Abstract
Allogeneic haematopoietic stem cell transplantation (HSCT) is an intensive medical treatment involving myeloablative chemo-radiotherapy followed by stem cell rescue using allogeneic haematopoietic stem cells harvested from HLA-matched donors, which is primarily used for the treatment of haematological malignancies. Cytomegalovirus (CMV) infection is one of the major causes of morbidity and death after HSCT. This focused research review highlights the advances made with research into CMV in the HSCT setting. It provides the reader with an overview of current CMV research into the prevention and management of CMV infection.
Keywords: CMV, Vaccine, Adoptive cellular therapy, Artificial antigen presenting cells
Haematopoietic stem cell transplantation
Allogeneic haematopoietic stem cell transplantation (HSCT) is an intensive medical treatment involving myeloablative chemo-radiotherapy followed by stem cell rescue using allogeneic haematopoietic stem cells harvested from HLA-matched donors, which is primarily used for the treatment of haematological malignancies. It is generally accepted that following ablation of the host marrow and immune system, the reconstitution of donor-derived immune system confers a graft versus leukaemia (GvL) or tumour effect which is associated with improvements in survival [1].
The main determinants of survival after HSCT are donor-recipient matching, the development of Graft versus Host disease (GvHD), the GvL effect, and viral infections. Although HSCT outcomes are improving, viral infections remain a significant cause of morbidity and mortality [2]. Although CMV infection is the main viral cause of morbidity and mortality after HSCT, the clinical importance of viruses, such as the adenovirus, bocavirus, coronovirus and HHV-6 is increasingly recognised [3].
The immune response to CMV infection
Around 50–85% of the general population are infected with CMV [4]. The virus is transmitted by salivary contact, sexual intercourse and by blood products. With the exception of pregnancy, infection with CMV is rarely pathogenic in immunocompetent individuals. After infection the virus establishes lifelong latency. The immune response against CMV is predominantly mediated by cellular immunity. After primary infection, CMV persists in the host in a delicate balance between the immune system and the virus, where the immune system actively prevents reactivation and replication of the virus. CMV reactivation occurs mainly in CMV seropositive patients receiving allogeneic HSCT, derived from CMV negative donors or in CMV positive recipients, where donor CMV-specific T cells have been depleted by conditioning chemotherapy and immunosuppression.
Over the last two decades, numerous studies have been conducted to identify the major CMV target antigens that induce cell mediated immune responses and the CMV peptides that are presented in association with different HLA molecules [5]. These studies have provided new information about the immune response to CMV and have led to the development of more sophisticated reagents, enabling studies of different aspects of the immune response. The majority of these studies have concentrated on cytotoxic T lymphocytes, mainly due to a lack of available reagents for helper T lymphocytes and evidence of the latter’s importance only becoming apparent in the last 5 years [6–8].
The recognition of virally infected cells by CD8+ T cells requires the viral proteins to be processed into peptides by the cell’s proteosome complex. The peptides that are generated are loaded onto major histocompatibility complex (MHC) class I molecules, which are typically 8–10 amino acids long. Virally derived peptides are transported into the endoplasmic reticulum for loading onto MHC class I molecules. The antigenic complex is then transported to the cell surface for display.
The major antigen targeted by CMV specific cytotoxic T lymphocytes (CTL) has been identified as the coat phosphoprotein 65 (pp65) [9]. The protein does not require viral gene expression and is one of the first antigens to be presented on the surface of infected cells [10]. Thus, pp65 is an ideal target for cytotoxic lymphocytes (CTL) as it is introduced into the cell during viral entry and is rapidly available for processing in the MHC class I pathway.
To date, most of the identified pp65 CD8+ T cell epitopes originate from HLA alleles that are predominant in the Caucasoid population, such as HLA-A*0201 [11]. Other target antigens that may enhance the immune response include the envelope protein glycoprotein B, pp28, IE1, and pp150 [12, 13]. The characterisation of the pp65 peptide epitopes for the HLA types A1, A2, A3, A24, B35 and B44 would potentially allow the formation of a multi-subunit anti-pp65 peptide vaccine that would cover 90% of the Caucasoid population.
Although CMV peptide epitope discovery is an active area of research, progress is slow, as the identification of CMV peptides is complex and laborious. This has led to the development of new rapid methods using both cellular techniques and computer generated models for predicting CMV epitopes [14], and has led to an increasing list of known CMV epitopes [5].
