Abstract
Recent studies have suggested that dendritic cell (DC)-based immunotherapy is one promising approach for the treatment of cancer. We previously studied the clinical toxicity, feasibility, and efficacy of cancer vaccine therapy with peptide-pulsed DCs. In that study, we used granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood monocytes as a cell source of DCs. However, previous investigations have suggested that G-CSF-mobilized peripheral blood monocytes produce reduced levels of proinflammatory cytokines such as interleukin (IL)-12 and tumor necrosis factor (TNF)-α. These T helper (Th)-1-type cytokines are thought to promote antitumor immune response. In this study, we assessed the functional abilities of DCs generated from G-CSF-mobilized monocytes obtained from 13 patients with CEA-positive advanced solid cancers. Peripheral blood mononuclear cells were obtained from leukapheresis products collected before and after systemic administration of G-CSF (subcutaneous administration of high-dose [5–10 μg/kg] human recombinant G-CSF for five consecutive days). In vitro cytokine production profiles after stimulation with lipopolysaccharide (LPS) were compared between monocytes with and without G-CSF mobilization. DCs generated from monocytes were also examined with respect to cytokine production and the capacity to induce peptide-specific T cell responses. Administration of G-CSF was found to efficiently mobilize peripheral blood monocytes. Although G-CSF-mobilized monocytes (G/Mo) less effectively produced Th-1-type cytokines than control monocytes (C/Mo), DCs generated from G/Mo restored the same level of IL-12 production as that seen in DCs generated from C/Mo. T cell induction assay using recall antigen peptide and phenotypic analyses also demonstrated that DCs generated from G/Mo retained characteristics identical to those generated from C/Mo. Our results suggest that G-CSF mobilization can be used to collect monocytes as a cell source for the generation of DCs for cancer immunotherapy. DCs generated in this fashion were pulsed with HLA-A24-restricted CEA epitope peptide and administered to patients safely; immunological responses were induced in some patients.
Keywords: Dendritic cell, Granulocyte colony-stimulating factor, Cancer immunotherapy, Cancer vaccine therapy, Cytokine
Introduction
Dendritic cells (DCs) are highly potent antigen-presenting cells (APCs), which play a crucial role in prime T cell-dependent immune responses specific for tumor-associated antigens [1, 2]. Recently, active immunotherapy using antigen-loaded DCs has been used to treat not only cancers relatively sensitive to immunotherapy, such as melanoma [2–5] and prostate cancer [6, 7], but also cancers considered insensitive to immunotherapy, such as colorectal and lung cancers [8–10], both of which are main causes of cancer-related mortality. In most previous studies, DCs were generated from monocytes harvested from peripheral blood by leukapheresis. Although the optimal number of DCs needed to induce antitumor immune response in vivo remains controversial, administration of large numbers of DCs and repeated vaccinations might lead to effective, sustained immunological responses specifically against less immunogenic antigens, such as tumor-associated antigens [11]. Thus, the development of ways to obtain larger numbers of monocytes that can differentiate into clinically competent DCs seems worth pursuing.
We have previously used granulocyte colony-stimulating factor (G-CSF)-mobilized leukapheresis products as a cell source for the generation of DCs in a clinical trial of DC-based immunotherapy for solid cancer [10]. Because G-CSF mobilizes monocytes as well as hematopoietic stem cells in peripheral blood [12–14], its use for obtaining monocytes appears reasonable. However, recent studies have suggested that G-CSF-mobilized monocytes have a cytokine production profile characteristic of T helper (Th)-2 type, not Th-1 type [15–17]. Indeed, the production of interleukin (IL)-12 and tumor necrosis factor (TNF)-α is apparently suppressed by G-CSF [17]. G-CSF has also been implicated in the inhibition of acute graft-versus-host (GVH) reactions in patients undergoing peripheral blood stem-cell transplantation (PBSCT) [18, 19]. Furthermore, another study has suggested that G-CSF treatment mobilizes DC2, which polarizes T cell response into Th-2 type [20]. Therefore, apheresis products obtained after G-CSF mobilization may not be useful as a cell source for DC-based cancer vaccine therapy, which requires Th-1-type immune response to eliminate tumor cells. We studied the functional abilities of DCs generated from G-CSF-mobilized monocytes to assess whether such cells could be used for cancer immunotherapy.
Materials and methods
Patients
Thirteen patients, who participated in a clinical study of carcinoembryonic antigen (CEA)-targeting DC-based immunotherapy [10], were enrolled in the present study after obtaining written informed consent. All patients had metastatic lesions from gastrointestinal or lung adenocarcinomas that were refractory to conventional chemotherapy or radiotherapy and met the following eligibility criteria for immunotherapy: (a) positive for HLA-A24; (b) metastatic lesions expressing CEA as confirmed by immunohistochemical analysis or elevated serum CEA levels; (c) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and (d) adequate cardiac, pulmonary, hepatic, renal, and hematological functions. Patients were excluded if they (a) had received chemotherapy, radiotherapy, or immunotherapy within 4 weeks before enrollment; (b) had severe infectious, hematological, cardiac, or pulmonary disease; (c) had autoimmune disease; (d) were receiving steroid therapy; or (e) were pregnant.
