Abstract
The anti-tumor properties of Toll-like receptor (TLR) 9 agonist CpG oligodeoxynucleotides (ODN) are enhanced by combinations with several cytotoxic chemotherapy regimens. The mechanisms of this added benefit, however, remain unclear. We now report that, similar to the depletion of regulatory T cells (Treg) using anti-CD25, paclitaxel increased the anti-tumor effect of the TLR9 agonist PF-3512676 in a CD8+ T cell-dependent fashion. Paclitaxel treatment decreased Treg numbers in a TLR4-independent fashion, and preferentially affected cycling Treg expressing high levels of FoxP3. The paclitaxel-induced reduction in Treg FoxP3 expression was associated with reduced inhibitory function. Adoptively transferred tumor-antigen specific CD8+ T cells proliferated better in mice treated with paclitaxel and their recruitment in the tumor was increased. However, the systemic frequency of PF-3512676-induced tumor-antigen specific effector CD8+ T cells decreased with paclitaxel, suggesting opposite effects of paclitaxel on the anti-tumor response. Finally, gene expression profiling and studies of tumor-associated immune cells revealed a complex modulation of the PF-3512676-induced immune response by paclitaxel, including a decrease of IL-10 expression and an increase in IL-17-secreting CD4+ T cells. Collectively, these data suggest that paclitaxel combined with PF-3512676 may not only promote a better anti-tumor CD8+ response though increased recruitment in the tumor, possibly through Treg depletion and suppression, but also exerts more complex immune modulatory effects.
Keywords: Regulatory T cell, TLR9, Chemotherapy, Tumor
Introduction
Toll-like receptors (TLR) recognize conserved molecular patterns expressed exclusively by microbes, as well as others which are expressed by host cells but which are not normally accessible to the TLRs. Together with other molecular sensors, TLRs serve as a first line of defense for the immune system, inducing soluble and cellular mediators of innate immunity and initiating key steps of the adaptive immune response [36]. The use of TLR agonists for therapeutic purposes relies on the ability of these compounds to induce, at least partially, some of the immune events that occur during natural infections. In particular, administration of synthetic CpG-containing oligodeoxynucleotide (ODN) TLR9 agonists mimics the stimulation of the immune system by bacterial or viral DNA. CpG ODNs are being developed for cancer vaccines and cancer therapy, owing to their capacity to stimulate potent Th1-like innate and adaptive anti-tumor responses in numerous preclinical models (reviewed in [20]).
In mice with relatively small tumors up to a few millimeters in diameter, CpG ODN monotherapy can be sufficient to induce T cell-mediated tumor regression [4, 25]. CpG ODN efficacy in mouse tumor models has been improved when combined with a wide variety of anti-tumor strategies, including monoclonal antibody therapy, other immune therapies, angiogenesis inhibitors, radiation therapy, surgery, cryotherapy, and chemotherapy (reviewed in [20]).
Although chemotherapy can suppress some immune functions, the immune enhancing effects of various chemotherapeutics have been identified in several animal studies [5, 7, 8, 12–16, 27, 28, 32, 33, 43]. However, the mode of action could differ according to the nature of the chemotherapeutic agent and, importantly, many of these studies were anti-tumor vaccination protocols in which the timing of chemotherapy relative to vaccine administration was crucial. A commonly evoked mechanism was a depletion of regulatory T cells (Treg) [7, 8, 15, 16, 27, 43], but other mechanisms such as an increase in the immunogenicity of tumor cells via calreticulin exposure [33], or a change in T cell homeostasis [13] were also proposed.
The TLR9 agonist PF-3512676 (formerly CPG 7909) is being developed for oncology indications and in particular has been clinically applied together with taxane-based chemotherapy for the treatment of advanced non-small cell lung cancer [20, 30]. The prototypical microtubule-stabilizing taxane paclitaxel has been shown to synergize with some tumor immune therapies [14, 45], but the underlying mechanism(s) remain unclear.
In the present study, we have examined the anti-tumor effects and modulation of the immune response of combinations of paclitaxel and PF-3512676 in mouse tumor models.
Materials and methods
Mice
Female BALB/c and C57BL/6 mice (18–22 g at start of study) were purchased from Charles River Canada (Quebec, QC, Canada). OT-1 and C3H/HeN mice were purchased from The Jackson Laboratory (Bar Harbor, MN) and C3H/HeJ mice from Taconic (Albany, NY, USA). All animals were housed in micro-isolator cages in Coley’s AAALAC accredited facility and all studies were conducted in accordance with the Animal Care Committee of Coley Canada under the guidance of the Canadian Council on Animal Care as well as with the “Principles of laboratory animal care” (NIH publication No. 85–23, revised 1985).
Culture medium
Cell culture medium consisted of RPMI 1640 supplemented with 10% heat-inactivated FBS, penicillin–streptomycin (final concentration of 1,000 U/ml and 1 μg/ml respectively), and 5 × 10−5 M β-mercaptoethanol (all from Life Technologies, Grand Island, NY, USA).
Treg in vitro inhibitory assay
Treg inhibitory function was assessed in vitro by the capacity of purified spleen CD4+CD25+ cells to inhibit naïve T cell proliferation [44]. CD4+CD25+ T cells and CD4CD62L+ naïve T cells were purified using magnetic bead isolation kits (Miltenyi Biotec, Auburn, CA, USA). Antigen-presenting cells (APC) were 20 Gy-irradiated spleen cells. Naïve T cells, 2 × 104, were cultured with 105 APC in 96-well round bottom plates in triplicate with various ratios of CD4+CD25+ cells in 200 μl culture medium containing 5 μg/ml anti-mouse CD3 antibody (BD Bioscience, Mississauga, ON, Canada). Cells were grown for 72 h and 1 μCi/well H3-thymidine (Amersham-GE Healthcare, Piscataway, NJ, USA) added for the last 18 h of culture to assess proliferation.
Tumor-associated dendritic cell induction of naive T cell proliferation in vitro
Dendritic cells from EG.7 tumors (harvested when reaching an average of 1 cm diameter) and from associated tumor-draining lymph nodes (TADC for tumor-associated dendritic cells) were enriched using CD11c+ magnetic beads according to the manufacturer’s protocol (Miltenyi Biotec) and as described previously [11]. Naive OT-I T cells were labeled with 7.5 μmol/L CFSE (Molecular Probes, Eugene, OR, USA) and cultured in 96-well plates at 1 × 106 cells/ml with 2 × 105/ml irradiated TADC, with or without 10 nmol/L OVA SIINFEKL peptide (Sigma Genosys, Oakville, ON, Canada). After 5 days of culture, cell division was analyzed based on CFSE labeling intensity using Modfit software (BD Biosciences).
