Abstract
Carbonic anhydrase IX (CA9) is a renal cell carcinoma (RCC)-specific tumor protein that is targeted using heat shock protein 110 (hsp110). The chaperoning ability of hsp110 can be utilized to form a complex with CA9 (hsp110 + CA9) in vitro, which can be administered as a highly concentrated tumor vaccine. In a tumor prevention model, hsp110 + CA9 prevented the growth of RENCA tumors in BALB/c mice, and produced IFN-γ response measured using ELISPOT and an antibody response measured using ELISA. To test a second vaccine strategy, hsp110 complexed to a previously described CA9 peptide prevented tumor growth and produced a very weak IFN-γ response, but no antibody response. A plasmid vector containing grp170, a member of the hsp110 family, linked to CA9 did not produce an antitumor response and produced no IFN-γ response or antibodies. In a model of metastatic RCC, RENCA cells were injected intradermally prior to vaccination. Hsp110 + CA9 decreased tumor growth compared to control vaccinations. These studies suggest that recombinant hsp110 complexed to CA9 should be evaluated for treatment of RCC.
Keywords: Renal cell carcinoma, Carbonic anhydrase IX, Heat shock protein, Tumor vaccine, RENCA, Heat shock protein 110
Introduction
Each year over 38,000 cases of renal malignancies are diagnosed, representing 2.8% of all newly diagnosed cancers. Renal malignancies result in over 12,000 deaths each year, which account for 2.3% of all cancer deaths [1]. Of all urologic malignancies, renal cell carcinoma (RCC) has the highest ratio of disease-related deaths to incidence. Metastatic RCC is resistant to standard chemo and radiation therapies. Cytokine therapy using interleukin-2 (IL-2) or interferon-α (IFN-α) remains a standard and is associated with response rates of 15–20% [2–4]. Despite the modest overall response rates, systemic cytokine therapy produces complete responses and durable remissions in 5–10% of patients with metastatic RCC. These responses to general immunostimulation underscore the immunoresponsive nature of RCC.
A major obstacle to developing more specific and more effective RCC tumor vaccines has been the lack of tumor-specific antigens [5]. Carbonic anhydrase IX (CA9) has been identified recently as a potential target for immunotherapy [6, 7]. CA9 is not expressed in normal renal tissue, but is present in more than 80% of primary and metastatic RCC, and 95–100% of the clear cell variant [8]. Results of phase I and II clinical trials of monoclonal antibodies directed against CA9 in patients with RCC show promise [9, 10]. Monoclonal antibodies against CA9 are being evaluated currently in large scale, phase III clinical trial as adjuvant therapy for RCC [11]. Alternate immunotherapeutic strategies targeting CA9 and stimulating a cellular immune response may prove more effective.
Heat shock proteins (HSPs) are abundant intracellular proteins [12]. HSPs are induced by a variety of environmental stresses, including heat, and function to bind and protect partially denatured proteins from further denaturation and aggregation. HSPs have been shown to stimulate an adaptive immune response against antigens bound to HSP. Therefore, this feature may be harnessed to develop a tumor vaccine [13, 14]. A complex of recombinant HSP and target antigen produces the same danger signal provided by intracellular HSPs released in various disease states associated with cellular damage.
Heat shock proteins can be categorized by size; hsp110 and grp170 are the largest members and share common sequences. The large HSPs are well suited for use in recombinant vaccines. Wang et al. [15] reported that tumor-derived hsp110 elicits a more potent antitumor response on a molar basis than hsp70. The enhanced immunogenicity has been attributed to hsp110’s more efficient chaperoning capability [14, 16, 17]. Several reports suggest that the affinity with which the chaperone binds antigen significantly contributes to its ability to generate a cytotoxic T-lymphocyte (CTL) response [18–20]. Therefore, this study evaluated three vaccine strategies that utilize large HSP family members to target CA9 in a murine RENCA model. Protein-based vaccines were created by complexing recombinant hsp110 to CA9, or a CA9 peptide that has previously been shown to be H-2K-restricted [21]. A DNA vaccine was created by constructing a plasmid containing grp170 linked to CA9.
Materials and methods
Mice and cell lines
Female BALB/c mice of 6–8 week old were purchased from NCI (Frederick, MD) and housed under pathogen-free conditions. All experiments involving animals were in compliance with federal and state standards, which include the federal Animal Welfare Act and the NIH guide for the care and use of laboratory animals. Parental RENCA cells and RENCA cells stably transduced to express human CA9 (RENCA-CA9) were provided by Dr. Arie Belldegrun (University of California, Los Angeles). CA9 expression in RENCA-CA9 was confirmed using western blot and monoclonal antibody against CA9, which was a gift from Dr. Egbert Oosterwijk (University Hospital of Nijmegen, Nijmegen, Netherlands). The RENCA lines were maintained in RPMI 1640, 10% heat-inactivated fetal calf serum, 1% glutamine, 1% nonessential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, and 1% HEPES buffer.
