Abstract
Experimental and clinical data demonstrate that ageing is associated with the gradual deterioration of the immune system, generally referred to as immunosenescence. Age-related immune dysfunction may have an impact not only on the incidence of cancer, but also on the preventive and therapeutic approaches, which are based on immune system activation. Over the last few years the use of immunological measures to prevent cancer in experimental mouse models involving preimmunisation with new vaccines against even a poor or apparently non-immunogenic tumour has yielded worse outcomes in older age than in young adults. Different mechanisms, which may be due to age-related numerical or functional dysfunction of immune cells and/or to tumour microenvironmental changes, could be responsible for this defect. This review summarises the impact of immunosenescence on the effectiveness of cancer vaccines, knowledge of cancer immunisation in old age and the potential mechanisms implicated in the poorer effectiveness of anticancer immune-based approaches in advanced age. Several approaches to, and possibilities of correcting the low effectiveness of immunisation procedures in old age are described.
Keywords: Immunosenescence, Cancer vaccines, Immunotherapy
Introduction
Immunoprevention and immunotherapy for tumour-associated antigens is now a major field of investigation for the treatment of cancer. Today, cancer vaccination represents the most intriguing means of activating an immune response capable of effectively hampering the progression of the preclinical stages of a tumour [11]. The emerging role of immune-based approaches in treating the disease is further emphasised by the fact that cancer vaccines which can be applied in both prevention and therapy are potentially less toxic than chemo- or radiotherapy and could be especially suitable for older more frail cancer patients [41]. In recent years, experimental data, mainly performed in mice transplanted with parental tumours or in transgenic mice, have shown the effectiveness of anticancer vaccination models, which can potentially elicit a potent immune response and induce immune memory against tumour antigens in a young/adult immunocompetent host [6, 34].
It has been suggested that the increased incidence of cancer present in the elderly is related to age-associated changes occurring in the immune system, namely immunosenescence [35]. This phenomenon is best described as the remodelling of the immune system, which appears early on and progresses throughout a person’s life. Immunosenescence may not only have an impact on the incidence of cancer, but also on the effectiveness of preventive and therapeutic approaches based on immune system activation. Indeed, in preclinical models cancer vaccines have been shown to be less effective at older age than in young adults, implying that vaccines may not be very effective in predominantly elderly cancer patients. The low efficacy of cancer vaccines in old age may be attributable to different mechanisms, which may act at the different steps of the immunisation process, and whose exact influence still remains unclear.
This paper analyses the impact of immunosenescence on the effectiveness of cancer vaccines and summarises the studies on cancer immunisation in old age, and examines the potential mechanisms implicated in the lower effectiveness of anticancer immune-based approaches in advanced age.
Immunosenescence and anti-tumoural responses
As introduced above, the remodelling of the immune system, which occurs during ageing, referred to as immunosenescence may seriously interfere with successful cancer immunoprevention and immunotherapy [40]. The age-dependent reduction of naive T cells, the shift from a Th1 to a Th2 phenotype, the defect of antigen presentation by antigen presenting cells (APCs) to T lymphocytes, the alteration of components of the innate immunity with potential damage to the innate-adaptive interrelationships, may determine an age-associated disadvantage with a multi-step defect in which different cell populations involved in the activation of anticancer immunity are all affected. The well-known immunological changes occurring during ageing at the component level of innate or adaptive immunity have been widely described elsewhere. The new light shed on the integration of innate with specific immune effectors collected over the past few years emphasises a further point, which might have consequences for the preventive approaches in the elderly. In this respect, the signals which are produced by the components of the innate system required to direct the adaptive immune response may be insufficient or erroneous in aged individuals and might thus adversely influence the specific clonal adaptive response. We have shown that T lymphocytes bearing the γδ T cell receptor (TCR) are affected by ageing [1], and as has been suggested, are thought to contribute to the definition of αβ T cell responses towards T helper cell type 1 (Th1) or type 2 (Th2) phenotype. In our study, an age-dependent numerical and functional alteration of γδ T lymphocytes was found in elderly people and in centenarians, with a lower frequency of circulating γδ T cells, a higher percentage of γδ T cells producing TNF-α, and an impaired in vitro expansion of these cells [1]. The decrease in the γδ T cell number and the reduced in vitro expansion both affected the Vδ2 T cell population, whereas the Vδ1 T cell subset was unaffected by age. In a further study, we demonstrated that the numerical decrease of γδ T cells in old subjects was due to the reduction of immature naive and central memory cells bearing CD27 and CCR7 antigens [43]. In contrast, the proportion of mature effector/memory γδ T cells lacking CD27 or CCR7 markers was increased in old subjects in comparison with younger donors. This age-related alteration of γδ T cell maturation and differentiation pathways may have direct implications in the defective activation of adaptive immune responses both in anti-infective and anti-tumour immunity. This evidence is further emphasised by the fact that γδ T cells are involved in coordinating the interplay between innate and adaptive immunity, and in particular in guiding the establishment of acquired immunity, thus contributing to the definition of αβ T cell responses towards T helper cell type 1 (Th1) or type 2 (Th2) phenotype [29]. Various mechanisms may be involved in this effect of γδ T cells, and one of the most important seems to be related to the cross-talk of γδ T cells with dendritic cells (DCs), which induces differentiation of DCs, increasing their migratory activity, up-regulating the chemokine receptors, and triggering the Th1 immune response [5]. In recent years, based on these mechanisms, γδ T cells have been proposed to be directly involved in immune control of cancer and infections, and in vitro and in vivo activation of γδ T cells are emerging as a rewarding target for immunotherapeutic strategies [10, 26].
