Abstract
Tumor immune escape plays a critical role in cancer, but the mechanisms involved in this process have still to be defined. In the recent years, progress has been made in understanding how peptides presented by MHC class I molecules were generated, in particular which proteases are involved in this process and how intracellular pathways influence antigen presentation in professional antigen-presenting cells and in various types of malignancies. Different MHC class I abnormalities have been found in solid tumors of distinct origin, but also in hematopoietic diseases. These include structural alterations such as total, haplotype and allelic loss of the MHC class I heavy chain, deletions and point mutations, in particular in β2-microglobulin and TAP1 as well as dysregulation of various components of the MHC class I antigen processing machinery (APM), which could occur at the epigenetic, transcriptional and posttranscriptional level. The lack or downmodulation of the expression of single or multiple components of the MHC class I antigen processing pathway may avoid the recognition of tumor cells by tumor-specific CD8+ cytotoxic T lymphocytes. This review will give an overview of the underlying molecular mechanisms of MHC class I abnormalities in human tumors of distinct histology, which also might have an impact on the design of T cell-based immunotherapies.
Keywords: Antigen processing, Gene regulation, Immune escape, MHC class I, Tumors
Introduction
Tumor development is a multifactorial process, which involves various genetic alterations including the activation of oncogenes, inactivation of tumor-suppressor genes as well as modification of immunosurveillance molecules resulting in an aberrant cell cycle control, instability of genomic integrity and induction of tolerance. Therefore, the tumor host interaction is regulated by a fine balance between professional antigen presenting cells (APC) like dendritic cells (DC), B cells and macrophages, other immune effector cells including T lymphocytes, NK cells, regulatory T cells and NKT cells as well as tumor cells [13]. Furthermore, the tumor stroma, in particular endothelial cells, and the tumor microenvironment play a key role in this process [14, 60].
So far, there exist three lines of evidence of cancer immunosurveillance. These include (1) an increased incidence of non-viral tumors in immunosuppressed transplanted patients, (2) a high frequency of immune cell infiltration within the tumor and (3) the development of innate and acquired immune responses leading to spontaneous tumor regression. However, it is noteworthy that the immune system has two activities. On one hand the tumor growth could induce anti-tumor immune responses due to the presentation of tumor antigens to CD8+ cytotoxic T lymphocytes (CTL), whereas on the other hand highly efficient immune responses could result in cancer progression due to selection of immune escape variants, which could not be recognized by immune effector cells [4, 10, 33, 57].
The complex MHC class I antigen processing pathway
MHC class I molecules display antigenic peptides on the cell surface, which are monitored by the immune system. When MHC class I surface antigens present self-antigens, immune tolerance is induced, whereas presentation of non-native antigens such as antigens derived from either transformed or viral proteins results in CD8+ CTL-mediated lysis. Total loss of MHC class I surface antigens caused an insensitivity of CTLs to recognize and lyse tumor cells, whereas downregulation of MHC class I surface expression decreased CTL efficacy. In contrast, NK cells have the capacity to eliminate MHC class I-negative tumor cells. Thus, for a proper T cell recognition, an effective MHC class I antigen processing and presentation is required.