Collection of peripheral blood mononuclear cells
Patients underwent two sessions of leukapheresis, before and 1 day after subcutaneous administration of high-dose human recombinant G-CSF (lenograstim) (Neutrogin®, Chugai, Tokyo, Japan; 5–10 μg/kg once daily for five consecutive days). Leukapheresis was done with a Blood Cell Separator CS-3000 (Baxter, Seattle, WA, USA). Peripheral blood mononuclear cells (PBMCs) were separated by density-gradient centrifugation using Ficoll-Paque Plus (Pharmacia Biotech, Stockholm, Sweden) and suspended in CP-1 (Kyokuto Seiyaku, Tokyo, Japan) mixed with an equal volume of RPMI 1640 (Nikken, Kyoto, Japan) for cryopreservation. The cell suspension was divided into vials so that each vial contained 2 ml of cell suspension at a concentration of 5 × 107 cells/ml. All vials were stored in a liquid nitrogen tank until use.
DC generation
Two or three vials were thawed, washed, and suspended in 20 ml of RPMI 1640 supplemented with 5% heat-inactivated pooled human AB plasma (Japanese Red Cross Society) and gentamicin (Fujisawa Pharmaceutical, Osaka, Japan). Cell suspensions were plated in 75 cm2 cell culture flasks and incubated in a 5% CO2 incubator at 37°C for 2 h; non-adherent cells were discarded. Adherent cells were then cultured in 30 ml of complete medium containing 1,000 U/ml each of recombinant human IL-4 (Genzyme, Minneapolis, MN, USA) and granulocyte/macrophage colony-stimulating factor (GM-CSF) (Kirin Brewery, Gunma, Japan) for 7 days. Cultured cells were harvested as DCs and used for subsequent experiments.
Flow-cytometric analysis of the cells
Phenotypical analyses of cells were performed by FACS analysis using FACSCalibur (Becton Dickinson, San Jose, CA, USA) after staining with the following monoclonal antibodies (mAbs): anti-CD14; anti-CD80 (Becton Dickinson); anti-CD83 (Immunotech, France); and anti-CD86 (Ancell, Bayport, MN, USA). Cells were washed and suspended in cell staining buffer (0.1% albumin phosphate-buffered saline) and then incubated with mAbs for 0.5 h at 4°C. Samples were washed, fixed with 4% paraformaldehyde, and subjected to FACS analysis.
Phytohemagglutinin-induced T cell proliferation assay
Phytohemagglutinin (PHA)-induced T cell proliferation was measured by 3H-thymidine incorporation into DNA. Cells (1 × 105/well) were plated onto flat-bottomed wells of microtiter plates in triplicate. PBMCs were cultured with or without (control) PHA (Sigma-Aldrich, Tokyo, Japan) at a final concentration of 1 μg/ml in the medium. On day 3, the wells were pulsed with 1 μCi of 3H-thymidine (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) for 18 h, and the cells were harvested on day 4 with an automatic multi-well harvester. The filters were allowed to air dry, scintillation cocktail was added, and the samples were counted in a multi-well beta-plate counter (Packard Instruments, Meriden, CT, USA).
Stimulation and detection of cytokines
CD14+ monocytes were isolated from PBMCs with the use of anti-CD14 mAb and a FACS Vantage (Becton Dickinson) cell sorter. DCs were purified using Human Dendritic Cell Enrichment Cocktail (Stem Cell Technologies, Vancouver, Canada) according to the manufacturer’s instruction. Cells were washed and cultured at 2.5 × 105 cells/ml (monocytes) or at 1.0 × 105 cells/ml (DCs) in 24-well flat-bottom plates for 12 h. Lipopolysaccharide (LPS) was then added to the culture at a concentration of 10 μg/ml. Supernatants were harvested 60 h after the initiation of cell incubation. The concentrations of cytokines in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA). The IL-10 and IL-12 ELISA kits were purchased and used according to the manufacturer’s instructions (Biosource International, Camarillo, CA, USA).
Antigen-specific cytotoxic T cell induction assay
To quantify the antigen-presenting capacity of DCs, a recall cytotoxic T lymphocyte (CTL) assay for the detection of flu peptide (RFYIQMCTEL, position 38–47), derived from influenza nucleoprotein restricted with HLA-A24, -specific killer cells was performed. Irradiated DCs used as APCs (5 × 105 cells/well) were seeded in the wells of 24-well plates and pulsed with 20 μg/ml of peptide for 2 h at 37°C. Autologous CD8+ T cells were sorted from non-mobilized leukapheresis products with the use of magnetic beads (Miltenyi Biotech, Germany) and then added to the wells and co-cultured with the peptide-pulsed APCs. On days 3 and 5, the cultures were fed with medium containing 20 U/ml IL-2 (Shionogi, Osaka, Japan). Effector cells were harvested on day 7 and tested for CTL activity in a standard 51Cr-releasing assay using peptide-pulsed TISI cells, an Epstein–Barr virus transformed B cell line expressing HLA-A24, as target cells. Percent lysis was determined by the following formula: % cytotoxicity = (experimental cpm − spontaneous cpm × 100)/(maximal cpm − spontaneous cpm).
