Abstract
Adjacent mucosa may reflect the conflicting of host factors in response to the establishment or invasion of cancers. Characterization of anti-tumor immunity in this region may add help in understanding the immune-related mechanisms of colorectal carcinoma (CRC). In this study, adjacent non-tumor mucosa from 36 patients with colorectal adenoma (CRA), 26 with CRC and normal mucosa from 15 health controls were included, immune cell populations of dendritic cell, lymphocyte and macrophage were characterized with immunohistochemistry (IHC) and tissue messenger RNA (mRNA) levels of Th1 cytokines interferon (IFN)-gamma and its upstream inducers interleukin (IL)-12 and IL-18 were quantified with real-time PCR; In addition, dendritic cell differentiation and function inhibitors cyclooxygenase-2 (COX-2) and IL-6 mRNA levels were also quantified. By IHC, a significant decreased dendritic cell density in the non-tumor mucosa adjacent to CRC was detected (P < 0.05) as compared to the normal controls or adjacent mucosa of CRA. The grading scores for lymphocyte number in the adjacent mucosa of CRA and CRC were gradually non-statistically increased, while the grading scores for macrophages number was not changed. By quantitative real-time PCR, distinct local cytokine gene expression profile was demonstrated. In which, the Th1 cytokines, particularly IL-12, were increased in adjacent mucosa of CRA, but all significantly decreased in adjacent mucosa of CRC. In addition, the mRNA levels of IL-6 and COX-2 were significantly higher in adjacent mucosa of CRC than that in adjacent mucosa of CRA (both P < 0.05). Therefore, dendritic cell functional changes could be one of the important mechanisms for altered anti-tumour immunity in the adjacent non-tumor mucosa throughout adenoma–carcinoma sequence. The increased COX-2 and IL-6 might contribute to dendritic cell funtional defect in adjacent mucosa of CRC.
Keywords: Premalignant lesions, Colorectal adenoma–carcinoma sequence, Anti-tumor immunity, Dendritic cell, Cytokine
Introduction
Colorectal carcinoma (CRC) is one of the most common human cancers all over the world. The occurrence of colorectal adenoma (CRA) is recognized as the earlier step in the carcinogenesis of normal mucosa. Normal-adenoma–carcinoma sequence is generally accepted as the description of a series of genetic and morphological changes during the development of CRC [1–3]. In response to the tumor initiation or invasion, there are reciprocal interactions between tumor cells and the host immune reacting components in the tumor microenvironment. The host immune system performs anti-tumor activity formed by cellular immunity. Tumor cells use diverse strategies to inhibit the local specific-tumor immunity in order to escape attack and prolong their survival [4]. This might reflect in disrupted host immune function including altered immune cell populations and dysregulated cytokine levels during the development of CRC [4]. Indeed, imbalanced cytokine network has been found and related to the progression of CRA to CRC [5, 6]; Recently, we were able to demonstrate a distinct microenvironmental cytokine gene profile between CRA and CRC, in which Th1 cytokines IL-12, IL-18 and IFN-gamma were slightly increased in CRA tissues, but all were significantly decreased in CRC tissues [7]. These results supported a suppressed host anti-tumor immunity in the tumor microenvironment.
In parallel to the multiple genetic changes in tumor site, a variety of alternations may also occur in surrounding normal tissues. Previously, it has been reported that the non-tumor mucosa adjacent to CRA or/and CRC shows histochemical abnormalities [8–11]. Filipe et al. [8] demonstrated changed mucin secretion in mucosa remote from adenoma and CRC. Shamsuddin et al. [12] showed abnormal morphology in this area. Furthermore, the alternation in adjacent mucosa may be reflected in genetic and molecular property changes compared with true normal mucosa [10], abnormalities have also been demonstrated in gene expressions of interleukin (IL)-8 and cyclooxygenase-2 (COX-2) in normal mucosa adjacent to CRC [13–18]. Thus, metabolic changes in the non-tumor mucosa adjacent to CRC may reflect the conflicting processes between the host and the arising or invasion of tumor. In view of these observations, we hypothesized that immune microenvironment in the adjacent non-tumor mucosa may be altered and reflected by abnormal immune cell presentations and cytokine expression profile.
Therefore, the aim of this study was to examine dynamic changes of immune response components in the non-tumor mucosa adjacent to adenoma–carcinoma sequence. Populations of dendritic cells, lymphocytes and macrophages were examined by immunohistochemistry (IHC). The expression of Th1 cytokines interferon gamma (IFN-gamma) and its upstream inducers IL-12 and IL-18 and dendritic cell differentiation and function inhibitors (COX-2 and IL-6) at messenger RNA (mRNA) level in the adjacent non-tumor mucosa were also measured with our improved real-time PCR method [19]. The results indicated that anti-tumor immunity was altered in the adjacent non-tumor mucosa throughout adenoma–carcinoma sequence.
Materials and methods
Patients and biopsies
Adjacent normal mucosa biopsies were collected from 36 patients (22 males, 14 females, age 43–90 years) with CRA, 26 patients (male 19, female 7, age 42–85 years) with CRC admitted to the Departments of Gastroenterology and Surgery, University Hospital of North Norway according to standardized diagnostic criteria. The definition of normal adjacent biopsies were: (1) taken from the normal appearing region 5∼7 cm far from pathological site and (2) confirmed to have grossly normal histology by heamatoxylin and eosin (H&E) staining. In addition, normal colorectal biopsies from 15 subjects without pathological evidence (11 males, 4 females, age 30–68 years) by colonoscopy were collected and used as heath controls. Detailed information for each group is presented in Table 1. The Norwegian Regional Ethical Committee of North Norway approved the study and the Norwegian Health Department approved the storage of human biological materials. Informed consent was obtained from the patients.