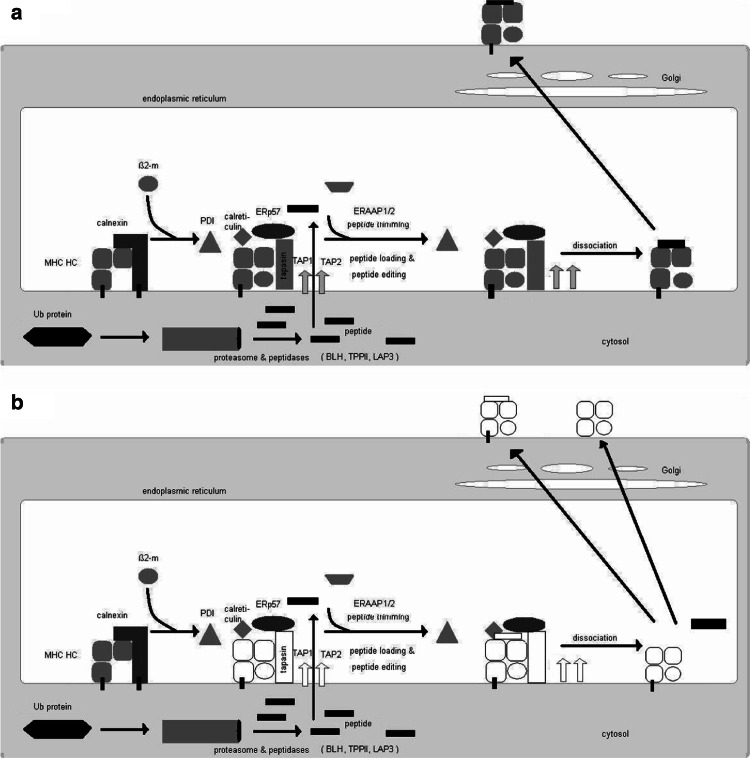
The processes relying on MHC class I surface expression such as T cell development, DC-mediated cross presentation and NK cell responses are pursued by the complex MHC class I antigen processing and presentation machinery (APM), which has been well characterized during the last two decades (Fig. 1). It consists of four major steps: (1) peptide generation/trimming; (2) peptide transport; (3) MHC class I assembly and (4) antigen presentation [26, 66]. In order to fit into most MHC class I molecules, antigenic peptides must have a length of 8–10 amino acids (aa) [35], although certain MHC class I molecules can admit peptides with a length of 11 and more aa residues [46]. It has been established that the multicatalytic proteasome is responsible for the generation of the majority of antigenic peptides [17], which is tightly controlled and plays an important role in protein homeostasis. Briefly, endogenously synthesized proteins were ubiquitinated and then subjected to degradation by the multicatalytic proteasome complex. In this process in particular, the interferon (IFN)-γ-inducible proteasome subunits, the low molecular weight proteins (LMP) 2, 7 and 10 are involved. The proteasome yields peptide fragments ranging from 2 to 25 residues with a correct C-terminus, whereas the N-termini are further trimmed by peptidases localized in the cytosol. Indeed, several cytosolic peptidases such as the tripeptidyl peptidase II (TPPII), the bleomycin hydrolase (BLH), the puromycin-sensitive aminopeptidase and the IFN-γ-inducible leucine aminopeptidase (LAP)3 play a role in trimming of the N-extended proteasome products [23]. In addition, a trimming could also occur in the endoplasmic reticulum (ER) mediated by the ER aminopeptidase associated with antigen processing (ERAP) 1 and 2 [9, 15, 21, 22, 53]. After cytosolic cleavage of antigens, the peptides are then transported via the ATP-dependent heterodimeric transporter associated with antigen processing (TAP)1 and TAP2 into the ER. TAP preferentially transports peptides with a length of 8–16 residues and is more efficient in translocation N-extended precursors than mature epitopes [18, 61]. In the ER, the MHC class I heavy chain (HC) and β2-microglobulin (β2-m) assembly occurs, which is coordinated by the chaperones calnexin, tapasin (tpn), calreticulin, protein disulfide isomerase (PDI) as well as the thiol oxidoreductase ERp57, a member of the PDI protein family that collectively form the multimeric peptide-loading complex (PLC). tpn, ERp57 and/or PDI are required for the stabilization of TAP and involved in peptide loading onto MHC class I molecules by regulating the redox state of a disulfide bound in the peptide-binding groove of the MHC class I HC [5, 7, 32, 52]. Upon peptide loading, the PLC dissociates and the trimer consisting of the MHC class I HC, β2-m and antigen is released and transported via the trans Golgi to the cell surface and there exposed to the CD8+ CTL.
Fig. 1.
