Abstract
The ability of cultured, antigen-loaded dendritic cells (DCs) to induce antigen-specific T cell immunity in vivo has previously been demonstrated and confirmed. Immune monitoring naturally focuses on immunity against vaccine antigens and may thus ignore other effects of DC vaccination. Here we therefore focused on antigen-independent responses induced by DC vaccination of renal cell carcinoma patients.
In addition to the anticipated response against the vaccine antigen KLH, vaccination with CD83+ monocyte-derived DCs resulted in a strong increase in the ex vivo proliferative and cytokine responses of PBMCs stimulated with LPS or BCG. In addition, LPS strongly enhanced the KLH-induced proliferative and cytokine response of PBMCs. Moreover, proliferative and cytokine responses of PBMCs stimulated with the homeostatic cytokines IL-7 and IL-15 were also clearly enhanced after DC vaccination. In contrast to LPS induced proliferation, which is well known to depend on monocytes, IL-7 induced proliferation was substantially enhanced after monocyte depletion indicating that monocytes limit IL-7 induced lymphocyte expansion.
Our data indicate that DC vaccination leads to an increase in the ex vivo responsiveness of patient PBMCs consistent with a DC vaccination induced enhancement of T cell memory. Our findings also suggest that incorporation of bacterial components and homeostatic cytokines into immunotherapy protocols may be useful in order to enhance the efficacy of DC vaccination and that monocytes may limit DC vaccination induced immunity.
Keywords: Dendritic cell vaccination, Renal cancer, LPS, BCG, IL-7, IL-15
Introduction
Dendritic cells (DCs) are professionally adapated antigen-presenting cells that induce and coordinate immune responses [2]. The clinical première of DCs, which was almost a decade ago, was when Hsu et al. administered DCs that had been isolated from peripheral blood to patients with B cell lymphoma [10]. This seminal work was followed by the comprehensive examination of monocyte-derived DCs (moDCs) in patients with various hematopoietic or solid tumors [1, 5, 16]. In these studies, immune monitoring naturally focused on immune responses against vaccine antigens in order to obtain proof of principle. DCs were loaded with tumor antigens for instance in the form of tumor lysate, tumor peptides, tumor RNA or recombinant tumor antigen [1, 16]. In addition, DCs were often loaded with immunogenic xenoantigens such as KLH or tetanus toxoid to check vaccine efficacy (tracer antigen) and to enhance antitumor immune responses (helper antigen) [7–9]. In immune monitoring experiments the same antigens that had been used for vaccination were used to stimulate peripheral blood mononuclear cells (PBMCs) obtained before and after vaccination. Using this approach, DC vaccination-induced increases of immune responses against tumor antigens and control antigens have frequently been reported [1–3, 7–10, 16]. Moreover, in some studies immune monitoring revealed immune responses against tumor antigens that were not part of the vaccine indicating that successful antitumor vaccination may also lead to antigen spreading [3, 21]. Besides antitumor immunity, anecdotal reports also described an increase in delayed-type hypersensitivity (DTH) against recall antigens [13] indicative of a more general improvement of immune responsiveness after DC vaccination.
The goal of DC-based T cell vaccines is the generation of memory T cells. One distinctive feature of memory T cells is their increased responsiveness to IL-7 and IL-15, which are both members of the common γ chain family of cytokines [12, 17]. IL-7 and IL-15 can induce T cell receptor-independent, homeostatic proliferation of human CD4+ memory T cells [6]. In addition, memory T cells can be activated in vitro by stimulation of PBMCs with LPS [14] or in vivo by LPS injection [19]. Along this line we now report that DC vaccination results in a substantial increase in the ex vivo PBMC responsiveness to stimulation with bacterial components and homeostatic cytokines indicative of a DC vaccination induced enhancement of T cell memory.
Patients, materials and methods
Bacteria, bacterial products and cytokines
Lipopolysaccharide (LPS) from E. coli O55:B5, E. coli O111:B4, S. abortus equi, S. minnesota, S. typhimurium were all purchased from Sigma-Aldrich (Vienna, Austria). Bacillus Calmette-Guérin (BCG, viable mycobacteria) were obtained from medac (Hamburg, Germany). IL-7 and IL-15 were from Strathmann Biotec (Hamburg, Germany).