Expression and purification of recombinant proteins
Mouse hsp110 and human CA9 cDNA (a gift from Dr. Belldegrun) were cloned into pBacPAK-his vector (BD Biosciences Clontech, Palo Alto, CA), transformed into monolayer Sf21 cells using replication defective virus, and expressed using the BacPAK baculovirus system. Proteins were purified using a nickel nitriloacetic acid-agarose column (Qiagen, Valencia, CA). Protein concentrations were measured using a Protein Assay Kit (Bio-Rad, Hercules, CA). Protein purity was assessed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie Blue staining. Endotoxin levels in recombinant proteins were assessed using a Limulus Amebocyte lysate kit (Biowhittaker, Walkersville, MD) and were 10–25 endotoxin units/mg protein.
Reconstitution of HSP and antigen complex
Recombinant hsp110 and CA9 were complexed at 1:1 molar ratio and incubated for 30 min at room temperature or at heat shock temperatures of 45 or 55°C. The complex was immunoprecipitated using anti-hsp110 antibody to verify noncovalent binding between hsp110 and CA9. After SDS-PAGE (10%) electrophoresis, western blot analysis was performed using anti-His antibody (Amersham, Piscataway, NJ). For the in vivo studies, hsp110 and CA9 were complexed at room temperature. CA9 peptide (A Y E Q L L S R L) was ordered from Alpha Diagnostics (San Antonio, TX) and similarly complexed to hsp110.
Construction of DNA vaccine
A DNA vaccine consisting of pcDNA3.1 vector (Invitrogen, Carlsbad, CA) carrying CA9 fused to the N terminus of grp170 was constructed. Control vaccines included pcDNA3.1 carrying CA9 alone or grp170 alone. All genes were inserted behind a CMV promoter and sequence was verified. Protein expression was verified using transfected COS cells.
Tumor prevention study
Mice (five per group) were immunized three times, 14 days apart, with 100 μl of vaccine. 2 × 105 RENCA-CA9 cells were injected intradermally, 7 days after the last immunization. Tumors were measured every 2 days using an electronic caliper [(shortest diameter2 ×longest diameter)/2). The complete set of experiments was repeated three times. The vaccination groups for the protein vaccines included PBS (control), CA9 (25 μg) alone, hsp110 (50 μg) alone, CA9 (25 μg) + 50 μl Freud’s Adjuvant (CA9 + FA), and hsp110 complexed to CA9 (hsp110 + CA9; 75 μg). The vaccination groups for the CA9 peptide-based vaccines included PBS (control), CA9 peptide (50 μg), hsp110 complexed to CA9 peptide (hsp110 + CA9 peptide; 100 μg). The vaccination groups for the DNA vaccine included pcDNA3.1 carrying CA9 (10 μg) alone, grp170 (10 μg) alone, and CA9-grp170 (10 μg). CA9 + FA vaccine was injected subcutaneously and all other vaccines were injected intradermally.
Tumor treatment study
The tumor treatment study was similar to the tumor prevention assay except that mice were injected intradermally with 2 × 105 RENCA-CA9 cells and the vaccines were injected on days 3, 9, and 14 after tumor implantation. Tumor growth was monitored as described previously.
ELISPOT
Splenocytes were harvested 2 weeks after immunization and stimulated in vitro using irradiated RENCA-CA9 for 5 days. Filtration plates (Millipore, Bedford, MA) were coated with 10 μg/ml rat antimouse INF-γ (clone R4-6A2; PharMingen, San Diego, CA) at 4°C overnight, washed and blocked. Splenocytes (5 × 105/well) were incubated with CA9 (20 μg/ml) at 37°C for 24 h, then washed. A biotinylated IFN-γ antibody (5 μg/ml; clone XMG1.2; PharMingen), avidin-alkaline phosphatase D (0.2 unit/ml; Vector Labs, Burlingame, CA) and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Boehringer Mannheim, Indianapolis, IN) were used to detect IFN-γ secretion. IFN-γ spots were counted using the KS Elispot System (version 4.3.56) from Zeiss Microscopy (Oberkochen, Germany).
ELISA
Fivefold serial dilutions starting at 1:200 of serial bleeds from immunized mice were tested for CA9-specific antibodies using CA9 coated microtiter plates (10 μg/ml). Antibodies were detected using biotinylated anti-mouse IgG1 or IgG2a, avidin-alkaline phosphatase D (0.2 unit/ml; Vector Labs) and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (Boehringer Mannheim). Binding specificity was assessed by testing the sera using a control protein coated on the microtiter plates and by testing preimmune sera. Optical densities (OD) were read at 450 nm using a Titertek Multiscan MCC/340 plate scanner. The OD at 450 nm and 1:200 dilution is reported for ELISA assays.
