Abstract
Therapeutic treatment with hu14.18-IL-2 immunocytokine (IC) or Flt3-L (FL) protein is initially effective at resolving established intradermal NXS2 neuroblastoma tumors in mice. However, many treated animals develop recurrent disease. We previously found that tumors recurring following natural killer (NK) mediated IC treatment show augmented MHC class I expression, while the tumors that recurred following T cell dependent Flt3-L treatment exhibited decreased MHC class I expression. We hypothesized that this divergent MHC modulation on recurrent tumors was due to therapy-specific immunoediting. We further postulated that combining IC and Flt3-L treatments might decrease the likelihood of recurrent disease by preventing MHC modulation as a mechanism for immune escape. We now report that combinatorial treatment of FL plus hu14.18-IL-2 IC provides greater antitumor benefit than treatment with either alone, suppressing development of recurrent disease. We administered FL by gene therapy using a clinically relevant approach: hydrodynamic limb vein (HLV) delivery of DNA for transgene expression by myofibers. Delivery of FL DNA by HLV injection in mice resulted in systemic expression of >10 ng/ml of FL in blood at day 3, and promoted up to a fourfold and tenfold increase in splenic NK and dendritic cells (DCs), respectively. Furthermore, the combination of FL gene therapy plus suboptimal IC treatment induced a greater expansion in the absolute number of splenic NK and DCs than achieved by individual component treatments. Mice that received combined FL gene therapy plus IC exhibited complete and durable resolution of established NXS2 tumors, and demonstrated protection from subsequent rechallenge with NXS2 tumor.
Keywords: Hydrodynamic gene delivery, Flt3-L, hu14.18-IL-2, Immunocytokine, Neuroblastoma
Introduction
Natural killer (NK) cell-mediated and T cell-mediated responses are complementary antitumor effector mechanisms against tumors expressing disparate levels of MHC class I molecules [2, 3]. Tumor cells expressing a relatively low level of MHC class I are generally more susceptible to NK-dependent antitumor effects [12, 15]. Higher levels of MHC class I expression on tumor cells are required for effective presentation of antigen and induce activation of cytolytic T-lymphocyte (CTL) responses [1, 31]. Immunotherapy has focused on either augmenting innate immunity by promoting NK-dependent antitumor responses, or triggering adaptive CTL responses through activating tumor-specific T cells using defined tumor associated antigens (TAAs). Unfortunately, approaches that rely predominantly on a single antitumor effector mechanism can favor development of tumor escape variants (TEVs), via “immunoediting” [5]. Naturally occurring innate [35] and adaptive immunity can eliminate spontaneously arising subclinical tumors through immunosurveillance. However, this also applies selective pressure to “sculpt” developing tumors that become resistant to immune recognition or destruction, with the eventual development of clinically detectable cancer [5, 7]. The process of immunoediting also generates tumors that are resistant to immunotherapy and facilitates further evolution and selection of TEVs.
In previous studies with the NXS2 murine neuroblastoma (NB) model, we treated mice with suboptimal doses of either hu14.18-IL-2 immunocytokine (IC; an antibody/human interleukin 2 (IL-2) fusion molecule that targets the GD2 disialoganglioside on NXS2 [32]) or Fms-like tyrosine kinase-3-ligand (FL). We showed that each treatment was effective at resolving established tumor in mice, but often failed to prevent tumor recurrence resulting from TEV [28]. Recurrent tumors that developed following IC treatment exhibited a fivefold increase in MHC class I expression, as compared to the MHC class I expression on NXS2 tumors excised from PBS-treated control mice. Conversely, tumors from FL-treated animals showed a decrease in MHC class I expression. IC treatment mediates an effective NK-dependent resolution of established NXS2 tumors when given early following tumor establishment and at a sufficient dose [22, 23], but fails to induce protective antitumor memory. FL is a hematopoietic stem cell growth and differentiation factor that acts on CD34+ progenitor cells and induces in vivo expansion of dendritic cells (DC) and NK cells when administered as protein [33] or by gene therapy [14]. FL treatment may induce an NK- or T cell-dependent antitumor response. The latter may result in durable antitumor memory [34], as shown in mice that were cured of NXS2 tumor [28]. The divergent modulation in MHC class I expression by NXS2 TEVs reflects the outcome of therapy-induced immunoediting. IC recruits and activates IFNγ-producing NK cells to the tumor microenvironment. IFNγ promotes up-regulation of antigen processing and MHC class I expression on many cell types [10]. The local increase in IFNγ induces elevated expression of MHC class I on NXS2 tumor cells and reduces their susceptibility to the antitumor effects of the NK effectors. These tumor cells progress and develop into recurrent TEVs. Alternatively, antitumor T cell responses elicited by FL treatment result in the outgrowth of TEVs expressing reduced MHC class I. The lowered MHC class I expression can aid in escape from antitumor T cell immunity by minimizing antigen presentation [18].
Recent evidence indicates that the interaction between NK cells and DCs results in cell-to-cell contact-dependent bidirectional “cross-talk.” This promotes DC maturation and NK cell activation [26, 38], which can lead to induction of antitumor T cell immunity [16, 17]. Therefore, we hypothesized that a combinatorial regimen of FL treatment to expand both NK and DC populations, in concert with the NK-dependent antitumor effect of IC against NXS2 tumors, could simultaneously enhance the IC-mediated NK-dependent antitumor response and induce tumor-specific T cell immunity. The larger pool of FL-expanded NK effectors would enable greater IC-mediated NXS2 tumor killing. This “immediate” IC-directed tumor resolution would also provide tumor antigen for presentation by the FL-expanded population of DCs. Such tumor-antigen-loaded DCs could subsequently activate tumor-specific T cell responses necessary for durable antitumor immunity. Given their complementary mechanisms of tumor recognition, a combination immunotherapeutic approach that includes concomitant NK- and T cell-dependent immune responses should result in increased antitumor efficacy against established tumor and reduce the potential development of TEVs and recurrent disease.
