Abstract
Immunostimulatory monoclonal antibodies are immunoglobulins directed toward surface proteins of immune system cells that augment the immune response against cancer in a novel therapeutic fashion. Exogenous administration of the recombinant humanized immunoglobulins is being tested in clinical trials with agents of this kind directed at a variety of immune-controlling molecular targets. In this study, the encapsulation of antibody-producing hybridoma cells was tested in comparison with the systemic administration of monoclonal antibodies. Hybridomas producing anti-CD137 and anti-OX40 mAb were encapsulated in alginate to generate microcapsules containing viable cells that secrete antibody. Immobilized cells in vitro were able to release the rat immunoglobulin produced by the hybridomas into the supernatant. Microcapsules were implanted by injection into the subcutaneous tissue of mice and thereby provided a platform for viable secreting cells, which lasted for more than 1 week. The pharmacokinetic profile of the rat monoclonal antibodies following microcapsule implantation was similar to that attained following an intraperitoneal administration of the purified antibodies. The rat–mouse hybridoma cells did not engraft as tumors in immunocompetent mice, while they lethally xenografted in immunodeficient mice, if not microencapsulated. The antitumor therapeutic activity of the strategy was studied on established CT26 colon carcinomas resulting in complete tumor eradication in an elevated fraction of cases and strong tumor-specific CTL responses with either anti-CD137 or anti-OX40 producing hybridomas, thus offering proof of the concept. This form of administration permitted combinations of more than one immunostimulatory monoclonal antibody to exploit the synergistic effects such as those known to be displayed by anti-CD137 and anti-OX40 mAb.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0888-z) contains supplementary material, which is available to authorized users.
Keywords: CD137, OX40, Encapsulated cell therapy
Introduction
Immunostimulatory monoclonal antibodies define a new category of agents in the treatment of cancer and chronic viral infections [1, 2]. The interplay of these antibodies with proteins of immune system cells upregulates or shapes the immune response to attain an overall therapeutic benefit. Some of these monoclonal antibodies tamper with receptors involved in immune inhibition [3] and some overstimulate receptors that enhance the intensity of the cellular immune response [1]. The preclinical efficacy of such treatments in a number of mouse models is unprecedented for immunotherapy.
A number of these antibodies have entered clinical testing in cancer patients [1]. Formulations of the purified proteins are delivered by intravenous infusions. Fully human immunoglobulin moieties are employed to avoid neutralizing antibody responses from the host and other complications. The pharmacokinetics of these agents generally involves half-lives of several weeks, while their on-target safety profile is frequently compromised by autoimmune inflammation [4, 5] or systemic inflammatory events [6, 7]. In many instances, combinations of various monoclonal antibodies show synergistic antitumor effects in animal models of cancer [8–10]. Nonetheless, such combinations will be of limited use in clinical trials until individual agents obtain approval for at least one indication, either as single agents or in combination with standard therapies. Regulatory and commercial concerns therefore make translational research difficult with combinations of these investigational products [11].
CD137 (4-1BB) and OX40 (CD134) are members of the TNF receptor family that provide costimulatory stimuli to T lymphocytes [12, 13]. Agonist antibodies directed at both moieties have been shown to enhance immune response to such an extent that established tumors in mice have been eradicated [14, 15]. Strategies targeting artificial costimulation with mAbs to both receptors for cancer have been currently undergoing clinical trials. Importantly, very powerful immune and therapeutic effects have been described on combined treatment with both mAb in rodents [10, 16, 17].
Encapsulation of cells is a promising field in cell therapy and it has been used in numerous types of diseases [18]. In addition, some clinical trials have been carried out or are being conducted at this moment based on cell microencapsulation technology [19, 20]. The semi-permeable membrane that surrounds the immobilized cells avoids immune rejection while at the same time allowing the diffusion of nutrients, oxygen, waste and therapeutic products [21]. By enclosing cells in semi-permeable membranes, the chronic administration of immunosuppressive drugs could be reduced or even avoided [22]. Another important advantage of this technology is that it allows a sustained and controlled delivery of the ‘de novo’ produced therapeutic product and thereby helps to reduce the number of shots required.
In this study, hybridoma cells secreting immunostimulatory antibodies were microencapsulated and implanted under the skin of mice. As a result, functional immunostimulatory anti-CD137 and anti-OX40 mAb circulated in the blood stream and gave rise to immune-mediated tumor rejections. This living “drug delivery technology” also permits the combined use of more than one antibody and can be refined so that safe antibody-producing cells are engineered.
Materials and methods
Mice and in vivo tumor experiments
Female BALB/c wild-type (WT) mice (5–6 weeks old) were purchased from Harlan Laboratories. Rag-1−/− BALB/c mice were obtained from the Jackson Laboratory and bred in our animal facility under specific pathogen-free conditions. For tumor implantation, mice were subcutaneously injected with CT26 cells in the right flank. Tumor growth was monitored by measuring two perpendicular diameters with a digital calliper every 2–4 days. All animal procedures were conducted under institutional guidelines (study approval #3/2007) that comply with national and local government laws and policies.
Cell culture
The murine colon carcinoma cell line CT26 (H-2d) was cultured in complete medium (RPMI 1640 + Glutamax medium supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin and 50 μmol/L 2-mercaptoethanol). Hybridoma cell lines 2A (kindly provided by Dr. Lieping Chen, Baltimore, USA) [23] and OX86 [24] (kindly provided by Dr. Mario Colombo, Milano, Italy) were cultivated in suspension in complete medium supplemented with 1% BM Condimed. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. Tumor cells were verified for identity by ATCC and cultures were periodically tested for lack of mycoplasma infection. All the components of the culture medium were purchased from Gibco BRL (Invitrogen S.A., Spain) except BM Condimed, which was obtained from Roche diagnostics (Indianapolis, USA).
