Abstract
Immunotherapy has been widely investigated for its potential use in cancer therapy and it becomes more and more apparent that the selection of target antigens is essential for its efficacy. Indeed, limited clinical efficacy is partly due to immune evasion mechanisms of neoplastic cells, e.g. downregulation of expression or presentation of the respective antigens. Consequently, antigens contributing to tumor cell survival seem to be more suitable therapeutic targets. However, even such antigens may be subject to immune evasion due to impaired processing and cell surface expression. Since development and progression of tumors is not only dependent on cancer cells themselves but also on the active contribution of the stromal cells, e.g. by secreting growth supporting factors, enzymes degrading the extracellular matrix or angiogenic factors, the tumor stroma may also serve as a target for immune intervention. To this end several antigens have been identified which are induced or upregulated on the tumor stroma. Tumor stroma-associated antigens are characterized by an otherwise restricted expression pattern, particularly with respect to differentiated tissues, and they have been successfully targeted by passive and active immunotherapy in preclinical models. Moreover, some of these strategies have already been translated into clinical trials.
Keywords: Endothelial cells, Fibroblast, Immune therapy, Macrophage, Stroma-associated antigens
Introduction
It was estimated that in the U.S. 1,368,030 cancer cases would be diagnosed and 563,700 people would die from cancer in 2004 [116]. While early, localized disease may effectively be treated by radical excision, metastatic cancer in most cases is fatal. Nevertheless, some patients show spontaneous regression of both primary tumors and metastases. This event is largely attributed to adaptive immune responses, and the presence of cytotoxic T cells (CTLs) infiltrating the tumor is associated with a better clinical prognosis [19, 37, 60]. In addition, the increased tumor incidence in immune suppressed individuals indicates that cancer, at least in part, can be controlled by the immune system [1]. Therefore, efforts are being made to stimulate the patient’s immune effector cells to recognize and destroy cancer cells. To this end, several different active immune therapies are currently under investigation, e.g. vaccination with whole cells [100, 114], protein, peptides [81, 99], nucleic acids encoding the respective antigens [35, 103, 112] or combinations thereof.
Antitumor immunotherapy targeting tumor-associated antigens
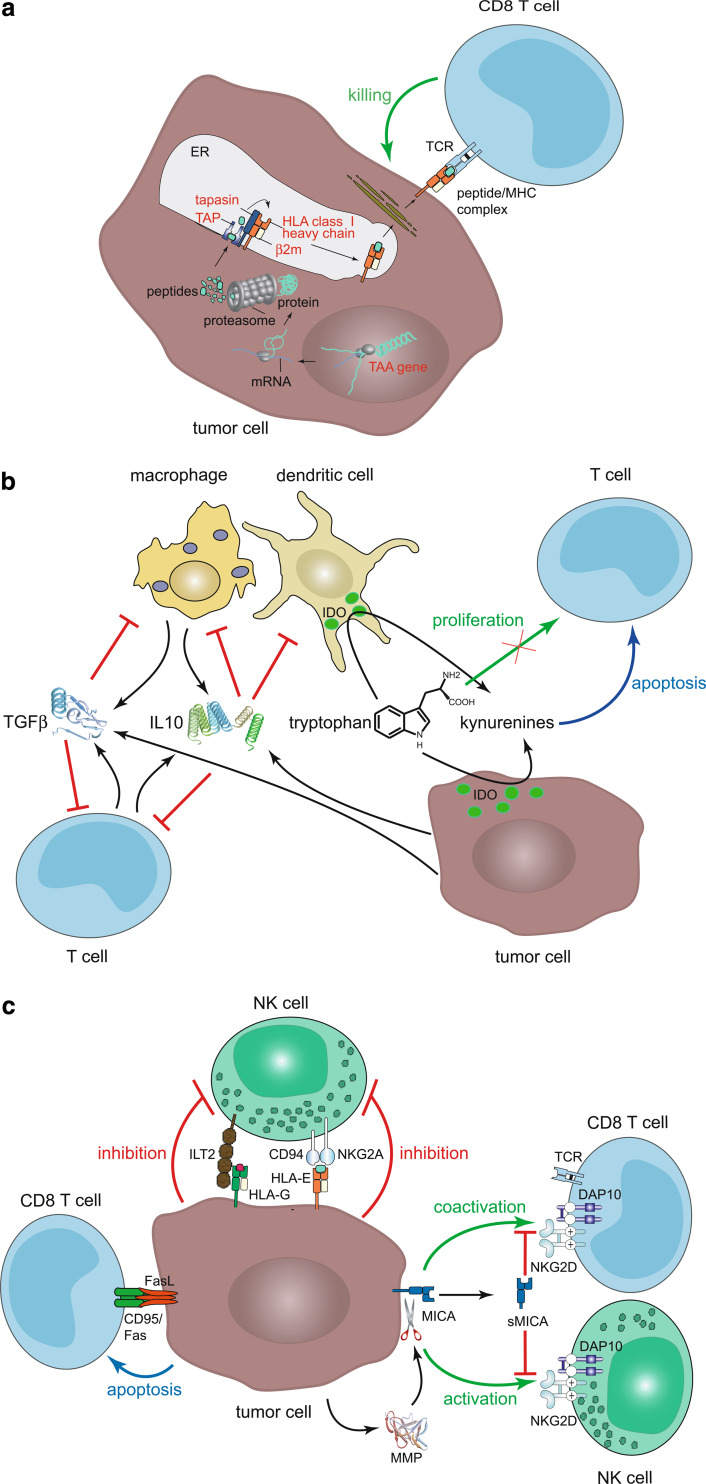
Despite the fact that immune-based treatments are able to induce objective tumor responses in selected patients, a cancer vaccine that can reliably induce tumor destruction or improve patient survival is yet to be established. In this respect, it is important to note that the majority of current vaccination strategies is based on tumor-associated antigens (TAAs). This strategy is actually prone to several pitfalls as cancer cells may be subject to several immune evasion mechanisms (Fig. 1) [2, 82, 111]. The presentation of TAA-derived peptides to CTLs can be lost by mutation, deletion or silencing of the TAA gene itself or defects in the antigen presentation pathway e.g. loss of MHC class I heavy chains, β2-microglobulin, TAP or tapasin expression (Fig. 1a). Tumor cells may promote anergy of T cells by a T cell receptor stimulation in the absence of adequate coactivation, e.g. lack of CD28 or ICOS, or active negative signaling. Secretion of immunomodulatory molecules such as TGF-β and IL-10 by tumor cells themselves or stroma cells may inhibit eradication of tumor cells by preventing T cell proliferation and differentiation, or T cell stimulatory functions of APCs (Fig. 1b) [33, 70]. A variety of human tumors express functionally active indoleamine 2,3-dioxygenase (IDO). Moreover, besides the tumor cells themselves, antigen presenting cells (APCs) infiltrating the tumor, its periphery or the tumor draining lymph node may also express IDO [29, 61, 73, 118]. Although IDO may inhibit tumor cell proliferation by tryptophan depletion, (as it has been demonstrated in vitro), this antiproliferative effect is outweighted in vivo by the immune suppressive effect inhibiting immune surveillance [118]. Indeed, tumor cells have been demonstrated to recruit IDO expressing cells, such as APCs which render the host tolerant to TAAs (Fig. 1b) [29, 61, 73]. Even in cases where recognition of cancer cells and activation of T cells is not prevented by these mechanisms, neoplastic cells may evade cell death by upregulation of endogenous apoptosis inhibitors such as survivin, secretion of decoy receptors neutralizing FasL, and impaired Fas (CD95) signaling or loss of its expression. In addition, many types of tumors express FasL; consequently T cells activated in the tumor micromilieu may be deleted as they express Fas shortly after activation and thereby become susceptible to FasL-mediated cell death (Fig. 1c) [49, 110]. Tumor cells have also developed mechanisms to evade an attack from NK cells. NK cells express activating receptors such as NKG2D which react with MHC class I chain-related A (MICA) or UL16-binding proteins (ULBPs). These antigens are upregulated as a consequence of neoplastic transformation [83]. MICA may be proteolytically cleaved from the cell surface by matrix metalloproteinases (MMPs) expressed in the tumor microenvironment and soluble MICA prevents activation of effector cells by binding to NKG2D (Fig. 1c) [94]. Reduced MHC class I antigen expression on tumor cells on one hand results in impaired recognition by T cells; on the other hand this is accompanied by decreased inhibitory signaling to NK cells, i.e. the activation of the innate immune system. Tumor cells, however, compensate for the loss of inhibitory signaling to NK cells provided by classical MHC molecules by upregulation of MHC class Ib antigens such as HLA-E and HLA-G interacting with the respective inhibitory receptors (Fig. 1c) [3, 122].
