Abstract
As the most potent antigen presenting cells, dendritic cells (DCs) play key roles in the immune response against tumors. Their density in the tumor tissue has been associated with prognosis in patients with various cancers. However, few studies have been aimed at the presence and maturation state of DCs in cutaneous melanoma, with regard to their potential clinical correlates. In this study, the density of DCs expressing CD1a and the maturation marker DC-LAMP was determined by immunohistochemistry in primary tumor samples from 82 patients with cutaneous malignant melanoma. Intratumoral and peritumoral cell densities were analyzed in relation to tumor thickness and the subsequent development of metastases, as well as to patients’ survival. CD1a+ DCs were found both infiltrating melanoma cell nests and in the surrounding stroma, while DC-LAMP+ mature DCs were generally confined to the peritumoral areas, associated with lymphocytic infiltrates. DC density values significantly correlated with the number of activated (CD25+ or OX40+) T lymphocytes (p < 0.001). The degree of infiltration by CD1a+ and DC-LAMP+ DCs showed strong inverse correlation with the thickness of melanomas (p < 0.001). High peritumoral density of mature DCs was associated with significantly longer survival (p = 0.0195), while density of CD1a+ cells had a prognostic impact of borderline significance (p = 0.0610). Moreover, combination of high peritumoral CD1a+ or DC-LAMP+ cell density with high number of CD25+ or OX40+ lymphocytes identified patient subgroups with more favorable survival compared to other subgroups. A multivariate survival analysis involving DC and activated T-cell densities alone and in combinations, as well as traditional prognostic factors, identified high DC-LAMP+ cell/high OX40+ cell density and Breslow index as independent predictors of good prognosis. These results suggest that the presence of CD1a+ DCs primarily depends on the thickness of melanomas, without direct relationship with the patients’ survival. On the other hand, the density of mature DCs, especially in association with that of activated T cells, proved of prognostic importance, suggesting that these parameters could be considered as signs of a functional immune response associated with better outcome of the disease.
Keywords: Melanoma, Metastasis, Dendritic cell, Activated T cell, Immunohistochemistry
Introduction
As the most potent antigen presenting cells, dendritic cells (DCs) are capable of initiating and maintaining primary and secondary immune responses. They originate from bone marrow precursors, and migrate via the bloodstream to peripheral tissues, where they reside as immature cells efficient in taking up antigens [5, 8]. DCs constitute a complex system of cells displaying considerable heterogeneity in phenotype, anatomical location and functional abilities [33]. Langerhans cells (LCs) are myeloid DCs found in the skin and mucosal surfaces, and are characterized by the presence of Birbeck granules and the expression of CD1a, Langerin and E-cadherin [8, 33, 42]. Interstitial DCs are also of myeloid origin; in the skin, they correspond to dermal DCs, which express CD1b and c, and partly CD1a and factor XIIIa [8, 25, 33, 42]. A third subset of dendritic cells, plasmacytoid DCs are suggested to derive from lymphoid precursors [33, 43]; they show plasma cell-like morphology, express IL-3Rα (CD123) and BDCA-2 (CD303), and secrete type I interferon upon viral infection [14, 34].
After acquiring antigens, DCs process them into peptides, which are subsequently presented in association with MHC molecules to specific CD4+ and CD8+ T cells. Upon exposure to microbial products, cytokines, CD40L, etc., they migrate to secondary lymphoid organs and undergo maturation, resulting in enhanced expression of MHC and costimulatory molecules, cytokine secretion, and ability to efficiently prime T cells. Beside costimulatory molecules, mature DCs express CD83 and CD208/DC-LAMP (DC-lysosome-associated membrane protein) markers [11, 46].
In tumor-bearing hosts, dendritic cells take up, process and present tumor-associated antigens to naive or memory T cells, therefore, are proposed to play a crucial role in the immune response against tumors. However, in cancer patients, as well as in animal tumor models, DCs found in blood, tumor tissues and draining lymph nodes are often functionally defective [2, 17, 18]. Tumor-infiltrating DCs have been shown to lack costimulatory molecules, and to possess poor T-cell stimulatory capacity or induce anergy [9, 12]. Tumor-derived factors, such as VEGF, TGF-β and IL-10, can induce apoptosis and inhibit differentiation or functional maturation of DCs [12, 13, 16, 19, 35]. It has been suggested that immature or incompletely matured DCs may mediate tolerance instead of immune activation, by inducing T-cell anergy or regulatory T cells [26, 27, 36].
The presence of a dense DC infiltration has been associated with prolonged survival and reduced incidence of metastases in patients with various human cancers including colorectal, gastric, esophageal, oral, and lung carcinoma [3, 20–22, 31]. In breast cancer, the number of CD83+ mature DCs, but not of CD1a+ or S100+ DCs, had prognostic relevance [23]. In the light of their importance in antitumor immunity, surprisingly few studies have been aimed at the presence of DCs and its potential clinical correlates in melanoma, most of them comprising data on a limited number of patients [37, 40]. The only study involving a considerable number of cases indicated a reduction of the amount of Langerhans cells in thicker tumors, without examining its correlation with metastatic potential or its prognostic role [7]. Other dendritic cell types, as dermal dendrocytes and plasmacytoid DCs have also been documented in melanomas [15, 44]. However, while it has been the subject of some studies on sentinel lymph nodes of melanoma patients [29, 45], the presence of mature DCs in primary melanoma has only been investigated in two studies, one involving 15 cases [44], and another performed on one regressive melanoma sample [29].
Recently, in a study examining the expression of T-cell activation markers in the infiltrate of human melanomas, we found a decreased peritumoral infiltration of T cells expressing CD25 or OX40 in melanomas developing distant metastases during the follow-up period (5 years), compared to nonmetastatic or lymph node metastatic tumors. Moreover, high peritumoral densities of CD25+ or OX40+ lymphocytes were associated with longer survival of the patients, suggesting that immune mechanisms at the primary site influence the outcome of the disease [24]. These findings prompted us to examine the role of the professional antigen presenting cells, DCs in these processes. In the present study, we investigated the density of DCs expressing CD1a by immunohistochemistry in primary tumor samples obtained from 82 patients with cutaneous melanoma. On a subset of cases (59 samples) we also determined the density of mature DCs defined by the expression of DC-LAMP. DC density results were evaluated with regard to their association with tumor thickness (the most powerful prognostic factor in localized melanomas), the development of metastases, patients’ survival, and other clinicopathological parameters, as well as with the density of activated T cells.