Patient treatment
Patients with metastatic renal cell carcinoma of the clear-cell type received DC-based immunotherapy [8, 9]. Peripheral blood mononuclear cells (PBMCs) were isolated from 150 ml heparinized venous blood of the patient by standard density gradient centrifugation using Lymphoprep™ (Life Technologies, Lofer, Austria). PBMCs (2 × 106/ml) were seeded in 6-well plates (Costar®, Corning Inc., NY) and after 2 h, the non-adherent cells were removed. Adherent monocytes were cultured in RPMI 1640 (Biowhittaker, Verviers, Belgium) supplemented with 2% heat-inactivated human serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, 10 mM Hepes, 0.1 mM nonessential amino acids, 1 mM pyruvate (all from Biowhittaker) and 5 × 10–5 M 2-mercaptoethanol in the presence of 1,000 units per ml of each recombinant human GM-CSF (Leucomax®, Novartis) and recombinant human IL-4 (Strathmann Biotech, Hannover, Germany) for 5 days. On day 5, moDCs were pulsed with autologous tumor lysate (10 μg/ml) or A-498 lysate (10 μg/ml) and KLH (Calbiochem-Novabiochem, San Diego, CA; 10–25 μg/ml). After 1 h at 37°C, 1,000 U/ml of recombinant human TNF-α, IL-1 (5 ng/ml), IL-6 (10 ng/ml) (all from R&D Systems, Minneapolis, MN) and 1 μM of Prostaglandin E2 (Prostin E2®, Pharmacia & Upjohn, Vienna, Austria) were added to induce maturation. After 24 h, moDCs were harvested, washed and resuspended in lactated Ringer’s solution containing 1% autologous serum. An aliquot of the cells was removed for phenotypic analysis and for sterility testing at the local Institute of Hygiene. Release criteria were typical DC morphology under phase contrast (veiled cells) and phenotype (>90% of the cells CD83+). The study protocols had been reviewed and approved by the institutional review board and all patients enrolled had given written informed consent.
Proliferation assay
PBMCs were harvested before the first (prevaccination PBMCs) and after three vaccinations (postvaccination PBMCs). PBMCs (1.5 × 106/ml) were stimulated with the substances indicated (KLH, LPS, BCG, IL-7, IL-15) in triplicates in flat-bottomed 96-well plates in AIM-V (Invitrogen, Grand Island, NY, USA). Cultures were pulsed during the last 16 h of a 5 day culture with 1 μCi [3H] thymidine (1 μCi/well = 37 kBq/well, ICN Biomedicals, Eschwege, Germany) per well. Cells were harvested with a Tomtec harvester and liquid scintillation counting was performed with a Chameleon Multi label reader. Results are mean cpm ± SD of triplicate wells.
Cytokine measurements
Cytokines were measured at 96 h in culture supernatants of pre- or postvaccination PBMCs stimulated with the substances indicated using the Th1/Th2 cytometric Cytokine Bead Array (CBA) from BD according the manufacturer’s instructions. Samples were analyzed using the FACSCalibur and the appropriate software from BD Biosciences (BD Biosciences Pharmingen, San Diego, CA and BD Immuncytometry Systems, San Jose, CA).
Monocyte depletion
Monocytes were depleted from PBMCs using CD14 microbeads and LD columns from Miltenyi Biotec (Bergisch Gladbach, Germany) according to the manufacturer’s instructions.
Statistical analysis
Data of continuous variables were normally distributed tested by Kolmogorov–Smirnov Test (P > 0.1). Group comparisons were preformed by using t test. All reported P values are results of two-sided tests. A P value equal to or less than 0.05 was considered statistically significant. Microsoft Excel and SPSS software was used for calculations.
