Abstract
Purpose
High-dose IFNα2b (HDI) was established as the first effective adjuvant therapy for patients with high-risk resected melanoma more than a decade ago, but its fundamental molecular mechanism of action remains unclear. STAT3 and the mitogen activated protein kinases (MAPKs), especially ERK (extracellular signal-regulating kinase) and MEK (MAPK/ERK kinase), play roles in melanoma progression and host immunity. We have therefore evaluated STAT3 and MEK/ERK MAP kinases in patients with regional lymph node metastasis (stage IIIB) of melanoma in the context of a prospective neoadjuvant trial of HDI (UPCI 00-008).
Patients and methods
In the context of this trial, HDI was administered daily for 20 doses following diagnostic biopsy, and prior to definitive surgery. Immunohistochemistry for pSTAT3, phospho-MEK1/2, phospho-ERK1/2, and EGFR was performed on paired fixed (nine patients) biopsies.
Results
HDI was found to down-regulate pSTAT3 (P = 0.008) and phospho-MEK1/2 (P = 0.008) levels significantly in tumor cells. Phospho-ERK1/2 was down-regulated by HDI in tumor cells (P = 0.015), but not in lymphoid cells. HDI down-regulated EGFR (P = 0.013), but pSTAT3 activation appeared not to be associated with EGFR expression and the MEK/ERK MAPK pathway.
Conclusion
We conclude that HDI regulates MAPK signaling differentially in melanoma tumor cells and host lymphoid cells in vivo. STAT3 activation is independent of the EGFR/MEK/ERK signaling pathway.
Keywords: IFNα2b, STAT3, MEK1/2, ERK1/2, Melanoma, Lymph node
Introduction
High-dose IFNα2b (HDI) prolongs both relapse-free and overall survival of patients with resected high-risk deep primary or lymph node metastatic melanoma. This therapy reduces the hazard of relapse and mortality by 22–33%, on the basis of the largest intergroup studies performed [17–19]. However, the mechanism of action for IFNα2b in melanoma remains uncertain. We have recently reported the results of the first neoadjuvant application of HDI, in which 11/20 patients demonstrated objective clinical response [23]. Understanding the molecular mechanism of HDI in the adjuvant therapy of melanoma would enable investigators to build more intelligently upon this modality, and would permit the application of this therapy in patients for whom the likelihood of response would be greater than for the population at large. The understanding of molecular biomarkers of response to HDI will facilitate the development of preventive applications of this and potentially other therapies.
The STAT3 signaling cascade is a central pathway of cancer progression in melanoma, and has also been associated with immunosuppression [6–8,10,12,15,25,28,40–42]. STAT3 activation has been linked to multiple protein kinase pathways in vitro [3,24]. Our previous studies have documented that HDI down-regulates pSTAT3 tyr705 in melanoma cells and lymphoid cells, while it up-regulates pSTAT1 tyr701 and TAP2 in tumor as well as lymphoid cells [34]. We therefore have considered how IFNα2b influences STAT phosphorylation and regulates host immunity.
MEK1 and MEK2 are dual-specificity protein kinases that function in the MAPK cascade to control cell growth and differentiation. Activation of MEK1 and MEK2 occurs through phosphorylation of two serine residues at positions 217 and 221 by Raf-like molecules. Then, phospho-MEK activates p44 and p42 MAP kinases (ERK1 and ERK2) by phosphorylation of both threonine and tyrosine residues at positions 202 and 204, respectively. ERK1 and ERK2 function as protein kinases in the MEK/ERK MAPK signal pathway that plays a critical role in the regulation of cell growth and differentiation [4,11,13,22]. Raf/MEK/ERK pathway activation in melanoma progression has been well documented [9], and the MEK/ERK MAPK pathway is also involved in the activation of effector cells that exhibit powerful processes to destroy malignant cells [5].
The effects of IFNα2b upon the MEK/ERK MAPK pathway have not been documented in melanoma in vivo. The response of MEK/ERK MAPKs to HDI is of interest in the clinical setting of neoadjuvant therapy of high-risk melanoma patients. The neoadjuvant approach has enabled us to analyze the impact of adjuvant therapy upon tumor tissue. High-dose IFNα2b intravenous (IV) induction therapy was administered to consenting patients following initial biopsy confirmation of disease, and before definitive surgery for patients with stage IIIB disease at presentation or for any patient with recurrent stage IIIB nodal disease. All patients gave written informed consent to this IRB-approved protocol, UPCI 00-008, prior to study entry. Initial biopsy specimens and the post-IFN completion lymphadenectomy tissue samples were evaluated by the pathologists of the Melanoma and Skin Cancer Program, and only released for the research studies specified in UPCI 00-008 after all diagnostic pathology requirements were satisfied. Both pre- and post-HDI treatment specimens from this clinical study of the signal transduction pathways in relation to IFNα2b are described in an earlier report [23,35–37]. HDI was found to differentially regulate pSTAT3, EGFR and MEK/ERK MAPKs in the lymph node tissue metastatic of melanoma.
