Abstract
Macrophages are considered a key component of the immunosuppressive environment present in solid tumors, where they support tumor growth through the production of pro-angiogenic factors and active suppression of effector immune responses. Zoledronic acid (ZA), an aminobisphosphonate clinically approved for treatment of symptomatic skeletal events, has recently been shown to have immunomodulatory properties that can be exploited in cancer immunotherapy. Here, we utilize an in vitro model of prostate cancer cell–macrophage interaction to dissect the effect of ZA, on the function of prostate cancer tumor-associated macrophages (PC-TAM). We show that prostate cancer cells recruit macrophages, which in turn express a variety of proangiogenic and immunosuppressive mediators. ZA selectively suppressed the expression of MMP-9 by PC-TAM, whereas the expression of other mediators was not limited. PC-TAM treated with ZA, on the other hand, could effectively drive the proliferation of activated Tγδ lymphocytes, which lysed bisphosphonate-pulsed prostate cancer cells. Moreover, ZA boosted the production of type-1 cytokines by PC-TAM in response to immunomodulators such as IL-12 and polyI:C, which are known to polarize macrophages towards an anti-tumoral M1 phenotype. Overall, we provide evidence that ZA shifts the balance of PC-TAM from a tumor promoting to a tumor-eliminating phenotype and also suggest a potential use of this pharmacological agent as an immunotherapeutic adjuvant.
Keywords: Tumor associated macrophages, Prostate cancer, Immunosuppression, Zoledronic acid, Immunotherapy
Introduction
Prostate cancer (PC) is frequently infiltrated by macrophages, which are more abundant than in benign gland, and constitute the majority of the cells of hemopoietic origin in the tumor [24, 39, 46]. Previously, macrophages were considered to contribute to the innate immune response against malignancy [17]. In fact, the tumor-eliminating capacity of tumor-associated macrophages (TAM) [17] is considerably compromised [5, 14, 41]. Although a certain degree of variability exists between tumors [23], TAM generally adopt a phenotype that is reminiscent of the one found in sites of chronic tissue inflammation and injury, characterized by the production of IL-1b, IL-10, TNF and VEGF [7]. The majority of clinical evidence also points to a poor prognosis of patients that have a high density of macrophages in their tumors [1], which further substantiates their role in supporting tumor growth. Indeed, PC patients with a high volume density of TAM have shorter median cancer specific survival and an increased macrophage cell profile area correlates with poor clinical outcome [24]. Moreover, ablation of macrophages in animal models results in a decreased capacity of the tumor to grow and metastasize [25, 45]. However, the function of PC-TAM has not thus far been characterized.
Zoledronic acid (ZA), a member of the aminobisphosphonate class of drugs, is approved for the treatment of bone disease in PC [40]. This agent targets the mevalonate pathway, thus interfering with the post-translational modification of G-proteins and cellular signaling [43]. The exact molecular mechanisms of this effect have not been described in detail. Among other functions, ZA has also been shown to specifically target angiogenesis pathways in the prostate [11] and to limit the production of MMP-9 by cervical cancer-TAM [9, 13, 32]. One of its documented effects is the modulation of monocyte/macrophage differentiation and functions [43]. It is also noteworthy that cells of the monocytic lineage such as monocytes and dendritic cells [10, 27] can present low molecular mass non-peptide antigens in a non-MHC dependent manner and effectively drive the proliferation and activation of Vγ9/Vδ2+ γδ T lymphocytes when treated with ZA. This can potentially be exploited in cancer immunotherapy as Tγδ lymphocytes in turn have been shown to exert direct cytotoxicity against malignant cells [20].
Here, we describe the key effect of ZA on TAM, in particular its potential use in reverting the immunosuppressive environment of the tumor in conjunction with immunotherapeutic modalities.
Materials and methods
Cell lines, drugs and conditioned media
PC3 and LNCaP prostate cancer cell lines were purchased from the American Type Culture Collection. All cell lines were cultured in RPMI-1640 (Gibco, Paisley, UK) supplemented with 2 mM l-glutamine (Sigma, St Louis, MO), 10 mM HEPES, 25 μg/ml gentamycin and 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA, USA). When confluency reached 80%, fresh culture medium was added to the cultures and collected after 24 h, filtered through a 0.22-μm filter (PALL Corporation, MI, USA), aliquoted and stored at −80°C. ZA (Zometa®) was purchased from Novartis.
Monocyte isolation, propagation of macrophages and induction of the PC-TAM phenotype
Monocytes were isolated from buffy coats of healthy donors, obtained from the Karolinska University Hospital Blood Bank. Mononuclear cells were isolated by centrifugation through a Ficoll gradient (Ficoll-PaqueTM Plus, GE Healthcare) and monocytes isolated either by adherence to plastic for 2 h or using CD14-microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). Monocytes were routinely cultured in vitro in culture medium for 10 days, until they differentiated to macrophages, as verified by their morphology. Staining for surface markers verified that macrophages were CD14+, MHC-I+, MHC-II+, CD86 low, CD83−. Day 10 macrophages were cultured for 48 h with 30% conditioned medium from the PC3 and LNCaP cell lines to induce the characteristic PC-TAM phenotype.
Monocyte migration
Monocytes isolated from buffy coats of healthy individuals were tested for their migratory capacity through a polycarbonate 5-μm-pore filter (Neuroprobe Inc., MD, USA) against CM from PC3 and LNCaP cells in a 96-well chemotaxis chamber (Neuroprobe). Briefly, CM in the lower chamber and monocytes in the upper chamber were incubated for 4 h at 37°C and the number of migrated cells in the lower chamber was evaluated either by counting in a hemocytometer or by the MTT cell proliferation assay (Cell Proliferation Kit I-MTT, Roche, IN, USA), according to the manufacturer’s instructions.