Results
Increase in the ex vivo proliferative and cytokine response of PBMCs stimulated with LPS or BCG after DC vaccination
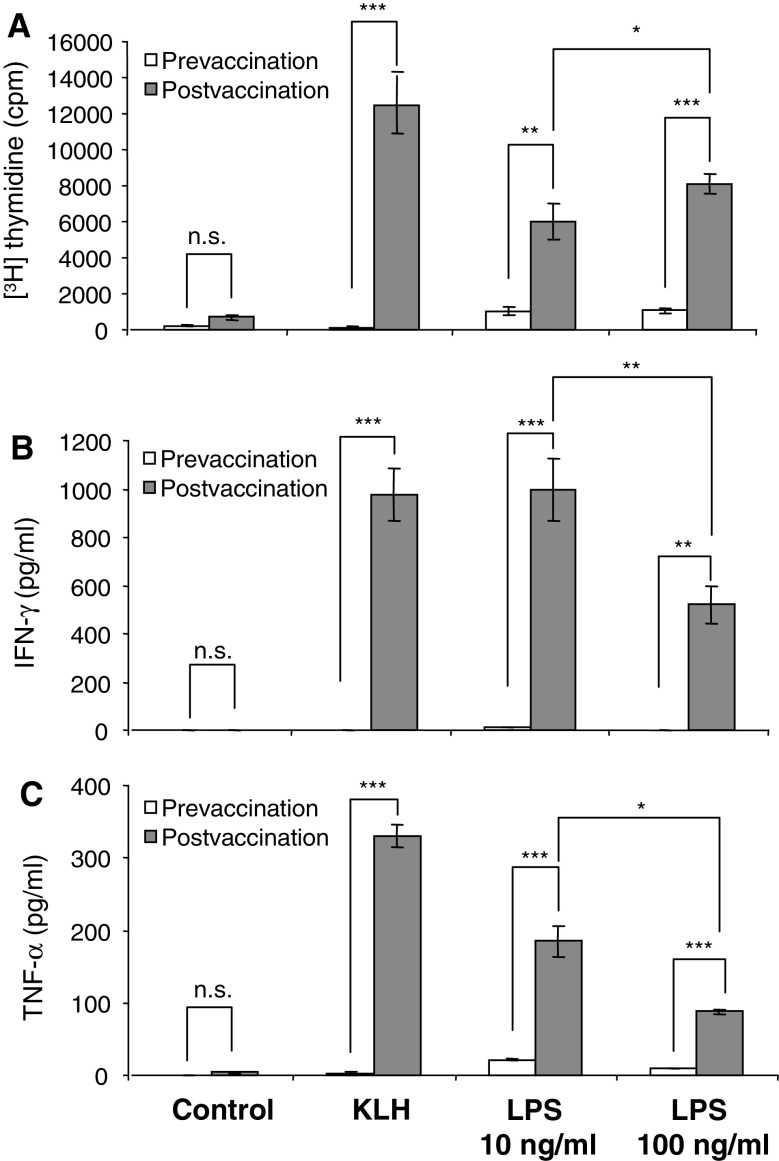
Immune monitoring of patients who had received immunotherapy with antigen-loaded, autologous, mature CD83+ moDCs [9] revealed the anticipated response against the vaccine control antigen keyhole limpet hemocyanin (KLH). Figure 1 demonstrates that KLH induced a proliferative (Fig. 1a) and cytokine response (Fig. 1b and c) in postvaccination but not in prevaccination PBMCs confirming that DC vaccination has induced KLH-specific immunity. Surprisingly, however, we also observed a DC vaccination-induced increase in the proliferative and cytokine response of PBMCs to stimulation with LPS derived from S. abortus equi (Fig. 1a–c). Increasing the LPS dose from 10 to 100 ng/ml resulted in a modest enhancement of the proliferative response (Fig. 1a), but in a diminished cytokine response (Fig. 1b and c). IL-10, IL-5, IL-4 and IL-2 were also enhanced in postvaccination PBMCs although at low levels (<40 pg/ml; data not shown).
Fig. 1.