To test this hypothesis, established NXS2 tumors were treated with a combination therapy of FL plus IC. FL was administered by non-viral hydrodynamic gene transfer to skeletal muscle [13], as an economical and practical alternative to protein delivery. We evaluated the level of FL production in the blood and the associated changes in the lymphocyte profile of the spleen following treatment. Mice that resolved their primary tumors following FL plus IC treatment were tested for induction of antitumor memory by NXS2 rechallenge. Tumors that failed to resolve following treatment were assessed for modulation in MHC class I expression.
Materials and methods
Animals
Female A/J and ICR strain mice (6–8 weeks of age) were obtained from the Jackson Laboratory, Bar Harbor, ME, or from Harlan Sprague Dawley, Indianapolis, IN. All animals were housed and handled in accordance with the NIH Guide for Care and Use of Laboratory Animals and Institutional Animal Care and Use Committee guidelines.
Cell lines and murine tumor models
GD+2 NXS2 NB cells were created by fusion of the GD− 2C1300 NB line from A/J mice, with dorsal root ganglion cells from C57BL/6J mice, followed by sorting for high GD2 expression [11, 22]. These hybrid cells are H2Kk and H2Dd positive and grow in A/J strain mice. The cells were maintained in Dulbecco’s minimal essential medium (DMEM), supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin at 5% CO2 and 37°C. Cells were used for tumor induction only if their viability exceeded 95%, as determined by eosin staining.
Intradermal tumors were induced by injection of 2 × 106 tumor cells in 100 μl of phosphate buffered saline (PBS) in the left lateral flank proximal to the spleen. Tumor growth was monitored by periodically measuring tumors with microcalipers and determining tumor volume using the formula: tumor size = width × length × width/2, where length is the larger of two perpendicular measurements. Tumor-bearing mice were sacrificed once tumor length reached 15 mm.
Expression vectors
Full length and a truncated (secreted) form of murine FL were expressed under transcriptional control of the elongation factor 1α (EF1α) and human cytomegalovirus (CMV) promoters, respectively. pORF9-mFL3L expresses the complete extracellular, transmembrane, and intracellular domains of the murine FL gene, and was obtained from InVivoGen (San Diego, CA). pUMVC3-mFLex (FLex) expresses only the extracellular domain of murine FL gene, and was obtained from Aldevron (Fargo, ND). All plasmid DNA for these studies was amplified and supplied endotoxin free by Aldevron.
Gene therapy
Plasmid DNA was administered in vivo by hydrodynamic limb vein (HLV) or tail vein (HTV) delivery [13, 21, 40].
Gene delivery by HLV
As described previously [13], mice were anesthetized with 1–2% isoflurane throughout each procedure. For each transfection procedure, pDNA was delivered into the great saphenous vein of the right hind limb in 1.0 ml of normal saline solution at a rate of 8 ml/min. Just prior to injection, blood flow to and from the limb was restricted by placing a tourniquet around the upper leg just proximal to, or partially over, the quadriceps muscle group. The tourniquet remained in place during the injection and for 2 min post-injection. In some experiments, both right and left hind legs were injected on the same day.
HTV gene delivery
Injection of the pDNA was performed as described before [40]. Briefly, pDNA was diluted in 1.6 ml of Ringer’s solution and injected into mice through their lateral tail vein over 4–8 s, using a 27-gauge needle.
Murine FL ELISA
FL levels in animal sera were measured using the Mouse Flt-3 Ligand DuoSet® ELISA (R&D Systems, Minneapolis, MN). Tests were performed in 96-well MaxiSorp™ immunoplates (Nalge Nunc, Rochester, NY) according to the manufacturer’s instructions. Sera were diluted 1:100–1:100,000 before testing.
Constant infusion of IL-2
Systemic IL-2 therapy was initiated by the surgical implantation of a subcutaneous (s.c.) osmotic pump (ALZET model 2001 pump; Alza Corporation, Palo Alto, CA) into the dorsal s.c. tissue of each mouse. These pumps delivered ∼143,000 IU/day of recombinant human IL-2 (rhIL-2) (TECIN; Hoffmann-La Roche, Inc., Nutley, NJ) for four days prior to spleen harvest.
Antitumor therapy
Mice bearing intradermal (i.d.) NXS2 tumors were treated by: (1) a single HLV delivery of 200 μg of FL or FLex pDNA to each hind limb on day 4 or 5 following tumor implantation; (2) four consecutive days with 10 μg of hu14.18-IL-2 IC (EMD Lexigen Research Center, Billerica, MA) in 100 μl of PBS given by tail vein injection starting on day 11 or 12 (suboptimal dose and schedule); or (3) the combination of (1) plus (2). Ten μg of hu14.18-IL-2 contains ∼ 30,000 IU of IL-2 [28, 29].
Flow cytometry
As described previously [28], a single cell suspension of spleen or tumor tissue was assessed by flow cytometry for expression of murine surface markers: CD4 (T cell), CD8 (T cell), DX5 (pan NK), CD11c (DC marker), or H2Dd (MHC class I) antigen with the appropriate commercially available fluorochrome-conjugated monoclonal antibodies (BD Biosciences, San Jose, CA).
The levels of H2Dd expression on NXS2 cells are represented as a specific mean fluorescence intensity ratio (sMFI-R) determined by the formula: sMFI-R = H2Dd antibody MFI/isotype control MFI [20]. sMFI-R values allow for comparison of staining levels between cells or tissues of differing origins.
Statistics
The results of tests of significance are reported as P values and are derived from Student’s two-tailed t test assuming equal variances.
