% lysis by CTLs obtained from mice immunized with CEA-CpG (Fig. 6b) and the difference in lysis was significant at all E:T ratios tested (P<0.005). Lysis of C15 cells with CTLs from all 3H1–CpG-immunized mice were significantly greater than that obtained with control targets (P<0.0008). Similar results were obtained when CEA–CpG-immunized mice were used (P<0.001). CTLs obtained from an additional six immunized mice from each individual group produced similar results. In contrast, only background lysis was observed with effector cells obtained from mice immunized with unrelated anti-Id mAb, 1A7 along with CpG (Fig. 6b). The nonspecific lytic activity was measured by lysis of natural killer-sensitive YAC-1 cells (Fig. 6c). These results suggested that CpG ODN are potent inducers of CTL response compared to other commonly used adjuvants.
Fig. 6.
Cytotoxic T-cell-mediated lysis of CEA-positive tumor cells. Spleens harvested from mice immunized with 3H1 or 1A7 were stimulated in vitro in the presence of 3H1 along with rhIL-2, and spleens harvested from mice immunized with CEA were stimulated in vitro in the presence of CEA along with rhIL-2. Five days later, cytolytic activity was determined by 6-h [51Cr]-release assay using C15 (open symbol) and MC-38 (closed symbol) as target cells (a, b). c YAC-1, a murine NK-sensitive cell line was also used as target in these experiments. All experiments were performed several times, and representative results are shown. (d) MHC class I antigen-restriction of the CTL response. 3H1–CpG-immunized mice splenocytes were stimulated in vitro in the presence of 3H1 along with rhIL-2 for 5 days and cytolytic activity was determined by 6-h [51Cr]-release assay using C15 as target cells. Antibody-blocking experiments were performed in the presence of anti-CD4/anti-CD8 mAb, anti-MHC class II (I-Ab)/anti-MHC class I (H-2Kb /H-2Db) mAb, or isotype-matched control mAb. Each experiment included six mice. Results are the representation of two experiments performed
To determine whether the antitumor cytolytic activity observed in 3H1–CpG-immunized mice was associated with the presence of tumor-specific CD8+ CTLs and/or CD4+ helper T lymphocytes, anti-CD8 or anti-CD4 mAb was incorporated as blocking reagent in cytotoxicity assay. The specific killing of C15 cells was significantly inhibited by preincubation of effector cells with anti-CD8 mAb (P<0.05), whereas, the antibody against CD4 was relatively less effective (P=0.58) compared to the isotype-matched control antibody at all E:T cell ratios tested (Fig. 6d). This result indicated that antitumor response observed was primarily mediated by CD8+ CTLs. Antibody-blocking experiments with anti-MHC class I, or anti-MHC class II mAb were also performed to determine the MHC class I antigen-restricted CTL specificity for C15 target cells. Inhibition of CTL activity was obtained by treatment with anti-H-2Kb/H-2Db mAb (Fig. 6d) and inhibition was significant (P<0.05) at all E:T cell ratios tested compared with the isotype-matched control antibody. In contrast, the inhibition of CTL activity with anti-I-Ab mAb was relatively less effective (P=0.6), which indicated MHC class I-restricted tumor cell lysis. Of interest, CEA–CpG-immunized mice splenocytes also lysed C15 target cells by MHC class I antigen-restricted manner (data not shown). These results therefore are consistent with the idea that CpG ODN induces Ag-specific effector CD8 T cells capable of displaying cytolytic activity [4, 10, 26, 41].
