Abstract
Natural cytotoxicity receptors (NCRs) are major activating receptors involved in NK cytotoxicity. NCR expression varies with the activation state of NK cells, and the expression level correlates with NK cells’ natural cytotoxicity. In this study, we found that Gö6983, a PKC inhibitor, induced a remarkable increase of NCR expression on primary NK cells, but other PKC inhibitors and NK cell stimulators such as IL-2 and PMA, did not. Gö6983 increased the expression of NCR in a time- and concentration-dependent manner. Furthermore, Gö6983 strongly upregulated the surface expression of death ligands FasL and TRAIL, but not cytotoxic molecules perforin and granzyme B. Unlike two other NK stimulating molecules, IL-2, and PMA, Gö6983 did not induce NK cell proliferation. Up-regulation of NCRs and death ligands on NK cells by Gö6983 resulted in a significant enhancement of NK cytotoxicity against various cancer cell lines. Most importantly, administration of Gö6983 effectively inhibited pulmonary tumor metastasis in mice in a dose-dependent manner. These results suggest that Gö6983 functions as an NK cell activating molecule (NKAM); this NKAM is a novel anti-cancer and anti-metastasis drug candidate because it enhances NK cytotoxicity against cancer cells in vivo as well as in vitro.
Keywords: Natural killer cell, Natural cytotoxicity receptor, NK activating molecule
Introduction
Natural killer (NK) cells are a distinct subset of large granular lymphocytes which have the ability to kill tumor cells and virus-infected cells, as well as certain bacteria and intracellular parasites [1–5]. NK cells were originally characterized by their ability to lyse certain tumor cells without prior immune sensitization [6]. Recent studies have shown that NK cells play a particularly important role in the suppression of tumor growth and metastasis. NK cells use three distinct mechanisms to kill tumor cells. First, targeted cell lysis by NK cells is mediated by secretory granules which contain perforin, granzymes, and other cytotoxic molecules. Perforin induces necrosis of target cells and granzymes induce apoptosis of target cells [7–9]. Second, the nonsecretory/apoptotic pathway which is mediated by FasL/Fas, TNF/TNF receptor, and TRAIL/TRAIL receptor interactions also plays an important role in NK cytotoxicity [8, 10–14]. Finally, NK cells also use certain cytokines to restrict tumor growth and stimulate adaptive immunity [15].
NK cytotoxicity is tightly regulated by a delicate balance between activating signals and inhibitory signals transmitted through NK cell-activating receptors and inhibitory receptors, respectively [15, 16]. Human inhibitory NK cell receptors (inhibitory NKRs) consist of the C-type lectin family members CD94/NKG2A and B, and Ig superfamily members such as the inhibitory killer Ig-like receptors (KIRs), leukocyte Ig-like receptors (LIRs) and leukocyte-associated inhibitory receptors (LAIRs). Inhibitory NKRs contain one or more immunoreceptor tyrosine based inhibition motifs (ITIMs) in the cytoplasmic region, and most inhibitory NKRs interact with class I MHC or its homologs, thus inhibiting lysis of target cells [5, 16, 17].
Natural cytotoxicity receptors (NCRs) and NKG2D are the major activating receptors which recognize susceptible target cells and directly mediate NK cytotoxicity [18–20]. Human NKG2D recognizes MICA, MICB, and the family of UL16-binding proteins (ULBP1-4) [19, 21, 22]. NK cells express three different NCRs [20]: NCR1, 2, and 3 (also called NKp46, NKp44, and NKp30, respectively). NCR1 and NCR3 are expressed on both resting and activated NK cells, while NCR2 is upregulated during in vitro culture with IL-2 [23, 24]. NCR1 has an extracellular portion characterized by two C2-type Ig-like domains, while NCR2 and NCR3 both have an extracellular region containing a single type V Ig-like domain [24–28]. NCR transmembrane regions contain positively charged amino acids that are thought to be crucial for their association with CD3ζ or DAP-12 [24, 25, 29]. Recent studies suggest that membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NCR1, 2, and 3 [30], but other cellular ligands recognized by NCRs have not yet been characterized. NCR1 and NCR2 have also been reported to recognize viral proteins such as influenza virus hemagglutinin, parainfluenza virus hemagglutinin–neuraminidase, as well as putative viral proteins [31, 32]. The main tegument protein (pp65) of human cytomegalovirus has also been reported as a ligand of NCR3 [33].
The surface density of NCRs appears to differ among NK cells, although the mechanisms of NCR expression and regulation are not well understood. Interestingly, NK cytolytic activity against susceptible tumor cells is proportional to the surface density of NCRs [23, 24]. NK cells with high NCR expression display stronger cytotoxic activity against target cells than cells with low NCR expression [16, 23]. Furthermore, prolactin-induced upregulation of NCR expression augmented NK cytotoxicity against tumor cells, while NCR downregulation by corticosteroids or TGF-β1 reduced NK cytotoxicity [34–36]. Similarly, NK cells purified from HIV-1-infected patients and AML patients expressed significantly decreased levels of NCRs, and this defective NCR expression was associated with the inability of NK cells to kill target cells [37, 38].
In this study, we found an NK cell-activating molecule (NKAM) which increased the surface expression of NCRs and death ligands responsible for NK cell-mediated cytotoxicity, and thereby enhanced the natural cytotoxicity of NK cells. We also demonstrated the in vitro and in vivo efficacy of this compound as an anti-cancer and anti-metastasis drug candidate.
Materials and methods
NK cell preparation
NK cells were purified from the whole blood of nine healthy volunteers by negative selection using the RosetteSep™ NK enrichment antibody cocktail (StemCell Technologies Inc., Vancouver, Canada), as previously described [39].