Materials and methods
Surgical specimens and patient treatment
Patient eligibility, treatment plan, and surgical biopsy schema are described for the clinical trial UPCI 00-008 separately [23]. The clinical protocol UPCI 00-008 was approved by the University of Pittsburgh IRB, and all patients who entered this trial gave written informed consent. The patient demographic details, and clinical response information have been reported [23]. Eligible patients with palpable regional lymph node disease underwent a pretreatment tumor biopsy after written informed consent. A portion of the biopsy was evaluated to confirm the diagnosis, and the remaining portions of the biopsy were evaluated in research described in protocol 00-008. Patients were treated with IFNα2b according to the HDI regimen developed in the E1684/E1690/E1694 ECOG and Intergroup trials and as approved in 1995, by the U.S. FDA [19]. IFNα2b, 20 MU/m² day, was administered IV for five consecutive days of seven for 4 weeks, followed by 10 MU/m² day subcutaneously (SC) every other day (M, W, F) three times each week × 48 weeks. Patients underwent definitive surgery with completion of lymphadenectomy after the induction IV therapy and before beginning maintenance SC therapy. All study interventions and assessments were performed at consistent time points as specified in the protocol UPCI 00-008 for this neoadjuvant trial. At the time of surgery, additional tumor and regional lymph node tissues were obtained for routine pathology and for the research studies described in the protocol. Specimens obtained from nine patients were adequate to permit analysis in this study. Four patients had not responded to therapy, while five patients had demonstrated partial clinical response to treatment (as detailed previously in Moschos et al., J. Clin. Oncol, 2006) [23].
Immunohistochemistry
Rabbit monoclonal anti-human phospho-MEK1/2 (Ser217/221)(166F8) antibody; rabbit polyclonal anti-human phospho-ERK1/2 (Thr202/Tyr204) and pSTAT3 tyr705 antibodies were purchased from Cell Signaling Technology® (Beverly, MA). Mouse anti-EGFR mAb 31G7 was purchased from Zymed Laboratories Inc. (South San Francisco, CA). Unconjugated goat anti-rabbit IgG (H + L) antibody was used as a blocking antibody (Vector Laboratories, Burlingame, CA).
Formaldehyde-fixed and paraffin-embedded tissues were unmasked with antigen retrieval reagent (Dako, S1669), then indirect immunohistochemistry was performed to detect specific antigens. The indirect immunohistochemistry was performed with Vector Laboratories’ Vectastain ABC system (alkaline phosphatase system) and double immunostain was performed according to the manufacturer’s instructions. Vector Red Substrate Kit (SK-5100) and Vector Blue Substrate Kit (SK-5300) were used. Vector Methyl Green (H-3402) was used as counter stain.
Data and statistical analysis
Two pathologists evaluated the whole tissue section using both 10X and 20X objectives, and were blinded in regard to treatment assignment. Quantitation was decided by consensus of the two pathologists and a research faculty member. Cells scored with staining intensity from 1+ to 4+ were considered as positive; cells with staining intensity 0 were considered as negative. The percentages of positive cells were evaluated. The intensity of staining was assessed for the entire cell, since the pSTAT reagent we utilized detects a minor cytoplasmic component of pSTAT as well. For accuracy, we tabulated pSTAT staining of the cytoplasm as well as the nucleus. The localization of pSTAT within the nucleus has separately been documented in the literature. Tumor cells and lymphocytes in each tumor biopsy section were separately evaluated. Lymphocytes observed in this study of lymph node metastases included lymphocytes that have been termed tumor infiltrated lymphocytes (TILs), although the scoring of these cells was performed throughout the entire node for this study. The percentage of pSTAT positive cells was enumerated for the same overall population of cells. Cell fractions of less than 1% were considered as zero. The formalin fixed paraffin embedded sections were used for this study.
The statistical analysis was performed by the UPCI Biostatistical Facility. Mean values of the percentage of positive cells are presented with standard errors (SE). Comparisons of the percentages of positive cells between pre- and post-therapy samples were performed using paired-sample permutation tests [32] with a two-sided significance level of 0.05. Two-sample permutation tests were used to compare those with objective clinical response (partial and complete) to those without clinical response, and in relation to whether patients had subsequent relapse and those with subsequent relapse at one year of follow-up, both in terms of the baseline percentages of positive cells and the change from pre- to post-therapy. No P-value adjustment has been performed to account for the multiple comparisons in this exploratory study.
