Abstract
The modes of action of the novel anti-skin tumor agent ingenol-3-angelate (PEP005) are incompletely understood. Crucially, the cytotoxic functions of neutrophils recruited to the tumor in response to topical application of PEP005 are necessary for effective ablation of the treated lesion. Here, we investigated the hypothesis that the phorbol ester-like properties of PEP005 and its ability to activate PKC could directly activate endothelial cells (EC) so that they support the recruitment of neutrophils. Exposure of EC to PEP005 induced mRNA and/or protein for E-selectin, ICAM-1 and IL-8 in a dose dependent manner, while in a flow based adhesion assay, PEP005 treated EC supported the recruitment of neutrophils at levels comparable to EC stimulated with TNF-α. Neutrophil adhesion was inhibited by antibody against E-selectin but not P-selectin. Activation of EC was inhibited by the PKC inhibitor bisindolylmaleimide-1 and confocal immuno-fluorescent studies demonstrated translocation of PKC-δ from the cytosol to the peri-nuclear membrane in response to PEP005. Importantly, the knock down of PKC-δ using siRNA completely abolished neutrophil recruitment to EC subsequently treated with PEP005. Thus, we describe a novel route by which the anti-tumor agent PEP005 regulates the recruitment of cytotoxic leukocytes by directly activating EC in a PKC-δ dependent manner.
Keywords: Skin cancer, Inflammation, Cytotoxic leukocytes, Leukocyte recruitment, Endothelial cells
Introduction
The diterpene ester ingenol-3-angelate (PEP005), derived from the tissues of the plant Euphorbia peplus, has been shown to posses potent tumoricidal capabilities against human and murine skin cancers [1, 2]. Topical application of the agent to the dermis results in tumor clearance with low rates of relapse and the compound is now in phase 2 trials for skin cancer and solar keratoses. The mechanisms of action of PEP005 are not fully elucidated, however, at high concentrations PEP005 directly induces tumor cell death by primary necrosis [2]. Secondary to this effect is the induction of a robust inflammatory response in which the leukocytic infiltrate is dominated by neutrophils [2, 3]. Importantly, we recently showed using in vivo models of tumor clearance, that the presence of intra-tumoral neutrophils and their production of cytotoxic agents was important for preventing tumor relapse [3].
The recruitment of neutrophils to sites of inflammation ordinarily occurs across the endothelial cells lining post capillary venules [4–6 for review]. This process can be initiated by inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β which are generated locally and stimulate transcriptional programs in EC which lead to the expression of the adhesion receptors and chemokines necessary for leukocyte recruitment. PEP005 has been shown to induce the expression of IL-1β and TNF-α, and the neutrophil chemotactic cytokine IL-8 (or the murine homologue MIP-2) in both normal mouse skin and in skin tumors, as well as in cultured human keratinocytes, fibroblasts and melanoma cells [3]. The production of these agents by cells of the skin and tumor thus provide a paracrine route by which EC stimulation can be achieved after application of PEP005 and it is possible that the phorbol-ester like properties of PEP005, which have been reported to lead to protein kinase-C (PKC) activation [7], underlie this pro-inflammatory capacity.
An alternative route by which PEP005 might promote inflammation is by directly activating vascular endothelial cells. Indeed, we have presented preliminary data using static adhesion assays demonstrating that cultured EC exposed to PEP005 support the adhesion of neutrophils [3]. The magnitude of this response was dependent upon the stimulating concentration of PEP005, but the mechanism of action of the agent and the identity of the adhesion receptors involved in neutrophil recruitment were not determined. Here, we demonstrate that PEP005 can promote the expression of adhesion receptors and chemokines on cultured EC. Moreover, PEP005-treated EC can recruit flowing neutrophils in an E-selectin (but not P-selectin) dependent manner. Importantly, PEP005 induced the rapid translocation of PKC-δ from a cytosolic to a membrane location. Furthermore, the PKC inhibitor, bisindolylmaleimide 1 could inhibit the PEP005 dependent expression of E-selectin and could also ablate the recruitment of flowing neutrophils. Knockdown of PKC-δ using si-RNA also greatly reduced neutrophil recruitment to PEP005 treated EC. Thus, we demonstrate a new, PKC-δ dependent mechanism by which PEP005 can promote endothelial inflammatory responses and could mediate the infiltration of cytotoxic neutrophils into tumor tissue.
Methods
Neutrophil preparation
Blood was collected from fully consented healthy adult volunteers into EDTA (1.6 mg/ml) in accordance with local ethical guidelines and with approval of the South Birmingham Local Research Ethics Committee. Neutrophils were separated using two-step density gradients of Histopaque 1119 and 1077 (all reagents are from Sigma, Poole, UK unless otherwise stated) as previously described [8]. Neutrophils were greater than 95% pure based on volume distribution using a Coulter Multisizer II. Neutrophils were diluted in PBS containing 0.1% bovine serum albumin (PBSA) at a final concentration of 1 × 106 cells/ml prior to adhesion assay.
Isolation and culture of endothelial cells
Human umbilical vein EC were enzymatically isolated as previously described [9] and cultured in medium which was Medium 199 (Invitrogen, Paisley, UK) containing 28 μg/ml gentomycin, 20% FCS, 1 μg/ml hydrocortisone and 10 ng/ml epidermal growth factor as previously described. EC were grown to confluence in 25 cm2 culture flasks (BD Falcon, Oxford, UK) pre-coated with 1% gelatin solution. Alternatively, the dermal microvascular cell line, HMEC-1, was cultured in the same medium.
Endothelial cell culture in microslides and 96 or 24-well plates
Adhesion experiments were conducted using first passage endothelial cultures grown in microslides as previously described [10], or HMEC-1 in 24-well plates. Microslides (Cam Lab, Cambridge, UK) are glass capillary tubes with a rectangular cross-section of 0.3 × 3 mm and a length of 5 cm, and good optical qualities. EDTA (0.02% in PBS) washed EC monolayers were dispersed in trypsin (2.5 mg/ml), washed and loaded into microslides coated first with 3-aminopropyltriethoxy-silane [10] and subsequently with 1% bovine gelatin. Microslides were seeded with EC at a concentration suitable to afford almost immediate confluence after sedimentation and spreading. After incubation (1 h, 37°C) to allow cell attachment to occur, the microslides were connected to a system allowing the perfusion of several volumes of sterile culture medium at regular intervals, and cultured for 24 h prior to experimentation [10].
Endothelial surface ELISA experiments were conducted using first passage HUVEC grown in proteinised (see above) 96-well culture plates. HUVEC for mRNA extraction were cultured in proteinised 24-well plates. Primary cultures were dispersed as previously described (see above), seeded at a density suitable to afford almost immediate confluence after sedimentation and spreading and cultured for 24 h prior to experimentation. HMEC-1 for static adhesion assays were grown in proteinised 24-well plates after dispersal as previously described (see above).
