Abstract
Increased evidence indicates that chemokines are involved in tumor growth. ITAC, a key member of chemokines, possesses the ability to recruit T cells and enhance immune responses. Therefore, ITAC might contribute to antitumor immunity. In this study, we evaluated the relationship between the expression of ITAC and human breast cancer advancement. We further investigated whether forced expression of ITAC in tumor sites could mediate enhanced antitumor immunity in a murine breast cancer model. Results showed that ITAC expression level was down-regulated in 31 breast cancer specimens compared to normal mammary tissues, and associated negatively with the stages of breast cancer. Contrarily, forced expression of ITAC in murine 4T1 tumor cells resulted in tumor regression after initial growth upon injection into naïve Balb/c mice. More lymphocytes were recruited to the site of tumor inoculated by 4T1-ITAC and more than 80% of these T cells expressed the ITAC receptor, CXCR3. ITAC-recruited TILs exhibited 4T1-specific proliferation and cytotoxicity, and an increased IFN-γ but decreased IL-4 production. Importantly, forced expression of ITAC in 4T1 tumor nodules inhibited tumor growth. These findings demonstrated that the decreased expression of ITAC is associated with the advancement of breast cancer in patients. Forced expression of ITAC in tumor site not only induces increased T cell-recruitment and elicits a specific antitumor immunity, but also mediates regression of established 4T1 tumors, indicating the potential application of ITAC-expressing tumor cells in cancer immunotherapy and vaccine designing.
Keywords: Chemokine, ITAC, Tumor regression, Antitumor immunity, 4T1 tumor
Introduction
Breast cancer is one of the most common cancers. There is an urgent need to develop effective therapies for breast cancer [1, 2]. Immunotherapy based on cancer vaccines is one of the most promising therapeutic strategies [3, 4]. However, an effective antitumor immunity depends on efficient tumor infiltration by antigen-specific lymphocytes [5, 6]. Therefore, molecules involved in the recruitment of lymphocytes may be strong candidates for designing new breast cancer vaccines.
The large chemokine family consists of more than twenty small, structurally related heparin-binding proteins, which are classified as C, CXC, CC, and CX3C chemokines according to the cysteine residues found in their primary amino acid sequences [7]. One of the characteristic biological effects of chemokines is their ability to recruit leukocytes into tissues [7, 8]. The IFN-γ-inducible T cell α chemoattractant (ITAC), a member of the CXC chemokine family, is produced by activated monocytes, fibroblasts, endothelial cells and keratinocytes [9]. It strongly induces migration of activated but not naïve T cells, because the only receptor of ITAC (CXCR3) is usually expressed solely on the surface of activated T lymphocytes [10–12]. It has been shown that ITAC plays a pivotal role in attracting effector T cells into the sites of Th1-type inflammation and is critically involved in the development of multiple Th1-type inflammatory diseases [13, 14]. It has also been reported that ITAC has potent antitumor activity by attracting CD8+ T lymphocytes [15]. However, the expression of ITAC in breast tumor masses of clinical specimens and the effects of its over-expression by tumor cells has not been carefully examined.
In this study, we analyzed the relationship between the expression of ITAC in breast cancer specimens and the magnitude of tumor growth. We found that ITAC expression is negatively associated with the stages of breast cancer. ITAC expression is lower both in human and murine breast cancer cell lines. Thus, we hypothesized that increased expression of ITAC by tumor cells may limit tumor progression. To test this hypothesis, we transfected the murine 4T1 breast cancer cell line with a plasmid vector encoding the ITAC gene (termed 4T1-ITAC), and studied tumor growth of 4T1-ITAC after it was injected into naïve Balb/c mice. We found a complete tumor regression of 4T1-ITAC tumors but of neither 4T1-pcDNA nor 4T1 tumor. More T lymphocytes were recruited into the tumor masses. An enhanced antitumor immune response against 4T1 tumor cell was induced in the ITAC-expressing tumor environment. Notably, inoculation of 4T1-ITAC into parental 4T1-tumor-bearing mice mediated the rejection of pre-existing tumor burden.
Materials and methods
Specimens, animals and cell lines
Tissue samples from patients with primary breast cancer were obtained during the period between September 2004 and April 2005, with informed consent from Zhongshan Hospital of Shanghai. The stage of each specimen was determined by histopathologic diagnosis. Patients were divided into two groups—early breast cancer (stage I and stage II) or advanced breast cancer (stage III and stage IV). Normal mammary tissue was derived from tissues adjacent to the cancer tissue. Female BALB/c mice aged 6–8 weeks were purchased from the Center of Experimental Animal, Fudan University (Shanghai, China). All animals were housed in pathogen-free conditions, and all animal experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals (Ministry of Health, Peoples Republic of China, 1998). 4T1 and EMT-6 are murine breast cancer cell lines (kindly provided by Dr. Hu, Earle A. Chiles Research Institute, Portland, OR, USA). SKBR3, MCF-7, MDA-MB-231 and MDA-MD-453 (kindly provided by Dr. Disis, Seattle, USA) are human breast cancer cell lines. THP-1 is a human monocytic cell line and L929 is a murine fibroblast cell line (ATCC, USA). All cell lines were cultured at 37°C under 5% CO2 in complete RPMI 1640 (GIBICO, Grand land, NY, USA) medium containing 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin sulfate.
Real-time PCR
Total cellular RNA from cell lines or tissues was prepared by the guanidinium thiocyanate-acid phenol method. Residual DNA was eliminated with DNase I. Reverse transcription was carried out as described by the manufacturer [16]. The resulting cDNA was quantitatively analyzed by real-time PCR using LightCycler Instrument (Roche Molecular Biochemicals, Mannheim, Germany). For relative quantitative analysis, housekeeper genes (human β-actin or murine GAPDH) were used as internal controls. Relative quantity of ITAC mRNA was represented by the ratio of the level of expression of ITAC to that of human β-actin or murine GAPDH (termed normalized expression of ITAC). DNA was amplified with specific primers (Table 1). The reaction mixture consists of 2 μl of LightCyclerTM DNA Master SYBR Green I (Roche Diagnostic Company, Mannheim, Germany). The cycling program comprises a 10 min initial denaturation at 95°C followed by 40 cycles of instant denaturation at 95°C, annealing at 60°C for 10 s and elongation at 72°C for 12 s, with a transition rate of 20°C/s between each temperature plateau. Quantification data was analyzed using the LightCycler analysis software version 4.5.
