Abstract
We generated a human/mouse chimeric antibody c-SN6j of human IgG1 isotype from a murine anti-human endoglin (EDG) monoclonal antibody (mAb) SN6j that suppressed angiogenesis, tumor growth and metastasis in mice. We determined pharmacokinetics (PKs) and immunogenicity of c-SN6j in monkeys after multiple i.v. injections. A dose-escalation study was performed by administration of c-SN6j into six monkeys at the dose of 1 mg, 3 mg and 10 mg per kg body weight. In addition, both c-SN6j (3 mg/kg) and doxorubicin (0.275 mg/kg) were injected into two monkeys. c-SN6j and doxorubicin were injected twice a week for 3 weeks. We developed a unique and sensitive ELISA by sequentially targeting the common and idiotypic epitopes of c-SN6j-Fv to quantify plasma c-SN6j. Application of the ELISA showed that increasing the c-SN6j dose resulted in a proportional increase in the circulating c-SN6j after the first injection. In addition, the estimated area under the curve (AUC) for the first injection of c-SN6j is proportional to dose. We carried out detailed analyses of PKs of c-SN6j during and after the repeated injections. Our model of PKs fitted the empirical data well. Addition of doxorubicin modulated the PK parameters. We developed two ELISAs to separately determine the immune responses to the murine part and the human part of c-SN6j in monkeys. Interestingly, the murine part induced a weaker immune response than the human part. Doxorubicin potentiated the immune responses. Increasing the dose of c-SN6j increased plasma levels of c-SN6j but did not increase the immune responses to c-SN6j.
Keywords: Anti-angiogenesis, Pharmacokinetics, Immune response, Endoglin, Chimeric antibody, Nonhuman primates
Introduction
Endoglin is a homodimer cell surface glycoprotein that is expressed on leukemia cells and endothelial cells [1–3]. The existence of two forms of EDG was reported, the smaller form (160 kD, termed S-EDG) and the larger form (170 kD, termed L-EDG); a small difference between the two EDGs was due to the difference in size of the cytoplasmic portions of the proteins [4]. EDG is a component of the TGF-β receptor complex [5]. It forms heterodimers with the signaling type I and type II receptors of TGF-β in the presence of ligand [6, 7], and can modulate cellular responses to TGF-β[7–9]. In addition, EDG may modulate cell migration and cytoskeletal organization [10, 11]. EDG is essential for angiogenesis and vascular development [12, 13], and is a proliferation/activation-associated antigen of endothelial cells [14–17] and leukemia cells [18]. EDG is strongly expressed on vascular endothelium of tumor tissues but less so on that of normal tissues [15, 19–21], and the high intratumoral microvessel density for EDG expression correlates with poor overall survival of patients with breast, lung and colorectal cancers [22–24].
We reported that systemic (i.v.) administration of anti-EDG monoclonal antibodies (mAbs) including SN6j and their immunoconjugates suppressed angiogenesis, tumor growth and metastasis without overt toxicity in mice [16, 21, 25–27]. The combination of SN6j with cyclophosphamide showed synergistic antitumor efficacy in human skin/SCID mouse chimeras when the drug was administered using a metronomic dosing schedule [26]. More recently we found that doxorubicin (adriamycin) also strongly potentiated antitumor efficacy of SN6j in the chimeras [28]. Doxorubicin has been widely used in the treatment of patients with a variety of cancers (reviewed in [29]). Therefore, we evaluated the preclinical properties of SN6j and doxorubicin in nonhuman primates in the present study. We generated a recombinant human/mouse chimeric mAb, termed c-SN6j, from SN6j, and c-SN6j or c-SN6j plus doxorubicin were administered six times, twice a week, into monkeys.
We developed a unique and sensitive ELISA to quantify plasma c-SN6j, and two additional ELISAs to separately determine the immune responses to the murine part and the human part of c-SN6j in monkeys. The results obtained from the present study are being utilized in the design of the therapeutic application of c-SN6j to cancer patients.
Materials and methods
Antibodies and materials
Murine Anti-human EDG mAb SN6j was generated in our laboratory [21]. Recombinant human/mouse chimeric mAbs of IgG1 and IgG3 isotypes were generated from SN6j. Details of the generation will be reported elsewhere 1. In the present study, the chimeric mAb of IgG1 isotype (termed γ1-c-SN6j or simply c-SN6j) was used. c-SN6j of GLP (good laboratory practice) grade was produced in the facility of Unisyn Division (Hopkinton, MA, USA) of Biovest International. F(ab’)2 fragment of SN6j was prepared by pepsin digestion of SN6j. Rabbit anti-SN6j-F(ab’)2 antibodies were generated in the Animal Facilities of our Institute by repeated injections of F(ab’)2 of SN6j with adjuvants (Freund’s complete and incomplete adjuvants) into two rabbits. Analysis of serially bled antisera demonstrated that early bleedings of both antisera from the two rabbits possessed strong anti-idiotypic antibody activity (data not shown). Purified Antibodies from the antisera were passed through serially connected human IgG-CL4B Sepharose column and SN6j-IgG-CL4B Sepharose column to obtain affinity purified antibodies that are free of any antibodies crossreative with human IgG. The materials bound to the SN6j-IgG column were eluted using glycine-HCl (pH 2.6) buffer. The resulting affinity-purified anti-SN6j-F(ab’)2antibodies were further passed through a normal human plasma-CL4B Sepharose column and a normal human plasma-AminoLink-agarose column (Pierce, Rockford, IL, USA) to remove any antibodies that crossreact with human plasma. Affinity-purified goat anti-mouse IgG-F(ab’)2 antibodies and goat anti-human IgG-Fc antibodies were purchased from Jackson Immuno Research (West Grove, PA, USA). Biotinylation of affinity-purified anti-SN6j-F(ab’)2 antibodies, SN6j and c-SN6j mAb were made using an EZ-Link Sulfo-NHS-Biotinylation Kit (Pierce) according to the manufacture’s protocol. Clinical grade doxorubicin (Novaplus, Irving, TX, USA) was obtained from the Pharmacy Department of Roswell Park Cancer Institute.