Results
Both pSTAT3 tyr705 and phospho-MEK1/2 (Ser217/221) are down-regulated by HDI in melanoma cells
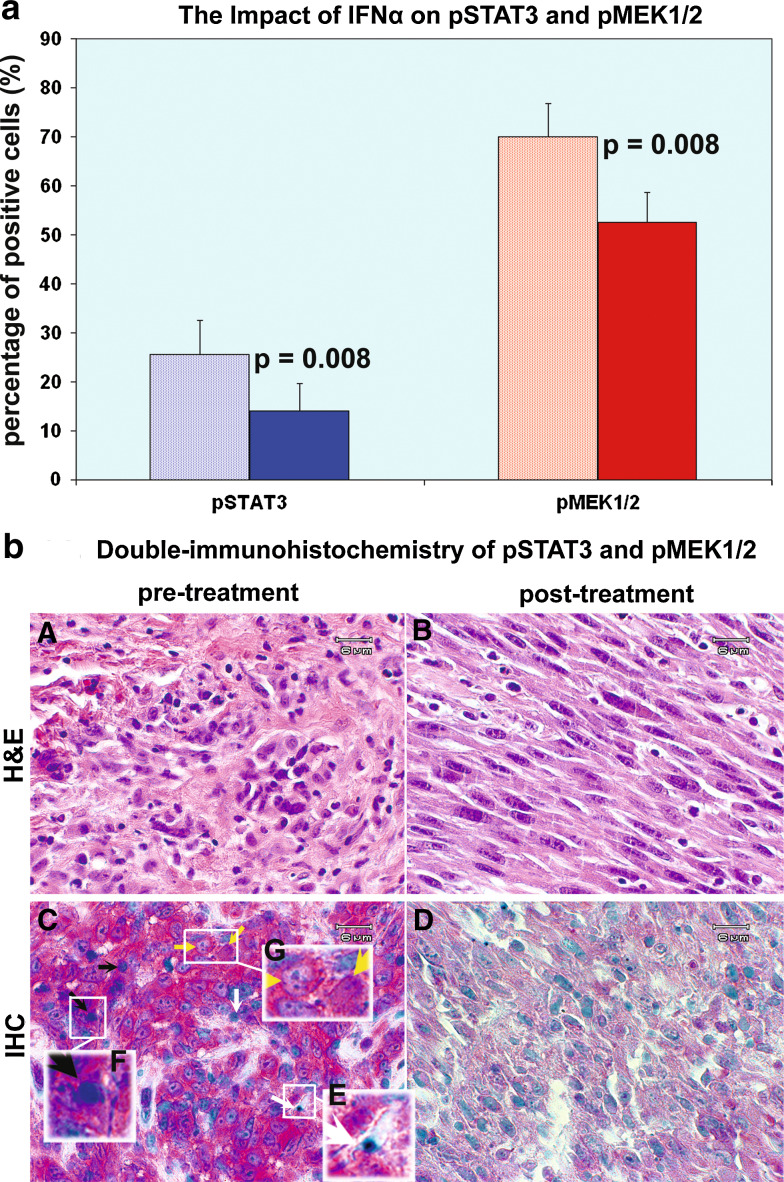
STAT3 is constitutively expressed and activated in melanoma cells, as are MEK1 and MEK2 [20,26,31,40]. pSTAT3 tyr705 and phospho-MEK1/2 (Ser217/221) were initially evaluated in this study. Double immunostaining was performed upon formaldehyde-fixed paraffin-embedded sections. pSTAT3 tyr705 (Vector blue) and phospho-MEK1/2 (Ser217/221) (Vector red) were stained with the Vector alkaline phosphatase system. Vector methyl green was applied for counter stain. The formaldehyde-fixed and paraffin-embedded sections of a total of eight cases pre- and post-HDI therapy were adequate to permit evaluation of pSTAT3 tyr705 and phospho-MEK1/2 (Ser217/221) in tumor cells. IFNα2b down-regulated pSTAT3 tyr705 in all of the eight cases. The mean percentage of positive cells was decreased from 25.63 ± 6.84 to 14.00 ± 5.64%, P = 0.008, as shown in Fig. 1a. Phospho-MEK1/2 (Ser217/221) was down-regulated by IFNα2b in all of the eight cases as well. The mean percentage of positive cells was decreased from 70.00 ± 6.75 to 52.50 ± 6.20%, P = 0.008, as shown in Fig. 1a. There was no significant difference between patients with clinical response and those without response in terms of pMEK1/2 and pSTAT3 tyr705.
Fig. 1.