Another cell population which may represent a functional link between innate and adaptive systems is that of antigen presenting cells (APCs). Several functions of APCs have been found to be affected by the ageing process [7]. A lower expression of the mRNA for the migratory CCR7 chemokine receptor was found in immature APCs from old mice, and a lower lymphocyte cytotoxicity, and a reduced number of CD8+ T cells producing IFN-γ were induced by APCs from aged mice in comparison to APCs from young animals [7]. The fact that CCR7 was greatly increased in mature APCs from old mice, cultured with GM-CSF and IFN-γ, up to the levels found in young animals and that in vivo migration of mature APCs to regional lymph nodes was higher in old than in young mice, suggests that an increased migratory capacity of old APCs may be required to balance their reduced antigen presentation to cytotoxic lymphocytes.
In recent years, particular attention has been paid to T regulatory cells and myeloid-derived suppressor cells, as well on inflammatory cytokines, since the changes they undergo in the elderly, which are discussed below, might play a relevant role in the success of cancer vaccination in advanced age.
Cancer vaccines and their effectiveness in old age
With regard to the evidence in support of an analysis of the effectiveness of cancer vaccines in old age, we firstly conducted a study on the efficacy of interleukin-2 (IL-2)-engineered mammary tumour cells to induce an immune response capable of rejecting the tumour and to activate a specific immune memory in young and old mice [38]. Young and old mice were immunised with the syngeneic mammary adenocarcinoma cell line TS/A engineered to release IL-2 (TS/A-IL-2) and subsequently challenged with the parental TS/A cell line. While TS/A-IL-2 protected 90% of the young mice, it only protected 10% of the old animals. Whereas, the rejection of IL-2-transduced cells was attributed to the good infiltration of neutrophils and macrophages, the defect in memory acquisition correlated with a reduced representation of both CD4+ and CD8+ lymphocytes in the tumoural infiltrate in old mice [38]. The age-related decreased effectiveness in inducing memory against tumour cells was recently confirmed in another study in which a different experimental approach was used. Immunisation with DNA plasmids codifying HER-2/neu in old Balb/c mice demonstrated that the effectiveness of inducing protective immunity against a lethal challenge with syngeneic tumour cells overexpressing HER-2/neu was lower in old mice than in young animals [39]. The reduced number of tumour-free mice observed in old age in this study was associated with an age-related impairment of humoral and cell-mediated immune responses. In another experimental model, preventive vaccination of young and old mice with a DNA vaccine encoding Mage-b protected 90% of the young mice from metastases, while only 60% of the old mice remained metastasis free [18]. Similarly, in this case the lower effectiveness of immunisation in old mice was associated with the impairment of protective immune responses. Although further evidence in other experimental models will have to be provided, this knowledge suggests that the application of an anticancer vaccination in ageing may not be as effective as it is in young age because of the existence of age-related defects in the activation of specific immune responses hence, necessitating the development of specific approaches for the immunoprevention of cancer in advanced age. Even though defects in the number and functionality of the effectors of specific immune responses are well established, additive defects may be present in old age which may determine flaws in immunisation procedures.
The data reported to date on the lower effectiveness of cancer vaccines in older age raises the important question on the causes involved in this defect in light of the possibility of correcting and possibly restoring it. Apart from the well-known age-related numerical and functional alterations in the innate and adaptive immune systems [40], other changes may occur in the old subjects which may cause an increase in the incidence of tumours, may favour tumour growth, and may determine the lower effectiveness of vaccinations.
Recent findings from our laboratory show that the defect in immunisation observed in old age may be related, at least in part, to the impaired effectiveness in the first steps of the vaccination procedure, with the consequent reduced presentation of tumour antigens by antigen presenting cells. Indeed, studying immunisation in Balb/c mice with plasmid DNA encoding HER-2/neu, we observed that combining an intramuscular injection of DNA plasmid with in vivo electroporation, allowed us to recover the defect observed by immunising old mice with the intramuscular injection of the plasmid alone, since in the electroporated group 100% of both young and old mice were able to reject a subsequent challenge with syngeneic TUBO tumour cells overexpressing HER-2/neu, (unpublished results). Besides demonstrating that the lower efficacy of immunisation at old age is not an irreversible phenomenon, these data suggest that different factors may be involved in the age-related defect in memory acquisition after administration of the vaccine. We previously demonstrated that experimental approaches aimed at rejuvenating T cell compartment through grafting a young thymus or the adoptive transfer of young memory T lymphocytes did not confer tumour-specific immune memory in old mice [38]. These data show that T cells per se do not seem to be the only cause of immune memory deficiency in old age and other steps of the immunisation process, such as antigen internalisation and presentation by antigen presenting cells, might be involved [7]. Since electroporation determines a transient increase of permeability of the plasma membrane, it is possible that the complete protection obtained in old mice after immunisation with DNA plasmids using this technique was dependent on the better uptake of plasmids into antigen presenting cells. This evidence is supported by the fact that plasma membrane fluidity diminishes during ageing, that the defect is reversible and that its correction ameliorates various immune functions impaired in old age [37]. In another experimental model we documented an age-related reduced transgene expression after intramuscular injection of a recombinant E1-deleted human type 5 adenovirus encoding β-gal, confirming the possibility that in the initial immunisation steps defects exist in the aged, and that these defects may be due to reduced plasma membrane fluidity, and/or to a decreased number of transducible cells, and/or to an impaired transcriptional function of single antigen presenting cells from aged donors [30].