Stimulation of endothelial cultures with PEP005 or TNFα
For flow based adhesion assays, EC in microslides were stimulated with ingenol-3-angelate (PEP005; Peplin Ltd, Brisbane, Australia) at a concentration of 100 ng/ml for 4 h. This concentration of reagent has previously been demonstrated to induce the adhesion of neutrophils to EC in static adhesion assays [3]. Control microslides were untreated or were stimulated with 100 U/ml TNF-α (R&D Systems, Abingdon, UK) for 4 h. For static adhesion assays using HMEC-1, EC were stimulated with PEP005 in the range of 1–100 ng/ml. For ELISA EC were treated with PEP005 at concentrations between 1 and 100 ng/ml for 4 h. Control wells were untreated or stimulated with TNF-α (100 U/ml). EC for RNA extraction were untreated or stimulated with PEP005 or TNF-α at concentrations of 100 ng/ml and 100 U/ml, respectively for 4 h.
Adhesion assay under conditions of shear stress or under static conditions
The flow based adhesion assay was based on that previously described [10]. Microslides were attached at one end, via flexible silicon tubing, to a Harvard syringe pump, and wash buffer or a suspension of neutrophils could selectively be drawn through the microslides by means of a microelectronic switching valve (Lee Products Ltd, Gerards Cross, UK). Microslides containing confluent EC were mounted on the stage of a microscope enclosed in a thermostatically controlled Perspex chamber which allowed assays to be conducted at 37°C. The microscope was fitted with a video camera, monitor and recorder. Neutrophil suspensions were flowed over the EC at a wall shear stress of 0.1 Pa for 3 min followed by 1 min of wash buffer to remove non-adherent cells. Video records of adhesive interactions were made from at least five microscope fields. Video records were digitised using analysis software (Image-Pro Plus, USA), adherent cells counted and data expressed as number/mm2/106 cells perfused. When counting adherent cells using phase contrast microscopy, distinction was made between those that were phase bright and attached to the apical surface of the endothelial cells, and those that were phase dark and spread, and had migrated through the endothelial layer. In some experiments EC monolayers were treated for 15 min prior to adhesion assay with function blocking antibodies against E-selectin (clone-ENA2 F(ab′)2; Bradshaw Biologicals, Chepshet, UK) or P-selectin (clone-G1; Centocor, Leiden, Netherlands).
Static adhesion assays were conducted with HMEC-1 cultured in 24-well plates. EC were washed prior to addition of PEP005 at concentrations ranging from 0 to 100 ng/ml. After 4 h of PEP005 stimulation EC were washed three times in culture medium and purified neutrophils added at a concentration of 1 × 106 cells/well. After 10 min of incubation at 37°C, non-adherent neutrophils were resuspended in culture medium and washed from the wells. EC with adherent and migrated neutrophils were fixed in 2% gluteraldehyde in PBS. Adhesion was assessed by counting the number of surface adherent cells and the number of transmigrated cells/mm2.
ELISA for endothelial adhesion molecules and IL-8
Endothelial cells in 96-well plates which had been treated with PEP005, TNFα or that were untreated were fixed with 2% gluteraldehyde for 15 min. After washing to remove fixative the cells were incubated with 1% albumin to block unreacted aldehyde groups. Monoclonal antibody 1.2B6 against E-selectin (DakoCytomation, Cambridge, UK), or 6.5B5 against ICAM-1 (Dako) or control IgG1 (Dako) were incubated with the endothelial cells for 1 h at room temperature. Unbound antibody was removed with serial washes in PBS/albumin. Primary antibody was labelled with P0447 (DAKO) a goat anti-mouse IgG/A/M, conjugated with horseradish peroxidase. Unbound secondary antibody was removed by serial washing with PBS/albumin. The substrate O-phenylene diamine was coincubated for 20 min with labelled cells, the colorimetric reaction quenched with 0.5 M H2SO4 and absorbance measured at 470 nm on Synergy HT plate reader (Biotech). Beadlyte® Human Multi-cytokine Beadmaster™ kit (Upstate, Lake Placid, NY, USA) was used to assay IL-8 (CXCL8) in culture supernatants.
Measuring mRNA expression for E-selectin and ICAM-1 by real time PCR
RNA for real time PCR was extracted from EC monolayers using TRIzol reagent (Invitrogen, Paisley, Scotland) according to the manufacturers’ instructions. Contaminating genomic DNA was removed using a DNA-free™ kit (Ambion, Huntingdon, UK). The reverse transcription of mRNA to first strand cDNA used Geneamp PCR reaction kit reagents (Perkin Elmer, Warrington, UK), i.e. 10 mM Tris HCl, pH 8.3; 50 mM KCl; 5 mM MgCl; 1 mM dGTP, dATP, dCTP, dTTP; 1 U/μl RNAse inhibitor; 2.5 U/μl Mu-MLV reverse transcriptase; 2.5 mM random hexamers. Quantitative real time PCR was performed using 500 ng of cDNA and Assays-on-Demand™ gene expression mix for the target genes ICAM-1 and E-selectin (using 6-FAM labelled probe; Applied Biosystems, Warrington, UK), 18-S endogenous control with forward and reverse primers [using VIC labelled probe (Eurogentec Ltd, Hampshire, UK)] and Taqman® Universal PCR master mix (Applied Biosystems). cDNA was amplified using a standard protocol of 40 cycles of 2 min at 50°C, 10 min at 95°C, 15 s at 95°C and 1 min at 60°C. Results were analysed using the SDS 2.2 software and results presented as relative quantity (RQ) normalised to the expression levels in unstimulated EC. In these experiments increase in E-selectin message was measured after EC had been stimulated with PEP005 in the presence of various PKC or MAP kinase inhibitors. Thus, Bisindolylmaleimide 1 and Go6976 (PKC inhibitors) were used at a concentration of 1 μM and 100 nM, respectively. We also assessed the effects of two MAP kinase inhibitors, PD098059 and SB202190, which have blocked this pathway in haemopoietic cells (Hampson, unpublished observations). These reagents were both utilised at concentration of 10 μM.
Immuno-fluorescent confocal microscopy for PKC-δ
Confluent EC grown in chamber slides (Nunc, NY, USA) were treated for 30 min with either medium alone, PEP005 (10 ng/ml) or PMA (20 nM). Cells were then fixed in 2% paraformaldehyde, permeabilised using PBS containing NP40 (0.4%) and blocked with PBS containing fish skin gelatine (0.2%) and casein (0.5%). Cells were then stained with a rabbit anti-PKC delta antibody (C20; Santa Cruz Biotechnology, CA, USA) or an irrelevant control antibody (Dako). Secondary antibody was Goat F(ab)2 anti-rabbit IgG (H + L)—FITC (Southern Biotechnology, AL, USA) and nuclei were counter stained with DAPI. Fluorescent staining was visualised on a Zeiss confocal microscope (LSM 510; Zeiss, Germany) with images captured and processed using the Zeiss LSM image examiner software.