Statistical analysis
Differences in tumor growth were assessed using repeating measure ANOVA. P values < 0.05 were considered significant. All statistical analyzes were performed using Stata 8.0 (StataCorp, College Station, Texas).
Results
Hsp110 binds CA9 in vitro
Recombinant hsp110 and CA9 synthesized using the BacPAK system and purified using a nickel nitriloacetic acid-agarose column produced highly pure recombinant protein (Fig. 1a).
Fig. 1.
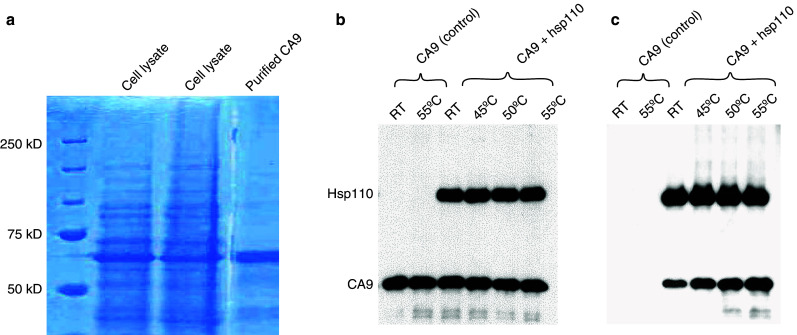
Noncovalent complexing of recombinant hsp110 and CA9. a Coomassie blue-gel staining of whole cell lysate (center two lanes), purified recombinant CA9 (right lane), and molecular weight markers (left lane). Each lane of a single gel was loaded with 4 μg of protein. b Hsp110 binds CA9 at room temperature and heat shock temperatures. Western blot analysis using anti-His antibody shows bands corresponding to hsp110 and CA9. Hsp110 and CA9 are staining are equal. c The same protein mixtures were immunoprecipitated with anti-hsp110 antibody. After SDS-PAGE (10%) electrophoresis, a western blot analysis was performed using anti-His antibody. CA9 staining increases with heat shock temperature, indicating greater hsp110 binding at higher temperatures
In prior studies from our laboratory, hsp110 has been shown to efficiently bind tumor proteins such as gp100 and Her2/neu at heat shock temperatures but not at room temperature [14, 22]. However, hsp110 efficiently binds CA9 even at room temperature. Recombinant hsp110 and CA9 were combined at 1:1 molar ratio and noncovalent binding was confirmed by immunoprecipitating the complex using anti-hsp110 antibody (Fig. 1b, c). After SDS-PAGE (10%) electrophoresis, western blot analysis was performed using anti-His antibody. When compared to room temperature, hsp110 binds more CA9 at 45, 50, and 55°C. However, in subsequent studies in mice, there was no difference in the antitumor effects comparing hsp110 and CA9 complexes formed at varying temperatures (data not shown). Therefore, hsp110 and CA9 were complexed at room temperature for the in vivo vaccine studies.
Hsp110 + CA9 was evaluated as a tumor vaccine in a RENCA murine model