Effects of vaccination on tumor growth and survival
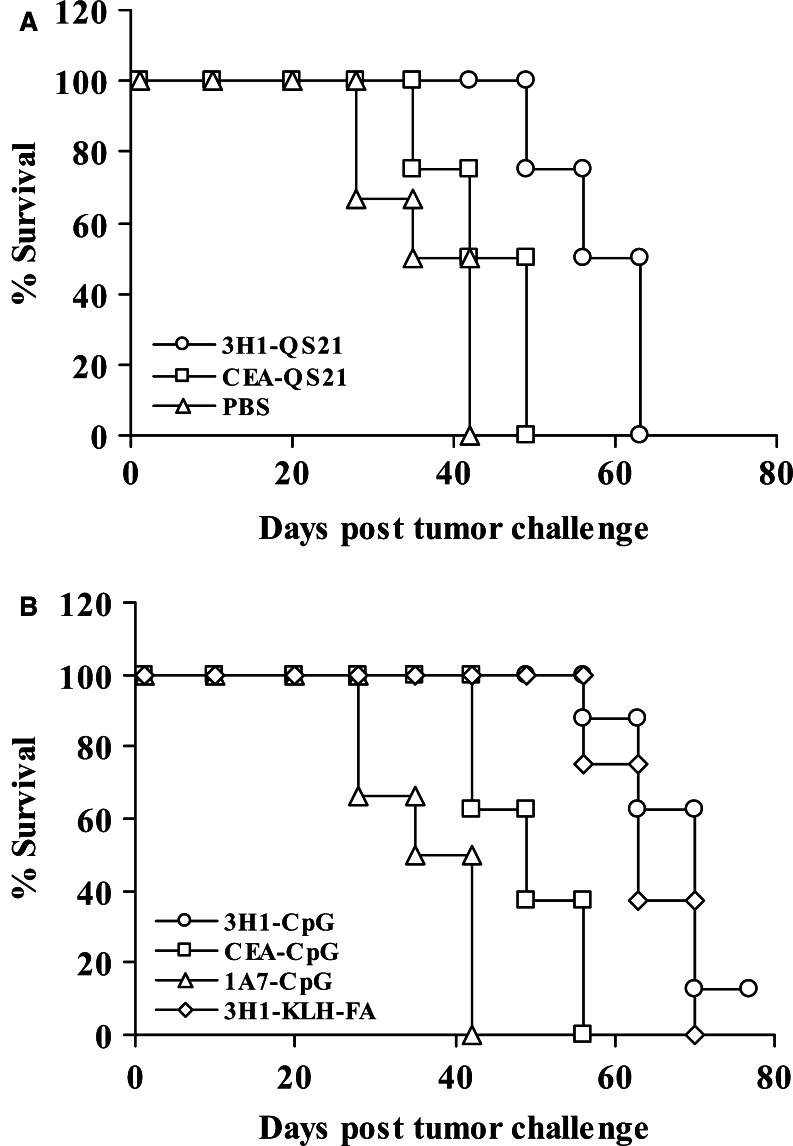
To evaluate the tumor protective role of anti-CEA immunity induced by protein vaccination in combination with different adjuvants, 2 weeks after the final immunization, each group of mice were further divided into two subgroups and one group was challenged with lethal doses of syngeneic CEA-transfected C15 cells, whereas the second group was challenged with nontransfected parental MC-38 cells. The anti-CEA immunity induced by 3H1-CpG vaccination led to increased survival from implanted CEA-tumors. The data presented in Fig. 7a, b suggest that the mice that were treated with PBS and those that received control vaccine 1A7 rapidly developed C15 tumors. All these mice died within 42 days (Fig. 8a, b). Whereas, tumor growth was relatively slower and mice survived relatively longer when immunization was performed with CEA. Mice immunized with CEA along with QS-21 survived for 49 days (Fig. 8a), and 56 days of survival was observed in the group of mice immunized with CEA-CpG (Fig. 8b). Thus, mice immunized with CEA-CpG survived 33% longer than the control vaccine-immunized mice. In contrast, tumor growth rate was significantly slower in mice vaccinated with 3H1 along with QS-21 (Fig. 7a) or CpG (Fig. 7b) compared with corresponding control vaccine (P<0.05). Also, there was a significant difference in tumor growth on day 42 when 3H1–CpG-immunized mice were compared with CEA–CpG-immunized littermates (P<0.01) or when CEA–CpG-immunized mice were compared with control 1A7-CpG immunized littermates (P<0.01). Mice immunized with 3H1-QS21 survived for 63 days (Fig. 8a), whereas mice immunized with 3H1-KLH in FA or 3H1-CpG survived for 70 days (Fig. 8b). Therefore, 3H1–CpG-immunized mice had 2 weeks of survival advantage over CEA-CpG-immunized littermates and one of eight mice that received 3H1-CpG vaccination remained tumor-free through out the study and survived until day 84, when the experiment was terminated (Fig. 8b). Furthermore, mice that developed tumor in the 3H1-CpG group, survived for a significantly longer period than the mice that received control vaccine, 1A7-CpG (P=0.03). Irrespective of immunization, different group of mice (n=8) challenged with MC-38 cells were not protected, and died between 30 days and 40 days (data not shown), indicating that protective immunity was antigen-specific, depending on CEA expression by the tumor target.