Quantitative PCR
RNA was isolated from PC-TAM using the RNeasy Mini Kit (Qiagen) and the RNase-Free DNase Set (both from Qiagen) according to manufacturer’s instructions. RNA concentration was measured using spectrophotometry (NanoDrop, Rockland, DE, USA). cDNA was synthesized using SUPERSCRIPT ™ II (Invitrogen) reverse transcriptase kit and oligodT18 as per manufacturer. The primer sequences are shown in Table 1. Real Time PCR was performed on Applied Biosystems’ ABI PRISM® 7700 Sequence Detection System (Foster City, CA, USA) using the SYBR Green PCR Master Mix (Applied Biosystems), and normalized using HPRT expression values.
Table 1.
Oligonucleotide primer sequences (5′–3′) for quantitative PCR analyses of macrophage gene expression
| Gene (abbreviation) | Forward primer | Reverse primer |
|---|---|---|
| Tumor Necrosis factor alpha (TNF-α) | AGCCCATGTTGTAGCAAACC | TGAGGTACAGGCCCTCTGAT |
| Matrix Metalloproteinase 9 (MMP-9) | CACTGTCCACCCCTCAGAGC | GCCACTTGTCGGCGATAAG |
| Interleukin 1 beta (IL-1 b) | CGACACATGGGATAACGA | CGCAGGACAGGTACAGATTC |
| Vascular Endothelial Growth factor (VEGF) | AGATCGAGTACATCTTCAAGCCATC | CGTCATTGCAGCAGCCC |
| chemokine (C-C motif) ligand 22 (CCL22) | GCGTGGTGTTGCTAACCTTCA | AAGGCCACGGTCATCAGAGT |
| Platelet Derived Growth factor (PDGF) | CGATCCGCTCCTTTGATGAT | TCCAACTCGGCCCCATCT |
| Hypoxanthine-guanine phosphoribosyltransferase (HPRT) | TGACACTGGCAAAACAATGCA | GGTCCTTTTCACCAGCAAGCT |
| Arginase 1 (Arg-1) | CAGAGCATGAGCGCCAAGT | ATCACACTCTTGTTCTTTAAGTTTCTCAA |
| Indoleamine 2,3-dioxygenase (IDO) | GCATTTTTCAGTGTTCTTCGCATA | TCATACACCAGACCGTCTGATAGC |
| Interferon-gamma (IFN-γ) | AGCTCTGCATCGTTTTGGGTT | GTTCCATTATCCGCTACATCTGAA |
| Tumor Growth factor beta (TGF-β) | GGACATCAACGGGTTCACTACC | AGCAGGAAAGGCCGGTTC |
| Interleukin 10 (IL-10) | ACGGCGCTGTCATCGATT | GGCATTCTTCACCTGCTCCA |
| Interleukin 6 (IL-6) | AAAGAGGCACTGGCAGAAAA | TTTCACCAGGCAAGTCTCCT |
Expansion of Tγδ lymphocytes
Expansion of Vγ9/Vδ2+ γδ T lymphocytes was carried out as previously described [10]. Briefly, PC-TAM were treated with different concentrations of ZA and washed extensively. Autologous PBMCs were then added to ZA-treated PC-TAM at a PBMC:PC-TAM ratio of 1:5, in medium supplemented with 10 U/ml IL-2. After 7-day incubation the percentage of γδ T lymphocytes among CD3+ lymphocytes was evaluated by flow cytometry (γδT cells were characterized as CD3+Vγ9TCR+ cells in the lymphocyte gate based on Forward—Side Scatter profile). The activation status of the expanded γδT cells was analyzed by surface staining for CD25 and intracellular staining for IFN-γ.
Flow cytometry
The antibodies used in the present study were mouse anti human Vγ9 TCR-FITC (Beckton-Dickinson, San Jose CA, USA), mouse anti-human CD3-PE (Beckton-Dickinson), mouse anti-human CD25-PE and CD25-APC (Beckton-Dickinson) and mouse anti-human IFNγ-PE (Beckton-Dickinson). Data collection and analysis was performed using a FACSCalibur flow cytometer and CELLQuest Pro software (Beckton-Dickinson).
In vitro cytotoxicity assays
A standard 4-h Cr-release assay was used to assess in vitro cytotoxicity of expanded γδ T lymphocytes, as previously described [33]. Briefly, LNCaP cells pretreated with 5 μM ZA overnight were labeled with 1–2 μCi/μl of Na51CrO4 (Amersham, Buckinghamshire, UK) at 37°C for the last 1.5 h and then washed extensively. Cells were then incubated at various cell ratios for 4 h with ex vivo expanded γδ T lymphocytes, prepared as described above. Following incubations, 100 μl of supernatant was collected and the amount of released 51Cr was measured using a gamma counter (Wallac, Upplands Vasby, Sweden). Specific lysis was calculated using the formula [(E − S)/(T − S)] × 100% where E represents the experimental release, S the spontaneous release, and T the total release. Total release was accomplished by incubating the targets in 5% Triton X-100.
Quantification of cytokine secretion
IFN-γ in the supernatants of ZA-treated PC-TAM was quantified by ELISA (Mabtech, Sweden), as per manufacturer. IL-12, IL-10 and TNF-α were quantified using the Cytometric Bead Array system (Beckton-Dickinson), following the manufacturer’s instructions, and analysis of the data was performed using the BD CBA Software.