Transfection of EC with siRNA
Endothelial cells were suspended by trypsin digestion and adjusted to 1 × 107 cells/ml in Nucleofector Solution (Amaxa AG, Cologne, Germany). To 100 μl of cell suspension (106 cells) 3 μg of either PKC-δ siRNA, scrambled siRNA control, 5 μl of the positive control vector pmaxGFP or phosphate buffered saline (to control for the effects of Nucleofector Solution on EC viability and function) were added. Samples were transferred to Amaxa certified cuvettes and electroporated using Amaxa apparatus on programme A-34. Following electroporation, 500 μl of prewarmed EC culture medium was added to the cuvettes and cells were transferred into 6-well plates to be incubated at 37°C with 5% CO2 for 48 h. Transfection efficiency was measured by flow cytometry using a Coulter epics XL flow cytometer and analysed using WinMDI software. PKC-δ expression in EC was measured by western blot 48 h after transfection. After blotting, membranes were incubated with a primary antibody against PKC-δ (Santa Cruz Biotechnology, Santa Cruz, CA) and an HRP-conjugated goat anti-rabbit secondary antibody (Amersham Pharmacia, Buckinghamshire, UK). Blots were developed using an enhanced chemiluminescence (ECL) method (Amersham Pharmacia).
Measuring PKC-δ activation
Translocation of PKC-δ between cellular compartments is an indication of enzyme activation and was detected by subcellular fractionation and western blotting. Briefly, confluent EC were treated with PEP005 (10 ng/ml) or with PMA (20 nM) for 30 min. Monolayers were then washed twice with ice cold PBS and then scraped into 20 mM Tris buffer (pH 7.5) containing MgC12 (2 mM), EGTA (10 mM), EDTA (2 mM), AEBSF (4 mM), leupeptin1 (10 μg/ml), and aprotonin (10 μg/ml). A lysate was prepared by snap freezing the cells in liquid nitrogen. Samples were then thawed at 37°C and homogenised in a Dounce homogeniser. Lysates were centrifuged at 100,000g for 30 min at 4°C (Optima Ultracentrifuge, Beckman Coulter). The recovered supernatant represents the cytosolic fraction. The remaining pellet was resuspended in 500 μl of lysis buffer (as above) with the addition of Triton X-100 (1%). Lysates were centrifuged at 100,000g for 15 min at 4°C. The recovered supernatant represented the cell membrane fraction. All fractions were re-suspended in an equal volume of SDS loading buffer and prepared for and subjected to western blotting (see above).
Results
PEP005 activates EC so that they support the adhesion of flowing neutrophils
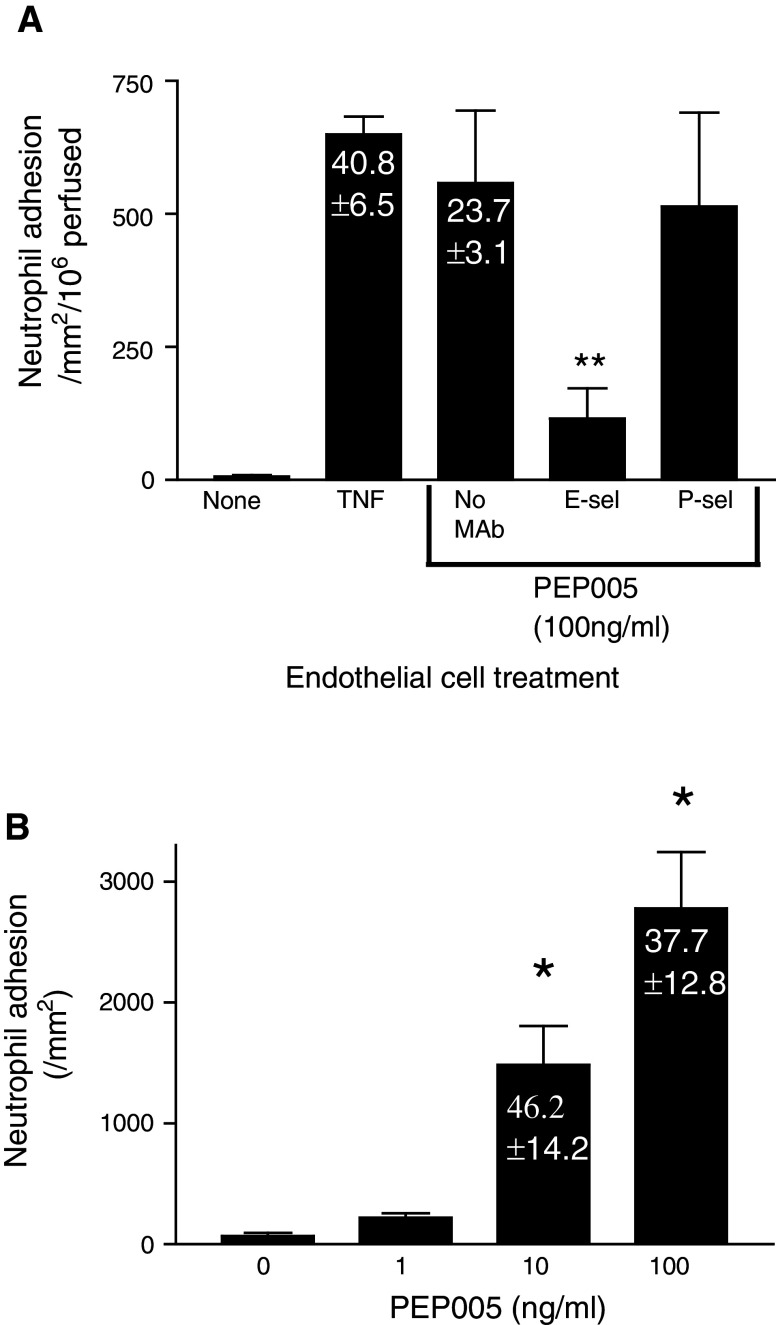
Using a static adhesion assay we have previously demonstrated that PEP005 could activate endothelial cells in a dose dependent manner so that they supported the adhesion of neutrophils [3]. Here, we utilised a more physiological flow based system to investigate the process in greater detail. Unstimulated HUVEC did not support the adhesion of flowing neutrophils (Fig. 1a). However, in the presence of 100 ng/ml PEP005, EC were activated so that they supported the recruitment of a substantial number of neutrophils. Indeed, there was no significant difference in the number of neutrophils recruited when compared to EC which had been stimulated with 100 U/ml TNF-α, a concentration of inflammatory cytokine which we routinely utilise to induce maximal levels of neutrophil recruitment in this assay [11] (Fig. 1a). Using function neutralising antibodies against EC selectins we ablated approximately 80% of neutrophil adhesion by blocking E-selectin, whereas an anti-P-selectin antibody (which was isotype matched and thus could operate as a control antibody) had no consistent effect on neutrophil recruitment (Fig. 1a). In addition, we were able to follow the behaviour of neutrophils once they were adherent to the EC monolayer. In both the TNF-α and PEP005 driven models of inflammation, a substantial number of neutrophils were activated and migrated into the sub-endothelial space (40.8 ± 6.5 and 23.7 ± 3.1%, respectively). In order to verify that dermal microvascular EC demonstrated the same response to PEP005, we incorporated these cells into a static adhesion assay in which their response to the agent could be assessed. Microvascular EC supported the adhesion of neutrophils in a manner dependent upon the stimulating concentration of PEP005 (Fig. 1b). Microvascular EC stimulated with PEP005 also supported the trans-endothelial migration of neutrophils with 37.7 ± 14.7% of adherent cells migrating across EC treated with 100 ng/ml PEP005.