Increase in the ex vivo PBMC response to LPS stimulation after vaccination with autologous, antigen-loaded, mature CD83+ moDCs. PBMCs harvested before the first (prevaccination) and after the third vaccination (postvaccination) were either left unstimulated (control) or stimulated with the vaccine control antigen KLH (2 μg/ml) or with LPS from S. abortus equi. Proliferative responses (a) were assessed as [3H] thymidine incorporation and cytokines (b and c) were determined in culture supernatants using a Th1/Th2 cytokine bead array (CBA) and flow cytometry. Results are mean values of triplicate measurements ± SD (*P < 0.05–0.01; **P < 0.01–0.001; ***P < 0.001)
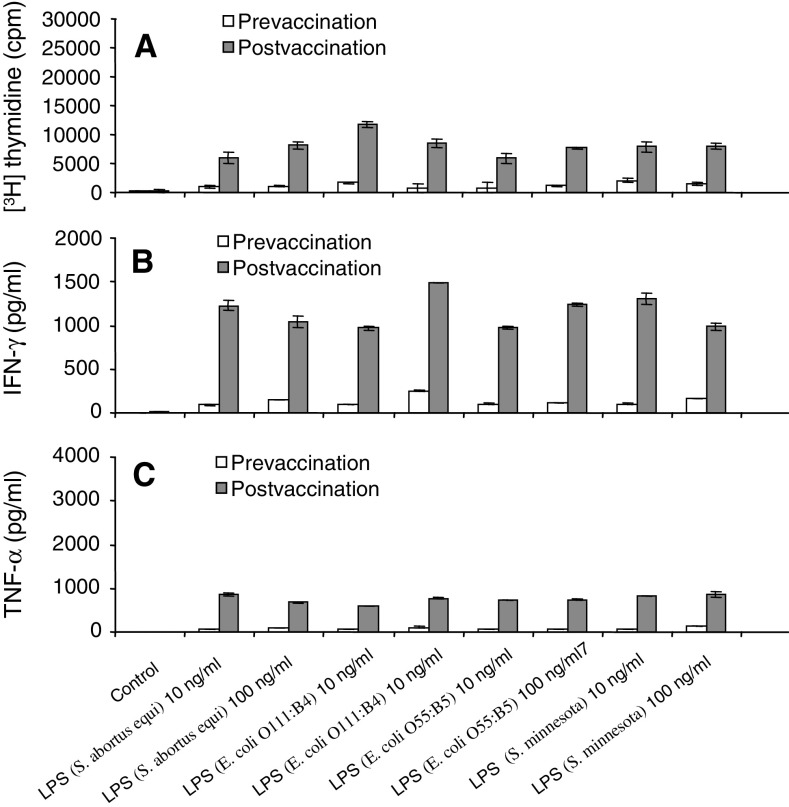
As a next step, pre- and postvaccination PBMCs were stimulated with LPS species derived from various Escherichia coli (O111:B4, O55:B5) and Salmonella strains (S. abortus equi, S. typhimurium, S. minnesota). All LPS species tested induced an increased proliferative response in postvaccination PBMCs as compared to prevaccination PBMCs (Fig. 2a). Consistent with the proliferation data, cytokine production in response to stimulation with the various LPS species was always increased in postvaccination PBMCs (Fig. 2b and c).
Fig. 2.