Schematic diagram of MHC class I antigen processing machinery (APM) and steps deficient in tumors. Ubiquitinated proteins are cleaved into peptides by the proteasome and further trimmed by cytosolic peptidases. The peptides are then transported via the TAP heterodimer from the cytosol into the ER. In the ER, MHC class I heavy chain (HC) and β2-m, assisted by the chaperones calnexin and calreticulin, are stabilized and associate. A multimeric peptide loading complex (PLC) consisting of the TAP subunits, MHC HC, β2-m, tpn, calreticulin and ERp57 is yielded. Upon peptide loading, the PLC dissociates and the peptide/MHC class I/β2-m complex is transported via the trans-Golgi to the cell surface (a). The open symbols mark the MHC class I APM components, which have been found to be structurally altered, or dysregulated in tumor cells (b)
Physiological expression and regulation of MHC class I APM components
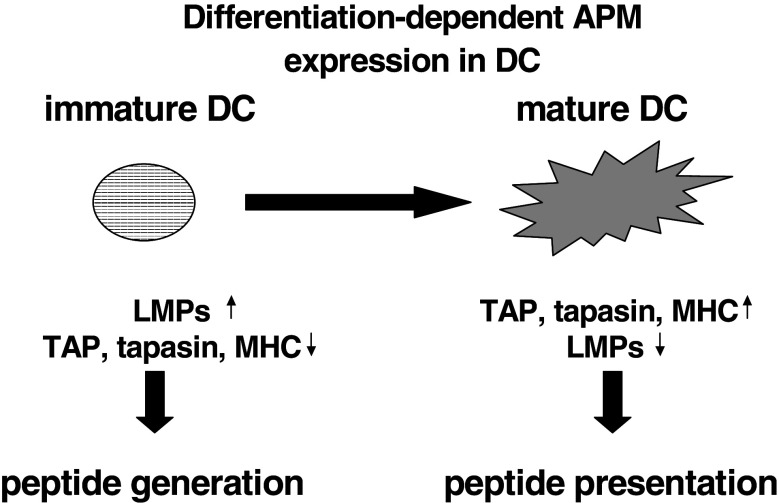
MHC class I antigens and the various APM constituents are constitutively, but heterogeneously, expressed in all normal tissues and cell lines, but not expressed in embryonic cells, testes and ovaries. Under physiological conditions, the expression of the MHC class I APM components might be regulated in a tissue-specific, differentiation-dependent, cell cycle-controlled manner and modulated by the microenvironment, thereby altering the repertoire of T cell epitopes presented. Indeed, an association between MHC class I APM component expression and cell cycle has been demonstrated for TAP1 and LMP2 mRNA [1], which might be due to p53 expression [68]. Regarding the differentiation dependence, there exist a distinct APM expression pattern in immature and mature DC. The LMP subunits were highly expressed in immature DC when compared to mature DC. In contrast, an inverse expression was found for the components mainly involved in antigen presentation, such as the MHC class I HC, β2-m, tpn and the TAP1/TAP2 heterodimer, which is more pronounced in the mature DC than in their immature counterparts [34]. This was further extended by a distinct expression pattern of TPPII, BLH and ERAP1 and 2. These data demonstrate a differentiation-dependent APM component regulation, reflecting the major functional activity of DCs during the maturation process (Fig. 2). In addition, there exists an extremely heterogeneous APM component expression in different embryonal carcinoma (EC) cell lines, which might be also linked to the distinct differentiation status of EC cells analyzed. Furthermore, the induction of differentiation of tumor cells and/or EC cells by retinoic acid (RA; [63, 64], Seliger, personal communication) as well as modulation of the epigenetic control mechanisms by demethylating and deacetylating agents, such as valproic acid (VPA) and trichostatin A (TSA), respectively, are able to alter the expression of some, but not all, MHC class I APM components. Besides the effect of different substances on the APM components, stress parameters including heat shock, irradiation and oxidative stress could also modulate their expression [12, 48].
Fig. 2.
Differential APM component expression during the differentiation process of DC and its functional association. Protein from immature and mature DCs was extracted and subjected to Western blot analysis using anti-APM component-specific antibodies [24]. A distinct APM component expression was found in immature and mature DCs
Furthermore, the major MHC class I APM component expression could be either upregulated by different cytokines like IFNs, tumor necrosis factor (TNF)-α, granulocyte macrophage colony stimulatory factor (GM-CSF) and interleukin (IL)-4 or downregulated by IL-10 [19, 20, 44, 67]. The most potent stimulator of the APM component expression is IFN-γ, which upregulates the expression of the LMP subunits, the proteasome activator (PA)28, TAP1, TAP2, tpn, MHC class I HC, β2-m, ERAP1, ERAP2 as well as LAP3. So far, no effect of this cytokine was found on the expression of calnexin, calreticulin, ERp57 and PDI. It is noteworthy that there exist also synergistic effects of treatment combinations in particular for IFN-α and IFN-γ, which strongly enhanced MHC class I APM component expression when compared to a single treatment.