The relevant role of defects in the antigen presentation step in age-related impaired immunisation is further suggested by the studies of the Lustgarten group, which reported that dendritic-cell based vaccination plus rIL-2 protected 60% of the young mice from challenge with syngeneic TRAMP-C2 tumour cells (adenocarcinoma of the prostate), while only a minimal effect was observed in the old mice [46]. However, when co-administered with anti-OX40 or anti-4-1BB mAbs a vigorous anti-tumour response in both young (85–90%) and in old (70–75%) mice was observed. Furthermore, administration of a syngeneic pre-B lymphoma cells line (BM-185) engineered to express the co-stimulatory molecule CD80 together with agonists of the co-stimulatory molecule OX40, restored or improved the lower memory response against tumour cells, suggesting that deficiencies in long-lasting memory responses may be secondary to ineffective cross-presentation of tumour antigens by endogenous antigen presenting cells [28].
Most data on the preventive potential of vaccines have been drawn from studies performed in mice transplanted with parental tumours or in transgenic mice. Although transgenic models have been shown to reflect human cancer more adequately than syngeneic models, they are not generally considered useful for the study of vaccines in old age since the development of cancer occurs early in young age. Nevertheless, the possibility of preventing the development of spontaneous tumours through cancer vaccination in young age and of tracking mice ageing renders transgenic models appealing even for studies in advanced age. The FVB/neuNT mice overexpress the rat HER-2/neu oncogene under the control of the MMTV promoter. These mice develop spontaneous tumours in their mammary glands with histological and clinical progression of the disease closely resembling what is seen in breast cancer patients. It has been demonstrated that intramuscular immunisation with plasmid DNA encoding HER-2/neu protects 100% of FVB/neuNT transgenic mice from the spontaneous development of mammary carcinomas. However, this vaccine does not permanently protect treated animals: preliminary data obtained in our laboratory in FVB/neuNT mice show that the vaccine protects 100% of the mice until about 45 weeks of age, with a progressive reduction of the percentage of tumour-free animals as their age increases, and with less than 20% tumour-free mice at more than 90 weeks of age (unpublished results). Although the mechanisms involved in the tumour relapses in old age in most of the mice where vaccination hampered tumour development in young age remains to be demonstrated, two main considerations can be made: immunisation against HER-2/neu remains protective until the onset of immunosenescence, and/or the cellular and humoral immunity activated by HER-2/neu vaccination does not confer long-term protection and booster shots are required to induce immunity into old age. Although immunosenescence certainly plays an important role in the age-related defect in cancer vaccines, several data suggest that the long-term persistence of protective immunity after a cancer vaccination largely depends on the number of immunisations. The immunisation of Balb/c mice with a single injection of TS/A cell clones engineered with IL-2 gene and producing intermediate (3,600 U, clone B6.3600) or high (6,000 U, clone B4.6000) amounts of IL-2 protected 25 or 88% of the mice 1 month after a challenge with TS/A parental cells (unpublished results). When the mice with no tumours were challenged again with TS/A-pc 5 months after their last immunisation, tumours developed in all the mice. Immunisation of the mice with three successive injections of IL-2-gene engineered clones at 1-month intervals followed by a challenge with TS/A-parental cells protected 87 or 88% of mice challenged with B6.3600 and B4.6000, respectively, 1 month after an injection of TS/A-pc. Five months after their last immunisation, 43 or 28% of the mice immunised with B6.3600 or B4.6000 clones, respectively, survived. In another tumour model, a single course of immunisation with DNA plasmid coding for HER-2/neu was unable to prevent the development of mammary tumours in transgenic Balb/c mice for the expression of the HER-2/neu oncogene. By contrast, progressive clearance of neoplastic lesions and complete protection of all 1-year-old mice were achieved when Balb/c mice were repeatedly immunised at 10-week intervals [42]. It is clear from these data that repeated immunisations prolong the length of time in which vaccines are protective and their efficiency. Whether repeated immunisations overcome the defect arising from immunosenescence and endow protective immunity up to old age remains to be demonstrated.