Fig. 7.
Antitumor immunity induced by vaccination is antigen-specific. Groups of mice were immunized as outlined in “Materials and methods.” Two weeks after the final immunization, mice were challenged s.c. with 1×106 CEA-transfected C15 tumor cells. Tumor growth was measured over time. All experiments included 6–8 mice per group. Results are the representation of two independent experiments
Fig. 8.
Vaccination with 3H1 increased overall survival in response to tumor challenge. Groups of mice were immunized as outlined in “Materials and methods.” Two weeks after the final immunization, mice were challenged s.c. with 1×106 C15 tumor cells. Mice were sacrificed when tumor became ulcerated or when tumor size reached >250 mm2 and survival was recorded accordingly. All experiments included 6–8 mice per group and data are representation of two independent experiments with similar results
Discussion
In the present study, we have evaluated the vaccine strategy in a murine model of colon carcinoma transgenic for human CEA. In this model, we used a murine monoclonal anti-Id antibody, 3H1, in combination with different adjuvants in an attempt to break peripheral tolerance to CEA and generate protective immune responses. An important approach to cancer immunotherapy involves the development of an effective immune adjuvant. FA is the standard adjuvant in animal models, although this adjuvant is not widely used in human vaccination protocols due to its undesirable side effects at the injection site. Other adjuvants such as Alugel, QS-21, and CpG ODN are currently being used in clinical trials. Recombinant cytokines also play a certain role as adjuvants. However, a suitable adjuvant that can induce activation of various immune subsets and production of various cytokines which participate in the development of an active response is thought to be more effective and less toxic than immunization with external cytokine as adjuvant.
CpG ODN are potent inducers of both innate and adaptive immunity and can serve as suitable vaccine adjuvants. CpG ODN trigger activation and maturation of dendritic cells (DCs) resulting in increased cell surface expression of CD40, CD80, and CD86 [1, 37]. DCs exposure to CpG ODN induces expression of proinflammatory cytokines including IL-1, IL-6, TNF-α, and type I IFNs as well as the Th1-promoting cytokine IL-12 [3, 37]. CpG has been shown to promote both B- and T-cell responses when combined with protein or peptide Ags. In addition, this adjuvant induces a large population of Ag-specific effector CD8 T cells capable of expressing IFN-γ, TNF-α and displaying cytolytic activity [10, 39, 41].
In our previous study [2], naïve C57BL/6 mice were immunized with 3H1-CpG, and 3–4 immunizations were required to obtain and maintain peak anti-CEA immunity. The immunity induced in mice resulted in a complete rejection of MC-38-CEA tumor cells in 100% of experimental mice, whereas no protection was observed when 3H1-CpG immunized mice were challenged with CEA-negative MC-38 cells. In the present study, we assessed whether a select CpG ODN 1826 could function as an immune adjuvant in the immunization of CEA.Tg mice with anti-Id 3H1. The vaccine potential of 3H1 was also compared in the presence of QS-21 and FA. 3H1 was coupled to KLH and used with FA as a gold standard in our previous studies in the murine model [31]. Hence, we also used 3H1-KLH-FA as a reference standard in this study. For comparison with the 3H1-QS21 or 3H1-CpG vaccine, it was possible that conjugation of 3H1 to KLH plus the use of QS-21 or CpG might well have produced higher immune responses. However, our goal was to find out an adjuvant in the murine model, which would be as potent as our gold standard 3H1-KLH-FA but without the conjugation to KLH. In all our clinical trials with anti-Id antibody vaccines, the antibody molecule was a foreign protein and was injected as an intact immunoglobulin. The Fc portion of the murine immunoglobulin probably served as a “carrier” to help promote the immune responses. In our experience, conjugation of anti-Id to KLH in combination with a strong adjuvant was necessary to raise optimal immunity in mice. Interestingly, as we moved to higher species, such as rabbits, we did not require KLH coupling, but only a strong adjuvant was needed; whereas in monkey and humans we could use anti-Id vaccines with a weak adjuvant without any conjugation to KLH [12–14].