Increase in the ex vivo PBMC response to LPS stimulation after autologous DC vaccination. Ex vivo PBMC responses were assessed before the first (prevaccination) and after the third vaccination (postvaccination). PBMCs were either left unstimulated (control) or stimulated with various LPS species derived from Salmonella or Escherichia strains. Proliferative responses were assessed as [3H] thymidine incorporation. Results are mean values of triplicate measurements ± SD. One patient representative of six
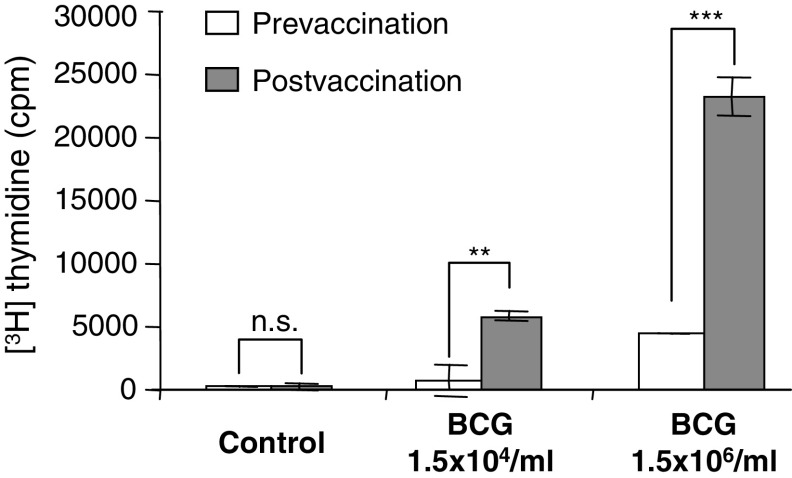
In addition to LPS, which is a component of the outer membrane of gram-negative bacteria, we also tested whole mycobacteria (BCG). BCG is a tuberculosis vaccine and has been used in cancer therapy for many years [15, 18]. BCG, like LPS, induced a much stronger response in postvaccination than in prevaccination PBMCs (Fig. 3) indicating that the observed effects are not restricted to gram-negative bacteria.
Fig. 3.
Increase in the ex vivo PBMC response to BCG stimulation after autologous DC vaccination. Ex vivo PBMC responses were assessed before the first (prevaccination) and after the third vaccination (postvaccination). PBMCs were either left unstimulated (control) or stimulated with BCG. Proliferative responses were assessed as [3H] thymidine incorporation. Results are mean values of triplicate measurements ± SD. One patient representative of three (*P < 0.05–0.01; **P < 0.01–0.001; ***P < 0.001)
Enhancement of the KLH-induced proliferative and cytokine response of PBMCs by LPS: synergistic enhancement of cytokine production
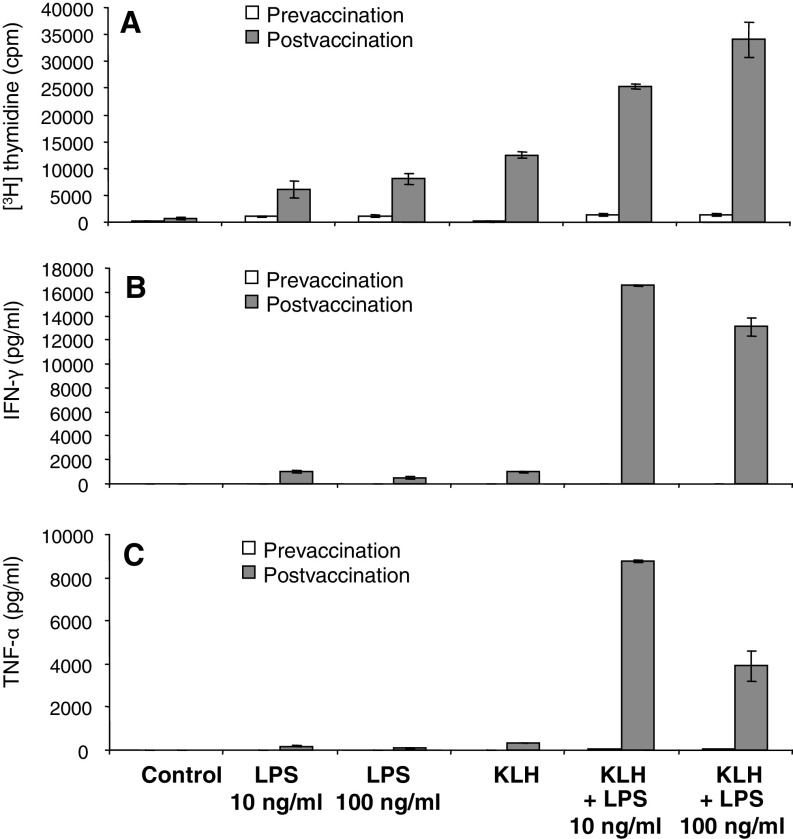
We then wanted to know whether the increased responses to LPS can affect the vaccination-induced responses against the control antigen KLH. Data shown in Fig. 4 confirm that DC vaccination induces KLH-specific immunity and increased LPS responsiveness. Importantly, concomitant addition of KLH and LPS resulted in a substantial increase of the proliferative response (Fig. 4a and b). The LPS effect on KLH-induced proliferation was dose-dependent (Fig. 4a). The effects of LPS on KLH-induced cytokine production were even more striking and clearly synergistic. Low dose LPS (10 ng/ml) induced a dramatic increase in KLH-induced IFN-γ and TNF-α production. In contrast to the proliferative response (Fig. 4a), the KLH-induced cytokine responses could not be further increased by high dose LPS (Fig. 5b and c) indicating that low dose LPS mediates optimum cytokine production.