Statistical analysis
All data are expressed as means ± SD. For comparison of two groups, Student’s t test was performed. The level of significance was set at P < 0.05.
Results
Patient characteristics
A total of 13 patients were enrolled in the study. All patients had metastatic lesions from gastrointestinal or lung adenocarcinomas that were refractory to conventional chemotherapy or radiotherapy. The study protocol was approved by the Institutional Ethics Review Committee, Kyoto Prefectural University of Medicine, and written informed consent was obtained from all subjects at the time of enrollment. Patients’ characteristics are shown in Table 1.
Table 1.
Patients’ characteristics
| Patient no. | Age/gender | Primary cancer | Metastatic lesion | Prior therapy |
|---|---|---|---|---|
| 1 | 55/F | Rectum | Lung, adrenal | S, C |
| 2 | 56/M | Rectum | Liver, LN | S, C |
| 3 | 52/F | Lung | Bone | S, C |
| 4 | 28/F | Colon | Liver, peritoneum | S, C |
| 5 | 42/F | Colon | Liver | S, C |
| 6 | 33/F | Stomach | Peritoneum | C |
| 7 | 60/F | Colon | Liver, peritoneum | S, C |
| 8 | 63/F | Colon | Liver, LN | S, C |
| 9 | 69/M | Lung | Pleura, LN | S, C |
| 10 | 46/F | Colon | Bone | S, C |
| 11 | 53/M | Stomach | Lung, brain | S, C |
| 12 | 65/F | Colon | Liver, lung | S, C |
| 13 | 56/M | Lung | Bone | S, C |
S surgery, C chemotherapy
Monocyte-dominant mobilization in leukapheresis products harvested from patients treated with G-CSF
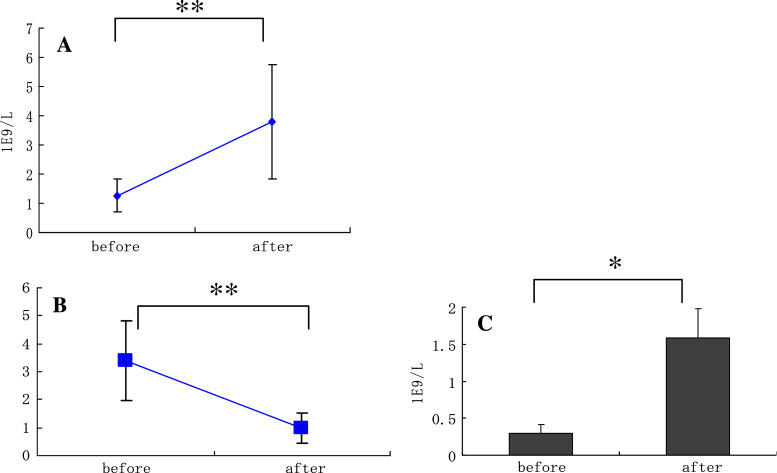
Ten liters of blood was processed for each session of leukapheresis, performed before and after administration of G-CSF. The average numbers of PBMCs obtained before and after G-CSF-induced mobilization were 1.3 × 109 and 3.6 × 109 cells/l, respectively (Fig. 1a). Mean lymphocyte/monocyte ratios decreased from 3.41 ± 0.56 to 1.18 ± 0.29 after mobilization (Fig. 1b), indicating that the absolute number of monocytes capable of being harvested from mobilized leukapheresis products was eight times higher than that without mobilization (Fig. 1c). As methods are now available to generate sizable numbers of DCs by culturing progenitor cells in the presence of various cytokines [21], we examined if the leukapheresis products contained sufficient CD34+ cells. However, the proportion of CD34+ cells were less than 0.3% in all subjects studied (data not shown). Thus, the optimal timing after G-CSF administration should be investigated further to obtain more hematopoietic stem/progenitor cells for DC generation.
Fig. 1.