Animals and blood samples
Eight female cynomolgus (Macaca fascicularis) monkeys weighing between 2.0 kg and 2.4 kg were used for this study. Captive bled monkeys were obtained from the Biomedical Resources Foundation, Inc. (Houston, TX, USA). Monkeys were housed and cared for at the Laboratory Animal Facilities, School of Medicine and Biomedical Sciences, State University of New York at Buffalo. Animal care was provided in accordance with the standards established by the Association for Assessment and Accreditation for Laboratory Animal Care. The experimental protocols were approved by the Institutional Animal Care and Use Committee. Eight monkeys were divided into four groups. Group 1 (monkeys A and B), group 2 (C and D) and group 3 (E and F) were given 1 mg, 3 mg and 10 mg/kg BW, respectively, of c-SN6j. Group 4 (monkeys G and H) received 3 mg/kg BW of c-SN6j and 0.275 mg/kg BW of doxorubicin. The dose of c-SN6j was chosen based on the results of our studies of SN6j in mouse models ([26, 27] and unpublished observation); c-SN6j at these doses is expected to be safe and effective for tumor suppression in cancer patients. Survey of clinical trial results [29] suggested that doxorubicin will be safe at the dose of 0.275 mg/kg BW after multiple injections into cancer patients. In addition, results of our animal studies suggest that doxorubicin will potentiate the antitumor activity of c-SN6j at a similar dose ([28], unpublished observation). c-SN6j in PBS was centrifuged at 100,000×g at 4°C for 1 h, and the supernatant was filtered through a sterile Millex-GV filter (0.22 mm; Millipore, Bedford, MA, USA) in a laminar flow hood before use. c-SN6j and doxorubicin were injected six times via the superficial vein of an arm on days 0, 4, 7, 11, 14, and 21. Blood was drawn via the superficial vein of the other arm. For the studies of pharmacokinetics (PKs) and immune response, blood samples were drawn in the following schedule: 0 (immediately before injection), 1, 2, 4, 24, 48 and 72 h after the first injection of c-SN6j; 0, 4, 24, 48 and 72 h after the third injection of c-SN6j; 0, 1, 2, 4, 24, 48 and 72 h, and on days 7, 11, 15, 18, 22, 25, 29, 32 and 36 after the sixth injection of c-SN6j. In addition, blood was drawn weekly for analyses of hematological indices and serum chemistry. The blood samples were centrifuged at 2,000×g for 20 min and the resulted serum samples were stored at −20°C until used.
ELISA to quantify circulating c-SN6j (ELISA-1)
We developed a unique double-antibody sandwich ELISA to quantify circulating plasma c-SN6j in monkeys that received multiple injections of c-SN6j. In this assay, c-SN6j was first captured onto the microtiter plates by pre-coated goat anti-mouse IgG-F(ab’)2 antibodies by targeting the common antigenic determinants of mouse IgG-Fv of c-SN6j. Then, the captured c-SN6j was detected by biotinylated rabbit anti-SN6j-F(ab’)2 antibodies (see above) by targeting the idiotypic epitopes of c-SN6j-Fv. Experimental procedures are described below. Individual wells in Maxisorp 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with goat anti-mouse IgG-F(ab’)2 antibodies by adding 100 μl of the antibodies (10 μg/ml in PBS) followed by overnight incubation at 4°C. The optimal concentration of 10 μg/ml was determined by preliminary titration experiments. The plates were washed twice with washing buffer (PBS containing 0.25% Tween 20). Microtiter plate wells were then filled with 200 μl of blocking buffer (PBS containing 2% BSA and 0.05% NaN3), incubated overnight at 4°C and washed twice with the washing buffer. Fifty micorliters of serial dilutions of c-SN6j (for a standard curve) or appropriate dilutions of sera from the c-SN6j-injected monkeys were added, in quadruplicate, to individual wells and incubated for 3 h at room temperature. Dilutions of c-SN6j and sera were made using a dilution buffer that consisted of RPMI 1640, or PBS containing 50 KIU/ml Aprotinin, 25 mM HEPES, 0.1% BSA, and 0.01% NaN3. Replacing RPMI 1640 with PBS in the dilution buffer did not affect the results of the ELISA. After washing three times with the washing buffer, 50 μl of biotinylated rabbit anti SN6j-F(ab’)2 antibodies (see above; diluted to 2.5 μg/ml with the dilution buffer) were added into individual wells and incubated for 1 h at room temperature. After washing three times with the washing buffer, 50 μl of ABC reagent (VECTASTAIN ABC Kits, Vector Labs, Burlingame, CA, USA) were added into individual wells, incubated for 30 min at room temperature and washed four times with washing buffer. Then, 100 μl of 0.1% 2,2′-azino-bis (3-ethylbenzthiazoline 6-sulfonic acid) (ABTS; Sigma, St Louis, MO, USA) in 0.05 M phosphate-citrate buffer (pH 5.0) containing 0.25 μl/ml hydrogen peroxide were added into wells followed by incubation for a suitable period of time (e.g., 5 min) at room temperature. The resulting color intensity was measured spectrophotometrically at 405/540 nm with a Microplate Autoreader (BIO-TEK, Winooski, VT, USA). Quadruplicate analyses were performed for each sample. The standard curve was constructed by generating a linear regression line using the assay results and Excel software (Micro Soft, Redmond, WA, USA). The measured serum c-SN6j values were presented as the mean ± SD.
ELISAs (ELISA-2 and ELISA-3) to measure immune responses to c-SN6j
We developed two double-antigen sandwich ELISAs to separately determine the immune responses to the mouse part (mouse IgG-Fv) and human part (human IgG-CL/CH) of c-SN6j. The assay procedures for ELISA-2 to measure the immune response to the mouse part of c-SN6j are as follows: individual wells of Maxisorp 96-well microtiter plates were coated with SN6j by adding 100 μl of SN6j (10 μg/ml in PBS) followed by overnight incubation at 4°C. The optimal SN6j concentration of 10 μg/ml was determined by preliminary titration experiments. The coated plates were washed twice with the washing buffer (see above ELISA-1) and incubated overnight at 4°C with the blocking buffer (see above). Fifty microliters of serial dilutions of rabbit anti-SN6j-F(ab’)2 antibodies (for a standard curve) or appropriate dilutions of monkey serum samples were added, in quadruplicate, into individual coated wells followed by incubation for 3 h at room temperature. Preimmune monkey serum was included as a negative control. After washing three times with the washing buffer, 50 μl of biotinylated SN6j (2 μg/ml) were added into individual wells followed by incubation for 1 h at room temperature. Preliminary titration experiments showed that 2 μg/ml is optimal for the biotinylated SN6j. The reactions with ABC reagent and 2,2′-azino-bis(3-ethylbenzthiazoline 6-sulfonic acid), absorption measurement, and construction of a standard curve were performed as described above for ELISA-1.
In ELISA-3 to measure the immune response to the human part of c-SN6j, the following modifications were performed compared with ELISA-2: microtiter plate wells were coated with c-SN6j (10 μg/ml) instead of SN6j. Serial dilutions of goat anti-human IgG-Fc antibodies, instead of rabbit anti-mouse IgG-F(ab’)2 antibodies, were added into the coated and blocked wells. Then biotinylated c-SN6j (4 μg/ml), instead of biotinylated SN6j, was added into microtiter plate wells.