High-dose IFNα2b down-regulates both pSTAT3 tyr705 and p-MEK in melanoma cells. a The Impact of IFNα on pSTAT3 and pMEK1/2. IFNα2b down-regulated pSTAT3 tyr705 in all of the eight cases. The mean percentage of positive cells was decreased from 25.63 ± 6.84 to 14.00 ± 5.64%, P = 0.008. Phospho-MEK1/2 (Ser217/221) was down-regulated by IFNα2b in all of the eight cases as well. The mean percentage of positive cells was decreased from 70.00 ± 6.75 to 52.50 ± 6.20%, P = 0.008. Data = mean ± SE. b Double-immunohistochemistry of pSTAT3 and pMEK1/2. Panels A and B represent H&E staining of pre- and post-treatment respectively; panels C and panel D are double immunostain sections of pre- and post-treatment sections, respectively. The blue color represents pSTAT3 tyr705, while the red color represents phospho-MEK1/2 (Ser217/221). Post IFNα2b treatment, both the density and intensity of the two stains are significantly reduced as shown in panel D. In panel C, white arrows point to cells which are pSTAT3 positive but pMEK negative; black arrows point to cells which are both pSTAT3 and pMEK1/2 positive; yellow arrows point to cells which are pSTAT3 negative but pMEK1/2 positive. Panel E amplifies the single cell that is pSTAT3 (blue) positive. Panel F amplifies the single cell that is both pSTAT3 (blue) and pMEK1/2 (red) positive. Panel G amplifies the single cells that are pMEK1/2 (red) positive but are pSTAT3 (blue) negative
Figure 1b demonstrates that IFNα2b down-regulates both pSTAT3 tyr705 and phospho-MEK1/2 (Ser217/221). Figure 1b panels A and B represent H&E staining pre- and post-treatment, respectively; panels C and D are double immunostain sections of pre- and post-treatment sections, respectively. The blue color represents pSTAT3 tyr705, while the red color represents phospho-MEK1/2 (Ser217/221). Post IFNα2b treatment, both the density and intensity of the two stains are significantly reduced as shown in panel D. It is also apparent that pSTAT3 positive cells are pMEK1/2 positive or negative, indicated by the arrow shown in Fig. 1b, panel C. Cells that are pSTAT3 (blue) positive but pMEK negative are designated with a white arrow; cells which are both pSTAT3 and pMEK1/2 positive are designated by a black arrow; cells that are pSTAT3 negative but pMEK1/2 positive are designated by a yellow arrow. Panel E amplifies the single cell that is pSTAT3 (blue) positive. Panel F amplifies the single cell that is both pSTAT3 (blue) and pMEK1/2 (red) positive. Panel G amplifies the single cells that are pMEK1/2 (red) positive but are pSTAT3 (blue) negative.