Fig. 4.
Enhancement of the ex vivo PBMC response to KLH by LPS. PBMCs were harvested before the first (prevaccination) and after the third vaccination (postvaccination) with autologous, antigen-loaded, mature CD83+ moDCs. PBMCs were either left unstimulated (control) or stimulated with LPS (S. abortus equi), the vaccine control antigen KLH (2 μg/ml) or with combinations of KLH and LPS. Proliferative responses were assessed as [3H] thymidine incorporation. Cytokine contents in culture supernatants were determined using a Th1/Th2 cytokine bead array (CBA) and flow cytometry. Results are mean values of triplicate measurements ± SD. One patient representative of three
Fig. 5.
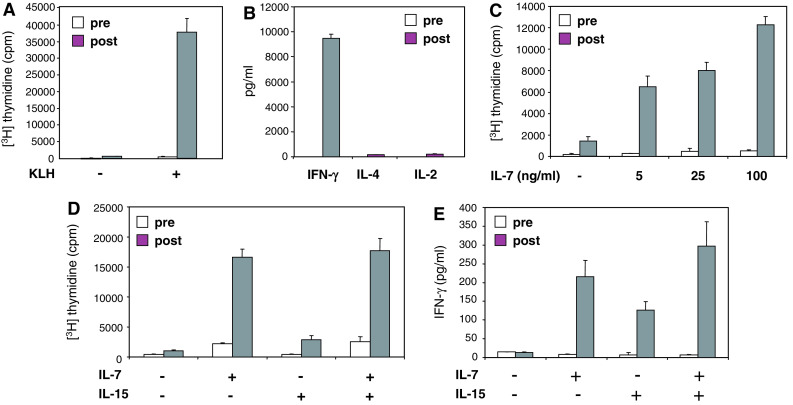
Increase in the ex vivo PBMC response to stimulation with homeostatic cytokines after DC vaccination. PBMCs were harvested before the first (prevaccination) and after the third vaccination (postvaccination) with autologous, antigen-loaded, mature CD83+ moDCs. PBMCs were stimulated with the vaccine control antigen KLH (2 μg/ml), IL-7, IL-15 or a combination of IL-7 and IL-15. Proliferative responses were assessed as [3H] thymidine incorporation. Cytokine contents in culture supernatants were determined using a Th1/Th2 cytokine bead array (CBA) and flow cytometry. Results are mean values of triplicate measurements ± SD. One patient representative of nine
Increase in the ex vivo proliferative and cytokine response of PBMCs stimulated with homeostatic cytokines after DC vaccination
The KLH-induced proliferation of postvaccination PBMCs is accompanied by strong IFN-γ production and by the relative absence of IL-4 and IL-2 (Fig. 5 a and b). Such a cytokine profile is characteristic of Th1 type effector memory T cells [12], which can be induced to proliferate by IL-7 and IL-15 [6]. As a final step we therefore tested the responsiveness of pre- and postvaccine PBMCs to IL-7 and IL-15, both common γ chain cytokines that can induce homeostatic proliferation of memory T cells.
Importantly, IL-7 dose-dependently induced much stronger proliferation in post- than in prevaccine PBMCs (Fig. 5c). In addition, IL-15 induced proliferation was also enhanced in postvaccine PBMCs, although less prominent compared to IL-7, and IL-15 did not cooperate with IL-7 (Fig. 5d). In contrast to IL-15, IL-7 also induced a modest proliferative response in prevaccine PBMCs (Fig. 5c, d). Both, IL-7 and IL-15 induced IFN-γ production in postvaccine PBMCs but not in prevaccine PBMCs (Fig. 5e). In accordance with the proliferative response, IL-7 appeared to be more potent than IL-15 in inducing IFN-γ production (Fig. 5e).