Cell lines and cell culture
Hepatocellular carcinoma cell lines HepG2 (ATCC HB 8065) and Hep3B (ATCC HB 8064) were used as target cells and maintained in MEM containing 10% FBS (Gibco BRL). HeLa cells (ATCC CCL 13) was cultured in DMEM containing 10% FBS.
Antibodies and reagents
For flow cytometry, PE-conjugated anti-NCR antibodies (anti-NKp46 mAb, anti-NKp44 mAb, and anti-NKp30 mAb) were purchased from Beckman Coulter (Fullerton, CA, USA). PE-conjugated anti-FasL mAb and anti-TRAIL mAb were purchased from Biolegend (San Diego, CA, USA). PE-conjugated anti-perforin and anti-granzyme B mAbs were purchased from Becton Dickinson Bioscience (Lincoln Park, NJ, USA). PMA, Lactacystin, PD150606, PD98059, Rottlerin, Gö6976, Gö6983 and BisIII were purchased from Calbiochem (San Diego, CA, USA). IL-2, IL-8, IL-12, IL-15, IL-18, IFN-α1, and IFN-α2b were purchased from ATGen (Sungnam, Korea). PKCζ pseudosubstrate was purchased from Biosource (Vamarillo, CA).
Flow cytometric analysis
Cell surface NCRs and death ligands were quantified by flow cytometric analysis. NK cells were washed twice with ice-cold PBS containing 0.05% BSA (BSA–PBS). Cells were incubated with specific antibodies for 30 min at 4°C. After two washes with BSA–PBS, cells were analyzed using a FACScalibur flow cytometer (Becton Dickinson Bioscience, Lincoln Park, NJ, USA). The data are expressed as relative mean fluorescence intensity (MFI) ratio (experimental MFI/MFI of the 0 h or the none-treated group). MFI ratio means MFI of PE-conjugated anti-receptor Ab stained cells/MFI of PE-conjugated anti-mouse IgG stained cells.
RT-PCR analysis
Total RNA was extracted from NK cells and tumor cell lines using an RNAeasy Kit (Qiagen, Santa Clara, CA, USA). The integrity of isolated total RNA was confirmed by 1.5% agarose gel electrophoresis. To synthesize cDNA, 1 μg of each RNA sample was mixed with 100 ng random hexamer, 6 μl of 5× first strand buffer, 12 μl of 2.5 mM dNTPs (TaKaRa, Shiga, Japan) and 200 U of murine Molony leukemia virus reverse transcriptase (MMLV-RT) (Invitrogen) and incubated at 42°C for 80 min. The reaction mixture was boiled at 95°C for 5 min, quickly chilled on ice, and then used for PCR without further manipulation. The PCR reaction mixture was prepared with 2.5 μl of cDNA, 2 μl of 2.5 mM dNTPs, 20 pmol primer, 2.5 μl of 10× PCR buffer, 13.8 μl of distilled water, and 1 U of Taq polymerase (TaKaRa). PCR reactions were performed with the appropriate primers. NCR1 was amplified with the sense primer, 5′-TATACGGAATTCATGTCTTCCACACTCCCTGCC-3′, and antisense primer, 5′-GACACCAAGCTTTCAAAGAGTCTGTGTGTTCAGCCTTCT-3′. NCR3 was amplified with the sense primer, 5′-ATCAATGAATTCATGGCCTGGATGCTGTTGCTCATC-3′, and antisense primer, 5′-GCCTTTAAGCTTCTAGGGACATCTGGGCTCTGGAATCAC-3′. Perforin was amplified with the sense primer, 5′-ATGTCACCTTGGGGGCCCACA-3′, and antisense primer, 5′-TTTCGTCCATAGGAGACAATG-3′. Granzyme B cDNAs were amplified with the sense primer, 5′-CGCCTACCTCAGGCTTATCTC-3′, and antisense primer, 5′-GGATGAAGGTCACCTCCAGCT-3′.
NK cytotoxicity assay
NK cell-mediated apoptotic target cell death was measured using a 2-h [3H]-thymidine release assay (the JAM test), as previously described [39]. NK cell-mediated target cell killing was also assessed using the standard 51Cr release assay, as previously described [39].
LDH assay
The cytotoxic effect of Gö6983 was assessed by LDH assay using the CytoTox 96 assay kit (Promega, Madison, USA). NK cells and PBMCs were prepared in 96-well microtiter plates for use in the assay. For the LDH positive control, 10 μl of Lysis solution (10×) was added to all wells to lyse cells and the cells were incubated for 45 min. Fifty microliter aliquots from all wells was transferred to a fresh 96-well flat-bottom (enzymatic assay) plate. Fifty microliters of reconstituted Substrate Mix was added to each well of the plate, and the plate was then incubated for 30 min in the dark. After 30 min, 50 μl of Stop Solution was added to each well and the absorbance was recorded at 490 nm within 1 h using an ELISA plate reader.
Animal studies: pulmonary tumor metastasis assay
Specific pathogen-free female C57BL/6 mice, 8 weeks of age, were purchased from Orient Bio (Seoul, Korea). Mice were maintained in a temperature-controlled, air-conditioned animal house with 14- to 10-h-light/dark cycles, and received food and water ad libitum. Animals were cared for and treated in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23). B16BL6 cells, a highly metastatic subline of murine B16 melanoma, were purchased from Korea Cell Line Bank (KCLB No., 80006, Seoul, Korea). B16BL6 cells were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin G, and 25 mg/ml streptomycin (Life Technologies) at 37°C under a humidified atmosphere of 5% CO2 in air. For tumor metastasis assays, cells were used between passages 5 and 8. Experimental metastasis cells were assessed following i.v. inoculation on day 0 of C57BL/6 mice with B16BL6 cells at a concentration of 1 × 105 cells in 0.2 ml of PBS. To evaluate the metastasis inhibition activity of Gö6983, mice receiving B16BL6 cells were intravenously injected a total of four times with 4.4 or 22.0 μg of Gö6983 in 0.2 ml of PBS on days −2, 0, 2, and 4 (n = 9).