Results
HLV-delivery of FL pDNA promotes expansion of splenic DC and NK cells
Both membrane-bound FL and its secreted form, FLex, are biologically active and induce in vivo expansion of DCs and NK cells in lymph nodes and spleens [24]. In these studies, we used the murine forms of FL and FLex. We determined the level of mFL in group-pooled sera obtained 24 h after hydrodynamic tail vein (HTV) delivery of 10 μg of pORF9-mFL3L or pUMVC3-mFLex into ICR mice (n = 3). FL levels of 11.8 μg/ml and 27.4 μg/ml were detected in the pooled sera from mice that received mFL and mFLex pDNA, respectively. These values are similar to those reported for human FLex delivered by HTV to mice [14].
While HTV gene delivery represents a highly efficient gene transfer procedure with research utility in rodents, hydrodynamic delivery of pDNA to skeletal muscle is currently attracting more interest for potential clinical application [39]. Therefore, we used the HLV procedure for all other experiments. The HLV procedure results in highly effective transgene expression by the transfected myofibers of the treated limb [13]. The results in Table 1 indicate that FL protein was systemically available in the blood vasculature following HLV gene delivery. Unlike HTV delivery, where maximal expression of transgene products is typically observed 12–24 h following gene transfer, the highest expression level was noted at 72 h following HLV delivery (evaluated at 1, 3, and 7 days post delivery). FL levels indicated a dose-response relation between the amount of DNA delivered and the level of FL expressed.
Table 1.
Time-course of FL expression
| HLV gene therapya | FL serum level (ng/ml) | ||
|---|---|---|---|
| Day 1 | Day 3 | Day 7 | |
| (1) No treatment | 0.56 | 0.66 | ND |
| (2) 25 μg mFLex DNA | 0.74 | 1.26 | 0.18 |
| (3) 100 μg mFLex DNA | 1.96 | 2.13 | 1.30 |
| (4) 200 μg mFLex DNA | 3.32 | 4.96 | 2.89 |
Not determined
a Groups (n = 3) of ICR strain mice received hydrodynamic limb vein gene delivery to the right hind limb on day 0
To evaluate the impact of HLV delivered FL on the immune system, we determined the cellular profile of the spleen. Groups of A/J strain mice received 200 μg of mFL or mFLex pDNA into either the right hind limb on day 0, into both the right and left hind limbs on day 0, or sequentially, the right limb on day 0 followed by the left limb on day 6. Sequential HTV delivery of human FLex pDNA has been shown to have a greater than additive biological effect [14]. FL serum levels were determined 72 h following HLV delivery and spleens harvested and analyzed on day 9 (previous studies with protein and HTV gene delivery of FL suggest that maximal biological impact is observed approximately 10 days following treatment initiation [14, 25]). The results in Table 2 indicate that HLV gene delivery to both hind limbs resulted in a near additive effect on the level of FL expressed in the sera, as compared to HLV delivery to a single limb. Delivery of mFLex pDNA resulted in 4.84 and 10.35 ng/ml of FL in the sera of mice that were single versus dual-limb treated, respectively. Likewise, mice that received mFL pDNA exhibited a similar additive pattern for FL expression, albeit at lower levels as compared to mFLex gene therapy. The frequency of CD4+ and CD8+ T cells remained relatively unchanged in all treatment groups. In contrast, the frequency of CD11c+ DCs and DX5+ NK cells were increased in all animals that received FL gene therapy. Given the >2.5-fold increase in the number of splenocytes from mice that received the dual-limb HLV gene therapy (76 and 70 × 106 cells/spleen: Groups 4 and 5, respectively), combined with the increased frequency of DC and NK cells in these treatment groups, a substantial expansion in the absolute number of DC and NK cells occurred. Whereas control mice possessed 0.3 and 1.0 × 106 DC and NK cells/spleen, respectively, spleens from animals that were dual-limb HLV treated with mFL DNA (Group 5) had 1.4 and 3.6 × 106 DC and NK cells/spleen. This gene therapy-induced expansion represented a >fourfold and >threefold increase in the absolute number of available DC and NK cells, respectively. In other studies, HLV or HTV delivery of the same amounts of control pDNA (having similar CpG content as the FL-expressing pDNA) indicated no appreciable change in the spleen profile (data not shown), further suggesting that the immunomodulation effects noted with FL gene therapy coincide with the amount of systemically available FL protein.
Table 2.