Materials and methods
Patient characteristics
Archival tissue samples were obtained from 82 patients with primary cutaneous melanoma who underwent surgery between 1980 and 2000 at the Institute of Dermato-Venerology, Semmelweis University, and at the National Institute of Oncology, Budapest. Patients were selected in order to obtain a study group involving a higher number of intermediate-thickness or thick (>1.0 mm) melanoma samples than their normal ratio, which have a more uncertain prognosis than thin tumors. The study was approved by the ethics committees of both institutions. Patients did not receive any anticancer treatment prior to surgery. Clinical and pathological characteristics are summarized in Table 1. The tumors were grouped into four thickness categories based on the current AJCC staging system [4] (≤1.0, 1.01–2.0, 2.01–4.0, >4.0 mm), and into three categories according to disease progression (nonmetastatic, lymph node metastatic and visceral metastatic). Distribution according to stages [4] was: st. IA, 17; IB, 10; IIA, 16; IIB, 21; IIC, 14; IIIA, 4. All patients received curative resection of their tumors. Four of 27 st. I patients and 33 of 55 st. II–III patients received postoperative adjuvant therapy consisting mostly of Dacarbazine monotherapy or radiotherapy (76 and 14%, respectively). In case of tumor progression, the majority of patients received chemotherapy, which was Dacarbazine-based in most of the cases (80%). Surviving patients had follow-up data for at least 5 years; none of the patients died of melanoma-unrelated causes within 5 years. Thirty-eight patients had no metastases developed during the follow-up period, while 13 had metastases confined to regional lymph nodes, which were excised. Thirty-one patients developed distant visceral metastases. Five-year survival of patients in both the nonmetastatic and the lymph node metastatic groups was 100%, while only two patients developing distant visceral metastases survived for more than 5 years (62 and 72 months). The majority (65/66) of tumors thicker than 1.0 mm, and 5/16 of thin melanomas entered vertical growth phase. Tumors with clinical regression and/or histological signs of late regression were not included in the study. There was no significant difference between thickness categories in the distribution according to patients’ age, sex, or localization of the tumor (Table 1). However, compared to thin tumors, a higher proportion of thick melanomas were of nodular type, and ulcerated tumors were also overrepresented in the higher thickness groups.
Table 1.
Patient and tumor characteristics
| Thickness (mm) | All groups | ≤1.0 | 1.01–2.0 | 2.01–4.0 | >4.0 |
|---|---|---|---|---|---|
| Age | |||||
| ≤50 | 38 | 11 | 8 | 10 | 9 |
| >50 | 44 | 7 | 6 | 19 | 12 |
| Sex | |||||
| Male | 34 | 5 | 6 | 12 | 11 |
| Female | 48 | 13 | 8 | 17 | 10 |
| Localization | |||||
| Extremities | 32 | 7 | 7 | 10 | 8 |
| Trunk | 44 | 10 | 6 | 18 | 10 |
| Head | 6 | 1 | 1 | 1 | 3 |
| Type | |||||
| SSM | 52 | 18 | 12 | 15 | 7 |
| NM | 27 | – | – | 13 | 14 |
| ALM | 2 | – | 1 | 1 | – |
| LMM | 1 | – | 1 | – | – |
| Ulceration | |||||
| Present | 37 | 1 | 5 | 16 | 15 |
| Absent | 45 | 17 | 9 | 13 | 6 |
| Metastasis | |||||
| Nonmet. | 38 | 16 | 5 | 10 | 7 |
| LN-met.a | 13 | 2 | 3 | 4 | 4b |
| Visceral met. | 31 | – | 6 | 15c | 10 |
| Five-year survival (%) | 53/82 (65) | 18/18 (100) | 10/14 (71) | 14/29 (48) | 11/21 (52) |
SSM superficial spreading melanoma, NM nodular melanoma, ALM acral lentiginous melanoma, LM lentigo maligna melanoma
aOnly regional lymph node metastases developed during the follow-up period (5 years)
bOne patient had LN metastasis at the time of diagnosis (st. IIIA)
cThree patients had LN metastasis at the time of diagnosis (st. IIIA)
Immunohistochemical detection of infiltrating cells in melanoma samples
Three-micrometer sections cut from formalin-fixed, paraffin-embedded cutaneous melanoma samples were used. Immunohistochemistry was performed as described earlier [24], using monoclonal anti-CD1a, anti-DC-LAMP (Coulter-Immunotech, Marseille, France) and anti-CD45R0 (DakoCytomation, Glostrup, Denmark) primary antibodies, followed by biotin/streptavidin-peroxidase method (LSAB2 System, HRP; DakoCytomation) and visualization with 3-amino-9-ethylcarbazole (Vector Laboratories Inc., Burlingame, CA, USA). Double staining for CD1a and CD25 or CD134 was performed in a subset of cases. Incubation with the first primary antibody (monoclonal anti-CD25, Novocastra Laboratories, Newcastle Upon Tyne, UK, or anti-CD134, PharMingen, San Diego, CA, USA) was followed by biotin/streptavidin-peroxidase treatment (LSAB2 System, HRP; DakoCytomation), using Vector SG as chromogen (Vector Laboratories). Then the second primary antibody was applied (monoclonal anti-CD1a, Coulter-Immunotech) and developed by streptavidin-alkaline phosphatase treatment (LSAB2 System, Alkaline Phosphatase; DakoCytomation), using fuchsin as chromogen (Vector Laboratories).