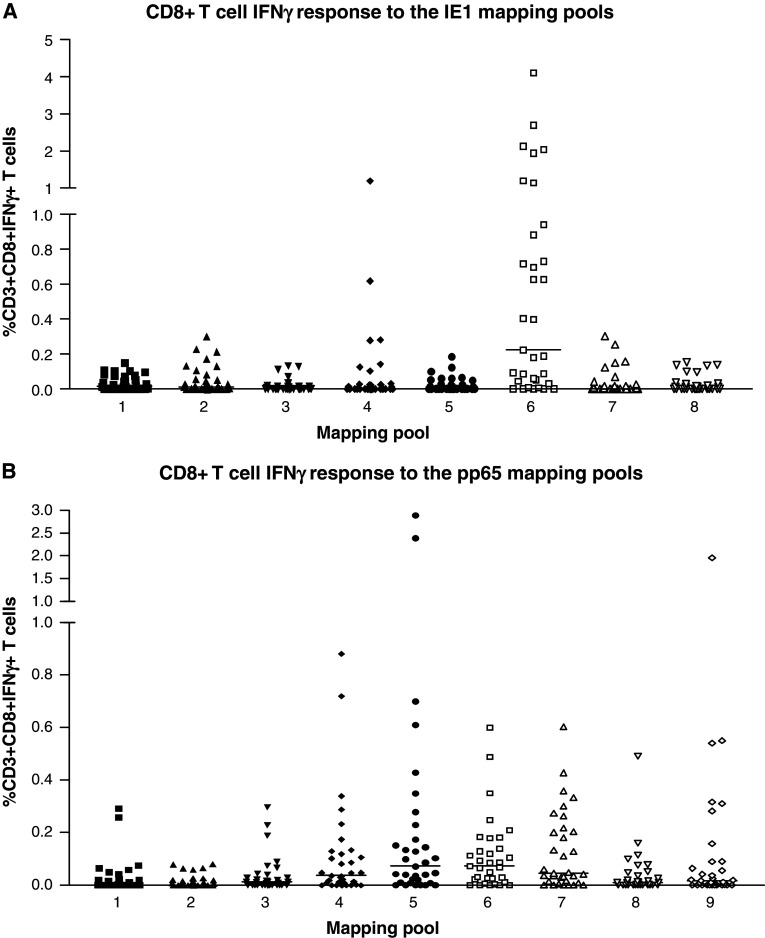
To scan for new CMV epitopes, Paston and Dodi [5] used peptide mapping pools, in which the amino acid sequence of the CMV antigens IE1 and pp65 was broken down into mapping pools, containing 15-mer peptides that overlap by 11 amino acids. To map the IE1 antigen there are eight mapping pools and nine mapping pools for pp65, with each mapping pool containing approximately 15 peptides. Each mapping pool spans 50 amino acids of the protein sequence, meaning that all potential peptides are included. In order to determine the portion of the antigen that elicits an immune response, peripheral blood mononuclear cells (PBMC) from thirty healthy donors were stimulated with both the IE1 and pp65 panel of mapping pools; IFN-γ production was measured by intracellular staining and flow cytometry.
The CD8+ T cell responses to the pp65 and IE1 antigens are mainly located towards the carboxyl end of the antigens (Fig. 1). The pp65 antigen showed the greatest diversity in response with the majority of the cohort responding to mapping pools 5–9. The CD8+ T cell responses to the IE1 antigen were less diverse and were mainly confined to mapping pools 4 and 6. The range of CD8+ T cell responses varied between donors, with some having a high level of responsiveness, while in others the response was just above background levels.
Fig. 1.
Scanning of IFNγ T cell responses to the IE1 and pp65 mapping pools for the study cohort: the responses to the IE1 (a) and pp65 (b) mapping pools were determined in a cohort of 30 healthy CMV seropositive individuals. PBMCs from each individual were stimulated overnight with each IE1 and pp65 mapping pool. The amount of IFNγ produced was determined by intracellular staining for IFNγ. The number of CD8+ T cells producing IFNγ in response to the mapping pools was represented as a percentage of the CD3+CD8+ population. The background (absence of antigen) level of IFNγ produced was subtracted from the result obtained for each pool. The bars indicate the median IFNγ T cell responses to each mapping pool. The amount of IFNγ produced in response to the mapping pools was below 0.1% in CMV seronegative healthy individuals (data not shown)
Paston and Dodi observed that CMV peptides are presented in an HLA allele hierarchy in relation to the relative dominance of the HLA-B*0702 molecule over HLA-A*0201. Tetramer staining has revealed that a number of CMV seropositive HLA-A*0201 individuals do not have any tetramer positive T cells to the dominant pp65 HLA-A*0201 peptide. Individuals that do not respond to the HLA-A*0201 peptide were found to be HLA-B*0702. These HLA-A*0201/B*0702 individuals manifest their CMV response in context of HLA-B*0702. The immunodominance of HLA-B*0702 over HLA-A*0201 has been observed by others [15] and described in EBV infection [16].