Defects of MHC class I antigens and β2-m in tumors
It has been demonstrated that human tumors of distinct histology express low or downregulated MHC class I surface antigens, which could be due to modulation and/or inhibition of the expression of various MHC class I APM components [2, 6, 58]. The distinct frequency of MHC class I abnormalities is caused by total HLA class I antigen loss, HLA class I down-regulation as well as loss or down-regulation of HLA class I allo-specificities [5, 40]. However, the frequency and mode of these defects significantly varied between the types of tumors analysed and could be associated in some cases with microsatellite instability [3, 25]. Mutations or deletions in β2-m were detected in colon carcinoma (21%), melanoma (15%) and other tumors (<5%) [58]. So far, no mutations in β2-m have been found in RCC lesions, bladder and laryngeal tumors despite MHC class I loss or downregulation [3, 15, 51]. The β2-m mutations described, in particular in melanoma and colon carcinoma, were detected on one copy of the gene due to a bp substitution or deletion, suggesting a hot spot in this region. This alteration is accompanied by a loss of the second wild-type gene due a total or partial loss of chromosome 15 [43]. Concerning the haplotype-specific loss, this has been associated with loss of heterozygosity (LOH) on chromosome 6p21. It has been detected in different tumors and tumor cell lines, but occur with a different frequency [27]. For example, haplotype loss was found in head and neck squamous cell carcinoma (HNSCC) with a frequency of 36%, whereas in renal cell carcinoma (RCC) LOH only occurs in approximately 12% of tumor lesions analyzed [36]. Since mainly total tumor lesions have been examined, the frequency of LOH might be underestimated. Using a combination of immunhistochemistry with molecular analyses and microdissection allowed the proper detection of such alterations [45]. The locus-specific MHC class I downregulation, which results in lack of the sensitivity to HLA-restricted CTL-mediated lysis, has been demonstrated in colorectal carcinoma, cervical and laryngeal carcinoma as well as in melanoma. It could be caused by the loss of transcription factor binding due to oncogenic or viral transformation by HPV16 E6, ras, HER-2/neu and myc, respectively [3, 49]. Allele-specific loss of MHC class I antigens in tumors can be mediated by structural alterations in the MHC class I HC, in particular in HLA-A11, -A2, -A42 and HLA-B15 and have been mainly described in colon carcinoma, melanoma and cervical cancer.
Altered MHC class I APM component expression
Besides abnormalities of MHC class I antigens, downregulation of APM components such as LMPs, TAP and tpn could result in deficient MHC class I surface expression. Whereas for example MHC class I HC and β2-m is not affected in RCC, a strong downregulation of LMP2, LMP7, TAP and tpn was found in this tumor entity. Furthermore, the frequency of APM deficiencies differed between the RCC subtypes analyzed and was more pronounced in metastatic lesions when compared to the primary tumor [54, 55]. In some cancers, MHC class I deficiencies correlated with increased disease stage and/or a reduced survival rate of patients suggesting that APM abnormalities are of clinical relevance [38, 39, 47, 65]. These defects are further associated with a diminished sensitivity to CTL-mediated lysis, but lack of destruction by NK cells. In most cases, this phenotype is IFN-γ inducible.
Concerning chaperones, only little information is available regarding the expression pattern of tpn, calnexin, calreticulin, ERp57 and PDI in human tumors compared to the normal counterpart. This could be reflected by either the lack of analyses of these components or by the low frequency and significance of such deficiencies. So far, calreticulin and PDI expression appeared to be unaltered in all tumor samples analyzed, whereas loss or downregulation of calnexin has only been detected in cervical carcinoma [49]. Monitoring of ERp57 has not yet been performed. A downregulation of tpn was detected in RCC, melanoma, neuroblastoma, hepatocellular carcinoma as well as in HNSCC.