Tumour-induced immunosuppression in advanced age
It still has to be clearly determined whether the increased incidence of tumours with age may primarily result from an increased number or accumulation of somatic mutation in cells and/or attenuated immunosurveillance leading to the development of tumours with age. In any case, it is well known that age-related alterations in immune effectors influence the ability of an aged subject to react against exogenous antigens and, in particular, reduce the capacity of anti-tumoural immune defences in the elderly. Besides this, other factors may contribute to triggering spontaneous tumour growth through avoiding detection by the immune system. In the last few years, it has become apparent that, in vivo, tumour cells have evolved multiple means of resisting attack from immune effector mechanisms. Either passive mechanisms, such as the lack of distinctive antigenic peptides or the adhesion and co-stimulatory molecules needed to elicit a primary T-cell response, or active mechanisms through which tumours can avoid or evade immune attack may be involved. Several mechanisms of active immune escape have been proposed to explain the incapacity of the immune response in rejecting tumours. It seems that these mechanisms of immune escape play an important role in the aged host even though the exact relevance of tumour-induced immunosuppression in the early phases of tumour development in the elderly remains to be proven. Some evidence suggests that at least some of the mechanisms used by cancer cells to escape immune clearance might be more effective in ageing.
The Fas ligand (FasL)/Fas receptor (FasR) interaction is a well-known mechanism in tumour immune escape. Malignant cells have been shown to escape immune recognition by developing resistance to Fas-mediated apoptosis and acquiring the expression of FasL which they may use for eliminating activated Fas+ lymphocytes. FasL is a key molecule in normal immune development, homeostasis, modulation, and function and acts by inducing apoptosis of sensitised cells through interaction with its own FasR receptor, expressed on their surface. To date, the expression of functional FasL has been reported in several distinct lineages of tumours [48]. Various studies have demonstrated significant increases in the FasR expression with age, either as percentages of T cells or as an intensity of mean fluorescence [9]. An increased FasR expression on aged leukocytes might facilitate the immune escape of tumours expressing FasL in elderly patients by promoting the apoptosis of tumour infiltrating leukocytes. The release of immunosuppressive cytokines by tumour cells represents another mechanism through which tumours evade immune rejection. Many tumours produce TGF-β, or IL-10, or other cytokines which tend to suppress inflammatory T-cell responses and cell-mediated immunity essential in controlling tumour growth and destroying tumour cells [40]. In old subjects, these suppressive cytokines released by tumour cells may synergise with immunosuppressive cytokines (TGF-β, IL-10, and others) which are already overproduced by leukocytes to elevated concentrations able to impair anti-tumour immune responses. The presence of prostaglandins is another factor which is involved in cancer-induced immune suppression. Tumour cells produce prostaglandins which can influence various immune functions and, in particular, may switch from Th1-type to Th2-type immune responses. The above examples lend weight to the idea that immune suppression induced by tumour cell-derived prostaglandins may have particular implications in ageing, since lymphocytes from elderly subjects are now known to be sensitive to inhibition by prostaglandins in comparison with lymphocytes from younger individuals [17]. Another molecule that has been reported to be involved in tumour immune escape is indoleamine-2,3-dioxygenase (IDO), an enzyme which catalyses the initial and rate-limiting step in the catabolism of tryptophan along the kynurenine pathway and which is endowed with immunosuppressive activity [51]. IDO activity was found to increase with older age in a group of healthy subjects aged 34–93 years [14]. In a study recently conducted in 284 nonagenarians and 309 controls, IDO activity was significantly higher in nonagenarian than in young subjects, and at enrollment predicted subsequent mortality in nonagenarians, suggesting that increased IDO activity might be a mechanism involved in the decline of T cell responses in immunosenescence [36].
Active suppression of tumour-specific T lymphocytes by immune cells
The induction of antigen-specific unresponsiveness is one of the mechanisms by which tumour cells evade the immune system. Two main cell populations have been implicated in the induction of T cell tolerance: T regulatory cells (Treg) and myeloid-derived suppressor cells (MSCs). Treg cells, which represent a CD4+ T cell population characterised by the expression of the forkhead/winged helix transcription factor (Foxp3), play an important role in the control of immune reactivity against self-antigens and non self-antigens. In humans, Treg cells have been found at higher frequencies in the peripheral blood of cancer patients and in several types of tumours, and may induce peripheral ignorance of tumour cells [52]. In vivo, depletion of Treg cells resulted in the suppression of tumour growth in various tumour models [25]. MSCs represent another leukocyte population endowed with suppressive activity towards T cells; MSCs have a CD11b+GR1+ phenotype and comprise different myeloid cells at various stages of differentiation, including granulocytes, monocytes, and a pool of immature cells of myelomonocytic lineage [16]. MCSs are generated in large numbers in patients with various types of cancers and have been shown to inhibit antigen-specific T cell responses in these patients.