Microcapsule elaboration
Alginate-poly-l-lysine-alginate (APA) microcapsules were elaborated using an electrostatic droplet generator following the procedure described by Lim and Sun with some modifications [25]. Briefly, cells (3 × 106 cells/mL) were suspended in 2% (w/v) low-viscosity high-guluronic acid alginate (FMC Biopolymer, Norway) and the cell–gel suspension was extruded into 55 mM anhydrous calcium solution. Then, the microbeads were suspended in 0.05% (w/v) poly-l-lysine (MW 15,000–30,000; Sigma, St. Louis, MO, USA) for 5 min and coated again with another layer of 0.1% (w/v) alginate for another 5 min. Subsequently, they were maintained under normal culture conditions. A detailed morphological characterization was carried out before implantation using an inverted optical microscope (Nikon TMS) equipped with a camera (Sony CCD-Iris) [26].
Microcapsule implantation
Before implantation, capsules were washed several times in PBS (Gibco BRL, Invitrogen S.A., Spain). For transplantation, animals were anesthetized by ketamine–xylazine solution. A total volume of 0.5 mL of cell-loaded microcapsules (about 5,000 capsules and 300 cells per capsule, approximately) suspended in PBS solution was implanted subcutaneously using an 18-gauge catheter (Nipro Europe N.V., Belgium). On recovery, animals had access to food and water ad libitum. No immunosuppressant protocol was applied to the animals during the study. The control group received 0.5 mL of 1H11.6 hybridoma.
Viability of the enclosed cells
The viability of encapsulated cells was measured using a hemocytometer and trypan blue exclusion system. As much as 50 μL of capsules were soaked in D-PBS containing alginate lyase. When capsules were completely degraded, the released cells were centrifuged at 1,000 rpm for 10 min, the alginate lyase solution was aspirated and the cell pellet was resuspended in PBS. Then, these cells were mixed with trypan blue and counted using a hemocytometer. Viable cells appeared round and clear, and non-viable cells asymmetrical and absorbed the dye, therefore appearing blue.
Determination of the metabolic activity of the enclosed cells
Metabolic activity was determined by tetrazolium assay. Briefly, 25 μl of 5 mg/ml solution of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO) in PBS was added to approximately 25 microcapsules placed in a 96-well cell culture cluster and incubated at 37°C for 4 h. Then, the MTT solution was removed by vacuum aspiration and 100 μl of dimethylsulfoxide was added. The purple solution was read 5 min later on a microplate reader (Titerteck Multiscan Lab Systems) at 560 nm test wavelength with reference at 690 nm.
Antibody detection in vitro and in vivo
Antibody production by encapsulated cells in media and in blood samples was measured by ELISA assay. 0X86 hybridoma production was measured with Rat IgG1 Elisa Quantitation Kit (Bethyl Laboratories Inc., Montgomery, USA) and 2A production with rat IgG2a Elisa Quantitation Kit (Bethyl Laboratories Inc., Montgomery, USA). To obtain antibody production in vitro, 100 μl of microcapsules were placed in a 24-well cell culture cluster with 1 mL of culture medium and maintained for 24 h. Afterward, the media were collected and samples assayed for antibody content.
Histologic analyses
At days 7 and 14 after implantation, animals were killed, and microcapsules were retrieved and fixed in a 4% paraformaldehyde solution in 0.1 M sodium phosphate of pH 7.2. Serial horizontal sections (14 μm) of paraffin-embedded tissue were processed for hematoxylin–eosin staining.
Enzyme-linked immunospot assays
T cells producing interferon-γ (IFNγ) were counted by Enzyme-linked immunospot (ELISPOT) assays using a kit from BD-Biosciences (San Diego, CA, USA), according to the manufacturer’s instructions. Briefly, plates (Multiscreen HTS; Millipore, Bedford, MA, USA) were coated overnight with anti-IFNγ antibody (clone R4-6A2), washed with phosphate-buffered saline (PBS) and blocked for 2 h with RPMI containing 10% fetal calf serum. Then, 5 × 105 splenocytes were cultured in triplicate in the absence or in the presence of AH1 peptide (1 μg/ml). After 36 h, plates were washed with PBS and incubated with biotinylated anti-IFNγ antibody (clone XMG 1.2). After 2 h, plates were washed and incubated with a 1/100 dilution of streptavidin-phosphatase alkaline (Pharmingen). After 2 h, plates were washed and developed with freshly prepared BCIP/NBT (Sigma) solution. The reaction was stopped with distilled water and spots were counted using an automated ELISPOT reader (CTL; Aalen, Germany).
In vivo killing assay
Splenocytes from naïve Balb/c mice were divided into two samples. One sample was pulsed with 10 μg/ml of the AH1 peptide [27, 28] or 30 min at 37°C in 5% CO2, washed extensively and subsequently labeled with a high concentration of 1.25 μM of CFSE (Sigma). The non-pulsed control sample was labeled with a low concentration of 0.125 μM of CFSE. Then, both CFSEhigh- and CFSElow-labeled cells were mixed in a 1:1 ratio and injected i.v. (5 × 106 cells of each population) into tumor-bearing mice [29]. At 24 h after transfer, spleens were harvested and specific cytotoxicity was analyzed by flow cytometry. Specific cytotoxicity was calculated as follows: 100 − [100 × (%CFSEhigh tumor-bearing mice/%CFSElow tumor-bearing mice)/(%CFSEhigh naïve mice/%CFSElow naïve mice)].