Underlying molecular mechanisms of MHC class I APM component abnormalities
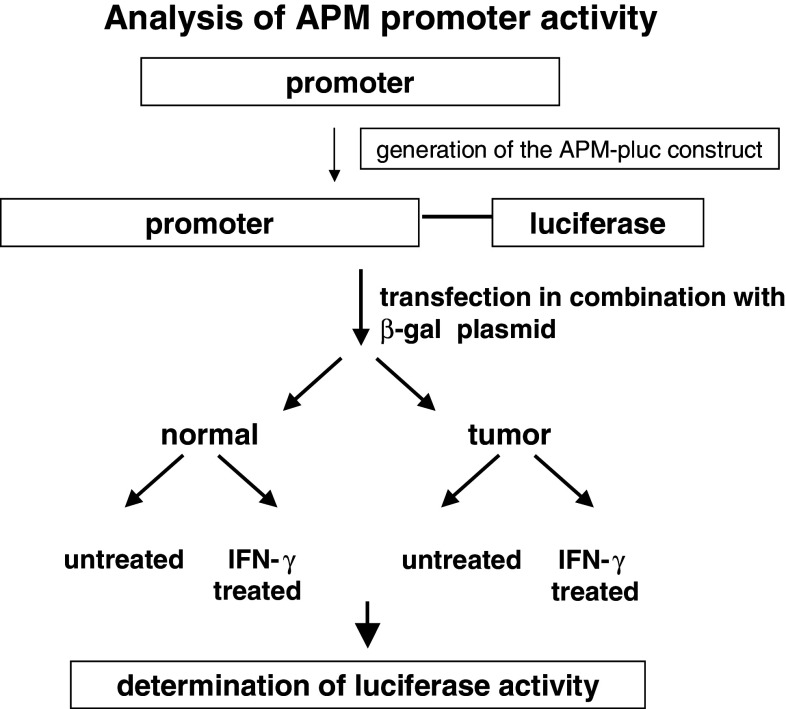
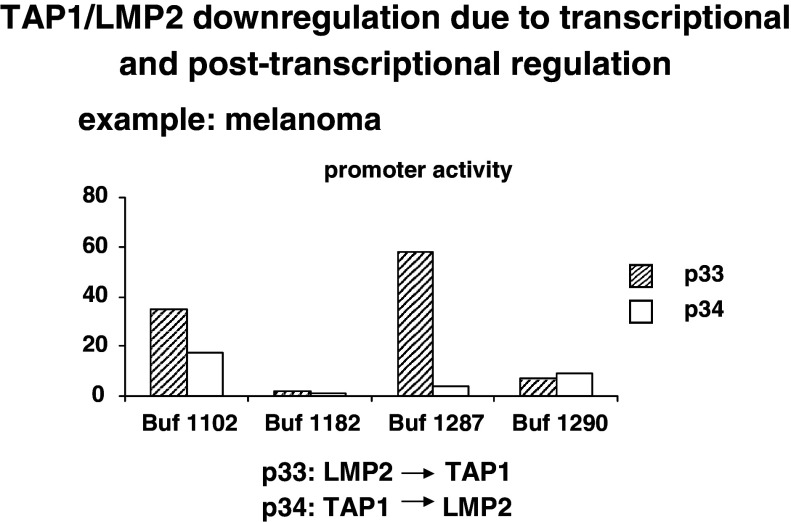
The molecular mechanisms underlying the MHC class I APM component downregulation could occur at different levels including structural alterations and dysregulation due to epigenetic control, and transcriptional and posttranscriptional modulation. So far, with the exception of the MHC class I HC and β2-m, structural alterations have been detected in the TAP1 subunit in small cell lung carcinoma, melanoma and cervical carcinoma [11, 56, 58], but appear to be a rare event. In addition, we have recently identified mutations in tpn and/or LMP subunits in neuroblastoma and in melanoma (Seliger et al., unpuplished data). These mutations were either point mutations or bp deletions. Based on the low frequency of sequence abnormalities, it has been suggested that APM components are mainly regulated at the epigenetic, transcriptional and/or posttranscriptional level in human tumors. In this context, it is also noteworthy that there can exist a loss of MHC class I APM inducibility by IFN, which could be caused by different defects in the IFN signal transduction cascade [50]. In order to determine the differential regulatory mechanisms of heterogeneous APM component expression, a number of distinct strategies were employed. First, the various APM promoters were cloned and hooked to the luciferase (luc) reporter gene (Fig. 3). These APM promoter luc constructs were then transiently transfected into human tumor cell lines of distinct origin. Heterogeneous APM promoter activities were detected in the tumor cell lines analyzed suggesting a transcriptional and/or a posttranscriptional regulation of APM component expression depending on the tumor cell line analyzed. This is representatively shown for the dual TAP1/LMP2 promoter activity in selected melanoma cell lines (Fig. 4). The differential promoter activity is in line with the downregulation or lack of APM component mRNA and/or protein expression. In addition, in some esophageal squamous cell carcinoma, colon carcinoma, RCC and melanoma cell lines, epigenetic changes like methylation and histone deacetylation of APM components and/or tumor antigens was found, which could be reverted by treatment with DAC or histone deacetylase inhibitors [31, 37, 42, 59] (Seliger, unpublished). Methylation of the tpn and/or TAP2 promoter has been demonstrated in melanoma and RCC cell lines. However, treatment of RCC and melanoma cells with DAC enhanced or even reconstituted not only TAP2 and tpn, but also TAP1 mRNA and protein expression suggesting that transcription factors regulating the TAP1 promoter activity become demethylated, but these factors still have to be defined.