Pharmacokinetic evaluation
Eight monkeys were given multiple bolus injections of c-SN6j, one injection scheduled every 3 or 4 days, except that the sixth injection came 7 days after the fifth injection, and 28 blood samples per subject were taken up to 57 days from the beginning of the schedule. The c-SN6j plasma concentration with the time data for each monkey was fit with a two-component (biexponential) model that was modified to account for the bolus infusion time and multiple injections. The equation was adapted from the standard equation for a two compartment model with immediate bolus injection [30] by the method of Loo and Riegelman [31] to account for the effect of the time of infusion, and for multiple injections. The model was parameterized first with four empirical parameters (A, B, t 1/2 α, and t1/2 β), and then with four physiological pharmacokinetic parameters (V C, V SS, CL, and CLD) [30]. The four estimatable empirical parameters include: A, the Y-intercept of the first exponential term; B, the Y-intercept of the second exponential term; t 1/2α, the time that it takes for the first exponential term to be reduced to 50% of A; and t 1/2β, the time that it takes for the second exponential term to be reduced to 50% of B. The four estimatable physiological pharmacokinetic parameters include: V C, the volume of the hypothetical central compartment, which includes as a minimum, the blood plasma; V SS, the volume at steady state, or the sum of the hypothetical central (plasma) and peripheral (tissue) compartments; CL, the systemic clearance of agent from the central (plasma) compartment; and CLD, the distributional clearance between the central (plasma) and peripheral (tissue) compartments. The model fitting approach was iteratively reweighed nonlinear regression, with weights equal to the reciprocal of one plus the square of the predicted c-SN6j plasma concentrations. This procedure was run with proc NLIN with SAS version 8.12 on an Intel Pentium IV-based microcomputer. Graphs of model fits were made with Sigma plot.
Results
Quantitation of plasma c-SN6j
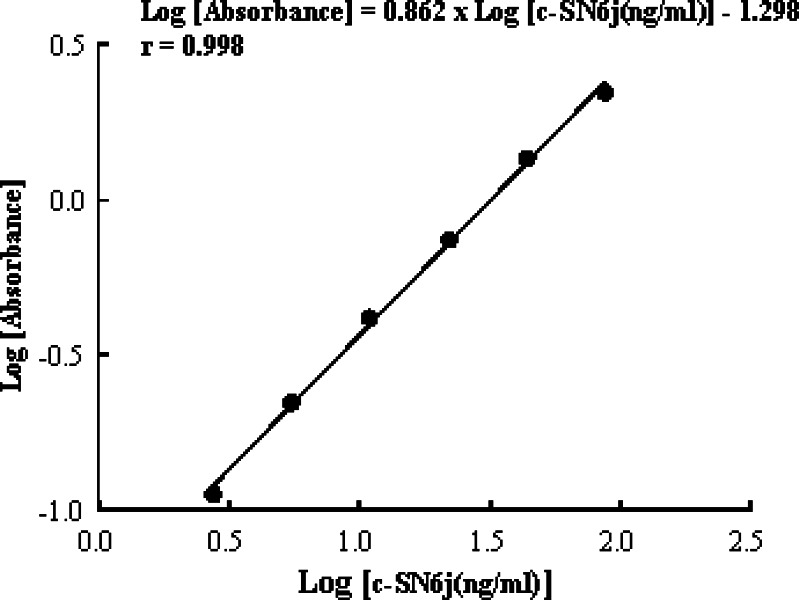
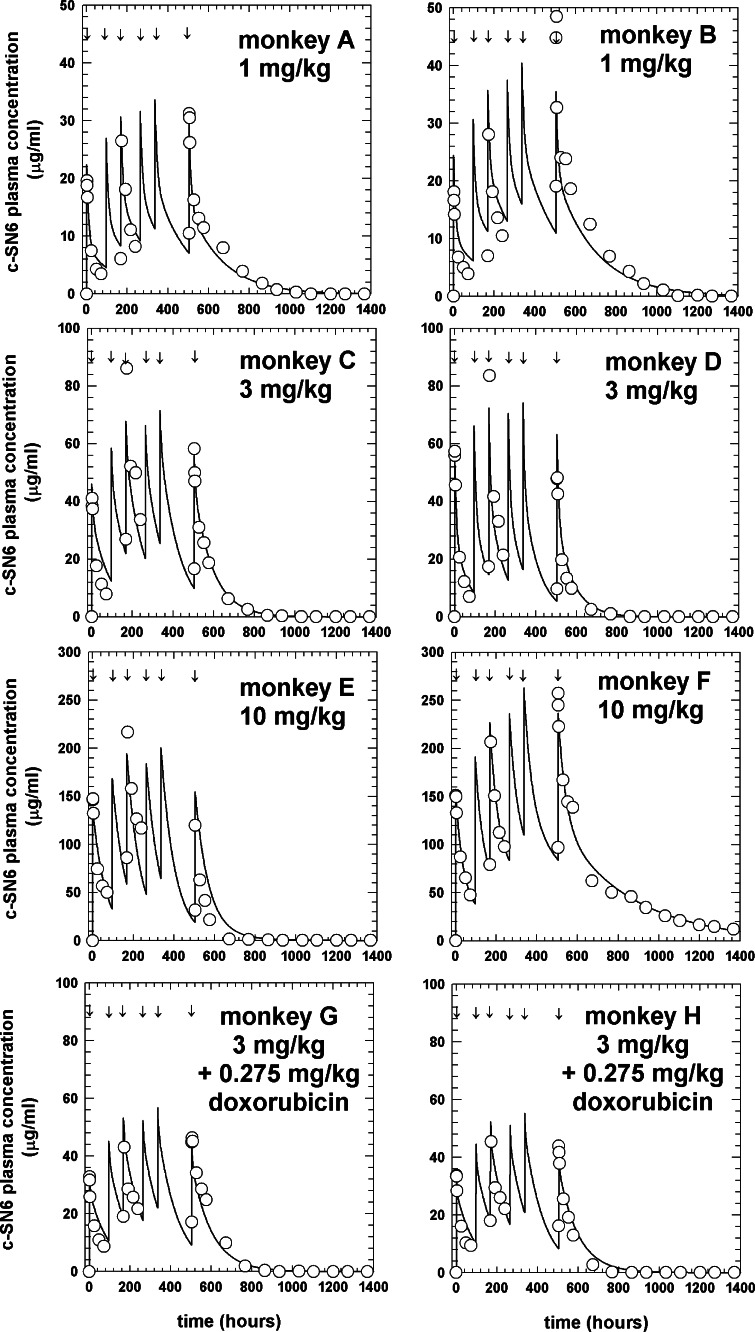
We developed a unique and sensitive ELISA (ELISA-1) to quantify circulating plasma c-SN6j in monkeys that received multiple bolus i.v. injections of c-SN6j (see Materials and methods for details). This assay was based on utilization of two types of antibodies, one targeting the common antigenic determinants of mouse IgG-Fv and the other targeting the idiotypic epitopes of SN6j-Fv. A standard curve was constructed to correlate the optical absorbance in ELISA with the concentration of circulating c-SN6j, and an example is shown in Fig. 1. An excellent linear relationship was observed in the range between 2.7 ng/ml and 87.5 ng/ml of c-SN6j (Fig. 1). In the six monkeys A – F, the measured peak c-SN6j concentration after the first injection was 19.6 ± 1.9, 18.1 ± 2.4, 41.1 ± 5.6, 57.3 ± 4.9, 147.1±11.9 and 151.2±5.8 μg/ml, respectively (Fig. 2). When the measured peak c-SN6j concentration for the first injection of c-SN6j was plotted against dose, the peak concentration was proportional to dose. In addition, it was evident that estimated (AUC) area under the curve for the first injection of c-SN6j is proportional to dose when the AUC was plotted against dose (data not shown). The AUC was calculated from the four empirical PK parameters (see below). In monkeys G and H that received both c-SN6j (3 mg/kg) and doxorubicin (0.275 mg/kg), the peak c-SN6j concentration after the first injection was 32.8±5.6 and 33.6±5.5 μg/ml, respectively (Fig. 2). The results suggest that doxorubicin decreases the levels of circulating c-SN6j. The rationale for the chosen doses of c-SN6j and doxorubicin is described in the Materials and methods. We were able to draw multiple blood samples for the pharmacokinetic study only after the first, third and sixth injections of c-SN6j (see Materials and methods for details) because of the consideration of the animal health. The results in Fig.2 show a biphasic kinetics pattern with a relatively rapid initial clearance (distribution) followed by a slower elimination phase. There were no clinically significant changes in blood counts or serum chemistries after repeated i.v. administration of c-SN6j 2.