Table 1.
Basic histological information of patients and normal individuals
| N | Position | Pathology | Dysplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Colon | Rectum | Tubular | Tubulovillous | Villous | L | M | H | ||
| Normal | 15 | 10 | 5 | ||||||
| CRA | 36 | 20 | 16 | 23 | 11 | 2 | 23 | 8a | 5b |
| Duke’s Class | |||||||||
| Adenocarcinoma | Mucinous | Signet-ring | A | B | C | ||||
| CRC | 26 | 10 | 16 | 23 | 2 | 1 | 8 | 9 | 9 |
L: lower dysplasia; M: moderate dysplasia; H: high dysplasia
aThree patients with mixed dysplasis of L/M
bFour patients with mixed dysplasia of L/H
Total RNA extraction and cDNA synthesis
Biopsies were collected in RNAlater solution (Invitrogen Life Tech., Carlsbad, MA, USA), this media has been demonstrated to preserve RNA quality at room temperature in our previous study [19]. Total RNA was extracted by the Trizol method (Invitrogen Life Tech.). Reverse transcription was performed with SuperScript II (Invitrogen Life Tech.) for cDNA synthesis according to our previous report [19].
The absolute mRNA levels of IL-12, IL-18 and IFN-gamma in the adjacent non-tumor mucosa quantified by real-time PCR with double stranded DNA based calibrator curves
Real-time PCR was performed on an ABI-prism 7900 sequence detector with TaqMan Gold™ PCR core reagents kit (Applied Biosystems/Roche, Branchburg, NJ, USA) in 25 μl format according to our previous published method [19]. In brief, to each reaction 2 μl unknown cDNA sample was added, and samples were run in duplicate. Reaction conditions were default TaqMan thermo-cycling (45 cycles). Primers and probes for cytokines and house keeping gene beta-actin (Table 2) were designed in Primer Express 1.0 (Perkin Elmer/Applied Biosystems, Foster City, CA, USA) and synthesized by Eurogentec. (S.A., Seraing, Belgium). The assays were designed to cross exon splicing points in order to avoid detection of genomic DNA.
Table 2.
Primer/probe sequences for real-time PCR
| Assay | Primer | Sequence | |
|---|---|---|---|
| Β-actin | TaqMan | Forward | 5′ TGCCGACAGGATGCAGAAG 3′ |
| Reverse | 5′ GCCGATCCACACGGAGTACT 3′ | ||
| Probe | FAM 5′ AGATCAAGATCATTGCTCCTCCTGAGCGC 3′ TAMRA | ||
| Calibrator | Forward | 5′ GCATGGAGTCCTGTGGCAT 3′ | |
| Reverse | 5′ GGGCCGGACTCGTCATACT 3′ | ||
| IFN-γ | TaqMan | Forward | 5′ TTTTAATGCAGGTCATTCAGATGT 3′ |
| Reverse | 5′ AAGTTTGAAGTAAAAGGAGACAATTTGG 3′ | ||
| Probe | FAM 5′ CATTTTGAAGAATTGGAAAGAGGAGAGTGACAGA 3′ TAMRA | ||
| Calibrator | Forward | 5′ TTTTAATGCAGGTCATTCAGATGT 3′ | |
| Reverse | 5′ TCATCTCGTTTCTTTTTGTTGCTAT 3′ | ||
| IL-12 | TaqMan | Forward | 5′ TGCAAAGCTTCTGATGGATCC 3′ |
| Reverse | 5′ AAAATCCGGTTCTTCAAGGGA 3′ | ||
| Probe | FAM 5′ AGCTGATGCAGGCCCTGAATTTCAACA 3′ TAMRA | ||
| Calibrator | Forward | 5′ ACCAGGTGGAGTTCAAGACCA 3′ | |
| Reverse | 5′ GCCCGAATTCTGAAAGCATG 3′ | ||
| IL-18 | TaqMan | Forward | 5′ ATCGCTTCCTCTCGCAACA 3′ |
| Reverse | 5′ CATTGCCACAAAGTTGATGCA 3′ | ||
| Probe | FAM 5′ CAGGAATAAAGATGGCTGCTGAACCAG 3′ TAMRA | ||
| Calibrator | Forward | 5′ TGCCACCTGCTGCAGTCTAC 3′ | |
| Reverse | 5′ CCAGGTTTTCATCATCTTCAGCT 3′ | ||
| IL-6 | TaqMan | Forward | 5′ CCAGGAGCCCAGCTATGAAC 3′ |
| Reverse | 5′ CCCAGGGAGAAGGCAACTG 3′ | ||
| Probe | FAM 5′ CCTTCTCCACAAGCGCCTTCGGT 3′ TAMRA | ||
| COX-2 | TaqMan | Forward | 5′GAATCATTCACCAGGCAAATTG 3′ |
| Reverse | 5′ TTTCTGTACTGCGGGTGGAAC 3′ | ||
| Probe | FAM 5′ TTCCTACCACCAGCATCCCTGCCA 3′ TAMRA |
Double stranded DNA based calibrator curves for cytokines (IL-12, IL-18 and IFN-gamma) and house keeping gene beta-actin were constructed from amplified regulated PCR products according to our previous report [19]. Cytokine mRNA levels were normalized to house keeping gene beta-actin mRNA level and expressed as copies/μg total RNA.