Although accumulating evidence suggests that Tregs and MCSs play an important role in tumour-mediated suppression, the role of these populations in the incidence and progression of cancer in elderly subjects has yet to be elucidated. The first paper to be published on the subject reports that the number of CD4+CD25+ T regulatory cells in peripheral blood progressively decreases with the increasing age of C57BL/6 mice [31]. More recently, another study conducted in the same mouse strain revealed that CD4+CD25+ T cells from the spleen of aged mice were functionally comparable in their suppressive activity to those in young mice, albeit slightly increased in number [33]. Moreover, functional changes to whole CD4+CD25− T cells were found in aged mice, the majority of these cells being hyporesponsive and present, inside this population, a Foxp3-positive population with suppressive activity, which does not seem to be present at young age. Another recent paper documents that old mice contain twice the amounts of CD4+CD25+Foxp3+ and CD8+CD25+Foxp3+ populations in spleen and lymph nodes when compared to spleen and lymp nodes from young mice [47]. The accumulation of these cells in old humans was able to inhibit the activation of immune responses, as demonstrated by the capacity of old mice to reject immunogenic tumour cells and to restore anti-tumour T cell cytotoxicity after depletion of CD25+ cells with anti-CD25 mAb [47]. In humans, the number of Treg cells, present as either CD4+CD25+ cells [20] or Foxp3+CD4+ cells [27], was found to be increased with age. Besides naturally occurring CD4+ T regulatory cells, other subsets of adaptive CD4+ Treg cells induced in the periphery have been described. Based on secreted cytokines at least two different groups may be distinguished: Tr1 cells, whose function relies on secreted IL-10 [22], and Th3 cells, which mainly produce TGFβ [15]. Both populations exert a suppressor function on T cells and can develop from conventional CD4+ T cells when exposed to specific stimulatory conditions such as the blockade of co-stimulatory signals, deactivating cytokines or different drugs [2]. Studies will be needed to clarify the role of these important regulatory populations in ageing. With regard to myeloid-derived suppressor cells, a recent paper reports that these cells, along with a CD11b+Gr1+ phenotype, accumulate in the spleen and in the tumours of old BXD12 mice previously challenged with TS/A mammary adenocarcinoma cells [21]. Interestingly, the increase of these cells correlated with a decline of T cell tumour cytotoxicity. The depletion of CD11b+Gr1+ cells in aged mice led to a slower growth of the tumour, while adoptive transfer of CD11b+Gr1+ cells from old mice resulted in an increase in susceptibility to tumour growth in young mice, thus demonstrating the immunosuppressive role played by this cell population in old age. Further studies will be required in order to better understand the role of both populations of suppressor cells in tumour tolerance in advanced age, as well as the interrelationships involved between Treg cells and MCSs in mediating suppression. Another mechanism of active suppression of immune cells which might be involved in tumour escape is represented by the PD-1/PD-L co-stimulatory pathway. Programmed cell death-1 (PD-1) is an immune inhibitory receptor belonging to the CD28/B7 family of co-stimulatory molecules, which is expressed on activated T cells, B cells, and myeloid cells [19]. PD-1 binds to two ligands, PD-L1 and PD-L2, which are expressed on immune cells and a variety of non-hematopoietic cell types. Engagement of PD-1 by its ligands has been shown to inhibit T cell proliferation and IL-2 production by arresting the cell cycle arrest in the GO/G1 phase [4]. Although the expression of PD-1 on CD4+ and CD8+ T cells does not change with age at the RNA or protein level, PD-1 seems to represent a good candidate for the regulation of the inhibition and fine tuning of T cell responses in ageing and further investigation of its function may provide to be of great therapeutic value in boosting anti-tumour immunity [24].
Inflammation, cancer and ageing
Inflammation is a response to acute tissue damage, whether resulting from physical injury, ischaemic injury, infection, exposure to toxins, or other types of trauma. In recent years, clinical and experimental studies have revealed that chronic inflammation predisposes to some forms of cancer and that the use of non-steroidal anti-inflammatory drugs (NSAIDS) may be associated with protection against various tumours [45]. The most thoroughly studied examples of the association between inflammation and cancer are the relationships between chronic inflammatory bowel disease and the increased risk of colorectal cancer, chronic gastritis resulting from Helicobacter pylori infection and gastric adenocarcinoma, and chronic hepatitis and liver cancer. It has been widely demonstrated in the last few years that ageing is often characterised by low-grade inflammation [12]. Altered cytokine profiles due to ageing of the innate immune system and/or of non-immune cell types, are hypothesised to contribute to age-related changes in the structure and function of tissues, pathophysiological changes, and the development of chronic diseases of ageing. Inflammatory cytokines and other mediators of inflammation can also serve as strong near-term predictors of mortality associated with age-related chronic diseases [23]. Chronic inflammation causes the release of a plethora of agents, such as cytokines, prostaglandins, chemotactic factors, reactive oxygen and nitrogen species. It also determines changes in gene expression which favour