Fig. 1.
PEP005 activates EC so that they support the adhesion of flowing neutrophils in an E-selectin dependent manner. a EC monolayers treated for 4 h with TNF-α or PEP005 were subject to flow based adhesion assay. Both agents induced high levels of neutrophil adhesion with a substantial proportion of recruited cells going on to transmigrate into the sub-endothelial environment (% migrated cells inset into relevant bars). The adhesion of neutrophils to PEP005 stimulated EC was significantly inhibited by an antibody against E-selectin where as an anti-P-selectin antibody had no consistent effect on adhesion. Data are mean ± SEM of three experiments; **P < 0.01 by t test for comparison between PEP005 treated cells in the presence or absence of antibody. b The adhesion of neutrophils to microvascular EC monolayers stimulated with PEP005 in the range of 1–100 ng/ml. Neutrophil adhesion increased in a dose dependent manner and a substantial proportion of adherent cells migrated across the EC monolayer (% migrated cells inset into relevant bars). Data are mean ± SEM of three experiments; *P < 0.05 by t test for comparison between PEP005 treated and untreated cells
PEP005 regulates the transcriptional activity and protein expression of EC adhesion receptors and chemokines
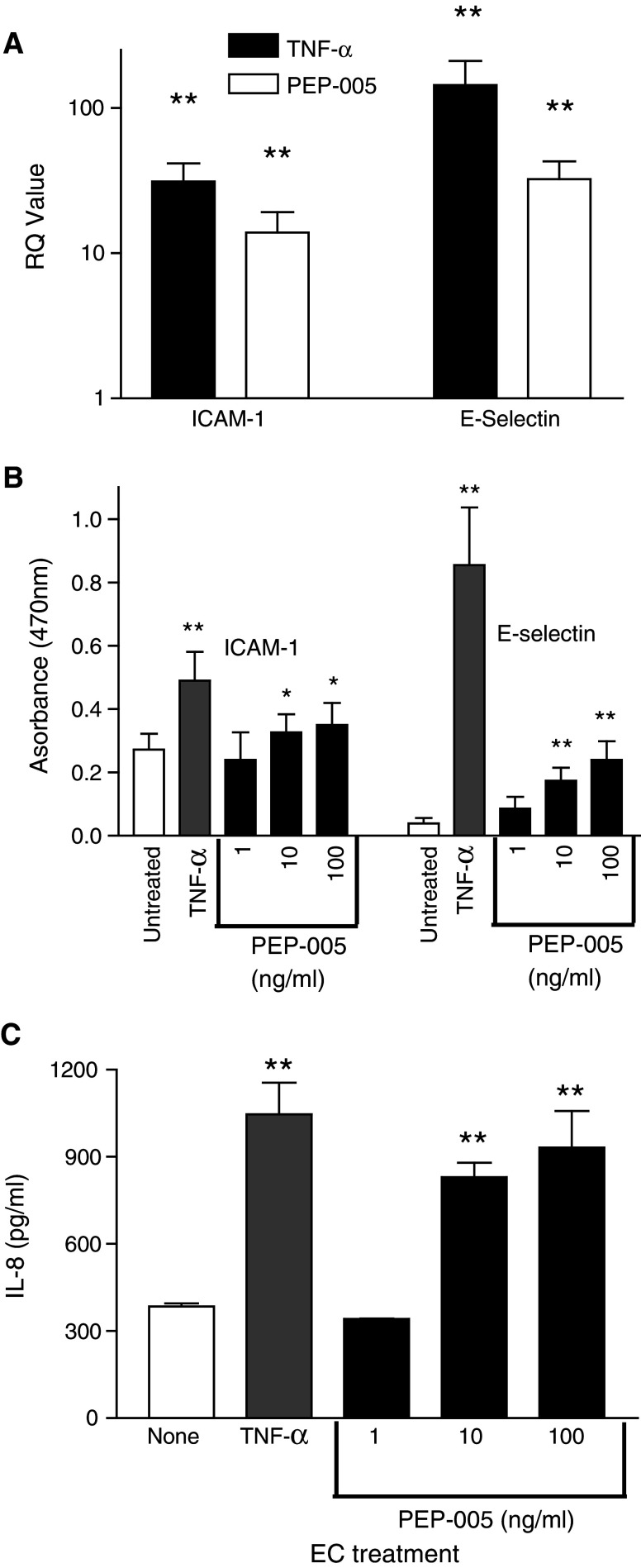
As PEP005 could promote the recruitment of neutrophils, we investigated its ability to up-regulate relevant adhesion receptors and chemokines in EC. Assessment by TaqMan PCR showed that the expression of mRNA for E-selectin and ICAM-1 was up-regulated by 4 h of exposure to either 100 U/ml TNF-α (≈140-fold and ≈30-fold, respectively) or by 100 ng/ml PEP005 (≈ 30-fold and ≈15-fold, respectively) (Fig. 2a). Protein levels were also increased as assessed by ELISA after 4 h of stimulation (Fig. 2b), with both E-selectin and ICAM-1 being induced in EC by PEP005 in a dose dependent manner. The screening of EC supernatants showed that the concentration of IL-8, a potent neutrophil chemoattractant, was also dramatically increased when EC were activated with either TNF-α or PEP005 for 4 h (Fig. 2c).
Fig. 2.