Table 1.
The primers for human and murine ITAC
| Genes | Primer sequence | PCR products (bps) |
|---|---|---|
| Human ITAC |
F: 5-GGGGTAAAAGCAGTGAAAGTGGC R: 5-GAAAGCACTTTGTAAACTCCGA |
492 |
| Human β-actin |
F:5′TCACCCACACTGTGCCCATCTACGA 3′ R:5′CAGCGGAACCGCTCATTGCCAATGG 3′ |
399 |
| Murine ITAC |
F: 5-CGGAATTCAAGAGAGATCTCCAAAGCCCA R:5-ATAAGAATGCGGCCGCCAATGCATCCACTGTAACCA |
594 |
| Murine GAPDH |
F:5-CTGCACCACCAACTGCTTAG-3 R:5-GTCTGGGATGGAAATTGTGA |
660 |
F forward, R reverse
ITAC gene transfection to 4T1 cell line
Total RNA was extracted from IFN-γ (1,000 U/ml) (R&D systems, Minneapolis, USA)-stimulated L929 cell line with Trizol (Invitrogen, San Diego, CA, USA). ITAC gene (EMBL AF178676) was amplified with RT-PCR subsequently. Primers were listed in Table 1. The ITAC gene was then subcloned into the EcoRI and XhoI sites of the mammalian expression vector pcDNA3 (Invitrogen, San Diego, USA). 1 × 106 4T1 cells were transfected with the pcDNA3 control vector or pcDNA3-ITAC target gene by electroporation (280 V and 7 ms) in a 400 μl medium and stable clones were selected in complete RPMI1640 medium containing 0.8 mg/ml G418 (Sigma, St Louis, USA). The individual G418-resistant, stably transfected clones obtained by limiting dilution were expanded.
ELISA
The detection of ITAC expression from the cultured supernatant of 4T1-ITAC, 4T1-pcDNA3 and 4T1 was performed by ELISA. All reagents for ELISA were purchased from R&D systems (Mineapolis, MN, USA) and were performed according to the manufacturer’s protocol. The results were determined by using a microplate reader set to 450 nm to determine the concentration of ITAC from the supernatant according to the mITAC standard curve.
Chemotaxis assay
Chemotaxis assay was performed as previously described [17]. In brief, supernatants harvested from the cultures of 4T1-ITAC, 4T1-pcDNA3 or parental 4T1 cells were added in triplicate into the lower chamber of a 48-well chemotaxis plate with 5-μm pores (Corning Costar, Corning, NY, USA). RPMI-1640 media containing 0.1% BSA was used as the negative control. Recombinant ITAC protein purchased from Pepro Tech (New Jersey, USA) was used as the positive control (1 ng/ml). The upper chambers of the chemotaxis plate were filled with 50 μl of IL-2-stimulated splenocytes (1 × 107 cells/ml) in the presence or absence of 10 μg/ml mITAC-specific Ab (R&D). The chemotaxis plate was incubated at 37°C. Four hours later, the numbers of cells that migrated into the lower chambers were numerated. Chemotaxis index (CI) was calculated by dividing the number of cells migrated in response to testing supernatants by the number of cells migrated in response to the negative control medium.
Experiment of tumorigenesis
BALB/c mice (n = 6 per group) were subcutaneously (s.c.) injected with 1 × 105 4T1-ITAC cells, 4T1-pcDNA3 or parental 4T1 cells, respectively. Mice were monitored for evidence of tumor growth by palpation and inspection, and tumor size was measured with a digital caliper every 2 or 3 days. The survival of the tumor-bearing mice was observed by daily assessment over 60 days.
Long-term protection experiments
Survived mice from several independent experiments that received the same treatment were pooled and re-challenged with 1 × 105 live 4T1 cells subcutaneously. Naïve mice that were injected with the same number of tumor cells on the same day served as control. Tumor growth was measured as described above. Mice survival was observed over 90 days.
Cell isolation from tumor tissue
Fourteen days after subcutaneous injection of 1 × 105 4T1-ITAC cells, 4T1-pcDNA3 or parental 4T1 cells, tumors were resected and cut into pieces, and then suspended in RPMI1640 containing 1.5 mg/ml collagenase IV for 2 h at room temperature with shaking. The resultant single cell suspension was loaded onto Ficoll gradient and tumor-infiltrating lymphocytes were isolated from the interface of gradient after centrifugation.
Flow cytometric assay
One million lymphocytes were stained with goat anti-mouse CXCR3 antibody and PE-conjugated hamster anti-mouse CD3 antibody for analysis of CXCR3+ CD3+ T cell population by flow cytometry. Goat anti-mouse CXCR3 antibody, FITC-conjugated donkey anti-goat IgG, PE-conjugated hamster anti-mouse CD8 antibody (53–6.7), and PerCP-conjugated hamster anti-mouse CD4 antibody (RM4-5) (BD, PharMingen, CA, USA) were used in some of the experiments.
Cell proliferation assay
5 × 105 lymphocytes were seeded in a 96-well plate and incubated for 3 days with Mitomycin C (MMC)-treated parental 4T1 cells for tumor-specific stimulation or with MMC-treated Renca cells to serve as negative control (at tumor:effector cell ratio of 0:1, 1:10, 1:100, 1:1,000). Lymphocytes stimulated with concanavalin A (ConA) were used as a positive control. 3H-thymidine (Shanghai Atomic Energy Institute, Chinese academy of Science) was added at 1 μCi per well for the last 18 h of the 3-day culture. 3H-thymidine incorporation was measured in a liquid scintillation counter (Shanghai Atomic Energy Institute, Chinese Academy of Science). Results are represented as count per minute (cpm).