Fig. 3.
Strategy used for determination of APM promoter activity. Upon cloning of the APM promoters, they were linked to the luciferase (luc) gene (APM-luc), and these APM promoter luc constructs were then transiently transfected into the respective tumor and/or control cell lines. The cells were either left untreated or treated with IFN-γ before the luciferase activity was determined. The results were expressed as luc activity
Fig. 4.
Transcriptional and posttranscriptional regulation of the TAP1/LMP2 promoter activity in distinct melanoma cells The dual TAP1/LMP2 promoter was transfected into different melanoma cell lines and the TAP1/LMP2 promoter activity was determined. A heterogeneous promoter activity was found in the melanoma cell lines tested exhibiting either a coordinated or discordant regulation of the TAP1/LMP2 activity. The striped bars represent the LMP2 → TAP1 promoter activity; the open bars the TAP1 → LMP2 promoter activity
Association of peptidase expression with HLA class I surface expression
It has been recently suggested that peptidases, in particular amino- and ectopeptidases, modulate the anti-tumor T cell responses. These peptidases could be found in the cytosol, in the ER as well as at the cell surface. A failure to customize the peptide MHC class I repertoire has profound immunological consequences [26]. A distinct expression pattern of both cytosolic as well as ER-resident peptidases have been described in some tumors, but so far only a few tumor samples and/or tumor cell lines have been analyzed [16]. The aberrant expression of these peptidases might have an influence on the antigen repertoire presented by MHC class I surface expression. Therefore, we determined the expression pattern of BLH, TPPII, THOP1, ERAP1 and ERAP2 in a large series of RCC as well as melanoma cell lines. A heterogeneous mRNA and/or protein expression profile of these peptidases was detected in the different tumor cell lines analyzed, but the role and the underlying molecular mechanisms of their differential expression pattern are still to be determined. Concerning the membrane-bound aminopeptidases, the expression of aminopeptidase N(APN)/CD13 in the context of MHC class I association was monitored. Besides its distinct expression in many human tumors [28–30, 41, 62], overexpression of APN in MHC class I-negative colon carcinoma cells results in an increased MHC class I surface expression, which is directly associated with the increase of the transcription and translation of the major APM components including MHC class I HC, β2-m, TAP1, TAP2, tpn as well as the LMP subunits (Seliger, personal communication). Furthermore, this APN-mediated upregulation of MHC class I surface expression was associated with an enhanced recognition by CD8+ CTL.