Increase in IL-7 induced proliferation of PBMCs after monocyte depletion
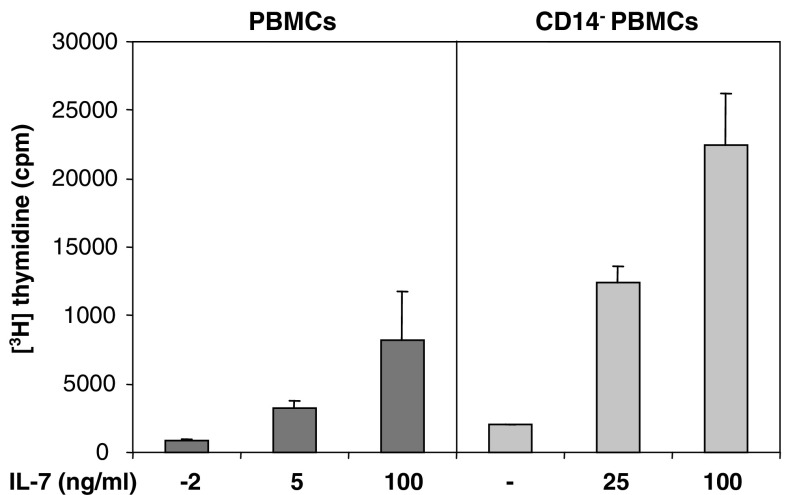
Previous work has demonstrated that LPS-induced T cell proliferation depends on monocytes. LPS acts on monocytes, which then acquire the ability to induce T cell proliferation even if LPS is absent during T cell stimulation [14]. In contrast, little is known about the role of monocytes in responses induced by homeostatic cytokines. We therefore stimulated postvaccine PBMCs with increasing concentrations of IL-7 with or without prior depletion of monocytes using MACS technology. In contrast to LPS induced responses, monocyte depletion resulted in a substantial enhancement of IL-7 induced proliferation (Fig. 6). Monocyte depletion also resulted in an enhancement of the proliferative response to IL-15, which was, however, less prominent compared to the IL-7 induced response (data not shown).
Fig. 6.
Increase in IL-7 induced proliferation of PBMCs after monocyte depletion. PBMCs were harvested after the third vaccination with autologous, antigen-loaded, mature CD83+ moDCs. PBMCs were depleted of monocytes using CD14 microbeads and LD columns (= CD14- PBMCs). PBMCs or CD14- PBMCs were stimulated with increasing concentrations of IL-7. Proliferative responses were assessed as [3H] thymidine incorporation. Results are mean values of triplicate measurements ± SD. One patient representative of three
Discussion
This is the first study demonstrating that vaccination with DCs induces an increase in the ex vivo proliferative and cytokine responses against bacterial components (Figs. 1, 2 and 4), whole bacteria (Fig. 3), and homeostatic cytokines (Fig. 5).
LPS acts via toll-like receptor (TLR)-4, which is a member of a family of pattern recognition receptors that detect pathogen-associated molecular patterns [11, 20]. LPS acts mainly on antigen-presenting cells such as macrophages, B cells and DCs. LPS has been shown to stimulate memory T cell proliferation both in vitro [14] and in vivo [19]. In contrast to B cells, which require quite high doses of LPS, very low doses of LPS are sufficient to cause T cell proliferation [19]. In vitro studies demonstrated that the activation of memory T lymphocytes by LPS requires direct cell-to-cell contact with viable accessory monocytes. This interaction was found to be MHC-unrestricted, but strongly dependent on costimulatory signals provided by B7/CD28 interactions [14]. Likewise, in vivo induction of memory T cell proliferation with LPS has been shown to operate indirectly via APC activation [19]. In contrast, little is known about the regulatory role of monocytes in the proliferative responses to homeostatic cytokines. To examine the role of monocytes in IL-7 induced responses, we depleted monocytes from PBMCs using anti-CD14 and MACS technology. Intriguingly, we found that monocyte depletion resulted in a substantial enhancement of the IL-7 induced proliferation (Fig. 6), which is in sharp contrast to LPS-induced responses, which require the presence of monocytes [14]. This finding may have important practical implications since it suggests that monocyte depletion can improve the IL-7 driven expansion of antigen-specific T cells induced by DC vaccination.