Quantitation of specific lysis by CFSE/7-AAD cytotoxicity assay
Cytotoxicity assay was performed as previously described with minor modification [18]. Briefly, 14 days after subcutaneous injection of 1 × 105 4T1-ITAC cells, 4T1-pcDNA3 or parental 4T1 cells, tumors were resected. Lymphocytes isolated from the tumors were stimulated with MMC-treated 4T1 parent tumor cells for 4 days and the resulting T cells were used as effector cells. Target 4T1 cells were labeled with 5 μM 5- and 6-carboxyfluorescein diacetate succinimydyl ester (CFSE, Fluka, Switzerland) as described before [19]. After that, the tumor infiltrating lymphocytes (TILs) were seeded with 4T1 cells (1 × 104 /well) in 96-well plates. The ratios of effector versus target cells (E:T) were 10:1, 20:1 and 40:1. In parallel, target cells were incubated alone to measure basal apoptosis. Six hours after culture, cells were collected and incubated in PBS/1% BSA containing 20 μg/ml 7-AAD (Sigma) for 20 min at 4°C in the dark. These cells were then fixed in 4% raformaldehyde (Sigma) followed by flow cytometry analysis. CFSE fluorescence and 7-ADD emission were detected in the FL-1 and FL-3 channels, respectively. For each E:T ratio, 20,000 target cells were acquired. Analysis was performed with Cell Quest software (BDIS). The formula for calculating the percentage of specific target cell lysis is: % specific lysis = 100 × (% sample lysis − % basal lysis)/(100 − % basal lysis).
Cytokine detection assay
Release of IFN-γ and IL-4 was determined by sandwich ELISA according to the manufacturer’s instruction (BD pharmingen, San Diago, USA). In brief, immunoassay plates, which had been coated with anti-mouse IFN-γ or IL-4 capture antibodies, were incubated with cell culture supernatants at room temperature for 2 h. Recombinant mouse IFN-γ and IL-4 were used for standard titration curves. Plates were subsequently incubated with HRP-conjugated streptavidin followed by addition of substrates (H2O2 and ABTS). Absorbance was measured at 450 nm in a microplate reader to analyze the generated data. The sensitivity of IFN-γ and IL-4 ELISA was 16 pg/ml.
Therapeutic vaccination with 4T1-ITAC
Naïve mice were subcutaneously injected with 1 × 105 live 4T1 tumor cells. Subsequently on day 6, after the tumor has established, either 1 × 106 4T1-ITAC tumor cells or 1 × 106 4T1-pcDNA3 were injected intratumorally. 4T1 tumor-bearing mice which do not receive intratumoral injection of 4T1-ITAC or 4T1-pcDNA3 were used as control. Tumor growth was measured as described above.
Statistical analysis
Statistical analyses of the data were performed with the aid of analysis programs (SPSS11.5 software). Statistical evaluation was performed using two-way analysis of variance ANOVA; using the program PRISM version 4 (GraphPad Software, Inc., San Diego, CA), a “P” value less than 0.05 is considered as significant.
Results
Expression of ITAC is down-regulated in both breast cancer specimens and cell lines
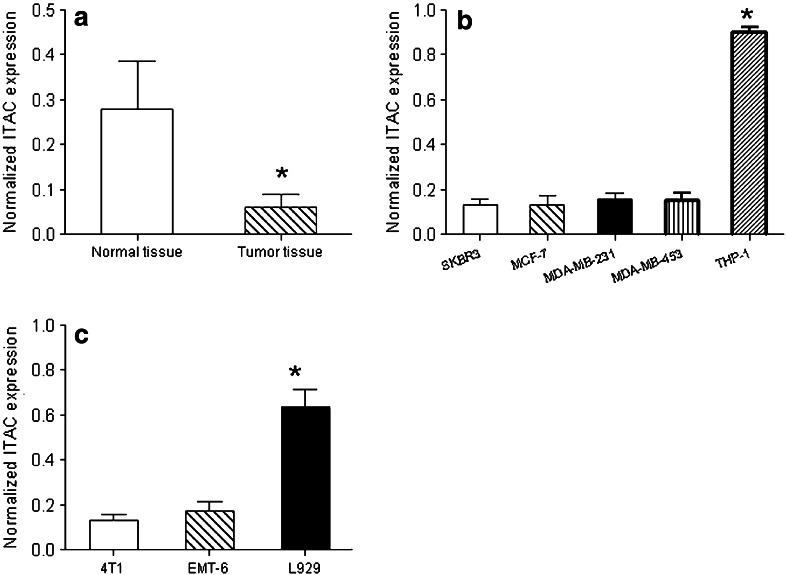
ITAC is a key chemokine that attracts T cells, especially activated T lymphocytes. To investigate whether ITAC was expressed in breast cancer specimens or cell lines, ITAC mRNA expression was evaluated by quantitative RT-PCR in 31 breast cancer specimens and 10 adjacent normal breast tissues. Results showed a significant down-regulation of ITAC mRNA in breast cancer specimens compared to normal breast tissues (Fig. 1a, P < 0.05). Moreover, a decreased expression of ITAC was associated with the advancement of breast cancer (Table 2).
Fig. 1.
Expression of ITAC in breast cancer specimens and cell lines. Total RNA was extracted from 31 human breast cancer tissues and 10 adjacent normal tissues, as well as 4 human and 2 murine breast cancer cell lines. ITAC expression was detected by RT-PCR and was normalized with GAPDH (mouse) or β-actin (human). a Human normal breast tissue and cancer tissue. *P < 0.05. b Human breast cancer cell lines: SKBR3, MCF-7, MDA-MB-231 and MDA-MD-453. Human monocytic cell line THP-1 served as a positive control. *P < 0.01. c Murine breast cancer cell lines: 4T1 and EMT-6. Murine fibroblast cell line L929 stimulated with IFN-γ served as a positive control. *P < 0.05
Table 2.