Tumor models and in vivo treatments
Mouse renal cell carcinoma (Renca) cells were obtained from Dr. Lu Anne Thompson-Snipes (McGill University, Montreal, QC, Canada). BALB/c mice were implanted with 1 × 105 Renca cells under the kidney capsule on day 0 (D0) and treated with weekly subcutaneous (SC) injections of 200 μg PF-3512676 (Coley, Wellesley, MA, USA) delivered as a split injection in four distinct sites (50 μg/site) from D7 to 3 months and/or weekly intraperitoneal (IP) injections of 36 mg/kg paclitaxel (Taxol®, Bristol-Myers Squibb, Montreal, QC, Canada) from D7 to D35. Control groups received injections of endotoxin-free PBS (Life Sciences). For CD4 and CD8 depletion, mice were further injected with 0.5 mg of purified no-azide low endotoxin anti-CD4 (clone GK1.5) or anti-CD8 (clone 53.6–7) antibodies (BD Biosciences) or purified rat IgG (Chemicon, Temecula, CA, USA) as control, on D3, D4, D5 and weekly for 3 months. For Treg depletion, 0.25 mg anti-CD25 antibody (clone PC61.5.3) or control purified rat IgG1 (Cedarlane, Burlington, ON, Canada) was injected IP at D-4. Tumor-free BALB/c mice were also treated with weekly IP injection(s) of 36 mg/kg paclitaxel or 2 mg cyclophosphamide (Sigma) for the analysis of Treg phenotype and function.
C57BL/6 mice were implanted subcutaneously with 1 × 105 E.G7 cells (EL-4 lymphoma cells stably expressing ovalbumin, ATCC, Manassas, VA) and received weekly treatments of 100 μg PF-3512676 injected peri-intratumorally and/or IP injections of 36 mg/kg paclitaxel starting on D7 post-tumor implantation. Tumor volume was monitored thrice weekly. In some experiments, mice were adoptively transferred with 2 × 107 CFSE-labeled OT-I naive T cells as previously described [11]. Cell division, surface phenotype and intra-cellular cytokine production were analyzed in spleen and/or tumor and tumor-draining lymph nodes by flow cytometry 3 days following transfer.
Flow cytometry analyses
All antibodies used in this study, except the FoxP3 antibody (clone FJK-16, e-Bioscience, San Diego, CA, USA), were from BD Biosciences: CD4 (L3T4), CD8α (Ly-3.2), CD25 (7D4), CD62L (MEL-14), CD11c (HL3), Vα2 (B20.1), IFN-γ (XMG1.2), IL-17 (TC11-18H10) and IL-10 (JES5-16E3). Fc receptors were blocked using Fc-Block (BD Biosciences) prior to cell surface staining. Fix-perm buffer (BD Biosciences) was used for intracellular staining, except for FoxP3 where the fixation and permeabilization buffers were from e-Bioscience. Cycling cells in vivo were analyzed using the BrdU flow kit (BD Bioscience) with BrdU being administered in drinking water for 3 days before drug treatment, following the manufacturer’s instructions. For analysis of tumor-infiltrating lymphocytes, tumors were minced and then incubated for 90 min at 37°C with 250 U/ml collagenase IV (InVitrogen, Burlington, Canada) and 50 U/ml DNase (Sigma) in DMEM (Life Sciences), strained on a 70 μm filter (BD Biosciences) to remove debris, washed with RPMI 1640, then resuspended in 40% Percoll (Sigma), and layered over 10 mL 80% Percoll and spun at 600 g for 15 min. Cells at the interphase were washed and resuspended in culture medium and incubated at 37°C for 4 h with 10 ng/ml PMA, 1 mg/ml ionomycin (both from Sigma), and 10 mg/ml Golgi Plug (BD Biosciences). All analyses were performed on an FC500 flow cytometer using Expo32 software (Beckman-Coulter). Treg were defined as CD4+CD25+FoxP3+ lymphocytes unless otherwise indicated.
In vivo CTL assay
Spleen cells from C57BL/6 mice were pulsed with 10 nmol/l OVA class I peptide and labeled with CFSE at a final concentration of 1 μmol/l; un-pulsed spleen cells were labeled at a final concentration of 10 μmol/l. The two cell populations were mixed at a 1:1 ratio and 2 × 107 total cells were injected intravenously (IV) into tumor-bearing or naïve mice. The percentage of antigen-specific lysis was determined 16 h following target cell injection as described [11].
Real-time PCR analysis of gene expression
Tumors and axillary lymph nodes were collected 24 h after the second treatment with PF-3512676 and/or paclitaxel and stored in RNALater stabilization reagent (Qiagen, Mississauga, ON, Canada). Tissue samples were homogenized and RNA was isolated from tumors using the Qiagen RNA midi kit according to the manufacturer’s instructions, with an additional step of DNase treatment on column (RNase free DNase Set, Qiagen). RNA was isolated from lymph nodes using the Qiagen RNA mini kit. One μg from each sample was tested for RNA integrity, the presence of inhibitors of reverse transcription and PCR amplification, and for genomic DNA contamination, using the RT2 RNA QC PCR Array (SuperArray Bioscience, Frederick, MD). All PCR experiments were conducted with an Mx3005P Quantitative PCR System (Stratagene, La Jolla, CA, USA). For PCR array experiments, an RT2 Profiler Custom PCR Array was used to simultaneously examine the mRNA levels of various genes, including three housekeeping genes (SuperArray Bioscience). cDNA was synthesized from 1 μg of purified RNA using the RT2 First Strand Kit (SuperArray). Quantitative PCR amplification was carried out in a total reaction volume of 25 μl containing 12.5 μl of 2× SuperArray RT2 qPCR master mix, 1 μl of diluted cDNA, and water. PCR reactions were cycled 40 times after initial denaturation (10 min at 95°C) under the following parameters: 15 s at 95°C and 1 min at 60°C. The raw Ct values were collected by the instrument and converted to relative expression values by the ΔΔCt method using MxPro QPCR software (Stratagene) for data analysis. In this analysis, the average Ct value for the housekeeping genes is subtracted from the Ct value of the target gene. The ΔCt value for the treated sample is then subtracted from the ΔCt value of the untreated (PBS) control sample (ΔΔCt). The fold difference in the expression of each gene between the treated and the control sample is then calculated as: 2−ΔΔCt.