The analysis of antibodies induced by 3H1 in the presence of various adjuvants showed that humoral responses differed quantitatively as reflected by the total antibody titers. Ab3 induced in 3H1-immunized mice, irrespective of adjuvants used, inhibited the Ab1–Ab2 binding in this system (Fig. 1) and binding of Ab1 to CEA was also inhibited (Fig. 2). Anti-CEA antibody developed in mice was detected by ELISA (Fig. 3). These results suggested the induction of Ab1′-like antibodies in the 3H1-immunized mice. We have also found in vitro ADCC mediated by immune effector cells obtained from mice immunized with 3H1. ADCC could be an additional important mechanism for tumor protection by 3H1 immunization. Several investigators have reported that CpG ODN can enhance ADCC and improve the in vivo efficacy of monoclonal antibody therapy [40].
We have demonstrated that Th1-type cellular immune response was important in mediating tumor protection in this model system. The proliferative response of splenocytes in the presence of 3H1 and CEA suggested that mice immunized with 3H1 induced anti-3H1 as well as anti-CEA immune responses. The T-cell proliferation was most significant when CpG ODN was used as an adjuvant. The phenotyping of proliferating splenocytes indicated that they are predominantly CD4+ T cells. Immunization of CEA.Tg mice with the self-antigen, CEA, also resulted in the induction of Ag-specific cellular immune response. The cytokine profile in cocultures of splenocytes with 3H1 indicated the induction of a Th1 immune response with moderate levels of IL-2 and IFN-γ production. The most likely explanation of these variable effects of different adjuvants on the vaccine is that, CpG ODN provided a “danger signal” to the immune system [16] that activated monocytes, macrophages, and DCs to express costimulatory molecules and to secrete Th1-like cytokines [3, 35, 37].
Our results have also demonstrated that vaccination with 3H1 using CpG ODN as an adjuvant resulted strong CTL responses that were not observed in the presence of other adjuvants studied (Fig. 6). Thus, it appears that the APC generated and stimulated by CpG administration were capable of capturing and processing soluble proteins into class I MHC CTL epitopes. In support of this, there are various examples that DCs pulsed in vitro with intact soluble proteins are capable of inducing CTL responses when injected into mice [29, 34], and it has also been reported that CpG can induce activation and maturation of DCs [1, 37]. In addition, there are reports of possible direct costimulatory effects of CpG ODN on T cells [5, 24]. The induction of CTL response in mice by 3H1 vaccination is supported by the evidence that there are number of peptides in 3H1, which have linear amino acid sequence homology to CEA [8]. One of these peptides (LCD-2) contain weak murine Kb binding motif [30]. Whether LCD-2 or any of these peptides constituted the CTL epitope in this system is currently under investigation.
The immune response developed in 3H1-immunized mice inhibited tumor growth after challenge with lethal dose of syngeneic CEA-transfected C15 tumor cells. This tumor-protective immunity is Ag-specific, because tumor development was not prevented in immunized mice when challenged with nontransfected parental MC-38 cells. The therapeutic effects were not observed in the mice vaccinated with an isotype-matched unrelated anti-Id antibody, 1A7. The survival benefit of 3H1–CpG-immunized mice was slightly better than the group of mice immunized with 3H1-KLH-FA. Furthermore, 3H1 did not need any conjugation to KLH to be used with CpG ODN as opposed to FA. In this regard, CpG ODN was more useful adjuvant than FA and our study demonstrates that adjuvant CpG ODN 1826 can orchestrate an immune response that leads to the formation of enhanced cellular immunity. Of interest, MC-38 murine colon adenocarcinoma and human CEA-transfected MC-38 cells are very aggressive in nature and grow rapidly both in vitro and in vivo. In most of the published studies [15, 17, 18, 20, 25, 27, 28, 42], prophylactic anti-CEA immunity led to minor protection against implanted tumors and the investigators had to terminate the experiments within 4–5 weeks of post-tumor challenge. In our study, mice were challenged with at least twice the lethal dose of MC-38-CEA cells for tumor growth as compared to the studies mentioned above, and yet we have clearly shown that using CpG ODN 1826 as an adjuvant, anti-Id 3H1 was capable of extending the survival benefit up to 10 weeks. 3H1 was also relatively more potent as compared to CEA to break immune tolerance and induced more effective CD4 and CD8 T-cell responses.