Histological grade and ITAC expression intensities
| Histological grade | ITAC expression intensities | P | |
|---|---|---|---|
| Low | High | ||
| I–II | 2 | 19 | <0.01 |
| III–IV | 9 | 1 | <0.05 |
Then we examined four human breast cancer cell lines (SKBR3, MCF-7, MDA-MB-231 and MDA-MD-453) for the expression of ITAC at a transcription level. Human monocytic cell line THP-1, which expresses ITAC served as positive control. All cell lines expresses less ITAC compared to the THP-1 control (Fig. 1b, P < 0.01). Likewise, we surveyed two murine breast tumor cell lines (EMT-6 and 4T1) for ITAC expression. Fibroblast cell line L929 stimulated with 1,000 U/ml IFN-γ served as positive control. Less expression of ITAC could be detected in these two breast cancer cell lines (Fig. 1c, P < 0.05). The results indicated that both breast cancer specimens and breast cell lines expressed less ITAC as compared to normal tissues.
Modification of 4T1 with ITAC-coding gene results in an increased expression of ITAC and a stronger chemoattractant ability
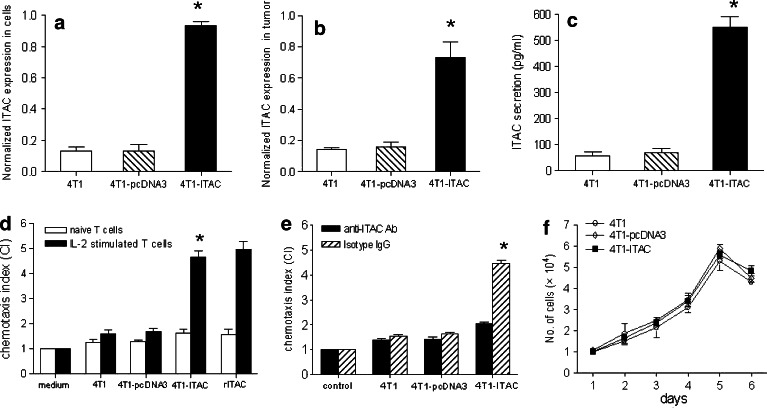
To determine whether the expression of ITAC in tumor cells could attract T cells, murine breast cancer 4T1 cells was transfected with a plasmid harboring the gene encoding for ITAC or with an empty vector plasmid pcDNA3. G418-resistant, ITAC-transfected 4T1 cell clones (4T1-ITAC) were selected, and the expression of ITAC was determined by RT-PCR. As shown in Fig. 2a, 4T1-ITAC tumor cells expressed a higher level of ITAC mRNA, compared to 4T1 and 4T1 transfected with pcDNA3 vector (4T1-pcDNA3) (P < 0.01). Moreover, 4T1-ITAC tumor xenograft derived from Balb/c mice also expressed a higher level of ITAC mRNA, compared to 4T1 or 4T1-pcDNA3 xenografts (Fig. 2b, P < 0.01). The supernatants from 1 × 106 4T1-ITAC cells cultured for 24 h in vitro were collected to measure their secretion of ITAC with ELISA. Approximately 550 ng/ml ITAC could be detected in the culture supernatant of 4T1-ITAC tumor cells. Less secretion of ITAC were detected in the culture supernatant from either 4T1-pcDNA3 or 4T1 parent tumor cells (Fig. 2c, P < 0.01). Next, the chemotactic activity of 4T1-ITAC cell culture supernatant was tested using IL-2-stimulated activated T lymphocytes that expressed high level of CXCR3 (the receptor of ITAC) as the responder cells in an in vitro chemotactic assay. Supernatants derived from 4T1-ITAC cells chemoattracted as many IL-2-stimulated CXCR3+ T lymphocytes as the positive control rITAC, and is about three times more than the supernatants derived from parental 4T1 or 4T1-pcDNA3 cells (Fig. 2d, P < 0.05). A significant decrease in chemotaxis index was observed after the addition of anti-ITAC neutralizing antibody (Fig. 2e, P < 0.05), indicating that the chemotatic activity of the supernatant derived from 4T1-ITAC cell was indeed mediated by ITAC.
Fig. 2.
Functional studies of ITAC on 4T1 tumor cells. Total RNA was extracted and reverse transcribed with random primers. The cDNA was subjected to RT-PCR. a ITAC expression in parental 4T1 cells (open bars), 4T1-pcDNA3 cells (hatched bars) and 4T1-ITAC cells (closed bars) which was normalized to the level of GAPDH. *P < 0.01. b 14 days after tumor cells injection, ITAC expression in 4T1 tumor tissue (open bars), 4T1-pcDNA3 tumor tissue (hatched bars) and 4T1-ITAC tumor tissue (closed bars) was detected *P < 0.01. c ITAC protein (pg/ml) from the supernatant of 1 × 106 4T1 (open bars), 4T1-pcDNA3 (hatched bar) and 4T1-ITAC (black bar) cultured for 48h. *P < 0.01. d Chemotaxis index (CI) of naïve (open bars) and IL-2-stimulated lymphocytes (closed bars) in response to cultured supernatants of parental 4T1, 4T1-pcDNA3,4T1-ITAC cells and recombinant ITAC (rITAC) was detected and calculated by chemotaxis assay. *P < 0.05. e Chemotaxis assay of IL-2-stimulated lymphocytes pre-incubated with anti-ITAC antibody (black bars), isotype IgG (hatched bars) in response to the cultured supernatants of parental 4T1, 4T1-pcDNA3 and 4T1-ITAC cells was performed. *P < 0.05. f The in vitro culture of parental 4T1, 4T1-pcDNA3 and 4T1-ITAC cells were harvested and cell numbers were counted at the designated time points (P > 0.05). These experiments were repeated for three times. Data represent mean and SD
To determine the influence of ITAC target gene transfection per se on the behavior of 4T1 cells growth, 4T1-ITAC, 4T1-pcDNA3 and 4T1 cells were cultured in vitro under the same culture condition. Expression of ITAC did not alter the growth pattern of 4T1 cells in vitro (Fig. 2f, P > 0.05). Thus, we have established a stable cell line, which expresses functional ITAC and the expression of ITAC did not change tumorigenecity of 4T1 tumor cells in vitro.