Evaluation of the immune reactions
Slides were examined using a graticule of 10 × 10 squares, calibrated as 0.25 mm2 at 200× magnification. Counting was performed independently by two investigators (AL and JK, both blinded to the clinical information), and the mean value of their separate counts was used for the analysis. Because the distribution of stained cells in the tumors was heterogeneous, the entire tumor area was analyzed in every case, and density of positive cells/mm2 is given. The number of CD1a+ and DC-LAMP+ dendritic cells was registered separately in intratumoral (infiltrating melanoma cell nests) and peritumoral areas (distributed in the infiltrate along the margin and the base of melanomas). In the case of ulcerated tumors, the areas of ulceration were not taken in account. The proportion of patients with significant densities of CD1a+ DCs was also calculated, using cutoff values set up separately for intra- and peritumoral localization (25 and 30 cells/mm2, respectively), based on the mean of the given variable in the whole patient group. In the case of DC-LAMP, cutoff level based on the median value was used (13 cells/mm2), for its higher discriminative power in the survival analysis. Density values of T cells expressing the activation markers CD25 or OX40 derived from our previous paper [24]. Cutoff levels used for peritumoral CD25+ and OX40+ cells were 75 and 20 cells/mm2, respectively.
Statistical analysis
Comparisons between cell densities in different tumor groups was made using the Mann–Whitney U-test and Kruskal–Wallis test, while χ 2-test was used for comparing the proportions of samples with high cell densities. Associations between tumor thickness and cell densities, and between the densities of the different cell types were evaluated by the Pearson test and Spearman rank correlation, respectively. Univariate analysis of survival was performed by the Kaplan–Meier method, and the statistical analysis was carried out by the generalized Wilcoxon test. In multivariate analysis, independent prognostic factors were determined by the Cox proportional hazards model. All statistics were calculated using the BMDP Statistical Software Pack.
Results
Detection of CD1a+ and DC-LAMP+ dendritic cells in melanoma
Compared to the normal epidermis adjacent to the tumors, a decrease was observed in the number of CD1a-positive Langerhans cells in the epidermis overlying the melanomas. This phenomenon was most pronounced in the epidermis above the VGP part of superficial spreading melanomas (SSMs) and above nodular melanomas (NMs), where a marked reduction was found in LC number in the majority of cases (higher than 50% decrease in 44 of 61 evaluable cases). In SSMs containing both radial and vertical growth phase (n = 24), a greater degree of depletion was observed above the VGP part of the tumor than above RGP (average reduction: 61.6% vs. 25.6%, p < 0.001). The epidermis overlying NMs showed a degree of DC reduction similar to that observed in the case of VGP (66.4%).
In melanoma tissue, CD1a+ DCs were detected both in the stroma surrounding tumor deposits (peritumoral), and interposed between the melanoma cells (intratumoral) (Fig. 1a and b). Peritumoral density of CD1a+ DCs was similar to their intralesional density (mean ± SD for the whole patient population: 26.6 ± 33.7 and 30.5 ± 37.0 cells/mm2, respectively). In SSMs containing both radial and vertical growth phase, the RGP contained numerous CD1a+ cells, while the VGP of the same tumor was generally characterized by a lower degree of DC infiltration (intratumoral, RGP: 47.0 ± 42.4 vs. VGP: 22.1 ± 30.3, p = 0.0049; peritumoral, RGP: 47.5 ± 37.6 vs. VGP: 22.3 ± 28.6, p = 0.0032).
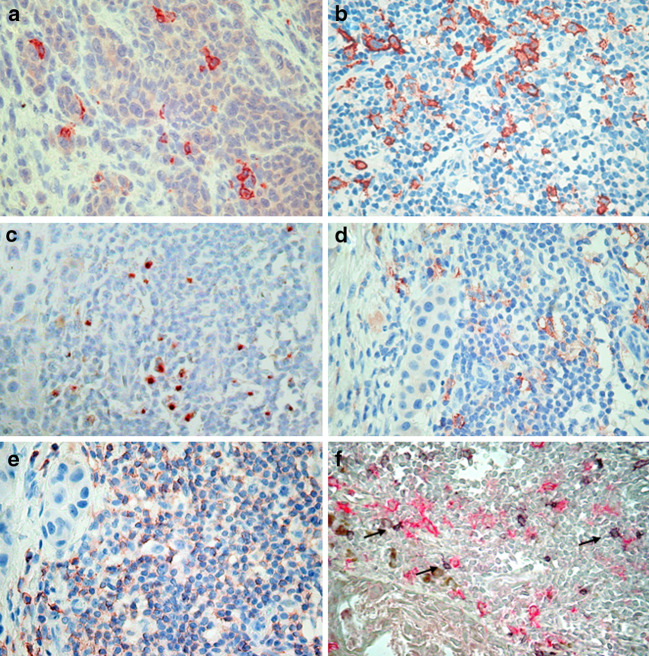
Fig. 1.
CD1a+ dendritic cells infiltrating melanoma cell nests (a) and in the peritumoral infiltrate of melanoma (b). Colocalization of DC-LAMP+ (c) or CD1a+ (d) dendritic cells in the lymphocytic infiltrate of melanoma (labeling for CD45R0, (e) in parallel slides of the same sample. (f) Double staining for OX40 (developed by Vector SG, gray signal), and CD1a (developed by fuchsin, red signal). CD1a+ DCs can be seen in close contact with OX40+ T cells (arrows). Pictures were taken using 40× objective
The DC maturation marker DC-LAMP was expressed as juxtanuclear dot-like staining. DC-LAMP+ cells were detected almost exclusively in the stromal compartment, associated with lymphocyte aggregates (Fig. 1c), with a density of 19.5 ± 23.3 cells/mm2. DC-LAMP+ cells infiltrating melanoma cell nests were found only in five cases (overall intratumoral density: 0.7 ± 3.1 cells/mm2). Staining of parallel slides showed colocalization of peritumoral CD1a+ and DC-LAMP+ DCs in T-cell-rich areas (Fig. 1c–e). Double staining for CD1a and CD25 or OX40 demonstrated the association of dendritic cells with lymphocytes expressing T-cell activation markers (Fig. 1f and not shown). Moreover, significant correlations were found between the density of DCs and activated T lymphocytes, where values for densities of CD25+ and OX40+ cells derived from our previous work [24]. The strongest associations were observed between peritumoral CD1a+ or DC-LAMP+ DCs and CD25+ cells (r = 0.4384, n = 73, p < 0.001 and r = 0.5637, n = 56, p < 0.001, respectively). For both intra- and peritumoral location, CD1a+ and DC-LAMP+ cell densities strongly correlated with each other (r = 0.6032 and 0.6321, respectively, p < 0.001).