Results
Hybridoma cells producing monoclonal antibodies can be readily microencapsulated in APA microcapsules
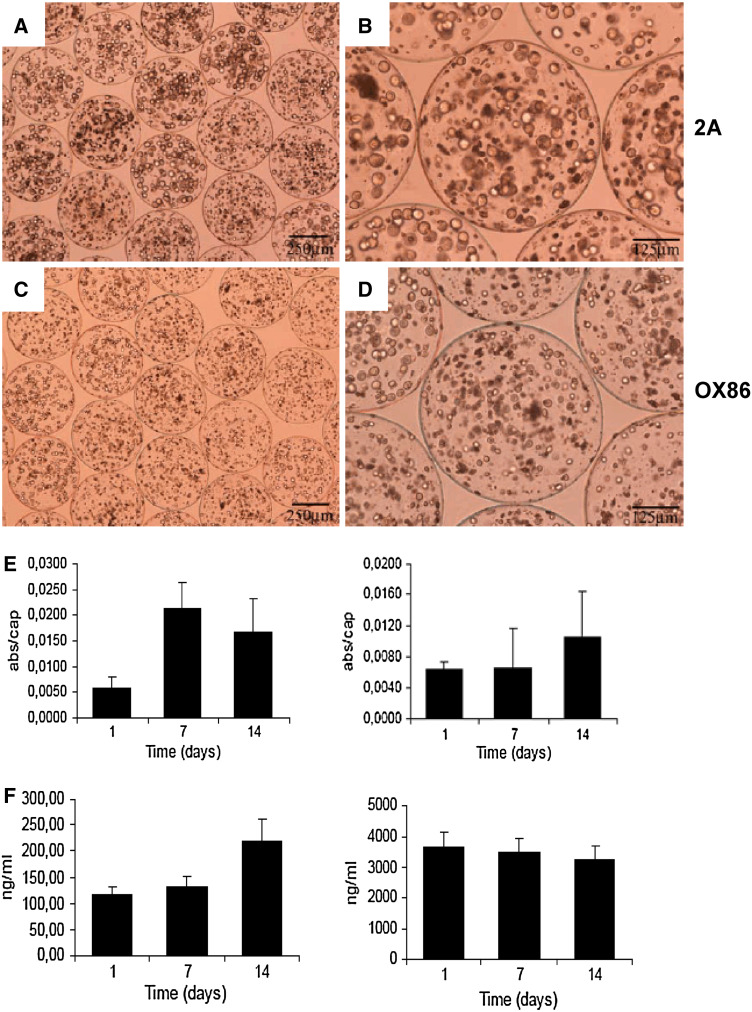
Cultured hybridoma cells producing rat monoclonal antibodies, anti-murine OX40 (clone OX86, rat IgG1) and CD137 (clone 2A, rat IgG2a) were encapsulated in spherical microcapsules of approximately 450–500 μm in diameter. Viable encapsulated cells at a density of 3 × 106 cells/ml could be visualized by inverted optical microscopy as shown in Fig. 1a–d. All microcapsules showed a nearly spherical and uniform shape with a small dispersion in diameter (0X86 489 ± 13 μm, 2A: 481 ± 15 μm). Cells were metabolically active for at least 2 weeks in culture (Fig. 1e). Cell viability, as measured by trypan blue exclusion assay, was 85%. Metabolic activity of the enclosed 41BB-2A cells increased during the first week, while it decreased slightly in the second week of the in vitro study. When 0X40 producing OX86 cells were enclosed, metabolic activity was maintained during the first week but increased only slightly in the second week. These data demonstrate the suitable adaptation of the cells to the microcapsule microenvironment.
Fig. 1.
Viable hybridoma cells producing immunostimulatory mAbs can be encapsulated in alginate-poly-l-lysine-alginate microcapsules and continue secreting antibody. a–d Inverted optical microscopy pictures showing encapsulated hybridomas 2A (anti-CD137) and OX86 (anti-OX40) as indicated at different magnifications of representative culture fields. e Metabolic activity by MTT assays on the microbead cultures from 2A (left) and OX86 (right) hybridomas on the indicated days following microencapsulation. f Concentration of immunoglobulin as assessed by ELISA in the culture supernatants from microcapsules containing 2A (left) and OX86 (right) that were replenished every 2 days
Microencapsulated cells delivered monoclonal antibody into the culture supernatant. Interestingly, the hybridoma cells producing anti-CD137 mAb secreted into the culture medium around 20-fold less antibody than anti-OX40-producing cells according to ELISA determinations of rat IgG1 and IgG2 in the respective culture supernatants, as assessed during a time course over a 2-week period (Fig. 1f). The media were renewed and replenished every 2–3 days and therefore graphs in Fig. 1f show sustained production of antibodies from the cell-loaded microcapsules. It is of note that over time, a minority of the proliferating cells contained in the microbeads passed through the microsphere coating and into the surrounding culture (data not shown).