Statistics
All experiments were repeated at least two times. Quantitative PCR data was analyzed using the 2−ΔΔCT method. Significance of gene expression levels relative to controls were determined using the Mann–Whitney U test (non-parametric). Statistical analysis comparing data from different groups was performed using two-tailed Student’s t test. All statistical analyses were performed using GraphPad Prism version 4.03 for Windows, GraphPad Software, San Diego California USA, http://www.graphpad.com.
Results
Prostate cancer cell lines expand macrophages in vitro and induce them into a protumorigenic phenotype
We initially studied the effect of PC tumor lines on bystander monocytes and macrophages in our in vitro culture system. Conditioned medium (CM) from androgen-dependent LNCaP cells had the capacity to chemoattract peripheral blood monocytes in standard migration assays, whereas migration towards CM from low-differentiation, androgen-independent PC3 cells was not significant (Fig. 1a). On the other hand, primary human macrophages that were exposed to CM from PC3 cells readily proliferated, in contrast to the absence of proliferation observed in macrophages exposed to factors secreted by LNCaP cells (Fig. 1b). Thus, PC cells of different grade of differentiation apparently use diverse mechanisms of enrichment in the vicinity of the tumor, namely the recruitment of monocytes and the proliferation of resident tissue macrophages.
Fig. 1.
a Monocytes isolated from peripheral blood mononuclear cells of healthy donors were tested in a transwell assay for their capacity to migrate towards conditioned medium from PC3 or LNCaP cell lines. b Proliferation of macrophages exposed to conditioned media derived from the PC3 and LNCaP prostate-cancer cell lines. Macrophages were propagated from peripheral blood mononuclear cells and exposed to conditioned medium from PC3 or LNCaP cells for 48 h. Their proliferation was evaluated by measuring 3H-thymidine incorporation in the DNA during the last 18 h of incubation. Asterisks denote significant difference over controls (*P < 0.05; **P < 0.01, Students’ t test). Bars represent mean ± SD. Data from three experiments is shown
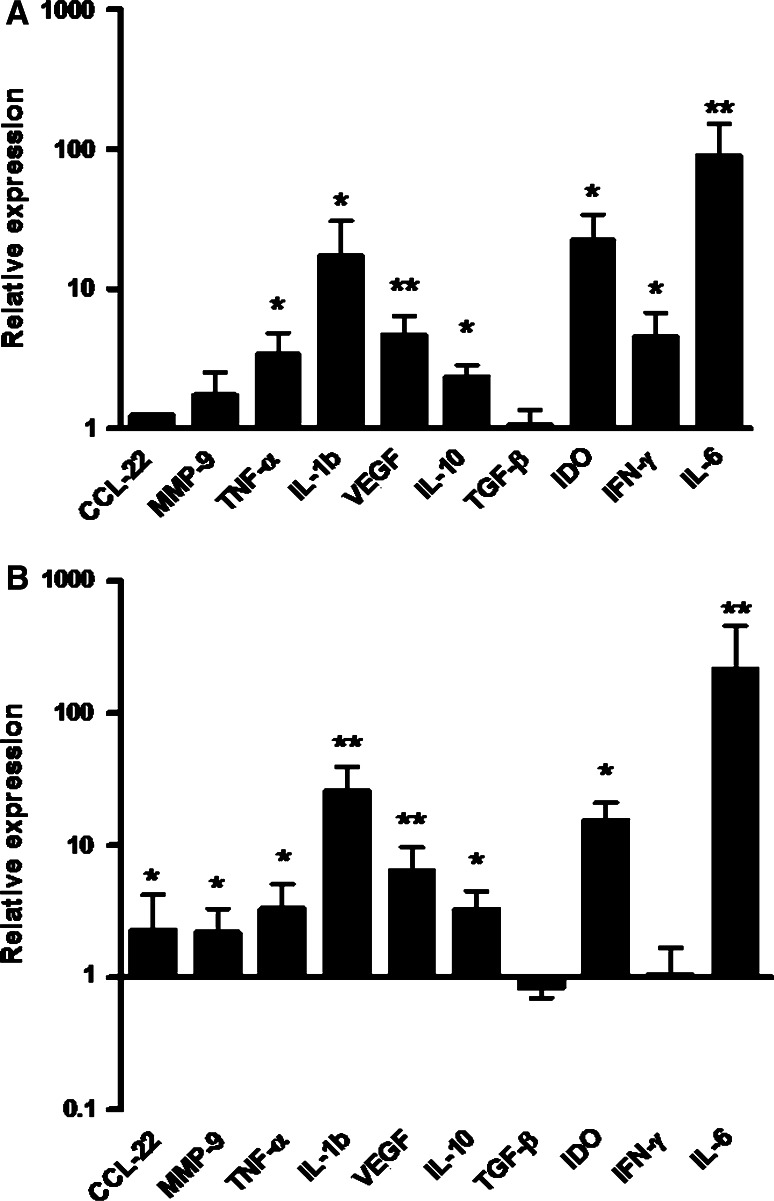
Furthermore, we characterized the phenotypic changes triggered by PC cell lines. Primary macrophages that were exposed for 48 h to CM from LNCaP cells expressed IDO, as well as IL-1b and TNF-α. VEGF and IL-10 were also overexpressed, but there was no considerable up-regulation of TGF-β (Fig. 2a). The majority of these genes have also been described as being up-regulated in macrophages associated with other forms of cancer. The pattern of gene up-regulation was similar in response to factors secreted by PC3 cells (Fig. 2b). Altogether, PC-TAM shared the features of the chronic, aberrant inflammatory response commonly observed amongst macrophages present in different types of tumors, although PC3 appeared to be the more potent inducers of this phenotype.