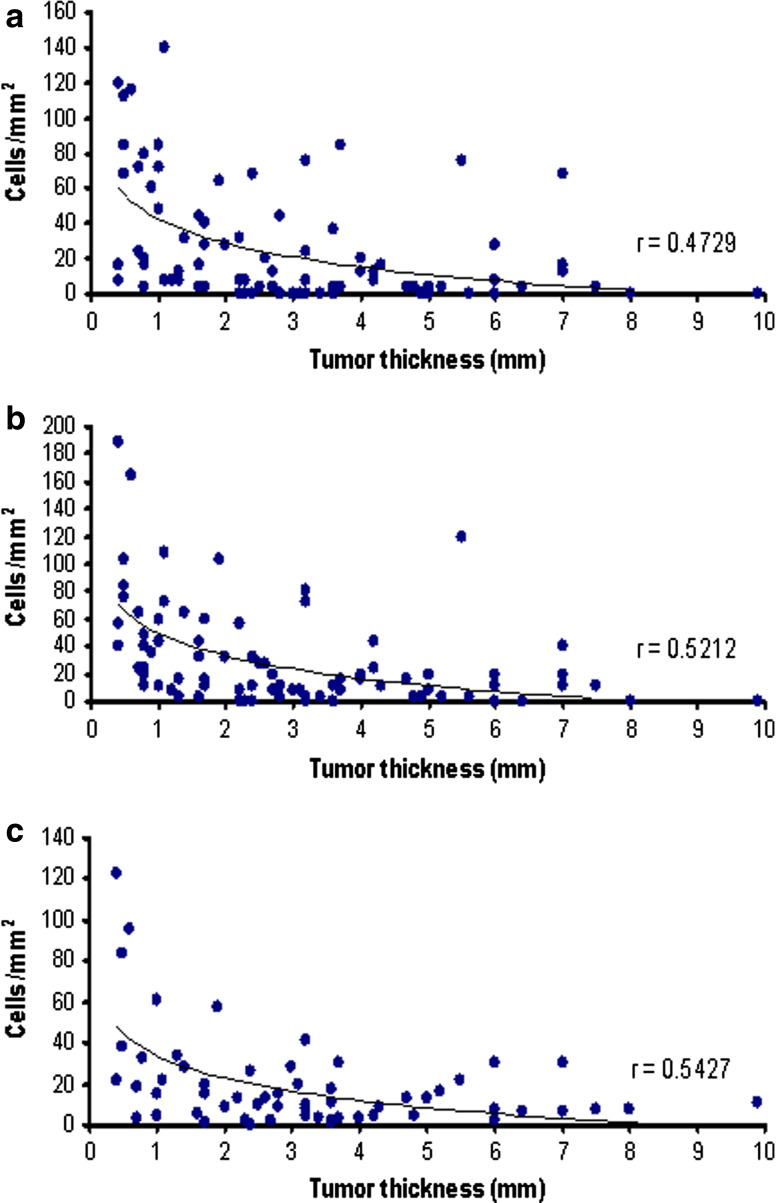
Correlation between DC density and tumor thickness
The degree of infiltration by CD1a+ and DC-LAMP+ DCs inversely correlated with melanoma thickness (p < 0.001; Fig. 2). For intra- and peritumoral CD1a+, and peritumoral DC-LAMP+ cell densities, cutoff values of 25, 30 and 13 cells/mm2 were introduced, respectively (see in “Materials and methods” section), and the proportion of melanomas with “significant cell density”, defined as higher than the cutoff value, was calculated. We evaluated the relationship between Breslow index and the proportion of patients with marked DC density. Similarly to the changes in the average cell densities, these values also decreased with the increasing thickness of melanomas (Table 2).
Fig. 2.
Correlation between the density of intratumoral CD1a+ (a), peritumoral CD1a+ (b), and DC-LAMP+ (c) dendritic cells with melanoma thickness. Each point represents a single tumor from an individual patient. r is the correlation coefficient
Table 2.
Proportion of patients with significant DC density in different Breslow categories
| Intratumoral CD1a+ (>25 cells/mm2) | Peritumoral CD1a+ (>30 cells/mm2) | Peritumoral DC-LAMP+ (>13 cells/mm2) | ||||
|---|---|---|---|---|---|---|
| Patient group | No. (%) | p | No. (%) | p | No. (%) | p |
| All thickness groups | 29/82 (35) | 28/82 (34) | 29/59 (49) | |||
| ≤1.0 mm | 12/18 (67) | 13/18 (72) | 9/11 (82) | |||
| 1.01–2.0 mm | 7/14 (50) | 8/14 (57) | 6/9 (67) | |||
| 2.01–4.0 mm | 7/29 (24) | 4/29 (14) | 9/23 (39) | |||
| >4.0 mm | 3/21 (14) | 0.0021 | 3/21 (14) | 0.0000 | 5/16 (31) | 0.0324 |
Data are expressed as number (%) of patients with significant cell density; p is the significance of difference between groups (χ 2-test)
Correlation between DC density and the development of metastases
The intensity of DC infiltration was studied in tumors that did not metastasize, and compared with those that gave regional lymph node metastases or visceral metastases during the follow-up period (5 years). When compared to visceral metastatic tumors, nonmetastatic ones showed more intense peritumoral CD1a+ DC infiltration (38.9 ± 44.1 cells/mm2 vs. 19.9 ± 24.9 cells/mm2, p = 0.0262). Similar tendency was observed in the case of both intratumoral CD1a+ and peritumoral DC-LAMP+ cell number, but the differences were not statistically significant (32.5 ± 38.1 cells/mm2 vs. 19.2 ± 28.3 cells/mm2 and 28.0 ± 32.7 cells/mm2 vs. 13.5 ± 12.5 cells/mm2, p = 0.1426 and 0.1850, respectively). Lymph node metastatic tumors showed intermediate values for each parameter, which were not significantly different from those of the other groups.