The relative mRNA levels of IL-6 and COX- in the adjacent non-tumor mucosa quantified by real-time PCR
Both IL-6 and COX-2 were demonstrated to inhibit dendritic cell differentiation and function in patients with cancers [20–23]. To examine the association of COX-2 and IL-6 with dendritic cell dysfunction in the non-tumor adjacent mucosa throughout adenoma–carcinoma sequence, the expression of IL-6 and COX-2 genes in adjacent mucosa were quantified with real-time PCR as described above, but without calibrator curves. Primers and probes for IL-6 and COX-2 were present in Table 2. The IL-6 and COX-2 mRNA levels in adjacent mucosa of CRA and CRC were expressed relative to that of normal mucosa as fold difference (N) = 2−ΔΔCT, ΔCT = CTtaget gene − CTbeta-actin, ΔΔCT = ΔCTCRA/CRC − average ΔCTnormal [24].
Immune cells present in the adjacent non-tumor mucosa identified by immunohistochemistry (IHC)
The biopsies from patients were prepared and embedded in paraffin routinely. Four micrometer sections were cut, and then stained with H&E. Immunohistochemistry was performed with LSAB-2 system-HRP kits (Dako, Carpinteria, CA, USA) according to the manufacturer’s instructions [25]. Antigen retrieval was achieved by boiling sections for 15 min in 0.01 M citrate buffer, pH 6.0. The following primary antibodies were used: Rabbit anti-human CD3, mouse anti-human CD68, mouse anti-human CD83 antibodies (1:100, all from Dako) to mark lymphocytes, macrophages and dendritic cells in the adjacent mucosa. Antibodies were added to the prepared tissue slides and incubated over night at 4°C, 3-amino-9-ethylcarbazole (AEC; Vector Laboratories, Burlingame, CA, USA) was used as chromogen and slides were counterstained with Mayer's hematoxylin.
Morphometric analysis
Well-oriented sections were examined with a light microscope (Reichert Inc, New York, USA). Semi-quantification of immunoreactive cells was done in normal, adjacent mucosa from adenoma and CRC, the density of immune cells in the mucosa were scored according to the method reported by Naito et al. [26]. In brief, immune cells (labeled by relative antibody immunoreactivities (IRs)) in each slide were graded as: nil (0), 1–19 cells/field (1+), 20–49 cells/field (2+) and >50 cells/field (3+) in at least 5 optical fields (×400) with abundant distribution and average values were taken.
Statistics
Results were expressed as mean ± SEM (standard error mean) unless otherwise stated. Mann-Whitney tests were used to compare differences between groups and one-way ANOVA (non-parametric Kruskal–Wallis test) was used to compare differences among three groups. P < 0.05 was considered as statistically significant.
Results
Different immune cell populations in the adjacent non-tumor mucosa throughout colorectal adenoma and carcinoma
In the normal mucosa from the health controls, lymphocytes, macrophages and dendritic cells were predominantly found in the stroma, most of them were present in the upper half region toward the lumen. Some intraepithelial lymphocytes and macrophages were also found, but dendritic cells were rarely shown in the epithelium (Fig. 1a).
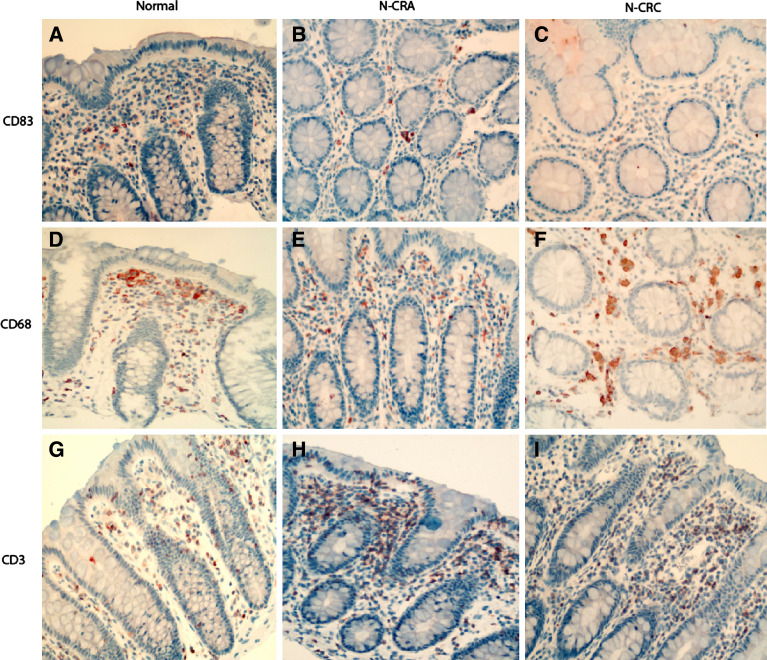
Fig. 1.