The development of HLA tetramer technology for detecting human T cells has added a new dimension to the study of cell mediated immunity [17, 18] as knowledge of HLA specificities allowed the identification of antigen-specific T cells by staining with HLA-tetramers. HLA tetramers can be used in conjunction with viral monitoring to study the relationship between CMV reactivation and CMV-specific CTL responses following CMV seropositive HSCT [19]. Studies using HLA tetramers have shown that CMV-specific CTLs are responsible for controlling CMV reactivation after allogeneic HSCT [20, 21]. Delayed or absent T cell expansion of CD+ T-cells after HSCT is associated with a high risk of CMV infection [22] and may lead to resistant or refractory CMV disease.
CMV infection in the setting of haematopoietic stem cell transplantation
Primary CMV infection or reactivation of CMV in the setting of HSCT causes pneumonitis, hepatitis, colitis and bone marrow suppression, amongst many other complications. Early CMV reactivation or primary CMV infection can be detected by regular monitoring for using quantitative polymerase chain reactions and pp65 antigeneamia. Risk factors for CMV reactivation include CMV viraemia, high-quantitative pp65 antigeneamia and high DNA load [23].
Most cases of CMV occur late, which can be defined as >100 days after transplant. Approximately 30% of patients receiving an allogeneic HSCT will experience a late CMV reactivation, which is associated with a mortality rate of approximately 46% [23]. Risk factors for late relapse include GvHD, persistent lymphopenia (>100 days) and a CMV-seronegative donor graft [24].
Strategies to prevent primary CMV infection include matching seronegative donors to seronegative recipients, prophylactic antiviral drugs and the search for a vaccine against CMV for use in donors and allograft recipients. There are only four licensed antiviral drugs for the treatment of CMV which target the viral DNA polymerase; these are ganciclovir and its prodrug valganciclovir, cidofovir, foscarnet and the antisense oligonucleotide Fomivirsen, which is only licensed for intra-ocular use. These drugs are characterised by poor oral bioavailability, significant drug toxicity and moderate efficacy [25]. The emergence of drug resistance is common, as most of these drugs share a similar mechanism of action [26]. Despite antiviral treatment, late onset reactivations are often resistant to treatment.
Prophylactic administration of ganciclovir during engraftment can prevent CMV reactivation within the first 3 months after HSCT [27]. However, ganciclovir is immunosuppressive and prevents the recovery of HLA restricted CMV-specific lymphocytes [28]. The use of prophylactic immunoglobulins in patients at high risk for CMV infection after HSCT does not reduce the incidence of CMV infection [29].
CMV vaccination
Vaccination offers the potential to prevent CMV infection in CMV negative HSCT recipients and to induce CMV immunity in CMV negative donors. The development of a CMV vaccine is a research priority as congenital CMV infection is a significant cause of cognitive, auditory and motor disabilities in newborn infants, as well as morbidity and mortality after solid organ transplantation.
CMV vaccine development is particularly challenging as repeat infections occur with new strains of the virus [30, 31]. Although the Towne vaccine can prevent CMV disease in renal allografts recipients, vaccinated patients may still acquire CMV infection [32] and the induced immunity is insufficient to prevent infection in the setting of HSCT.
As the viral tegument protein pp65 is expressed in all strains of CMV virus, the identification of the nanomere peptides for HLA-A*0201 [11] derived from the viral tegument protein pp65 may allow the development of a peptide vaccine against CMV. Since this early work, epitopes for up to 90% of Caucasian tissue types have been identified [5] which will help to pave the way for trials of peptide-based vaccines against CMV infection.
Vaccines against other CMV proteins such as the viral envelope portent glycoprotein B and DNA vaccines containing plasmids encoding pp65 or glycoprotein B are in development (Table 1). Results from a recent phase II placebo-controlled, randomised, double-blind study of recombinant CMV envelope glycoprotein B with MF59 adjuvant have recently been reported [33]. In the study, 464 patients were randomised to receive either CMV envelope glycoprotein B with MF59 adjuvant vaccination or placebo. The patients were all CMV negative antibody postpartum women who were considered to be at risk of CMV infection.
Table 1.