Biological impact of HLV FL gene therapy
| Gene therapya | FL levelb | Cells/spleen (× 106)c | Splenic phenotypic profile (%)d | Total DCs (× 106)e | Total NKs (× 106)f | |||
|---|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD11c+ | DX5+ | |||||
| (1) No treatment | 0.1 | 28 | 12.9 | 8.7 | 1.1 | 3.5 | 0.3 | 1.0 |
| (2) mFLex (RT limb) | 4.84 | 64 | 12.3 | 9.0 | 3.3 | 5.2 | 2.1 | 3.3 |
| (3) mFL (RT limb) | 0.87 | 56 | 13.8 | 9.7 | 1.7 | 4.8 | 0.9 | 2.7 |
| (4) mFLex (Both limbs) | 10.35 | 76 | 12.2 | 9.3 | 4.3 | 5.5 | 3.3 | 4.2 |
| (5) mFL (Both limbs) | 1.70 | 70 | 12.4 | 9.4 | 2.0 | 5.1 | 1.4 | 3.6 |
| (6) mFLex (Sequential) | 5.06, 5.65 | 80 | 11.5 | 9.4 | 3.4 | 4.8 | 2.7 | 3.8 |
| (7) mFL (Sequential) | 1.69, 1.01 | 66 | 12.4 | 8.6 | 1.6 | 4.4 | 1.1 | 2.9 |
a Groups (n = 4) of A/J strain mice received gene delivery as follows: (1) no treatment, (2) hydrodynamic limb vein injection (HLV) of 200 μg murine FL extracellular secreted (mFLex) DNA to right limb on day 0, (3) HLV of 200 μg mFL DNA to right limb day 0, (4) HLV of 200 μg mFLex DNA to both limbs on day 0, (5) HLV of 200 μg mFL DNA to both limbs on day 0, (6) HLV of 200 μg mFLex DNA to right limb on day 0 plus 200 μg mFLex DNA to left limb on day 6, and (7) HLV of 200 μg mFL DNA to right limb on day 0 plus 200 μg mFL DNA to left limb on day 6
b Serum murine FL concentration (ng/ml) of pooled sera 72 h following HLV gene delivery on day 0 as determined by ELISA (R&D Systems, Minneapolis, MN). Additional values for Groups 6 and 7 represents serum levels 72 h following second HLV injection on day 6
c Spleens were harvested on day 9 and pooled for each group. Following erythrocyte lysis by hypotonic shock, the number of viable cells was determined. Values are × 106 cells/spleen
d Isolated splenocytes were pooled from all four animals per group and stained with primary-conjugated mAbs (BD Biosciences, San Diego, CA) to murine CD4, CD8, CD11c, and DX5. Value represents the percent of viable splenocytes positive for specific cell-surface staining
e The total number of CD11c+ DCs per spleen and is determined by the formula: number of viable cells/spleen × %CD11c+ cells. Values are × 106 cells/spleen
f The total number of DX5+ NKs per spleen and is determined by the formula: number of viable cells/spleen × %DX5+ cells. Values are × 106 cells/spleen
FL gene therapy augments hu14.18-IL-2-mediated antitumor effects against NXS2 tumors
To evaluate the antitumor potential of a combinatorial regimen consisting of FL HLV gene therapy plus hu14.18-IL-2 IC, mice with NXS2 tumors received FL HLV gene therapy on day 3 and an IC treatment course on days 11 through 14. The sequential administration of FL gene therapy followed by IC treatment 8 days later permits a sufficient time delay for the gene-expressed FL to induce the expansion of NK effectors, known to be involved in the IC mediated antitumor effect against NXS2 [23, 28].
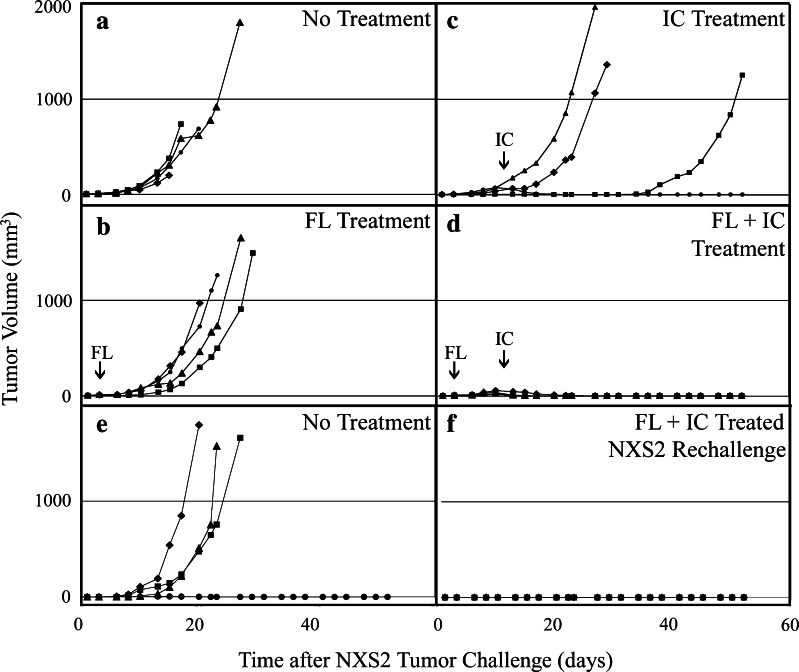
Groups (n = 4) of NXS2 tumor-bearing A/J mice received: no treatment (Fig. 1a), four consecutive daily treatments of 10 μg of IC beginning on day 11 (Fig. 1b), HLV delivery of 200 μg of mFL pDNA in both hind limbs on day 3 (Fig. 1c), or the combination of FL gene therapy plus IC treatment (Fig. 1d). All no-treatment and HLV mFL gene therapy treated mice were euthanized by day 27 as a result of progressive NXS2 tumor growth. Some IC-treated mice (2 of 4) also exhibited progressive tumor growth and were euthanized by day 29. The remaining two IC-treated mice displayed resolution of any grossly detectible tumor, followed by NXS2 tumor recurrence in one of these animals. In contrast, all mice that received the combinatorial regimen of FL gene therapy plus IC exhibited durable cure of their existing NXS2 tumors.
Fig. 1.
HLV FL plus IC effect on NXS2 tumor growth. Groups (n = 4) of A/J mice were injected with 5 × 106 NXS2 cell i.d. on day 0 and received a no treatment, b 200 μg mFL DNA by HLV in both hind limbs on d 3, c 10 μg/d hu14.18-IL-2 IC (day 11–14), or d both FL DNA + IC. Small (FL) and large (IC) arrows indicate HLV gene delivery and hu14.18-IL-2 treatment initiation dates, respectively. f Mice that had received FL + IC (i.e., same animals use in panel d) were rechallenged with NXS2 tumor 70 days later and compared to e naïve mice for tumor progression. Data represent NXS2 tumor growth for individual animals
To determine whether mice successfully treated with the combinatorial FL gene therapy plus IC regimen possessed protective antitumor immunity, the mice from Fig. 1d were rechallenged with NXS2 tumor 70 days after the initial tumor challenge. As shown in Fig. 1f, all (4 of 4) rechallenged mice were completely protected, as compared to tumor challenge in naïve animals (Fig. 1e). This result indicates that the original combination therapy induced lasting anti-tumor memory.