Statistical analyses
The appropriate statistical tests are indicated in the legends of figures or results and were all performed using Graphpad software (San Diego, CA, USA).
Results
Anti-tumor effect of the TLR9 agonist PF-3512676 and paclitaxel
In the mouse metastatic Renca model, we observed that both SC administration of PF-3512676 and IP administration of paclitaxel had a significant anti-tumor effect over control (P = 0.0007 and P < 0.0001, respectively, by log-rank analysis) (Fig. 1a). In addition, the combination of PF-3512676 and paclitaxel showed a better anti-tumor effect than either treatment alone (P < 0.0001) (Fig. 1a).
Fig. 1.
Anti-tumor effect of paclitaxel and PF-3512676 in a mouse orthotopic renal cell carcinoma model. BALB/c were implanted under the kidney capsule with Renca tumor cells at D0 and treated with weekly IP injections of paclitaxel and SC injections of PF-3512676 or control, with or without various antibody treatments as described in "Materials and methods"; survival was monitored. Symbols representing each treatment group are indicated for each panel. a Paclitaxel enhanced the anti-tumor effect of PF-3512676 (P < 0.0001 by logrank analysis for combination therapy compared to either treatment alone). b Anti-CD25 antibody enhanced the anti-tumor effect of PF-3512676 (P = 0.015). c Anti-CD4 and anti-CD8 antibodies alone had no effect on survival. d Paclitaxel anti-tumor effect was CD8-dependent (P = 0015); anti-CD4 effect non-significant. e PF-3512676 anti-tumor effect was increased with anti-CD4 (P = 0.0027), anti-CD8 effect non-significant. f Paclitaxel plus PF-3512676 anti-tumor effect was CD8-dependent (P = 0.0044); anti-CD4 effect non-significant. a Results are representative of more than three independent experiments (n = 10 per group). b–f Results are representative of two independent experiments (n = 5 per group)
The role of CD4 or CD8 T cells in the metastatic Renca model were then analyzed using specific antibody treatments. Either CD4 or CD8 depletion had little effect on mice treated with PBS (Fig. 1c). However, CD8 depletion partly or completely abrogated the anti-tumor effects of PF-3512676, paclitaxel or PF-3512676 + paclitaxel (Fig. 1d–f). Conversely, CD4 depletion improved the anti-tumor effects of PF-3512676, paclitaxel or PF-3512676 + paclitaxel to some degree (Fig. 1d–f). Since CD4+ cells encompass both conventional T cells and regulatory T cells (Treg), we also administered to mice an anti-CD25 antibody which has been shown to more specifically affect the CD4+CD25+ Treg population [19, 34]. The anti-tumor effects of PF-3512676 were improved with anti-CD25 antibody treatment, while the antibody alone had a modest effect which did not reach statistical significance (P = 0.09) (Fig. 1b). Collectively, these data indicate that CD8+ cells, presumably CD8+ T cells, play an important positive role in the anti-tumor effect of PF-3512676, paclitaxel, or paclitaxel + PF-3512676, while CD4+ cells play a negative role. Results obtained with anti-CD25 antibody would further support a role for CD4+ Treg in negatively modulating the effects of PF-3512676.
Paclitaxel affects both Treg numbers and function
The number of CD4+CD25+FoxP3+ Treg cells in the spleen of normal and tumor-bearing mice was analyzed 48 h after a single injection of paclitaxel. Treg numbers were significantly reduced in the spleen of normal mice, the average decrease being around 20% (Fig. 2a). The 48 h time-point was determined as optimal when performing kinetic studies of Treg numbers in the spleen following paclitaxel administration, from 24 h to 1 week (data not shown). However, the number of conventional T cells was also reduced in similar proportions (Fig. 2b). Since paclitaxel has been shown to possibly trigger TLR4 [6], and TLR4 is possibly involved in Treg biology [35], the effect of paclitaxel in C3H/HeJ mice deficient in TLR4 signaling [37] was analyzed. In C3H/HeJ mice, Treg decrease following paclitaxel injection was observed, ruling out TLR4-dependence for the effect of paclitaxel on Treg numbers in our model (Fig. 2c). A similar decrease was also observed in TLR4-competent C3H/HeN mice, (data not shown). In EG.7 tumor-bearing mice, a significant decrease of Treg numbers in the spleen 48 h following the last of 3 weekly injections of paclitaxel was also observed (Fig. 2d). Importantly, 3 weekly SC injections of PF-3512676 in this tumor model had no effect on spleen Treg numbers, and mice injected with both paclitaxel and PF-3512676 had reductions in Treg number similar to those seen in mice treated with paclitaxel alone (Fig. 2d). When treating mice with cyclophosphamide (CTX), another chemotherapeutic drug reported to decrease Treg numbers, it was shown that the cycling fraction of Treg was preferentially affected [15]. We confirmed this observation by showing a decrease in the proportion of BrdU+ cells within CD4+CD25+ spleen cells following in vivo BrdU administration and CTX treatment, and found that paclitaxel resulted in a similar pattern (Fig. 2e). Interestingly, both chemotherapies also affected cycling conventional T cells, but the proportion of cycling cells within spleen Treg at the steady state was higher than within conventional T cells, as previously reported [15].
Fig. 2.
Paclitaxel decreased Treg numbers in normal and tumor-bearing mice in a TLR4-dependent fashion and preferentially affected cycling cells. CD4+CD25+ FoxP3+ Treg (a) as well as CD4+CD25 T cell (b) numbers were analyzed in spleens from BALB/c mice 48 h after paclitaxel (black bars) or PBS (white bars) treatment. Results combined five different independent experiments (n = 5 per group). c CD4+CD25+ FoxP3+ Treg numbers were analyzed in spleens from TLR4-deficient C3H/HeJ mice 48 h after paclitaxel (black bars) or PBS (white bars) treatment. Results are representative of three independent experiments (n = 5 per group). d CD4+CD25+FoxP3+ Treg numbers were analyzed in spleens from EG.7 tumor-bearing C57BL/6 mice 48 h after 3 weekly cycles of PBS (white bars), PF-3512676 (black bars) paclitaxel (gray bars) or both treatments (hatched bars). Results are representative of three independent experiments (n = 6 per group). e The percentage of cycling BrdU + cells within Treg (defined here as CD4+CD25+ cells, black bars) and CD4+CD25 cells (gray bars) was determined in BALB/c mice previously treated for 3 days with BrdU 48 h following paclitaxel or cyclophosphamide (CTX) treatment. Results are representative of two independent experiments (n = 5 per group). Statistical analyses: Mann–Whitney test
Although significant, the effect of paclitaxel on Treg numbers remained modest. We therefore analyzed features of the remaining population that could account for a Treg functional defect greater than that which could be deduced based on cell numbers only. We first observed that the mean fluorescence intensity of FoxP3 staining within the remaining CD4+CD25+FoxP3+ cells after paclitaxel treatment was lower (Fig. 3a), strongly suggesting a lower expression of FoxP3. Furthermore, the inhibitory capacity of the remaining CD4+CD25+ cells, tested for in a classical in vitro naïve T cell proliferation assay, was decreased following paclitaxel treatment when comparing to equivalent numbers of CD4+CD25+ cells from naïve animals (Fig. 3b).