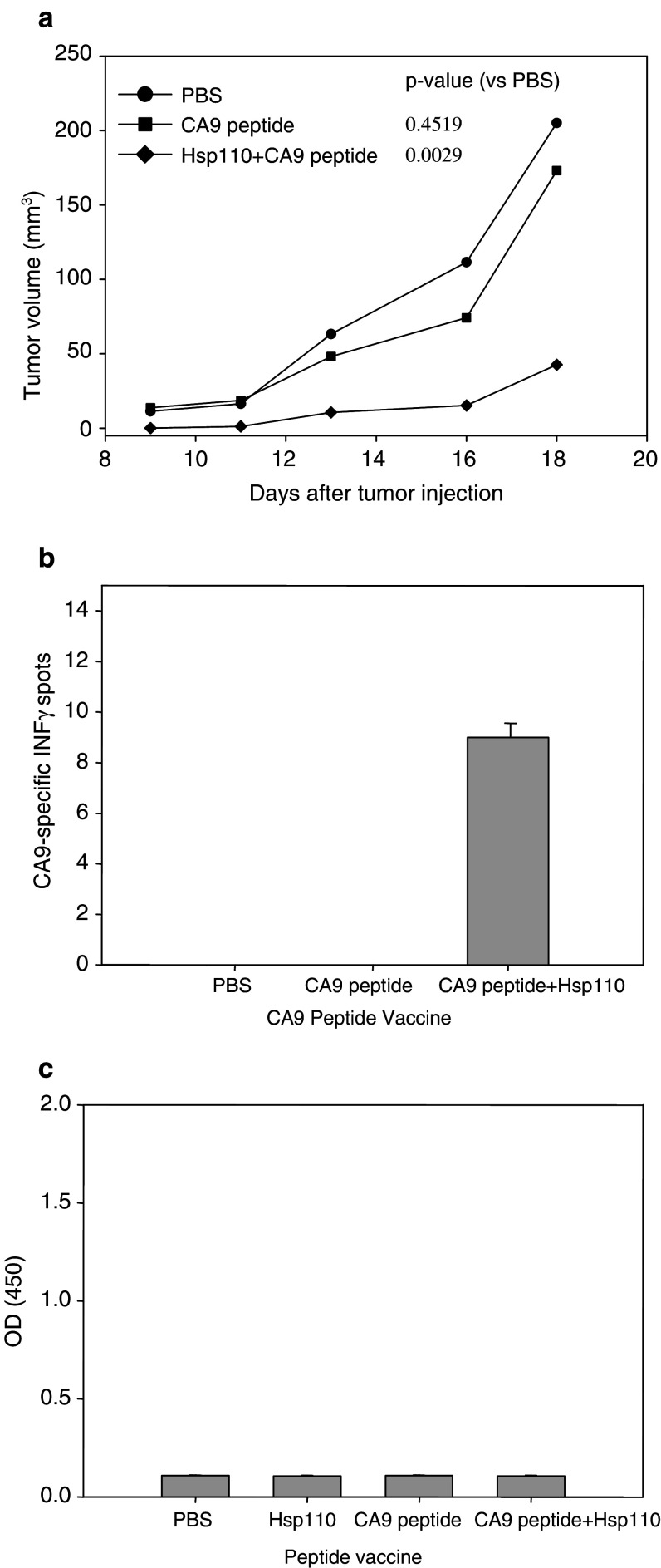
In a tumor prevention assay, BALB/c mice were immunized prior to injection of RENCA-CA9 (Fig. 2a). Hsp110 + CA9 prevented tumor growth in all animals. Although both CA9 alone and hsp110 alone decreased tumor growth, the effect was not statistically significant when compared to the control group that was immunized with PBS. When the mice challenged with RENCA-CA9 were observed for 40 days, none of the mice immunized with hsp110 + CA9 developed tumors (Fig. 2b). At 40 days, one of five mice immunized with hsp110 was tumor free. All other mice developed palpable tumors at the site of tumor injection.
Fig. 2.
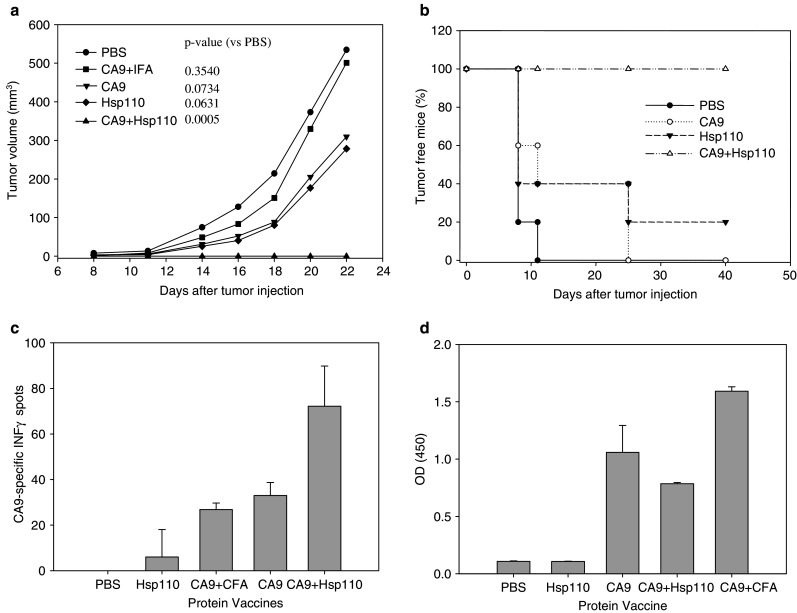
Immunization with hsp110 + CA9 had an antitumor effect. a 2 × 105 RENCA-CA9 tumor cells were injected intradermally 7 days after three immunizations administered 14 days apart (see Materials and methods for dosing information). Each line represents the mean tumor growth in five mice. P values based on repeating measure ANOVA, which takes into account the variability within each group, are provided comparing each group to the control mice injected with PBS. b Immunization with hsp110 + CA9 prevented tumor formation in mice with extended followup. After a total of 40 days, none of the mice immunized with has110 + CA9 developed palpable tumor. c Immunization with hsp110 + CA9 elicited CA9-specific immune responses measured using ELISPOT assay. d Immunization with hsp110 + CA9 elicited CA9-specific humoral response measured using ELISA
Immunization with hsp110 + CA9 produced both cellular and humoral immune responses. Hsp110 + CA9 generated a CA9-specific IFN-γ response measured using Elispot assay (Fig. 2c). CA9 alone and CA9 with complete FA produced lesser IFN-γ responses. Vaccination with hsp110 + CA9 also produced CA9 specific antibodies as measured using Elisa assay (Fig. 2d). To address the question of specificity of the immune response, mice vaccinated with hsp110 + CA9 were evaluated for IFN-γ response to TRP2 (an irrelevant antigen) using ELISPOT assay. TRP2 stimulation of splenocytes produced an average of 42 spots.