Immunohistochemical examination of dendritic cells (labeled by anti-human CD83), lymphocytes (labeled by anti-human CD3) and macrophages (labeled by anti-human CD68) in the non-tumor mucosa adjacent to colorectal adenoma (CRA) and carcinoma (CRC). It shows that dendritic cells in the non-tumor muocsa adjacent to CRA (N-CRA) were mostly distributed in the upper part of stroma b, but the number of dendritic cells was remarkably decreased as compared with the non-tumor adjacent mucosa of CRC (N-CRC) c or normal colorectal mucosa from health controls a. In contrast, the number of lymphocytes was gradual slightly increased and the number of macrophages was comparable to that in normal controls in both N-CRA (e for macrophages and h for lymphocytes) and N-CRC (f for macrophages and i for lymphocytes) as compared with the normal colorectal mucosa from health controls (d for macrophages and g for lymphocytes). (Immunohistochemistry, counterstained with hematoxylin, original magnification 200×)
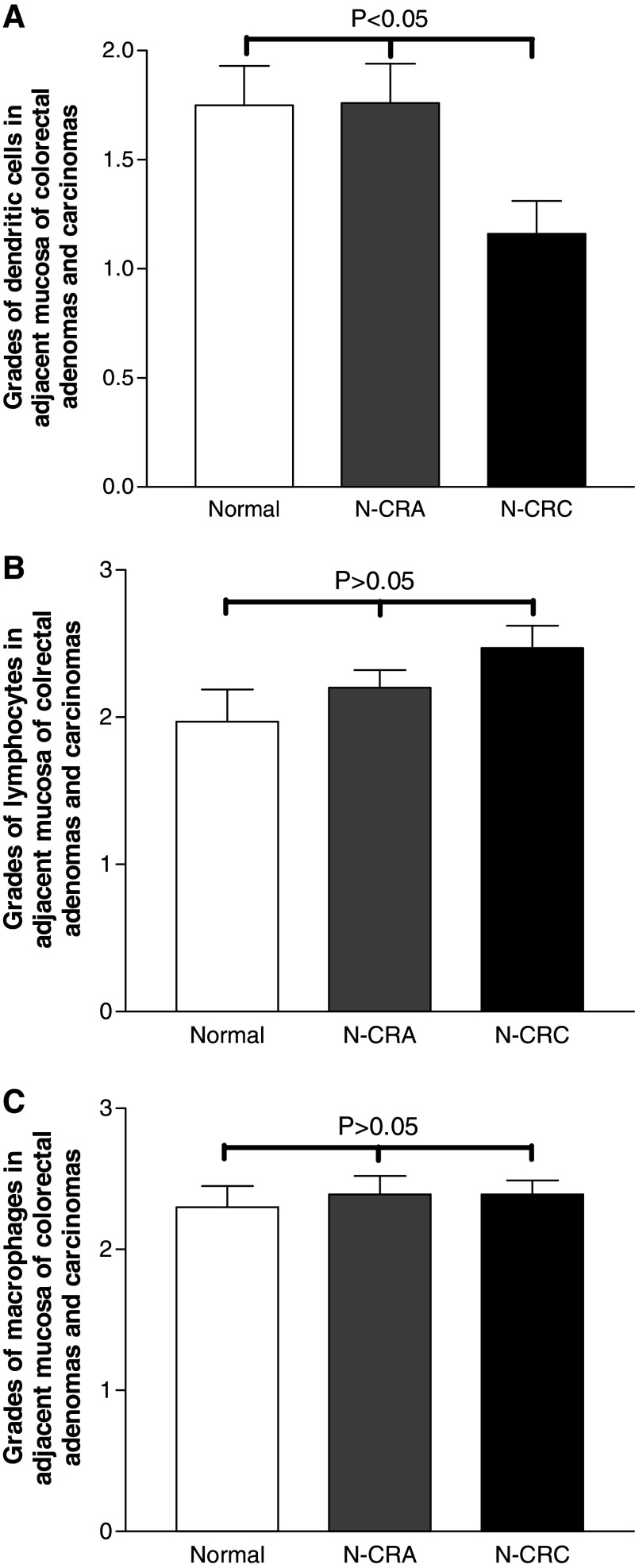
The number of dendritic cells present in the non-tumor mucosa adjacent to CRA and CRC was distinctly different. As compared with normal mucosa from health control subjects, the number of dendritic cells in the stroma of adjacent mucosa from adenoma was slightly and non-significantly increased, most were ∼score 2 (Fig. 2a) with a high abundance in the part of stroma close to the epithelium (Fig. 1b). Among different dysplasia grades, the grading scores of dendritic cell number did not significantly differ (low vs. moderate vs. high dysplasia: 1.8 vs. 1.2 vs. 1.7, P < 0.05). In the adjacent mucosa from CRC, the dendritic cells were distributed in the stroma (Fig. 1c) and no intraepithelial dendritic cell could be found. The grade of dendritic cell population in the stroma of adjacent mucosa was significantly lower as compared with adjacent mucosa from either normal controls or adenoma (Fig. 2a). However, the grades of dendritic cell number did not statistically differ among Duke’s classes (Duke’s A vs. B vs. C: 0.8 vs. 1.4 vs. 1.3; P > 0.05).
Fig. 2.
Morphometrical population grades of dendritic cell, lymphocyte and macrophage in the non-tumor mucosa adjacent to CRA (N-CRA) and CRC (N-CRC). The grading scores for dendritic cell number in the stroma of N-CRC (Black bar in a) was significantly lower as compared with N-CRA (Grey bar in a) and normal colorectal mucosa from health controls (White bar in a) (P < 0.05, nonparametric Kruskal–Wallis test). However, the grading scores for lymphocyte number in both N-CRA (Grey bars in b) and N-CRC (Black bars in b) were slightly increased as compared with normal colorectal mucosa from health controls (P > 0.05, non-parametric Kruskal–Wallis test), while the grading scores for macrophage number in both N-CRA (Grey bars in c) and N-CRC (Black bars in c) were at the same level as that in normal controls (P > 0.05, non-parametric Kruskal–Wallis test)
The numbers of lymphocytes present in adjacent mucosa to CRA and CRC was slightly gradual increased (Fig. 2b), but the differences were not statistically significant (P > 0.05). The number of macrophages was comparable to that in normal controls (Fig. 2c). In adjacent mucosa from either CRA or CRC, most of lymphocytes and macrophages were scattered in the stroma (Fig. 1e, f, h, i). In adjacent mucosa from different dysplasia grades of CRA, the difference for lymphocyte number and macrophage number were not statistically significant (for lymphocytes: lower dysplasia vs. moderate vs. high dysplasia = 2.4 vs. 1.7 vs. 2.5 and for macrophages: lower dysplasia vs. moderate vs. high dysplasia = 2.4 vs. 2.2 vs. 2.7; both P > 0.05). Similarly, the scores for lymphocyte number and macrophage number among different Duke’s stages did not differ significantly (Duke’s A vs. B vs. C, for lymphocytes: 2.4 vs. 2.6 vs. 2.3; for macrophages: 2.3 vs. 2.5 vs. 2.5; both P > 0.05).