PEP005 stimulated EC demonstrate increased mRNA and protein expression for ICAM-1, E-selectin and IL-8. EC monolayers stimulated with TNF-α or PEP005 were assayed for; a mRNA for ICAM-1 or E-selectin by TaqMan PCR. Both reagents significantly increased the expression of mRNA compared to unstimulated control monolayers. Data are mean ± SEM of five experiments; **P < 0.01 by t test for comparison of TNF-α or PEP005 treated cells with unstimulated cells. b Protein expression of ICAM-1 and E-selectin by ELISA. Data are mean ± SEM of eight experiments. ANOVA demonstrated a significant effect of the concentration of PEP005 on ICAM-1 (P < 0.03) and E-selectin (P < 0.01). *P < 0.05; **P < 0.01 by t test for comparison of TNF or PEP005 treated EC to untreated cells. c Release of IL-8 into culture supernatant assessed by luminex assay. Both PEP005 and TNF-α increased IL-8 release from EC compared to untreated EC. Data are mean ± SEM of three experiments. **P < 0.01 by t test for comparison between TNF-α or PEP005 treated cells with untreated cells
Inhibition of PKC prevents EC activation by PEP005
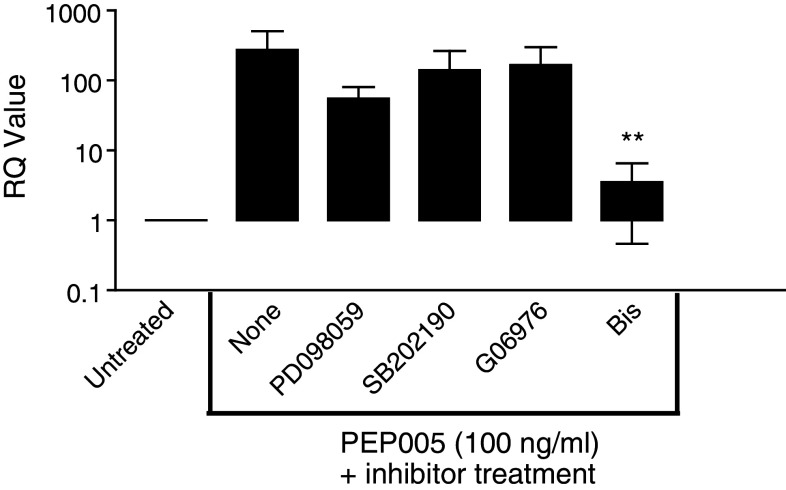
As PEP005 has been reported to activate PKC isoforms in a phorbol ester like manner [7], we used two different pharmacological PKC inhibitors, bisindolylmaleimide 1 and Go6976, to determine whether the pro-inflammatory actions of PEP005 were due to activation of endothelial PKC. Bisindolylmaleimide 1 is a broad range inhibitor able to block the activity of both the classical (α, βI, βII, γ) and novel (δ, ε, η, θ) PKC isoenzymes, whereas Go6976 inhibits only the classical PKC isoenzymes. Using the induction of E-selectin mRNA by TaqMan PCR as a sensitive indicator of endothelial activation, we found that bisindolylmaleimide 1 dramatically inhibited EC activation, whereas Go6976 had no effect on E-selectin mRNA expression (Fig. 3). These data suggest that PEP005 effects on EC activation are mediated by modulation of a member of the novel PKC family. Moreover, in a flow based adhesion assay where PEP005 (100 ng/ml) was able to induce high levels of neutrophil adhesion to HUVEC (558 ± 136 cells/mm2/106 perfused), bisindolylmaleimide 1 was able to abolish the recruitment of neutrophils (9 ± 3 cells/mm2/106 perfused data are mean ± SEM of three experiments), which was a greater than 95% inhibition. In static adhesion assays using microvascular EC bisindolylmaleimide-1 also reduced leukocyte adhesion by greater than 90% (from 2,775 ± 469 to 238 ± 22 neutrophils/mm2; data is mean ± SEM of three experiments) when 100 ng/ml of PEP005 was used to stimulate the EC. We also assessed the effects of two MAP kinase inhibitors, PD098059 and SB202190, as PEP005 has also been shown to activate this signalling pathway in haematopoietic cells (Hampson, unpublished observations). These inhibitors did not affect endothelial cell activation by PEP005 (Fig. 3) supporting the notion that the effects of PEP005 are mediated primarily via activation of novel PKCs.
Fig. 3.
E-selectin expression is inhibited by the PKC inhibitor bisindolylmaleimide-1. EC were treated with PEP005 in the presence of a number of PKC and MAP kinase inhibitors. Only bisindolylmaleimide 1 consistently reduced the expression E-selectin mRNA as assessed by TaqMan PCR. Data are mean ± SEM of five experiments; **P < 0.01 by t test for comparison of PEP005 treated cells in the presence or absence of PKC inhibitors
PEP005 induces translocation of PKC-δ from the cytosol to a membrane fraction
A number of avenues of evidence lead us to the belief that PKC-δ was the most likely target for PEP005 in EC activation. Briefly, various PKC isoforms have been implicated in the upregulation of E-selectin during EC activation, specifically PKC-α, βI and δ [12–15] and PEP005 shows highest affinity for the PKC isoforms-α and -δ in vitro [7]. However, Go6976 which inhibits PKC-α and β, but not PKC-δ, was ineffective in blocking PEP005 induced upregulation of E-selectin, whereas Bisindolylmaleimide 1 which inhibits both PKC-α and -δ was effective (Fig. 3).
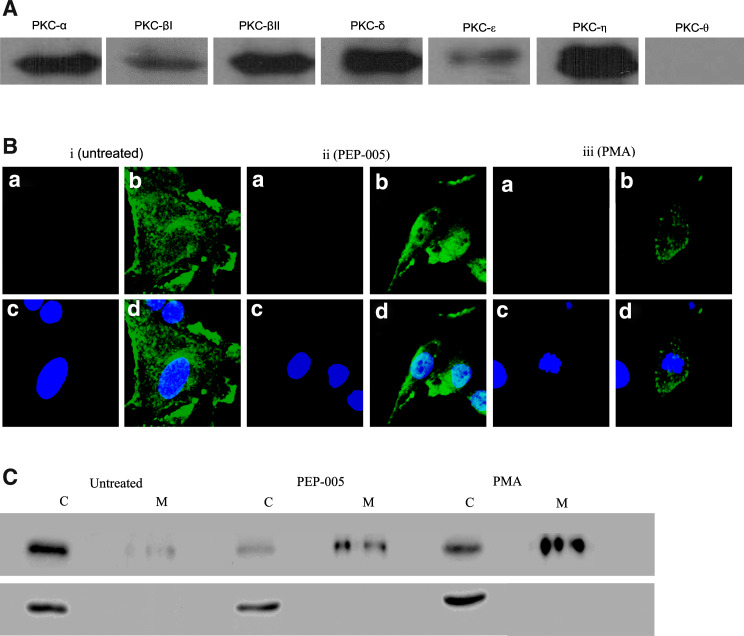
A screen of EC PKC isoenzyme expression by western blot revealed that EC expressed six classical and novel PKC isoforms, namely cPKC- α, βI and βII and n-PKC-δ, η and ε (Fig. 4a). The dominant PKCs were PKC-α, βII, δ and η. Atypical PKCs were not determined as these are not activated by PEP005 [7], though EC have been reported to express PKC-ζ previously [15]. Using confocal immuno-fluorescent microscopy, endothelial PKC-δ could be seen to translocate from a cytoplasmic to a perinuclear location in response to PEP005, a pattern of activation mirroring that seen in response to the phorbol ester PMA (Fig. 4b). In addition, detergent extraction followed by western blot of EC stimulated with PEP005 or PMA, showed that PKC-δ translocated from a detergent insoluble fraction (cytosol) to a detergent soluble fraction (membrane), indicative of activation by these agents (Fig. 4c).
Fig. 4.