Alternative HSP-based tumor vaccine targeting CA9 were evaluated in a RENCA murine model
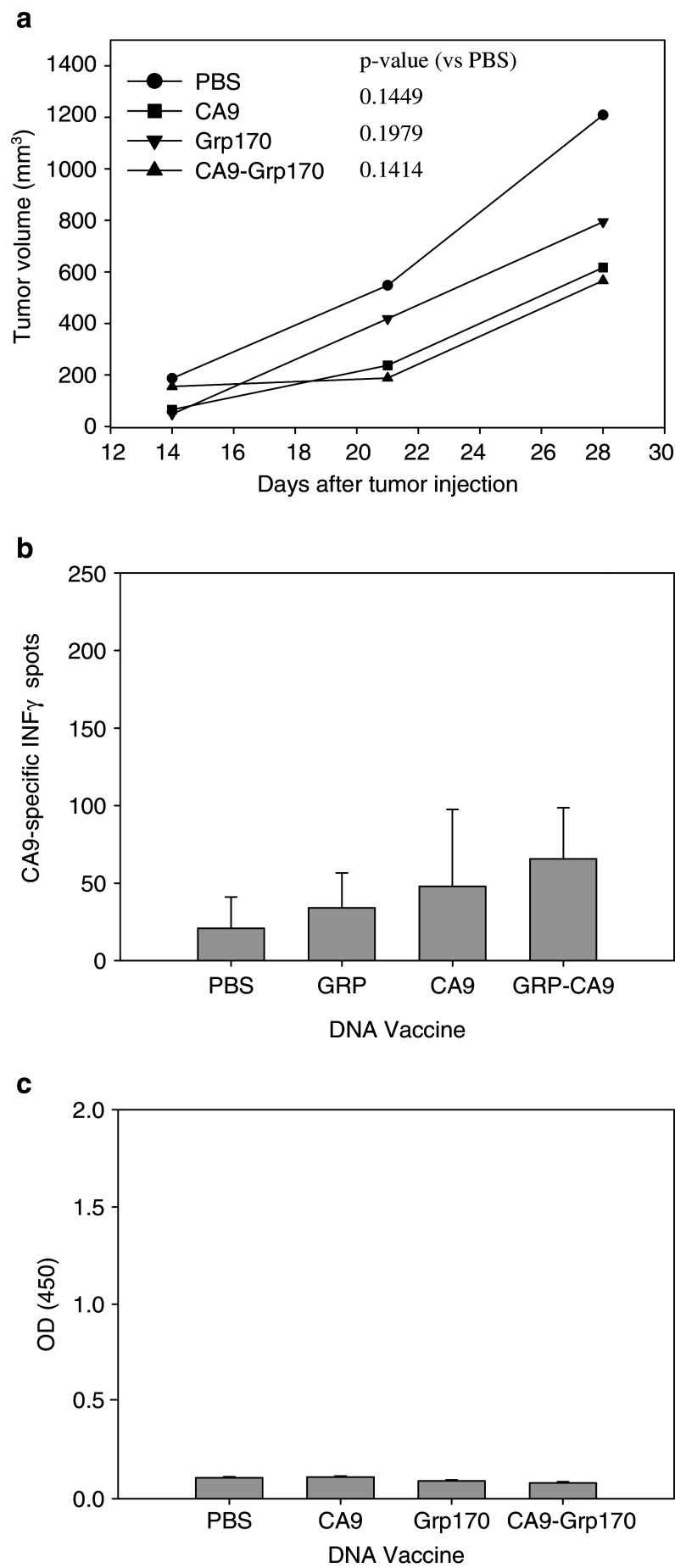
A vaccine consisting of hsp110 complexed to an immunodominant CA9 peptide decreased growth of RENCA-CA9 in a tumor prevention assay (Fig. 3a). Hsp110 + CA9 peptide vaccination generated a very weak CA9-specific IFN-γ response, but produced no CA9-specific antibodies (Fig. 3b, c). A DNA vaccine consisting of pcDNA3.1 vector carrying CA9 fused to the N terminus of grp170 produced no antitumor effects in a tumor prevention assay (Fig. 4a). The vaccine produced no CA9 specific IFN-γ response or CA9-specific antibodies (Fig. 4b, c).
Fig. 3.
Immunization with hsp110 + CA9 peptide (A Y E Q L L S R L) had an antitumor effect. a 2 × 105 RENCA-CA9 tumor cells were injected intradermally 7 days after three immunizations administered 14 days apart. Each line represents the mean tumor growth in five mice. P values are provided comparing each group to the control mice injected with PBS. b Immunization with hsp110 + CA9 peptide elicited a weak CA9-specific immune responses measured using ELISPOT assay. c Immunization with hsp110 + CA9 peptide elicited CA9-specific humoral response measured using ELISA
Fig. 4.