Fig. 3.
Treg from paclitaxel-treated mice have lower FoxP3 expression and inhibitory activity. a The mean fluorescence intensity of FoxP3 expression was analyzed among CD4+CD25+FoxP3+ spleen and lymph node cells 48 h after PBS (open symbols) or paclitaxel (closed symbols) treatment. Results are expressed as individual data and mean and are representative of three independent experiments. Statistical analysis: Mann–Whitney test. b In vitro inhibition of naïve T cell proliferation was assessed using purified Treg (CD4+CD25+) from the spleen of BALB/c mice 48 h after three weekly cycles of paclitaxel (black bars) or PBS (gray bars) and CD4+CD62L naïve T cells from untreated mice. White bars: naïve T cells alone. Results are representative of three independent experiments
Taken together, this data shows not only that paclitaxel decreases Treg numbers in normal and tumor-bearing mice in a TLR4-independent fashion, but also that remaining Treg are impaired in their inhibitory functions.
Effect of paclitaxel on CD8+ tumor antigen-specific responses: priming phase
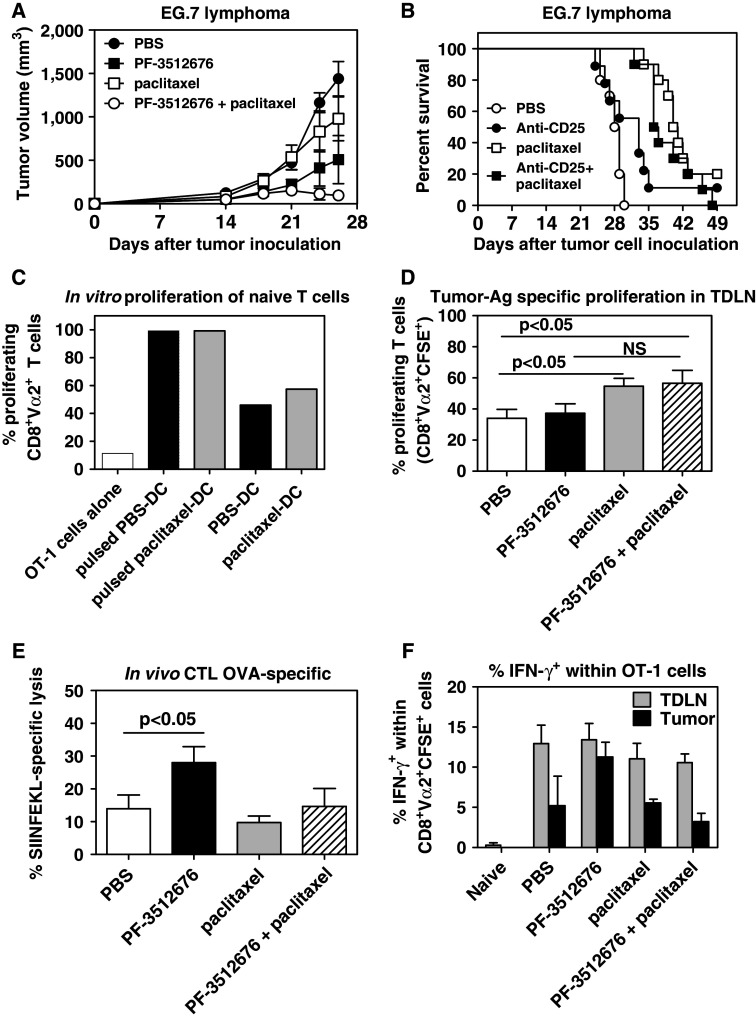
Since we showed that CD8+ cells played an important role in the anti-tumor effects of the combination of paclitaxel and PF-3512676, we sought to analyze how paclitaxel could affect antigen-specific CD8+ T cell responses, by using ovalbumin (OVA) as a model tumor-associated antigen (TAA) and the EG.7 OVA-expressing lymphoma system. A similar additive effect between PF-3512676 and paclitaxel was observed in the EG.7 model, where tumor-bearing mice were treated with peri/intra-tumoral injections of PF-3512676 and IP paclitaxel (Fig. 4a). In this model, we did not observe any additive effect between paclitaxel and anti-CD25 depletion (Fig. 4b), while the additive effect between anti-CD25 and CpG ODN was reported elsewhere [11].
Fig. 4.