Perspectives
As summarized in this article MHC class I abnormalities occur at a high frequency in human tumors and were often associated with the metastatic potential of disease and with patients’ survival. This might be due to a reduced host anti-tumor immune response based on the resistance of tumor cells to T cell-mediated lysis. The underlying molecular mechanisms of such deficiencies are diverse and include structural alterations and/or dysregulation, which could occur at each different step of the MHC class I APM. The data suggest that the assessment of the structure, expression and/or regulation of MHC class I APM components might identify patients at risk for metastatic dissemination and might allow the proper selection of patients undergoing specific immunotherapies. Therefore, prospective studies have to be performed to validate the prognostic value as well as the study inclusion criteria of patients selected for immunotherapies for MHC class I abnormalities. However, this might be difficult to perform since in most studies the patients′ follow-up is often not available and only small numbers of tumor samples have been analyzed in this context, minimizing the statistical power of such approaches.
Acknowledgments
This work was sponsored by the DFG projects (SE581/9-1, SE581/9-2, SE581/11-1). We would like to thank Anne Wasilewski for excellent secretarial help.
Abbreviations
- Aa
Amino acid
- APC
Antigen-presenting cell
- APM
Antigen processing machinery
- APN
Aminopeptidase N
- BLH
Bleomycin hydrolase
- β2-m
β2-microglobulin
- LOH
Loss of heterozygosity
- CSF
Colony-stimulating factor
- CTL
Cytotoxic T lymphocytes
- DAC
2′5′Desoxyazacytidine
- DC
Dendritic cells
- ER
Endoplasmic reticulum
- ERAP
ER aminopeptidase associated with antigen processing
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- HC
Heavy chain
- HNSCC
Head and neck squamous cell carcinoma
- IFN
Interferon
- IL
Interleukin
- LAP
Leucin aminopeptidase
- LMP
Low molecular weight proteins
- LOH
Loss of heterozygosity
- Luc
Luciferase
- MHC
Major histocompatibility complex
- NK
Natural killer
- PA
Proteasome activator
- PDI
Protein disulfide isomerase
- PLC
Peptide loading complex
- RCC
Renal cell carcinoma
- TAP
Transporter associated with antigen processing
- TGF
Transforming growth factor
- Tpn
Tapasin
- TPPII
Tripeptidyl peptidase II
- TSA
Trichostatin A
- VPA
Valproic acid
Footnotes
This article is a symposium paper from the conference “Progress in Vaccination against Cancer 2007 (PIVAC 7)”, held in Stockholm, Sweden, on 10 and 11 September 2007.
References
- 1.Alpan RS, Zhang M, Pardee AB. Cell cycle-dependent expression of TAP1, TAP2, and HLA-B27 messenger RNAs in a human breast cancer cell line. Cancer Res. 1996;56:4358–4361. [PubMed] [Google Scholar]
- 2.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 3.Atkins D, Ferrone S, Schmahl GE, Storkel S, Seliger B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma? J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 4.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera CM. The double role of the endoplasmic reticulum chaperone tapasin in peptide optimization of HLA class I molecules. Scand J Immunol. 2007;65:487–493. doi: 10.1111/j.1365-3083.2007.01934.x. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying mechanisms. Tissue Antigens. 2007;69:264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 7.Chambers JE, Jessop CE, Bulleid NJ. Formation of a MHC Class I-tapasin mixed disulfide indicates a change in spatial organization of the peptide loading complex during assembly. J Biol Chem. 2007;283(4):1862–1869. doi: 10.1074/jbc.M708196200. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 9.Chang SC, Momburg F, Bhutani N, Goldberg AL. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci USA. 2005;102:17107–17112. doi: 10.1073/pnas.0500721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13:210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 12.Deng XL, Chen W, Cai MY, Wei DP. Expression of class I MHC molecule, HSP70 and TAP in human hepatocellular carcinoma. World J Gastroenterol. 2003;9:1853–1855. doi: 10.3748/wjg.v9.i8.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 15.Fernández MA, Ruiz-Cabello F, Oliva MR, Cabrera T, Jimenez P, López Nevot MA, Garrido F. Beta2-microglobulin gene mutation is not a common mechanism of HLA class I total loss in human tumors. Int J Clin Lab Res. 2000;30:87–92. doi: 10.1007/BF02874164. [DOI] [PubMed] [Google Scholar]