Immunization with plasmid vector (pcDNA 3.1) containing grp170 linked to CA9 did not prevent tumor growth. a 2 × 105 RENCA-CA9 tumor cells were injected intradermally 7 days after three immunizations (five mice per group) administered 14 days apart. Each line represents the mean tumor growth in five mice. P values are provided comparing each group to the control mice injected with PBS. b Immunization with plasmid vector (pcDNA 3.1) containing grp170 linked to CA9 did not elicit CA9-specific immune responses measured using ELISPOT assay. c Immunization with plasmid vector (pcDNA 3.1) containing grp170 linked to CA9 did not elicit CA9-specific humoral response measured using ELISA
Hsp110 + CA9 is effective against established RENCA tumors
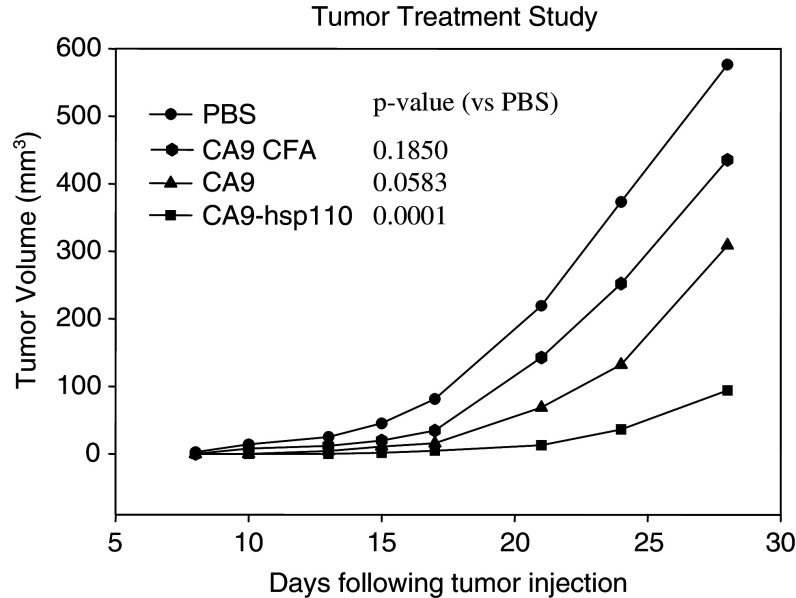
Of the three vaccine strategies evaluated, a complex of recombinant hsp110 and CA9 produced the most antitumor effect. Therefore, the vaccine employing full-length proteins was evaluated in a tumor treatment assay. Balb/c mice were injected intradermally with RENCA-CA9 to establish palpable tumors prior to treatment. Immunization with hsp110 + CA9 significantly decreased tumor growth when compared to immunization with PBS (Fig. 5). CA9 alone decreased tumor growth; however, the difference compared to PBS control did not reach statistical significance.
Fig. 5.
Immunization with hsp110 + CA9 decreased the growth of established tumor. 2 × 105 RENCA-CA9 tumor cells were injected intradermally and vaccines were injected 3, 9, and 14 days after tumor implantation. Each line represents the mean tumor growth in five mice. P values are provided comparing each group to the control mice injected with PBS
Discussion
The most common histologic subtype of RCC is the clear cell variant. Von Hippel-Lindau (VHL) mutations and deletions are found in more than 50% of sporadic clear cell RCCs [23, 24]. Hypermethylation represents an additional mechanism for VHL inactivation [24, 25]. In clear cell RCC, overexpression of CA9 is the direct consequence of the defect in VHL function, which normally functions to degrade and suppress HIF-1α. CA9 expression is positively regulated by HIF-1α. Therefore, in the majority of clear cell RCCs, both HIF-1α and CA9 are constitutively expressed and no longer regulated by oxygen tension. CA9 expression is found in 95% of clear cell renal tumors with no expression in normal kidney [6, 8]. Expression in other normal tissue is limited to basal cells of hair follicles, gonadal epithelium, choroid plexus, and some gastrointestinal mucosa [8].
HSPs normally function as molecular chaperones, assisting with protein folding and formation of multi-subunit complexes [26, 27]. Experiments performed in the early half of the 1900s demonstrated that tumor cells and lysates can protect mice against subsequent tumor challenges [28]. Follow up experiments using tumor fractions identified HSPs as the “active ingredient” providing immune protection [29]. The HSPs are promiscuously bound to a large repertoire of tumor antigens and produce tumor-specific immune responses [30]. These HSPs can be isolated and administered as tumor-specific, autologous vaccines. In two different phase II trials for metastatic kidney cancer, approximately 35% of patients had clinical responses. No significant toxicities were observed, and no autoimmune effects were noted [31, 32]. Phase II clinical trials have been promising, however, technical and procedural problems in phase III trials have lead to difficulties.