Current CMV vaccines in clinical trials
| Vaccine | ClinicalTrials.gov identifier | Clinical trial aim | Status |
|---|---|---|---|
| VCL-CB01 | NCT00285259 | To evaluate the safety, immunogenicity, and clinical benefit in donors and CMV-seropositive recipients undergoing allogeneic matched HCT | This study is ongoing, but not recruiting participants |
| gB/MF59 Vaccine | NCT00133497 | To evaluate gB/MF59 vaccine in preventing CMV Infection in healthy adolescent females | This study is currently recruiting participants |
| Recombinant CMV gB Vaccine | NCT00125502 | To evaluate the immunogenicity of CMV gB vaccine in postpartum women | This study is ongoing, but not recruiting participants |
| CMV gB Sub-Unit Vaccine GSK1492903A | NCT00435396 | To evaluate the safety and immunogenicity in CMV-seronegative healthy adult males. | Completed enrolment and results are awaited |
| PADRE-CMV fusion peptide vaccine or tetanus-CMV fusion peptide vaccine | NCT00722839 | A phase I dose escalation study of the peptide vaccine in healthy participants | This study is currently recruiting participants |
| Drug: ALVAC-CMV (vCP260) | NCT00353977 | To evaluate the induction of CMV-specific immunity in stem cell allotransplant donors and healthy volunteers | This study has been completed. Results are awaited |
| pDNA CMV Vaccine (VCL-CT02) Followed by Towne CMV Vaccine (Towne) Challenge | NCT00373412 | To evaluate safety and CMV-specific immune response to pDNA CMV Trivalent Vaccine (VCL-CT02) followed by Towne CMV Vaccine (Towne) in Healthy, CMV-seronegative volunteers | This study has been completed. Results are awaited |
| CMV gB vaccine | NCT00299260 | To evaluate immunogenicity in solid organ allograft candidate recipients | Trial has completed enrolment. Results are awaited |
| Towne Strain of CMV | NCT00201448 | To evaluate the immunogenicity and efficacy of the vaccine in seronegative women | This study has been suspended |
A full list of all active and completed trials can be found at http://www.clinicaltrials.gov
The subjects were vaccinated postpartum with either CMV glycoprotein B vaccine with MF59 adjuvant or placebo at 0, 1 and 6 months. The trial met the criteria for early termination because of a vaccine efficacy of 50% (95% CI 7–73: infection rates per 100 person-years) and a phase III trial is planned. A phase II trial (NCT00299260) investigating the immunogenicity of a CMV Glycoprotein B vaccine in solid organ allograft recipients has just completed enrolment in the United Kingdom and results are expected later this year.
Other vaccines in development include the CMV DNA vaccine, VCL-CB01, which contains plasmids encoding pp65 and glycoprotein B formulated with the poloxamer CRL1005 and benzalkonium chloride to enhance immune responses. In a recent phase I trial, the vaccine induced in immunogenicity in 45.5% of CMV-seronegative subjects and in 25.0% of fully vaccinated CMV-seropositive subjects. Also 68.1% of CMV-seronegative subjects developed memory IFN-gamma T cell responses at 32 weeks after vaccination [34]. A larger Phase 2 trial (NCT00285259) evaluating the vaccine in donors and CMV-seropositive recipients undergoing allogeneic HSCT is in progress.
Adoptive cell transfer therapies for the treatment of viral infections
The lack of effective non-toxic antiviral therapies has lead to considerable research into cellular treatments for CMV. Understanding of T cell responses to CMV could lead to the development of targeted adoptive cell transfer (ACT) therapies for the treatment of both viral infections and malignances. ACT is the isolation of autologous or allogeneic lymphocytes with specific anti-viral or anti-tumour activity. The principle advantage of ACT is that a few highly specific lymphocytes can be expanded in vivo or in vitro and can be used as a treatment for a disseminated disease.
Achievements in adoptive immunotherapy have been seen primarily in the fields of melanoma and in haematopoietic stem cell transplantation. In the latter, adoptive immunotherapy has evolved from the recognition of GvL effect transferred from the donor to the recipient of bone marrow transplant in the late 1980s, to the use of non-specific donor derived T cells in the 1990s for the treatment of viral infections.
An early successful example was antigen-targeted CTL immunotherapy for Epstein Barr Virus (EBV) associated post-transplant lymphoproliferative disorder [35, 36]. In these studies, EBV transformed lymphoma, resulting from iatrogenic immunosuppression of HSCT patients, regressed after infusing in vitro EBV-specific donor CTLs, which had been expanded using EBV-transformed donor B cells as simulators. However, this approach is costly and labour–intense as establishing the simulators and expanding the CTLs requires months of preparation and although effective, this approach has not been universally adopted.