To determine whether DC density values could distinguish between tumors of different metastatic properties in intermediate thickness or thick tumors, which are generally characterized by a more uncertain outcome than thin melanomas, we performed the analysis with the exclusion of tumors ≤1.0 mm. This group contained mostly nonmetastatic cases (Table 1) and, on the other hand, showed the highest DC densities (Fig. 2 and Table 2), which could have a major effect on the correlations with metastatic potential in the whole patient group. Indeed, when analyzed only in the remaining 64 cases of melanomas >1.0 mm, no difference was found in these values between metastatic and nonmetastatic tumors. Similarly, evaluation of DC densities within the Breslow categories did not reveal significant variance between tumors of different metastatic pattern (not shown).
When analyzing the proportion of melanomas with high DC density in tumors with different metastatic behavior, no significant difference was found in the case of intratumoral CD1a+ cell infiltration. On the other hand, the frequency of samples with high peritumoral CD1a+ or DC-LAMP+ DC number was significantly lower in visceral metastatic cases compared to nonmetastatic and lymph node metastatic tumors (Table 3). The latter two categories were evaluated together because of the similar percentage of samples with marked infiltration in the case of each cell type (Table 3), and the similar biological behavior reflected by survival data (100% 5-year survival). However, when only melanomas thicker than 1.0 mm were included in the analysis, there was no difference in the density of DCs between these groups (Table 3).
Table 3.
Proportion of patients with significant DC density in different metastasis categories
| Intratumoral CD1a+ (>25 cells/mm2) | Peritumoral CD1a+ (>30 cells/mm2) | Peritumoral DC-LAMP+ (>13 cells/mm2) | ||||
|---|---|---|---|---|---|---|
| Patient group | No. (%) | p | No. (%) | p | No. (%) | p |
| All thickness groups | 29/82 (35) | 28/82 (34) | 29/59 (49) | |||
| Nonmetastatic | 14/38 (37) | 17/38 (45) | 14/23 (61) | |||
| LN metastatic | 5/13 (38) | 5/13 (38) | 5/8 (62) | |||
| Nonmet. + LN met. | 19/51 (37) | 22/51 (43) | 19/31 (61) | |||
| Visceral metastatic | 10/31 (32) | n.s. | 6/31 (19) | 0.0277 | 10/28 (36) | 0.0497 |
| >1.0 mm tumors | 17/64 (27) | 15/64 (23) | 20/48 (42) | |||
| Nonmetastatic | 3/22 (14) | 5/22 (23) | 6/13 (46) | |||
| LN metastatic | 4/11 (36) | 4/11 (36) | 4/7 (57) | |||
| Nonmet. + LN met. | 7/33 (21) | 9/33 (27) | 10/20 (50) | |||
| Visceral metastatic | 10/31 (32) | n.s. | 6/31 (19) | n.s. | 10/28 (36) | n.s. |
Data are expressed as number (%) of patients with significant cell density; p is the significance of difference from nonmetastatic + LN metastatic tumors (χ 2-test); n.s. not significant
Relationship between DC density and clinicopathologic parameters
Using the cutoff values described above, the distribution of melanomas with significant DC densities was analyzed according to clinicopathologic factors. There was no significant difference in the ratio of patients with high CD1a+ or DC-LAMP+ cell densities when the tumors were distinguished according to localization, or patient age or gender. Concerning histological type, SSM and NM cases were compared only in the >2.0 mm thickness categories, since no nodular melanomas were included in the thinner tumor groups; in these cases no difference in DC content was found. The frequency of samples with intense infiltration by intratumoral CD1a+, peritumoral CD1a+ or DC-LAMP+ DCs was lower in ulcerated tumors (p = 0.0579, 0.0004 and 0.0011, respectively).
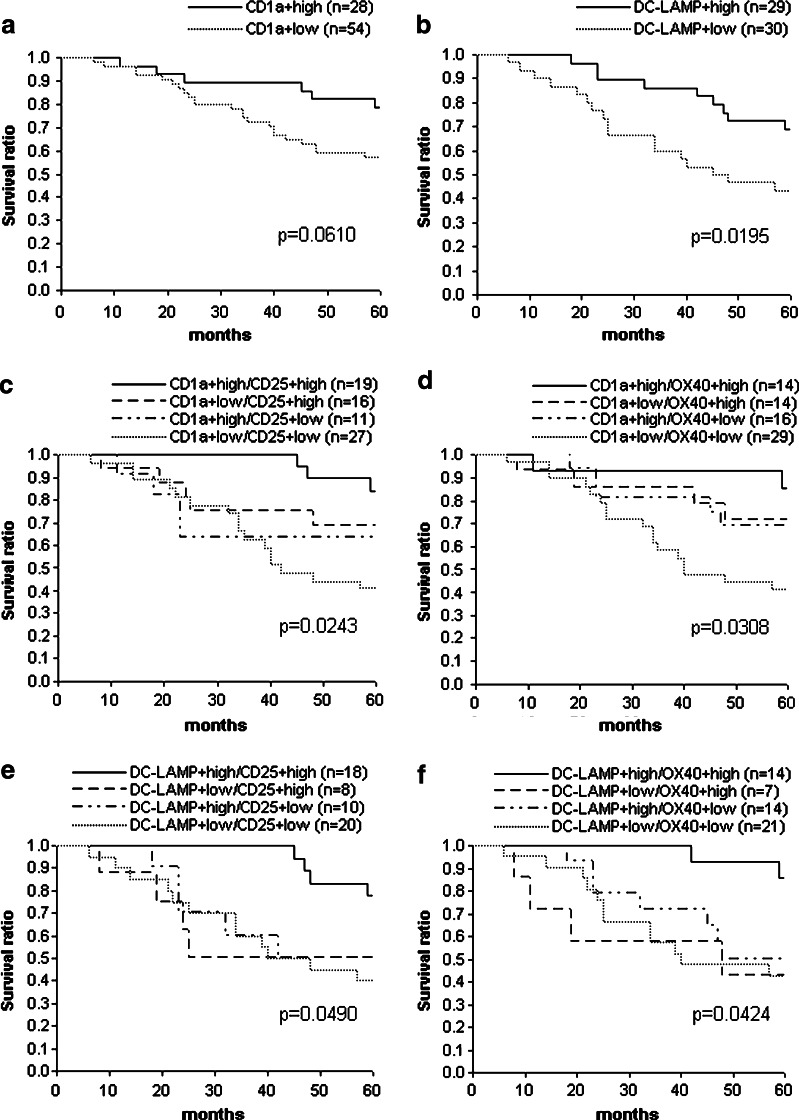
Survival analysis according to DC density
To evaluate the prognostic impact of the density of DCs, we performed Kaplan–Meier analysis using the same cutoff levels as in the comparisons described above. The intratumoral density of CD1a+ cells did not show association with the survival of the patients. On the other hand, intense peritumoral CD1a+ cell infiltration provided survival advantage of borderline significance (p = 0.0610), while high density of DC-LAMP+ cells was associated with significantly better prognosis (p = 0.0195) (Fig. 3a and b). The percentage of patients with more than 5 years survival was higher in the case of tumors characterized by significant numbers of peritumoral CD1a+ or DC-LAMP+ cells (79% vs. 57%, p = 0.0573 and 69% vs. 43%, p = 0.0472, respectively). However, similarly to the correlation with the occurrence of visceral metastases, differences in survival between the groups with different DC densities decreased when patients with ≤1.0 mm tumors were excluded, although in the case of high DC-LAMP+ cell density a statistically not significant trend for longer survival was found in thicker tumors as well (p = 0.1191).
Fig. 3.