PEP005 or PMA mobilise PKC-δ to the perinuclear membrane. a EC were assessed for classical and novel PKC isoform content by western blotting and found to express high levels of six isoforms. b EC were assessed for PKC-δ activation by immuno-fluorescent staining and confocal microscopy in i untreated cells or cells treated with either ii PEP005 or iii PMA. a Shows cells stained with an irrelevant isotype matched antibody; b with anti-PKC-δ antibody; c with the nuclear stain DAPI; d a merged image of b and c. c EC were assessed for translocation of PKC-δ by western blot after extraction of cytoplasmic (C) and membrane (M) associated fractions after no treatment (untreated) or treatment with PEP005 or PMA. Upper panel shows PKC-δ immunoreactivity and the lower panel is the loading control β-actin. Both means of assessment showed that PKC-δ was translocated from a cytoplasmic to membrane location in response to PEP005 or PMA
The knockdown of PKC-δ using siRNA inhibits neutrophil recruitment in response to PEP005
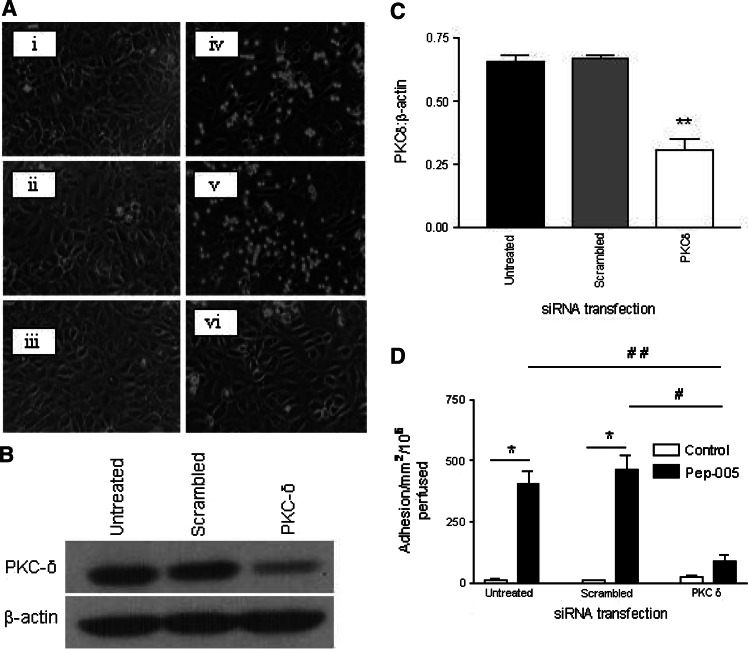
The experiments above strongly imply a role for PKC-δ in the activation of EC by PEP005 and subsequent enhanced recruitment of neutrophils. To confirm this, an siRNA construct designed to reduce PKC-δ expression (or a scrambled control sequence) was transfected into EC by electroporation. Figure 5a shows that EC subjected to electroporation remained viable and formed confluent monolayers that were indistinguishable from untreated EC when they had been sub-cultured into microslides. Importantly, siRNA treatment effectively reduced EC PKC-δ protein expression when assessed by western blot, while a scrambled sequence, or EC suspended in electroporation buffer but receiving no current, showed no response to treatment (Fig. 5b, c). PKC-δ siRNA treated EC (or cells treated under control conditions) were also utilised in a flow based adhesion assay. The knockdown of PKC-δ effectively inhibited EC responses to PEP005 and abolished the recruitment of flowing neutrophils (Fig. 5c). Importantly, the responses of EC subject to control treatments were not affected.
Fig. 5.
Knockdown of PKC-δ using siRNA inhibits neutrophil recruitment to PEP005 stimulated EC. a Integrity of EC monolayers was unaffected in EC that had been i untransfected or ii transfected by electroporation with a scrambled siRNA sequence or iii transfected with PKC-δ siRNA. In response to PEP005 untransfected EC supported the adhesion of flowing neutrophils iv. Transfection with scrambled sequence did not affect the levels of neutrophil adhesion v, but siRNA for PKC-δ abolished neutrophil recruitment vi. Images are taken from representative monolayers of EC cultured in microslides and subject to flow based neutrophil adhesion assay. b When assessed by western blot, PKC-δ expression was reduced in EC transfected with siRNA but not in untransfected cells or cells transfected with scrambled sequence. Data are from a single experiment representative of four performed. c Densitometry of western blots showed that PKC-δ was significantly reduced after transfection with siRNA but not with scrambled sequence or in untransfected cells. Data are mean ± SEM of four experiments. **P < 0.01 for comparison between cells transfected with siRNA or scrambled sequence by t test. d EC that were untransfected, transfected with scrambled sequence or transfected with PKC-δ siRNA were incorporated into flow based adhesion assay. The process of electroporation did not activate EC and monolayers did not support the adhesion of neutrophils in the absence of PEP005. Untransfected cells supported the adhesion of flowing neutrophils in response to PEP005 and levels of adhesion were unaffected by transfection with a scrambled sequence. Transfection with PKC-δ siRNA virtually abolished neutrophil recruitment. Data are mean ± SEM of three experiments; *P < 0.05 for comparison of neutrophil adhesion to EC monolayers with or without treatment with PEP005; #P < 0.05, ##P < 0.01 for comparison of PKC-δ siRNA transfected cells with untreated or scrambled sequence transfected cells by t test. Data are mean ± SEM of three experiments
Discussion
Although it is a relatively new concept, it appears that a growing number of cancer treatments rely on mobilisation of the innate immune system to mediate efficient tumor destruction [16, 17]. For example, the efficacy of bacillus Calmette-Geurin (BCG) in the treatment of bladder cancer appears to require the release of pro-apoptotic granule constituents from neutrophils (e.g. TRAIL/Apo-2L) [18]. Induction of neutrophil mediated tumour cell apoptosis in response to IL-12 gene therapy has also been reported in a metastatic model of prostate cancer [19]. In a number of in vitro and in vivo studies, the efficacy of antibodies targeting tumours (e.g. Rituximab which targets CD20 on malignant B-cells) relies on a neutrophil response mediated through ligation of Fc receptors [20–23]. Indeed, it is possible that antibody therapies designed specifically to promote interactions with the Fc receptors borne on neutrophils may make such therapies more effective [23]. We have previously shown that neutrophils are important for the tumoricidal functions of PEP005. Although high concentrations of PEP005 induce primary necrosis of skin tumor cells [2], tumors are prone to relapse in the absence of secondary inflammation and the recruitment of neutrophils [3].