The experience in immunotherapy to date has shown that HSCT offers a unique immunological environment for introducing adoptive CTL. In a profoundly lymphopenic environment, as seen after HSCT conditioning, the ‘vacated space’ allows rapid homeostatic expansion of lymphocytes driven by cytokines such as IL-7, and IL-15 [37]. The induction of lymphopenia, using fludarabine and cyclophophamide, allows massive expansion of in vitro selected autologous T cell clones and improves responses to ACT.
Similar lymphoid expansions are seen post-HSCT where the initial rapid expansion of post-thymic donor T cells, derived from donor’s peripheral T cell compartment, consisting of central memory and effector memory T cells which are crucial for the success or failure of HSCT due to their impact on engraftment, GvHD, GvL and antiviral activity [38, 39].
In later post-transplant stages (up to a year), the normalisation of the immune system requires the emergence of newly tolerised T cells processed from precursors through the recipient’s thymus. Initially, in the early stages post-HSCT, the mature donor repertoire interacts with the new recipient environment, and leads to clonal expansion to diverse antigens, driven by lymphopenia and cytokines. It is this window of opportunity, post-HSCT, that may allow rapid homeostatic expansion of immunotherapeutic CTLs.
Early clonal expansion of CMV-specific CTLs is dependent on the presence of cells with these specificities in the donor. Therefore, the most effective immunotherapy approach may be to introduce unmanipulated ex vivo antigen experienced cells or in vitro expanded epitope-specific T cells into the patient in the early stages following HSCT in order for the patient to benefit from the physiological in vivo homeostatic expansion.
The most dramatic expansion of epitope specific T cells was seen in a phase I clinical trial of ACT for CMV reactivation in stem cell transplant recipients. Antigen-experienced CMV-specific T cells, isolated using HLA tetramers and nanomagnetic beads, were transferred directly to post-HSCT patients without further manipulation or expansion [40]. Eight out of nine patients treated had a complete resolution of CMV viraemia without any adverse events or GvHD attributable to the cell therapy. Only a small number of T cells of a mean of 0.5 × 106 were required to achieve complete resolution of the viraemia, which can be obtained from a single whole blood donation. Cells selected with this method are up to 99% pure and able to expand 250 fold in vivo following infusion. Non-specific potentially alloreactive cells account for less than a total of 104 cells, which is below the generally accepted cell dose threshold for GvHD of 104 per kilogram.
The use of directly selected ex vivo antigen-specific CTL for transfer to recipient is currently the most promising strategy for ACT. Patients can have almost immediate access to cell therapy selection procedures, which can be undertaken by routine cell therapy laboratories with experience in immunomagnetic selection. In addition, efficacy and costs are comparable to prolonged pharmacological antiviral therapy. A recent study in which donor derived CMV specific CTLs, stimulated with Ad5f35pp65 gene-modified dendritic cells, were administered prophylactically to 12 patients after allogeneic HSCT showed promising results. In the study, no patient required treatment for CMV disease and immune reconstitution to CMV was demonstrated in all patients [41]. Clinical trials are currently in progress of CMV pp65-specific CTLs (Table 2), generated using pp65 peptides, in patients who have undergone allogeneic HSCT and have persistent CMV infections despite antiviral treatment with ganciclovir or foscarnet.
Table 2.
Current clinical trials of ACT for the treatment or prevention of CMV and other HSCT related viral infections
| ClinicalTrials.gov identifier | Clinical trial aim | Status |
|---|---|---|
| NCT00674648 | The safety and efficacy of donor T Cells sensitised with pentadecapeptides of PP65 for the treatment of CMV after allogeneic HSCT | This study is currently recruiting participants |
| NCT00611637 | The efficacy of pp65 CTLs in preventing after allogeneic HSCT | This study is currently recruiting participants |
| NCT00159055 | The efficacy pre-emptive treatment of seronegative patients at risk or patients with documented viremia or CMV disease with CMV-specific CTLs | This study is currently recruiting participants |
| NCT00078533 | To evaluate the efficacy and safety of CMV-specific CTLs for prevention of CMV after allogeneic HSCT | This study is currently recruiting participants |
| NCT00682864 | To evaluate emergency treatment of persistent or therapy refractory CMV infection with pp65/I.E-1 Specific CTLs after HSCT | This study is currently recruiting participants |
| NCT00590083 | Administration of CMV-specific CTLs for the prophylaxis and therapy of adenovirus infection post-allogeneic stem cell transplant | This study is ongoing, but not recruiting participants |
| NCT00058812 | Giving EBV virus specific CTLs after HSCT | This study is currently recruiting participants |
| NCT00711035 | Closely HLA matched allogeneic virus specific CTLs for the treatment of persistent reactivation or infection with adenovirus, CMV and EBV after HSCT | This study is currently recruiting participants |
| NCT00111033 | To evaluate CMV specific CTLs for adenovirus infection following an HSCT | This study has been completed and results are awaited |
A full list of all active and completed trials can be found at http://www.clinicaltrials.gov
On top of CMV infection and reactivation, adenovirus infection after HSCT is estimated to occur in around 25% of patients after HSCT due to delayed immune reconstitution. Humans are susceptible to over 51 serotypes of adenovirus, which can cause heamorrhagic cystitis, pneumonitis, colitis, hepatitis, pancreatitis and nephritis. Paediatric recipients of donor allogeneic bone marrow transplants are at particularly high risk of adenovirus infection [42].