Forced expression of ITAC enhances the recruitment of CXCR3+ T lymphocytes into tumor site
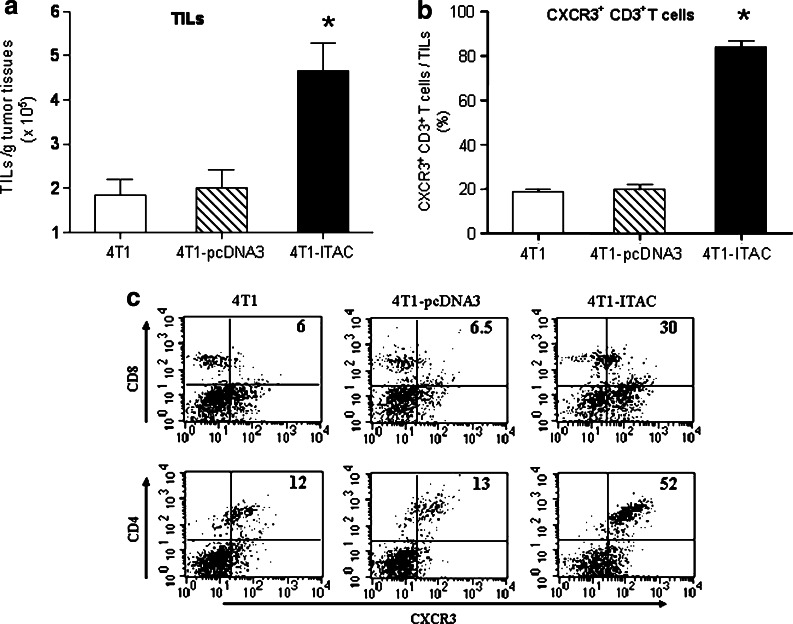
To determine whether 4T1-ITAC tumor enhanced the recruitment of CXCR3+ T lymphocytes into tumor site, TILs were isolated from 4T1-ITAC, 4T1-pcDNA3 and 4T1 tumors at day 15 after tumor cells inoculation. The number of TILs per gram weight of tumor was measured. Compared to the 4T1-pcDNA3 and 4T1 inoculated tumors, a great increase in the number of TILs per gram of tumor was found in 4T1-ITAC tumor (Fig. 3a, P < 0.05). Almost 83% of TILs found in 4T1-ITAC tumors were CXCR3+CD3+ T cells; however, less than 20% of TILs in either 4T1 or 4T1-pcDNA3 tumors were CXCR3+CD3+ T cells, suggesting that expression of ITAC by tumor cells enhanced the recruitment of CXCR3+CD3+ T lymphocytes into the tumor sites (Fig. 3b, P < 0.05). On analysis of the subsets of CXCR3+CD3+ T cells by staining these cells with CD4+ and CD8+ antibodies, we found that both CD4+ and CD8+ T cells were remarkably increased in the 4T1-ITAC tumor sites compared to the 4T1 or 4T1-pcDNA3 tumors. These results also indicate that both CD4+ and CD8+ T cells might play roles in antitumor immunity (Fig. 3c).
Fig. 3.
In situ expression of ITAC enhanced the recruitment of CXCR3+ T lymphocytes into tumors. Day 14 after tumor cell inoculation, the amount of TILs isolated from 4T1 (open bars), 4T1-pcDNA3 (hatched bar) and 4T1-ITAC (black bar) per gram tumor were counted and normalized to tumor mass (a). *P < 0.05. The CXCR3+CD3+ T cells were further stained and counted with flow cytometry (b). *P < 0.05. The percentages of CXCR3+CD4+ and CXCR3+CD8+ T cells in TILs were assessed by flow cytometry (c). Experiments were performed for three times
Tumor growth is reduced in 4T1-ITAC inoculated tumor
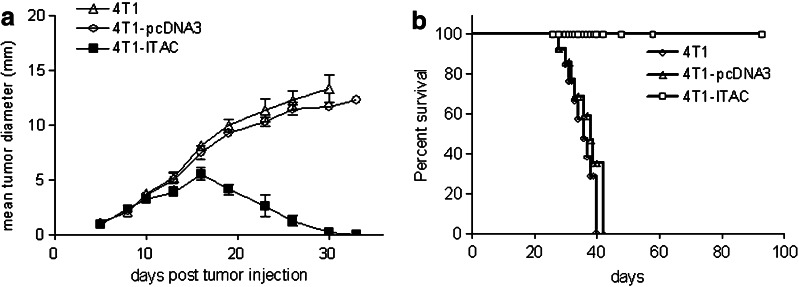
To examine the effect of forced expression of ITAC by 4T1 tumor cells, naïve Balb/c mice were injected with 1 × 105 4T1-ITAC, 4T1-pcDNA3 or 4T1 tumor cells and tumor size was recorded every 2 or 3 days after tumor inoculation. Mice receiving either 4T1 or 4T1-pcDNA3 cells developed progressively growing tumors, whereas the growth of 4T1-ITAC tumor cells in all mice was dramatically inhibited around 15 days and totally regressed 33 days post tumor inoculation (Fig. 4a, P < 0.01). All mice receiving 4T1-ITAC tumor cells survived over 90 days without tumors, whereas all control mice that received either parental 4T1 or 4T1-pcDNA3 succumbed to the tumor by day 38 after tumor inoculation (Fig. 4b, P < 0.01). To further determine whether those mice that rejected 4T1-ITAC developed antitumor memory responses, 4T1 parental tumor cells were inoculated into those mice that survived after 4T1-ITAC challenge. Tumor grew in 0/6 of the mice in the experiment group with previous 4T1-ITAC challenge, while tumor grew in 6/6 of the control mice that did not receive 4T1-ITAC challenge (Table 3, P < 0.01). These results demonstrated that 4T1-ITAC not only induced complete rejection of tumor but also a long-term antitumor memory response.