the activation of oncogenes and down-regulation of tumour suppression genes [32]. These factors also change the responses of cells to apoptosis signals and up-regulate angiogenesis factors as well as factors favouring the growth of tumour cells. Moreover, some of the same factors cause impairments in immune surveillance, which facilitates the escape of tumour cells from surveillance and their clonal expansion [49]. In this respect, very recent studies have suggested the role of inflammatory cytokines in the protection of cancer stem cells. One hypothesis propounded to explain the lack of treatment efficacy and tumour relapse is the presence of cancer stem cells in the tumour itself. Recent advances in the study of tumour biology have led to the identification of a sub-lineage of cells retaining key stem cell properties [50]. These properties include self-renewal, which drives tumourigenesis, and differentiation, albeit aberrant, which contributes to cellular heterogeneity. By virtue of their very nature, cancer stem cells represent an unlimited tumour reservoir and are resistant to treatment therapies directed against primary tumour cells. Important efforts are being made to identify oncogenic alterations which could drive the initiation and maintenance of this cell subpopulation. These alterations can be produced at a genetic or epigenetic level and may affect different cellular functions. The biology of tumour stem cells has been shown to be strictly affected by the pro-inflammatory milieu of the tumour: recent evidence shows that inflammatory cytokines, such as interleukin-6 (IL-6), play primary roles in the pathogenesis of breast cancer by sustaining the survival and proliferative capacity of tumour stem cells [44]. Another cytokine which was very recently discovered to play a crucial role in the survival of cancer stem cells, and in particular of stem cells from colon carcinoma, is interleukin-4 (IL-4) [13]. Since the up-regulation of the inflammatory response is a major characteristic of the remodelling process of the immune system during ageing, further research is needed to evaluate the possibility that the “aged” microenvironment may constitute a preferential niche for the survival of cancer stem cells. The inflammatory microenvironment present in old age may be further involved in another mechanism of evasion of tumour cells from immunosurveillance. It has been reported that inflammatory cytokines enhance the accumulation of myeloid-derived suppressor cells which inhibit tumour immunity and accelerate tumour progression thereby supporting the hypothesis that the induction of suppressor cells which down-regulate tumour immunity is one of the mechanisms linking inflammation and cancer [3]. The dysregulation of pro-inflammatory cytokine production seems to be mainly due to an intrinsic alteration present in cells from aged individuals. At least in mice, hematopoietic stem cells from old animals differentiate in vivo in CD4+ T cells producing IL-4 levels characteristic of old age, even when these stem cells are injected in a young host [8]. Thus cells from animals in old age retain their capacity to produce higher levels of inflammatory cytokines independently of the microenvironment in which they proliferate and differentiate.
Concluding remarks and future prospects
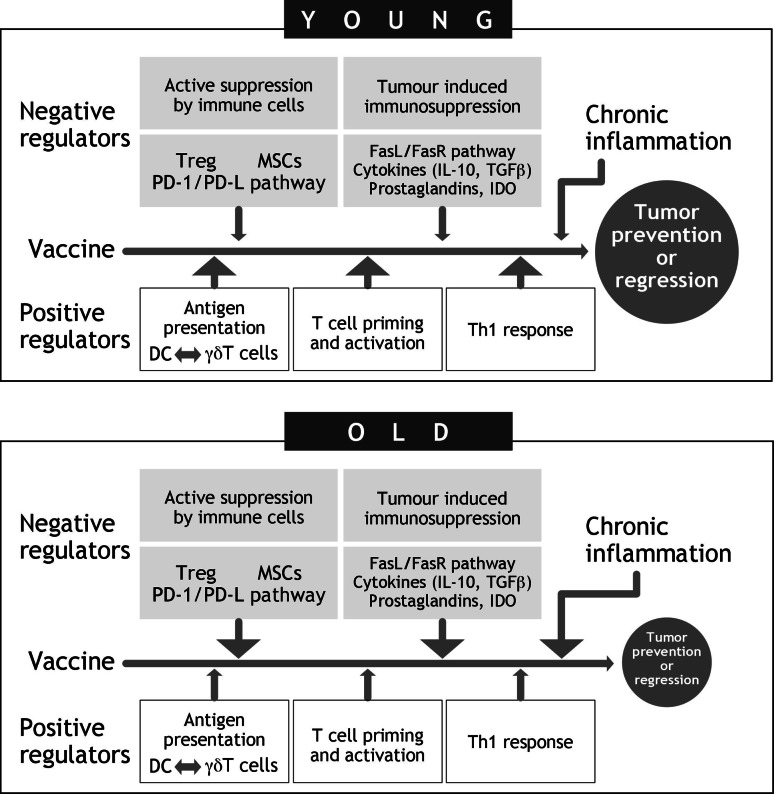
The alteration of both innate and adaptive immune responses which occur during ageing, the phenomenon of immunosenescence, may have an impact on the incidence of cancer and on the effectiveness of preventive and therapeutic approaches based on immune system activation. Cancer vaccines have been shown to be less effective in older age than in young adults, implying that vaccines may not be very effective in cancer patients who are for the most part elderly. The imbalance between positive and negative regulators of immune responsiveness may be involved in the poorer effectiveness of anticancer immune-based approaches in advanced age (Fig. 1). Various mechanisms, which may act at the different steps of immunisation, from antigen presentation to activation and maintenance of protective tumour-specific immune responses, may be involved and may be particularly relevant in the aged host. These include tumour-induced immunosuppression, suppression of tumour-specific responses by subsets of immune cells and the effect of chronic inflammation on the tumour microenvironment. Experimental approaches able to correct some of these mechanisms have been reported, increasing the effectiveness of immune-based preventive and therapeutic strategies. The defect of antigen presentation by APCs present in old age has been overcome: (a) by inducing APCs to express co-stimulatory molecules which render APCs effective in self-presentation, and (b) by optimising the immunisation approach, using for example electroporation to increase plasmid uptake by APCs. The lower activation of memory T cells in old age may be corrected if different co-stimulatory signals are utilised or increased in intensity. Another approach may be related to the route of immunisation, since long-term persistence of protective immunity after a cancer vaccination has been shown to depend on the number of immunisations. The correction of the age-related numerical and functional alteration of Treg and myeloid-derived suppressor cells has been shown to restore the anti-tumour immune effectiveness. Other, as yet, unexplored approaches may enhance the efficacy of vaccines in inhibiting immunosuppressive factors produced by tumour cells, such as inflammatory cytokines, prostaglandins, PD1-ligand, or IDO, or in recovering the age-related alteration of γδ Τ cells.