Results
Regulation of NCR expression in primary NK cells
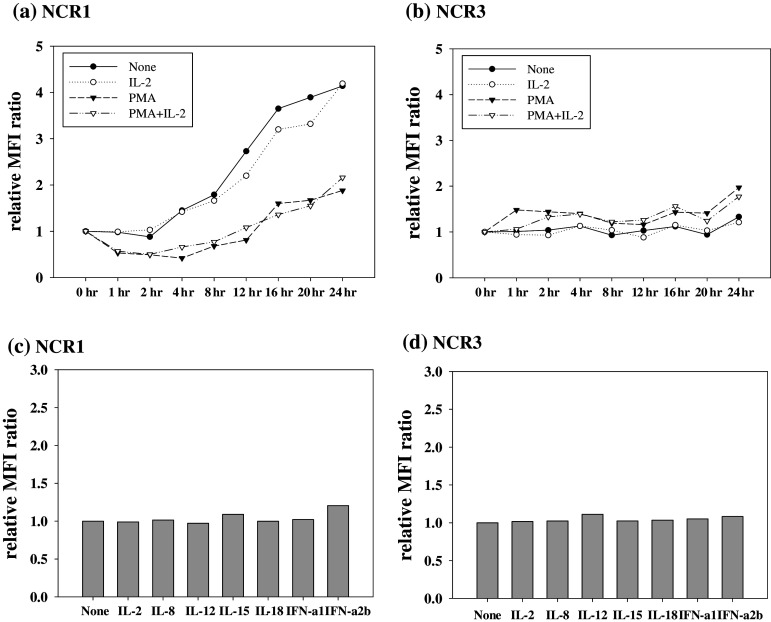
To understand how NCR expression is regulated in NK cells, we first investigated the effects of PMA and IL-2, which are known to activate NK cells, on the expression of NCR. Flow cytometric analysis showed that NCR1 (NKp46) expression on primary NK cells increased as a function of incubation time in untreated cultures (Fig. 1a). As shown in Fig. 1a, treatment of cells with IL-2 did not affect the expression of NCR1 on NK cells compared to untreated control group cells. In the presence of PMA, however, increase of NCR1 on NK cells was less profound compared to control group cells (Fig. 1a). Unlike NCR1, NCR3 (NKp30) expression on primary NK cells appeared to increase slightly after addition of PMA to the culture while IL-2 treatment did not appear to increase NKp30 expression compared to untreated cells (Fig. 1b). We next examined the effects of several cytokines which known activators of NK cells or other immune cells. As shown in Fig. 1c, d, treatment of cells with IL-2, IL-8, IL-12, IL-15, IL-18, IFN-α1, or IFN-α2b for 24 h did not significantly change either NCR1 (Fig. 1c) or NCR3 (Fig. 1d) expression on primary NK cells. Inhibitors of MAP kinase (MEK), calpain, and proteosomes (PD98059, PD150606, and lactacystin, respectively) also did not appear to induce NCR expression on primary NK cells (data not shown).
Fig. 1.
Regulation of NCR expression on NK cells by IL-2, PMA, and cytokines. a NCR1 and b NCR3 surface expression on primary NK cells after indicated treatments (1 ng/ml of IL-2 and 100 ng/ml of PMA) are represented by the relative MFI ratio. NCR expression was investigated as a function of time. c NCR1 and d NCR3 expression on primary NK cells 24 h after cytokine (50 ng/ml of IL-8, IL-12, IL-15, IL-18, IFN-α1 and IFN-α2b) treatment were determined by flow cytometry and are presented as the relative MFI ratio. Similar results were obtained from at least three independent experiments using NK cells from different donors
Effects of PKC inhibitors on the surface expression of NCRs
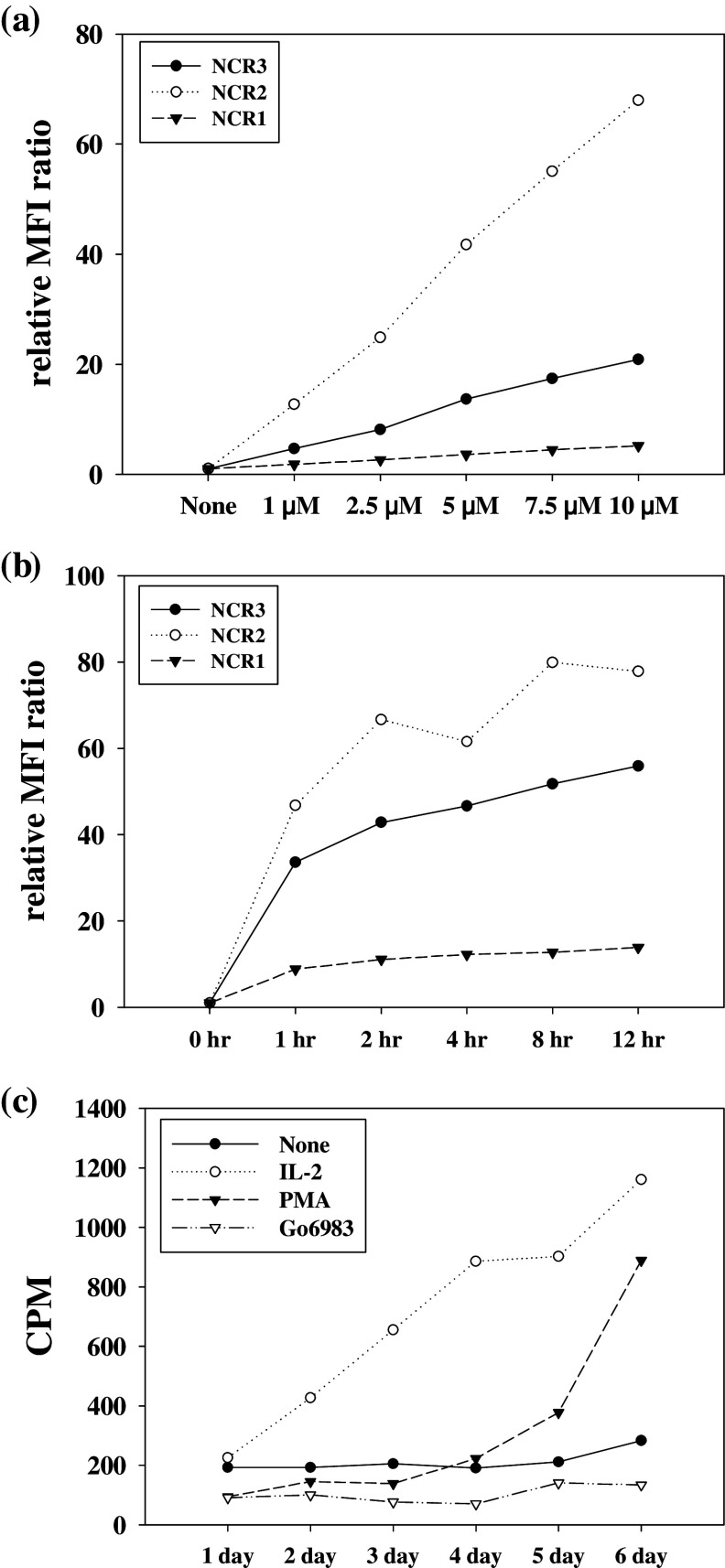