Fig. 1.
A standard curve for the circulating c-SN6j as measured by a double-antibody sandwich ELISA. Serial dilutions of the purified c-SN6j were used in the assay
Fig. 2.
Pharmacokinetic profiles for plasma c-SN6j in eight monkeys. The fitted curve for the whole time course and raw data points (indicated by circles) are displayed for each monkey. All monkeys were injected six times, at the time points indicted by the arrows, with the indicated amount of c-SN6j. Monkeys G and H received doxorubicin in addition to c-SN6j. c-SN6j and doxorubicin were given i.v
Pharmacokinetics
The four estimated empirical parameters, A (the Y-intercept for the first exponential term), B (the Y-intercept for the second exponential term), halftime for the first exponential term (t 1/2α), and halftime for the second exponential term (t 1/2β), are listed in Table 1. We were able to use only two monkeys per treatment group for practical reasons including costs and ethical considerations. Nevertheless, the results provide us with valuable information about PKs and immunogenicity of c-SN6j in primates. The values of the four parameters generally agree well within each group for the first (Monkeys A and B), second (C and D) and fourth (G and H) group. However, there are consistent differences in the values of the four parameters between monkeys E and F in the third group. These differences may be related to the fact that monkey E is different from monkey F and other monkeys in that monkey E showed a stronger anti-cSN6j immune response (see below). This notion is supported by the fact that the measured peak c-SN6j concentration after the first injection is very similar between monkeys E and F, i.e., 147.1±11.9 versus 151.2 ±5.8 μg/ml (see above). Despite the questions concerning the parameter estimates of monkeys E and F, it is apparent that the Y-intercept parameters (A and B) tend to increase with increasing dose of c-SN6j. The data suggest that addition of doxorubicin decreases the A parameter and t 1/2α. The data also suggest that increasing the dose from 1 mg/kg to 3 mg/kg decreases the alpha and beta halftimes, but that the increase to 10 mg/kg increases the alpha halftime.
Table 1.
Pharmacokinetic parameters of c-SN6j
| Monkey | BW (kg) | c-SN6j dose (mg/kg) | IT (min) | DOX dose (mg/kg) | A (μg/ml) | t1/2 α (h) | B (μg/ml) | t1/2 β (h) | CL (ml/h/kg) | CLD (ml/h/kg) | VC (ml/kg) | VSS (ml/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 2.2 | 1 | 1 | 0 | 14.2 | 7.24 | 8.20 | 115 | 0.669±0.038 | 2.54±1.8 | 42.7±6.9 | 100±8.9 |
| B | 2.3 | 1 | 1 | 0 | 14.4 | 7.01 | 10.1 | 135 | 0.481±0.040 | 5.55±5.4 | 34.1±15 | 89.5±11 |
| C | 2.3 | 3 | 2 | 0 | 11.2 | 4.73 | 34.7 | 64.7 | 0.902±0.063 | 3.50±9.7 | 61.4±20 | 85.8±8.9 |
| D | 2.1 | 3 | 2 | 0 | 30.3 | 5.82 | 27.5 | 55.6 | 1.16±0.080 | 1.58±1.7 | 53.4±8.1 | 85.5±9.7 |
| E | 2.4 | 10 | 2 | 0 | 36.3 | 46.1 | 95.9 | 47.2 | 1.09±0.13 | 0.216±6.7 | 74.5±23 | 77.1±14 |
| F | 2.3 | 10 | 2 | 0 | 113 | 25.5 | 39.7 | 246 | 0.550±0.010 | 0.820±0.11 | 65.6±2.9 | 155±5.0 |
| G | 2.6 | 3 | 2 | 0.275 | 8.87 | 3.25 | 27.4 | 70.3 | 1.06±0.081 | 3.49±12 | 77.7±25 | 106±12 |
| H | 2.6 | 3 | 2 | 0.275 | 9.69 | 1.96 | 28.0 | 65.7 | 1.12±0.10 | 4.00±18 | 78.4±33 | 102±14 |
BW Body weight, IT Infusion time, DOX doxorubicin, AY-intercept for the first exponential term, BY-intercept for the second exponential term, CL Clearance from the central compartment, CLDf, Distribution clearance, Vc Volume of distribution of the central compartment, Vss volume at steady state
Four physiological pharmacokinetic parameters (V C, V SS, CL and CLD) are also listed in Table 1. The volumes and clearances are small. There is a tendency for CL to increase from the 1 mg/kg group to the 3 mg/kg group. The CLD seems to decrease as the dose is increased from 3 mg/kg to 10 mg/kg. The V C seems to increase as the dose is increased from 1 to 3 mg/kg, but the V SS seems to be relatively uniform across the four groups. Doxorubicin appears to increase volumes of distribution and decrease the overall plasma levels of c-SN6j. From Fig. 2 it can be seen that the model fits the data well. Increasing the dose of c-SN6j increases plasma levels of c-SN6j. The PK analysis indicates that the administered c-SN6j circulates in monkeys in a similar manner to other reported chimeric and humanized mAbs of IgG1 isotype in humans [32].