It is important to point out that the overall immune response to CEA in the CEA.Tg mice, following CEA vaccination, was relatively weak when compared with that generated in mice immunized with 3H1. CEA is an extremely heterogeneous molecule and CEA-based vaccines presenting multiple epitopes could potentially invoke a mixture of counterproductive immune responses. Similar phenomenon was observed when intact HER-2/neu antigen was used as an immunogen. Of interest, we did not observe any autoimmune side effects during immunizations with 3H1 or CEA, and mice appeared healthy and maintained normal weight when compared with PBS-vaccinated littermates. Other studies have also documented that autoimmunity was not observed in CEA.Tg mice treated with different immunogenic forms of CEA [15, 17].
In summary, we have shown that a potent tumor antigen-specific immune response can be induced by exploiting and amplifying normal host immune mechanisms in vivo. We found that among the three different adjuvants studied, 3H1-KLH-FA induced the highest humoral immune response. The conjugation of 3H1 to KLH plus the use of CpG or QS-21 might well have produced higher antibody responses. Overall, for antibody response, QS-21 was comparable to CpG. However, CpG ODN served as the most potent adjuvant compared to QS-21 or FA at inducing cellular immune responses, and 3H1–CpG-vaccinated mice survived somewhat longer compared to the 3H1–QS21-vaccinated mice against s.c. implanted tumors. Also, the demonstration of the adjuvant effect of CpG ODN on the induction of CTL response has important implications in vaccine development. Additional manipulation of the antitumor immune response may be required to enhance the T-cell responses, so these can last sufficiently long to control tumor progression. To our knowledge, this is the first report in the literature describing the combination of CEA or anti-Id 3H1 with CpG ODN for the generation of specific and reproducible immunity to CEA in CEA.Tg mice. It is clear that anti-Id 3H1 along with a select CpG ODN 1826 may serve as a potential tumor vaccine in future clinical studies in human patients with CEA-positive tumors.
Acknowledgements
We would like to thank Dr. Jeffrey Schlom (NIH) for providing the MC-38 cell lines. We also thank Mary B. Palascak and Peter Ciraolo for flow cytometry and Audrey Morrison for typing the manuscript. This work was supported in part by NIH grants RO1 CA86025, RO1 CA91878, and RO1 CA104804.
Abbreviations
- Id
Idiotype
- CEA
Carcinoembryonic antigen
- ODN
Oligodeoxynucleotide
- KLH
Keyhole limpet hemocyanin
- TAA
Tumor-associated antigen
- CEA.Tg
CEA transgenic
- ADCC
Antibody-dependent cellular cytotoxicity
- IL
Interleukin
- IFN
Interferon
- rh
Recombinant human
References
- 1.Askew D, Chu RS, Krieg AM, Harding CV. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J Immunol. 2000;165:6889–6895. doi: 10.4049/jimmunol.165.12.6889. [DOI] [PubMed] [Google Scholar]
- 2.Baral RN, Saha A, Chatterjee SK, Foon KA, Krieg AM, Weiner GJ, Bhattacharya-Chatterjee M. Immunostimulatory CpG oligonucleotides enhance the immune response of anti-idiotype vaccine that mimics carcinoembryonic antigen. Cancer Immunol Immunother. 2003;52:317–327. doi: 10.1007/s00262-002-0351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behboudi S, Chao D, Klenerman P, Austyn J. The effects of DNA containing CpG motif on dendritic cells. Immunology. 2000;99:361–366. doi: 10.1046/j.1365-2567.2000.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beloeil L, Tomkowiak M, Angelov G, Walzer T, Dubois P, Marvel J. In vivo impact of CpG 1826 oligodeoxynucleotide on CD8 T cell primary responses and survival. J Immunol. 2003;171:2995–3002. doi: 10.4049/jimmunol.171.6.2995. [DOI] [PubMed] [Google Scholar]