Because PMA, a PKC activator, appeared to be more effective in regulating NCR expression than any other cytokine or inhibitor tested (Fig. 1), we further studied the effects of various PKC inhibitors, Gö6976, Gö6983, Rottlerin, and Bisindolylmaleimide III (BisIII), on NCR expression. Interestingly, Gö6983 induced a dramatic increase in NCR1 expression on primary NK cells, while the other compounds did not (Fig. 2a). The relative MFI ratio of Gö6983-treated NK cells was about fourfold higher than that of the control, while NK cells treated with the other PKC inhibitors had ratios similar to that of untreated NK cells. Gö6983 also increased the expression of NCR3 about 20-fold compared to the control (Fig. 2b). Next, we measured NCR mRNA expression in primary NK cells after Gö6983 treatment. Consistent with the surface expression of NCRs, only Gö6983 induced prominent transcription of NCR1 and NCR3 mRNA, and these mRNA transcripts gradually increased until 12 h (Fig. 2c). In addition, the mRNA levels of NCR1 and NCR3 remained increased until 24 h (data not shown). Furthermore, Gö6983 also increased the cell surface expression of NCR2 (NKp44) (Fig. 2d), which is expressed only on activated NK cells [23, 24, 29]. However, PMA treatment did not induce the expression of NCR2 (Fig. 2d).
Fig. 2.
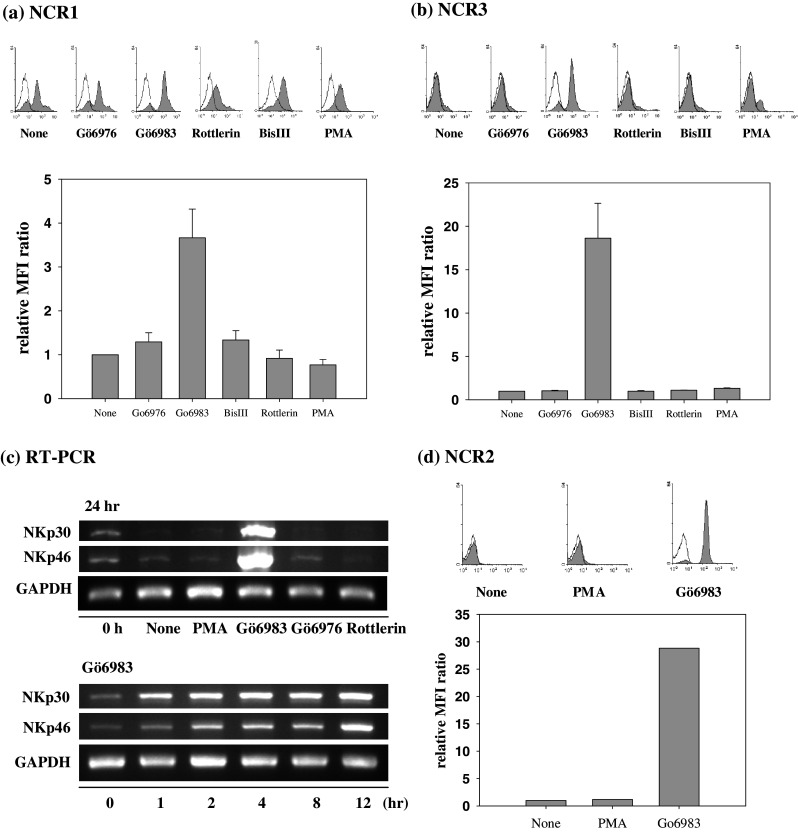
Effects of PKC inhibitors on NCR expression a NCR1 and b NCR3 expression in primary NK cells were determined by flow cytometry and are presented by histograms (upper panel) and relative MFI ratio (lower panel). The inhibitors were used at 5 μM for 24 h. The data are presented as the mean of at least three independent experiments (mean ± SD). c Levels of NCR mRNA in primary NK cells after the indicated treatments were determined by RT-PCR. The internal control was GAPDH. d NCR2 expression on primary NK cells after PMA and Gö6983 treatment was determined by flow cytometry and is presented by histograms (upper panel) and relative MFI ratio (lower panel). PMA was used at 100 ng/ml and Gö6983 at 5 μM for 24 h. Black line isotype control, Filled NCR expression following indicated treatment
We next investigated the effects of concentration and incubation time of Gö6983 on the surface expression of NCRs on NK cells (Fig. 3). The expression of NCR1 increased in a time-dependent manner (Fig. 3b) and was directly related to the concentration of Gö6983 used (Fig. 3a). Expression of NCR2 and NCR3 also increased in a dose and time-dependent manner (Fig. 3a, b). NCR expression reached almost maximum level within 2 h (Fig. 3b), and the expression level remained elevated for 24 h (data not shown).