Treatment for adenovirus infection is limited to cidofovir, reduction in immunosuppression and intravenous immunoglobulin. ACT has a potential role in the management of adenoviral infections as antiviral drugs are of limited efficacy and infection can be prevented by the recovery of virus specific T cells [43]. Clinical trials of closely matched allogeneic virus specific CTLs to treat and prevent reactivation or infection with adenovirus, CMV and EBV after HSCT are ongoing (Table 2).
The role of artificial antigen presenting cells in adoptive cellular therapies
Adoptive immunotherapy is evolving towards the infusion of selected subsets of T cells to prevent the induction of anti-host responses. It is clear that small numbers of unmanipulated CMV specific donor T cells can effectively control CMV reactivation in patients undergoing bone marrow transplantation due to the empty “privileged” immune environment in these patients which allows the rapid expansion and reconstitution of adoptively transferred T cells. However, finding matched donors to provide cells can be difficult and current methods for the generation of antigen-specific T cells are laborious and difficult to replicate.
HSCT is just one scenario where adoptive immunotherapy plays an important role. In other forms of cancer, patients do not posses the empty “privileged” environment and anti tumour specific T cells are simply not available from healthy donors. The in vitro generation of anti tumour specific T cells can be challenging in these patients. Since natural antigen presenting cells (APCs) have fundamental roles in mounting, organising and suppressing immune responses, there has been a large drive to create artificial antigen presenting cells (aAPCs) to manipulate the immune system in diseases such as cancer and viral infections, which have overwhelmed, suppressed and/or evaded the immune system.
These aAPCs can be considered as reagents with “off the shelf” availability, avoiding the need to harvest and expand natural APCs from patients and/or donors. The aAPCs vary in their potency, biosafety, speed of expansion and the specificity and quality of the T cells generated. The development of aAPCs, which mimic in vitro and in vivo dendritic cell functions, is an area of active research, for example, the generation of artificial exosomes (explained below). Natural dendritic cell derived exosomes are able to mediate and modulate immune responses in vivo by semi-direct T cell activation [44, 45]. Importantly novel artificial exosomes can form a targeted and traceable nanotechnology system for expanding T cells for adoptive and active immunotherapy [46].
In vivo artificial exosomes have been created by coating liposomes with an optimised number of MHC Class I/II peptide complexes and a specific range of ligands for adhesion, early activation, late activation and survival T cell receptors. These targeted artificial exosomes (also known as immunoliposomes) are super para-magnetic (magnetoliposomes) and are traceable in vitro and in vivo via fluorescent and magnetic resonance imaging and can be focused to specific areas by applying external magnetic attraction [47, 48]. Artificial exosomes can activate and expand functional antigen specific T cells at sufficient levels and exemplify one of the many uses of aAPCs from the treatment of viral infections to cancer nanotechnology and immunotherapy.
Other aAPCs include coated beads, constructed using soluble an HLA antigen-immunoglobulin fusion protein and a CD28 antibody, can induce and expand CMV specific CTLs [49]. aAPCs expressing the full length pp65 are able to expand and produce clinically relevant numbers of CMV specific CTLs from the blood of HLA A2.1 donors, which can kill CMV infected fibroblasts [50]. Importantly, these cells were generated from only 100 ml of blood and had a memory phenotype identical to those generated with autologous APCs.
The aAPC systems, which activate T cells by either polyclonal stimulation or by antigenic specificity and are starting to revolutionise the way immunotherapy is delivered to patients. A limitation of aAPCs is that the mounting of ‘in vivo’ immune responses is dependent on memory or naive T cells, which may have been deleted, killed or anergised as a consequence of immune evasion and suppression mechanisms or by transplant conditioning regimens. Therefore, the latest generation of artificial APCs must be capable of mounting immune responses both in vitro and in vivo depending on the patient’s requirements.