Paclitaxel increased the priming of tumor-antigen specific CD8 T cells but decreased the systemic frequency of tumor-antigen specific effector CD8 T cells. a Effect of weekly administration (D7, D14, D21) of PBS (closed circles), IP paclitaxel (open squares), peri-tumoral PF-3512676 (closed squares) or both (open circles) on tumor growth in the EG.7 tumor model. Results are representative of three independent experiments. b Effect of weekly administration (D7, D14, D21) of PBS (open circles), IP anti-CD25 (closed circles), IP paclitaxel (open squares) or both (closed squares) on tumor survival in the EG.7 tumor model. P < 0.001 for paclitaxel or paclitaxel plus anti-CD25 versus PBS; anti-CD25 versus PBS NS. c In vitro proliferation of OT-1 cells over 72 h, measured by CFSE staining dilution, in the presence of tumor-associated dendritic cells purified from mice treated with IP paclitaxel (gray bars) or PBS (black bars) 48 h before, and with (pulsed) or without additional OVA SIINFEKL peptide. White bars: OT-1 cells alone. Results are representative of two independent experiments. d Proliferation of adoptively transferred OT-1 cells, measured by CFSE staining dilution, in tumor draining lymph nodes from EG.7 tumor-bearing mice 3 days after transfer and 5 days after treatment with PBS (white bars), peritumoral PF-3512676 (black bars), IP paclitaxel (gray bars) or both (hatched bars). Results are cumulative of three independent experiments (n = 4–5 per experiment). e OVA SIINFEKL-specific in vivo CTL activity assessed in spleen as described in "Materials and methods" in EG.7 tumor-bearing mice 2 days after treatment with PBS (white bars), peritumoral PF-3512676 (black bars), IP paclitaxel (gray bars) or both (hatched bars). Results are representative of 3 independent experiments (n = 5 per experiment). f Intra-cellular IFN-γ expression within adoptively transferred OT-1 cells in EG.7 tumor draining lymph nodes (TDLN, gray bars) or tumors (black bars) 3 days after transfer and 5 days after treatment with PBS, peritumoral PF-3512676, IP paclitaxel or both. Expression in OT-1 cells transferred into naïve animals is also shown. Results are representative of two independent experiments (n = 5 per experiment). Statistical analyses: Mann–Whitney test
The dose of antigen available for the priming of the immune response to tumors is thought to be a limiting factor [10]. Tumor-associated dendritic cells (TADC) from EG.7 tumors (around 1 cm in diameter) and their draining lymph nodes were isolated and co-cultured with naïve OVA-specific OT-1 CD8+ T cells, 48 h following paclitaxel or PBS treatment. The stimulating capacity of TADC from PBS-treated mice was sufficient to induce the proliferation of OT-1 cells in vitro, albeit to a much lesser degree than the same TADC further pulsed with the OVA SIINFEKL peptide (Fig. 4c), confirming our previous observations that tumor antigen dose is also a limiting factor in this model [11]. However, no significantly higher proliferation was observed when TADC were isolated from paclitaxel-treated mice.
The in vivo proliferation of OT-1 cells adoptively transferred into EG.7 tumor-bearing mice treated with paclitaxel, PF-3512676, or both was then analyzed in tumor-draining lymph nodes. OT-1 cells were transferred 3 days after treatment, since paclitaxel could affect the survival of the OT-1 cells through direct killing of proliferating cells if administered simultaneously. Three days following adoptive transfer, a significant increase in the proportion of proliferating OT-1 cells in mice treated with paclitaxel or paclitaxel plus PF-3512676 compared to mice treated with PBS was observed; there was also an increase in the paclitaxel plus PF-3512676 group compared to PF-3512676 alone, which did not reach statistical significance (Fig. 4d). By comparing these results with the in vitro data obtained with TADC, we hypothesize that paclitaxel treatment may enhance the priming of tumor-antigen specific CD8+ T cells in vivo, possibly by relieving the inhibitory effect of Treg rather than by increasing the tumor antigen dose available for priming.
Effect of paclitaxel on CD8 tumor antigen-specific responses: effector phase
The development of effector anti-tumor CD8+ T cells in mice bearing EG.7 tumors and treated with PF-3512676, paclitaxel or both was next examined using an in vivo CTL assay specific for the SIINFEKL peptide. As we previously reported [11], untreated mice showed a basal level of specific CTL activity of around 15% in the spleen, likely owing to the high immunogenicity of the EG.7 tumor (Fig. 4e). Treatment with PF-3512676 alone significantly increased tumor antigen-specific CTL activity, whereas mice treated with paclitaxel alone or paclitaxel + PF-3512676 had a CTL activity similar to that of control mice (Fig. 4f). Similar results were found by measuring the frequency of IFN-γ-secreting SIINFEKL-specific CD8+ T cells by Elispot (data not shown).
We also analyzed, by intra-cellular staining, IFN-γ production by transferred OT-1 cells within the tumor and the draining lymph node, following in vitro re-stimulation with PMA-ionomycin. We found that OT-1 cells within the draining lymph nodes had all been primed to become effector cells to a similar degree since 10–15% of them expressed IFN-γ while OT-1 cells transferred into naïve recipients did not (Fig. 4f). In tumors from PBS-treated animals, transferred cells also expressed IFN-γ but less than in the lymph nodes (Fig. 4f). IFN-γ expression was increased by PF-3512676 treatment alone, but not by paclitaxel or the combination therapy (Fig. 4f).
Since the efficiency of tumor-specific CD8+ T cells is ultimately related to their migration into the tumor, we then investigated the migration of adoptively transferred OT-1 cells into EG.7 tumors in vivo. In mice treated with PBS, the level of OT-1 cells recruited into the tumor was invariably low (Fig. 5a). In mice treated with PF-3512676, higher levels of OT-1 cells were recruited within the tumor of a few animals (Fig. 5a). Surprizingly, the recruitment of OT-1 cells in tumors from mice treated with paclitaxel alone or with the combination therapy was consistently stronger (Fig. 5a).
Fig. 5.

Paclitaxel induced higher recruitment into tumor and a higher proportion of CD62lo cells in spleen for tumor antigen-specific CD8+ T cells. a Percentage of OT-1 cells recovered from tumors 3 days after adoptive transfer and 5 days after treatment with PBS (closed circles), peritumoral PF-3512676 (open circles), IP paclitaxel (closed diamonds) or both (open diamonds). Results are expressed as individual data and mean and are cumulative of three independent experiments. b Percentage of CD62lo cells within adoptively transferred OT-1 cells in spleen from EG.7 tumor-bearing mice 3 days after transfer and 5 days after treatment with PBS (white bars), peritumoral PF-3512676 (black bars), IP paclitaxel (gray bars) or both (hatched bars). Results are representative of three independent experiments (n = 5 per experiment). c Percentage of CD62lo cells within normal mouse spleen CD8+ T cells 48 h after treatment with PBS (white bars) or anti-CD25 antibody (black bars). Results are representative of two independent experiments (n = 5 per experiment). Statistical analyses: Mann–Whitney test
The expression of surface molecules known to be important for recruitment into effector sites was analyzed in transferred isolated OT-1 CD8+ T cells from mice undergoing the different treatments. No significant changes in the expression of CXCR3 were observed (data not shown) and, as expected, OT-1 cells isolated from lymph nodes or the tumor were all CD62Llow/− (data not shown). However, the proportion of CD62Llow/− among transferred OT-1 cells recovered from spleen was higher in mice treated with paclitaxel, with or without PF-3512676 relative to PBS (Fig. 5b). Indeed, this was also true for resident CD8+ T cells (data not shown). Interestingly, CD25 antibody treatment modified the profile of CD62L expression in spleen CD8+ T cells in a similar fashion (Fig. 5c).