Fig. 2.
a Macrophages were exposed to LNCaP conditioned medium for 48 h, RNA was isolated and the expression of specific genes associated with TAM functions was evaluated by quantitative PCR. b Quantification of the aforementioned gene expression in the case of macrophages exposed to PC3 conditioned medium for 48 h. Values, normalized to GAPDH, depict fold-expression as compared to normal macrophages raised from the same donor. Asterisks denote significant difference in gene expression (*P < 0.05; **P < 0.01, Mann–Whitney U test). Bars represent mean ± SD. Shown is data from up to eight experiments
Zoledronic acid selectively suppresses MMP-9 expression but does not inhibit the acquisition of a PC-TAM phenotype
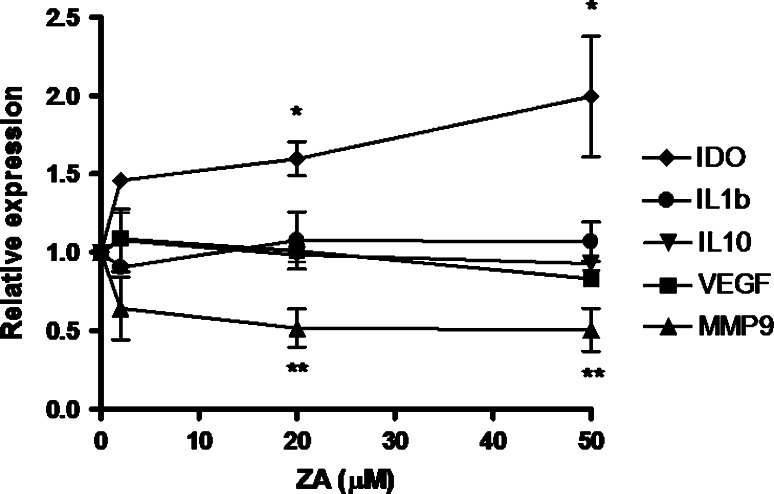
Since ZA has been proposed as a potential agent with antitumoral and immunomodulatory activity [13], we sought to study in detail the effect of ZA on TAM in prostate cancer. In quantitative PCR experiments where we exposed PC-TAM to graded concentrations of ZA and measured the expression of immunosuppressive, proangiogenic and prometastatic genes, we observed that ZA selectively suppressed the expression of MMP-9 (Fig. 3). This observation suggests that the effect observed in a mouse ovarian cancer model also applies in humans and is conserved among different types of malignancies. Importantly, MMP-9 suppression was evident in the high range of ZA concentration. The majority of TAM-associated genes, however, did not show any considerable modulation by ZA. Actually, the expression of IDO was up-regulated in the presence of increasing levels of ZA (Fig. 3). Similar results were obtained after exposure to PC3-conditioned medium and ZA (data not shown). In preliminary experiments, concentrations up to 200 μM of ZA were found to have no cytotoxic effect on macrophages (data not shown). Collectively, these results suggest that ZA has a capacity to limit the expression of particular genes such as MMP-9 in PC-TAM, but does not by itself revert their immunosuppressive/proangiogenic gene expression pattern.
Fig. 3.
Macrophages were cultured with LNCaP conditioned medium for 48 h, in the presence of various concentrations of ZA. RNA was extracted and the expression of specific genes known to be implicated in PC-TAM function was evaluated by quantitative PCR. Values depict fold-expression as compared to non-ZA treated TAM raised from the same donor after normalization to GAPDH. Asterisks indicate significant difference in gene expression (*P < 0.05; **P < 0.01, Mann–Whitney U test). Error bars are representative of SD. Shown is data from three experiments
PC-TAM during ZA treatment drive the proliferation of activated Tγδ lymphocytes
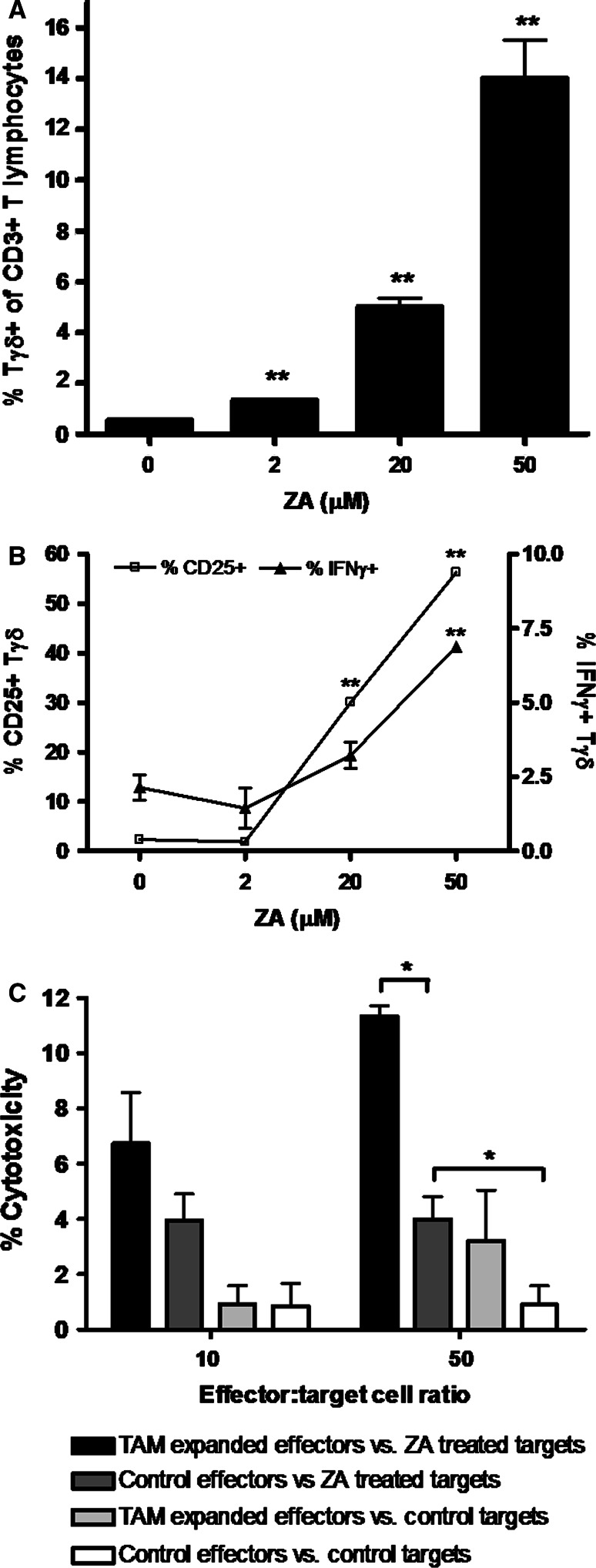
Tγδ lymphocytes are important arms of the immune response against tumors [20]. To investigate if PC-TAM during ZA treatment enhances Tγδ lymphocyte expansion, autologous PBMCs were co-incubated for 1 week with PC-TAM previously treated with various concentrations of ZA, and the percentage of Tγδ lymphocytes, their surface markers and intracellular IFN-γ levels were evaluated. As shown in Fig. 4a, ZA treatment of PC-TAM resulted in a robust expansion of Tγδ lymphocytes, which was maximal during treatment of PC-TAM with a high dose of the drug. Moreover, Tγδ lymphocytes