Mice implanted with microcapsules containing hybridoma cells release immunostimulatory monoclonal antibodies into circulation
Microcapsule suspensions were injected under the skin of BALB/c mice with the help of an 18 G syringe. The microcapsules with anti-CD137- or OX40 mAb-producing hybridomas remained in the subcutaneous tissue by forming a plug, as depicted in Fig. 2a and as seen on H&E staining of paraffin-embedded sections taken 7 and 14 days after implantation of microcapsules. The microcapsules became integrated into the subcutaneous tissue where they could be identified and were found to contain the hybridoma cells (Fig. 2a). A moderate inflammatory/fibrotic reaction was observed around the microcapsules and antibody secreted by encapsulated cells, which reached the systemic circulation. To address this point, groups of mice were injected with 0.5 mL of microcapsules containing either hybridoma cells producing anti-CD137 or anti-OX40 mAb. Subsequently, serial samples of serum were examined by ELISA to determine the concentrations of rat immunoglobulin. As seen in Fig. 2b, injected animals showed circulating rat immunoglobulin for a period of time that lasted approximately around 1–2 weeks. The concentrations were compared to those corresponding to mice without microcapsules, which had received an intraperitoneal dose of 100 μg of monoclonal antibody in a regimen that was typically used in immunotherapy (Fig. 2b). It is of note that regardless of the lower production of anti-CD137 mAb in vitro, the serum levels were comparable. In fact, the level of anti-CD137 2A antibody lasted longer.
Fig. 2.
Subcutaneously implanted microcapsules containing 2A hybridoma cells producing anti-CD137 or anti-OX40 mAbs release monoclonal antibody into the systemic circulation and elicit mouse antibodies anti-rat immunoglobulins. a Microphotographs at different magnifications of H&E-stained sections from the subcutaneous tissue implanted with microcapsules containing 2A and OX86 hybridomas as indicated at different magnifications. b Concentration of rat IgG2a (left) and rat IgG1 (OX86) in serial sera samples drawn at different time points from mice implanted with the indicated hybridoma-containing microcapsules or injected with an intraperitoneal dose of 100 μg of the corresponding mAb as a purified protein. c ELISA results detecting anti-rat immunoglobulin antibodies in the serum of groups of mice harboring the indicated capsules. In the graph on the left, the absorbance at three different time points is plotted for each hybridoma at a 1/5 serum dilution using sera pooled from five mice in each condition. The graph on the right shows ELISA results with serial dilutions of the sera samples taken on day 14 following microcapsule implantation
Elimination of the rat antibodies from the mice is probably related to a humoral immune response directed at the xenogenic rat immunoglobulin determinants. Anti-CD137 mAb are known to inhibit antibody responses [30] via a mechanism that involves T helper cell attrition [30–32]. Indeed, our study confirms that the antibody response against rat IgG is delayed and weakened in mice treated with anti-CD137 capsules when compared to those treated with OX40 hybridoma-containing microcapsules (Fig. 2c). These inhibitory effects of CD137 mAb on the humoral response have also been previously shown in combinations of monoclonal antibodies, anti-CD137 and anti-CTLA-4 [9], and are considered advantageous for the pharmacokinetics of the antibody combinations.
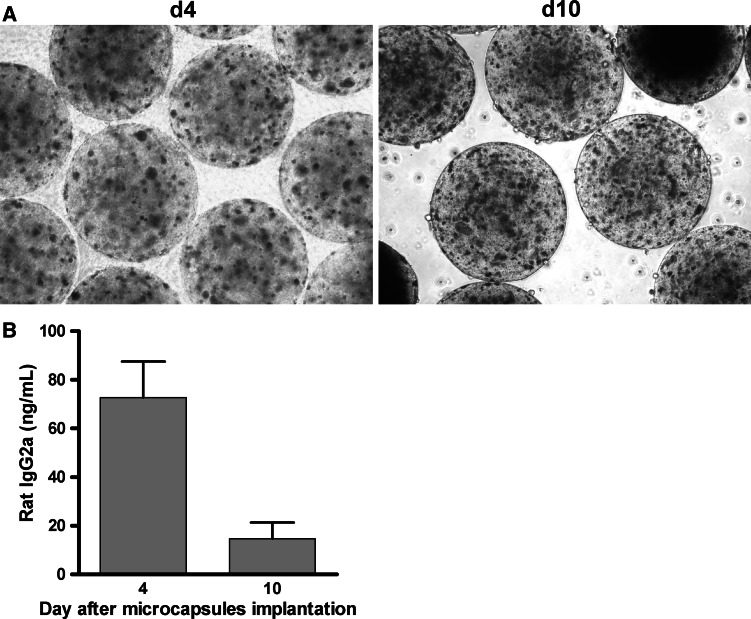
To further understand these phenomena, we implanted microcapsules in mice and explanted them 4 and 10 days later. As seen in Fig. 3, viable antibody-producing cells were detected in the explanted capsules seeded in culture media (100 µL of explanted capsules per mL of medium) (Fig. 3a). The 48-h supernatants of these cultures contained rat immunoglobulin that indicated that the encapsulated cells were still producing their monoclonal antibody (Fig. 3b).
Fig. 3.