Combined FL gene therapy plus IC treatment regimen results in enhanced expansion of NK and DC cells
To delineate the mechanism of the enhanced treatment effect, we investigated the changes in total spleen cellularity, as well as NK and DC cell numbers, that were induced in combination treated mice. In Table 3, groups of A/J strain mice received: no treatment (Group 1), 10 μg/day of hu14.18-IL-2 on days 7–10 (Group 2), HLV delivery of 200 μg of mFLex pDNA in right hind limb and 200 μg of mFL pDNA in the left hind limb on day 0 (Group 3), the combination of HLV gene therapy plus IC treatment (Group 4), or 140,000 IU/day of rhIL-2 by osmotic pump on days 8–11 (Group 5). Spleens were harvested 24 h following completion of the IC treatment schedule (day 11) and DC and NK cells analyzed as in Table 2. Mice that received the combinatorial treatment exhibited the highest degree of splenomegaly, with a near fourfold increase in the number of splenocytes (149 × 106 cells/spleen) as compared to no-treatment controls (38 × 106 cells/spleen). The combinatorial treatment induced NK expansion (16.1 × 106 cells/spleen) that was greater than IC or mFL alone, and comparable to that induced by 4 d of constant infusion IL-2 (Group 5). Furthermore, the combinatorial treatment induced the highest observed increases in the frequency of splenic DCs, reaching 4.3%. Thus, the biological effect of combining FL gene therapy with IC treatment induced an even greater expansion in the absolute number of DC (6.4 × 106 cells/spleen) than all other treatments in Tables 2 and 3.
Table 3.
Combined treatment
| Treatmenta | Cells/spleen (× 106)b | Splenic phenotypic profile (%)c | Total DCs (× 106)d | Total NKs (× 106)e | |||
|---|---|---|---|---|---|---|---|
| CD4+ | CD8+ | CD11c+ | DX5+ | ||||
| (1) No Treatment | 38 | 14.5 | 6.3 | 1.8 | 4.5 | 0.7 | 1.7 |
| (2) hu14.18-IL-2 | 113 | 12.6 | 5.8 | 2.6 | 9.6 | 2.9 | 10.8 |
| (3) mFL (Both) | 79 | 13.6 | 7.1 | 3.2 | 5.9 | 2.5 | 4.6 |
| (4) mFL + hu14.19-IL-2 | 149 | 14.8 | 8.4 | 4.3 | 10.8 | 6.4 | 16.1 |
| (5) rhIL-2 | 130 | 13.3 | 9.3 | 1.8 | 12.8 | 2.3 | 16.6 |
a Groups (n = 4) of A/J strain mice received treatment as follows: (1) no treatment, (2) 10 μg/d of hu14.18-IL-2 IC days 7–10, (3) HLV delivery of 200 μg mFLex DNA to right limb plus 200 μg mFL DNA to left limb on day 0, (4) combination of treatments described in (2) and (3), and (5) 140,000 I.U./d of rhIL-2 by constant infusion osmotic pump on days 8–11
b Spleens were harvested on day 11 and pooled for each group. Following erythrocyte lysis by hypotonic shock, the number of viable cells was determined. Values are × 106 cells/spleen
c Isolated splenocytes were pooled for each group and stained with primary-conjugated mAbs (BD Biosciences, San Diego, CA) to murine CD4, CD8, CD11c, and DX5. Value represents the percent of viable splenocytes positive for specific cell-surface staining
d The total number of CD11c+ DCs per spleen and is determined by the formula: number of viable cells/spleen × %CD11c+ cells. Values are × 106 cells/spleen
e The total number of DX5+ NKs per spleen and is determined by the formula: number of viable cells/spleen × %DX5+ cells. Values are × 106 cells/spleen
MHC class I expression on NXS2 tumors
Our previous studies with NXS2 tumors showed that TEVs that developed following initial resolution of the primary tumor displayed profoundly disparate MHC class I expression levels, which correlated with the immunotherapeutic employed [28]. We wondered whether tumors that progressed during FL gene therapy or FL plus IC combination treatment exhibited a similar modulation in MHC class I expression. Progressively growing primary NXS2 tumors were excised and evaluated for MHC class I H2Dd expression by flow cytometry.
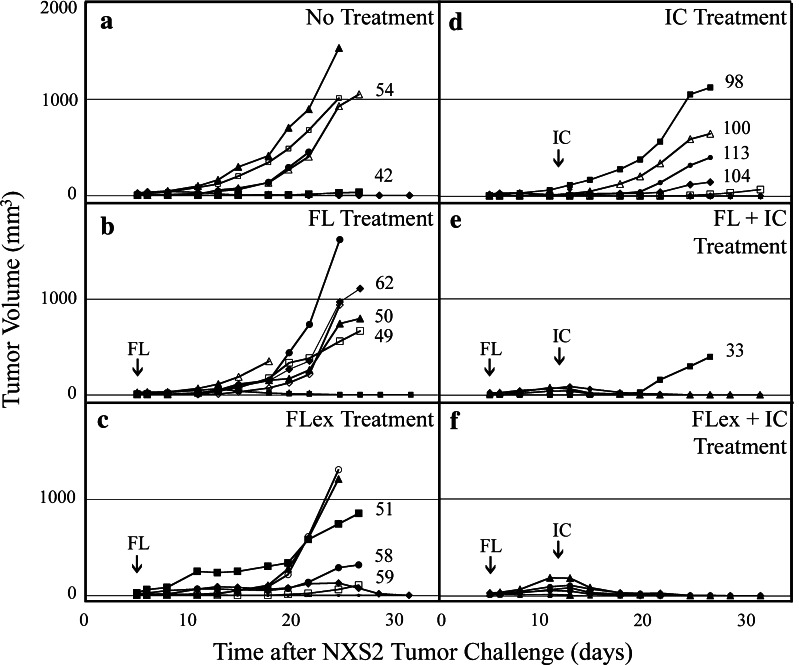
Six different treatment groups bearing NXS2 tumors were compared for tumor growth and H2Dd expression (Fig. 2): no treatment (Fig. 2a), HLV delivery of 200 μg of mFL pDNA in both hind limbs on day 5 (Fig. 2b), HLV delivery of 200 μg of mFLex pDNA in both hind limbs on day 5 (Fig. 2c), four consecutive daily treatments of 10 μg of IC beginning on day 12 (Fig. 2d), the combination of mFL gene therapy plus IC (Fig. 2e), or the combination of mFLex gene therapy plus IC (Fig. 2f). Single-agent treatment with mFL gene therapy, mFLex gene therapy, or IC treatment (Fig. 2b, c, d) resulted in progressive tumor growth in 6 of 8, 5 of 8, and 5 of 8 treated mice, respectively, and failed to demonstrate statistical difference when compared to no-treatment control mice (comparison of day 25 tumor volumes). Furthermore no statistical differences in tumor growth were noted in comparisons between these three single-agent treatment groups. In contrast, the combinatorial regimen was the most effective with 7 of 8 FL gene therapy plus IC-treated mice (Fig. 2e), and 8 of 8 FLex gene therapy plus IC-treated mice (Fig. 2f), exhibiting complete resolution of their NXS2 tumor. Animals that were treated with mFL + IC or mFLex + IC showed significant differences in tumor growth compared to animals receiving mFL or mFLex alone. (P = 0.007 and 0.029, respectively; day 25 comparison).