expanded during co-culture with ZA-treated PC-TAM were functionally activated as they overexpressed surface CD25 and intracellular IFN-γ (Fig. 4b) in a concentration dependent manner. CD25 expression on Tγδ lymphocytes was relatively dim (data not shown). These Tγδ cell-enriched autologous PBMCs also exhibited a significantly enhanced cytotoxicity against ZA-treated PC cells (Fig. 4c) when compared to control effectors. PC cells pre-treated with ZA were more vulnerable to cytotoxic lysis even by non-ZA expanded effectors. This finding is in line with previous observations, which suggest that tumor cells treated with ZA accumulate intracellular metabolites, which are recognized by cytotoxic Tγδ lymphocytes, thus leading to the lysis of the tumor cell.
Fig. 4.
a PC-TAM were exposed to different concentration of ZA for 48 h and subsequently cultured with autologous PBMCs in the presence of low-dose IL-2. After 7 days cells were collected and the percentage of Vγ9/Vδ2+ γδ T lymphocytes among CD3+ T lymphocytes was evaluated by flow cytometry, and depicted for each particular ZA concentration used to treat PC-TAM. Bars represent mean ± SD. b Activation status of γδ T lymphocytes expanded during co-culture with ZA-treated PC-TAM. After 7 days of co-culture with ZA-treated PC-TAM, surface staining for CD25 and intracellular staining for IFN-γ was performed on γδ T lymphocytes to assess the functional activation. The percentage of T γδ cells that stained positive for CD25 (open squares, left Y-axis) and IFN-γ (closed triangles, right Y-axis) is depicted for each particular ZA concentration used to treat PC-TAM. Error bars are representative of SD. Asterisks indicate significant difference compared to non-ZA treated TAM (**P < 0.01, Students’ t test). c Cytotoxicity of PC-TAM expanded γδ T lymphocytes against ZA-treated PC cells. PBMCs enriched in γδ T lymphocytes as a result of co-culture with ZA-treated PC-TAM (macrophages exposed to 30% LNCaP conditioned medium and 20 μM ZA) were tested for their capacity to lyse LNCaP cells pulsed with 5 μM ZA or untreated control cells, at different effector/target ratios during a standard 4 h 51Cr release assay. Autologous PBMCs, co-cultured for the same time period with PC-TAM that were not treated with ZA served as a control. Spontaneous release was 18%. * Significant difference (P < 0.05, Students’ t test). Bars show mean ± SD. One representative experiment is shown
Taken together, these results reveal a critical aspect of the interaction of ZA with TAM, the expansion of activated Tγδ cells, which represent a powerful arm of the innate immune response against tumors, as they have the ability to lyse ZA-treated malignant cells.
Zoledronic acid synergizes with immunomodulators in shifting TAM to a M1 phenotype
TAM activated by cytokines or Toll-like receptor (TLR) ligands have been shown to drive type-1 immune responses and exert potent anti-tumor effects [3, 15, 42]. To evaluate if ZA synergizes with immunomodulating agents in conferring a M1 phenotype on TAM, we elaborated on the combined use of ZA with IL-12, a prototypical type-1 cytokine with well-established anti-tumor properties. PC-TAM were screened for their IFN-γ and IL-10 expression, which was validated by measuring cytokine protein production by ELISA and Cytometric Bead Assay.