Immune responses to c-SN6j
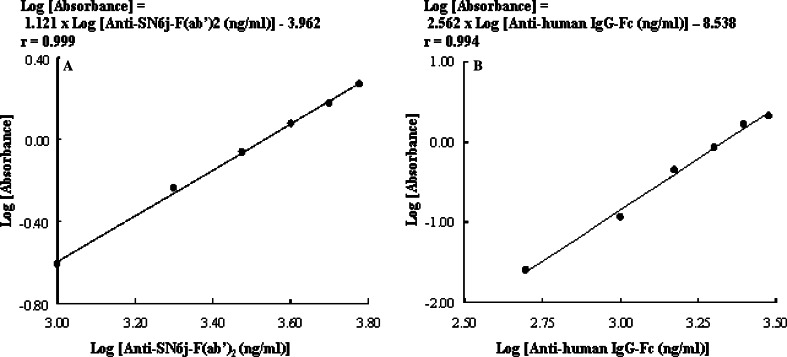
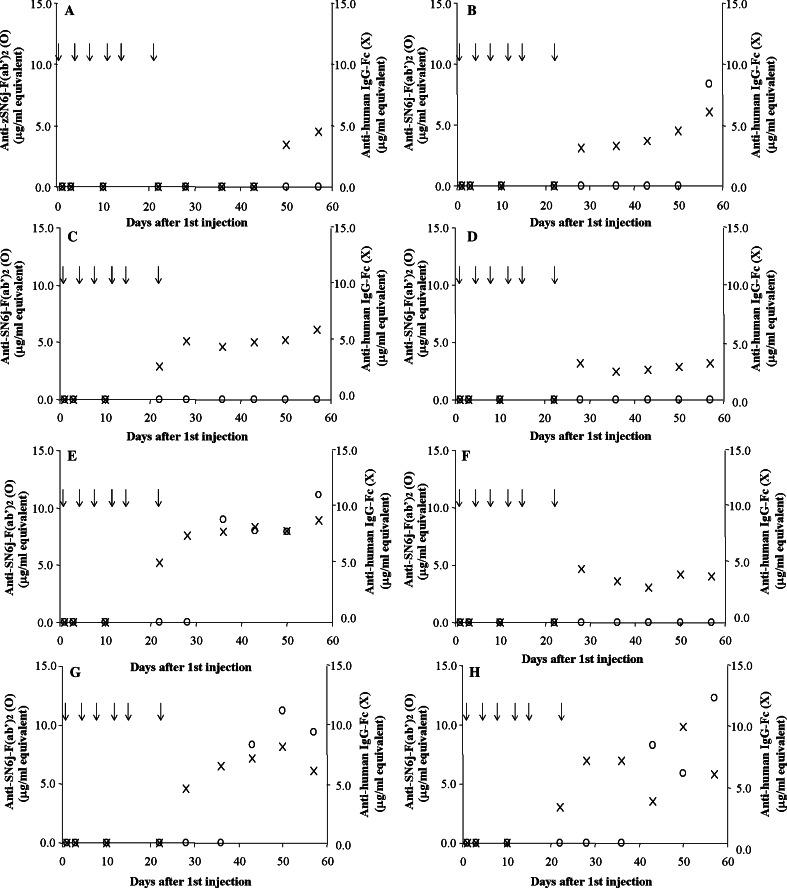
We developed two double-antigen ELISAs, ELISA-2 and ELISA-3, to separately determine the immune responses to the mouse part (mouse IgG-VL/VH, i.e., Fv) and human part (human IgG-CL/CH) of c-SN6j. Examples of the standard curves for ELISA-2 and ELISA-3 are shown in Fig. 3a, b respectively. A good linear relationship is observed between the concentration of anti-mouse Ig antibodies and the optical absorbance in ELISA in the range between 1.0 μg/ml and 6.0 μg/ml of affinity-purified anti-mouse SN6j-F(ab’)2 antibodies (Fig. 3a). Similarly, a good linear relationship is observed between the concentration of anti-human Ig antibodies and the optical absorbance in ELISA in the range between 0.5 μg/ml and 3.0 μg/ml of affinity-purified anti-human IgG-Fc antibodies (Fig. 3b). Serial dilutions of serum were used in the assay of monkey serum samples. The immune responses to the mouse and human parts of c-SN6j were separately measured using ELISA-2 and ELISA-3, respectively, during and after the multiple injections of c-SN6j into the monkeys. The results are presented in Fig. 4. No immune response to the murine part of c-SN6j was detected in monkeys A, C, D and F throughout the test period (Fig. 4). In monkey B, only the serum sample on day 57 showed detectable anti-mouse SN6j-Fv antibody activity that is equivalent to 8.4 μg/ml of affinity-purified rabbit anti-SN6j-F(ab’)2 antibodies (termed 8.4 μg/ml equivalent). In monkey E, serum samples on days 36, 43, 50 and 57 showed anti-mouse SN6j-Fv activities of 7.9– 11.1 μg/ml equivalents. Monkeys G and H, that received doxorubicin in addition to c-SN6j (3 mg/kg), showed mutually similar anti-mouse IgG-Fv (i.e., IgG-VL/VH) activities, and samples on days 43, 50 and 57 showed antibody titers of 5.9– 12.3 μg/ml equivalents (Fig. 4). Comparison of monkeys G and H with monkeys C and D, that received c-SN6j (3 mg/kg) only, indicates that doxorubicin appears to potentiate the immune response to SN6j-Fv in monkeys.
Fig. 3.
Standard curves for anti-c-SN6j antibodies as measured by two double-antigen sandwich ELISAs. Serial dilutions of the affinity-purified anti-SN6j-F(ab’)2 antibodies (a) and anti-human IgG-Fc antibodies (b) were used in the assays to separately evaluate the immune responses to the mouse part and human part of c-SN6j, respectively
Fig. 4.