Fig. 3.
Effects of Gö6983 upon NCR expression and NK cell proliferation NCR expression was determined by flow cytometry and is presented are the relative MFI ratio. NK cells were treated (a) for 4 h with indicated concentration of Gö6983 and (b) for the indicated times with 5 μM of Gö6983. c Proliferation was examined by a [3H]-thymidine uptake assay. NK cells were treated for the indicated times with IL-2 (1 ng/ml), PMA (100 ng/ml), or 5 μM Gö6983. The radioactivity was measured using a β-counter. Similar results were obtained from at least three independent experiments
We also examined the effect of Gö6983 on NK cell proliferation using a [3H]-thymidine uptake assay. In contrast to NK cells treated with IL-2 or PMA, thymidine uptake did not increase in Gö6983-treated NK cells (Fig. 3c). The ratio represents the CPM of experimental time point to the CPM of 1 day. These results indicate that IL-2 and PMA induced NK cell proliferation but Gö6983 did not.
Effect of Gö6983 on the expression of death ligands and cytotoxic molecules
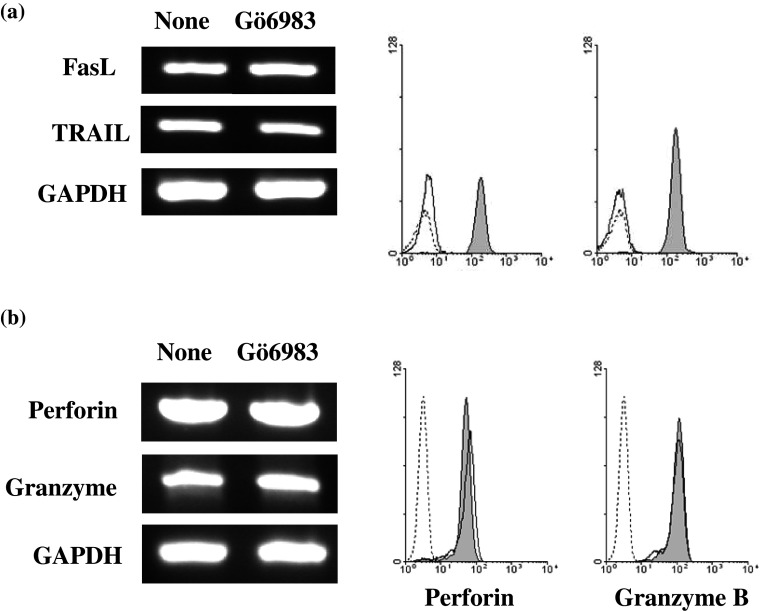
The effects of Gö6983 on the expression of death ligands and cytotoxic molecules were investigated following Gö6983 treatment of NK cells. As shown in Fig. 4a, FasL and TRAIL were minimally expressed by untreated NK cells, while Gö6983 treatment dramatically increased the surface expression of FasL and TRAIL. However, RT-PCR analysis indicated that Gö6983 treatment did not significantly increase expression of FasL and TRAIL transcripts, suggesting that the increase of FasL and TRAIL surface expressions by Gö6983 were not transcriptionally regulated. Unlike the cases of NCRs and death ligands, Gö6983 treatment did not cause a significant increase in perforin and Granzyme mRNA transcripts, and it did not increase the expression of those proteins in NK cells (Fig. 4b).
Fig. 4.