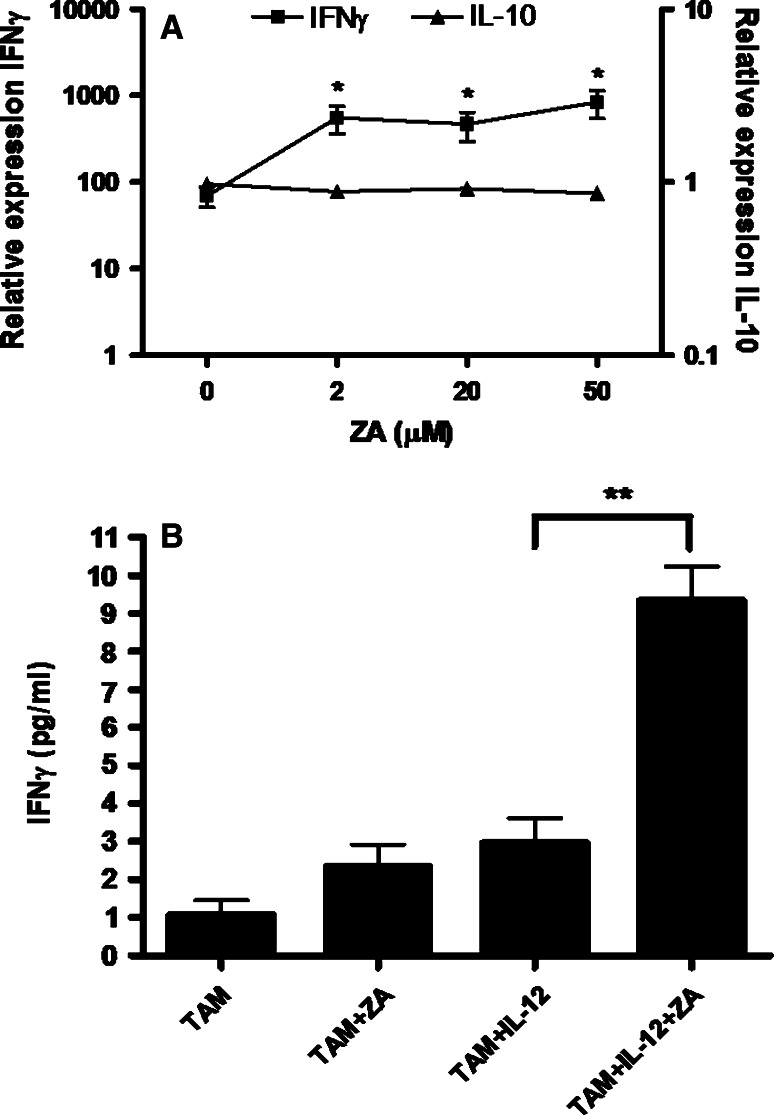
Both IL-12 and ZA induced expression of IFN-γ. Their combined function had a marked, synergistic effect, which was observed over a wide range of concentrations of ZA, including those where we previously documented maximal MMP-9 suppression and Tγδ lymphocyte expansion (20–50 μM). There was also a lower IL-10 expression by ZA/IL-12 treated macrophages, which further enhanced their M1 polarization (Fig. 5a). Likewise at the protein level, an enhanced secretion of IFN-γ in the supernatant of PC-TAM cultures was evident (Fig. 5b). Secretion of the type-2 cytokine IL-10 was lower in the presence of ZA, whereas type-1 cytokines such as TNF-α, as well as IL-12, exhibited a similar increase as IFN-γ. Proinflammatory cytokines such as IL-8, IL-6 and IL-1b were not significantly modified by the combination treatment (data not shown). In analogous experiments where a potent TLR3 agonist, polyI:C, was used, the same immunopotentiating phenomenon was observed, with approximately 10-times boost of IFN-γ gene expression and doubling of IFN-γ protein secretion (data not shown).
Fig. 5.
a PC-TAM were incubated with IL-12 (20 ng/ml), in the absence or in the presence of various concentrations of ZA. After 24 h total mRNA was collected and the expression of IFN-γ (filled squares, left Y-axis) and IL-10 (filled triangles, right Y-axis) was measured by quantitative PCR. Shown is expression relative to non-treated macrophages after normalization to GAPDH and data are depicted relative to the concentration of ZA. Asterisks indicate significant difference compared to IL-12-treated/non-ZA treated TAM (*P < 0.05, Students’ t test). Error bars represent SD. b IFN-γ protein secretion in the supernatant of PC-TAM during combination treatment with IL-12 and ZA. Supernatants were collected at 24 h from cultures of PC-TAM incubated with IL-12 (20 ng/ml), ZA (20 μM), or a combination of IL-12 and ZA, and tested for the presence of IFN-γ by ELISA. ** Significant difference (P < 0.01, Students’ t test). Bars show mean ± SD. One representative experiment is shown
It is thus evident that macrophage diversion towards a M1 antitumoral phenotype is particularly amenable to combination treatment utilizing ZA with other Th1-promoting immunomodulatory mediators.
Discussion
Macrophages comprise a prominent cell component of the stroma in a variety of solid tumors. They probably constitute, along with T regulatory cells [26], the major immunosuppressive cell populations infiltrating PC. Existence of TAM in high numbers in PC can be attributed to both migration of blood monocytes and to in situ expansion of macrophages. Both mechanisms were observed in our in vitro model, as androgen-dependent LNCaP cells induced monocyte migration, while PC3 cells supported proliferation of macrophages. The difference could be due to the fact that PC3 cells, but not LNCaP, produce M-CSF [35], and secrete significant levels of biologically active GM-CSF, which supports macrophage proliferation [31]. On the other hand, androgen-dependent LNCaP cells were superior regarding their ability to attract monocytes. Overall, it is likely that different types of prostate tumors employ either or both of these mechanisms to sustain TAM accumulation.