Immune responses to the mouse part (open circle) and human part (crosses) of c-SN6j during and after six injections of c-SN6j into monkeys A – H. c-SN6j was given i.v. at a dose of 1 mg (monkeys A and B), 3 mg (monkeys C, D, G and H) or 10 mg (monkeys E and F) per kg BW. Monkeys G and H received i.v. administration of doxorubicin (0.275 mg/kg BW) in addition to c-SN6j. Preimmune serum of each monkey was included as a negative control in the assays
Interestingly, repeated administration of c-SN6j into monkeys induced a stronger immune response to human IgG-CL/CH than to mouse IgG-VL/VH (Fig. 4). A theoretical basis for this finding is presented in the Discussion. It should be noted that assay sensitivity is similar between ELISA-2 and ELISA-3 (Fig. 3). In monkey A, serum samples on days 50 and 57 showed anti-human Ig activities that are equivalent to 3.4 and 4.5 μg/ml, respectively, of affinity-purified anti-human IgG-Fc antibodies (termed 3.4 and 4.5 μg/ml equivalents). In monkey B, anti-human Ig activity became detectable after day 28 and the activity remained detectable until the end of the assay (i.e., day 57). The antibody titer ranged between 3.1 and 6.1 μg/ml equivalents. In monkeys C and D, anti-human Ig antibodies became detectable after days 22 and 28, respectively. The antibody titer ranged between 2.9 μg/ml and 6.1 μg/ml equivalents for monkey C and between 2.5 μg/ml and 3.2 μg/ml equivalents for monkey D. Monkey E showed a stronger immune response to the human part as well as to the mouse part of c-SN6j compared with monkey F (Fig. 4). Anti-human Ig titer in monkey E was 5.2 – 8.9 μg/ml equivalents during days 22 – 57, whereas the titer in monkey F was 3.0 – 4.7 μg/ml equivalents during days 28–57. An increase in c-SN6j dose from 1 mg/kg to either 3 mg/kg or 10 mg/kg was not accompanied by an increased immune response except for monkey E (Fig. 4); monkey E appears to behave differently from the other seven monkeys in both the pharmacokinetic profile of the administered c-SN6j and in the elicited immune response (see above).
No immune response to either the mouse or human part of c-SN6j was detected in any monkeys until 10 days after the first injection of c-SN6j. The peak values of anti-mouse Ig antibodies in monkeys A - H were 0.0, 8.4, 0.0, 0.0, 11.1, 0.0, 9.4, and 12.3 μg/ml equivalents, respectively. The peak values of anti-human Ig antibodies in monkeys A – H were 4.5, 6.1, 6.1, 3.2, 8.9, 4.0, 6.1, and 5.8 μg/ml equivalents, respectively. There were no obvious relationships between the titers of the induced antibodies (either anti-mouse antibodies or anti-human antibodies) and the dose of administered c-SN6j into monkeys.
Discussion
Antiangiogenic therapy of cancer is highly attractive for several reasons (reviewed in [33]). For instance, it could overcome the problems of drug resistance [34, 35], poor delivery [36, 37] and tumor heterogeneity [21]. Among the variety of known antiangiogenic agents, antibody-based antiangiogenic agents will be particularly attractive because of the highly selective specificity and multifaceted functions of an appropriately chosen mAb; these functions may include induction of cell signaling, apoptosis and cell-cycle arrest as well as antibody-dependent cell-mediated cytotoxicity (ADCC), complement-mediated cytolysis and antibody-induced peroxide lysis. This notion was corroborated by a recent report of phase III clinical trials of bevacizumab (Avastin), a humanized anti-VEGF (vascular endothelial growth factor) mAb, in patients with metastatic colorectal cancer [38]. We have been targeting EDG of angiogenic vascular endothelium to treat solid tumors and metastasis [16, 21, 25–28]. EDG was also targeted by others as a marker for radioimaging of tumor vessels in tumor-bearing animals [39, 40] or in the resected malignant human kidneys [41]. EDG is an integral cell membrane antigen of leukemia cells and endothelial cells [1], whereas VEGF is a soluble proangiogenic factor that is primarily released by tumor cells and tumor-associated stroma cells in solid tumors [42, 43]. Therefore, bevacizumab and anti-EDG mAbs are anticipated to utilize different mechanisms in suppression of angiogenesis.
In mouse models, certain anti-EDG mAbs and their immunoconjugates (conjugates with deglycosylated ricin A-chain or with 125I) induced regression/suppression of established tumors [21, 26] and inhibited growth of pre-established tumors [16, 25] in spite of the fact that these anti-human EDG mAbs cross-react very weakly with murine endothelial cells [16, 21]. Anti-EDG immunotoxins showed a stronger antiangiogenic activity and antitumor efficacy compared with the naked anti-EDG mAbs. However, these immunotoxins showed a stronger undesirable toxicity and LD50 values were 14.8 – 16.6 μg/g BW in mice [16, 21]. In contrast, the naked anti-EDG mAb SN6j showed no detectable toxicity in mice in a dose escalation study, up to 344 μg/g BW (unpublished observation). Our data collectively showed that the therapeutic window is wider for the naked anti-EDG mAbs compared with the immunotoxins. Therefore, we chose the naked anti-EDG mAbs in our initial attempt for clinical application of anti-EDG mAbs. Among the four anti-EDG mAbs that were evaluated for the in vivo antitumor efficacy in our laboratory ([16, 21, 25, 26], unpublished observation), SN6j was selected based on its strong in vivo antitumor efficacy [21, 25, 26] and strong in vitro suppressive activity against proliferating endothelial cells [9]. To facilitate clinical application of SN6j, we generated recombinant human/mouse chimeric mAb of IgG1 isotype from SN6j in the present study. Human/mouse chimeric mAbs including an anti-CD20 mAb rituximab (or termed Rituxan) and an anti-EGF receptor mAb cetuximab (or termed C225, IMC-C225 or Erbitux) have been successfully used to treat cancer patients by repeated administration (e.g., [44]). In view of these results, we are hoping to test antitumor efficacy and safety of c-SN6j in cancer patients. Before such a clinical trial, we performed studies on PKs and immune response of c-SN6j in nonhuman primates as reported here.
To obtain clinically relevant information, c-SN6j was administered i.v. multiple times into monkeys. Furthermore, doxorubicin (adriamycin) was added to c-SN6j in a set of the test. We have recently demonstrated that combination of SN6j with cyclophosphamide or doxorubicin strongly potentiates antitumor efficacy of SN6j in human skin/SCID mouse chimeras bearing human MCF-7 breast tumors [26, 28]. Therefore, we anticipate that combination of c-SN6j with these drugs will improve antitumor efficacy of c-SN6j in cancer patients. Doxorubicin was used in the present study for the reason described above (see Introduction).
During and after injections of c-SN6j with or without doxorubicin, we evaluated pharmacokinetic parameters and immune response. To quantify the circulating plasma c-SN6j in monkey serum samples, we developed a unique and sensitive double-antibody sandwich ELISA (ELISA-1) by sequentially targeting the common epitopes and the idiotypic epitopes in the murine part of c-SN6j. In this assay, no significant background was detected and an excellent correlation was observed between the optical absorbance in ELISA and the concentration of c-SN6j in the sample. This design of ELISA may be applied for quantifying other chimeric antibodies in plasma and other tissues of patients who received a chimeric mAb for therapy.
We determined four empirical pharmacokinetic parameters A, B, t 1/2α and t 1/2β. In addition, four physiological pharmacokinetic parameters CL, CLD, V C and V SS were determined. The c-SN6j plasma concentration with time data for each monkey was fit with a two-compartment model with multiple bolus injections. Overall the model fitted the empirical data well.