Fig. 1.
Immune evasion mechanisms of solid tumors. a Alterations in expression of TAA and MHC class I abrogate antigen presentation. Production of TAA-derived peptides can be prevented by mutation or deletion of the TAA gene. The formation of MHC class I/peptide complexes can be hindered by loss or mutation of HLA class I heavy chains, β2-microglobulin or components of the protein processing machinery, e.g. TAP1/TAP2 and tapasin. Cellular components that can contribute to immune evasion by mutation or altered expression are highlighted in red. b Secretion of inhibitory molecules such as IL-10 and TGF-β by tumor and stromal cells may reduce anti-tumoral activity of effector cells. These molecules inhibit proliferation and differentiation of T cells to CTLs and helper T cells and they prevent the T cell stimulatory functions of APCs by downregulation of MHC class I and II expression and their maturation. IDO expression in tumor cells and/or TAMs or APC in the tumor draining lymph nodes may inhibit T cell proliferation due to lack of tryptophan and may induce their apoptosis by tryptophan metabolites. c Activation of cytotoxic effector cells may be prevented by enhanced negative and/or reduced activating signaling. (1) Expression of FasL by tumor cells may induce apoptosis of tumor-reactive T cells. (2) Upregulation of MHC class Ib antigens, e.g. HLA-E and HLA-G inhibit cytotoxicity of NK and T cells by delivering inhibitory signals via CD94/NKG2A and ILT2, respectively. (3) Activation or coactivation of NKG2D expressing NK and T cells respectively may be prevented by loss of MIC and ULBP expression or the cleavage of MICA by MMPs; soluble MICA binds to and thereby blocks activating effects of NKG2D on effector cells
Melanoma may serve as an example to illustrate yet another immune evasion of solid tumors to TAA-directed vaccination. The most frequently used TAAs for vaccinations to treat this tumor are melanocyte differentiation antigens such as gp100, Melan-A/Mart-1 or tyrosinase, and cancer testis antigens, e.g. the MAGE family [99, 109]. The expression of these proteins, however, is irrelevant for the progression of the tumor. Thus, their expression can be downregulated without impacting tumor progression and subsequent immunoselection results in antigen negative variants [52]. In fact, expression of melanocyte differentiation antigens not only differs from patient to patient but also may vary between metastases of individual patients with a trend towards reduced expression with disease progression [93]. However, even mutated, oncogenetic proteins opt for loss due to immune selection. Activating mutations of BRaf, for example, are present in a large percentage of human melanoma, the majority consisting of the single missense substitution of a valine for a glutamic acid at position 600 (V600E). In some patients V600EBRaf-specific CTL responses have been detected. The efficacy of immune selective pressure is demonstrated by the fact that this V600EBRaf genotype may be lost during progression from primary to metastatic melanoma [5]. The possible growth disadvantage of these loss variants is compensated by the fact that they are not eradicated by the immune system.
The tumor stroma as target for immune intervention
Carcinogenesis is a process depending on genetic and epigenetic alterations accumulating in transforming cells. Nevertheless, many steps necessary for tumor progression e.g. proliferation, invasion, angiogenesis, and metastasis are influenced by microenvironmental factors such as growth factors, angiogenic factors, cytokines, and proteolytic enzymes. During transformation, reciprocal interactions occur between neoplastic and adjacent normal cells, i.e. fibroblasts, endothelial, and immunocompetent cells. In general, stroma cells contribute 20–50% to the tumor mass, but the stromal compartment may account for up to 90% in several carcinomas. The microenvironment influences the stroma cells in such a way that they rather promote tumor progression than inhibit it by allowing vasculo- and angiogenesis, recruitment of reactive stromal fibroblasts, lymphoid and phagocytic infiltrates, secretion of peptide mediators, and proteolytic enzymes, as well as the production of a modified extracellular matrix (ECM). In contrast to cancer cells, tumor stroma cells are genetically more stable so that at least some immune evasion mechanisms of tumors do not apply for these cells. Nevertheless, stroma cells differ from their normal counterparts by upregulation or induction of various antigens. Some of the tumor stroma-associated antigens (TSAAs) are highly selective for the tumor microenvironment. It should be noted that some TSAAs may be expressed in the neoplastic cells as well and that they are not confined to one histiotype, indeed, they may be expressed by a broad spectrum of solid tumors. Thus, therapies designed to target the tumor stroma are not restricted to a selected tumor entity.
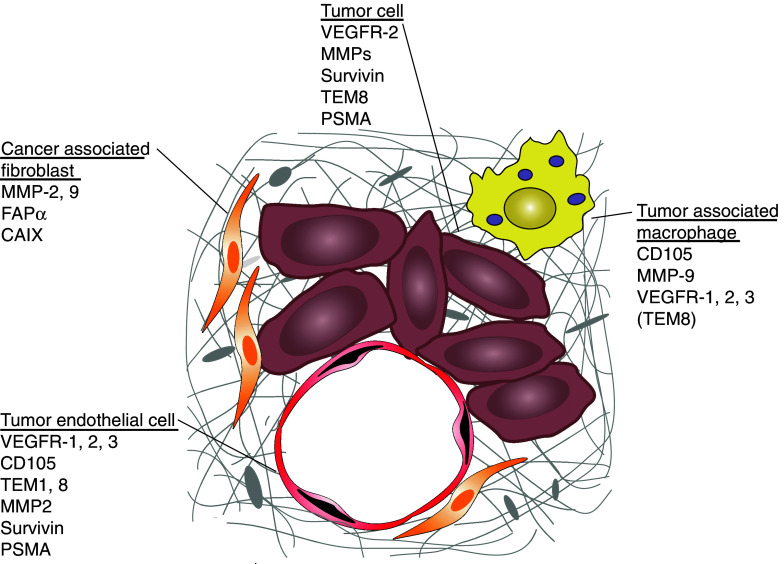
The most promising cellular targets for stroma-directed cancer are probably stromal fibroblasts, endothelial cells, and tumor-associated macrophages. Cancer-associated fibroblasts (CAFs) are reactive fibroblasts with a phenotype differing from that of quiescent fibroblasts in normal adult tissue. CAFs contribute to the development of cancer by secreting growth promoting factors such as TGF-β, matrix degrading enzymes, and angiogenic factors, e.g. MMPs or vascular endothelial growth factor (VEGF). Endothelial cells have a major part in tumor progression since they are necessary for angiogenesis. For tumor growth beyond a few cubic millimeter new blood vessels are required to deliver oxygen and nutrients, and to remove waste products. Tumor endothelial cells (TECs) express surface receptors and secrete factors that sustain their own growth by an autocrine pathway. Moreover, tumor cells contribute to the growth of the TECs by paracrine secretion of the respective growth factors. Vice versa, growth factors produced by TECs support the oncogenic phenotype of tumor cells. Another prime target cell population for immune intervention present in the tumor microenvironment is tumor-associated macrophages (TAMs). Although TAMs may contribute to tumor control, clinical and experimental evidence strongly suggest that their presence rather results in enhanced proliferation, invasion, and angiogenesis. Due to the various interactions of tumor and stroma cells in the tumor microenvironment, agents used for antistromal therapy fall into different categories, e.g. enzyme and protease inhibitors [92], antiadhesive molecules, such as anti-integrin peptides or antibodies [36], inhibitors of signaling pathways, i.e. antibodies to VEGF or VEGFRs [102], and active immunotherapy. In the following we will focus on the relevance of TSAAs as possible targets for anticancer immune therapy aiming at the destruction of the tumor stroma. For a cytotoxic approach directed at antigens overexpressed in tumor stroma, the expression level of the targeted antigen in a normal tissue should be absent or at least low. Otherwise targeting vascular endothelial cells may for example disturb physiological angiogenesis in wound healing or the female reproductive cycle, particularly pregnancy. Therefore, ideal target proteins are selectively induced or upregulated in the tumor micromilieu, and they confer a growth or survival advantage for the tumor. An additional expression on the tumor cells is even more advantageous allowing the simultaneous targeting of tumor and stroma cells (fig. 2).
Fig. 2.