Distinct IL-12, IL-18 and IFN-gamma mRNA levels in the adjacent non-tumor mucosa throughout CRA and CRC
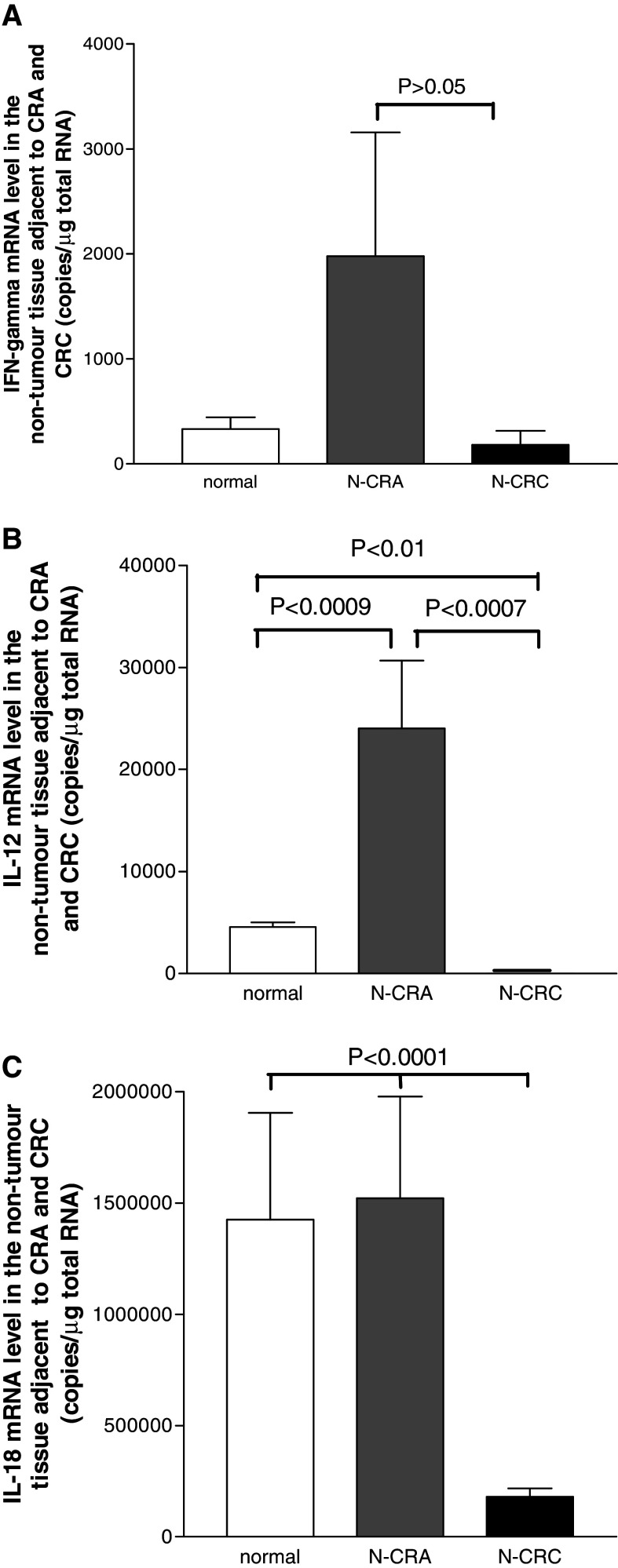
The mRNA expressions of cytokines IL-12, IL-18 and IFN-gamma in normal mucosa of the health controls, the adjacent non-tumor mucosa of adenoma and CRC are presented in Fig. 3a–c. As compared to normal controls, the tissue mRNA levels of IFN-gamma, IL-12 and IL-18 in the non-tumor mucosa adjacent to adenoma were all elevated (Fig. 3a–c) and particularly IL-12 was significantly increased (Fig. 3b). To assess whether the cytokine levels in adjacent mucosa were associated with dysplasia grading in adenoma, the cytokine mRNA levels were compared between low and moderate dysplasia (high dysplasia adenomas was excluded for analysis, because cytokines were only detected in 2 of 5 cases). A decreased trend of the cytokine levels was found following the dysplasia degree grading increase in CRA (low vs. moderate: IL-18, 3904000 ± 2091000 copies/μg total RNA vs. 977785 ± 293257 copies/μg total RNA; IL-12, 32364 ± 12796 copies/μg total RNA vs. 9318 ± 1800 copies/μg total RNA; IFN-gamma, 4790 ± 4061 copies/μg total RNA vs. 231 ± 105 copies/μg total RNA), though not significantly (all P > 0.05).
Fig. 3.
The mRNA levels of IL-12, IL-18 and IFN-gamma in the non-tumor mucosa adjacent to CRA (N-CRA) and CRC (N-CRC) measured with quantitative real-time PCR. Compare to the normal colorectal mucosa from health controls (White bars in a–c), all three Th1 cytokines IFN-gamma a, IL-12 b and IL-18 c were increased in N-CRA (Grey bars in a–c), but significant decreased in colorectal carcinoma (CRC) (Black bars in a–c). Notably, IL-12 and IFN-gamma mRNA levels were only detectable in 20% (for IL-12) and 28% (for IFN-gamma) of N-CRC
In contrast to the cytokine profile in the non-tumor mucosa adjacent to adenoma, the mRNA of IL-18 in adjacent mucosa from CRC was only detected in 65% cases and the level was significantly decreased (Fig. 3c). Both IFN-gamma and IL-12 became undetectable in most cases of adjacent mucosa from CRC (80% undetectable for IL-12 and 78% undetectable for IFN-gamma). Even in those detectable cases (20% for IL-12; 22% for IFM-gamma), the expression level of IL-12 was remarkably decreased as compared with that in adjacent non-tumor mucosa from CRA (Fig. 3b). The mRNA level of IFN-gamma was also decreased in adjacent mucosa from CRC (Fig. 3a), though not significantly. Since these cytokine mRNAs in adjacent mucosa from CRC were undetectable in many cases, thus, it was hard to compare the difference among different Duke’s class groups.