Taken together, these results support the role of the TLR9 agonist PF-3512676 as a potent enhancer of antigen-specific CD8+ T effector cells but, on the other hand, suggest that paclitaxel has a detrimental effect on the expansion and/or functional differentiation of tumor-specific CD8+ T cells induced by PF-3512676. However, better recruitment of CD8+ effector T cells within the tumor site may compensate for this immunosuppressive aspect.
Gene expression profiles induced by paclitaxel and/or PF-3512676 in tumor-bearing mice
Given the presumably wide range of effects of paclitaxel on anti-tumor immune responses, we performed gene expression analysis for 49 genes known to be positively or negatively involved in TLR9-mediated immune responses and/or tumor control (Table 1), either in the tumor or tumor-draining lymph node, in mice treated with paclitaxel, PF-3512676 or both. Since one injection may not be sufficient to substantially modulate the anti-tumor response, gene analysis was performed 24 h after the second of two injections. Only genes with a threefold or greater increase or decrease in expression compared to their relative control are presented (Fig. 6). When compared to PBS control, PF-3512676 induced in tumors and/or draining lymph nodes a broad array of genes related to type I or type II interferon and Th1 responses (such as IFN-β, IFN-γ, Mx, 2′,5′ OAS, CXCL9, CXCL10, CXL11, IL-12, IL-15) and cytotoxic killing (TRAIL, Granzyme B). Interestingly, IL-10 was also one of the most strongly induced genes in both tumors and lymph nodes, and PF-3512676 induced the Th17-specific transcription factor RORγt in lymph nodes. Very few genes were down-regulated by PF-3512676 and the role of these down-regulated genes is unclear. In contrast, paclitaxel treatment induced little changes in gene expression compared with PBS with the noticeable exception of the up-regulation of RORγt and IL-17 gene expression. Interestingly, when compared to PF-3512676 alone, the combination PF-3512676 plus paclitaxel resulted in little gene up-regulation in the tumor (CXCL11 and IL-1RA), and although the pattern of interferons/Th1 gene up-regulation was present in the lymph nodes, it was not as pronounced as for PF-3512676 alone. Strikingly, the up-regulation of both RORγt and IL-17 was the most pronounced change in gene expression. With PF-3512676 plus paclitaxel, we observed in the tumor that not only IFN-γ, perforin and granzyme B expression but also IL-10 expression was markedly decreased compared to PF-3512676 alone. In lymph nodes, much stronger expression of not only RORγt and IL-17 but also of indoleamine 2,3-dioxygenase (IDO) was observed with the combination treatment.
Table 1.
List of genes analyzed in tumor and tumor-draining lymph nodes
| Interferon pathways | Inflammation/costimulation | Th1/Th2/Th17 | Migration/angiogenesis | Killing/cell death | Regulation |
|---|---|---|---|---|---|
| TLR9 | IL-1α | Tbet | CCL19 | Perforin | IL-2Rα |
| IFN-α-Rα | IL-1β | IL-27 | CXCL9 | Granzyme B | IDO |
| IFN-α-Rβ | IL-1RA | RORγt | CXCL10 | Fas | Foxp3 |
| IFN-α4 | IL-2 | IL-4 | CXCL11 | FasL | TGFβ |
| IFN-α11 | IL-6 | IFN-γ | CXCL12 | Caspase-1 | IL-10 |
| IFN-β | IL-12p40 | IL-17 | CCR7 | Caspase-3 | |
| 2′,5′-OAS | IL-15 | IL-12p35 | ICAM-1 | Lymphotoxin | |
| Mx | IL-18 | VCAM-1 | TNF | ||
| IRF-3 | CD80 | VEGF | TRAIL | ||
| CD86 |
Fig. 6.

Modification of gene expression induced by paclitaxel and/or PF-3512676 in tumor bearing mice. Gene expression analyses were performed by real-time RT-PCR in tumors (left panels) and tumor-draining lymph nodes (right panels) 24 h after a second cycle of treatment with IP paclitaxel and peritumoral PF-3512676 and results calculated as fold expression relative to the indicated controls as outlined in "Materials and methods". Results combined nine different tumors from three independent experiments and six lymph nodes from two independent experiments. From all genes analyzed (Table 1), only those expressed (mean) at least threefold higher (black bars) or threefold lower (gray bars) compared to their respective controls are represented. a Gene expression in PF-3512676-treated mice compared to PBS-treated mice. b Gene expression in paclitaxel-treated mice compared to PBS-treated mice. c Gene expression in paclitaxel plus PF-3512676-treated mice compared to PBS-treated mice. d Gene expression in paclitaxel plus PF-3512676-treated mice compared to PF-3512676-treated mice
The pattern of IFN-γ gene expression within the whole tumor (Fig. 6) was consistent with the IFN-γ expression observed using intra-cellular staining (Fig. 4e). Interestingly, intra-cellular staining also showed that tumor-infiltrating DCs expressed higher IL-10 in mice treated with PF-3512676 compared to controls (P = 0.008, Mann–Whitney test), and this was reduced in the combination treatment with paclitaxel (P = 0.05 compared to PF-3512676 alone) (Fig. 7a). Given the prominence of RORγt and IL-17 gene up-regulation, we also analyzed IL-17 protein expression by intra-cellular staining in tumors and observed a marked increase in IL-17 positive CD4 T cells for the paclitaxel plus PF-3512676 combination, confirming the gene expression data (Fig. 7b). No difference was observed in lymph nodes (data not shown), perhaps due to a non-optimal sampling time.
Fig. 7.
Modulation of IL-17 and IL-10 expression in tumors by paclitaxel and PF-3512676 treatment. Intra-cellular expression of IL-10 within CD11c+ DC (a) and IL-17 within CD4+ T cells (b) was analyzed in EG.7 tumors 24 h after a second cycle of treatment with either PBS (closed circles), peritumoral PF-3512676 (open circles), IP paclitaxel (closed diamonds), or both (open diamonds) as indicated in “Materials and methods”. Results are expressed as individual data and mean and are representative of two independent experiments
Collectively, the gene expression data, supported by protein data for IFN-γ, IL-10 and IL-17, confirm that the combination of PF-3512676 plus paclitaxel not only dampens some of the immunostimulatory properties of PF-3512676, but also profoundly affects the tumor milieu.