Effects of Gö6983 on the expression of death ligands and cytotoxic molecules in primary NK cells. a Expression of death ligands on primary NK cells were determined by RT-PCR and flow cytometry analysis. b Expression levels of perforin and granzyme B mRNAs and proteins in primary NK cells after 5 μM Gö6983 treatment for 4 h were determined by RT-PCR and intracellular flow cytometry. Dotted line isotype control, Black line none, Filled Gö6983 treatment)
Gö6983 enhanced NK cell-mediated tumor cell killing
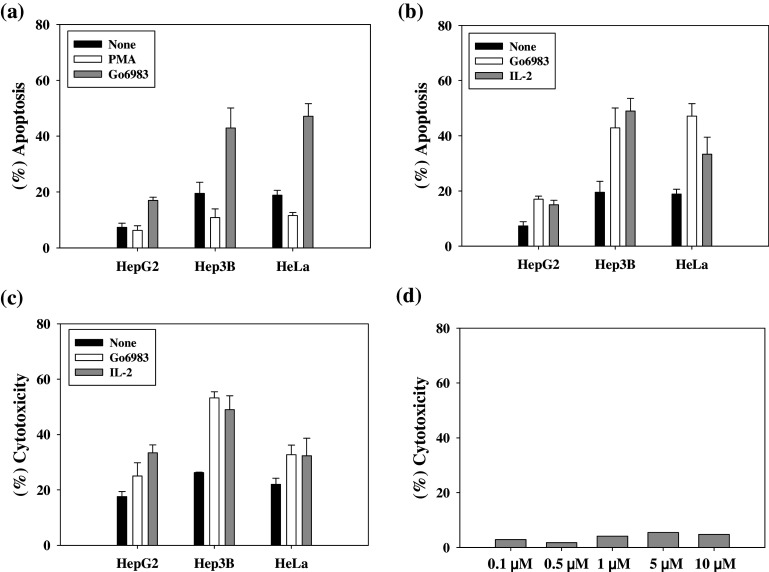
To test the role of increased expression of NCRs and death ligands in NK cytotoxicity, we studied the lysis of tumor cells by NK cells pretreated with Gö6983. NK cytotoxicity in three cancer cell lines, HepG2, Hep3B, and HeLa, was first assessed by the JAM test, a [3H]-thymidine release assay (Fig. 5a). When target cells were co-incubated with NK cells for 2 h, at an optimum E:T ratio of 3:1 [39], Hep3B and HeLa cells exhibited a significant amount of DNA fragmentation, whereas HepG2 cells showed a relatively small amount of DNA fragmentation (Fig. 5a, black bars). When the target cells were co-incubated with NK cells pretreated with Gö6983, all three cancer cell lines exhibited about a twofold increase in apoptotic cell death (gray bars). Although HepG2 targets were resistant to apoptotic cell death induced by untreated NK cells, Gö6983-treated NK cells successfully induced apoptosis in HepG2. In contrast, when the target cells were co-incubated with NK cells pretreated with PMA, all three cancer cell lines exhibited a slightly decreased amount of apoptotic cell death (white bars).
Fig. 5.
Effects of a PKC inhibitor (Gö6983) upon NK cytotoxicity The NK activity of target cell lysis was determined by a, b a JAM test and c a 51Cr release assay. Primary NK cells were incubated with indicated materials for 24 h and added to target cells at an E:T ratio of 3:1. NK cells and target cells were cocultured for 2 h (JAM test) or 4 h (51Cr release assay). The data are presented as a mean of at least three independent experiments (mean ± SD) using NK cells from one donor, and the effects of Go6983 on NK cytotoxicity appeared to be statistically significant. d Gö6983 is not cytotoxic by itself. Cytotoxic effects of Gö6983 against HeLa cells were measured by LDH assays. Primary NK cells and PBMCs were treated with 5 μM Gö6983, and HeLa cells were treated with 0.1, 0.5, 1.0, 5.0, and 10 μM Gö6983 for 4 h
We next compared the effect of Gö6983 on NK cytotoxicity against tumor cells with that of IL-2 treatment of NK cells, which is well known to activate NK cells. Interestingly, in the JAM test (Fig. 5b) and 51Cr release assay (Fig. 5c), Gö6983 treatment appeared to enhance NK cytotoxicity to a similar degree as IL-2 in all target cell lines, although the known mechanisms of action for Gö6983 and IL-2 are quite different.
To determine if Gö6983 itself is able to kill cancer cells; the cytotoxicity of Gö6983 was assessed by LDH assay in HeLa cells. As shown in Fig. 5d, Gö6983 did not appear to kill a significant number of HeLa cells. Similar results were obtained in PBMCs or NK cells treated with increasing concentrations of Gö6983 (data not shown), suggesting that Gö6983 is not itself cytotoxic.
Inhibition of tumor metastasis by Gö6983 administration
Finally, we investigated the in vivo efficacy of Gö6983 administration in a melanoma mouse model. In vivo activation of NK cells by Gö6983 administration and its effects on experimental metastases were assessed by counting melanoma foci on the lung surface following i.v. inoculation of C57BL/6 mice with B16BL6 cells on day 0. Mice were injected once per day on days −2, 0, 2, and 4 with either 4.4 or 22.0 μg of Gö6983. As shown in Fig. 6a, melanoma foci on the lung surface were decreased in mice treated with Gö6983. Administration of 4.4 μg Gö6983 caused a statistically significant inhibition of tumor metastasis as measured by a 43.2% decrease in pulmonary foci compared with control mice (p < 0.01) (Fig. 6b). Furthermore, higher doses of Gö6983 (22.0 μg) more strongly inhibited tumor metastasis in this pulmonary tumor metastasis model (51.2%, p < 0.01) (Fig. 6b).
Fig. 6.
Inhibition of tumor metastasis by Gö6983 in a mouse model B16Bl6 cells (1 × 105 cells/mouse) were injected into the tail veins of C57BL/6 mice on day 0. Mice were treated with PBS or NKAMs (4.4 or 22.0 μg per mouse) on days −2, 0, 2, and 4 a total of four times. On day 18, the melanoma foci on the lung surface were counted macroscopically. a The representative photographs show that the number of pulmonary melanoma foci was reduced by Gö6983 treatment. b The graph shows the mean number of metastatic foci counted on day 18 for each treatment ±SD (n = 9, p < 0.01)
Discussion
We have demonstrated that a bisindolylmaleimide compound, Gö6983, functions as a NK cell activating molecule. Unlike other PKC inhibitors, Gö6983 increased the expression of NCRs and death ligands such as FasL and TRAIL, on the surface of NK cells. Gö6983 also slightly increased the expression of perforin and granzyme B in NK cells. However, unlike two other NK stimulating molecules, IL-2 and PMA, Gö6983 did not induce NK cell proliferation. As a consequence of the upregulation of NCRs, death ligands and cytotoxic granules, NK cytotoxicity against HCCs, and HeLa cells appeared to be increased after Gö6983 treatment in vitro, despite Gö6983 having no cytotoxic properties itself against normal and cancer cells. Most importantly, Gö6983 appeared to inhibit pulmonary tumor metastasis in a mouse model. This finding suggests that Gö6983 could be utilized to enhance NK cytotoxicity in vivo to increase host immunity against cancers.