Monocytes were efficiently mobilized into peripheral blood by G-CSF. Peripheral blood mononuclear cells (PBMCs) were harvested by leukapheresis before and after administration of G-CSF in 13 patients. Lymphocytes (CD3+) and monocytes (CD14+) were then measured by FACS analysis. The numbers of PBMCs were counted (a). The ratios of the numbers of lymphocytes to monocytes (b) and absolute numbers of monocytes harvested (c) were calculated from the results of FACS analyses. Data represent the means ± SD of 13 cases. *P < 0.01, **P < 0.03
Suppressed proliferative response caused by PHA stimulation in T cells obtained from G-CSF-mobilized apheresis products
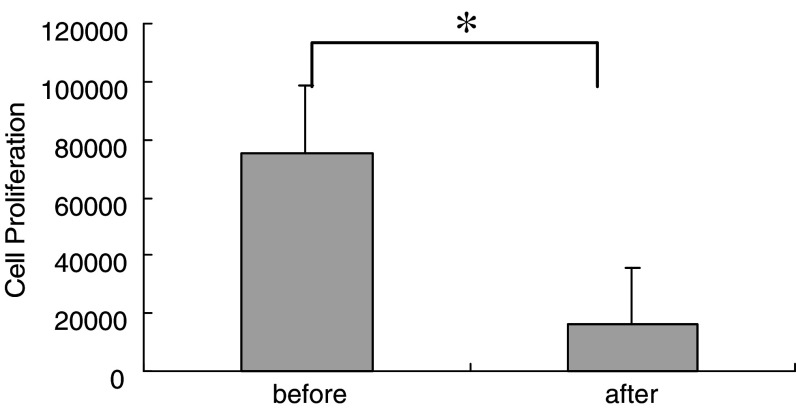
To determine if administration of G-CSF affects immunological responses, especially T cell proliferative responses in patients, we performed PHA-induced T cell proliferation assay. Consistent with the results of a previous study suggesting that G-CSF mobilization decreases the proliferative activity of T cells [22], our data indicated that G-CSF mobilization attenuated T cell proliferation activity in response to PHA stimulation. Proliferative responses expressed as 3H incorporation decreased from 75,027 to 15,949 cpm after treatment with G-CSF (Fig. 2). Controls for each condition were less than 500 cpm (data not shown).
Fig. 2.
Decreased T cell proliferation response against phytohemagglutinin (PHA) stimulation after G-CSF mobilization. T cells were cultured in the presence of PHA and then pulsed with 3H-thymidine for 18 h. Cell proliferation activity was measured by counting 3H incorporation with a scintillation counter. Data represent the means ± SD of 13 cases. *P < 0.02
Th-2-type cytokine production by monocytes mobilized with G-CSF
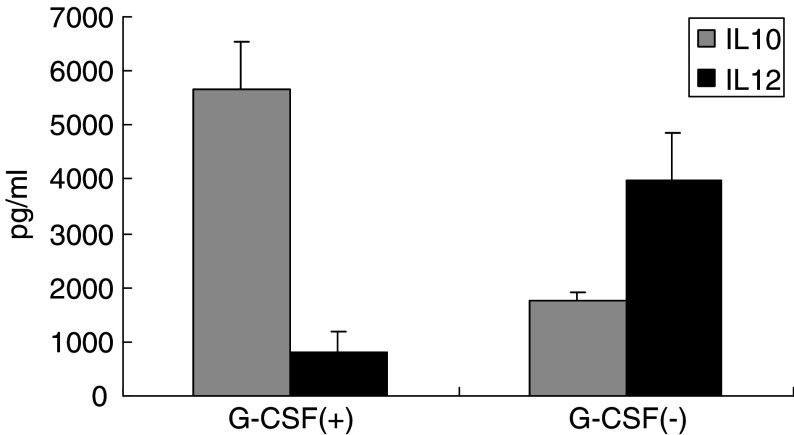
As previous studies have demonstrated that G-CSF-mobilized PBMCs had a higher level of constitutive IL-10 mRNA expression than control PBMCs, suggesting that T cell hyporesponsiveness occurs in patients undergoing PBSCT who receive G-CSF [16], we assayed cytokine production. We specifically assessed the production of IL-10 and IL-12 in response to LPS stimulation in vitro from monocytes obtained before and after G-CSF administration to assess the capacity of monocytes to promote Th-1 response. CD14+ monocytes were isolated by cell sorter using anti-CD14 mAb and were then stimulated in vitro with LPS. As shown in Fig. 3, the production of IL-10 by G-CSF-mobilized monocytes was three times higher than that of non-mobilized monocytes. In contrast, IL-12 production from G-CSF-mobilized monocytes was five times lower than that from non-mobilized monocytes. In conclusion, G-CSF treatment mobilized monocytes whose cytokine production profiles were skewed to Th-2-type response.
Fig. 3.