Target antigens on tumor cells, CAFs, TECs, and TAMs. Tumor cells are in close contact with fibroblasts (CAFs), endothelial cells (TECs), and macrophages (TAMs). In the tumor microenvironment antigens are induced or upregulated on stroma cells that can be targeted by immune therapeutic approaches
Cancer-associated fibroblasts (CAFs)
Cancer-associated fibroblasts are present in the close vicinity of tumor cells and contribute to the progression of cancer. They secrete growth and angiogenic factors supporting the proliferation and survival of the neoplastic cells. The selective destruction of CAFs should therefore interfere with the progression of cancer. For this purpose several antigens expressed on CAFs may be considered.
The carbonic anhydrase IX (CAIX, MN, G250) is important for the pH regulation. It is upregulated under hypoxic conditions in fibroblasts e.g. within the tumor micromillieu. To this end CAIX overexpression has been demonstrated in CAFs of renal cell, colorectal, cervix, NSCL, bladder, and kidney cancer as well as on some malignant cells themselves [21, 50]. High CAIX expression in tumors is associated with an unfavorable prognosis [21]. It should be noted, however, that CAIX is also expressed in normal gastric epithelium [63]. In vitro CAIX-specific antibodies mediate ADCC that can be enhanced by cytokines [67]. Interestingly, treatment of renal cell carcinoma with a CAIX-specific antibody resulted in clinical responses [10]. Spontaneous T cell responses against CAIX, however, are only rarely detectable in cancer patients even if TILs (tumor-infiltrating lymphocyte) are used for analysis [34]. Nevertheless, DCs loaded with MHC-restricted peptide epitopes derived from CAIX are able to stimulate both CD8+ and CD4+ T cells which are capable of recognizing naturally processed CAIX [119, 120]. Upon vaccination of renal cancer patients with DCs transfected with whole tumor RNA, CTL expansion, and cytotoxicity to a broad set of tumor antigens, including CAIX was detected [103]. In animal models transduction of DCs with a recombinant adenovirus encoding, a GM–CSF/CAIX fusion protein induced specific CTL responses which inhibited growth of tumor cells in vivo [41]. These results suggest that an induction of anti-CAIX-immune responses may be a feasible approach for anticancer therapy.
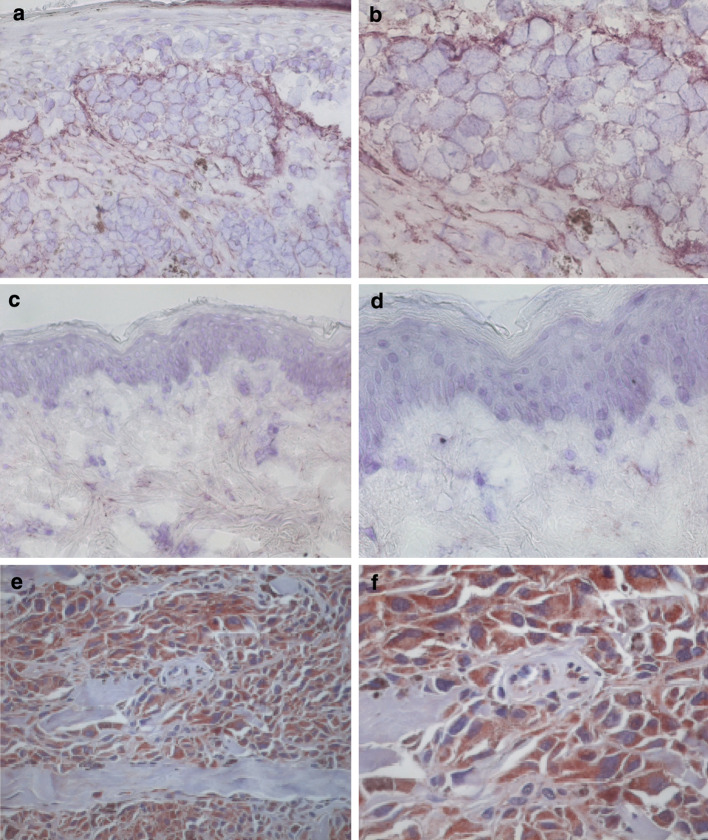
The fibroblast activation protein α (FAPα, seprase) is a surface glycoprotein selectively expressed on reactive stromal fibroblasts of solid tumors, whereas it is hardly present in adult tissue or benign epithelial tumors (Fig. 3a-d). It should be noted, however, that FAPα is not only induced during carcinogenesis, but also during embryonic development and wound healing. Still, FAPα is overexpressed in the stroma of more than 90% of common solid cancers [30, 47, 56, 72] and this overexpression is associated with enhanced tumor growth, invasion, angiogenesis, and metastasis [46, 51]. Patients afflicted by FAPα+ gastric carcinoma have a reduced survival time compared to patients with FAPα− tumors [72]. However, the role of FAPα in cancerogenesis is still controversial as in some studies a reversed association has been described; e.g. while in a mouse model for human breast cancer FAPα promotes tumor growth, in human breast carcinoma expression of FAPα is associated with better survival [8, 46]. Interestingly, a similar apparently paradoxical situation has been described for bcl-2 expression; a notion which has been attributed to protective immune responses in humans [9]. FAPα expression has also been identified in neoplastic cells [32, 56]. Thus, FAPα has been used as a target for therapeutic interventions either aiming at inhibiting its function or mediating the destruction of FAPα+ cells. To this end, several antibody constructs have been tested. A 131 I-labeled FAPα-specific antibody was used to characterize its biodistribution in man, demonstrating that it selectively accumulated in primary colorectal carcinoma and corresponding liver metastases [121] and a FAPα-CD3-bispecific single chain antibody was used to target CTLs to the tumor stroma and induced cytotoxicity was demonstrated in vitro [123]. The direct harnessing of the adaptive immune system to attack FAPα+ cells is the subject of ongoing research efforts (Hofmeister and Becker, unpublished results).
Fig. 3.
Expression of FAPα and survivin in tumors. FAPα is expressed by fibroblasts and tumor cells of melanoma, whereas staining of normal skin is negative. Immunohistochemical staining of melanoma (a, b) and normal skin (c, d) with the FAPα-specific antibody F11-24 is shown. Staining of a pancreas carcinoma with a survivin-specific polyclonal rabbit antiserum (e, f) demonstrates that survivin is not only expressed on tumor cells but also on endothelial cells in the tumormicromilieu. Magnification of 20× (a, c, e) and 40× (b, d, f)
Structural changes in the ECM are necessary for neoplastic cell invasion and metastasis. The matrix metalloproteinases (MMPs) and their inhibitors have been shown to be critical modulators of ECM composition. Several members of this family of membrane-anchored and secreted zinc-dependent proteases (e.g. MMP-1, −2, and −9) have been shown to be overexpressed in neoplasia [44]. Both tumor and stroma cells, particularly fibroblasts, but also endothelial cells and macrophages, express MMPs. In contact coculture experiments of human fibroblasts and melanoma cells the expression of MMP-2 in membrane extracts was enhanced compared with those in individual cultures [78]. MMP expression correlates with an invasive phenotype of tumor cells and tumor progression [43]. Thus, this upregulated expression of MMPs on both stromal and tumor cells suggest them as possible candidates for active vaccination therapies. First attempts using MMP-encoding RNA transfected DCs to induce T cells are currently in progress 1.