- 5.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co- stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–1218. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya-Chatterjee M, Mukherjee S, Biddle W, Foon KA, Kohler H. Murine monoclonal anti-idiotype antibody as a potential network antigen for human carcinoembryonic antigen. J Immunol. 1990;145:2758–2765. [PubMed] [Google Scholar]
- 7.Chakraborty M, Foon KA, Kohler H, Bhattacharya-Chatterjee M. Preclinical evaluation in nonhuman primates of an anti-idiotypic antibody that mimics the carcinoembryonic antigen. J Immunother Emphasis Tumor Immunol. 1995;18:95–103. doi: 10.1097/00002371-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee SK, Tripathi PK, Chakraborty M, Yannelli J, Wang H, Foon KA, Maier CC, Blalock JE, Bhattacharya-Chatterjee M. Molecular mimicry of carcinoembryonic antigen by peptides derived from the structure of an anti-idiotype antibody. Cancer Res. 1998;58:1217–1224. [PubMed] [Google Scholar]
- 9.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1469–1477. [PubMed] [Google Scholar]
- 10.Davila E, Celis E. Repeated administration of cytosine-phosphorothiolated guanine- containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol. 2000;165:539–547. doi: 10.4049/jimmunol.165.1.539. [DOI] [PubMed] [Google Scholar]
- 11.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 12.Foon KA, Chakraborty M, John WJ, Sherratt A, Kohler H, Bhattacharya-Chatterjee M. Immune response to the carcinoembryonic antigen in patients treated with anti-idiotype antibody vaccine. J Clin Invest. 1995;96:334–342. doi: 10.1172/JCI118039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foon KA, John WJ, Chakraborty M, Das R, Teitelbaum A, Garrison J, Kashala O, Chatterjee SK, Bhattacharya-Chatterjee M. Clinical and immune responses in resected colon cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. J Clin Oncol. 1999;17:2889–2895. doi: 10.1200/JCO.1999.17.9.2889. [DOI] [PubMed] [Google Scholar]
- 14.Foon KA, John WJ, Chakraborty M, Sherratt A, Garrison J, Flett M, Bhattacharya-Chatterjee M. Clinical and immune responses in advanced colorectal cancer patients treated with anti-idiotype monoclonal antibody vaccine that mimics the carcinoembryonic antigen. Clin Cancer Res. 1997;3:1267–1276. [PubMed] [Google Scholar]
- 15.Greiner JW, Zeytin H, Anver MR, Schlom J. Vaccine-based therapy directed against carcinoembryonic antigen demonstrates antitumor activity on spontaneous intestinal tumors in the absence of autoimmunity. Cancer Res. 2002;62:6944–6951. [PubMed] [Google Scholar]
- 16.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 17.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–1849. [PubMed] [Google Scholar]
- 18.Hodge JW, Poole DJ, Aarts WM, Gomez Yafal A, Gritz L, Schlom J. Modified vaccinia virus ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res. 2003;63:7942–7949. [PubMed] [Google Scholar]
- 19.Horig H, Wainstein A, Long L, Kahn D, Soni S, Marcus A, Edelmann W, Kucherlapati R, Kaufman HL. A new mouse model for evaluating the immunotherapy of human colorectal cancer. Cancer Res. 2001;61:8520–8526. [PubMed] [Google Scholar]
- 20.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676–683. [PubMed] [Google Scholar]