Th-2-type cytokine production profiles observed in monocytes mobilized with G-CSF. PBMCs were obtained by leukapheresis before and after G-CSF mobilization. Monocytes were then purified with cell sorter using anti-CD14 mAb and subjected to cytokine production assay. Isolated cells (2.5 × 105/ml) were stimulated with 10 μg/ml LPS. After 48 h of incubation at 37°C, released cytokines were measured in the cell-free supernatants. The concentrations of IL-10 and IL-12 were measured by ELISA. Data represent the means ± SD, derived from two independent experiments using PBMCs obtained from patient nos. 1 to 3 and 5
Phenotypic and morphological analyses of DCs generated from monocytes with or without G-CSF mobilization
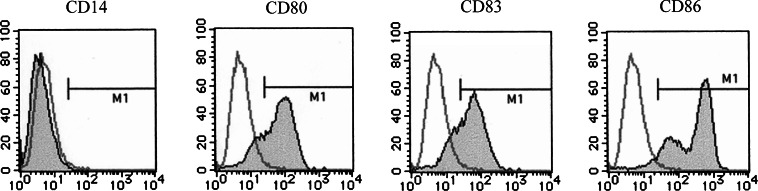
Dendritic cells were generated in vitro from monocytes obtained with or without G-CSF mobilization as described previously [10, 23]. The microscopic findings of G/DCs were identical to those of C/DCs characterized by the cells with faintly veiled structure and prominent dendrites making small cell clumps (data not shown). FACS analyses demonstrated that G/DC expressed CD80, CD83, and CD86, and lost CD14 on their surface (Fig. 4). These phenotypic findings were also identical to those of C/DC (data not shown). As for the expression of CD83, which is a maturation marker of DCs, mean distribution of CD83-positive cells in the cultured cells from each donor represented about 35% without maturation process (data not shown).
Fig. 4.
Surface phenotypic analysis of DCs generated from G-CSF-mobilized monocytes. G-CSF-mobilized monocytes were cultured in the presence of IL-4 and GM-CSF for 7 days and then subjected to phenotypic analysis. The expression of CD14, CD80, CD83, and CD86 was assessed with the use of mAbs (closed histogram). Isotype immunoglobulins were used for controls (open histogram). Results represent the data from patient no. 11
Cytokine production analyses of DCs generated from G-CSF-mobilized monocytes
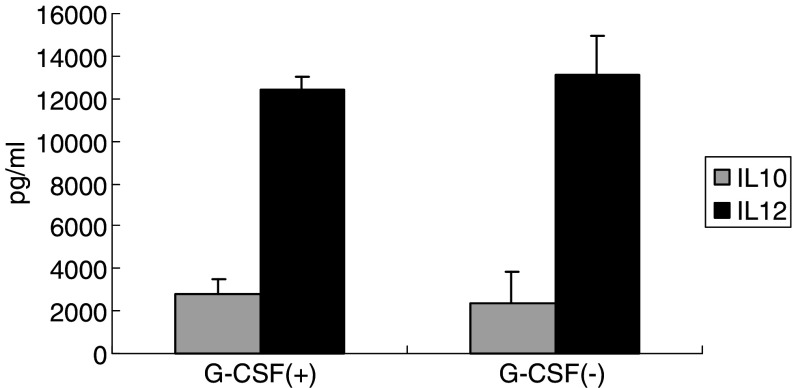
Production of Th-1-type cytokines, such as IL-12, IFN-γ, and TNF-α, in response to antigen stimulation is considered essential for effective antitumor immune responses [24, 25]. To determine whether DCs generated from monocytes mobilized with G-CSF (G/DC) can be used for cancer vaccine therapy, we analyzed and compared the cytokine production profiles and phenotypic markers of G/DC with those of DCs generated from control monocytes without G-CSF mobilization (C/DC). The cytokine production profile of C/DC, characterized by a high level of IL-12 and low level of IL-10, was also retained by G/DC (Fig. 5).
Fig. 5.