Fig. 2.
Modulation of MHC class I expression. Groups of A/J mice (6 mice in group A and 8 mice in all other groups) were injected with NXS2 cell i.d. on day 0 and received a no treatment, b 200 μg mFL DNA by HLV in both limbs on day 5, c 200 μg mFLex DNA by HLV in both limbs on day 5, d 10 μg/d hu14.18-IL-2 IC (day 12–15), e both FL DNA + IC, or f both FLex DNA + IC. Data represents NXS2 tumor growth for individual animals. Primary tumors were harvested on day 27 following NXS2 cell injection and individually profiled for H2Dd expression. The specific MFI ratio for H2Dd expression is represented numerically and is adjacent to tumor growth graph for that particular tumor lesion. Due to progressive tumor growth, some mice were euthanized prior to the tumor harvest date. Small (FL) and large (IC) arrows indicate HLV gene delivery and hu14.18-IL-2 treatment initiation dates, respectively
At day 27, all detectable NXS2 tumors were collected and assessed for MHC class I H2Dd expression by flow cytometry. The sMFI ratio for the H2Dd expression level on each individually excised NXS2 tumor is represented as a numerical value adjacent to the graph showing the growth of that specific harvested tumor in Fig. 2. In some instances, animals with more progressively growing tumors had to be sacrificed prior to day 27 and their tumors were not analyzed. The sMFI ratio for H2Dd expression on cultured NXS2 cells was 15. Tumors from untreated mice exhibited a relative increase in H2Dd expression (Fig. 2a: sMFI ratio of 54 and 42 for two individually excised NXS2 tumors; mean sMFI ratio value of 48) compared to cultured NXS2 cells, consistent with results obtained in our earlier study [28]. Although the number of tumor samples was limited, the sMFI H2Dd ratios for tumors from the mFL (Fig. 2b: sMFI ratios = 49, 50, and 65; mean = 55) or mFLex (Fig. 2c: sMFI ratios = 51, 58, and 59; mean = 56) gene therapy treatment groups were similar to the sMFI H2Dd ratios for tumors from the control group (mean = 48). In contrast, the ratios for tumors from the IC treatment group were notably elevated (Fig. 2d: sMFI ratios = 98, 100, 104 and 113; mean = 104). There was a significant statistical difference (P = 3 × 10−6) in sMFI H2Dd ratio values from tumors obtained from the mFL and mFLex gene therapy treated mice when compared to those obtained from the IC treated animals. The single recurrent tumor that developed from an animal in the mFL gene therapy plus IC treatment group exhibited the lowest sMFI ratio (33) from any of the recovered tumors.
Discussion
The impact of immunoediting on the evolution of tumor progression requires novel strategies that elicit several distinct and complementary antitumor mechanisms of action in order to successfully treat cancers [5–7]. Such multifaceted approaches should provide better tumor destruction (and greater clinical benefit) by involving a larger accompaniment of the antitumor immune response machinery to provide a more comprehensive antitumor response. The results presented here support our hypothesis that the combination of FL plus IC provides a greater immediate therapeutic antitumor response, induces tumor-specific T cell immunity, and thwarts TEV development.
In our earlier study with NXS2 tumors [28], treatment with FL protein induced a T cell-dependent antitumor response, which promoted the development of H2-depressed TEVs. Conversely, IC treatment mediated an NK-dependent antitumor response [22, 23], which resulted in H2-elevated TEVs. Tumor escape from IC treatment involved the pro-inflammatory molecule IFNγ. This factor was released in the tumor microenvironment by IC-activated NK effectors to mediate the increased MHC class I expression on TEVs, making them less sensitive to the antitumor effects of NK cells. In contrast, attenuation of H2 expression on NXS2 tumors may have helped orchestrate their escape from the FL-induced T cell response as low or absent MHC class I expression by tumor cells results in evasion from T cell immunity [1].
In our present study, we sought to elicit the complementary antitumor activity of NK- and T cell-dependent responses with a combined immunotherapeutic regimen as an attempt to thwart TEV development. We delivered FL, administered as HLV gene therapy, plus IC as a combinatorial approach against established NXS2 tumors. The combined regimen prompted a > ninefold increases in the number of both splenic DC and NK cells, facilitated durable resolution of established NXS2 tumors in 19 of 20 treated mice, and induced long-term prophylactic antitumor memory in all animals tested. Alternatively, FL gene delivery or IC treatment, given as single-agent modalities, failed to evoke a tumor-resolving response in the majority of mice.