Phospho-ERK1/2 (Thr202/Tyr204) is down-regulated by HDI in melanoma but not lymphoid cells
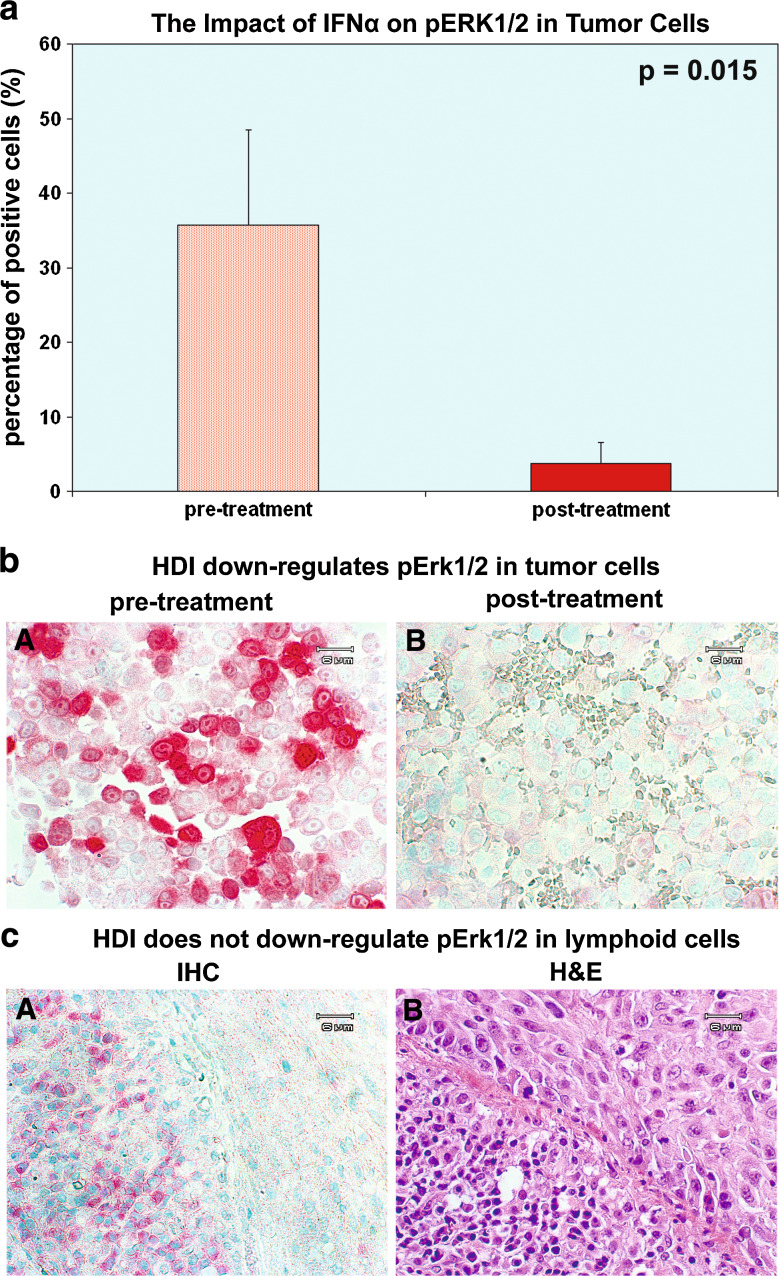
IFNα2b down-regulates phospho-MEK1/2 (Ser217/221); since ERK is the downstream phosphorylation target of phospho-MEK1/2 (Ser217/221), we further evaluated phospho-ERK1/2 to establish whether IFNα2b down-regulates the whole MEK/ERK MAPK signal pathway. Phospho-ERK (Thr202/Tyr204) (vector red) was immunostained indirectly with the Vector alkaline phosphatase system. Vector methyl green was applied for counter stain. Formaldehyde-fixed and paraffin-embedded sections of a total of seven cases pre- and post-HDI therapy were adequate to probe phospho-ERK1/2 in tumor cells. IFNα2b down-regulated phospho-ERK1/2 (Thr202/Tyr204) in all of the seven cases studied. The mean percentage of positive cells was decreased from 35.71 ± 12.74 to 3.71 ± 2.80%, P = 0.015, as shown in Fig. 2a. There was no significant difference between patients with clinical response and those with non-response in terms of pERK1/2.
Fig. 2.
High-dose IFNα2b regulates pERK1/2 in melanoma and lymphocytes differentially. a The impact of IFNα on pERK1/2. IFNα2b down-regulates phospho-ERK1/2 (Thr202/Tyr204) in all of the seven cases. The mean percentage of positive cells was decreased from 35.71 ± 12.74 to 3.71 ± 2.80%, P = 0.015. Data = mean ± SE. b HDI down-regulates phospho-ERK1/2 in tumor cells. IFNα2b down-regulated phospho-p44/42 (Thr202/Tyr204) in the post-treatment section, panel B. c HDI did not down-regulate phospho-ERK1/2 in lymphoid cells. Panel A, tumor cells of the post-treatment biopsies are on the right side, in which phospho-ERK1/2 is stained weakly 0–1+; however, lymphoid cells in the post-treatment sample, left side of panel A, strongly demonstrate positive (3–4+) phospho-ERK1/2
Figure 2b demonstrates that IFNα2b down-regulates phospho-ERK1/2 (Thr202/Tyr204) in the post-treatment section, panel B. Post IFNα2b treatment, both the density and intensity of the stain for phospho-ERK1/2 (Thr202/Tyr204) were reduced significantly. We did not find that IFNα2b regulated phospho-ERK1/2 (Thr202/Tyr204) in lymphoid cells, but as demonstrated in Fig. 2c lymphoid cells are strongly positive (3+ to 4+) in the post-treatment biopsies. In Fig. 2c panel A, tumor cells of the post-treatment biopsies are located on the right side of the panel, in which phospho-ERK1/2 is stained only weakly 0–1+; however, lymphoid cells in the post-treatment sample on the left side of panel A demonstrate strongly positive phospho-ERK1/2 staining (3–4+). Macrophages are also strongly stained (+4) (not shown) in the post-treatment sample.