Conclusions
The promising findings from recent CMV vaccination trials and clinical trials of adoptive cellular transfer therapies for CMV and adenovirus suggest that decades of research are starting to yield tangible results. There is great potential for artificial antigen presenting cells to provide ‘off the shelf’ access to ACT for the treatment of viral infections and cancer which should be a priority for clinical development. Although ACT therapies are showing great promise, the prevention of CMV infection will make HSCT safer and more successful. To prevent and treat CMV infections, improved antiviral therapies are needed together with a better understanding of CMV immunity.
Footnotes
This paper is a Focussed Research Review from the meeting which took place 28–29 May 2008 in Nottingham, UK, celebrating the contribution of Prof. I. A. “Tony” Dodi (+29.1.2008) to the EU project “Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumour immunology (ENACT)”.
References
- 1.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36:757–769. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 3.Fischer SA. Emerging viruses in transplantation: there is more to infection after transplant than CMV and EBV. Transplantation. 2008;86:1327–1339. doi: 10.1097/TP.0b013e31818b6548. [DOI] [PubMed] [Google Scholar]
- 4.Alford CA, Stagno S, Pass RF, Britt WJ. Congenital and perinatal cytomegalovirus infections. Rev Infect Dis. 1990;12:S745–S753. doi: 10.1093/clinids/12.supplement_7.s745. [DOI] [PubMed] [Google Scholar]
- 5.Paston SJ, Dodi IA, Madrigal JA. Progress made towards the development of a CMV peptide vaccine. Hum Immunol. 2004;65:544–549. doi: 10.1016/j.humimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Gamadia LE, Remmerswaal EB, Weel JF, et al. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 7.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pourgheysari B, Piper KP, McLarnon A et al (2008) Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant [Epub ahead of print] [DOI] [PubMed]
- 9.Wills MR, Carmichael AJ, Mynard K, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell SR, Rabin M, Geballe AP, et al. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J Immunol. 1991;146:2795–2804. [PubMed] [Google Scholar]
- 11.Solache A, Morgan CL, Dodi AI, et al. Identification of three HLA-A*0201-restricted cytotoxic T cell epitopes in the cytomegalovirus protein pp65 that are conserved between eight strains of the virus. J Immunol. 1999;163:5512–5518. [PubMed] [Google Scholar]
- 12.Kern F, Surel IP, Faulhaber N, et al. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J Virol. 1999;73:8179–8184. doi: 10.1128/jvi.73.10.8179-8184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyulai Z, Endresz V, Burian K, et al. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J Infect Dis. 2000;181:1537–1546. doi: 10.1086/315445. [DOI] [PubMed] [Google Scholar]
- 14.Stanley SM, Dodi IA, Evans CR, et al. Layer guided-acoustic plate mode biosensors for monitoring MHC-peptide interactions. Analyst. 2006;131:892–894. doi: 10.1039/b604812a. [DOI] [PubMed] [Google Scholar]
- 15.Lacey SF, Villacres MC, La Rosa C, et al. Relative dominance of HLA-B*07 restricted CD8+ T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum Immunol. 2003;64:440–452. doi: 10.1016/S0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 16.Höllsberg P. Contribution of HLA class I allele expression to CD8+ T-cell responses against Epstein-Barr virus. Scand J Immunol. 2002;55:189–195. doi: 10.1046/j.0300-9475.2001.01043.x. [DOI] [PubMed] [Google Scholar]
- 17.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 18.Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen FE, Aubert G, Travers P, et al. HLA tetramers and anti-CMV immune responses: from epitope to immunotherapy. Cytotherapy. 2002;4:41–48. doi: 10.1080/146532402317251518. [DOI] [PubMed] [Google Scholar]
- 20.Aubert G, Hassan-Walker AF, Madrigal JA, et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis. 2001;184:955–963. doi: 10.1086/323354. [DOI] [PubMed] [Google Scholar]
- 21.Cwynarski K, Ainsworth J, Cobbold M, et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood. 2001;97:1232–1240. doi: 10.1182/blood.V97.5.1232. [DOI] [PubMed] [Google Scholar]
- 22.Ganepola S, Gentilini C, Hilbers U, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:293–299. doi: 10.1038/sj.bmt.1705585. [DOI] [PubMed] [Google Scholar]