Significant increased expression levels of COX-2 and IL-6 in the adjacent non-tumor mucosa of CRC as compared with CRA
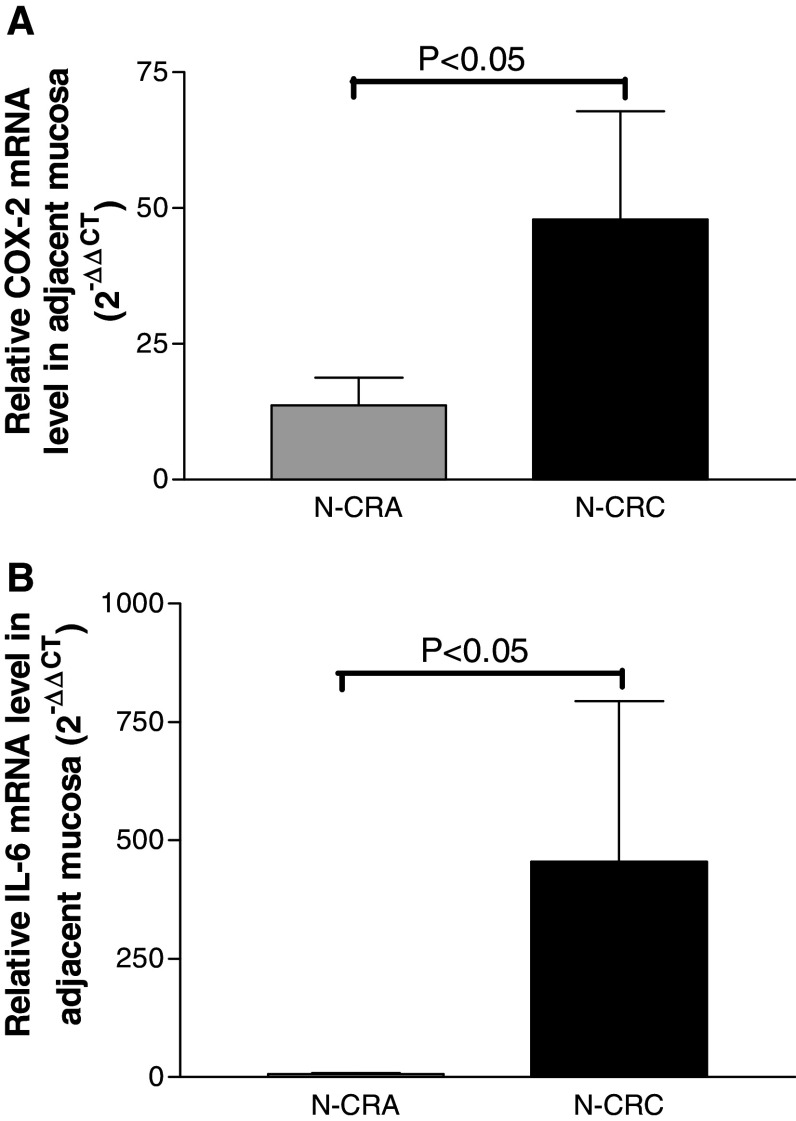
To examine the possible role of COX-2 and IL-6 in inducing dendritic cell dysfunction in the non-tumor adjacent mucosa throughout adenoma–carcinoma sequence, their mRNA expression levels were examined with real-time PCR. The results showed that both the relative mRNA expression levels of COX-2 (Fig. 4a) and IL-6 (Fig. 4b) in adjacent mucosa of CRC were significantly higher than that in adjacent mucosa of CRA (both P < 0.05).
Fig. 4.
The mRNA levels of COX-2 and IL6 in the non-tumor mucosa adjacent to CRA (N-CRA) and CRC (N-CRC) measured with quantitative real-time PCR, the results were expressed a relative folder referred to normal controls. It showed that the relative expression mRNA level of COX-2 a and IL-6 b in the adjacent mucosa of CRC (Black bars) were significantly increased as compared with the adjacent mucosa of CRA (Grey bars) (both P < 0.05)
Discussion
Previously, we have demonstrated an altered microenvironmental Th1 cytokine gene expression pattern accompanies the adenoma–carcinoma sequence of colorectum [7], in which Th1 cytokines IL-12, IL-18 and IFN-gamma were remarkably decreased in CRC tissues. In this study, we were able to demonstrate a reduced dendritic cell number and a similar Th1 cytokine mRNA expression trend, particular low IL-12 mRNA levels, in morphological normal mucosa adjacent to CRC.