Kaplan–Meier survival curves for melanoma patients subdivided according to the peritumoral density of CD1a+ (a) and DC-LAMP+ (b) DCs, or combined density values of CD1a+ and CD25+ (c), CD1a+ and OX40+ (d), DC-LAMP+ and CD25+ (e) or DC-LAMP+ and OX40+ cells (f). p values denote significance between the two (a, b) or all four (c–f) groups, evaluated by the generalized Wilcoxon test
Survival analysis according to DC and activated T-cell density
In a previous paper [24], we have reported significant association between patients’ survival and peritumoral infiltration by activated T lymphocytes expressing CD25 or OX40 markers. Although density values for DCs and activated T cells showed strong correlations (as shown above), high density samples for the different cell populations did not completely overlap. Therefore, it was possible to evaluate survival curves for patient subgroups characterized by high or low peritumoral DC densities combined with high or low activated T-cell densities. Cutoff levels for DC densities were the same as applied in the studies described above, and for CD25+ and OX40+ T cells 75 and 20 cells/mm2 levels were used, respectively. For all combinations of DC markers and T-cell activation markers, high peritumoral DC density values combined with high activated T lymphocyte numbers identified a subgroup of patients with more favorable survival compared to other subgroups (p = 0.0243, 0.0490, 0.0308 and 0.0424 for CD1a/CD25, DC-LAMP/CD25, CD1a/OX40 and DC-LAMP/OX40 combinations, respectively (Fig. 3c–f). Interestingly, the DC-LAMPhigh/activated Thigh combinations demonstrated the most discriminant survival advantage compared to all other groups, which were not significantly different from each other (Fig. 3e and f; p = 0.0057 and 0.0097, respectively, for DC-LAMPhigh/CD25high and DC-LAMPhigh/OX40high subgroups vs. all other subgroups combined). On the other hand, CD1alow/activated Tlow combinations defined patient populations with worse prognosis than either CD1alow/activated Thigh or CD1ahigh/activated Tlow combinations (Fig. 3c and d; p = 0.0086 and 0.0044, respectively, for CD1alow/CD25low and CD1alow/OX40low subgroups vs. all other subgroups combined).
When all these parameters (density values for DCs and activated T cells, as well as DC/T-cell marker combinations) were tested in multivariate analysis, together with other prognostic factors (tumor thickness, localization, histological type, ulceration, patients’ age and sex), high DC-LAMP+/OX40+ combination (relative risk: 0.215, p = 0.010) and tumor thickness (relative risk: 1.238, p = 0.001) proved significant independent predictors of good prognosis.
Discussion
In the study presented here, we analyzed the density of dendritic cells expressing CD1a and the maturation marker DC-LAMP in malignant melanoma, in relation to clinicopathological parameters. We found similar numbers of CD1a+ DCs in melanoma cell nests and in the stromal compartment, while DC-LAMP+ cells were detected almost exclusively in the peritumoral areas. The degree of both intratumoral and peritumoral infiltration by CD1a+ DCs, as well as peritumoral density of DC-LAMP+ mature DCs showed strong inverse correlation with the thickness of the tumors. With regard to CD1a+ DCs, these results corroborate those of an earlier report demonstrating decreased percentage of tumors with high DC number in thicker melanomas [7]. To the best of our knowledge, however, ours is the first study that analyzed the density of mature DCs in association with tumor parameters, on a substantial number of primary melanoma samples.
The peritumoral localization of mature DCs, observed in our study, corresponds to that described in other tumor types [6, 41]. DCs expressing maturation markers were predominantly found located to lymphocyte aggregates in the cancer stroma, sometimes forming clusters with T cells, which could be considered as evidence of an ongoing immune response. Consistent with these findings, the presence of mature DCs within the tumor tissue suggests that antigen presentation and T-cell priming can take place in the tumor microenvironment. Moreover, we found significant associations between the densities of DCs and activated T lymphocytes, suggesting some degree of functional activity of DCs in these tumors. The preferential localization of mature DCs in lymphocyte-rich peritumoral areas of melanomas could be due to the resemblance of these areas to secondary lymphoid organs where mature DCs are normally found. In fact, these cells can be detected in much higher numbers in tumor-draining lymph nodes, and in their study, although performed on a limited number of melanoma patients, Movassagh et al. [29] demonstrated an association between a high density of mature DCs in melanoma-positive sentinel nodes and the absence of metastases in non-sentinel lymph nodes.