Tumor endothelial cells (TECs)
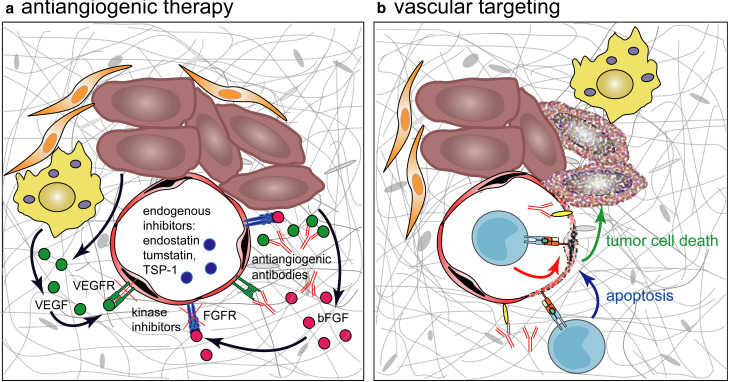
Tumor vascularization includes angiogenesis and vasculogenesis. Prevention of the development of new blood vessels is mostly achieved by antiangiogenic therapy (Fig. 4a). Extracellular angiogenic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) may be blocked by neutralizing antibodies or soluble receptors. Another approach also takes advantage of endogenous angiogenesis inhibitors e.g. angiostatin or tumstatin. In contrast, vascular targeting agents are designed to cause selective destruction of existing tumor blood vessels (Fig. 4b). This strategy takes advantage of antigens expressed on activated endothelial cells not necessarily directly implicated in vasculogenesis. The endothelium lining the blood vessels of differentiated normal tissue is quiescent. In contrast, the vascular endothelial cells in tumors exist in an environment that is rich with pro-angiogenic factors and inflammatory cytokines. These factors cause TECs to express or upregulate activation and proliferation markers, markers of hypoxic stress, growth factor receptors, and yet undefined endothelial cell antigens. However, a phenotype which is absolutely specific for tumor endothelial cells has not been identified to date and it probably does not exist. Nevertheless, side effects of therapies targeting the vascular endothelial cells are expected to be low, if the attack is restricted to proliferating endothelial cells. Angiogenesis is infrequent in adults, except during wound healing, ovulation, pregnancy, and certain chronic diseases. Immunological targeting of endothelial cells is an attractive approach as they are highly accessible to antibodies or lytic effector cells. The resulting immunological destruction of tumor blood vessels should lead to widespread necrosis in solid tumors, hence inflicting its effect upon hundreds of tumor cells. On tumor endothelial cells several molecules are expressed that have potential as immunotherapeutic targets: (1) growth factor receptors such as VEGF receptors and CD105 (TGF-β receptor), (2) cell surface antigens with enzymatic activity e.g. prostate-specific membrane antigen (PSMA), and (3) functionally uncharacterized antigens as tumor endothelial markers (TEMs).
Fig. 4.
Antiangiogenic therapy versus vascular targeting. a Antiangiogenic therapy addresses the signals mediating growth and invasion of endothelial cells by capture of soluble growth factors with antibodies or soluble receptors, by use of specific endogenous inhibitors such as endostatin, tumstatin or thrombospondin-1 (TSP-1) or kinase inhibitors that block signals generated by growth factor binding. Redundancy of signaling pathways may allow evasion of therapeutic interventions by upregulation of alternative interactions, e.g. signaling by EGFRs while targeting VEGF/VEGFR. b Immunotherapeutic vascular targeting approches directly address endothelial cells via activated T cells or antibodies reacting with TSAAs thereby mediating destruction of endothelial cells. In consequence of thrombosis the blood supply for the tumor is abrogated leading to necrosis of the cancer cells
It should be noted, that in humans most of the antiangiogenic approaches based on blocking endothelial growth factors to date failed to give the same inhibition of tumor growth seen in animal models [13]. This lack of efficacy in human patients in comparison with animal models is partly due to the heterogeneity of human tumors. As the genome of the cancer cells is unstable, the tumor cells accumulate mutations over time and form heterogenous subpopulations that differ in their production of growth factors. For example, in early-stage breast cancer VEGF is the only angiogenic factor produced, during progression, however, tumors acquire the capacity to secrete many angiogenic factors such as bFGF, TGF-β1, PIGF, PDGF, and pleiotrophin [87]. Moreover, it has been demonstrated that TECs also acquire genetic and epigenetic changes which may render them self-sufficient of growth factors [12, 42]. In contrast, in animal models a high number of relatively homogenous tumor cells are injected. These develop to sizeable tumors within a few weeks. Due to a low genetic variation they have a smaller chance to evade the therapeutic effect of the applied intervention by selection of cell populations. These differences aggravate the translation of therapeutic approaches from preclinical models to the clinic and stress the necessity of developing appropriate tumor models such as spontaneously occurring tumors. In addition, these observations demonstrate the advantage of an active immunisation approach. In contrast to interfering with a single growth factor, actively immunising with tumor endothelial-derived antigens directly aims at the destruction of the endothelial cells. In addition, the induced vascular injury is likely to cause a thrombosis in the respective vessels, thereby synergistically augmenting the antitumor effect (Fig. 4b).
Vascular endothelial growth factor is a potent pro-angiogenic growth factor abundantly expressed in most tumors. Tumor cells secrete VEGF in consequence of genetic and epigenetic changes as well as hypoxic conditions [68]. Hypoxia and increased VEGF levels induce enhanced expression of VEGF receptors, such as the receptor tyrosine kinases VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1). Consequently, several VEGF- and VEGFR-antagonists have been developed, e.g. VEGF antisense oligonucleotides, VEGF ribozymes, anti-VEGF and anti-VEGFR antibodies, VEGFR inhibitors, and soluble VEGFRs. Anti-VEGFR-2 antibodies selectively bind to tumor endothelial cells and reduce proliferation and migration of tumor cells in the mouse system [57]. In fact some of these inhibitors have already entered the clinic. Bevacizumab, a recombinant humanised anti-VEGF mab was approved by the FDA for the treatment of colorectal cancer in combined regimens [53, 54] (http://www.nci.nih.gov/clinicaltrials). Apart from these passive immunotherapies, active immunisation to VEGFRs has been tested. Vaccination with a DNA-based VEGFR-2 vaccine induced specific CTL responses and inhibited angiogenesis in a mouse model. The growth of different tumors, e.g. melanoma, colon carcinoma, and lung carcinoma, was significantly reduced, stressing the potential of VEGFR vaccine for broad antitumor therapy [77]. In addition, immune responses to VEGFR-2, i.e. the generation of neutralising antibodies and CTLs, were elicited in mice by immunisation with dendritic cells pulsed with a soluble VEGFR-2 protein. Tumor angiogenesis and the development of metastases were suppressed as well as the survival of immunised mice was prolonged [66]. Immune responses to this self-antigen were associated with only minor side effects such as delayed wound healing [77]. Therefore, VEGFR-2 seems to be a feasible candidate for vaccination studies. However, VEGFR-2 is not only upregulated on endothelial cells in tumors but also on T cells under hypoxia [71]. This may reduce the efficacy of vaccination if activated T cells are targeted, too. In contrast, VEGFR-1 is upregulated only on tumor endothelium, but not on T cells.
Another endothelial marker scrutinized for its role in vascular targeting is CD105 (endoglin). It is a 95 kD cell surface protein expressed as homodimer, which functions as an accessory receptor for transforming growth factor (TGF-) β1 and β3. CD105 modulates TGF-β signaling, i.e. it antagonises the inhibitory effects of TGF-β1 [65]. Although expression of CD105 is not restricted to TECs—actually it is present on activated monocytes, differentiated macrophages, early B cells, erythroid precursor cells, fibroblasts, follicular DCs, melanocytes, heart smooth muscle cells, and trophoblasts—only on these cells the expressed amount appears relevant for immune recognition. CD105 is upregulated on activated endothelium via TGF-β2 and by hypoxia and has been reported to be expressed on TECs of a plethora of solid tumors, i.e. breast, prostate, gastric, colorectal, renal cell, cervix, and endometrial carcinoma, as well as for melanoma, and glioblastoma [24, 106]. Expression of CD105 in these tumors is correlated with vascular density and most importantly, poor prognosis [24, 106]. Thus, CD105 appears as an attractive target for therapeutic interventions. Passive immunotherapy using antibodies specific for CD105 either alone or conjugated with different effector molecules demonstrated in preclinical models specific binding to proliferating endothelial cells and fast accumulation in tumors, whereas the antibodies react only weakly or not at all with quiescent endothelium [28, 104]. More important, angiogenesis, tumor growth, and metastases were inhibited and side effects were low [69, 95]. To test the effect on human blood vessels transplantation of human foreskin onto immunodeficient mice resulted in a chimeric vasculature partly of human and murine origin. In these tissues tumors can be induced and their growth is reduced by an anti-CD105 antibody [105]. Recently the pharmacokinetics and immunogenicity of an anti-CD105 antibody were analyzed in a primate model demonstrating its safety [96]. With respect to active vaccination, it has been demonstrated that in a murine tumor model immunisation with swine endoglin provoked specific anti-CD105 antibodies that inhibited angiogenesis and enhanced apoptosis in subsequently induced tumors. Notably, this antitumor response was dependent on CD4+ T cells and the combination of this active immunisation with cisplatin revealed a synergistic antitumor activity [107, 108]. These data demonstrate that for an effective antitumor immune response functional CD4+ T cells are essential [27].