The pro-inflammatory activities of PEP005 that support tumor infiltration by neutrophils are not fully described. However, it seems probable that its mode of inflammatory action is multi-factorial. For example, PEP005 can induce the production of inflammatory cytokines such as TNF-α and IL-1β in skin and tumor cells [3], a process that appears to depend upon its ability to directly activate PKC isoforms in these cells [3, 7]. Such cytokines are well documented to be able to orchestrate the recruitment of neutrophils by activating vascular endothelial cells so that they express the requisite adhesion receptors and chemotactic signals for neutrophil recruitment [4–6]. In addition, PEP005 induces the production of chemokines such as IL-8 from the stromal cells of the skin and tumor [3]. Chemokines do not ordinarily activate endothelial cells directly, however, endothelial cells have the capability to assimilate stromal derived chemokines and to present them to leukocytes recruited to the vessel wall [24]. By so doing they regulate the process of leukocyte activation and trans-endothelial migration in a manner that is influenced by the inflammatory status of the stromal environment. Thus, through the production of stromal cytokines and chemokines, PEP005 appears capable of establishing a complex network of agents that interact with EC and neutrophils in a paracrine fashion, thereby contributing to the recruitment of neutrophils into the tumor.
As PEP005 can activate novel and classical isoforms of PKC [7], it seemed likely to us that an alternative route of pro-inflammatory signalling might derive from the direct action of PEP005 on endothelial cell PKCs [7]. In accordance with this hypothesis, we have been able to demonstrate that PEP005 mobilises endothelial PKC-δ, thereby upregulating E-selectin, ICAM-1 and IL-8 and inducing the recruitment of neutrophils. This is an important and additional route of EC activation over and above the paracrine routes described above, as the expression of adhesion receptors and chemokines by some inflammatory cytokines (i.e. IL-1β) is reported to occur in a PKC independent manner [25, 26]. Indeed, there is some indication that activation of PKC with agents such as PMA can potentiate the activity of cytokines such as IL-1β in a process where cytokine and PKC mediated signalling pathways synergise [25, 26]. In the current study however, the use of suboptimal concentrations of TNF (0.1 U/ml) and PEP005 (1 ng/ml) did not result in such a synergistic response (data not shown), indicating that there is little interaction between PKC-δ and TNF-α signalling pathways, as has previously been reported [26]. However, these observations do not preclude synergism between PKC-δ and other signalling pathways. For example, Shimamura and colleagues [12] report that the histamine mediated activation of PKC-δ increases the expression of VCAM-1, ICAM-1 and E-selectin synergistically in HUVEC in response to a secondary stimulus of sphingosine 1-phosphate.
Here, we have demonstrated an essential role for PKC-δ in the induction of EC adhesion receptors and chemokines in response to a therapeutic agent (PEP005). As stated above, and in the case of IL-1β, PKC activation is not essential for EC expression of these molecules [26]. However, there is substantial data demonstrating that various patho-physiological stimuli do operate through the activation of various PKC isoforms and not just through PKC-δ, to induce adhesion molecule expression in EC. Early experiments utilising agents such as PMA to activate HUVEC demonstrated up-regulation of E-selectin, which could be attenuated with inhibitors of PKC activation [25]. However, these authors did not identify the PKC isoform(s) involved in EC activation. Later studies demonstrated that broad spectrum PKC activating agents such as PMA up-regulated E-selectin in a PKC-β1 dependent manner in HUVEC, although interestingly, EC derived from other sources (e.g. the dermal vasculature) could upregulate E-selectin in response to PMA in a manner reliant upon PKC signalling but independent of PKC-β1 [13]. In response to sphingosine-1-phosphate, E-selectin was upregulated in a PKC-α dependent manner [12], while thrombin upregulated E-selectin through PKC-δ [14, 15] and VCAM-1 through PKC-ζ [15]. In a model of diabetic hyperglycemia, high and/or fluctuating levels of glucose were shown to induce E-selectin and ICAM-1 in HUVEC through PKC-β [27]. Thus, it is apparent that during the activation of EC with inflammatory stimuli, PKC signalling can be completely redundant (e.g. in response to IL-1β). However, expression of adhesion receptors for leukocyte recruitment can be induced in a PKC dependent manner, although there is substantial redundancy in the isoform(s) of PKC which can mediate this activation process.
The inflammatory functions of PEP005 are also likely to extend to direct stimulatory activity of neutrophil functions, possibly in a PKC dependent manner. Indeed, we have previously demonstrated that PEP005, at concentrations of approximately 100 nM, induced the neutrophil respiratory burst leading to increased superoxide production [3]. In addition, PEP005 at a concentration of 5 nM or less could induce IL-8 release from human neutrophils [3]. Others have shown that PMA activates neutrophil PKC-δ [28] and that components of the neutrophil NADPH oxidase complex are translocated to membrane locations by this stimulus, a requirement for superoxide generation [29, 30].
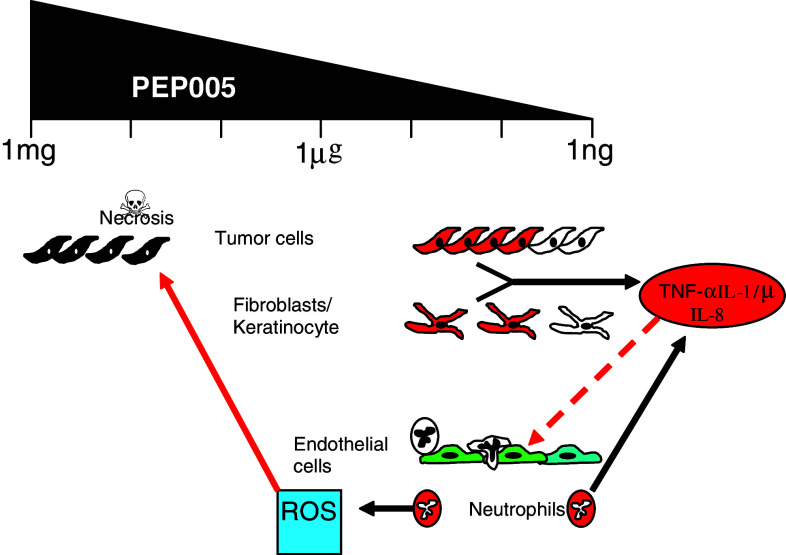
Thus in summary, PEP005 is now known to regulate the inflammatory process by stimulating a number of cellular targets over a broad range of concentrations that are achievable by topical application to the skin (Fig. 6). Besides inducing primary necrosis of tumour cells at high concentration it can induce a network of inflammatory cytokines and chemokines from cells of the tissue stroma that promote the recruitment of neutrophils by paracrine interaction with both EC and neutrophils. In addition, PEP005 directly regulates the inflammatory status of EC by inducing adhesion receptor and chemokine expression in these cells and thereby, promoting neutrophil recruitment. Finally, it can also stimulate the release of cytotoxic agents from neutrophils once they have been recruited to the tumor mass. The broad spectrum of cells targeted by PEP005 and the diversity of the inflammatory and cytotoxic pathways that it stimulates may explain why this agent is so effective for the topical treatment of skin tumors [2].
Fig. 6.