Since PMA, a PKC activator, appeared to modulate NCR expression in primary NK cells (Fig. 1), we examined how PKC inhibitors affect NCR expression. Typical inhibitors of PKC isotypes including Rottlerin for novel PKCs, Gö6976 for conventional PKCs, Gö6983 for broad PKC isotypes, and BisIII for PKC/PKA [40], were selected and tested in this study. Among these PKC inhibitors, only Gö6983 induced a significant increase of NCR expression in primary NK cells (Fig. 2). The PKC isotypes inhibited by Gö6983 are PKC α, β, γ, δ, and ζ [40]. Among these PKC isotypes, PKC α, β, γ, and δ are also inhibited by Gö6976 and rottlerin. Thus, PKC ζ is the only isotype that might be inhibited by Gö6983 alone, suggesting that PKC ζ might be involved in the upregulation of NCR expression upon Gö6983 treatment, if PKC inhibition is responsible for the Gö6983-induced upregulation of NCRs. However, the pseudosubstrate for PKC ζ (from Biosource), a PKC ζ specific inhibitor [41], did not significantly induce the surface expression of NCR (data not shown). More elaborate studies are needed to elucidate the specific molecular mechanism of NCR upregulation by Gö6983 observed in this study; however, it seems highly likely that NK cell activation by Gö6983 occurs via an unknown mechanism other than PKC inhibition.
Many studies have shown that NK cells can kill a variety of different mouse and human tumor cells in vitro and in vivo, and are also involved in the removal of experimentally induced and spontaneously developing tumors in mice [3, 15, 42]. Human NK cells adoptively transferred into mice also appeared to be able to kill human tumor grafts. Recently, clinical hematopoietic stem cell transplantation and adoptive NK cell therapy studies have also shown that NK cells play an important role in the destruction of human tumor cells [43, 44]. Furthermore, many lines of evidence indicate that increased NK activity by cytokine treatment is well correlated with increased antitumor responses [45, 46]. These findings suggest that NK cells can be used to cure cancers if properly modulated by NK activating molecules.
Cytokines, such as IL-2, IL-12, IL-15, and IFNs, and nonspecific immunostimulatory molecules, such as BCG and certain DNA complexes, are known to activate NK cells [15, 47]. Immunomodulatory drugs, such as thalidomide and lenalidomide, are also able to activate NK cells, although their mechanisms of action are not well known [15]. Interestingly, many studies have suggested that NK activating molecules, such as cytokines and immunostimulatory DNA complexes, can be used to control cancer immunologically and to inhibit metastases to other organs [47, 48]. For example, several clinical studies have been performed to assess the effect of IL-2, IL-12, and IL-15 on the treatment of cancer by activation and expansion of NK cells in cancer patients [5, 46, 49, 50]. The NKAM found in this study, Gö6983, is quite different from these immunomodulating molecules, and it activates NK cells via distinct mechanisms, including upregulation of NCRs and expression of death ligands. In fact, this NKAM enhanced NK cytotoxicity against cancer cell lines twofold in vitro and effectively inhibited tumor metastasis to lungs in a mouse model. These results suggest that Gö6983 could be a novel anti-cancer drug candidate that enhances the tumor immunity of NK cells.
Preclinical and clinical studies with NK cells are more focused on the cure of various cancers, although NK cells play an important role in immune defense against viral infections, in tumor control, and in graft-versus-leukemia reactions [1]. These studies have revealed that NK cells play an important role in killing of not only circulating tumor cells, but also well-established micrometastases [47]. Many lines of evidence also indicate that NK cells are efficient at removing metastasizing cells and small tumor grafts, but not larger solid tumors [15]. Therefore, NK cell immunotherapy is most likely to be effective in situations with small tumor burdens [48]. In this sense, Gö6983 or its derivatives might be most effective for cancer patients with minimum residual diseases, e.g., following surgery, chemotherapy or radiotherapy. NK cell activation by NKAM administration may prevent cancer relapses after surgery or chemotherapy because they can kill residual cancer cells and even drug-resistant cancer cells.
Certain tumor types are known to be more sensitive to NK cells, and these tumor types should be primary targets for the therapeutic application of the NKAM or its derivatives. For example, myeloid leukemia is more sensitive to NK-mediated lysis than lymphoblastic leukemia [51]. It has been demonstrated that while organ resistance to metastases correlates well with NK activity of the host, a clear correlation between NK activity and tumor cell clearance is found only in the lungs [47]. Takeda and coworkers have shown that liver NK cells play an important role in anti-metastasis to this organ by TRAIL-mediated tumor cell killing [14]. Taken together, the NKAM developed in this study and its optimized derivatives could be primarily tested for the treatment of leukemia and lung cancer, as well as for the prevention of metastases to various organs.
Acknowledgments
This study was supported in part by a basic research grant (R01-2007-000-20089-0) and by a medical research center grant (R13-2002-054-03003-0) of KOSEF.