Discussion
In the present study we demonstrate that paclitaxel treatment increases the anti-tumor activity of the TLR9 agonist PF-3512676 and that this anti-tumor activity requires CD8+ T cells. The effective destruction of tumors by CD8+ T cells relies on a series of events from the priming of a few naïve CD8+ T cells efficiently by antigen-loaded and activated antigen-presenting cells (APC), presumably in the tumor draining-lymph node, to the proliferation of the activated naïve T cells, their differentiation into effector cells and their migration into the tumor. In principle, all these different checkpoints could be modulated to increase or decrease the CD8+ T cell-mediated anti-tumor response. TLR9 agonists including PF-3512676 have been shown to be potent activators of CD8+ T cell responses—in particular when administered with tumor antigens in a vaccine setting—in mice [9, 24, 41, 52] and humans [2, 3, 42, 46]. This is likely due to their capacity to induce the functional maturation of dendritic cells through the up-regulation of co-stimulation molecules for T lymphocytes, the secretion of cytokines important for the initiation of adaptive responses such as type I interferons and IL-12, and the modulation of the chemokine receptor repertoire allowing migration into lymphoid organs (reviewed in [1, 29]). In addition, as demonstrated here and elsewhere [21, 49, 50], TLR9 agonists administered locally can trigger the expression of chemokines such as the CXCR3 ligands CXCL9, CXCL10 and CXCL11, which favor the recruitment of CXCR3-expressing activated T cells [38], provided those T cells express the right pattern of addressins to allow for their migration into the tumor.
One of the first limiting factors in the onset of anti-tumor immunity may be the dose of antigen available at a given time for priming [31, 54]. Indeed, artificial release of tumor antigens by cryotherapy can increase the tumor antigen load in dendritic cells isolated from tumor-draining lymph nodes and improve the efficacy of TLR9 agonists [10]. However, although paclitaxel is a cytotoxic drug, we did not find any increase in the intrinsic capacity of tumor-associated DC from paclitaxel-treated mice to prime naïve T cells. This may be specific to the EG.7 model, in which the surrogate tumor antigen ovalbumin is abundantly expressed and highly immunogenic.
On the other hand, several natural or tumor-induced counter-inhibitory mechanisms exist that could limit the efficiency of TLR9 stimulation for the generation of an efficient tumor-specific CD8 T cell response. Secreted tumor factors such as IL-10, prostaglandins, VEGF or TGF-β are known as inhibitors of anti-tumor immune responses (reviewed in [47]) and, more recently, Treg have appeared as major regulators of anti-tumor immune responses (reviewed in [53, 55]). Because treatment with anti-CD25 led to an increased efficiency of PF-3512676, as we showed before with another TLR9 agonist [11], and since chemotherapies such as cyclophosphamide can decrease Treg numbers and function, we investigated the effect of paclitaxel on Treg cells. We found that, similar to what was reported for cyclophosphamide, paclitaxel was able to reduce the numbers of Treg, in particular by affecting their cycling fraction [15]. Interestingly, we further demonstrated that the remaining Treg had lower FoxP3 expression together with lower inhibitory function in vitro. Previous studies have demonstrated that small changes in the level of FoxP3 expression could dramatically affect the Treg function [51]. Thus, even though the decrease in Treg numbers was modest, the paclitaxel-mediated suppression of FoxP3 expression could significantly enhance the immune response, as we observed.
Treg have been shown to affect many check-points of the adaptive immune response (reviewed in [40]). The fact that we observed significantly more proliferation of antigen-specific naïve T cells in mice treated with paclitaxel, together with a similar observation in mice treated with anti-CD25 [11], would suggest that paclitaxel may prevent the inhibition of T cell proliferation by Treg. The lack of additive effect between paclitaxel and anti-CD25 in the same model also supports the hypothesis that the modes of action of the two agents are redundant. However, this did not translate into increased frequency of tumor-specific CD8 T effector cells in the periphery. Indeed, this proportion was decreased in mice treated with paclitaxel + PF-3512676 compared to PF-3512676 alone. Interestingly, it was reported that CpG ODN could enhance the CD8 T cell dependent efficacy of a tumor adenovirus vaccine, while the frequency of CD8+ effector cells in the periphery was decreased, and that this effect was independent of the tumor [18, 26]. Hence, anti-tumor activity cannot be simply correlated to the systemic frequency of effector CD8 T cells. We do not know why this frequency was reduced following paclitaxel treatment, but hypothesize that it may be related to the overall cytotoxic effect of paclitaxel on cycling cells. In this respect, the identification of specific doses and regimens of chemotherapeutic agents that preferentially affect Treg with less effect on anti-tumor immune cells may be an important step in the clinical development of such approaches [17].
The negative impact of paclitaxel on the generation of antigen-specific effector CD8 T cells in the spleen might be mitigated by other effects that still make its overall contribution to the anti-tumor response positive. First, we observed that the recruitment of a cohort of antigen-specific CD8 T cells within the tumor itself was higher in mice treated with paclitaxel. This correlated with a lower expression of CD62L in spleen CD8 T cells, an observation we also made in mice treated with anti-CD25. A role of Treg in preventing the migration of effector T cells into tissues was demonstrated in another model [39]. Although much remains to be done to demonstrate whether paclitaxel affects T cell recruitment within the tumor directly through Treg inhibition, it is a tempting hypothesis. Second, we observed that one of the genes whose expression was most profoundly affected in mice treated with PF-3512676 plus paclitaxel was IL-10. We have previously shown that neutralization of IL-10 could dramatically increase the anti-tumor effect of TLR9 agonists [11, 48]. A role for Treg in modulating IL-10 expression in APC and dampening anti-tumor immunity through B7-H4 has been shown elsewhere [23]. Hence, several observations point to possible multiple modes of action of paclitaxel via an effect on Treg. However, it is likely that the immune effects of paclitaxel go beyond Treg, as exemplified by the fact that we observed a slightly enhanced anti-tumor effect when paclitaxel was given together with anti-CD4 antibodies (although this was not statistically significant). Indeed, we observed, unexpectedly, a very strong increase in RORγT and IL-17 expression with the paclitaxel + PF-3512676 combination. The role of IL-17-producing cells in tumors is largely unexplored, and it is not clear whether they could have beneficial or detrimental effects [22]. Our observations could provide a model to study the role of Th17 cells or IL-17 in the tumor setting.
In conclusion, we reveal that paclitaxel can profoundly affect the immunological milieu, in particular through its action on Treg, and enhance the anti-tumor effect of the TLR9 PF-3512676 through several mechanisms. This confirms the potential therapeutic value of the combination of chemotherapies with TLR9 agonists, although it also suggests that the full spectrum of the effects of non-targeted chemotherapies may be difficult to appraise in the clinic.
Acknowledgments
The authors thank Dr. Thompson-Snipes for her kind gift of the Renca cells.