TAM share a relatively conserved phenotype across various cancer types [7], with a definite degree of differentiation between cancer types, or even particular microenvironments in the same tumor [23]. In our experiments, PC-TAM were found to overexpress the immunosuppressive cytokine IL-10, along with prominent expression of VEGF, IDO, IL-1b and TNF-α. VEGF is a molecule exhibiting a dual profile, which apart from its classic proangiogenic function shares a well-defined immunosuppressive potential [12, 30]. IDO is a key enzyme that depletes the aminoacid tryptophan from the tumor milieu, thus hindering T lymphocytes from exerting their anti-tumoral effect [29]. IL-1b on the other hand is a pro-inflammatory cytokine, which contributes to PC cell resistance against anti-androgen treatment [46]. The observed TNF-α overexpression also corroborates the pro-angiogenic phenotype of PC-TAM, and might also be a factor crucial for the induction of matrix metalloproteinase activity in bystander cells, as is the case of breast cancer [16].
Apart from its classical application in the treatment of symptomatic bone metastases, ZA attracts interest as a potent immunomodulator, which can be exploited in cancer immunotherapy. In this context, we observed that ZA suppressed the expression of MMP-9, whilst it did not exhibit such effect on the rest of the genes tested. A previous study in a mouse model of cervical carcinoma also depicted reduced expression of MMP-9 by TAM [13], pointing to conserved patterns of ZA function among man and mouse, where particular genes are more readily suppressed. The dose-dependent effect of ZA should be taken into consideration as far as expression of TAM-genes is concerned, since the suppression was optimal in the high, 50-μM level. Typically, peak plasma ZA levels are 1–3 μM [6, 22], but the actual local levels in the tumor are difficult to evaluate. Concentrations in bone and bone metastases can possibly be as high as 1 mM [28], since ZA accumulates locally and is very slowly released.
ZA-pulsed TAM expanded a cell population enriched in activated Tγδ lymphocytes. The mechanism likely involves both cell contact and humoral factors. It has been previously shown that pamidronate-pulsed THP-1 monocytes/macrophages induced active engagement of the γδ TCR on Tγδ lymphocytes, and their subsequent clustering, activation and proliferation. On the other hand IL-12, a key cytokine up-regulated by ZA, has been shown to be an important cytokine in the activation/proliferation process of Tγδ lymphocytes [37]. We also show that activated Tγδ lymphocytes had a significantly increased capacity to lyse malignant PC cells, corroborating previous data showing that ZA-exposed cells are more vulnerable to Tγδ lymphocyte-mediated lysis [27]. This pathway involves secretion of molecules such as perforin, granzyme and granulysin [2]. We propose that ZA thus restores a potent innate immunity pathway operating in the stroma of the tumor, since some reports point to a diminished frequency of Tγδ lymphocytes in the tumor [8, 21]. Induction of activated Tγδ lymphocytes in the tumor stroma may bypass any potential disability of this cellular population to home to malignant sites. Furthermore, it provides a pathway through which Tγδ lymphocytes recruited from the peripheral blood receive survival stimuli and expand locally in the tumor milieu.
Novel approaches targeting TAM by using cytokines such as IL-12 or TLR-agonists such as CpG, have demonstrated very promising results, since they seem to be able to re-educate TAM back to a M1-like anti-tumoral phenotype [3, 4, 42]. The relevance of this approach has also been tested in preclinical models of PC [34]. Our data suggest that ZA boosts such responses, so that combined use of ZA with TLR-agonists or cytokines should be taken into consideration in future studies. In this context, we documented that ZA significantly enhanced the IL-12-dependent production of IFN-γ by PC-TAM, a classic marker of a TH1 type immune response [18]. Suppression of IL-10 production and up-regulation of IL-12 and TNF-α further substantiated this M1 shift. This synergistic effect was also observed during treatment with polyI:C, a TLR agonist commonly used to mature human dendritic cells. Of note, this effect was present over a wide range of ZA levels, including the high 20–50 μM ones where suppression of MMP-9 was evident and expansion of Tγδ lymphocytes was optimal. An underlying mechanism of this cytokine shift might be through STAT signaling, since a recent study [38] in mice showed that aminobisphosphonates may prolong phosphorylation of STAT1, a key molecule in the polarization of macrophages towards an M1 phenotype [36].
In summary, we propose that ZA selectively suppresses critical genes, such as MMP-9, involved in PC-TAM function, and expands biologically active Tγδ lymphocytes, which target ZA-treated malignant cells. At the same time, ZA exhibits a synergistic effect in combination with immunomodulating agents, such as polyI:C and IL-12, and enhances their efficacy. Overall, our study introduces new aspects of ZA function on PC-TAM, and given the relatively conserved phenotype of TAM, it opens new scenarios for its therapeutic use in other malignancies where these cells have a prominent role, e.g. breast [25] and lung cancer [5, 19]. Such an approach may improve the outcome of current immunotherapeutic strategies, which are hindered by the presence of potent immunosuppressive pathways in the tumor stroma [44].
Acknowledgments
This work was supported in part by grants from the Cancer Society in Stockholm, the Swedish Cancer Society, the EU 6-FP “ALLOSTEM” (LSHB-CT-2004-502219) and U.S. Department of Defense Prostate Cancer Research Program (PC030958).