Restored production of IL-12 in monocytes obtained from PBMCs mobilized with G-CSF by culturing with IL-4 and GM-CSF. To assess the ability to produce Th-1-type cytokines from DCs generated from monocytes obtained after G-CSF mobilization, DCs were stimulated with 10 μg/ml LPS. DCs were purified with the use of Human Dendritic Cell Enrichment Cocktail (Stem Cell Technologies), which is a depletion cocktail containing several mAbs (anti-CD3, anti-CD14, anti-CD16, anti-CD19, anti-CD56, anti-CD66b, and Glycophorin A), according to the manufacturer’s instruction. Cells were then cultured at the concentration of 1 × 105/ml for 48 h. The concentrations of IL-10 and IL-12 were measured by ELISA using the harvested cell-free supernatants. Data represent the means ± SD, derived from two independent experiments using PBMCs obtained from patient nos. 1 to 3 and 5. There were no significant differences with regard to the production levels of IL-10 and IL-12 between PBMCs obtained before and after G-CSF mobilization
In vitro cellular immune responses induced by DCs generated from monocytes with or without G-CSF mobilization
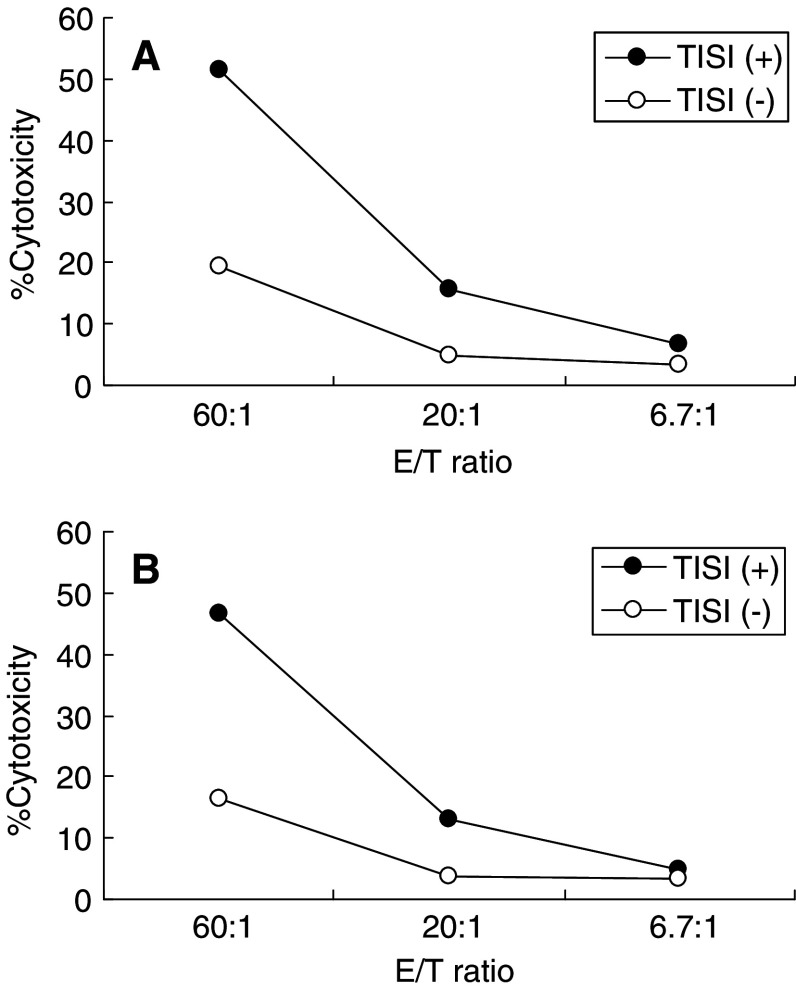
To further analyze the ability of generated DCs to effectively induce cellular immune response, T cells were stimulated with a recall antigen. DCs were pulsed with CTL epitope peptide from influenza nucleoprotein and used for T cell stimulation in vitro. After 7 days’ culture, the cytotoxicity of effector T cells stimulated with cognate peptide was measured to assess CTL responses. As shown in Fig. 6, G/DC-induced cytotoxicity was equivalent to C/DC-induced cytotoxicity, indicating that G/DC as well as C/DC could effectively induce T cell responses.
Fig. 6.
Dendritic cells generated from G-CSF-mobilized monocytes induced efficient cytotoxic T cell response against peptide antigen in vitro. To assess the functional ability of DCs, T cells were stimulated by DCs generated from monocytes before (a) and after (b) G-CSF mobilization, pulsed with HLA-A24-restricted CTL epitope peptide derived from influenza nucleoprotein, and cultured for 7 days. Cytotoxic activities of effector T cells were measured by standard 51Cr-releasing assay using TISI cells (HLA-A24 positive) pulsed with or without cognate peptide at three different effector-to-target ratios. Data represent one of the two independent experiments using PBMCs obtained from patient nos. 1 to 3
Discussion
Our results showed that administration of G-CSF efficiently mobilized CD14+ monocytes in peripheral blood. This technique may facilitate the collection of PBMCs as a cell source for the generation of DCs for cancer immunotherapy. Previous clinical studies have yet to clarify the optimal way to administer antigen-loaded DCs to patients, i.e., the cell number per vaccination, the route of injection, and the number of vaccinations. Because available evidence suggests that repeated vaccinations may promote sustained immunological response [11], the development of optimal techniques to obtain large numbers of DCs is warranted.