Tumor endothelial markers (TEMs), among them TEM1 and TEM8, are overexpressed during tumor angiogenesis [101]. Despite the fact that their functions have not been characterized in detail so far, it is well established that they are strongly expressed on vascular endothelial cells in developing embryos and tumors [14]. TEM1 (endosialin) is a 165 kD highly sialylated C-type lectin-like transmembrane molecule [20]. Although TEM1 mRNA is ubiquitously expressed on endothelial cells in normal human and murine somatic tissues, TEM1 protein is largely restricted to corpus luteum and high angiogenic tissues such as the granular tissue of healing wounds or tumors [80, 89]. TEM1 protein expression is upregulated on tumor endothelial cells of carcinomas (breast, kidney, lung, colorectal, colon, pancreas mesothelioma), sarcomas, and neuroectodermal tumors (melanoma, glioma, neuroblastoma) [89]. In addition, TEM1 is expressed at a low level on a subset of tumor stroma fibroblasts [11, 80]. TEM8 (ATR = anthrax-toxin-receptor) is a transmembrane receptor existing in three splice variants. It binds to the collagen subunit α3 (VI) and its expression on endothelial cells is associated with enhanced cell-matrix-interaction and migration [45, 75]. TEM8 protein is highly expressed in endothelial cells of the stroma of breast, esophagus, lung, and bladder cancer [75]. Even if TEM8 is also found on malignant cells of colorectal cancer, it has the most restricted expression pattern of all the TEMs [90]. Notably, in contrast to other TEMs, TEM8 is not detectable during wound healing or in the corpus luteum [14, 101]. Nevertheless, TEM8 is induced in most cell types by in vitro propagation. Recently, TEM1 and TEM8 mRNA have been detected in a cell population, coexpressing DC and endothelial markers, capable of generating functional blood vessels; thus supporting the function of these molecules during vasculogenesis [22].
Prostate-specific membrane antigen (PSMA) is another endothelial cell surface molecule of particular interest for vascular targeting. PSMA is a 110 kD glycoprotein with enzymatic activity as glutamate carboxypeptidase. It is upregulated on malignant prostate cells as well as on TECs of many solid tumors including breast, renal cell, bladder, non-small cell lung, prostate and rectal carcinoma, glioblastoma multiforme, melanoma, and soft tissue sarcoma [17]. However, PSMA is also expressed on some normal tissues e.g. prostate epithelial cells, epithelia of the large intestine, and the kidney tubules. PSMA has been widely used as a therapeutic target in prostate cancer in preclinical models. Antibodies reacting with PSMA have been demonstrated to selectively bind to PSMA positive cells [76]. While PSMA-specific antibodies alone did not inhibit tumor growth, immunotoxins significantly delayed tumor progression [40]. Furthermore, a DNA vaccine encoding PSMA in combination with CpG oligodeoxynucleotides induced both anti-PSMA antibodies and cytotoxic T cell responses resulting in reduced tumor growth [88]. Clinical trials testing the efficacy of vaccination of prostate cancer patients with a DNA-based vaccine demonstrated humoral immune responses in the majority of patients [112]. In addition, T cell responses to PSMA can be induced by HLA-restricted PSMA-derived peptides; HLA-restricted, antigen-specific IFNγ production and cytotoxicity were observed among PBL of vaccinated patients [39].
Survivin is a member of the inhibitor of apoptosis (IAP) protein family. It is involved in cell cycle control and apoptosis. Overexpression of survivin has been described for virtually all tested tumors and its expression is associated with accelerated progression, higher recurrence rates, abbreviated survival, and increased therapy resistance [15, 31]. Interestingly, its overexpression in tumors is not restricted to neoplastic cells, but it is also present in TECs (Fig. 3e-f). Survivin is an especially attractive target for cancer therapy due to its universal expression in cancer, its contribution to tumor progression and its absence from most terminally differentiated normal tissues [4]. The few exceptions are thymus cells, CD34+ bone marrow-derived stem cells, and basal colonic epithelial cells [15, 31, 58]. In addition, survivin is expressed on endothelial cells after vascular injury as it is present in newly formed blood vessels during tumor angiogenesis. This is in part due to the fact that survivin is upregulated on endothelial cells by vascular endothelial growth factors [79, 113]. In several studies cellular and humoral responses to survivin have been demonstrated [6, 16, 91]. Such immune responses may actually refer protection to tumors since in murine model systems vaccination with survivin mRNA transfected DCs induced protection to subsequent tumor challenges [126]. Moreover, a DNA vaccine encoding survivin elicited effective CTL responses leading to suppression of angiogenesis, tumor apoptosis, and finally suppression or eradication of established pulmonary metastases [124]. Accordingly, in cancer patients survivin-specific T cells have been described within TIL of breast and colorectal cancer, leukemia as well as melanoma [6, 16, 48, 86]. Human survivin-specific CTLs were also obtained after stimulation of PBMCs with DCs transfected with the survivin DNA or mRNA; these effector cells were cytotoxic for malignant hemopoietic cells [85, 126]. Vaccination with survivin-derived peptides has also been evaluated in patients. In a colorectal cancer study even poor immunological responses to vaccination correlated with clinical efficacy [115]. In another trial, heavily pretreated melanoma patients mounted strong survivin-specific T cell responses if vaccinated with affinity improved survivin-derived peptides. A prolonged survival of these patients suggested an association of this survivin-specific T cell response with an improved prognosis [81]. The efficacy of targeting survivin can be explained by the concomitant attack on transformed and vascular cells. Combination with other tumor therapies as chemotherapy are especially promising, as downregulation or loss of survivin would severely inflict the growth potential of the tumor cells rendering them more susceptible to the other forms of therapy [4].
Tumor-associated macrophages (TAMs)
Tumor-associated macrophages represent a major component of the leukocyte infiltrate of solid tumors; in some tumors they can constitute up to half of the tumor’s mass. TAMs originate from circulating blood monocytes and are recruited to the tumor microenvironment by chemo- and cytokines e.g. chemotactic protein-1 (MCP-1), macrophage colony stimulating factor (M-CSF), and VEGF produced by tumor and stromal cells [26, 74]. As macrophage migration is suppressed by hypoxic conditions, TAMs preferentially accumulate in regions characterized with low oxygen tensions, i.e. the tumor stroma. In consequence, several hypoxia-inducible molecules are found to be upregulated in TAMs. These include the above-discussed antigens VEGFR-1, CD105, and MMP-9. Furthermore, additional non-hypoxia induced molecules such as TEM8 may also be expressed on TAMs. Immune targeting of these antigens on TECs and CAFs may therefore synergistically address TAMs. This is very likely to be of advantage as extensive TAM infiltration correlates with poor prognosis [18, 59], albeit some controversial evidence for their role in several tumors. In contrast to other tumor stroma cell populations, TAMs are endowed with the potential capability to kill transformed cells (by ADCC, reactive oxygen and nitrogen intermediates), if appropriately triggered or activated. Nonetheless, in most cases their cytotoxic function is ineffective during tumor initiation and progression [55]. On the other hand, TAMs may elicit functions to support tumor growth and dissemination: (1) they express a repertoire of substances promoting angiogenesis including growth factors, and proteolytic enzymes such as MMPs and the uPA system [64, 84], (2) they produce immune modulatory molecules such as IDO and IL-10 (Fig. 1b) [61, 97], and (3) they condition the extracellular matrix to promote tumor cell survival and dissemination [38].
Urokinase plasminogen activator (uPA) is a serine protease converting plasminogen to plasmin that is able to degrade many ECM proteins (e.g. collagen IV, laminin, fibronectin) either directly or through activation of other proteases [7]. uPA and its inhibitor PAI-1 and receptor uPAR are involved in cell migration and tissue degradation in normal and pathological conditions as in cancer invasion and metastasis. High-expression levels of uPA and uPAR have been detected in various malignant human cancers, e.g. breast, colorectal, renal, and prostate, and are associated with poor prognosis [25, 117, 125]. Thereby uPA is mainly synthesized by TAMs, but also by CAFs, endothelial, and some cancer cells, whereas uPAR is found in tumor cells [117, 125]. Consequently, the uPA system has been addressed for anticancer therapy, by interfering with plasminogen activation or uPA/uPAR interaction, by reducing the expression of uPA and/or uPAR, or by inhibition of uPA activity. Several natural (PAI-1, PAI-2) and synthetic inhibitors of the uPA system, ranging from soluble receptors and catalytically inactive uPA fragments to synthetic peptides, have been used [98], and their effect on tumor invasion and metastasis has been demonstrated in animal models [23, 62]. These studies implicate that targeting of uPA/uPAR by an active immunological approach may be possible without major side effects as the uPA system seems not to be essential for fertility or survival in treated animals.