Fig. 4.
The effect of 4T1-ITAC tumor cells on inhibiting tumor growth in vivo. a BALB/c immunocompetent mice (n = 6 per group) were injected with 1 × 105 parental 4T1 cells (open triangle), 4T1-pcDNA3 cells (open circle) or 4T1-ITAC cells (closed square). Tumor diameters were expressed as an average of the longest and perpendicular diameter ±SD. P < 0.01. b Survival of tumor-bearing immunocompetent mice was observed by daily assessment for 90 days. P < 0.01. Experiments were repeated for three times
Table 3.
Mice that experienced complete 4T1-ITAC tumor regression were protected against 4T1 parent tumor rechallenge
| Mice | 4T1 rechallenge | Incidences of tumor growth (%)* |
|---|---|---|
| Navie | 1 × 105 | 10/10 (100) |
| Survival | 1 × 105 | 0/20 (0) |
*P<0.01
ITAC-recruited TILs dominates 4T1-specific cellular immune responses
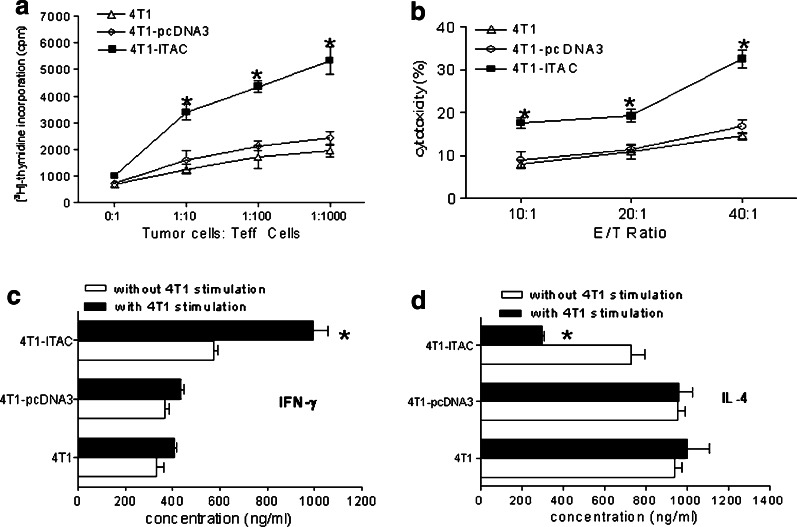
To determine whether the T cells recruited by 4T1-ITAC could exhibit tumor-specific antitumor immune responses, tumor infiltrating lymphocytes were isolated from 4T1-ITAC, 4T1-pcDNA3, and 4T1 respectively and cultured in the presence of MMC-treated 4T1 tumor cells for measurement of cell proliferation, cytokine secretion and cytotoxic activity in vitro. As shown in Fig. 5a, TILs from 4T1-ITAC tumors proliferated significantly faster than TILs from parental 4T1 cells or control 4T1-pcDNA3 cells in response to MMC-treated 4T1 tumor cells (P < 0.05). Specific cytotoxicity assay showed that TILs from 4T1-ITAC tumors displayed more potent cytolytic killing activity against 4T1 target cells compared to TILs derived from parental 4T1 tumors or 4T1-pcDNA3 tumors (Fig. 5b, P < 0.05). Furthermore, a higher level of IFN-γ was detected in the culture supernatant of TILs isolated from 4T1-ITAC cells than those of TILs derived from 4T1 or control 4T1-pcDNA3 tumor cells (Fig. 5c, P < 0.05). Interestingly, a reduced level of IL-4 was found in the culture supernatant of TILs isolated from 4T1-ITAC tumors compared to those found in parental 4T1 or 4T1 pcDNA3 tumors (Fig. 5d, P < 0.05). These results suggest that ITAC derived from 4T1-ITAC tumor cells not only enhances the trafficking of tumor-specific TILs into tumor sites, but also augmented Th1 while diminished Th2 cytokine responses in tumor sites.
Fig. 5.
TILs recruited by 4T1-ITAC elicited anti-4T1 specific cellular immune responses in vitro. Fourteen days after 4T1, 4T1-pcDNA3 and 4T1-ITAC tumor cell inoculation, TILs were isolated. a 5 × 105 TILs were cultured with 5 × 103 inactivated 4T1 in vitro for 72 h, proliferation of the TILs was determined by [3H]-thymidine incorporation. *P < 0.05. b Cytotoxic activity of TILs against specific target 4T1 cells at different ratio of effector versus target cells was assessed with CFSE/7-AAD method. *P < 0.05. c IFN-γ and d IL-4 release from the 72 h-cultured supernatant were measured by ELISA. *P < 0.05. Experiments were repeated twice
Inoculation of 4T1-ITAC cells in tumors leads to rejection of pre-existing 4T1 tumor
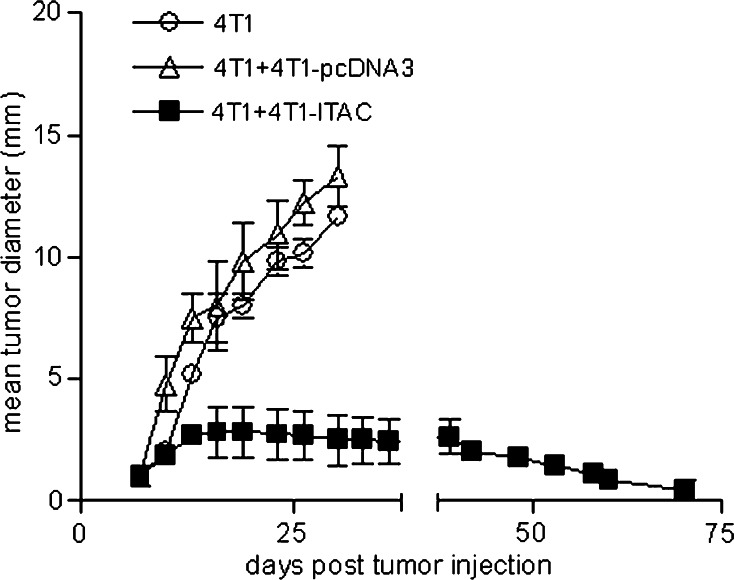
To examine if ITAC-transfected 4T1 tumors have impacts on pre-existing parental 4T1 tumors, 1 × 105 4T1 parental tumor cells were subcutaneously injected into Balb/c mice and allowed to establish into tumor xenografts for 14 days. Subsequently, 1 × 106 4T1-ITAC tumor cells were injected into the parental 4T1 tumors. After injection of 4T1-ITAC, tumors grew for another 5–8 days before the onset of regression and tumors gradually disappeared in all mice around 70 days post inoculation of 4T1 parental tumors (Fig. 6). On the contrary, tumor grew aggressively if tumor-bearing mice were injected with 1 × 106 4T1-pcDNA3 tumor cells (Fig. 6, P < 0.01). The result showed that ITAC-transfected tumor cells can mediate the rejection of established parental tumors when introduced into the tumor xenografts. Therefore, ITAC-expressing tumor cell lines could serve as a potential tumor vaccine to facilitate the rejection of parental tumors.