We found reduced number of CD1a-positive Langerhans cells in the epidermis overlying most melanomas, as it has also been observed in some [37, 40], but not all [44] earlier reports. According to our results, this phenomenon was found more pronounced in nodular melanomas, and in the vertical growth phase of superficial spreading melanomas than in the radial growth phase. The cause of this depletion is not known yet, although it could be explained in part by the fact that even non-ulcerated epidermis above melanomas is often atrophic or hyperkeratotic. Several other potential mechanisms have been suggested, including the effect of factors deriving from cells in the tumor microenvironment, which inhibit the migration or differentiation of dendritic cells. Indeed, such factors, as IL-10, TGF-β and gangliosides have been detected in the tumor tissue in melanoma [10, 12, 30], and were found to reduce DC development or cause their death [12, 30]. However, the effect of a diffusible factor could not explain the abrupt decline in epidermal LC number at the border of the tumor, leaving these cells intact in the immediate vicinity of the tumors (our study and [37]). As another potential cause of LC depletion from the epidermis above melanomas, the migration of mature LCs in the tumor, due to stimuli by the tumor microenvironment, has also been considered [40]. However, in our study most tumors with a decrease in epidermal LC number simultaneously showed low intra- and peritumoral DC densities. Nevertheless, melanomas in average demonstrated higher DC density than the histologically normal adjacent dermis [37, 44]. Similarly, an accumulation of DCs compared to normal tissues has been observed in several tumor types [3, 6, 32, 39], and their density positively correlated with the expression of different chemokines in the tumor tissue, as GM-CSF, MIP-3 α or others, known as chemoattractants for DCs [6, 32, 39]. Beside factors produced by the tumor cells themselves, molecules secreted by other cell types composing the lymphocytic infiltrate (mostly T cells) probably contribute to the development of an environment promoting DC recruitment, in accordance with the preferential localization of peritumoral DCs in T-cell infiltrates. The actual density of DCs in a tumor is influenced by the balance of the local concentrations of chemo- and cytokines with opposing effects on DC migration and differentiation. Whether they derive from epidermal LCs has not been determined yet; as opposed to their LC origin, DCs in the tumor tissue may be derived from immigration of blood progenitors, as it has been suggested for most dermal DCs [28].
In our cohort of cutaneous melanoma samples, there was no significant variation in DC densities when tumors were distinguished according to histological type, localization, or patient age or sex. Ulcerated tumors contained lower numbers of infiltrating DCs, probably due to the increasing frequency of ulceration in thicker tumors. Finally, tumors that gave visceral metastases during the follow-up period were characterized with lower peritumoral DC density. Accordingly, intense peritumoral infiltration of DCs, especially of mature ones expressing DC-LAMP, was associated with longer survival of patients. In the case of CD1a+ DCs, these associations were lost when ≤1.0 mm cases were omitted, suggesting that the density of these cells is dependent on tumor thickness, without direct relationship with metastatic potential or the patients’ survival. In contrast, intense infiltration by DC-LAMP+ mature DCs proved of prognostic significance in univariate analysis, and also in multivariate analysis involving DC density values and traditional prognosticators (not shown). Moreover, combination of DC and activated T lymphocyte density values yielded patient subgroups with markedly different survival where all combinations of high DC/high activated T lymphocyte density predicted significantly better prognosis compared to other subgroups. In our earlier work analyzing the prognostic value of activated T cells in melanoma, peritumoral density of OX40+ lymphocytes proved an independent predictor of favorable disease outcome. In accordance with data supporting their role as independent prognostic factors alone, the combination of high density of cells expressing DC-LAMP and of those expressing OX40 markers defined a patient subgroup with significant survival advantage over all other subgroups, which proved independent factor also in multivariate analysis involving all DC- and T-cell activation markers as well as their combinations, beside other, more traditional prognosticators. These observations suggest that the prevalence of an “immunological functional unit” of antigen presenting dendritic cells and activated T cells in primary melanoma carries important prognostic information. Their role is supported in two ways, the combination of low density or absence of any DCs with low density of activated T cells predicting the worst, while high density of mature DCs and activated T cells predicting the best disease outcome within 5 years. This also suggests that the presence of antigen presenting DCs at the primary site, especially of mature ones together with that of activated T cells, could be a marker of a functional immune response against melanoma progression. Beside their prognostic value, evaluation of these parameters could prove useful in patient selection for immunotherapeutical protocols or monitoring the response to these treatment modalities.
Acknowledgments
We thank Katalin Derecskei, Violetta Piurkó and Miklós Kónya (National Institute of Oncology) for their excellent technical assistance, and to Eric Angevin (Institut Gustave-Roussy, Villejuif, France) for helpful suggestions. The study was supported by Hungarian Ministry of Health grant ETT 308/2003, grants GVOP-3.11.-2004-05-0090.3.0 and NKFP1a-0024-05.