Implanted encapsulated hybridoma cells persist in their ability to secrete anti-CD137 mAb. Encapsulated hybridomas implanted as in Fig. 2 were surgically removed at days 4 and 10 post-implant. a Microphotographs obtained by phase-contrast microscopy of explanted capsules at the indicated days showing persistence of viable cells in their interior after seeded in culture (100 μL of capsules per mL of culture medium). b Concentration of rat IgG2a in the supernatant of the explanted capsule cultures assessed 48 h after culture initiation
Encapsulated hybridoma cells producing immunostimulatory mAbs can induce tumor rejection
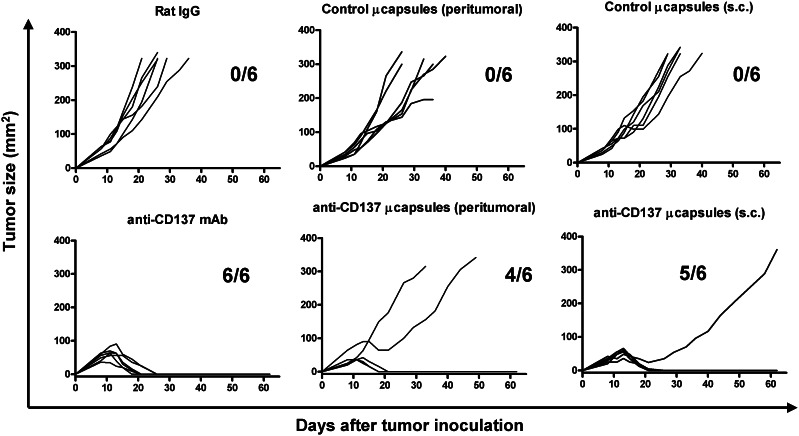
We had previously shown that transplanted CT26 colon carcinomas can be successfully treated by two doses of anti-CD137 agonist antibodies given intraperitoneally on days 6 and 8 following tumor cell inoculation by enhancing a cytotoxic T cell response [15, 23]. Control mice treated with polyclonal rat IgG or microcapsules in which cells have not been introduced develop lethal CT26-derived tumors in 3–4 weeks (Fig. 4). However, implantation of microcapsules on day 9 with the hybridoma producing anti-CD137 (4-1BB 2A) induced complete rejection in 9 out of 12 mice with a noticeable retardation in those tumors that finally progressed (Fig. 4). In a separate group of animals, treatment with purified mAbs given on day 9 eradicated six out of six tumors [33] (Fig. 4). It is of note that the microcapsules were effective regardless of whether they were implanted in the immediate neighborhood of the established tumor (peritumoral) or far away at the back of the cervical region (referred to as sc in Fig. 4).
Fig. 4.
Implantation of microcapsules producing anti-CD137 mAb results in therapeutic immunity against CT26 colon established carcinomas. Sequential individual follow-up of tumor sizes from mice implanted with 5 × 105 CT26 cells implanted 8 days later with APA microcapsules containing 2A hybridoma cells or with one single 100 μg dose of the purified 2A mAb given also on day 8. Fractions of mice completely rejecting their tumors are included in each graph. In the indicated graphs, microcapsules were implanted in the subcutaneous tissue surrounding the tumor lesion (peritumoral), while when indicated the microcapsules were implanted in the retrocervical region far from the area implanted with the tumor cells (sc)
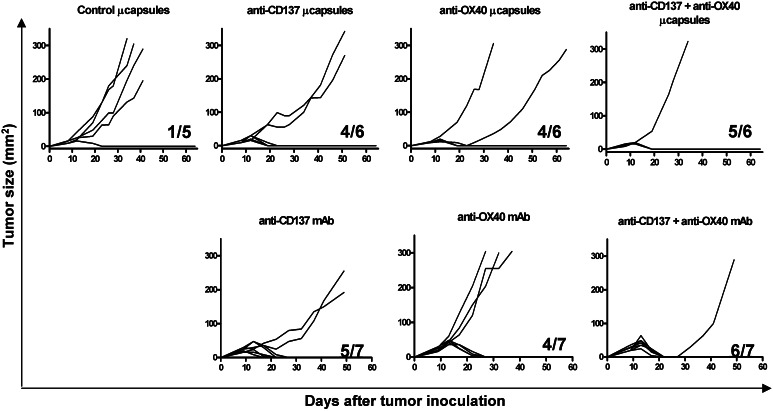
This type of effect is not exclusive to CD137 mAb, since in a separate series of experiments an antitumor effect was achieved with microcapsules containing OX86 hybridoma cells producing an anti-OX40 monoclonal antibody (Fig. 5). The antitumor effects of the capsules containing the anti-OX40 and the anti-CD137 hybridoma cells given on day 8 were comparable to those elicited by the purified monoclonal antibodies given i.p. From a therapeutic point of view, it is important that mice be simultaneously treated with anti-CD137 and anti-OX40 monoclonal antibodies. This was achieved by providing both types of capsules to the same mouse (Fig. 4). One of the animals rejected a tumor in the control group treated with capsules containing an unrelated hybridoma (Fig. 5), whereas untreated mice developed progressive tumor in every case (data not shown).
Fig. 5.
Anti-CD137 and anti-OX40-producing microcapsules can be employed concomitantly. Mice with established CT26 tumors for 8 days were treated with the indicated subcutaneous microcapsules or purified monoclonal antibodies given as single doses. Individual follow-up of tumor sizes and the fraction of mice in each group that underwent complete rejection are given
This combination of immunostimulatory monoclonal antibodies has already been reported to be synergistic [10, 16, 17]. The relative simplicity of the procedures indicates that combinations of a higher number of different hybridomas producing more than two monoclonal antibodies could be feasible. Capsules containing the negative control hybridoma and that containing the immunostimulatory hybridomas did not graft as tumors in immunocompetent BALB/c mice (data not shown) or in immunodeficient Rag−/− syngenic mice (supplementary Fig. 1). However, hybridoma cells gave rise to lethal tumors in the immunodeficient Rag−/− mice, if injected as a cell suspension (supplementary Fig. 1). The reason why hybridomas do not develop tumors from the capsules is unknown, while the lack of engraftment in immunocompetent mice even as cell suspensions is probably related to the rat lymphocyte component of the hybridomas that prevents xenografting.