Several groups reported PK parameters of human/mouse chimeric mAbs or humanized mAbs (reviewed in [32]). These mAbs include cetuximab (anti-EGF receptor), rituximab (anti-CD20) and trastuzumab (anti-HER2). Although PK parameters were only partially characterized in many of these mAbs, the mAbs of human IgG1 isotype showed long half-lives, small clearance rates and small distribution values which indicate that these mAbs are relatively tightly bound shortly after injection. The PK parameters of these mAbs in humans are similar to those of c-SN6j in monkeys. It should be noted that these mAbs including c-SN6j react with individually different target antigens. Therefore, the major factors for the relatively tight binding and long half-lives of c-SN6j in monkeys and other chimeric/humanized mAbs in humans may be common. These common factors are likely to be related to the binding of these chimeric/humanized mAbs to the MHC class I-related neonatal Fc receptor, termed FcRn [45], through their common Fc derived from the human IgG1.
We established two new ELISAs (ELISA-2 and ELISA-3) to measure the immune responses to the murine part (mouse IgG-Vκ/Vγ) and the human part (human IgG-Cκ/Cγ) of c-SN6j during and after repeated i.v. injections of c-SN6j with or without doxorubicin. As a whole, the immune response to the mouse part of c-SN6j is weak in monkeys. It is interesting to note that repeated injections of c-SN6j induced weaker immune response to the mouse part than to the human part in monkeys. This finding may be explained as follows:
It is important to note that the murine part of c-SN6j consists of the variable regions (V-regions) of SN6j (a mouse IgG-κ mAb), i.e., Vκ (κ light chain V-region) and Vγ (γ heavy chain V-region) while the human part of c-SN6j consists of the constant regions (C-regions) of a human IgG1-κ, i.e., Cκ and Cγ1. The cynomolgus monkey Cκ region possesses 83% amino acid sequence indentity to its human counterpart, and the monkey Cγ region possesses 95% amino acid sequence indentity to the human Cγ1 region [46]. These amino acid sequence differences between humans and cynomolgus monkeys in the Cκ and Cγ regions are likely the major cause of the immune response to the human part of c-SN6j in cynomolgus monkeys.
The degree of homology between murine IgG V-regions and human IgG V-regions is similar to that between a human IgG V-region and another human IgG V-region [47]. This finding is likely applicable to the homology between murine IgG V-regions and monkey IgG V-regions and that between different monkey IgG V-regions. Therefore, murine IgG V-regions will be weak immunogens in both humans and monkeys.
In the present study, we performed detailed analyses of PK parameters and immune response during and after multiple i.v. injections of a chimeric mAb in nonhuman primates. Few studies reported such detailed analyses of repeatedly injected chimeric/humanized mAbs in nonhuman primates or humans. Multiple injections of mAbs will be more clinically relevant for cancer therapy compared with single injection.
The data presented in this report and a subsequent manuscript (see foot note 2) appear to support our hypothesis that c-SN6j can be safely applied to cancer patients. Addition of doxorubicin to c-SN6j did not cause major problems in monkeys although it modulated pharmacokinetic parameters as well as the immune responses. The latter observation may be related to the potential immunomodulatory activity of doxorubicin (reviewed in [48]).
Acknowledgements
We wish to thank Drs. Lisa Martin and Sandra Buitrago for help in the primate study, and Dr. Youcef Rustum for useful discussions and help. We thank Tom Spence for help in the preparation of the manuscript. This work was supported by Translational Research Grant DAMD17-97-1-7197 and Clinical Translational Research Grant DAMD17-03-1-0463 from the Breast Cancer Research Program of US Department of Defense, a grant from the New York Center for Advanced Technology, and Roswell Park Cancer Center Support Grant P30 CA16056 from the National Cancer Institute.
Footnotes
Haba A, Norderhaug L, and Seon BK. Generation of IgG1 and IgG3 human/mouse chimeric anti-endoglin monoclonal antibodies and comparative studies with the IgG1, IgG3 and parental monoclonal antibodies. Manuscript in preparation
Harada N, Shiozaki KA, Martin L, Buitrago S, and Seon BK. Toxicity Tests of an Antiangiogenic and Chimeric Anti-Endoglin (CD105) Monoclonal Antibody in Nonhuman Primates after Repeated Systemic Injections. Manuscript in preparation
Ken shiozaki and Naoko Harada have contributed equally to the present work.
References
- 1.Haruta Y, Seon BK. Distinct human leukemia-associated cell surface glycoprotein GP160 defined by monoclonal antibody SN6. Proc Natl Acad Sci USA. 1986;83:7898–7902. doi: 10.1073/pnas.83.20.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol. 1988;141:1925–1933. [PubMed] [Google Scholar]
- 3.Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265:8361–8364. [PubMed] [Google Scholar]
- 4.Bellon T, Corbi A, Lastres P, Cales C, Cerbrian M, Vera S, Cheifetz S, Massague J, Letarte M, Bernabeu C. Identification and expression of two forms of the human transforming growth factor-beta-binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol. 1993;23:2340–2345. doi: 10.1002/eji.1830230943. [DOI] [PubMed] [Google Scholar]
- 5.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 6.Yamashita H, Ichijo H, Grimsby S, Moren A, ten Dijke P, Miyazono K. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem. 1994;269:1995–2001. [PubMed] [Google Scholar]
- 7.Guerrero-Esteo M, Sanchez-Elsner T, Letamendia A, Bernabeu C. Extracellular and cytoplasmic domains of endoglin interact with the transforming growth factor-beta receptors I and II. J Biol Chem. 2002;277:29197–29209. doi: 10.1074/jbc.M111991200. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor beta1 on human vascular endothelial cells. Faseb J. 2000;14:55–64. doi: 10.1096/fasebj.14.1.55. [DOI] [PubMed] [Google Scholar]
- 9.She X, Matsuno F, Harada N, Tsai H, Seon BK. Synergy between anti-endoglin (CD105) monoclonal antibodies and TGF-beta in suppression of growth of human endothelial cells. Int J Cancer. 2004;108:251–257. doi: 10.1002/ijc.11551. [DOI] [PubMed] [Google Scholar]
- 10.Conley BA, Koleva R, Smith JD, Kacer D, Zhang D, Bernabeu C, Vary CP. Endoglin controls cell migration and composition of focal adhesions: function of the cytosolic domain. J Biol Chem. 2004;279:27440–27449. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- 11.Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CHP, Bernabeu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem. 2004;279:32858–32868. doi: 10.1074/jbc.M400843200. [DOI] [PubMed] [Google Scholar]