Abbreviations
- NK cells
Natural killer cells
- NCRs
Natural cytotoxicity receptors
- HCCs
Hepatocellular carcinoma cells
- FasL
Fas ligand
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- PKC
Protein kinase C
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/S0065-230X(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 5.Miller JS. The biology of natural killer cells in cancer, infection, and pregnancy. Exp Hematol. 2001;29:1157–1168. doi: 10.1016/S0301-472X(01)00696-8. [DOI] [PubMed] [Google Scholar]
- 6.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 7.Podack ER, Hengartner H, Lichtenheld MG. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 8.Screpanti V, Wallin RP, Ljunggren HG, Grandien A. A central role for death receptor-mediated apoptosis in the rejection of tumors by NK cells. J Immunol. 2001;167:2068–2073. doi: 10.4049/jimmunol.167.4.2068. [DOI] [PubMed] [Google Scholar]
- 9.Yagita H, Nakata M, Kawasaki A, Shinkai Y, Okumura K. Role of perforin in lymphocyte-mediated cytolysis. Adv Immunol. 1992;51:215–242. doi: 10.1016/S0065-2776(08)60488-5. [DOI] [PubMed] [Google Scholar]
- 10.Arase H, Arase N, Saito T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J Exp Med. 1995;181:1235–1238. doi: 10.1084/jem.181.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashii Y, Giorda R, Herberman RB, Whiteside TL, Vujanovic NL. Constitutive expression and role of the TNF family ligands in apoptotic killing of tumor cells by human NK cells. J Immunol. 1999;163:5358–5366. [PubMed] [Google Scholar]
- 12.Lee RK, Spielman J, Zhao DY, Olsen KJ, Podack ER. Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol. 1996;157:1919–1925. [PubMed] [Google Scholar]
- 13.Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995;166:236–246. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 15.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 16.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–660. doi: 10.1016/S0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 18.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–234. doi: 10.1016/S0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 19.Watzl C. The NKG2D receptor and its ligands-recognition beyond the “missing self”? Microbes Infect. 2003;5:31–37. doi: 10.1016/S1286-4579(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 20.Biassoni R, Cantoni C, Marras D, Giron-Michel J, Falco M, Moretta L, Dimasi N. Human natural killer cell receptors: insights into their molecular function and structure. J Cell Mol Med. 2003;7:376–387. doi: 10.1111/j.1582-4934.2003.tb00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/S1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 22.Chalupny NJ, Sutherland CL, Lawrence WA, Rein-Weston A, Cosman D. ULBP4 is a novel ligand for human NKG2D. Biochem Biophys Res Commun. 2003;305:129–135. doi: 10.1016/S0006-291X(03)00714-9. [DOI] [PubMed] [Google Scholar]
- 23.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster CE, Colonna M, Sun PD. Crystal structure of the human natural killer (NK) cell activating receptor NKp46 reveals structural relationship to other leukocyte receptor complex immunoreceptors. J Biol Chem. 2003;278:46081–46086. doi: 10.1074/jbc.M308491200. [DOI] [PubMed] [Google Scholar]
- 27.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantoni C, Ponassi M, Biassoni R, Conte R, Spallarossa A, Moretta A, Moretta L, Bolognesi M, Bordo D. The three-dimensional structure of the human NK cell receptor NKp44, a triggering partner in natural cytotoxicity. Structure. 2003;11:725–734. doi: 10.1016/S0969-2126(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 29.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershkovitz O, Jivov S, Bloushtain N, Zilka A, Landau G, Bar-Ilan A, Lichtenstein RG, Campbell KS, van Kuppevelt TH, Porgador A. Characterization of the recognition of tumor cells by the natural cytotoxicity receptor, NKp44. Biochemistry. 2007;46:7426–7436. doi: 10.1021/bi7000455. [DOI] [PubMed] [Google Scholar]
- 31.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 32.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::AID-IMMU2680>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Arnon TI, Achdout H, Levi O, Markel G, Saleh N, Katz G, Gazit R, Gonen-Gross T, Hanna J, Nahari E, Porgador A, Honigman A, Plachter B, Mevorach D, Wolf DG, Mandelboim O. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 34.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitale C, Chiossone L, Cantoni C, Morreale G, Cottalasso F, Moretti S, Pistorio A, Haupt R, Lanino E, Dini G, Moretta L, Mingari MC. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur J Immunol. 2004;34:3028–3038. doi: 10.1002/eji.200425418. [DOI] [PubMed] [Google Scholar]
- 36.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin Exp Immunol. 2005;139:287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 38.Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D, Gastaut JA, Pende D, Olive D, Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 39.Kim HR, Park HJ, Park JH, Kim SJ, Kim K, Kim J. Characteristics of the killing mechanism of human natural killer cells against hepatocellular carcinoma cell lines HepG2 and Hep3B. Cancer Immunol Immunother. 2004;53:461–470. doi: 10.1007/s00262-003-0461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332(Pt 2):281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.RES.0000017631.28815.8E. [DOI] [PubMed] [Google Scholar]
- 42.Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- 43.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 44.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 45.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/S1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 46.Farag SS, Caligiuri MA. Cytokine modulation of the innate immune system in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:295–318. doi: 10.1016/S1054-3589(04)51013-X. [DOI] [PubMed] [Google Scholar]
- 47.Yang Q, Goding SR, Hokland ME, Basse PH. Antitumor activity of NK cells. Immunol Res. 2006;36:13–25. doi: 10.1385/IR:36:1:13. [DOI] [PubMed] [Google Scholar]
- 48.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 49.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/S1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 50.Barao I, Murphy WJ. The immunobiology of natural killer cells and bone marrow allograft rejection. Biol Blood Marrow Transpl. 2003;9:727–741. doi: 10.1016/j.bbmt.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R, Bacigalupo A, Mingari MC, Moretta A, Moretta L. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]