Both pSTAT3 tyr705 and EGFR are down-regulated by HDI; STAT3 activation is independent of EGFR expression
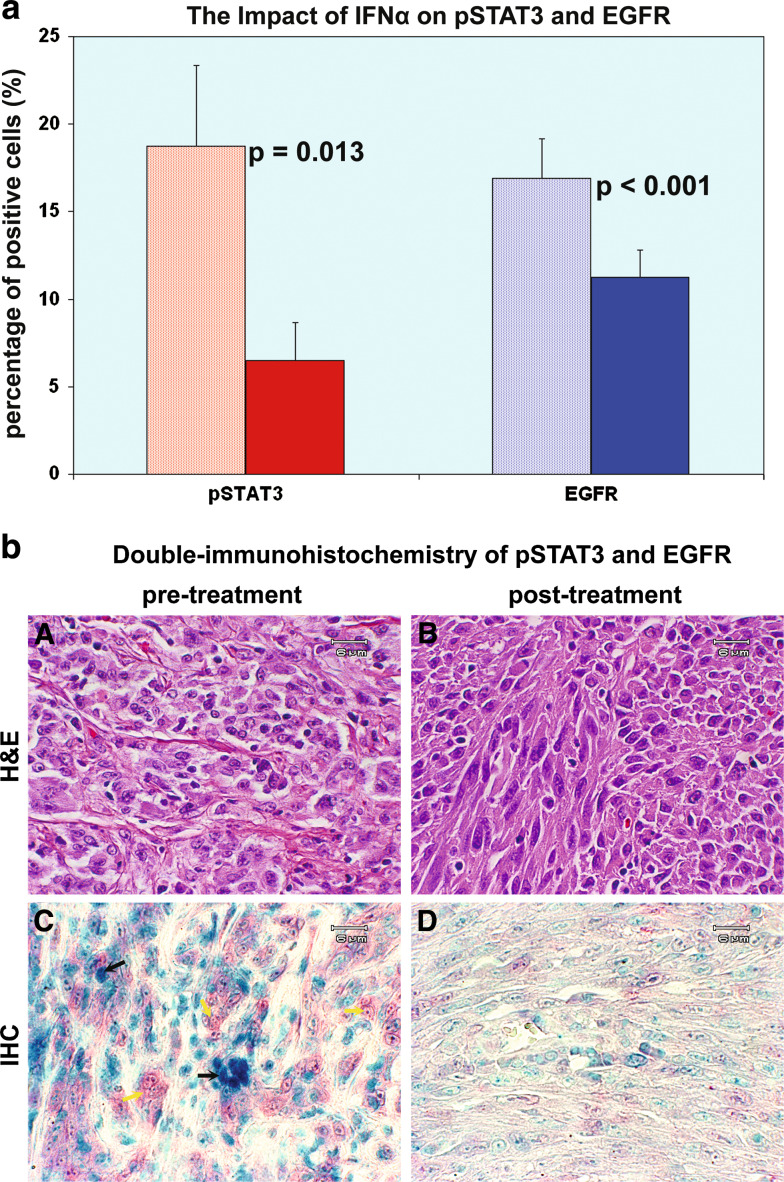
EGFR expression has been correlated with the degree of melanocytic dysplasia in atypical nevi, and its interaction with the JAK-STAT signaling pathway was therefore of interest. We evaluated EGFR and the JAK-STAT pathway using double immunostaining of formaldehyde-fixed, paraffin-embedded tissue sections. pSTAT3 tyr705, EGFR and nuclear staining were indicated by blue, red and green chromogens, respectively. HDI down-regulated pSTAT3 tyr705 in tumor cells in all eight cases. The mean percentage of cells strongly expressing pSTAT3 was decreased from 18.75 ± 4.61 to 6.50 ± 2.17%, P = 0.013, as shown in Fig. 3a. This adds to the previous evidence that HDI down-regulates pSTAT3 tyr705 expression in melanoma metastases to regional lymph nodes as noted earlier. HDI down-regulated EGFR in tumor cells in seven out of eight cases. The mean percentage of positive cells was decreased from 16.88 ± 2.30% to 11.25 ± 1.57%, P < 0.001, as shown in Fig. 3a. There were no significant differences between clinical responders and non-responders in terms of pSTAT3 tyr705 or EGFR. Despite the evidence that HDI down-regulates both pSTAT3 tyr705 and EGFR levels concurrently in tumor samples, as shown in Fig. 3b panels C and D, pSTAT3 tyr705 levels were not found to exhibit a pattern correlated with EGFR expression, as shown in Fig. 3b, panel C. It was observed that pSTAT3 tyr705 negative cells (yellow arrow) exhibited high EGFR expression levels; conversely, cells denoted by the black arrow exhibited strongly positive staining for pSTAT3 tyr705, but are negative for EGFR.
Fig. 3.