- 23.Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 24.Ozdemir E, Saliba RM, Champlin RE, et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant. 2007;40:125–136. doi: 10.1038/sj.bmt.1705699. [DOI] [PubMed] [Google Scholar]
- 25.Andrei G, De Clercq E, Snoeck R. Novel inhibitors of human CMV. Curr Opin Investig Drugs. 2008;9:32–45. [PubMed] [Google Scholar]
- 26.Mercorelli B, Sinigalia E, Loregian A, Palù G. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev Med Virol. 2008;18:177–210. doi: 10.1002/rmv.558. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich JM, Bowden RA, Fisher L, et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993;118:173–178. doi: 10.7326/0003-4819-118-3-199302010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Li CR, Greenberg PD, Gilbert MJ, et al. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 29.Schmidt-Hieber M, Schwarck S, Stroux A et al (2009) Prophylactic i.v. Igs in patients with a high risk for CMV after allo-SCT. Bone Marrow Transplant [Epub ahead of print] [DOI] [PubMed]
- 30.Bale JF, Jr, Petheram SJ, Souza IE, Murph JR. Cytomegalovirus reinfection in young children. J Pediatr. 1996;128:347–352. doi: 10.1016/S0022-3476(96)70279-2. [DOI] [PubMed] [Google Scholar]
- 31.Chandler SH, Handsfield HH, McDougall JK. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J Infect Dis. 1987;155:655–660. doi: 10.1093/infdis/155.4.655. [DOI] [PubMed] [Google Scholar]
- 32.Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet. 1984;10:528–530. doi: 10.1016/S0140-6736(84)90930-9. [DOI] [PubMed] [Google Scholar]
- 33.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. NEJM. 2009;360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197:1634–1642. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heslop HE, Brenner MK, Rooney C, et al. Administration of neomycin-resistance-gene-marked EBV-specific cytotoxic T lymphocytes to recipients of mismatched-related or phenotypically similar unrelated donor marrow grafts. Hum Gene Ther. 1994;5:381–397. doi: 10.1089/hum.1994.5.3-381. [DOI] [PubMed] [Google Scholar]
- 36.Rooney CM, Smith CA, Ng C, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/S0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 37.Madrigal JA, Travers PJ, Dodi IA. Immunotherapeutic aspects of stem cell transplantation. Hematology. 2005;10(Suppl 1):289–292. doi: 10.1080/10245330512331390131. [DOI] [PubMed] [Google Scholar]
- 38.Dolstra H, Preijers F, Van de Wiel-van Kemenade E, et al. Expansion of CD8+ CD57+ T cells after allogeneic BMT is related with a low incidence of relapse and with cytomegalovirus infection. Br J Haematol. 1995;90:300–307. doi: 10.1111/j.1365-2141.1995.tb05150.x. [DOI] [PubMed] [Google Scholar]
- 39.Fallen PR, Duarte RF, McGreavey L, et al. Identification of non-naïve CD4+CD45RA+ T cell subsets in adult allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32:609–616. doi: 10.1038/sj.bmt.1704185. [DOI] [PubMed] [Google Scholar]
- 40.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 42.Myers GD, Krance RA, Weiss H, et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath) Bone Marrow Transplant. 2005;36:1001–1008. doi: 10.1038/sj.bmt.1705164. [DOI] [PubMed] [Google Scholar]
- 43.Fujita Y, Rooney CM, Heslop HE. Adoptive cellular immunotherapy for viral diseases. Bone Marrow Transplant. 2008;41:193–198. doi: 10.1038/sj.bmt.1705906. [DOI] [PubMed] [Google Scholar]
- 44.André F, Chaput N, Schartz NE, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 45.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 46.De La Peña H, Madrigal JA, Rusakiewicz S et al (2009) Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods [Epub ahead of print] [DOI] [PubMed]
- 47.Babincová M, Altanerová V, Lampert M, et al. Site-specific in vivo targeting of magnetoliposomes using externally applied magnetic field. Z Naturforsch [C] 2000;55:278–281. doi: 10.1515/znc-2000-3-422. [DOI] [PubMed] [Google Scholar]
- 48.Fortin-Ripoche JP, Martina MS, Gazeau F, et al. Magnetic targeting of magnetoliposomes to solid tumors with MR imaging monitoring in mice: feasibility. Radiology. 2006;239:415–424. doi: 10.1148/radiol.2392042110. [DOI] [PubMed] [Google Scholar]
- 49.Oelke M, Maus MV, Didiano D, et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 50.Papanicolaou GA, Latouche JB, Tan C, et al. Rapid expansion of cytomegalovirus-specific cytotoxic T lymphocytes by artificial antigen-presenting cells expressing a single HLA allele. Blood. 2003;102:2498–2505. doi: 10.1182/blood-2003-02-0345. [DOI] [PubMed] [Google Scholar]