Analysis of MHC class I expression on tumors that progressed following immunotherapy, while limited in sampling size, indicates that the effects of IC-induced immunoediting may be occurring during or shortly following IC treatment. Tumors harvested 12 days after completion of IC treatment were already exhibiting evidence of immunoediting as their group mean H2Dd sMFI value was 104, as compared to the mean value of 48 for the two tumors from non-treatment control animals. This coincides with our earlier report regarding MHC class I modulation and tumor escape from IC treatment [28]. Conversely, HLV FL gene therapy did not induce a detectible MHC class I immunoediting response as the group mean H2Dd sMFI value of tumors from these treated animals was similar to non-treatment controls (55 and 48, respectively). These results do not reflect our earlier observations with FL protein therapy where recurrent NXS2 tumors exhibited depressed H2Dd expression [28], and may be attributed to differences in the time interval between therapy and tumor harvest, or an apparent lack of detectable antitumor effect of the HLV FL gene therapy alone.
How the HLV FL gene therapy plus IC regimen provides greater therapeutic antitumor benefit remains speculative. We favor a model involving an IC-mediated NK-dependent resolution of the primary tumor mass (early innate response), NK-DC cross-talk (innate-adaptive bridging response), and the development of a tumor-specific T cell response able to provide antitumor memory (late adaptive response). Recent evidence has clearly demonstrated that the close interface between DCs and NK cells may be instrumental in fully activating NK-dependent innate immune responses, as well as inducing DC maturation events critical in orchestrating the development of adaptive T cell-dependent immunity [16, 27, 38]. HLV delivery of FL resulted in a dramatic increase in DC and NK cells (Table 2), which was even more pronounced when followed by IC treatment (Table 3). When IC is administered following the FL-induced expansion of DC and NK cells, several events may occur. IC should interact with the expanded pool of NK cells to mediate a greater early antitumor response, due in part, to antibody dependent cellular cytotoxicity (ADCC) by the increased number of NK effectors available [28]. The IL-2 component of the IC molecule activates these NK effectors to mediate ADCC and to produce the proinflammatory factor, IFNγ [28]. This IFNγ increases MHC class I expression on any viable tumor targets still remaining and should cause them to become less susceptible to NK-dependent antitumor effects. Yet, it also increases the MHC class I expressed in the tumor microenvironment, and would boost tumor immunogenicity if DCs are present. The prior FL HLV should have expanded DC numbers near the tumor and in the tumor draining lymph nodes. Additionally, the NK-derived IFNγ should promote maturation of the expanded pool of DCs [38] and contribute to NK-DC cross-talk. The maturing DCs would be expected to produce IL-12, further activating the IFNγ-producing NK effectors involved in the early antitumor response. It is known that NK-mediated tumor rejection can induce tumor-specific T cell memory [17]. In our proposed model, the destruction of the primary tumor is mostly accomplished by NK effectors and provides a reservoir of antigenic tumor material for accumulation and presentation by the maturing pool of DCs. This should elicit a more effective adaptive tumor-specific CTL and T cell memory response, which is able to break tolerance for tumor-expressed self-antigens. Those tumor cells which escaped the ensuing NK-dependent response by up-regulating their MHC class I expression should also become more immunogenic and susceptible to recognition and elimination by the adaptive T cell response [8]. Furthermore, and although not evaluated in this study, the CpG content of the mFL and mFLex pDNAs likely provided additional in vivo adjuvant effects by stimulating immature DCs [41] and NK cells [36].
Importantly for human therapeutic relevance, the HLV gene delivery procedure works equally well in rodents and larger animals, including non-human primates. The HLV delivery method did provide important antitumor benefit in this animal model when combined with IC, and represents a feasible option for the delivery of FL clinically [39]. As we have recently demonstrated that HLV gene delivery is highly effective in administration of a genetic cancer vaccine in mice [30], we envision a “next generation” FL (HLV delivered) plus IC regimen to also include HLV delivery of a genetic cancer vaccine targeting tumor-specific antigens or universal tumor antigens such as telomerase [37]. This study did not compare the relative effectiveness of FL protein and gene therapy. Yet, is interesting to speculate that the constant and prolonged (metronomic) dosing capable with hydrodynamic gene delivery, and with HLV FL gene therapy in particular, may have greater pharmacologic effects, and potentially enhanced therapeutic benefit.
The immunocytokines KS-IL-2 [targeting the epithelial cell adhesion molecule (EpCAM)] [4] and hu14.18-IL-2 [19] are presently being evaluated in Phase I and Phase II clinical trials for patients with EpCAM or GD2 expressing tumors. The results presented in this preclinical study demonstrating an increased antitumor benefit of including FL pDNA delivery may be an important consideration in subsequent clinical evaluation of IC therapy, or other monoclonal antibody based treatments that are designed to induce ADCC in vivo. The HLV gene delivery procedure will soon be evaluated in patients and is anticipated to reinvigorate the utility of gene therapy in the clinical setting [39]. As such, additional studies to confirm the therapeutic antitumor benefit of a HLV FL gene therapy plus IC regimen in other murine tumor models, as well as deciphering the antitumor mechanisms of action should be pursued in developing this combinatorial strategy for future clinical testing. In addition, these data suggest proceeding with preclinical development of combinatorial regimens testing IC plus FL protein, as both have been evaluated already as single agent treatments in clinical trials [9, 19].
Acknowledgments
The authors thank Kathy Schell (Department of Human Oncology, University of Wisconsin-Madison, WI) for assistance with the flow cytometric analyses of excised tumors, and Drs. Jackie Hank, Ilia Buhtoiarov, Alexander Rakhmilevich, Ralph Reisfeld and Jon Wolff for helpful discussions. This work was supported in part by grants R01-063285 and R01-087025 from the National Institutes of Health and research support from The Midwest Athletes Against Childhood Cancer (MACC) Fund (PI, P.M. Sondel).