Abbreviations
- APC
Antigen presenting cell
- BrdU
Bromodeoxyuridine
- CTX
Cyclophosphamide
- CTL
Cytotoxic T lymphocyte
- IP
Intraperitoneal
- IV
Intravenous
- ODN
Oligodeoxynucleotide
- OVA
Ovalbumin
- PCR
Polymerase chain reaction
- SC
Subcutaneous
- TADC
Tumor-associated dendritic cell
- TLR
Toll-like receptor
- Treg
Regulatory T cell
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC, Romero P, Leyvraz S, Rufer N, Speiser DE. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 3.Appay V, Speiser DE, Rufer N, Reynard S, Barbey C, Cerottini JC, Leyvraz S, Pinilla C, Romero P. Decreased specific CD8+ T cell cross-reactivity of antigen recognition following vaccination with Melan-A peptide. Eur J Immunol. 2006;36:1805–1814. doi: 10.1002/eji.200535805. [DOI] [PubMed] [Google Scholar]
- 4.Baines J, Celis E. Immune-mediated tumor regression induced by CpG-containing oligodeoxynucleotides. Clin Cancer Res. 2003;9:2693–2700. [PubMed] [Google Scholar]
- 5.Bourquin C, Schreiber S, Beck S, Hartmann G, Endres S. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer. 2006;118:2790–2795. doi: 10.1002/ijc.21681. [DOI] [PubMed] [Google Scholar]
- 6.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::AID-IMMU2448>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y, Wang LX, Yang G, Ross HJ, Urba WJ, Prell R, Jooss K, Xiong S, Hu HM. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J Immunother. 2006;29:367–380. doi: 10.1097/01.cji.0000199198.43587.ba. [DOI] [PubMed] [Google Scholar]
- 8.Correale P, Cusi MG, Tsang KY, Del Vecchio MT, Marsili S, Placa ML, Intrivici C, Aquino A, Micheli L, Nencini C, Ferrari F, Giorgi G, Bonmassar E, Francini G. Chemo-immunotherapy of metastatic colorectal carcinoma with gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte macrophage colony-stimulating factor and interleukin-2 induces strong immunologic and antitumor activity in metastatic colon cancer patients. J Clin Oncol. 2005;23:8950–8958. doi: 10.1200/JCO.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 9.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- 10.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, Ruers TJ, Adema GJ. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 11.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65:8479–8486. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 12.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 14.Eralp Y, Wang X, Wang JP, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neu in a murine mammary carcinoma model. Breast Cancer Res. 2004;6:R275–R283. doi: 10.1186/bcr787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 17.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karan D, Krieg AM, Lubaroff DM. Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. Int J Cancer. 2007;121:1520–1528. doi: 10.1002/ijc.22873. [DOI] [PubMed] [Google Scholar]
- 19.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 20.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 23.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, Zou W. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Li WM, Dragowska WH, Bally MB, Schutze-Redelmeier MP. Effective induction of CD8 + T-cell response using CpG oligodeoxynucleotides and HER-2/neu-derived peptide co-encapsulated in liposomes. Vaccine. 2003;21:3319–3329. doi: 10.1016/S0264-410X(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 25.Lonsdorf AS, Kuekrek H, Stern BV, Boehm BO, Lehmann PV, Tary-Lehmann M. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J Immunol. 2003;171:3941–3946. doi: 10.4049/jimmunol.171.8.3941. [DOI] [PubMed] [Google Scholar]
- 26.Lubaroff DM, Karan D, Andrews MP, Acosta A, Abouassaly C, Sharma M, Krieg AM. Decreased cytotoxic T cell activity generated by co-administration of PSA vaccine and CpG ODN is associated with increased tumor protection in a mouse model of prostate cancer. Vaccine. 2006;24:6155–6162. doi: 10.1016/j.vaccine.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 28.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 29.McCluskie MJ, Krieg AM. Enhancement of infectious disease vaccines through TLR9-dependent recognition of CpG DNA. Curr Top Microbiol Immunol. 2006;311:155–178. doi: 10.1007/3-540-32636-7_6. [DOI] [PubMed] [Google Scholar]
- 30.Murad YM, Clay TM, Lyerly HK, Morse MA. CPG-7909 (PF-3512676, ProMune): toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7:1257–1266. doi: 10.1517/14712598.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 31.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 32.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 33.Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67:7941–7944. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 34.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 35.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 36.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 37.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 38.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK, Walker LS. CD4+ CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 41.Sparwasser T, Vabulas RM, Villmow B, Lipford GB, Wagner H. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur J Immunol. 2000;30:3591–3597. doi: 10.1002/1521-4141(200012)30:12<3591::AID-IMMU3591>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8 + T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jeze G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25 + CD4 + naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda N, Chang DZ, Mine T, Efferson C, Garcia-Sastre A, Wang X, Ferrone S, Ioannides CG. Taxol increases the amount and T cell activating ability of self-immune stimulatory multimolecular complexes found in ovarian cancer cells. Cancer Res. 2007;67:8378–8387. doi: 10.1158/0008-5472.CAN-07-0327. [DOI] [PubMed] [Google Scholar]
- 46.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicari AP, Caux C, Trinchieri G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- 48.Vicari AP, Chiodoni C, Vaure C, Ait-Yahia S, Dercamp C, Matsos F, Reynard O, Taverne C, Merle P, Colombo MP, O’Garra A, Trinchieri G, Caux C. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vicari AP, Schmalbach T, Lekstrom-Himes J, Morris ML, Al Adhami MJ, Laframboise C, Leese P, Krieg AM, Efler SM, Davis HL. Safety, pharmacokinetics and immune effects in normal volunteers of CPG 10101 (ACTILON), an investigational synthetic toll-like receptor 9 agonist. Antivir Ther. 2007;12:741–751. [PubMed] [Google Scholar]
- 50.Vollmer J, Jurk M, Samulowitz U, Lipford G, Forsbach A, Wullner M, Tluk S, Hartmann H, Kritzler A, Muller C, Schetter C, Krieg AM. CpG oligodeoxynucleotides stimulate IFN-gamma-inducible protein-10 production in human B cells. J Endotoxin Res. 2004;10:431–438. doi: 10.1179/096805104225006534. [DOI] [PubMed] [Google Scholar]
- 51.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 52.Whitmore MM, Li S, Falo L, Jr, Huang L. Systemic administration of LPD prepared with CpG oligonucleotides inhibits the growth of established pulmonary metastases by stimulating innate and acquired antitumor immune responses. Cancer Immunol Immunother. 2001;50:503–514. doi: 10.1007/s002620100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]