Conclusion
The potential of targeting the tumor stroma as a cancer therapeutic approach has been established in experimental studies. Possible cellular targets of active immune interventions include cancer-associated fibroblasts, infiltrating macrophages/histiocytes, and tumor endothelial cells. In particular, vascular targeting approaches can be expected to be effective even in large well-established tumors. However, targeting the stroma but not the tumor cells themselves may render tumor cells only quiescent and they may be reactivated if conditions ameliorate. Therefore, active immunisation leading to persistent immune response against antigens on tumor stroma cells appears to be more suitable than passive therapeutic strategies.
Acknowledgements
This work was supported by the DFG (Klinische Forschergruppe 124).
Abbreviations
- ADCC
Antibody-dependent cellular cytotoxicity
- CAF
Cancer-associated fibroblast
- CTL
Cytotoxic T cell
- ECM
Extracellular matrix
- IDO
Indoleamine 2,3-dioxygenase
- MHC
Major histocompatibility complex
- PSMA
Prostate specific membrane antigen
- TAA
Tumor-associated antigen
- TAM
Tumor-associated macrophage
- TEC
Tumor endothelial cell
- TEM
Tumor endothelial marker
- TIL
Tumor-infiltrating lymphocyte
- TSAA
Tumor stroma-associated antigen
- uPA
Urokinase plasminogen activator
- VEGF
Vascular endohelial growth factor
Footnotes
E. Gilboa, J. Lee, M. Fassnacht, S.K. Nair. Poster presented at the Keystone symposium “Cancer immunotherapy targeting the tumor stroma” in february 2005 in Fairmont Banff Springs, Canada with the title “The role of microenvironment in tumor induction and progression”, Abstract 138
References
- 1.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen MH, Becker JC, Straten P. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov. 2005;4:399–409. doi: 10.1038/nrd1717. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH, Fensterle J, Ugurel S, Reker S, Houben R, Guldberg P, Berger TG, Schadendorf D, Trefzer U, Brocker EB, Straten P, Rapp UR, Becker JC. Immunogenicity of constitutively active V599EBRaf. Cancer Res. 2004;64:5456–5460. doi: 10.1158/0008-5472.CAN-04-0937. [DOI] [PubMed] [Google Scholar]
- 6.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Can Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 7.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariga N, Sato E, Ohuchi N, Nagura H, Ohtani H. Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int J Cancer. 2001;95:67–72. doi: 10.1002/1097-0215(20010120)95:1<67::AID-IJC1012>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Becker JC, thor Straten P, Andersen MH. The paradoxical association between Bcl-2 expression and prognosis: does the immune system make the difference. Cancer Res. 2005;65:2983. doi: 10.1158/0008-5472.CAN-05-0092. [DOI] [PubMed] [Google Scholar]
- 10.Bleumer I, Knuth A, Oosterwijk E, Hofmann R, Varga Z, Lamers C, Kruit W, Melchior S, Mala C, Ullrich S, De Mulder P, Mulders PF, Beck J. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90:985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady J, Neal J, Sadakar N, Gasque P. Human endosialin (tumor endothelial marker 1) is abundantly expressed in highly malignant and invasive brain tumors. J Neuropathol Exp Neurol. 2004;63:1274–1283. doi: 10.1093/jnen/63.12.1274. [DOI] [PubMed] [Google Scholar]
- 12.Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–1161. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y. Antioangiogenic cancer therapy. Sem Canc Biol. 2004;14:139–145. doi: 10.1016/j.semcancer.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 15.Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784–2790. doi: 10.1182/blood.V97.9.2784. [DOI] [PubMed] [Google Scholar]
- 16.Casati C, Dalerba P, Rivoltini L, Gallino G, Deho P, Rini F, Belli F, Mezzanzanica D, Costa A, Andreola S, Leo E, Parmiani G, Castelli C. The apoptosis inhibitor protein survivin induces tumor-specific CD8+ and CD4+ T cells in colorectal cancer patients. Cancer Res. 2003;63:4507–4515. [PubMed] [Google Scholar]
- 17.Chang SS, O‘Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 18.Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, Tsai MF, Chen CH, Yang PC. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23:953–964. doi: 10.1200/JCO.2005.12.172. [DOI] [PubMed] [Google Scholar]
- 19.Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Murakami S, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 20.Christian S, Ahorn H, Koehler A, Eisenhaber F, Rodi HP, Garin-Chesa P, Park JE, Rettig WJ, Lenter MC. Molecular cloning and characterization of endosialin, a C-type lectin-like cell surface receptor of tumor endothelium. J Biol Chem. 2001;276:7408–7414. doi: 10.1074/jbc.M009604200. [DOI] [PubMed] [Google Scholar]
- 21.Colpaert CG, Vermeulen PB, Fox SB, Harris AL, Dirix LY, Van Marck EA. The presence of a fibrotic focus in invasive breast carcinoma correlates with the expression of carbonic anhydrase IX and is a marker of hypoxia and poor prognosis. Breast Cancer Res Treat. 2003;81:137–147. doi: 10.1023/A:1025702330207. [DOI] [PubMed] [Google Scholar]
- 22.Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- 23.Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levinson AD. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA. 1993;90:5021–5025. doi: 10.1073/pnas.90.11.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dales JP, Garcia S, Andrac L, Carpentier S, Ramuz O, Lavaut MN, Allasia C, Bonnier P, Charpin C. Prognostic significance of angiogenesis evaluated by CD105 expression compared to CD31 in 905 breast carcinomas: correlation with long-term patient outcome. Int J Oncol. 2004;24:1197–1204. [PubMed] [Google Scholar]
- 25.Duffy MJ, Maguire TM, McDermott EW, O’Higgins N. Urokinase plasminogen activator: a prognostic marker in multiple types of cancer. J Surg Oncol. 1999;71:130–135. doi: 10.1002/(SICI)1096-9098(199906)71:2<130::AID-JSO14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Duyndam MC, Hilhorst MC, Schluper HM, Verheul HM, van Diest PJ, Kraal G, Pinedo HM, Boven E. Vascular endothelial growth factor-165 overexpression stimulates angiogenesis and induces cyst formation and macrophage infiltration in human ovarian cancer xenografts. Am J Pathol. 2002;160:537–548. doi: 10.1016/s0002-9440(10)64873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer WH, thor Straten P, Terheyden P, Becker JC. Function and dysfunction of CD4(+) T cells in the immune response to melanoma. Cancer Immunol Immunother. 1999;48:363–370. doi: 10.1007/s002620050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6:2037–2043. [PubMed] [Google Scholar]
- 29.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 30.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianani R, Jarboe E, Orlicky D, Frost M, Bobak J, Lehner R, Shroyer KR. Expression of survivin in normal, hyperplastic, and neoplastic colonic mucosa. Hum Pathol. 2001;32:119–125. doi: 10.1053/hupa.2001.21897. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein LA, Chen WT. Identification of an alternatively spliced seprase mRNA that encodes a novel intracellular isoform. J Biol Chem. 2000;275:2554–2559. doi: 10.1074/jbc.275.4.2554. [DOI] [PubMed] [Google Scholar]
- 33.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 34.Grabmaier K, Vissers JL, De Weijert MC, Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH, Noessner E, Mulders PA, Merkx G, Figdor CG, Adema GJ, Oosterwijk E. Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer. 2000;85:865–870. doi: 10.1002/(SICI)1097-0215(20000315)85:6<865::AID-IJC21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Grünebach F, Muller MR, Brossart P. New developments in dendritic cell-based vaccinations: RNA translated into clinics. Cancer Immunol Immunother. 2005;54:517–525. doi: 10.1007/s00262-004-0605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 37.Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, von Blomberg BM, Bloemena E, Scheper RJ, van Ham SM, Pinedo HM, van den Eertwegh AJ (2005) Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother (in print) [DOI] [PMC free article] [PubMed]
- 38.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 39.Harada M, Matsueda S, Yao A, Ogata R, Noguchi M, Itoh K. Prostate-related antigen-derived new peptides having the capacity of inducing prostate cancer-reactive CTLs in HLA-A2+ prostate cancer patients. Oncol Rep. 2004;12:601–607. [PubMed] [Google Scholar]