High-dose IFNα2b down-regulates both pSTAT3 tyr705 and EGFR. a The impact of IFNα on pSTAT3 and EGFR. HDI down-regulated pSTAT3 tyr705 in tumor cells in all eight cases. The mean percentage of cells strongly expressing pSTAT3 was decreased from 18.75 ± 4.61 to 6.50 ± 2.17%, P = 0.013. HDI down-regulated EGFR in tumor cells in seven out of eight cases. The mean percentage of positive cells was decreased from 16.88 ± 2.30 to 11.25 ± 1.57%, P < 0.001. Data = mean ± SE. b Double-immunohistochemistry of pSTAT3 and EGFR. Panels A and B are H&E stain. HDI down-regulates both pSTAT3 tyr705 (blue) and EGFR levels concurrently in tumor samples as shown in panels C and D. It was observed that pSTAT3 tyr705 negative cells (yellow arrow) exhibited higher EGFR expression level; conversely, cells denoted by the black arrow are strongly positive for pSTAT3 tyr705, but negative for EGFR
Discussion
Because of the importance of the MEK/ERK MAPK pathway in melanoma progression, phospho-MEK1/2 (Ser217/221) was probed in lymph node metastases of patients prior to and following high-dose IV IFNα2b treatment in the context of a neoadjuvant trial. HDI given in this manner was found to down-regulate phospho-MEK1/2 (Ser217/221), and studies of the downstream MEK1/2 protein kinase, the phospho-ERK1/2 (Thr202/Tyr204), confirm down-regulation of this member of the cascade in tumor cells. HDI therefore down-regulates the MEK/ERK MAPK pathway in melanoma cells in vivo. These findings are consistent with findings in hepatocellular carcinoma that demonstrate that IFNα diminishes the phosphorylation of both ERK and MEK [14]; these findings represent the first evaluation of these targets in vivo in patient tumor tissues prior to and following HDI therapy. Accumulated evidence documents that the MEK/ERK MAPK pathway activation is important to tumor cell motility [16,33,38], and our data shows that down-regulation of MEK/ERK MAPK activity may be correlated with inhibition of tumor cell metastasis.
The combined disruption of both the IL-6R/STAT3 and MEK/ERK pathways is required to induce apoptosis of multiple myeloma cells [2]. MEK and ERK inhibitors enhance the anti-proliferative effect of IFNα2b [27]. Src tyrosine kinase, rather than EGFR or JAK family kinases, lead to STAT3 activation in melanoma cell lines [24]. We have found that some pSTAT3 positive melanoma cells are pMEK1/2 negative; and that EGFR positive melanoma cells are pSTAT3 negative in vivo. MEK/ERK MAPK pathway is the downstream signal of EGFR [29]. Our findings suggest that STAT3 phosphorylation occurs independent of the EGFR/MEK/ERK pathway in vivo.
HDI down-regulates both MEK/ERK MAPK and pSTAT3 activation, but not the ERK MAPK in lymphoid cells. ERK pathway activation is crucial for T thymocyte activation, differentiation and function in immune responses [1,39,43]. IFNα2b maintains MEK/ERK activation in lymphoid cells in order to initiate antitumor immune response. Moreover, IFNs have been documented to mediate survival and development of cells of hemopoietic origins [21,30], which is consistent with our data.
Acknowledgments
This research study was supported by the Grant Channel Memorial Fund of the University of Pittsburgh Cancer Institute’s Melanoma Program. We thank the generous support of the Grant Channel Memorial Fund of the UPCI Melanoma Program.
References
- 1.Adachi S, Iwata M. Duration of calcineurin and Erk signals regulates CD4/CD8 lineage commitment of thymocytes. Cell Immunol. 2002;215:45–53. doi: 10.1016/S0008-8749(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee M, Stuhmer T, Herrmann P, Bommert K, Dorken B, Bargou RC. Combined disruption of both the MEK/ERK and the IL-6R/STAT3 pathways is required to induce apoptosis of multiple myeloma cells in the presence of bone marrow stromal cells. Blood. 2004;104:3712–3721. doi: 10.1182/blood-2004-04-1670. [DOI] [PubMed] [Google Scholar]
- 3.Coppo P, Flamant S, De Mas V, Jarrier P, Guillier M, Bonnet ML, Lacout C, Guilhot F, Vainchenker W, Turhan AG. BCR-ABL activates STAT3 via JAK and MEK pathways in human cells. Br J Haematol. 2006;134:171–179. doi: 10.1111/j.1365-2141.2006.06161.x. [DOI] [PubMed] [Google Scholar]
- 4.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 5.Djeu JY, Jiang K, Wei S. A view to a kill: signals triggering cytotoxicity. Clin Cancer Res. 2002;8:636–640. [PubMed] [Google Scholar]
- 6.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Gamero AM, Young HA, Wiltrout RH. Inactivation of Stat3 in tumor cells: releasing a brake on immune responses against cancer? Cancer Cell. 2004;5:111–112. doi: 10.1016/S1535-6108(04)00028-5. [DOI] [PubMed] [Google Scholar]
- 8.Gao SP, Bromberg JF (2006) Touched and moved by STAT3. Sci STKE 2006: pe30 [DOI] [PubMed]