There are limitations to using tumor derived HSPs. Surgically obtained tumor tissue is not available for all patients. Even when tumor tissue is available, a vaccine cannot be prepared in approximately 10% of cases [33]. Finally, only a small fraction of relevant tumor peptides in the vaccine produce an antitumor effect. In the approach described in this study, recombinant HSP and a target tumor protein are combined in vitro to produce a noncovalent complex. This complex provides the same danger signal provided by intracellular HSPs released in various disease states associated with cellular damage. Advantages to this approach include the following:
This preparation is a highly concentrated vaccine directed at a known tumor target.
This preparation is produced in unlimited quantity.
Although this vaccine would not be patient-specific, it has the potential to be effective against all tumors expressing the target.
By using the full-length protein, the vaccine can be used in all patients, regardless of HLA restrictions.
In this study, three HSP based tumor vaccines targeting CA9 were evaluated. All three strategies were screened using an in vivo tumor prevention model where vaccination was followed by tumor challenge. A vaccine using full-length, recombinant CA9 and hsp110 was most effective in preventing tumor growth, and produced robust cellular and humoral immune responses. A vaccine combining hsp110 and a CA9 peptide also prevented tumor growth. As expected, a peptide-based vaccine produced a weak cellular response but no humoral response. More effective antitumor effects may be possible with use of multiple immunodominant peptides.
The final vaccine strategy we evaluated was a DNA vaccine. A DNA vaccine obviates the technical challenges associated with production of recombinant proteins. However, a plasmid vector designed to express grp170 linked to CA9 had no antitumor effects and failed to produce a cellular or humor immune response. In unpublished work from our laboratory, a plasmid vector linking hsp110 and HPV 16-E7 was not effective as a tumor vaccine in a murine, cervical cancer model. Therefore, in the present study, a plasmid containing grp170 was constructed. Both large members of the hsp70 superfamily (i.e. hsp110 and grp170) failed to produce an immune response against the linked antigen.
Several other laboratories have examined genetically engineered fusion proteins consisting of tumor antigen fused to either the N or C terminal of the HSP and reported antitumor activity [34–36]. However, in most cases the HSP used in these vaccines were of microbial origin, and therefore, the HSP itself is highly immunogenic. In addition, a possible explanation for lack antitumor activity associated with our DNA vaccine is that a fusion protein is an unnatural construct and interactions with APCs depend on proper positioning and steric changes associated with noncovalent complexing of HSP and tumor antigen.
A tumor prevention model is analogous to the clinical setting in which adjuvant therapy is utilized. Adjuvant therapy is provided to patients at high risk for recurrence following resection of clinically localized RCC. These patients have no radiographically detectable disease after surgery, and the goal of adjuvant therapy is to prevent disease recurrence. This study suggests that a HSP-based tumor vaccine targeting CA9 may be an effective adjuvant therapy. This vaccine strategy may also be effective in the treatment of metastatic RCC. To explore this possibility, the vaccine strategy shown to be the most effective in the tumor prevention model was tested in a tumor treatment model where vaccine was administered after palpable, intradermal tumors were established. The recombinant protein vaccine combining hsp110 and CA9 decreased the growth of established tumor.
A limitation of this study is the use of human CA9 in a mouse model. However, this system is useful for screening various strategies for targeting CA9 using HSP. The results indicate that hsp110 complexed to full-length CA9 produces a more robust immune response than hsp110 complexed to a CA9 peptide or a DNA vaccine composed of CA9 fused to a HSP. In additional, the results highlight the potent adjuvant properties of hsp110. Although CA9 alone produced a modest immune response, the complex of CA9 and hsp110 produced a significantly more potent immune response. Prior to investigation of vaccine in a clinical trial, future studies will need to determine if hsp110 and CA9 can generate human antitumor immunity.
Conclusion
In an animal model, recombinant hsp110 complexed to CA9 was effective treatment for RCC, and produced a more effective antitumor effect than HSP-based strategies using CA9 peptide. This work suggests that hsp110–CA9 heat shock complex may be effective for the treatment of RCC.
Acknowledgment
Funding for the study is from The Edwin Beer Program of the New York Academy of Medicine (no sponsor number) and individual allocation from American Cancer Society Institutional Research Grant (IRG 02-197-01).