- 40.Henry MD, Wen S, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004;64:7995–8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez JM, Bui MH, Han KR, Mukouyama H, Freitas DG, Nguyen D, Caliliw R, Shintaku PI, Paik SH, Tso CL, Figlin RA, Belldegrun AS. Novel kidney cancer immunotherapy based on the granulocyte-macrophage colony-stimulating factor and carbonic anhydrase IX fusion gene. Clin Cancer Res. 2003;9:1906–1916. [PubMed] [Google Scholar]
- 42.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann UB, Eggert AA, Blass K, Brocker EB, Becker JC. Expression of matrix metalloproteinases in the microenvironment of spontaneous and experimental melanoma metastases reflects the requirements for tumor formation. Cancer Res. 2003;63:8221–8225. [PubMed] [Google Scholar]
- 44.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–344. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 45.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Wang S, Kelly T. Seprase promotes rapid tumor growth and increased microvessel density in a mouse model of human breast cancer. Cancer Res. 2004;64:2712–2716. doi: 10.1158/0008-5472.CAN-03-3184. [DOI] [PubMed] [Google Scholar]
- 47.Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, Garin-Chesa P. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol. 2003;120:182–188. doi: 10.1046/j.1523-1747.2003.12035.x. [DOI] [PubMed] [Google Scholar]
- 48.Idenoue S, Hirohashi Y, Torigoe T, Sato Y, Tamura Y, Hariu H, Yamamoto M, Kurotaki T, Tsuruma T, Asanuma H, Kanaseki T, Ikeda H, Kashiwagi K, Okazaki M, Sasaki K, Sato T, Ohmura T, Hata F, Yamaguchi K, Hirata K, Sato N. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res. 2005;11:1474–1482. doi: 10.1158/1078-0432.CCR-03-0817. [DOI] [PubMed] [Google Scholar]
- 49.Igney FH, Krammer PH (2005) Tumor counterattack: fact or fiction? Cancer Immunol Immunother (in print) [DOI] [PMC free article] [PubMed]
- 50.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwasa S, Jin X, Okada K, Mitsumata M, Ooi A. Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colorectal cancer. Cancer Lett. 2003;199:91–98. doi: 10.1016/S0304-3835(03)00315-X. [DOI] [PubMed] [Google Scholar]
- 52.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1996;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 53.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 54.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Randolph Hecht J, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 55.Kataki A, Scheid P, Piet M, Marie B, Martinet N, Martinet Y, Vignaud JM. Tumor infiltrating lymphocytes and macrophages have a potential dual role in lung cancer by supporting both host-defense and tumor progression. J Lab Clin Med. 2002;140:320–328. doi: 10.1067/mlc.2002.128317. [DOI] [PubMed] [Google Scholar]
- 56.Kelly T, Kechelava S, Rozypal TL, West KW, Korourian S. Seprase, a membrane-bound protease, is overexpressed by invasive ductal carcinoma cells of human breast cancers. Mod Pathol. 1998;11:855–863. [PubMed] [Google Scholar]
- 57.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi Y, Yukiue H, Sasaki H, Fukai I, Yokoyama T, Kiriyama M, Yamakawa Y, Maeda M, Fujii Y. Developmentally regulated expression of survivin in the human thymus. Hum Immunol. 2002;63:101–107. doi: 10.1016/S0198-8859(01)00369-X. [DOI] [PubMed] [Google Scholar]
- 59.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99:1667–1674. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 60.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T Lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 61.Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest. 2003;83:1457–1466. doi: 10.1097/01.LAB.0000090158.68852.D1. [DOI] [PubMed] [Google Scholar]
- 62.Lefesvre P, Attema J, van Bekkum D. Adenoviral gene transfer of angiostatic ATF-BPTI inhibits tumor growth. BMC Cancer. 2002;2:17–31. doi: 10.1186/1471-2407-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leppilampi M, Saarnio J, Karttunen TJ, Kivela J, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila S. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol. 2003;9:1398–1403. doi: 10.3748/wjg.v9.i7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. FASEB J. 2000;14:55–64. doi: 10.1096/fasebj.14.1.55. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Wang MN, Li H, King KD, Bassi R, Sun H, Santiago A, Hooper AT, Bohlen P, Hicklin DJ. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med. 2002;195:1575–1584. doi: 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Z, Smyth FE, Renner C, Lee FT, Oosterwijk E, Scott AM. Anti-renal cell carcinoma chimeric antibody G250: cytokine enhancement of in vitro antibody-dependent cellular cytotoxicity. Cancer Immunol Immunother. 2002;51:171–177. doi: 10.1007/s00262-002-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5:237–256. doi: 10.1023/A:1024532022166. [DOI] [PubMed] [Google Scholar]
- 69.Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin Cancer Res. 1999;5:371–382. [PubMed] [Google Scholar]
- 70.Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61–76. doi: 10.1016/j.cytogfr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 72.Mori Y, Kono K, Matsumoto Y, Fujii H, Yamane T, Mitsumata M, Chen WT. The expression of a type II transmembrane serine protease (Seprase) in human gastric carcinoma. Oncology. 2004;67:411–419. doi: 10.1159/000082926. [DOI] [PubMed] [Google Scholar]
- 73.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 75.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.CAN-03-2408. [DOI] [PubMed] [Google Scholar]
- 76.Nanus DM, Milowsky MI, Kostakoglu L, Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, Bander NH. Clinical use of monoclonal antibody HuJ591 therapy: targeting prostate specific membrane antigen. J Urol. 2003;170:84–88. doi: 10.1097/01.ju.0000095151.97404.7c. [DOI] [PubMed] [Google Scholar]
- 77.Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, Eliceiri BP, Reisfeld RA. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–1375. doi: 10.1038/nm794. [DOI] [PubMed] [Google Scholar]
- 78.Ntayi C, Hornebeck W, Bernard P. Influence of cultured dermal fibroblasts on human melanoma cell proliferation, matrix metalloproteinase-2 (MMP-2) expression and invasion in vitro. Arch Dermatol Res. 2003;295:236–241. doi: 10.1007/s00403-003-0429-0. [DOI] [PubMed] [Google Scholar]
- 79.O’Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Opavsky R, Haviernik P, Jurkovicova D, Garin MT, Copeland NG, Gilbert DJ, Jenkins NA, Bies J, Garfield S, Pastorekova S, Oue A, Wolff L. Molecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal tissues in vivo. J Biol Chem. 2001;276:38795–38807. doi: 10.1074/jbc.M105241200. [DOI] [PubMed] [Google Scholar]
- 81.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, Bock M, Brocker EB, Straten PT, Kampgen E, Becker JC. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Pawelec G. Tumour escape: antitumour effectors too much of a good thing. Cancer Immunol Immunother. 2004;53:262–274. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pende D, Rivera P, Marcenaro S, Chang CC, Biassoni R, Conte R, Kubin M, Cosman D, Ferrone S, Moretta L, Moretta A. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 84.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 85.Pisarev V, Yu B, Salup R, Sherman S, Altieri DC, Gabrilovich DI. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;9:6523–6533. [PubMed] [Google Scholar]
- 86.Reker S, Meier A, Holten-Andersen L, Svane IM, Becker JC, thor Straten P, Andersen MH. Identification of novel survivin-derived CTL epitopes. Cancer Biol Ther. 2004;3:173–179. doi: 10.4161/cbt.3.2.611. [DOI] [PubMed] [Google Scholar]
- 87.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 88.Ren J, Zheng L, Chen Q, Li H, Zhang L, Zhu H. Co-administration of a DNA vaccine encoding the prostate specific membrane antigen and CpG oligodeoxynucleotides suppresses tumor growth. J Transl Med. 2004;2:29–38. doi: 10.1186/1479-5876-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci USA. 1992;89:10832–10836. doi: 10.1073/pnas.89.22.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rmali KA, Watkins G, Harrison G, Parr C, Puntis MC, Jiang WG. Tumour endothelial marker 8 (TEM-8) in human colon cancer and its association with tumour progression. Eur J Surg Oncol. 2004;30:948–953. doi: 10.1016/j.ejso.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 91.Rohayem J, Diestelkoetter P, Weigle B, Oehmichen A, Schmitz M, Mehlhorn J, Conrad K, Rieber EP. Antibody response to the tumor-associated inhibitor of apoptosis protein survivin in cancer patients. Cancer Res. 2000;60:1815–1817. [PubMed] [Google Scholar]