Fig. 6.
Therapeutic vaccination with 4T1-ITAC. 1 × 105 4T1 parental tumor cells were subcutaneously injected into Balb/c mice and allowed to establish into tumors for 14 days. Subsequently, 1 × 106 4T1-pcDNA3 or 4T1-ITAC tumor cells were injected into the established 4T1 tumor. Tumor growth was measured by averaging the longest and perpendicular diameter ± SD. The experiment was repeated three times. P < 0.01
Discussion
In clinical settings, the major obstacle to successful immunotherapy is the lack of efficient T-cell infiltration and activation in the tumor site [19–21]. A recent study has also established that the density and type of T cells in tumor sites is a better predictor of survival than the traditional staging systems [22], suggesting that it may have prognostic impacts by carefully analyzing local antitumor immune responses. The trafficking of lymphocytes from the systemic circulation into tumor sites is governed by the chemoattractant activity of various chemokines [23]. In this study, we found that the expression of the chemokine ITAC is down-regulated both in primary breast specimens and breast cancer cell lines, and that the level of ITAC expression is inversely correlated with histological grading. Our results indicate that down-regulation of ITAC in tumor tissues might diminish the infiltration of tumor-reactive lymphocytes and lead to tumor progression. The number of infiltrating CXCR3+CD3+lymphocytes is less in breast cancer tissues without ITAC expression compared to the number of lymphocytes in breast tumor tissue with ITAC expression (Fig. 3b), strongly suggesting that ITAC expression in tumor sites played an important role in the recruitment T cells into the tumor site. These results is consistent with other reports which showed that the CXCR3-targeting chemokine ITAC has potent antitumor activity in vivo by attracting CD8+ T lymphocytes into tumor sites [15] and that ITAC has a distinctive CXCR3 binding site to chemoattract CXCR3-expressing T lymphocytes [24].
T cell stimulation in the absence of costimulation can induce energy and apoptosis of antigen-specific T cells [25–27]. Thus, it was strongly believed that priming of tumor-specific T cells does not occur in tumor sites [25, 27]. However, direct priming of tumor-specific T cells has been shown before when tumor cells were forced to express certain cytokines (Light) or chemokines (CCL7) that are capable to induce de novo generation of tertiary lymphoid-like tissues or organs [5, 28, 29]. In our study, we found that aside from the rejection of ITAC-expressing tumors when injected into naïve mice, ITAC also induced specific immune responses against 4T1 parent tumor and rejection of pre-existing tumors when injected directly into tumors. 4T1-ITAC induced an enhanced proliferation and cytotoxicity against 4T1 tumor cells in addition to a high level of IFN-γ secretion in response to 4T1 cells (Fig. 5). Because these effects were exhibited only in 4T1-ITAC inoculated mice and not in 4T1 or 4T1-pcDNA3 inoculated mice, our results indicated that ITAC could potentially act like Light or CCL7 in priming and expanding the CXCR3+ lymphocytes aggregating in tumor environment. However, such possibility remains to be examined in mice lacking secondary lymphoid tissues.
Tumor vaccination is a useful tool in antitumor immunotherapy [30–32]. Tumor cells were modified with various immune modulating molecules to generate effective antitumor vaccine [33, 34]. Our findings demonstrated that 4T1 transfected with ITAC could eradicate the parent 4T1 tumor in 4T1 tumor-bearing mice, indicating that ITAC expressing tumor cells could serve as tumor vaccine to eliminate established parent tumor. This result was confirmed by intratumoral injection of purified ITAC protein (data not shown). It will be worth exploring whether the local expression of ITAC achieved by various delivery systems at tumor sites may induce better immune responses in cancer patients.
In summary, we demonstrated that injection of ITAC-expressing tumor cells can significantly augment T cell-mediated antitumor effect in vivo. These findings suggest that, in addition to the recruitment of CXCR3+ T cells into tumor sites, ITAC also acts as a stimulator to promote the proliferation of recruited T cells and sustain the tumor-specific T cells in local tissues, leading to tumor regression. Identification of the multiple functions of ITAC in T cell-mediated antitumor immunity should yield further insight into novel chemokine-based tumor therapy, and the molecular and cellular mechanisms by which tumor-specific T cells eliminate solid tumor.
Acknowledgments
We thank Prof. Hong-Ming Hu (EACRI, Portland, OR, USA) for his helpful suggestions, Yi Lin (Department of Immunology, Fudan University, Shanghai, China) for her assistance in the paper, Prof. Chong-Xian Pan (Department of Internal Medicine, UC Davis Cancer Center, USA) and Jin-Di Wen (Shanghai Medical College, Fudan University, Shanghai, China) for proofreading the paper.