References
- 1.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 2.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 3.Ambe K, Mori M, Enjoji M. S-100 protein-positive dendritic cells in colorectal adenocarcinomas. Distribution and relation to the clinical prognosis. Cancer. 1989;63:496–503. doi: 10.1002/1097-0142(19890201)63:3<496::AID-CNCR2820630318>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Buzaid AC, Soong S-J, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1425. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bröcker EB, Zwadlo G, Holzmann B, Macher E, Sorg C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int J Cancer. 1988;41:562–567. doi: 10.1002/ijc.2910410415. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/S0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 9.Chaux P, Moutet M, Faivre J, Martin F, Martin M. Inflammatory cells infiltrating human colorectal carcinomas express HLA class II but not B7-1 and B7-2 costimulatory molecules of the T-cell activation. Lab Invest. 1996;74:975–983. [PubMed] [Google Scholar]
- 10.Conrad CT, Ernst NR, Dummer W, Bröcker EB, Becker JC. Differential expression of transforming growth factor beta 1 and interleukin 10 in progressing and regressing areas of primary melanoma. J Exp Clin Cancer Res. 1999;18:225–232. [PubMed] [Google Scholar]
- 11.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/S1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 12.Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309–316. doi: 10.1002/(SICI)1097-0215(19971104)73:3<309::AID-IJC1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Esche C, Lokshin A, Shurin GV, Gastman BR, Rabinowich H, Watkins SC, Lotze MT, Shurin MR. Tumor’s other immune targets: dendritic cells. J Leukoc Biol. 1999;66:336–344. doi: 10.1002/jlb.66.2.336. [DOI] [PubMed] [Google Scholar]
- 14.Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443:703–717. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 15.Fullen DR, Headington JT. Factor XIIIa-positive dermal dendritic cells and HLA-DR expression in radial versus vertical growth-phase melanomas. J Cutan Pathol. 1998;25:553–558. doi: 10.1111/j.1600-0560.1998.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovich DI, Chen HL, Girgis KR, Cunningham T, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Ciernik F, Carbone DP. Dendritic cells in antitumor immune responses I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 19.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-β1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- 20.Inoshima N, Nakanishi Y, Minami T, Izumi M, Takayama K, Yoshino I, Hara N. The influence of dendritic cell infiltration and vascular endothelial growth factor expression on the prognosis of non-small cell lung cancer. Clin Cancer Res. 2002;8:3480–3486. [PubMed] [Google Scholar]
- 21.Ishigami S, Aikou T, Natsugoe S, Hokita S, Iwashige H, Tokushige M, Sonoda S. Prognostic value of HLA-DR expression and dendritic cell infiltration in gastric cancer. Oncology. 1998;55:65–69. doi: 10.1159/000011837. [DOI] [PubMed] [Google Scholar]
- 22.Ishigami S, Natsugoe S, Matsumoto M, Okumura H, Sakita H, Nakashima S, Takao S, Aikou T. Clinical implications of intratumoral dendritic cell infiltration in esophageal squamous cell carcinoma. Oncol Rep. 2003;10:1237–1240. [PubMed] [Google Scholar]
- 23.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–97. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 24.Ladányi A, Somlai B, Gilde K, Fejös Z, Gaudi I, Tímár J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10:521–530. doi: 10.1158/1078-0432.CCR-1161-03. [DOI] [PubMed] [Google Scholar]
- 25.Lenz A, Heine M, Schuler G, Romani N. Human and murine dermis contain dendritic cells. J Clin Invest. 1993;92:2587–2596. doi: 10.1172/JCI116873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz M, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/S1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 27.Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 28.McLellan AD, Heiser A, Sorg RV, Fearnley DB, Hart DNJ. Dermal dendritic cells associated with T lymphocytes in normal human skin display an activated phenotype. J Invest Dermatol. 1998;111:841–849. doi: 10.1046/j.1523-1747.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- 29.Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, Rimoldi D, Liénard D, Gugerli O, Ferradini L, Robert C, Avril M-F, Zitvogel L, Angevin E. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–2198. doi: 10.1158/0008-5472.CAN-03-2969. [DOI] [PubMed] [Google Scholar]
- 30.Péguet-Navarro J, Sportouch M, Popa I, Berthier O, Schmitt D, Portoukalian J. Gangliosides from human melanoma tumors impair dendritic cell differentiation from monocytes and induce their apoptosis. J Immunol. 2003;170:3488–3494. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- 31.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and ζ-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. doi: 10.1002/1097-0142(20010601)91:11<2136::AID-CNCR1242>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 32.Scarpino S, Stoppacciaro A, Ballerini F, Marchesi M, Prat M, Stella C, Sozzani S, Allavena P, Mantovani A, Ruco LP. Papillary carcinoma of the thyroid: Hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. Am J Pathol. 2000;156:831–837. doi: 10.1016/S0002-9440(10)64951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;21:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 34.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu Y-J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 35.Steinbrink K, Jonuleit H, Müller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 36.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 37.Stene MA, Babajanians M, Bhuta S, Cochran AJ. Quantitative alterations in cutaneous Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1988;91:125–128. doi: 10.1111/1523-1747.ep12464142. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki A, Masuda A, Nagata H, Kameoka S, Kikawada Y, Yamakawa M, Kasajima T. Mature dendritic cells make clusters with T cells in the invasive margin of colorectal carcinoma. J Pathol. 2002;196:37–43. doi: 10.1002/path.1018. [DOI] [PubMed] [Google Scholar]
- 39.Tazi A, Bouchonnet F, Grandsaigne M, Boumsell L, Hance AJ, Soler P. Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J Clin Invest. 1993;91:566–576. doi: 10.1172/JCI116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toriyama K, Wen D-R, Paul E, Cochran AJ. Variations in the distribution, frequency, and phenotype of Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1993;100:269S–273S. doi: 10.1111/1523-1747.ep12470135. [DOI] [PubMed] [Google Scholar]
- 41.Troy AJ, Summers KL, Davidson PJT, Atkinson CH, Hart DNJ. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–593. [PubMed] [Google Scholar]
- 42.Udey MC. Skin dendritic cells in immunity and autoimmunity. J Invest Dermatol Symp Proc. 2004;9:15–17. doi: 10.1111/j.1087-0024.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 43.Vandenabeele S, Wu L. Dendritic cell origins: puzzles and paradoxes. Immunol Cell Biol. 1999;77:411–419. doi: 10.1046/j.1440-1711.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 44.Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, Berenzi A, Cella M, Colonna M. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–268. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- 45.Vuylsteke RJCLM, Molenkamp BG, Gietema HA, van Leeuwen PAM, Wijnands PGJTB, Vos W, van Diest PJ, Scheper RJ, Meijer S, de Gruijl TD. Local administration of granulocyte/macrophage colony-stimulating factor increases the number and activation state of dendritic cells in the sentinel lymph node of early-stage melanoma. Cancer Res. 2004;64:8456–8460. doi: 10.1158/0008-5472.CAN-03-3251. [DOI] [PubMed] [Google Scholar]
- 46.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]