- 92.Rowinsky EK, Humphrey R, Hammond LA, Aylesworth C, Smetzer L, Hidalgo M, Morrow M, Smith L, Garner A, Sorensen JM, Von Hoff DD, Eckhardt SG. Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12–9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J Clin Oncol. 2000;18:178–186. doi: 10.1200/JCO.2000.18.1.178. [DOI] [PubMed] [Google Scholar]
- 93.Saleh FH, Crotty KA, Hersey P, Menzies SW, Rahman W. Autonomous histopathological regression of primary tumours associated with specific immune responses to cancer antigens. J Pathol. 2003;200:383–395. doi: 10.1002/path.1369. [DOI] [PubMed] [Google Scholar]
- 94.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–4102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 95.She X, Matsuno F, Harada N, Tsai H, Seon BK. Synergy between anti-endoglin (CD105) monoclonal antibodies and TGF-beta in suppression of growth of human endothelial cells. Int J Cancer. 2004;108:251–257. doi: 10.1002/ijc.11551. [DOI] [PubMed] [Google Scholar]
- 96.Shiozaki K, Harada N, Greco WR, Haba A, Uneda S, Tsai H, Seon BK (2005) Antiangiogenic chimeric anti-endoglin (CD105) antibody: pharmacokinetics and immunogenicity in nonhuman primates and effects of doxorubicin. Cancer Immunol Immunother (in print) [DOI] [PMC free article] [PubMed]
- 97.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 98.Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev. 2003;22:205–222. doi: 10.1023/A:1023099415940. [DOI] [PubMed] [Google Scholar]
- 99.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, Bissonette EA, Barnd DL, Deacon DH, Patterson JW, Parekh J, Neese PY, Woodson EM, Wiernasz CJ, Merrill P. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 100.Sondak VK, Sosman JA. Results of clinical trials with an allogenic melanoma tumor cell lysate vaccine: Melacine. Semin Cancer Biol. 2003;13:409–415. doi: 10.1016/j.semcancer.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 101.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 102.Starling N, Cunningham D. Monoclonal antibodies against vascular endothelial growth factor and epidermal growth factor receptor in advanced colorectal cancers: present and future directions. Curr Opin Oncol. 2004;16:385–390. doi: 10.1097/01.cco.0000128278.15371.e4. [DOI] [PubMed] [Google Scholar]
- 103.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected Dendritic Cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 104.Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999;82:737–742. doi: 10.1002/(SICI)1097-0215(19990827)82:5<737::AID-IJC18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:7846–7854. [PubMed] [Google Scholar]
- 106.Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, Seon BK. Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res. 2001;7:524–532. [PubMed] [Google Scholar]
- 107.Tan GH, Tian L, Wei YQ, Zhao X, Li J, Wu Y, Wen YJ, Yi T, Ding ZY, Kan B, Mao YQ, Deng HX, Li HL, Zou CH, Fu CH. Combination of low-dose cisplatin and recombinant xenogeneic endoglin as a vaccine induces synergistic antitumor activities. Int J Cancer. 2004;112:701–706. doi: 10.1002/ijc.20449. [DOI] [PubMed] [Google Scholar]
- 108.Tan GH, Wei YQ, Tian L, Zhao X, Yang L, Li J, He QM, Wu Y, Wen YJ, Yi T, Ding ZY, Kan B, Mao YQ, Deng HX, Li HL, Zhou CH, Fu CH, Xiao F, Zhang XW. Active immunotherapy of tumors with a recombinant xenogeneic endoglin as a model antigen. Eur J Immunol. 2004;34:2012–2021. doi: 10.1002/eji.200424933. [DOI] [PubMed] [Google Scholar]
- 109.Terheyden P, Schrama D, Pedersen LO, Andersen MH, Kampgen E, thor Straten P, Becker JC. Longitudinal analysis of MART-1/HLA-A2-reactive T cells over the course of melanoma progression. Scand J Immunol. 2003;58:566–571. doi: 10.1046/j.1365-3083.2003.01324.x. [DOI] [PubMed] [Google Scholar]
- 110.Terheyden P, Siedel C, Merkel A, Kampgen E, Brocker EB, Becker JC. Predominant expression of Fas (CD95) ligand in metastatic melanoma revealed by longitudinal analysis. J Invest Dermatol. 1999;112:899–902. doi: 10.1046/j.1523-1747.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 111.thor Straten P, Becker JC, Guldberg P, Zeuthen J. In situ T cells in melanoma. Cancer Immunol Immunother. 1999;48:386–395. doi: 10.1007/s002620050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Todorova K, Ignatova I, Tchakarov S, Altankova I, Zoubak S, Kyurkchiev S, Mincheff M. Humoral immune response in prostate cancer patients after immunization with gene-based vaccines that encode for a protein that is proteasomally degraded. Cancer Immun. 2005;5:1–8. [PubMed] [Google Scholar]
- 113.Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA. 2002;99:4349–4354. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sherev T, Sparbier K, Sterry W, Walden P. Vaccination with hybrids of tumor and dendritic cells induces tumor-specific T-cell and clinical responses in melanoma stage III and IV patients. Int J Cancer. 2004;110:730–740. doi: 10.1002/ijc.20191. [DOI] [PubMed] [Google Scholar]
- 115.Tsuruma T, Hata F, Torigoe T, Furuhata T, Idenoue S, Kurotaki T, Yamamoto M, Yagihashi A, Ohmura T, Yamaguchi K, Katsuramaki T, Yasoshima T, Sasaki K, Mizushima Y, Minamida H, Kimura H, Akiyama M, Hirohashi Y, Asanuma H, Tamura Y, Shimozawa K, Sato N, Hirata K. Phase I clinical study of anti-apoptosis protein, survivin-derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med. 2004;2:19–29. doi: 10.1186/1479-5876-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.U.S. Cancer Statistics Working Group. United States Cancer Statistics (1999–2001) Incidence and Mortality Web-based Report Version. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2004. Available at: http://www.cdc.gov/cancer/npcr/uscs. Accessed November 20th, 2004
- 117.Usher PA, Thomsen OF, Iversen P, Johnsen M, Brunner N, Hoyer-Hansen G, Andreasen P, Dano K, Nielsen BS. Expression of urokinase plasminogen activator, its receptor and type-1 inhibitor in malignant and benign prostate tissue. Int J Cancer. 2005;113:870–880. doi: 10.1002/ijc.20665. [DOI] [PubMed] [Google Scholar]
- 118.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 119.Vissers JL, De Vries IJ, Engelen LP, Scharenborg NM, Molkenboer J, Figdor CG, Oosterwijk E, Adema GJ. Renal cell carcinoma-associated antigen G250 encodes a naturally processed epitope presented by human leukocyte antigen-DR molecules to CD4(+) T lymphocytes. Int J Cancer. 2002;100:441–444. doi: 10.1002/ijc.10518. [DOI] [PubMed] [Google Scholar]
- 120.Vissers JL, De Vries IJ, Schreurs MW, Engelen LP, Oosterwijk E, Figdor CG, Adema GJ. The renal cell carcinoma-associated antigen G250 encodes a human leukocyte antigen (HLA)-A2.1-restricted epitope recognized by cytotoxic T lymphocytes. Cancer Res. 1999;59:5554–5559. [PubMed] [Google Scholar]
- 121.Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ, Rettig WJ. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 122.Wiendl H, Mitsdoerffer M, Hofmeister V, Wischhusen J, Bornemann A, Meyermann R, Weiss EH, Melms A, Weller M. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 123.Wuest T, Moosmayer D, Pfizenmaier K. Construction of a bispecific single chain antibody for recruitment of cytotoxic T cells to the tumour stroma associated antigen fibroblast activation protein. J Biotechnol. 2001;92:159–168. doi: 10.1016/S0168-1656(01)00355-8. [DOI] [PubMed] [Google Scholar]
- 124.Xiang R, Mizutani N, Luo Y, Chiodoni C, Zhou H, Mizutani M, Ba Y, Becker JC, Reisfeld RA. A DNA vaccine targeting survivin combines apoptosis with suppression of angiogenesis in lung tumor eradication. Cancer Res. 2005;65:553–561. [PubMed] [Google Scholar]
- 125.Xu Y, Hagege J, Doublet JD, Callard P, Sraer JD, Ronne E, Rondeau E. Endothelial and macrophage upregulation of urokinase receptor expression in human renal cell carcinoma. Hum Pathol. 1997;28:206–213. doi: 10.1016/S0046-8177(97)90108-8. [DOI] [PubMed] [Google Scholar]
- 126.Zeis M, Siegel S, Wagner A, Schmitz M, Marget M, Kuhl-Burmeister R, Adamzik I, Kabelitz D, Dreger P, Schmitz N, Heiser A. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–5397. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]