Peripheral blood monocytes are often used as a cell source for DC induction, perhaps because of their accessibility. We have used G-CSF mobilization protocols for DC-based immunotherapy, as leukapheresis products mobilized with G-CSF contain large numbers of PBMCs [12–14], especially monocytes, as demonstrated by many studies. Several investigators have studied whether G-CSF-mobilized blood can serve as a cell source for DC generation [26–28]. Syme et al. found that DCs were successfully induced from leukapheresis products obtained after G-CSF mobilization from patients with lymphoma or breast cancer. These DCs were functional in mixed lymphocyte reaction [26]. Moreover, Choi et al. have shown that G-CSF treatment mobilized PBSCs as well as DC precursors, which could differentiate into DCs by in vitro culture with cytokines. They proposed a combination of PBSCT and DC-based immunotherapy as a new strategy for the treatment of cancer [27]. However, previous studies have also suggested that G-CSF mobilization has immunosuppressive effects on monocytes and may be responsible for the low incidence of graft-versus-host disease (GVHD) in PBSCT [16, 18, 29, 30]. G-CSF-mobilized PBSC grafts are not associated with a higher incidence of acute GVHD than BM grafts, although they contain about tenfold more T cells than BM grafts [18]. Boneberg et al. [17] have demonstrated that blood from healthy volunteers pretreated with G-CSF showed decreased release of proinflammatory cytokines, including TNF-α, IFN-γ, and IL-12, in response to LPS stimulation in vitro. Furthermore, enhanced secretion of immunosuppressive cytokines such as IL-10 by monocytes pretreated with G-CSF in vitro has been observed [30]. Besides these studies using in vitro cell culture systems, similar investigations have been performed using peripheral blood mobilized with G-CSF in vivo. Constitutive IL-10 mRNA levels were found to be tenfold higher in G-CSF-mobilized monocytes (G/Mo) than in control (C/Mo), suggesting that G/Mo may suppress T cell responsiveness by secreting IL-10 [16]. Consistent with the findings of these previous studies, our results indicated that G/Mo produced a higher amount of IL-10 and a lesser amount of IL-12 than C/Mo on stimulation with LPS in vitro (Fig. 3). These results caused concern about the use of G/Mo for DC-based cancer immunotherapy and suggest the need to investigate whether DCs generated from G/Mo promote Th-1-type immune responses. In contrast to the results obtained with monocytes, we found no difference between DCs generated from G/Mo and that generated from C/Mo in cytokine production profiles (Fig. 5) or in the ability to induce antigen-specific T cell responses (Fig. 6). These findings suggested that in vitro culture in the presence of IL-4 and GM-CSF enabled both types of monocytes (G/Mo and C/Mo) to differentiate into DCs capable of potently inducing effective CTL responses. Our results are supported by the findings of a previous study suggesting that DCs generated from G/Mo are phenotypically and functionally equivalent to DCs generated from control monocytes [26]. Indeed, we have demonstrated that some patients vaccinated with antigen-loaded DCs generated from G/Mo showed immunological responses associated with progression-free status of disease [10]. Taken together, we conclude that although DCs obtained from peripheral blood of patients administered G-CSF has been reported to be DC2, DCs generated from G/Mo in vitro under the adequate environment with required cytokines could induce Th-1-type response and thus may be useful for DC-based tumor immunotherapy.
As recent reports have suggested that DCs generated without maturation process seem to induce immunological tolerance rather than efficient CTL response [31], we should start conducting clinical trial using mature DCs. Although the majority of the cell population used for vaccination in our clinical study was immature DC with regard to the expression of CD83, they appeared to contain substantial proportion of mature DCs without maturation step. This can be attributable to the serum used for cell culture which might have contained humoral factors having some effects for maturation step.
The mechanism by which G-CSF pretreatment inhibits the production of proinflammatory cytokines from monocytes remains unknown. Activation of STAT3 by G-CSF may be involved. Studies using STAT3 knockout mice have suggested that the production of TNF-α and IL-12 in response to LPS stimulation is negatively regulated by STAT3 [32]. Therefore, STAT3 activated by G-CSF may inhibit the production of proinflammatory cytokines in monocytes. However, as our results indicated that the decreased production of IL-12 and increased production of IL-10 seen in G/Mo was not retained by DCs generated from G/Mo (Fig. 5), culture conditions in the presence of IL-4 and GM-CSF may somehow alter the mechanism of signal transduction in G/Mo after LPS stimulation.
Our findings also suggested that G-CSF administration had another effect on host immunological functions. The results of T cell proliferation assay indicated that G-CSF administration suppressed the responsiveness of T cells to PHA stimulation (Fig. 2) indicating that these T cells may have Th-2 characteristics. This decreased responsiveness may also be attributed to the suppressed function of monocytes, as shown in a previous study [33]. As optimal effectiveness of a vaccine is unlikely in the presence of suppressed T cell function, vaccination should be withheld until T cell function is restored. The optimal timing for vaccination after G-CSF mobilization should be confirmed by further studies.
In conclusion, although administration of high-dose G-CSF mobilizes monocytes (G/Mo) which induce Th-2-type response in the peripheral blood, DCs generated from G/Mo in vitro seemed to have an ability to induce efficient Th-1-type response. This G-CSF mobilization protocol enabled us to generate eightfold more monocyte-derived DCs per unit of PBMCs, as compared with non-mobilized monocytes. Therefore, treatment of patients with G-CSF before leukapheresis for the collection of monocytes to generate DCs may have an important role in cancer immunotherapy.
Acknowledgments
This work was supported by the following Grants-In-Aid for Scientific Research from the Japan Society for the Promotion of Science: no. 13470259 (2001–2003, to H.Y.), no. 14571152 (2002–2003, to Y.U.), no. 16591341 (2004–2005, to N.F.).