Fig. 1.
Positive and negative regulators of immune responsiveness which may condition the poorer effectiveness of anticancer immune-based approaches in advanced age
Greater insight into the mechanisms underlying immunosenescence, not only at a cellular, but also at a molecular level, will be indispensable for the future of tumour vaccines for old subjects. Adopting “old”-tailored procedures of immunisation for conferring both adequate antigen presentation and long-term activation of protective immune responses seems to represent an indispensable requisite for the planning of specific immunopreventive and immunotherapeutic anticancer approaches for the elderly. However, it is to be regarded as unfortunate that the enormous effort required to accomplish this will almost certainly be offset by the fact that cancer vaccines which can be applied in both prevention and therapy are potentially less toxic than chemotherapy or radiation and could be especially suitable for older more frail cancer patients. The way forward requires devising effective tumour vaccines for the old which should include: (a) improved delivery systems for a better expression of the vaccine; (b) greater knowledge of the factors which exert suppression of immune-surveillance and which are up-regulated in old, and, in particular, T and myeloid suppressor cells, and inflammatory cytokines, the latter being implicated in the survival of cancer stem cells.
Consequently, as cancer predominantly afflicts the old and any adjuvant approaches which yielded promising results in the young but generally failed to do so in old individuals subjects, the time has come to usher in a new era of immune-based anticancer research and clinical trials primarily in the old to design effective cancer vaccines.
Footnotes
This article is part of the symposium in writing on “Impact of ageing on cancer immunity and immunotherapy”.
References
- 1.Argentati K, Re F, Donnini A, Tucci MG, Franceschi C, Bartozzi B, Bernardini G, Provinciali M. Numerical and functional alterations of circulating γδ T lymphocytes in aged people and centenarians. J Leukoc Biol. 2002;72:65. [PubMed] [Google Scholar]
- 2.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T-cells. Nat Rev Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 3.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4+ and CD8+ T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Casetti R, Martino A. The plasticity of γδ T cells: innate immunity, antigen presentation and new immunotherapy. Cell Mol Immunol. 2008;5:161. doi: 10.1038/cmi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ, Colombo MP, Forni G. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 7.Donnini A, Argentati K, Mancini R, Smorlesi A, Bartozzi B, Bernardini G, Provinciali M. Phenotype, antigen-presenting capacity, and migration of antigen-presenting cells in young and old age. Exp Gerontol. 2002;37:1097. doi: 10.1016/S0531-5565(02)00087-6. [DOI] [PubMed] [Google Scholar]
- 8.Donnini A, Re F, Orlando F, Provinciali M. Intrinsic and microenvironmental defects are involved in the age-related changes of Lin−c-kit+ hematopoietic progenitor cells. Rejuvenation Res. 2007;10:459. doi: 10.1089/rej.2006.0524. [DOI] [PubMed] [Google Scholar]
- 9.Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P. Shortage of circulating naive CD8+ T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860. [PubMed] [Google Scholar]
- 10.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human γδ T cells: a nonreduntant system in the immune-surveillance against cancer. Trends Immunol. 2002;23:14. doi: 10.1016/S1471-4906(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 11.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Immunol. 2003;3:630. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 13.Francipane MG, Perez Alea M, Lombardo Y, Todaro M, Medema JP, Stassi G. Crucial role of interleukin-4 in the survival of colon cancer stem cells. Cancer Res. 2008;68:4022. doi: 10.1158/0008-5472.CAN-07-6874. [DOI] [PubMed] [Google Scholar]
- 14.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factorbeta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosoppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin JS, Messner RP. Sensitivity of lymphocytes to prostaglandin E2 increases in subjects over age 70. J Clin Invest. 1979;64:434. doi: 10.1172/JCI109480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravekamp C. Cancer vaccines in old age. Exp Gerontol. 2007;42:441. doi: 10.1016/j.exger.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 20.Gregg R, Smith CM, Clark FJ, Dunnion D, Khan N, Chakravertry R, Nayak L, Moss PA. The number of human peripheral blood CD4+CD25high regulatory T cells increases with age. Clin Exp Immunol. 2005;140:540. doi: 10.1111/j.1365-2249.2005.02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev. 2007;128:672. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 23.Haugen E, Li-Ming G, Isic A, Skommevik T, Fu M. Increased interleukin-6 but not tumour factor necrosis-alpha predicts mortality in the population of elderly heart failure patients. Exp Clin Cardiol. 2008;13:19. [PMC free article] [PubMed] [Google Scholar]