Abbreviations
- CTL
cytolytic T-lymphocyte
- DC
dendritic cell
- Flt3-L
fms-like tyrosine kinase 3 ligand
- IC
immunocytokine
- NB
neuroblastoma
- NK
natural killer
- TAA
tumor associated antigen
- TEV
tumor escape variants
References
- 1.Algarra I, Cabrera T, Garrido F. The HLA crossroad in tumor immunology. Hum Immunol. 2000;61:65–73. doi: 10.1016/S0198-8859(99)00156-1. [DOI] [PubMed] [Google Scholar]
- 2.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubenik J. Tumour MHC class I downregulation and immunotherapy (review) Oncol Rep. 2003;10:2005–2008. [PubMed] [Google Scholar]
- 4.Connor JP, Felder M, Hank J, Harter J, Gan J, Gillies SD, Sondel P. Ex vivo evaluation of anti-EpCAM immunocytokine huKS-IL2 in ovarian cancer. J Immunother. 2004;27:211–219. doi: 10.1097/00002371-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–245. doi: 10.1385/IR:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, Kavanagh JJ, Hicks ME, Levy LB, Folloder JK, Garcia ME. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin Cancer Res. 2003;9:5228–5237. [PubMed] [Google Scholar]
- 10.Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11:76–81. doi: 10.1016/S0952-7915(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 11.Greene LA, Shain W, Chalazonitis A, Breakfield X, Minna J, Coon HG, Nirenberg M. Neuronal properties of hybrid neuroblastoma X sympathetic ganglion cells. Proc Natl Acad Sci USA. 1975;72:4923–7. doi: 10.1073/pnas.72.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumperz JE, Parham P. The enigma of the natural killer cell. Nature. 1995;378:245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- 13.Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL, Herweijer H, Wolff JA. A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Mol Ther. 2004;10:386–398. doi: 10.1016/j.ymthe.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Pimenov AA, Nayak JV, Plowey J, Falo LD, Jr, Huang L. Intravenous injection of naked DNA encoding secreted flt3 ligand dramatically increases the number of dendritic cells and natural killer cells in vivo. Hum Gene Ther. 2000;11:547–554. doi: 10.1089/10430340050015734. [DOI] [PubMed] [Google Scholar]
- 15.Imboden M, Murphy KR, Rakhmilevich AL, Neal ZC, Xiang R, Reisfeld RA, Gillies SD, Sondel PM. The level of MHC class I expression on murine adenocarcinoma can change the antitumor effector mechanism of immunocytokine therapy. Cancer Res. 2001;61:1500–1507. [PubMed] [Google Scholar]
- 16.Kalinski P, Mailliard RB, Giermasz A, Zeh HJ, Basse P, Bartlett DL, Kirkwood JM, Lotze MT, Herberman RB. Natural killer–dendritic cell cross-talk in cancer immunotherapy. Expert Opin Biol Ther. 2005;5:1303–1315. doi: 10.1517/14712598.5.10.1303. [DOI] [PubMed] [Google Scholar]
- 17.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 18.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King DM, Albertini MR, Schalch H, Hank JA, Gan J, Surfus J, Mahvi D, Schiller JH, Warner T, Kim K, Eickhoff J, Kendra K, Reisfeld R, Gillies SD, Sondel P. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanza F, Latorraca A, Moretti S, Castagnari B, Ferrari L, Castoldi G. Comparative analysis of different permeabilization methods for the flow cytometry measurement of cytoplasmic myeloperoxidase and lysozyme in normal and leukemic cells. Cytometry. 1997;30:134–144. doi: 10.1002/(SICI)1097-0320(19970615)30:3<134::AID-CYTO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 22.Lode HN, Xiang R, Varki NM, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89:1586–1594. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 23.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 24.Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 25.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 27.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal ZC, Imboden M, Rakhmilevich AL, Kim KM, Hank JA, Surfus J, Dixon JR, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer Immunol Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neal ZC, Yang JC, Rakhmilevich AL, Buhtoiarov IN, Lum HE, Imboden M, Hank JA, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 30.Neal ZC, Bates MK, Albertini MR, Herweijer H. Hydrodynamic limb vein delivery of a xenogeneic DNA cancer vaccine effectively induces antitumor immunity. Mol Ther. 2007;15:422–430. doi: 10.1038/sj.mt.6300046. [DOI] [PubMed] [Google Scholar]
- 31.Rees RC, Mian S. Selective MHC expression in tumours modulates adaptive and innate antitumour responses. Cancer Immunol Immunother. 1999;48:374–381. doi: 10.1007/s002620050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisfeld RA. Potential of genetically engineered monoclonal antibodies for cancer immunotherapy. Pigment Cell Res Suppl. 1992;2:109–112. doi: 10.1111/j.1600-0749.1990.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 33.Shaw SG, Maung AA, Steptoe RJ, Thomson AW, Vujanovic NL. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161:2817–2824. [PubMed] [Google Scholar]
- 34.Silver DF, Hempling RE, Piver MS, Repasky EA. Flt-3 ligand inhibits growth of human ovarian tumors engrafted in severe combined immunodeficient mice. Gynecol Oncol. 2000;77:377–382. doi: 10.1006/gyno.2000.5782. [DOI] [PubMed] [Google Scholar]
- 35.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 36.van Ojik HH, Bevaart L, Dahle CE, Bakker A, Jansen MJ, van Vugt MJ, van de Winkel JG, Weiner GJ. CpG-A and B oligodeoxynucleotides enhance the efficacy of antibody therapy by activating different effector cell populations. Cancer Res. 2003;63:5595–5600. [PubMed] [Google Scholar]
- 37.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 38.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 39.Wells DJ. Opening the floodgates: clinically applicable hydrodynamic delivery of plasmid DNA to skeletal muscle. Mol Ther. 2004;10:207–208. doi: 10.1016/j.ymthe.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]