- 21.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 22.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JJ, Janetzki S, Schaed S, Panageas KS, Wang S, Williams L, Meyers M, Butterworth L, Livingston PO, Chapman PB, Houghton AN. Evaluation of CD8+ T-cell frequencies by the ELISPOT assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer. 2000;87:391–398. doi: 10.1002/1097-0215(20000801)87:3<391::AID-IJC13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, O’Hagan D, Zhou H, Singh M, Ulmer J, Reisfeld RA, James Primus F, Xiang R. Plasmid DNA encoding human carcinoembryonic antigen (CEA) adsorbed onto cationic microparticles induces protective immunity against colon cancer in CEA-transgenic mice. Vaccine. 2003;21:1938–1947. doi: 10.1016/S0264-410X(02)00821-6. [DOI] [PubMed] [Google Scholar]
- 26.Miconnet I, Koenig S, Speiser D, Krieg A, Guillaume P, Cerottini JC, Romero P. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol. 2002;168:1212–1218. doi: 10.4049/jimmunol.168.3.1212. [DOI] [PubMed] [Google Scholar]
- 27.Mizobata S, Tompkins K, Simpson JF, Shyr Y, Primus FJ. Induction of cytotoxic T cells and their antitumor activity in mice transgenic for carcinoembryonic antigen. Cancer Immunol Immunother. 2000;49:285–295. doi: 10.1007/s002620000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niethammer AG, Primus FJ, Xiang R, Dolman CS, Ruehlmann JM, Ba Y, Gillies SD, Reisfeld RA. An oral DNA vaccine against human carcinoembryonic antigen (CEA) prevents growth and dissemination of Lewis lung carcinoma in CEA transgenic mice. Vaccine. 2001;20:421–429. doi: 10.1016/S0264-410X(01)00362-0. [DOI] [PubMed] [Google Scholar]
- 29.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 31.Pervin S, Chakraborty M, Bhattacharya-Chatterjee M, Zeytin H, Foon KA, Chatterjee SK. Induction of antitumor immunity by an anti-idiotype antibody mimicking carcinoembryonic antigen. Cancer Res. 1997;57:728–734. [PubMed] [Google Scholar]
- 32.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone- KLH conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(SICI)1097-0215(20000301)85:5<659::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha A, Chatterjee SK, Foon KA, Primus FJ, Bhattacharya-Chatterjee M. Murine dendritic cells pulsed with an anti-idiotype antibody induce antigen-specific protective antitumor immunity. Cancer Res. 2003;63:2844–2854. [PubMed] [Google Scholar]
- 35.Sandler AD, Chihara H, Kobayashi G, Zhu X, Miller MA, Scott DL, Krieg AM. CpG oligonucleotides enhance the tumor antigen-specific immune response of a granulocyte macrophage colony-stimulating factor-based vaccine strategy in neuroblastoma. Cancer Res. 2003;63:394–399. [PubMed] [Google Scholar]
- 36.Sen G, Chakraborty M, Foon KA, Reisfeld RA, Bhattacharya-Chatterjee M. Preclinical evaluation in nonhuman primates of murine monoclonal anti-idiotype antibody that mimics the disialoganglioside GD2. Clin Cancer Res. 1997;3:1969–1976. [PubMed] [Google Scholar]
- 37.Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 39.Vabulas RM, Pircher H, Lipford GB, Hacker H, Wagner H. CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses. J Immunol. 2000;164:2372–2378. doi: 10.4049/jimmunol.164.5.2372. [DOI] [PubMed] [Google Scholar]
- 40.van Ojik HH, Bevaart L, Dahle CE, Bakker A, Jansen MJ, van Vugt MJ, van de Winkel JG, Weiner GJ. CpG-A and B oligodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res. 2003;63:5595–5600. [PubMed] [Google Scholar]
- 41.Warren TL, Bhatia SK, Acosta AM, Dahle CE, Ratliff TL, Krieg AM, Weiner GJ. APC stimulation by CpG oligodeoxynucleotide enhance activation of MHC class I-restricted T cells. J Immunol. 2000;165:6244–6251. doi: 10.4049/jimmunol.165.11.6244. [DOI] [PubMed] [Google Scholar]
- 42.Xiang R, Primus FJ, Ruehlmann JM, Niethammer AG, Silletti S, Lode HN, Dolman CS, Gillies SD, Reisfeld RA. A dual-function DNA vaccine encoding carcinoembryonic antigen and CD40 ligand trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J Immunol. 2001;167:4560–4565. doi: 10.4049/jimmunol.167.8.4560. [DOI] [PubMed] [Google Scholar]