The tumoricidal modes of action of PEP005. PEP005 shows tumoricidal activity over a wide range of concentrations. At high concentration (≈100 μg/ml) PEP005 induces primary necrosis of tumor cells. At much lower concentrations the effects of PEP005 are mainly pro-inflammatory. Thus between 5 and 100 ng/ml, PEP005 induces the release of TNF-α, IL-1β and IL-8 from tumor cells, cells of the dermal stroma (fibroblasts and keratinocytes) and from neutrophils. It is probable that the release of these agents into the tumor mass regulate the process of neutrophil recruitment through paracrine interaction with vascular EC. In addition, PEP005 can directly activate EC at concentrations in the order of 10 ng/ml, resulting in the recruitment of neutrophils. At concentrations of ≈100 ng/ml PEP005 also directly induces the neutrophil respiratory burst with consequent production of ROS, which are believed to be important for tumor cell killing and appear to be essential for preventing tumor relapse
Acknowledgments
The work reported here was supported by funding from the European Commission (Integrated project LSHB-CT-2004-503467).
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- 1.Weedon D, Chick J. Home treatment of basal cell carcinoma. Med J Aust. 1976;1:928. [PubMed] [Google Scholar]
- 2.Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833–2839. doi: 10.1158/0008-5472.CAN-03-2837. [DOI] [PubMed] [Google Scholar]
- 3.Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are key component of the anti-tumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman GA, Prescott SM, McIntyre TM. Leukocyte–endothelial cell interactions. Immunol Today. 1992;13:93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 5.Imhof BA, Dunon D. Leukocyte migration and adhesion. Adv Immunol. 1995;58:345–416. doi: 10.1016/S0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- 6.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 7.Kedei N, Lundberg DJ, Toth A, et al. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64:3243–3255. doi: 10.1158/0008-5472.CAN-03-3403. [DOI] [PubMed] [Google Scholar]
- 8.Buckley CD, Ross EA, McGettrick HM, et al. Identification of a phenotypically and functionally distinct population of long lived neutrophils in a model of reverse endothelial migration. J Leuk Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 9.Lally F, Smith E, Filer A, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arth Rheum. 2005;52:3460–3469. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke BM, Usami S, Perry I, et al. A simplified method for culture of endothelial cells and analysis of blood cells under conditions of flow. Microvasc Res. 1993;45:33–45. doi: 10.1006/mvre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 11.Luu NT, Rainger GE, Nash GB. Kinetics of the different steps during neutrophil migration through cultured endothelial monolayers treated with tumour necrosis factor-α (TNF) J Vasc Res. 1999;36:477–485. doi: 10.1159/000025690. [DOI] [PubMed] [Google Scholar]
- 12.Shimamura K, Takashiro Y, Akiyama N, et al. Expression of adhesion molecules by sphingosine 1-phosphate and histamine in endothelial cells. Eur J Pharmacol. 2004;486:141–150. doi: 10.1016/j.ejphar.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Mason JC, Yarwood H, Sugars K, et al. Human umbilical vein and dermal microvascular endothelial cells show heterogeneity in response to PKC activation. Am J Physiol. 1997;273:C1233–1240. doi: 10.1152/ajpcell.1997.273.4.C1233. [DOI] [PubMed] [Google Scholar]
- 14.Rahman A, Anwar KN, Uddin S, et al. Protein kinase C-δ regulates thrombin-induced ICAM-1 gene expression in endothelial cells via activation of p38 mitogen activated protein kinase. Mol Cell Biol. 2001;21:5554–5565. doi: 10.1128/MCB.21.16.5554-5565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami T, Abid MR, Zhang J, et al. Thrombin stimulation of vascular adhesion molecule-1 in endothelial cells is mediated by protin kinase C (PKC)-δ-NF-κB and PKC-ζ-GATA signalling pathways. J Biol Chem. 2003;278:6976–6984. doi: 10.1074/jbc.M208974200. [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo E, Forni G, Lollini P, et al. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.V97.2.339. [DOI] [PubMed] [Google Scholar]
- 17.Di Carlo E, Forni G, Musiani P. Neutrophils in the antitumoral immune response. Chem Immunol Allergy. 2003;83:182–203. doi: 10.1159/000068929. [DOI] [PubMed] [Google Scholar]
- 18.Kemp TJ, Ludwig AT, Earel JK, et al. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 2005;106:3474–3482. doi: 10.1182/blood-2005-03-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanford MA, Yan Y, Canfield SE, et al. Independent contributions of GR-1+ leukocytes and Fas/FasL interactions to induce apoptosis following interleukin-12 gene therapy in a metastatic model of prostate cancer. Hum Gene Ther. 2001;12:1485–1498. doi: 10.1089/10430340152480221. [DOI] [PubMed] [Google Scholar]
- 20.Stockmeyer B, Beyer T, Neuhuber W, et al. Polymorphonuclear granulocytes induce antibody-dependent apoptosis in human breast cancer cells. J Immunol. 2003;171:5124–5129. doi: 10.4049/jimmunol.171.10.5124. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–5873. [PubMed] [Google Scholar]
- 22.Niitsu N, Khori M, Hayama M, et al. Phase I/II study of the rituximab-EPOCT regimen in combination with granulocyte colony-stimulating factor in patients with relapsed or refractory follicular lymphoma including evaluation of its cardiotoxicity using B-type natriuretic peptide and troponin T levels. Clin Cancer Res. 2005;11:697–702. [PubMed] [Google Scholar]
- 23.Otten MA, Rudolph E, Dechant M, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- 24.Middleton J, Neil S, Wintle J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/S0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 25.May MJ, Pearson JD (1994) The role of protein kinase C (PKC) in Eselectin expression by human umbilical vein endothelial cels (HUVEC): comparison of the effects of IL-1 and PMA. J Physiol 475P
- 26.Tamaru M, Narumi S. E-selectin gene expression is induced synergistically with rthe cocexistence of activated classic protein kinase C and signals elicited by interleukin-1β but not tumour necrosis factor-α. J Biol Chem. 1999;274:3753–3763. doi: 10.1074/jbc.274.6.3753. [DOI] [PubMed] [Google Scholar]
- 27.Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005;183:259–267. doi: 10.1016/j.atherosclerosis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Hendy B, Zhu CL, Greenstein S. Fas activation opposes PMA-stimulated changes in the localisation of PKC delta: a mechanism for reducing neutrophil adhesion to endothelial cells. J Leuk Biol. 2002;71:863–870. [PubMed] [Google Scholar]
- 29.Umei T, Ohhara N, Okamura S, et al. Activation of neutrophils NADPH oxidase by PMA—cytosol activity is translocated in phorbol-primed neutrophils. Int J Biochem. 1993;25:631–633. doi: 10.1016/0020-711X(93)90346-G. [DOI] [PubMed] [Google Scholar]
- 30.El Benna J, Dang PMC, Andrieu V, et al. P40(phox) associates with neutrophil Triton X-100-insoluble cytoskeletal fraction and PMA-activated membrane skeleton: a comparative study with P67(phox) and P47(phox) J Leuk Biol. 1999;66:1014–1020. doi: 10.1002/jlb.66.6.1014. [DOI] [PubMed] [Google Scholar]