CTL responses are enhanced by anti-CD137 mAb produced from microencapsulated hybridomas cells
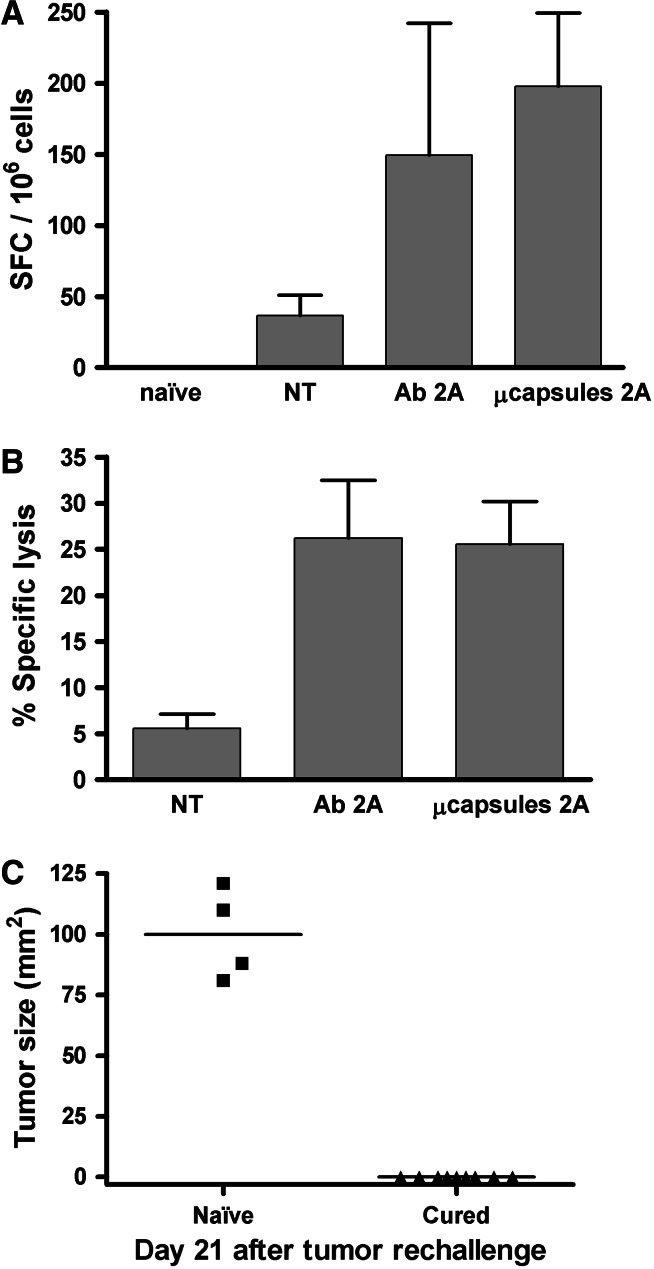
To study if treatment with microencapsulated cells producing immunostimulatory monoclonal antibodies resulted in enhanced T cell-mediated cytotoxic activity against tumors, mice were treated with soluble antibody or microcapsules as shown in Fig. 4. However in this case, mice were killed on day 14 and their spleen lymphocytes tested for the presence of specific CTLs recognizing the AH1 tumor antigen. As seen in Fig. 6a, the number of lymphocytes responding to the AH1 epitope [27] increased on CD137 treatment, both by soluble 2A antibody and the implanted microcapsules containing 2A-secreting hybridoma cells to a comparable extent. Moreover, similar results were obtained on quantification of FACS intracellular stainings for IFNγ (data not shown).
Fig. 6.
Anti-CD137 produced by implanted microcapsules enhances antitumor CTL activity. Groups of mice bearing CT26 tumors were treated as in Fig. 4, and on day 14 subjected to assays measuring the CTL response against the H-2Ld restricted epitope AH1. a IFNγ ELISPOTs of spleen lymphocytes stimulated with AH1 synthetic peptides were quantitated comparing tumor-naïve mice, non-treated animals and animals treated with anti-CD137 mAb 2A intraperitoneally or mice implanted with the 2A-producing microcapsules. Data represent mean ± SD of spot-forming cells in a total of 106 spleen lymphocytes. A representative experiment with six animals per group is shown. b In vivo cytotoxicity was tested in mice treated in parallel and the specific cytotoxic activity against autologous splenocytes pulsed with the AH1 synthetic peptide was assessed. Data represent the mean ± SD of a representative experiment with six animals per group. c Mice cured of tumor (n = 9), as those shown in Fig. 4, were challenged with CT26 cells once they were tumor free for 4–5 weeks. Sequential follow-up of the tumor size is presented. As a control, tumor-naïve mice (n = 4) were used
Moreover, in CT26-bearing groups of mice subjected to similar treatments, in vivo killing assays with splenocytes pulsed with AH1 synthetic peptide as target cells showed that the animals treated with microencapsulated 2A cells had similarly increased cytotoxic activity as compared to mice treated with injections of 2A mAb. The efficiency of the antitumor immunity is in accordance with the ability of cured mice to reject a rechallenge with CT26 cells as can be seen in Fig. 6c. Mice treated with encapsulated 2A hybridomas, which had completely rejected CT26 tumors 4–5 weeks before tumor cell reinjection (as those presented in supplementary Fig. 2), also rejected the rechallenge inoculi in contrast to tumor-naïve mice. Taken together along with the results presented in Fig. 6, we can conclude that immunostimulatory anti-CD137 mAb released from the implanted hybridoma microcapsules enhances antitumor cellular immunity to a degree comparable with intraperitoneal injections of the monoclonal antibody.