- 24.Henson SM, Macaulay R, Kiani-Alikhan S, Akbar AN. The use of the inhibitory receptors for modulating the immune responses. Curr Pharm Des. 2008;14:2643. doi: 10.2174/138161208786264124. [DOI] [PubMed] [Google Scholar]
- 25.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 26.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 27.Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lustgarten J, Dominguez AL, Thoman M. Aged mice develop protective antitumor responses with appropriate costimulation. J Immunol. 2004;173:4510. doi: 10.4049/jimmunol.173.7.4510. [DOI] [PubMed] [Google Scholar]
- 29.Mak TW, Ferrick DA. The γδ T cell bridge: linking innate and acquired immunity. Nat Med. 1998;4:764. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 30.Massari I, Donnini A, Argentati K, Straino S, Mangoni A, Gaetano C, Viticchi C, Capogrossi MC, Provinciali M. Age-dependent effects of repeated immunization with a first generation adenovirus vector on the immune response and transgene expression in young and old rats. Exp Gerontol. 2002;37:823. doi: 10.1016/S0531-5565(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. PNAS. 2002;99:8832. doi: 10.1073/pnas.132254399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mumm JB, Oft M. Cytokine-based transformation of immune surveillance into tumor-promoting inflammation. Oncogene. 2008;27:5913. doi: 10.1038/onc.2008.275. [DOI] [PubMed] [Google Scholar]
- 33.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25−Foxp3* T cells in aged mice. J Immunol. 2006;176:6586. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 34.Oshikawa K, Shi F, Rakhmilevich AL, Sondel PM, Mahvi DM, Yang NS. Synergistic inhibition of tumor growth in a murine mammary adenocarcinoma model by combinational gene therapy using IL-12, pro-IL 18, and IL-1 beta converting enzyme cDNA. Proc Natl Acad Sci. 1999;96:13351. doi: 10.1073/pnas.96.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawelec G, Solana R. Immunosenescence. Trends Immunol. 1997;11:514. doi: 10.1016/s0167-5699(97)01145-6. [DOI] [PubMed] [Google Scholar]
- 36.Pertovaara M, Raitala A, Lehtimaki T, Karhunen PJ, Oja SS, Jylha M, Hervonen A, Hurme M. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Provinciali M, Fabris N, Pieri C. Improvement of natural killer cell activity by in vitro active lipids (AL 721) administration in old mice. Mech Ageing Dev. 1990;52:245. doi: 10.1016/0047-6374(90)90128-3. [DOI] [PubMed] [Google Scholar]
- 38.Provinciali M, Argentati K, Tibaldi A. Efficacy of cancer gene therapy in aging: adenocarcinoma cells engineered to release IL-2 are rejected but do not induce tumor specific immune memory in old mice. Gene Ther. 2000;7:624. doi: 10.1038/sj.gt.3301131. [DOI] [PubMed] [Google Scholar]
- 39.Provinciali M, Smorlesi A, Donnini A, Bartozzi B, Amici A. Low effectiveness of DNA vaccination against HER-2/neu in ageing. Vaccine. 2003;21:843. doi: 10.1016/S0264-410X(02)00530-3. [DOI] [PubMed] [Google Scholar]
- 40.Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in aging. Cancer Immunol Immunother. 2005;54:93. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Provinciali M, Donnini A, Smorlesi A, Gatti C (2008) Breast cancer and immunosenescence. In: Fulop T, Franceschi C, Hirokawa K, Pawelec G (eds) Immunosenescence handbook. Springer, Berlin (in press)
- 42.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa S, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musini P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammari carcinomas in Her-2/neu transgenic mice. Cancer Res. 2004;64:2858. doi: 10.1158/0008-5472.CAN-03-2962. [DOI] [PubMed] [Google Scholar]
- 43.Re F, Poccia F, Donnini A, Bartozzi B, Bernardini G, Provinciali M. Skewed representation of functionally distinct populations of Vγ9 Vδ2 T lymphocytes in aging. Exp Gerontol. 2005;40:59. doi: 10.1016/j.exger.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Sansone P, Storci G, Tavolati S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seth R-N. Why cancer and inflammation? Yale J Biol Med. 2006;79:123. [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma S, Dominguez A, Lustgarten J. Aging affect the anti-tumor potential of dendritic cell vaccination, but it can be overcome by co-stimulation with anti-OX40 or anti-4-1BB. Exp Gerontol. 2006;41:78–84. doi: 10.1016/j.exger.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Dominguez AL, Justgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- 48.Walker PR, Saas P, Dietrich P-Y. Role of Fas ligand (CD95L) in immune escape. The tumor cell strikes back. J Immunol. 1997;158:4521. [PubMed] [Google Scholar]
- 49.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea-a paradigm shift. Cancer Res. 2006;66:1883. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 51.Zamanakou M, Germenis AE, Karanikas V. Tumor immune escape mediated by indole amine 2,3-dioxygenase. Immunol Lett. 2007;111:69. doi: 10.1016/j.imlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Zou W. Regulatory T cells, tumor immunity and immunotherapy. Nat Rev Immunol. 2006;6:295. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]