Another interesting observation in our study was that LPS could substantially enhance anti-KLH responses induced by DC vaccination (Fig. 4) suggesting that low doses of bacterial products are required for the induction of optimal immune responses [4]. The cooperative effect between LPS and KLH was particularly evident at the level of cytokine production. Low dose LPS (10 ng/ml) induced a synergistic enhancement of KLH-mediated IFN-γ and TNF-α production (Fig. 4b and c). This finding also reveals an important control mechanism. The massive production of IFN-γ and TNF-α, which are both cytotoxic cytokines, occurs only in the presence of bacterial products signaling infection and thus danger. An obvious clinical implication of this finding is that T cell memory generated by DC vaccination can be enhanced by low doses of bacterial components.
Another way to enhance the efficacy of DC vaccination may be via homeostatic cytokines. In the present study we found an enhanced ex vivo responsiveness of patient PBMCs to IL-7 and IL-15, both members of the common γ chain family of cytokines (Fig. 5). One possible explanation for the enhanced ex vivo responsiveness of patient PBMCs to IL-7 and IL-15 is a DC vaccination induced generation of memory T cells, which are characterized by an increased sensitivity to IL-7 and IL-15 [12, 17].
Taken together, our findings suggest that IL-7 and IL-15 may be useful to expand memory T cells induced by DC vaccination either in vivo or ex vivo for subsequent adoptive immunotherapy and that low doses of bacterial products may be used to potentiate effector cytokine production. Future studies are required to clarify whether antigen-independent responses may serve as a useful surrogate for successful vaccination and favorable clinical course.
Acknowledgments
This work was supported by a grant awarded to Martin Thurnher from the kompetenzzentrum medizin tirol (KMT), a center of excellence. We thank Gordon Koell (KMT) and the Tilak GesmbH, the holding company of our hospital for continuous support.
Footnotes
Supported by a grant to Martin Thurnher from the kompetenzzentrum medizin tirol (kmt), a center of excellence.
References
- 1.Banchereau Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 4.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 5.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 6.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilliet M, Kleinhans M, Lantelme E, Schadendorf D, Burg G, Nestle FO. Intranodal injection of semimature monocyte-derived dendritic cells induces T helper type 1 responses to protein neoantigen. Blood. 2003;102:36–42. doi: 10.1182/blood-2002-07-2274. [DOI] [PubMed] [Google Scholar]
- 8.Holtl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet. 1998;352:1358. doi: 10.1016/S0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 9.Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–3376. [PubMed] [Google Scholar]
- 10.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 12.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Marten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, Caspari R, Flieger D, Hanfland P, Von Ruecker A, Eis-Hubinger AM, Muller S, Schwaner I, Lohmann U, Heylmann G, Sauerbruch T, Schmidt-Wolf IG. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2003;14:483–494. doi: 10.1089/104303403321467243. [DOI] [PubMed] [Google Scholar]
- 14.Mattern T, Thanhauser A, Reiling N, Toellner KM, Duchrow M, Kusumoto S, Rietschel ET, Ernst M, Brade H, Flad HD, et al. Endotoxin and lipid A stimulate proliferation of human T cells in the presence of autologous monocytes. J Immunol. 1994;153:2996–3004. [PubMed] [Google Scholar]
- 15.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 16.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 17.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Thurnher M, Ramoner R, Gastl G, Radmayr C, Bock G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–134. doi: 10.1002/(SICI)1097-0215(19970106)70:1<128::AID-IJC19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 21.Wierecky J, Muller MR, Wirths S, Halder-Oehler E, Dorfel D, Schmidt SM, Hantschel M, Brugger W, Schroder S, Horger MS, Kanz L, Brossart P. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]