Discussion
Immunostimulatory monoclonal antibodies are yet in their infancy as a therapeutic tool. Antibodies are given at doses that act on the immune system receptors and mediate biological functions, but little is known of the pharmacokinetics and pharmacodynamics that optimize such events [34]. Receptor occupancy dynamics and surrogate biomarkers are largely absent. Monoclonal antibody pharmacology with purified humanized or fully human immunoglobulins has undergone many technical improvements in the recent past [35]. In the case of anti-CD137 mAb, the therapeutic effect is thought to be mediated via an enhancement of CD8 T cell-mediated immunity with a contribution of NK cells in some models [23, 29, 34, 36, 37]. In the case of anti-OX40 monoclonal antibodies, the mechanisms involve enhancement of the antitumor performance of CD4 and CD8 T cells [38] with a very interesting inhibition of suppressive Treg functions in mice [39], without deleting this subset [24, 40].
APA capsules could provide an alternative to the exogenous infusion of the immunoglobulins. The concept, as raised in this study, is clearly in need of optimization to become clinically worthwhile, both with regard to the antibody-producing cells and to the monoclonal antibody moieties. Safer cells transfected with genes encoding an immunostimulatory mAb are yet to be engineered. The antibodies to be produced should be devoid of xenotypic differences and be fully compatible with the species of the treated tumor host. In the case of mouse studies, primary cells would have to be transfected with the genes of the light and heavy chain of fully murine immunoglobulins. If translated to humans, this strategy must be performed with fully human immunoglobulin sequences.
Instead of the hybridomas, cells to be encapsulated should not pose problems of malignant engraftment or transformation. We have seen these hybridoma tumor engraftments in immunodeficient mice, but not in immunocompetent mice; even in the latter, engraftment occurs only when hybridoma cells are injected as a suspension, but not if enclosed in microcapsules. The cell to be engineered must be chosen taking into account that the protein biosynthesis machinery must be extremely active as it is in plasmacytoma or hybridoma cells. Fibroblasts [41], muscle fibers [42] or stem cells [43] might be suitable alternatives that are currently being investigated. The choice of the encapsulating polymer must fulfill the following features. It must be biocompatible [44], must not affect the viability of the enclosing cells and must provide sufficient mechanical stability to the microcapsules.
This study as a whole provides the proof of the concept that viable antibody-secreting cells can be encapsulated while maintaining the production of antibody that reaches the blood stream and enhances cancer immunity. Persistence of the action of encapsulated cells for at least 10 days is a particularly promising feature. Our histology data indicate the formation of a fibrotic reaction around the hybridoma-containing beads. However, in spite of this, immobilized cells continue producing the therapeutic product [45] as determined in blood circulation and in cultured explanted microcapsules. The location of the capsules near the tumor was not a therapeutic advantage in our case with the antibodies studied. However, in other cases such as anti-CD40 mAb or anti-CTLA-4 mAb, it may prove advantageous because of a postulated mechanism of action on the dendritic cells presenting tumor antigen [46] or their actions at the immunological synapse [47]. These issues deserve future research because it is somehow counterintuitive that fostering bioavailability at the local tumor draining lymph nodes does not enhance therapeutic performance in the case of anti-CD137 mAb, the mechanism of action of which involves dendritic cell cross-presentation of tumor antigens at these sites [29].
Alginate polymers are very stable and appropriate for all these purposes. They have been employed with a great number of cells producing proteins that are bioactive, such as insulin [48], erythropoietin [49], angiogenic factors [50], neurotrophic factors [51] and endostatin [52]. Our data with 10-day capsule explants are in agreement with these results.
In our opinion, it is very important that encapsulated antibody-producing cells provide the possibility of combining various monoclonal antibodies known to be synergistic in a simultaneous or sequential fashion. Outstandingly efficient results have been achieved by combinations of more than two agents of this kind [8]. In this regard, the ability of CD137 to minimize the antibody-neutralizing response may be useful for the combinations by diminishing the immune response to the partner antibody [9].
Separate administration of purified proteins is probably a feasible and more familiar alternative than encapsulated cells, because it complies with traditional pharmaceutical wisdom. However, the use of encapsulated cells introduced into the body as a bio-factory that produces a combination of synergistic therapeutic agents could be a reasonable alternative if further refined and developed. Moreover, combinations could involve other proteins apart from monoclonal antibodies such as antitumor cytokines including IL-12 [53].
Overall, these in vivo tumor treatment results prove four concepts: (i) when implanted, anti-CD137 and anti-OX40 mAb-producing cells elicit antitumor therapeutic efficacy; (ii) combinations of various immunostimulatory monoclonal antibodies are feasible by co-injecting microencapsulated cells producing more than one mAb; (iii) entrapped cells are able to produce the antibody continuously for at least 2 weeks when implanted subcutaneously; and (iv) immunostimulatory monoclonal antibodies secreted by implanted encapsulated hybridoma cells enhance tumor-specific cellular immunity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Drs. Lieping Chen for the kind gift of the 2A hybridoma and Mario Colombo for OX86 cells. Elena Ciordia and Eneko Elizalde are acknowledged for their excellent animal facility management. We received financial support from MEC/MICINN (SAF2005-03131 and SAF2008-03294), Departamento de Educación del Gobierno de Navarra, Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri), Redes temáticas de investigación cooperativa RETIC (RD06/0020/0065), Fondo de investigación sanitaria (FIS PI060932), European Commission FP7 (ENCITE) and SUDOE-IMMUNONET (FEDER) which supports JD, Fundacion Mutua Madrileña and “UTE for project FIMA”. M S-H received a Ramon y Cajal contract from Ministerio de Ciencia y tecnología and A P a scholarship from FIS.