In human colorectal cancers, reduced dendritic cell numbers were already reported in tumour tissue [27], but few studies examined the dendritic cells present in the morphological normal mucosa adjacent to adenoma and CRC. In the current study, we demonstrated a grossly normal number of dendritic cell in the adjacent mucosa of adenoma, but remarkably decreased in the adjacent mucosa from CRC. Since dendritic cells play a crucial role in response to the uptake, processing and cross-presentation of tumor associated antigens to T cells necessary for generating tumor-specific immunity [28], a decreased dendritic cell number may indicate decreased immune response to tumor antigens and increased chance of survival of tumor cells. IL-12 is a strong inducer for tumor-specific immunity and IFN-gamma is its downstream actor [32]. The dendritic cell is the main cellular source of IL-12. Previously, we have found that IL-12 mRNA level in CRC was over hundred times lower as compared with CRAs and normal controls [7]. This may indicate a heavy functional damage of dendritic cells in the tumor microenvironment. Interestingly, IL-12 mRNA level was also significantly downregulated and over several hundred times lower in non-tumor mucosa adjacent to CRC as compared with CRA or normal controls in the current study. Our current data suggest that a suppression of anti-tumor immunity also exists in adjacent mucosa from CRC, since IL-12 plays a critic role in generating anti-tumor immunity [32–35]. In this study, we have also shown a different anti-tumor cytokine regulatory network including IL-18 and IFN-maga mRNAs in adjacent mucosa accompanies with CRA and CRC. Throughout adenoma–carcinoma sequence, a distinct different Th1 cytokine mRNA level in the adjacent non-tumor mucosa was demonstrated: the Th1 cytokines were increased in the former, but significantly decreased in later. This altered Th1 cytokine expression profile may represent a prerequisite dysfunction of the local anti-tumor immunity and favourite tumor invasion.
The mechanisms behind the reduced number of dendritic cell number and IL-12 level are currently unclear. It could be related to many factors released from cancer cells. It have been found that COX-2, IL-6 and vascular endothelial growth factor (VEGF) produced by cancer cells may interfere with dendritic cell differentiation, maturation and recruitment [29], the suppressing effect could be both locally and systemically [20]. COX-2 and IL-6 are the two major inhibitors of dendritic cell differentiation and functions in patients with cancers [21–23, 30, 31]. To extend the findings of significantly increased COX-2 in the non-tumor “normal” mucosa adjacent to CRC by Hao et al. [13], our current study further demonstrated remarkably increased COX-2 and IL-6 mRNA expression levels in adjacent mucosa of CRC as compared with adjacent mucosa of CRA (Fig. 4). This could be a potential explanation for the great difference of DC density in the adjacent mucosa throughout adenoma–carcinoma sequence. In addition, we have previously found that cancer cells can produce IL-10 to inhibit Th1 cytokines in CRC [7]. Thus, a suppressed anti-tumor immunity in adjacent mucosa from CRC that is not seen in CRA could result from several factors [36].
Previously, lymphocytes and macrophages infiltrated in CRC tissues have been frequently reported [27, 37–39]. Interestingly, we also found a slightly increased number of lymphocytes and a normal density of macrophages in the adjacent mucosa throughout adenoma–carcinoma sequence. However, whether these immune cells function as tumor-killers or tumor-promoters are still questionable. Recent findings suggest that macrophages infiltrated in the tumor microenviroment may inhibit host immunity and stimulate tumor cell growth via release of various molecules [33–35].
Taken together, our current study revealed a suppressed local anti-tumor immunity in the adjacent non-tumor mucosa from CRC and which might reflect a host–tumor interaction status and explain the lack of efficient immune response in CRC invasion.
Acknowledgments
This work was financially supported by grant from Medical Research Program, Northern Norway Regional Health Authority to Cui G (SFP-44-04). We express our sincere gratitude to our colleagues at Departments of Gastroenterology and Gastrointestinal Surgery for supplying biopsies, and to Ingrid Christiansen, Marian Remijn and Line Wilsgaard for their technical assistance.
Abbreviation
- IFN-gamma
Interferon gamma
- IL
Interleukin
- real-time PCR
Real-time polymerase chain reaction
- RT-PCR
Reverse-transcription polymerase chain reaction
- IHC
Immunohistochemistry
References
- 1.Fearon ER. Molecular genetic studies of the adenoma–carcinoma sequence. Adv Intern Med. 1994;39:123–147. [PubMed] [Google Scholar]
- 2.Fodde R. The APC gene in colorectal cancer. Eur J Cancer. 2002;38(7):867–871. doi: 10.1016/S0959-8049(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 3.Muto T, Nagawa H, Watanabe T, Masaki T, Sawada T. Colorectal carcinogenesis: historical review. Dis Colon Rectum. 1997;40(10 Suppl):S80–85. doi: 10.1007/BF02062026. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Contasta I, Berghella AM, Pellegrini P, Adorno D. Passage from normal mucosa to adenoma and colon cancer: alteration of normal sCD30 mechanisms regulating TH1/TH2 cell functions. Cancer Biother Radiopharm. 2003;18(4):549–557. doi: 10.1089/108497803322287628. [DOI] [PubMed] [Google Scholar]
- 6.Contasta I, Pellegrini P, Berghella AM, Beato TD, Adorno D. Colon cancer and gene alterations: their immunological implications and suggestions for prognostic indices and improvements in biotherapy. Cancer Biother Radiopharm. 2006;21(5):488–505. doi: 10.1089/cbr.2006.21.488. [DOI] [PubMed] [Google Scholar]
- 7.Cui G, Goll G, Olsen T, Steigen S, Husebekk A, Vonen B, Florholmen J (2006) Reduced expression of microenvironmental Th1 cytokines accompanies adenomas–carcinomas sequence of colorectum. Cancer Immunol Immunother Dec 8; (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- 8.Filipe MI, Mughal S, Bussey HJ. Patterns of mucus secretion in the colonic epithelium in familial polyposis. Invest Cell Pathol. 1980;3(4):329–343. [PubMed] [Google Scholar]