References
- 1.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 4.Buhtoiarov IN, Sondel PM, Eickhoff JC, Rakhmilevich AL. Macrophages are essential for antitumour effects against weakly immunogenic murine tumours induced by class B CpG-oligodeoxynucleotides. Immunology. 2007;120:412–423. doi: 10.1111/j.1365-2567.2006.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- 7.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Fajac I, Tazi A, Hance AJ, Bouchonnet F, Riquet M, Battesti JP, Soler P. Lymphocytes infiltrating normal human lung and lung carcinomas rarely express gamma delta T cell antigen receptors. Clin Exp Immunol. 1992;87:127–131. doi: 10.1111/j.1365-2249.1992.tb06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B, D’Agosto G, Bordignon V, Trento E, Cognetti F. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69:35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 10.Fiore FCB, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M BM, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110:921–927. doi: 10.1182/blood-2006-09-044321. [DOI] [PubMed] [Google Scholar]
- 11.Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clezardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- 12.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 13.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon IO, Freedman RS. Defective antitumor function of monocyte-derived macrophages from epithelial ovarian cancer patients. Clin Cancer Res. 2006;12:1515–1524. doi: 10.1158/1078-0432.CCR-05-2254. [DOI] [PubMed] [Google Scholar]
- 15.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 16.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 17.Hamdy FC, Fadlon EJ, Cottam D, Lawry J, Thurrell W, Silcocks PB, Anderson JB, Williams JL, Rees RC. Matrix metalloproteinase 9 expression in primary human prostatic adenocarcinoma and benign prostatic hyperplasia. Br J Cancer. 1994;69:177–182. doi: 10.1038/bjc.1994.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/S1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 19.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 20.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 21.Kowalczyk D, Skorupski W, Kwias Z, Nowak J. Flow cytometric analysis of tumour-infiltrating lymphocytes in patients with renal cell carcinoma. Br J Urol. 1997;80:543–547. doi: 10.1046/j.1464-410x.1997.00408.x. [DOI] [PubMed] [Google Scholar]
- 22.Legay F, Gauron S, Deckert F, Gosset G, Pfaar U, Ravera C, Wiegand H, Schran H. Development and validation of a highly sensitive RIA for zoledronic acid, a new potent heterocyclic bisphosphonate, in human serum, plasma and urine. J Pharm Biomed Anal. 2002;30:897–911. doi: 10.1016/S0731-7085(02)00218-2. [DOI] [PubMed] [Google Scholar]
- 23.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 24.Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445–451. doi: 10.3892/ijo.17.3.445. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, Xiang R. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 27.Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol. 2001;166:5508–5514. doi: 10.4049/jimmunol.166.9.5508. [DOI] [PubMed] [Google Scholar]
- 28.Morgan C LP, Jones RM, Bertelli G, Thomas GA, Leonard RC. The in vitro anti-tumour activity of zoledronic acid and docetaxel at clinically achievable concentrations in prostate cancer. Acta Oncol. 2007;46:669–677. doi: 10.1080/02841860600996447. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 31.Rokhlin OW, Griebling TL, Karassina NV, Raines MA, Cohen MB. Human prostate carcinoma cell lines secrete GM-CSF and express GM-CSF-receptor on their cell surface. Anticancer Res. 1996;16:557–563. [PubMed] [Google Scholar]
- 32.Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9:2893–2897. [PubMed] [Google Scholar]
- 33.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 34.Satoh T, Saika T, Ebara S, Kusaka N, Timme TL, Yang G, Wang J, Mouraviev V, Cao G, Fattah el MA, Thompson TC. Macrophages transduced with an adenoviral vector expressing interleukin 12 suppress tumor growth and metastasis in a preclinical metastatic prostate cancer model. Cancer Res. 2003;63:7853–7860. [PubMed] [Google Scholar]
- 35.Savarese DM, Valinski H, Quesenberry P, Savarese T. Expression and function of colony-stimulating factors and their receptors in human prostate carcinoma cell lines. Prostate. 1998;34:80–91. doi: 10.1002/(SICI)1097-0045(19980201)34:2<80::AID-PROS2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 38.Takagi K, Takagi M, Kanangat S, Warrington KJ, Shigemitsu H, Postlethwaite AE. Modulation of TNF-alpha gene expression by IFN-gamma and pamidronate in murine macrophages: regulation by STAT1-dependent pathways. J Immunol. 2005;174:1801–1810. doi: 10.4049/jimmunol.174.4.1801. [DOI] [PubMed] [Google Scholar]
- 39.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer (see comment) J Urol. 1998;160:214–219. doi: 10.1016/S0022-5347(01)63093-3. [DOI] [PubMed] [Google Scholar]
- 40.Valdespino V, Tsagozis P, Pisa P. Current perspectives in the treatment of advanced prostate cancer. Med Oncol. 2007;24:273–286. doi: 10.1007/s12032-007-0017-9. [DOI] [PubMed] [Google Scholar]
- 41.van Ravenswaay Claasen HH, Kluin PM, Fleuren GJ. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992;67:166–174. [PubMed] [Google Scholar]
- 42.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–1362. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 43.Wolf AM, Rumpold H, Tilg H, Gastl G, Gunsilius E, Wolf D. The effect of zoledronic acid on the function and differentiation of myeloid cells (see comment) Haematologica. 2006;91:1165–1171. [PubMed] [Google Scholar]
- 44.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. (see comment) Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]