- 9.Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol. 2006;33:392–406. doi: 10.1053/j.seminoncol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Haura EB, Turkson J, Jove R. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 11.Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 12.Homsi J, Cubitt C, Daud A. The Src signaling pathway: a potential target in melanoma and other malignancies. Expert Opin Ther Targets. 2007;11:91–100. doi: 10.1517/14728222.11.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–36. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 14.Inamura K, Matsuzaki Y, Uematsu N, Honda A, Tanaka N, Uchida K. Rapid inhibition of MAPK signaling and anti-proliferation effect via JAK/STAT signaling by interferon-alpha in hepatocellular carcinoma cell lines. Biochim Biophys Acta. 2005;1745:401–410. doi: 10.1016/j.bbamcr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Itoh M, Murata T, Suzuki T, Shindoh M, Nakajima K, Imai K, Yoshida K. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25:1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- 16.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta. cooperatively regulate epithelial cell plasticity and metastasis dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 18.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.CCR-1103-3. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 21.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol. 2000;164:1286–1292. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]
- 22.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 23.Moschos SJ, Edington HD, Land SR, Rao UN, Jukic D, Shipe-Spotloe J, Kirkwood JM. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J Clin Oncol. 2006;24:3164–3171. doi: 10.1200/JCO.2005.05.2498. [DOI] [PubMed] [Google Scholar]
- 24.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 25.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 26.Park S, Yeung ML, Beach S, Shields JM, Yeung KC. RKIP downregulates B-Raf kinase activity in melanoma cancer cells. Oncogene. 2005;24:3535–3540. doi: 10.1038/sj.onc.1208435. [DOI] [PubMed] [Google Scholar]
- 27.Romerio F, Zella D. MEK and ERK inhibitors enhance the anti-proliferative effect of interferon-alpha2b. Faseb J. 2002;16:1680–1682. doi: 10.1096/fj.02-0120fje. [DOI] [PubMed] [Google Scholar]
- 28.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 29.Soreide K, Janssen EA, Korner H, Baak JP. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–156. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]
- 30.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–926. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas NE. BRAF somatic mutations in malignant melanoma and melanocytic naevi. Melanoma Res. 2006;16:97–103. doi: 10.1097/01.cmr.0000215035.38436.87. [DOI] [PubMed] [Google Scholar]
- 32.Tibshirani Ea . An introduction to the Boostrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 33.Vial E, Sahai E, Marshall CJ. ERK-MAPK signaling coordinately regulates activity of Rac1 and RhoA for tumor cell motility. Cancer Cell. 2003;4:67–79. doi: 10.1016/S1535-6108(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Edington HD, Rao UN, Jukic DM, Land SR, Ferrone S, Kirkwood JM. Modulation of Signal Transducers and Activators of Transcription 1 and 3 Signaling in Melanoma by High-Dose IFN{alpha}2b. Clin Cancer Res. 2007;13:1523–1531. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Edington H, Rao UN, Jukic D, Mascari R, Sander C, Ferrone S, Moschos S, Kirkwood JM (2005) Effects of neoadjuvant high-dose interferon (IFNα2b) upon STAT signaling, IFNαRβ, MHC and Tap expression in lymph node metastatic melanoma (UPCI 008). Proc AACR 2005 (Abstr. 6003)
- 36.Wang W, Edington HD, Rao UN, Jukic D, Land S, Mascari R, Sander C, Kirkwood JM (2006) Impact of neoadjuvant high-dose IFNα2b (HDI) upon STAT and MEK/ERK MAPK pathways in lymph node metastatic melanoma. Proc AACR 2006 (Abstr. 6198)
- 37.Wang W, Edington H, Rao UN, Jukic D, Wang H, Mascari R, Sander C, Kirkwood JM (2006) Dose-depended modulation of STAT signaling in the atypical/dysplastic nevus by exposure to interferon α. Proc AACR (Abstr. 5815)
- 38.Webb CP, Van Aelst L, Wigler MH, Woude GF. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J, Luo H. Recent advances on T-cell regulation by receptor tyrosine kinases. Curr Opin Hematol. 2005;12:292–297. doi: 10.1097/01.moh.0000166497.26397.9f. [DOI] [PubMed] [Google Scholar]
- 40.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 42.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YL, Dong C. MAP kinases in immune responses. Cell Mol Immunol. 2005;2:20–27. [PubMed] [Google Scholar]