- 9.Filipe MI, Cooke KB. Changes in composition of mucin in the mucosa adjacent to carcinoma of the colon as compared with the normal: a biochemical investigation. J Clin Pathol. 1974;27(4):315–318. doi: 10.1136/jcp.27.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson PA, Filipe MI. An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer. 1976;37(5):2388–2398. doi: 10.1002/1097-0142(197605)37:5<2388::AID-CNCR2820370531>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Filipe MI, Branfoot AC. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::AID-CNCR2820340211>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Shamsuddin AK, Weiss L, Phelps PC, Trump BF. Colon epithelium. IV. Human colon carcinogenesis. Changes in human colon mucosa adjacent to and remote from carcinomas of the colon. J Natl Cancer Inst. 1981;66(2):413–419. [PubMed] [Google Scholar]
- 13.Hao CY, Moore DH, Wong P, Bennington JL, Lee NM, Chen LC. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin Cancer Res. 2005;11(4):1400–1407. doi: 10.1158/1078-0432.CCR-04-1942. [DOI] [PubMed] [Google Scholar]
- 14.Hao CY, Moore DH, Chiu YS, Wong P, Bennington JL, Smith AP, Chen LC, Lee NM. Altered gene expression in normal colonic mucosa of individuals with polyps of the colon. Dis Colon Rectum. 2005;48(12):2329–2335. doi: 10.1007/s10350-005-0153-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen LC, Hao CY, Chiu YS, Wong P, Melnick JS, Brotman M, Moretto J, Mendes F, Smith AP, Bennington JL, et al. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64(10):3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 16.Kuniyasu H, Yasui W, Shinohara H, Yano S, Ellis LM, Wilson MR, Bucana CD, Rikita T, Tahara E, Fidler IJ. Induction of angiogenesis by hyperplastic colonic mucosa adjacent to colon cancer. Am J Pathol. 2000;157(5):1523–1535. doi: 10.1016/S0002-9440(10)64790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, Fidler IJ. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res. 2003;9(13):4802–4810. [PubMed] [Google Scholar]
- 18.Fox SH, Whalen GF, Sanders MM, Burleson JA, Jennings K, Kurtzman S, Kreutzer D. Angiogenesis in normal tissue adjacent to colon cancer. J Surg Oncol. 1998;69(4):230–234. doi: 10.1002/(SICI)1096-9098(199812)69:4<230::AID-JSO7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Cui G, Olsen T, Christiansen I, Vonen B, Florholmen J, Rasmus G. Improvement of real-time PCR for quantifying TNF-a mRNA expression in inflamed colorectal mucosa-An approach to optimize procedures for clinical use. Scand J Clin Lab Invest. 2006;66(3):249–259. doi: 10.1080/00365510600590472. [DOI] [PubMed] [Google Scholar]
- 20.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–1766. [PubMed] [Google Scholar]
- 21.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100(1):230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 22.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168(9):4333–4343. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Stolina M, Yang SC, Baratelli F, Lin JF, Atianzar K, Luo J, Zhu L, Lin Y, Huang M, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9(3):961–968. [PubMed] [Google Scholar]
- 24.Suzuki N, Yoshida A, Nakano Y. Quantitative analysis of multi-species oral biofilms by TaqMan Real-Time PCR. Clin Med Res. 2005;3(3):176–185. doi: 10.3121/cmr.3.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui G, Koh TJ, Chen D, Zhao CM, Takaishi S, Dockray GJ, Varro A, Rogers AB, Fox JG, Wang TC. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer Res. 2004;64(22):8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- 26.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494. [PubMed] [Google Scholar]
- 27.Schwaab T, Weiss JE, Schned AR, Barth RJ., Jr Dendritic cell infiltration in colon cancer. J Immunother. 2001;24(2):130–137. doi: 10.1097/00002371-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Dalerba P, Maccalli C, Casati C, Castelli C, Parmiani G. Immunology and immunotherapy of colorectal cancer. Crit Rev Oncol Hematol. 2003;46(1):33–57. doi: 10.1016/S1040-8428(02)00159-2. [DOI] [PubMed] [Google Scholar]
- 29.Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4(3):585–593. [PubMed] [Google Scholar]
- 30.Botella-Estrada R, Dasi F, Ramos D, Nagore E, Herrero MJ, Gimenez J, Fuster C, Sanmartin O, Guillen C, Alino S. Cytokine expression and dendritic cell density in melanoma sentinel nodes. Melanoma Res. 2005;15(2):99–106. doi: 10.1097/00008390-200504000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Torisu-Itakara H, Cochran AJ, Kadison A, Huynh Y, Morton DL, Essner R. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin Cancer Res. 2005;11(1):107–112. [PubMed] [Google Scholar]
- 32.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl):S52–S61. doi: 10.1007/PL00014051. [DOI] [PubMed] [Google Scholar]
- 33.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86(3):231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 34.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19(3):247–258. doi: 10.1023/A:1015587423262. [DOI] [PubMed] [Google Scholar]
- 35.O'Sullivan C, Lewis CE. Tumour-associated leucocytes: friends or foes in breast carcinoma. J Pathol. 1994;172(3):229–235. doi: 10.1002/path.1711720302. [DOI] [PubMed] [Google Scholar]
- 36.Lenahan C, Avigan D. Dendritic cell defects in patients with cancer: mechanisms and significance. Breast Cancer Res. 2006;8(1):101. doi: 10.1186/bcr1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underwood JC. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dave BJ, Hopwood VL, Hughes JI, Jackson GL, Melillo D, Pathak S. Cytogenetic abnormalities in colon cancer patients: a comparison of T- and B-lymphocytes. Anticancer Res. 1993;13(2):433–438. [PubMed] [Google Scholar]
- 39.Chaux P, Moutet M, Faivre J, Martin F, Martin M. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 costimulatory molecules of the T-cell activation. Lab Invest. 1996;74(5):975–983. [PubMed] [Google Scholar]