Abbreviations
- mAb
Monoclonal antibody
- DC
Dendritic cell
- TNF
Tumor necrosis factor
- NK
Natural killer
- APA
Alginate-poly-l-lysine-alginate
Footnotes
I. Melero and J. L. Pedraz equally share credit for senior authorship.
Contributor Information
Jose Luis Pedraz, Email: joseluis.pedraz@ehu.es.
Ignacio Melero, Email: imelero@unav.es.
References
- 1.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 2.Murillo O, Arina A, Tirapu I, et al. Potentiation of therapeutic immune responses against malignancies with monoclonal antibodies. Clin Cancer Res. 2003;9:5454–5464. [PubMed] [Google Scholar]
- 3.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 6.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870, 893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 7.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 8.Uno T, Takeda K, Kojima Y, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 9.Kocak E, Lute K, Chang X, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 10.Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–2511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 12.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 15.Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 16.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–566. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuadros C, Dominguez AL, Lollini PL, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–943. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 18.Murua A, Portero A, Orive G, Hernandez RM, de Castro M, Pedraz JL. Cell microencapsulation technology: towards clinical application. J Control Release. 2008;132:76–83. doi: 10.1016/j.jconrel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Calafiore R, Basta G, Luca G, et al. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: first two cases. Diabetes Care. 2006;29:137–138. doi: 10.2337/diacare.29.01.06.dc05-1270. [DOI] [PubMed] [Google Scholar]
- 20.Lohr M, Hoffmeyer A, Kroger J, et al. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet. 2001;357:1591–1592. doi: 10.1016/S0140-6736(00)04749-8. [DOI] [PubMed] [Google Scholar]
- 21.Orive G, Hernandez RM, Gascon AR, et al. Cell encapsulation: promise and progress. Nat Med. 2003;9:104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev. 2008;60:124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 25.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 26.Ponce S, Orive G, Hernandez RM, et al. In vivo evaluation of EPO-secreting cells immobilized in different alginate-PLL microcapsules. J Control Release. 2006;116:28–34. doi: 10.1016/j.jconrel.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Huang AY, Gulden PH, Woods AS, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casares N, Lasarte JJ, de Cerio AL, et al. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur J Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::AID-IMMU1780>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Murillo O, Dubrot J, Palazon A, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–2436. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 30.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med. 1999;190:1535–1540. doi: 10.1084/jem.190.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo SK, Choi JH, Kim YH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Blink SE, Chen JH, Fu YX. Regulation of follicular dendritic cell networks by activated T cells: the role of CD137 signaling. J Immunol. 2005;175:884–890. doi: 10.4049/jimmunol.175.2.884. [DOI] [PubMed] [Google Scholar]
- 33.Mazzolini G, Murillo O, Atorrasagasti C, et al. Immunotherapy and immunoescape in colorectal cancer. World J Gastroenterol. 2007;13:5822–5831. doi: 10.3748/wjg.v13.i44.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 36.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 37.Murillo O, Arina A, Hervas-Stubbs S, et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008;14:6895–6906. doi: 10.1158/1078-0432.CCR-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberg AD. OX40: targeted immunotherapy–implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/S1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
- 39.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 40.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grandoso L, Ponce S, Manuel I, et al. Long-term survival of encapsulated GDNF secreting cells implanted within the striatum of parkinsonized rats. Int J Pharm. 2007;343:69–78. doi: 10.1016/j.ijpharm.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Murua A, de Castro M, Orive G, Hernandez RM, Pedraz JL. In vitro characterization and in vivo functionality of erythropoietin-secreting cells immobilized in alginate-poly-l-lysine-alginate microcapsules. Biomacromolecules. 2007;8:3302–3307. doi: 10.1021/bm070194b. [DOI] [PubMed] [Google Scholar]
- 43.McLenachan S, Sarsero JP, Ioannou PA. Flow-cytometric analysis of mouse embryonic stem cell lipofection using small and large DNA constructs. Genomics. 2007;89:708–720. doi: 10.1016/j.ygeno.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Orive G, Tam SK, Pedraz JL, Halle JP. Biocompatibility of alginate-poly-l-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691–3700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 45.Orive G, De Castro M, Kong HJ, et al. Bioactive cell-hydrogel microcapsules for cell-based drug delivery. J Control Release. 2009;135:203–210. doi: 10.1016/j.jconrel.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 46.French RR, Taraban VY, Crowther GR, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 47.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Orive G, De Castro M, Ponce S, et al. Long-term expression of erythropoietin from myoblasts immobilized in biocompatible and neovascularized microcapsules. Mol Ther. 2005;12:283–289. doi: 10.1016/j.ymthe.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Zhu SJ, Wang W, Wei YJ, Hu SS. Transplantation of microencapsulated genetically modified xenogeneic cells augments angiogenesis and improves heart function. Gene Ther. 2008;15:40–48. doi: 10.1038/sj.gt.3303049. [DOI] [PubMed] [Google Scholar]
- 51.Tobias CA, Han SS, Shumsky JS, et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J Neurotrauma. 2005;22:138–156. doi: 10.1089/neu.2005.22.138. [DOI] [PubMed] [Google Scholar]
- 52.Teng H, Zhang Y, Wang W, Ma X, Fei J. Inhibition of tumor growth in mice by endostatin derived from abdominal transplanted encapsulated cells. Acta Biochim Biophys Sin (Shanghai) 2007;39:278–284. doi: 10.1111/j.1745-7270.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 53.Sangro B, Melero I, Qian C, Prieto J. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573–581. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.