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Bukowski RM. Cytokine combinations: therapeutic use in patients with advanced renal cell carcinoma. Semin Oncol. 2000;27:204–212. [PubMed] [Google Scholar]
- 3.Figlin RA. Renal cell carcinoma: management of advanced disease (discussion 386–387) J Urol. 1999;161:381–386. doi: 10.1016/S0022-5347(01)61897-4. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Fyfe G. High-dose aldesleukin in renal cell carcinoma: long-term survival update. Cancer J Sci Am. 1997;3(Suppl1):S70–S72. [PubMed] [Google Scholar]
- 5.Neumann E, Engelsberg A, Decker J, Storkel S, Jaeger E, Huber C, Seliger B. Heterogeneous expression of the tumor-associated antigens RAGE-1, PRAME, and glycoprotein 75 in human renal cell carcinoma: candidates for T-cell-based immunotherapies? Cancer Res. 1998;58:4090–4095. [PubMed] [Google Scholar]
- 6.Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, Horvath S, Leibovich BC, Chopra S, Liao SY, Stanbridge E, Lerman MI, Palotie A, Figlin RA, Belldegrun AS. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 7.Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J, Warnaar SO. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oosterwijk E, Bander NH, Divgi CR, Welt S, Wakka JC, Finn RD, Carswell EA, Larson SM, Warnaar SO, Fleuren GJ, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11:738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 10.Bleumer I, Knuth A, Oosterwijk E, Hofmann R, Varga Z, Lamers C, Kruit W, Melchior S, Mala C, Ullrich S, De Mulder P, Mulders PF, Beck J. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90:985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HL, Pantuck AJ, Zomorodian N, Belldegrun AS. Monoclonal antibody (MAB) in patients with advanced cancer. Curr Urol Rep. 2003;4:11–12. doi: 10.1007/s11934-003-0051-x. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 13.Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, Subjeck JR. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 14.Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63:2553–2560. [PubMed] [Google Scholar]
- 15.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 16.Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem. 1997;272:31636–31640. doi: 10.1074/jbc.272.50.31636. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Easton DP, Chen X, MacDonald IJ, Wang XY, Subjeck JR. The chaperoning properties of mouse grp170, a member of the third family of hsp70 related proteins. Biochemistry. 2003;42:14893–14902. doi: 10.1021/bi030122e. [DOI] [PubMed] [Google Scholar]
- 18.Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, Rothman JE, Houghton AN. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci USA. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacAry PA, Javid B, Floto RA, Smith KG, Oehlmann W, Singh M, Lehner PJ. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity. 2004;20:95–106. doi: 10.1016/S1074-7613(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 20.Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- 21.Vissers JL, De Vries IJ, Engelen LP, Scharenborg NM, Molkenboer J, Figdor CG, Oosterwijk E, Adema GJ. Renal cell carcinoma-associated antigen G250 encodes a naturally processed epitope presented by human leukocyte antigen-DR molecules to CD4(+) T lymphocytes. Int J Cancer. 2002;100:441–444. doi: 10.1002/ijc.10518. [DOI] [PubMed] [Google Scholar]
- 22.Manjili MH, Henderson R, Wang XY, Chen X, Li Y, Repasky E, Kazim L, Subjeck JR. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742. [PubMed] [Google Scholar]
- 23.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Yoshida M, Kishida T, Nakaigawa N, Baba M, Kobayashi K, Miura T, Moriyama M, Nagashima Y, Nakatani Y, Kubota Y, Kondo K. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–1575. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 25.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 27.Haas IG. BiP—a heat shock protein involved in immunoglobulin chain assembly. Curr Top Microbiol Immunol. 1991;167:71–82. doi: 10.1007/978-3-642-75875-1_4. [DOI] [PubMed] [Google Scholar]
- 28.Klein G, Sjogren HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561–1572. [PubMed] [Google Scholar]
- 29.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- 30.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate antimelanoma T cells. Cancer Res. 2001;61:222–227. [PubMed] [Google Scholar]
- 31.Amato R, Wood L, Savary C, Wood C, Hawkins E, Reitsma D, Srivastava PK. (2000) Patients with renal cell carcinoma (RCC) using autologous tumor-derived heat shock protein–peptide complex (HSPPC-96) with or without interleukin-2 (IL-2). Presented at: ASCO A1782
- 32.Assikis VJ, Daliani D, Pagliaro L, Wood C, Perez C, Logothetis C, Papandreou C, Hawkins ES, Srivastava PK (2003) Phase II study of an autologous tumor derived heat shock protein–peptide complex vaccine (HSPPC-96) for patients with metastatic renal cell carcinoma (mRCC). Presented at: ASCO A1552
- 33.Wood C, Escudier B, Gorelov S, Krajka K, Lacombe L, Fossa S, Hoos A, Flanigan R, Figlin RA, Srivastava PK. (2004) A multicenter randomized study of adjuvant heat-shock protein peptide-complex 96 (HSPPC-96) vaccine in patients with high-risk of recurrence after nephrectomy for renal cell carcinoma (RCC)—a preliminary report. Presented at: ASCO A2618
- 34.Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol. 2001;13:1233–1242. doi: 10.1093/intimm/13.10.1233. [DOI] [PubMed] [Google Scholar]
- 35.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony LS, Wu H, Sweet H, Turnnir C, Boux LJ, Mizzen LA. Priming of CD8+ CTL effector cells in mice by immunization with a stress protein-influenza virus nucleoprotein fusion molecule. Vaccine. 1999;17:373–383. doi: 10.1016/S0264-410X(98)00199-6. [DOI] [PubMed] [Google Scholar]