Abbreviations
- ITAC
IFN-γ-inducible T cell α chemoattractant
- CXCR3
Cys-X-Cys receptor 3
- TIL
Tumor infiltrating lymphocyte
- CI
Chemotaxis index
- MMC
Mitomycin C
- CFSE
5- and 6-carboxyfluorescein diacetate succinimydyl ester
- 7-AAD
7-Amino actinomycin D
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Grant Support: The program of Science and Technology Commission of Shanghai Municipality (STCSM) (04XD14003, 04DZ14902, 045407038), the National Natural Science Foundation of China (NSFC) (30571713) and the program for Outstanding Medical Academic Leader.
Yiwei Chu and Xiuli Yang are contributed equally to this work.
References
- 1.Bondy ML, Newman LA. Assessing breast cancer risk: evolution of the Gail Model. J Natl Cancer Inst. 2006;98:1172–1173. doi: 10.1093/jnci/djj365. [DOI] [PubMed] [Google Scholar]
- 2.Adams SA, Matthews CE, Hebert JR, Moore CG, Cunningham JE, Shu XO, Fulton J, Gao Y, Zheng W. Association of physical activity with hormone receptor status: the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2006;15:1170–1178. doi: 10.1158/1055-9965.EPI-05-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer NG, Pestalozzi BC, Knuth A, Renner C. Potential use of humanized antibodies in the treatment of breast cancer. Expert Rev Anticancer Ther. 2006;6:1065–1074. doi: 10.1586/14737140.6.7.1065. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer Res. 2006;66:6826–6833. doi: 10.1158/0008-5472.CAN-05-3529. [DOI] [PubMed] [Google Scholar]
- 5.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2000;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/S1074-7613(00)80165-X. [DOI] [PubMed] [Google Scholar]
- 8.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 9.Widney DP, Xia YR, Lusis AJ, Smith JB. The murine chemokine CXCL11 (IFN-inducible T cell alpha chemoattractant) is an IFN-gamma- and lipopolysaccharide-inducible glucocorticoid-attenuated response gene expressed in lung and other tissues during endotoxemia. J Immunol. 2000;164:6322–6331. doi: 10.4049/jimmunol.164.12.6322. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xanthou G, Williams TJ, Pease JE. Molecular characterization of the chemokine receptor CXCR3: evidence for the involvement of distinct extracellular domains in a multi-step model of ligand binding and receptor activation. Eur J Immunol. 2003;33:2927–2936. doi: 10.1002/eji.200324235. [DOI] [PubMed] [Google Scholar]
- 12.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, Rosa R, Di Salvo J, Mudgett J, Peterson LB, Wicker LS, DeMartino JA. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–780. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 13.McColl SR, Mahalingam S, Staykova M, Tylaska LA, Fisher KE, Strick CA, Gladue RP, Neote KS, Willenborg DO. Expression of rat I-TAC/CXCL11/SCYA11 during central nervous system inflammation: comparison with other CXCR3 ligands. Lab Invest. 2004;84:1418–1429. doi: 10.1038/labinvest.3700155. [DOI] [PubMed] [Google Scholar]
- 14.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::AID-PATH899>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 16.Dumoulin FL, Nischalke HD, Leifeld L, von dem Bussche A, Rockstroh JK, Sauerbruch T, Spengler U. Semi-quantification of human C–C chemokine mRNAs with reverse transcription/real-time PCR using multi-specific standards. J Immunol Methods. 2000;241:109–119. doi: 10.1016/S0022-1759(00)00210-6. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli R, Sabroe I, LaRosa G, Williams TJ, Pease JE. The CC chemokine eotaxin (CCL11) is a partial agonist of CC chemokine receptor 2b. J Biol Chem. 2001;276:42957–42964. doi: 10.1074/jbc.M103933200. [DOI] [PubMed] [Google Scholar]
- 18.Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–187. doi: 10.1016/S0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 19.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J Exp Med. 2003;197:985–995. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu HM, Urba WJ, Fox BA. Gene-modified tumor vaccine with therapeutic potential shifts tumor-specific T cell response from a type 2 to a type 1 cytokine profile. J Immunol. 1998;161:3033–3041. [PubMed] [Google Scholar]
- 21.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci USA. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Xiang J. Synergistic effect of lymphotactin and interferon gamma-inducible protein-10 transgene expression in T-cell localization and adoptive T-cell therapy of tumors. Int J Cancer. 2004;109:817–825. doi: 10.1002/ijc.20043. [DOI] [PubMed] [Google Scholar]
- 24.Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, Hipkin RW. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- 25.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 27.Chen L. Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunol Today. 1998;19:27–30. doi: 10.1016/S0167-5699(97)01180-8. [DOI] [PubMed] [Google Scholar]
- 28.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon KB, Seddon YT, Andrew DL. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 30.Chu Y, Wang LX, Yang G, Ross HJ, Urba WJ, Prell R, Jooss K, Xiong S, Hu HM. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J Immunother. 2006;29:367–380. doi: 10.1097/01.cji.0000199198.43587.ba. [DOI] [PubMed] [Google Scholar]
- 31.Morris E, Hart D, Gao L, Tsallios A, Xue SA, Stauss H. Generation of tumor-specific T-cell therapies. Blood Rev. 2006;20:61–69. doi: 10.1016/j.blre.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Subjeck J, Yang G, Repasky E, Wang XY. Generation of anti-tumor immunity using mammalian heat shock protein 70 DNA vaccines for cancer immunotherapy. Vaccine. 2006;24:5360–5370. doi: 10.1016/j.vaccine.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Huang H, Saxena A, Xiang J. Intratumoral coinjection of two adenoviral vectors expressing functional interleukin-18 and inducible protein-10, respectively, synergizes to facilitate regression of established tumors. Cancer Gene Ther. 2002;9:533–542. doi: 10.1038/sj.cgt.7700466. [DOI] [PubMed] [Google Scholar]
- 34.Pilon-Thomas S, Verhaegen M, Kuhn L, Riker A, Mule JJ. Induction of anti-tumor immunity by vaccination with dendritic cells pulsed with anti-CD44 IgG opsonized tumor cells. Cancer Immunol Immunother. 2006;55:1238–1246. doi: 10.1007/s00262-005-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]