- 12.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science. 1999;284:1534–1537. doi: 10.1126/science.284.5419.1534. [DOI] [PubMed] [Google Scholar]
- 13.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGF beta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 14.Westphal JR, Willems HW, Schalkwijk CJM, Ruiter DJ, de Waal RMW. A new 180-kDa dermal endothelial cell activation antigen: In vitro and in situ characteristics. J Invest Dermatol. 1993;100:27–34. doi: 10.1111/1523-1747.ep12349946. [DOI] [PubMed] [Google Scholar]
- 15.Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, Letarte M, Vitetta ES, Thorpe PE. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res. 1995;1:1623–1634. [PubMed] [Google Scholar]
- 16.Seon BK, Matsuno F, Haruta Y, Kondo M, Barcos M. Long-lasting complete inhibition of human solid tumors in SCID mice by targeting endothelial cells of tumor vasculature with antihuman endoglin immunotoxin. Clin Cancer Res. 1997;3:1031–1044. [PubMed] [Google Scholar]
- 17.Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, Sedlacek HH, Muller R, Adamkiewicz J. Elevated expression of endoglin, a component of the TGF-beta-receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer. 1999;81:568–572. doi: 10.1002/(SICI)1097-0215(19990517)81:4<568::AID-IJC11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki H, Haruta Y, Seon BK. Effect of induced transformation of human leukemia cells on the expression of GP160, a novel human leukemia-associated cell surface glycoprotein. Fed Proc. 1987;46:1056. [Google Scholar]
- 19.Wang JM, Kumar S, Pye D, van Agthoven AJ, Krupinski J, Hunter RD. A monoclonal antibody detects heterogeneity in vascular endothelium of tumours and normal tissues. Int J Cancer. 1993;54:363–370. doi: 10.1002/ijc.2910540303. [DOI] [PubMed] [Google Scholar]
- 20.Seon BK, Matsuno F, Haruta Y, Barcos M, Spaulding B. CD105 Workshop: immunohistochemical detection of CD105 in the vascular endothelium of human malignant and non-malignant tissues. In: Kishimoto T, Kikutani H, van dem Borne AEGKr, Goyert S, Mason D, Miyasaka M, Moretta L, Okumura K, Show S, Springer TA, Sugamura K, Zola H, editors. Leucocyte typing VI: white cell differentiation antigens. New York: Garland Publishing; 1998. pp. 709–110. [Google Scholar]
- 21.Matsuno F, Haruta Y, Kondo M, Tsai H, Barcos M, Seon BK. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin Cancer Res. 1999;5:371–382. [PubMed] [Google Scholar]
- 22.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, Bundred N. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 23.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H. Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7:3410–3415. [PubMed] [Google Scholar]
- 24.Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, O’Dwyer ST, Haboubi N, Kumar S. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88:1424–1431. doi: 10.1038/sj.bjc.6600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999;82:737–742. doi: 10.1002/(SICI)1097-0215(19990827)82:5<737::AID-IJC18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Haba A, Matsuno F, Seon BK. Antiangiogenic therapy of established tumors in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res. 2001;61:7846–7854. [PubMed] [Google Scholar]
- 27.Haba A, Kondo M, Takahashi N, Harada N, Tsai H, Seon BK. Suppression of metastases of colon-26 tumors by anti-endoglin (CD105) monoclonal antibodies and detection of a novel epitope for monitoring micrometastases and metastases. Proc Am Assoc Cancer Res. 2002;43:524–525. [Google Scholar]
- 28.Harada N, Shiozaki K, Takahashi N, Tsai H, Seon BK. Antiangiogenic and vascular targeting therapy of established tumors in human skin/SCID mouse chimeras by anti-endoglin (CD105) monoclonal antibody, cyclophosphamide and doxorubicin. Proc Am Assoc Cancer Res. 2003;44:693. [Google Scholar]
- 29.Riggs CE., Jr . Antitumor antibiotics and related compounds. In: Perry MC, editor. The Chemotherapy source book. 3rd edn. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 227–252. [Google Scholar]
- 30.Jusko WJ (1992) Guidelines for collection and analysis of pharmacokinetic data. In: Evans WE, Schentag JJ, Jusko WJ (eds) Applied pharmacokinetics, 3rd edn. Therapeutics Inc, Vancouver, pp 2–1–2–43
- 31.Loo JC, Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970;59:53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- 32.Lobo ED, Hansen RJ, Balthasar JP. Minireview: Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 33.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 34.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays. 1991;13:31–36. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 35.Boehm T, Folkman J, Browder T, O’Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak HF, Nagy JA, Dvorak AM. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cells. 1991;3:77–85. [PubMed] [Google Scholar]
- 37.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 38.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 39.Fonsatti E, Jekunen AP, Kairemo KJ, Coral S, Snellman M, Nicotra MR, Natali PG, Altomonte M, Maio M. Endoglin is a suitable target for efficient imaging of solid tumors: in vivo evidence in a canine mammary carcinoma model. Clin Cancer Res. 2000;6:2037–2043. [PubMed] [Google Scholar]
- 40.Bredow S, Lewin M, Hofmann B, Marecos E, Weissleder R. Imaging of tumour neovasculature by targeting the TGF-beta binding receptor endoglin. Eur J Cancer. 2000;36:675–681. doi: 10.1016/S0959-8049(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 41.Costello B, Li C, Duff S, Butterworth D, Khan A, Perkins M, Owens S, Al-Mowallad AF, O’Dwyer S, Kumar S. Perfusion of 99Tcm-labeled CD105 Mab into kidneys from patients with renal carcinoma suggests that CD105 is a promising vascular target. Int J Cancer. 2004;109:436–441. doi: 10.1002/ijc.11699. [DOI] [PubMed] [Google Scholar]
- 42.Senger DR, Vande Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 44.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 45.Ghetie V, Ward ES. FcRn: the MHC class I-related receptor that is more than an IgG transporter. Immunol Today. 1997;18:592–598. doi: 10.1016/S0167-5699(97)01172-9. [DOI] [PubMed] [Google Scholar]
- 46.Lewis AP, Barber KA, Cooper HJ, Sims MJ, Worden J, Crowe JS. Cloning and sequence analysis of kappa and gamma cynomolgus monkey immunoglobulin cDNAs. Dev Comp Immunol. 1993;17:549–560. doi: 10.1016/S0145-305X(05)80010-2. [DOI] [PubMed] [Google Scholar]
- 47.Clark M. Antibody humanization: a case of the ‘Emperor’s new clothes’. Immunol Today. 2000;21:397–402. doi: 10.1016/S0167-5699(00)01680-7. [DOI] [PubMed] [Google Scholar]
- 48.Ehrke MJ, Mihich E, Berd D, Mastrangelo MJ. Effects of anticancer drugs on the immune system in humans. Semin Oncol. 1989;16:230–253. [PubMed] [Google Scholar]