References
- 1.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med. 1999;50:507. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 2.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysates-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 3.Schuler TB, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paneli MC, Wunderlich J, Jeffries J, Wang E, Mixon A, Rosenberg SA, et al. Phase 1 study in melanoma-associated antigens MART-1 and gp100. J Immunother. 2000;23:487. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Lau R, Wang F, Jeffery G, Marty V, Kuniyoshi J, Made E, et al. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother. 2001;24:66. doi: 10.1097/00002371-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Murphy GP, Tjoa BA, Simmons SJ, Jarisch J, Bowes VA, Radge H, et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate. 1999;38:73. doi: 10.1002/(SICI)1097-0045(19990101)38:1<73::AID-PROS9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Horiguchi Y, Nukaya I, Okazawa K, Kawashima I, Fikes J, Sette A, et al. Screening of HLA-A24-restricted epitope peptides from prostate-specific membrane antigen that induce specific antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3885. [PubMed] [Google Scholar]
- 8.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadanaga N, Nagashima H, Mashino K, Tahara K, Yamaguchi H, Ohta M, et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin Cancer Res. 2001;7:2277. [PubMed] [Google Scholar]
- 10.Ueda Y, Itoh T, Nukaya I, Kawashima I, Okugawa K, Yano Y, et al. Dendritic cell-based immunotherapy of cancer with carcinoembryonic antigen-derived, HLA-A24-restricted CTL epitope: clinical outcomes of 18 cases with metastatic gastrointestinal or lung adenocarcinomas. Int J Oncol. 2004;24:909. [PubMed] [Google Scholar]
- 11.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessinger A, Armitage JO. The evolving role of autologous peripheral stem cell transplantation following high-dose therapy for malignancies. Blood. 1991;77:211. [PubMed] [Google Scholar]
- 13.Teshima T, Harada M, Takamatsu Y, Makino K, Taniguchi S, Inaba S, et al. Cytotoxic drug and cytotoxic drug/G-CSF mobilization of peripheral blood stem cells and their use for autografting. Bone Marrow Transplant. 1992;10:215. [PubMed] [Google Scholar]
- 14.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;18:175. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 15.Saito M, Kiyokawa N, Taguchi T, Suzuki K, Sekino T, Mimori K, et al. Granulocyte colony-stimulating factor directly affects human monocytes and modulates cytokine secretion. Exp Hematol. 2002;30:1115. doi: 10.1016/S0301-472X(02)00889-5. [DOI] [PubMed] [Google Scholar]
- 16.Mielcarek M, Graf L, Johnson G, Torok-Storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215. [PubMed] [Google Scholar]
- 17.Boneberg EM, Hareng L, Gantner F, Wendel A, Hartung T. Human monocytes express functional receptors for granulocyte colony-stimulating factor that mediate suppression of monokines and interferon-gamma. Blood. 2000;95:270. [PubMed] [Google Scholar]
- 18.Bensinger WI, Clift RA, Anasetti C, Appelbaum FA, Demirer T, Rowley S, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony stimulating factor. Stem Cells. 1996;14:90. doi: 10.1002/stem.140090. [DOI] [PubMed] [Google Scholar]
- 19.Pan L, Delmonte J, Jr, Jalen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422. [PubMed] [Google Scholar]
- 20.Sloand EM, Kim S, Maciejewski JP, VanRhee F, Chaurhuri A, Barrett J, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95:2269. [PubMed] [Google Scholar]
- 21.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4, downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutella S, Rumi C, Lucia MB, Sica S, Cauda R, Leone G. Serum of healthy donors receiving granulocyte colony-stimulating factor induces T cell unresponsiveness. Exp Hematol. 1998;26:1024. [PubMed] [Google Scholar]
- 23.Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett DL, et al. Natural killer-dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bender A, Sapp M, Schuler G, Steinman RM, Bhardwaji N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 26.Syme RM, Duggan P, Stewart D, Gluck S. Generation of dendritic cells ex vivo: differences in steady state versus mobilized blood from patients with breast cancer, with lymphoma, and from normal donors. J Hematother Stem Cell Res. 2001;10:621. doi: 10.1089/152581601753193832. [DOI] [PubMed] [Google Scholar]
- 27.Choi D, Perrin M, Hoffmann S, Chang AE, Ratanatharaorn V, Uberti J, et al. Dendritic cell-based vaccines in the setting of peripheral blood stem cell transplantation: CD34+ cell-depleted mobilized peripheral blood can serve as a source of potent dendritic cells. Clin Cancer Res. 1998;4:2709. [PubMed] [Google Scholar]
- 28.Reichardt VL, Okada CY, Liso A, Benike CJ, Stockerl KE, Engelman EG, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood. 1999;93:2411. [PubMed] [Google Scholar]
- 29.Mielcarek M, Martin PJ, Torok-Storb B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 1997;89:1629. [PubMed] [Google Scholar]
- 30.Tanaka J, Mielcarek M, Torok-Storb B. Impaired induction of the CD28-responsive complex in granulocyte colony-stimulating factor mobilized CD4 T cells. Blood. 1998;91:347. [PubMed] [Google Scholar]
- 31.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 33.Ino K, Singh RK, Talmadge JE. Monocytes from mobilized stem cells inhibit T cell function. J Leukoc Biol. 1997;61:583–591. doi: 10.1002/jlb.61.5.583. [DOI] [PubMed] [Google Scholar]