- 16.Fruci D, Ferracuti S, Limongi MZ, Cunsolo V, Giorda E, Fraioli R, Sibilio L, Carroll O, Hattori A, van Endert PM, et al. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–4879. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–164. doi: 10.1016/S0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 18.Gubler B, Daniel S, Armandola EA, Hammer J, Caillat-Zucman S, van Endert PM. Substrate selection by transporters associated with antigen processing occurs during peptide binding to TAP. Mol Immunol. 1998;35:427–433. doi: 10.1016/S0161-5890(98)00059-5. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Yang T, Liu X, Lu S, Wen J, Durbin JE, Liu Y, Zheng P. Cis elements for transporter associated with antigen-processing-2 transcription: two new promoters and an essential role of the IFN response factor binding element in IFN-gamma-mediated activation of the transcription initiator. Int Immunol. 2002;14:189–200. doi: 10.1093/intimm/14.2.189. [DOI] [PubMed] [Google Scholar]
- 20.Hallermalm K, Seki K, Wei C, Castelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98:1108–1115. doi: 10.1182/blood.V98.4.1108. [DOI] [PubMed] [Google Scholar]
- 21.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 22.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 23.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Hicklin DJ, Kageshita T, Ferrone S. Development and characterization of rabbit antisera to human MHC-linked transporters associated with antigen processing. Tissue Antigens. 1996;48:38–46. doi: 10.1111/j.1399-0039.1996.tb02603.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirata T, Yamamoto H, Taniguchi H, Horiuchi S, Oki M, Adachi Y, Imai K, Shinomura Y. Characterization of the immune escape phenotype of human gastric cancers with and without high-frequency microsatellite instability. J Pathol. 2007;211:516–523. doi: 10.1002/path.2142. [DOI] [PubMed] [Google Scholar]
- 26.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez P, Canton J, Collado A, Cabrera T, Serrano A, Real LM, Garcia A, Ruiz-Cabello F, Garrido F. Chromosome loss is the most frequent mechanism contributing to HLA haplotype loss in human tumors. Int J Cancer. 1999;83:91–97. doi: 10.1002/(SICI)1097-0215(19990924)83:1<91::AID-IJC17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura J, Shimada Y, Kitaichi H, Komoto I, Hashimoto Y, Kaganoi J, Miyake M, Yamasaki S, Kondo K, Imamura M. Clinicopathological significance of aminopeptidase N/CD13 expression in human gastric carcinoma. Hepatogastroenterology. 2007;54:36–40. [PubMed] [Google Scholar]
- 29.Kehlen A, Gohring B, Langner J, Riemann D. Regulation of the expression of aminopeptidase A, aminopeptidase N/CD13 and dipeptidylpeptidase IV/CD26 in renal carcinoma cells and renal tubular epithelial cells by cytokines and cAMP-increasing mediators. Clin Exp Immunol. 1998;111:435–441. doi: 10.1046/j.1365-2249.1998.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kehlen A, Lendeckel U, Dralle H, Langner J, Hoang-Vu C. Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res. 2003;63:8500–8506. [PubMed] [Google Scholar]
- 31.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2007;57(5):647–654. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol. 2007;8:864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 33.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Schuler-Thurner B, Schuler G, Huber C, Seliger B. Bipartite regulation of different components of the MHC class I antigen-processing machinery during dendritic cell maturation. Int Immunol. 2001;13:1515–1523. doi: 10.1093/intimm/13.12.1515. [DOI] [PubMed] [Google Scholar]
- 35.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 36.Maleno I, Lopez Nevot MA, Seliger B, Garrido F. Low frequency of HLA haplotype loss associated with loss of heterozygocity in chromosome region 6p21 in clear renal cell carcinomas. Int J Cancer. 2004;109:636–638. doi: 10.1002/ijc.20000. [DOI] [PubMed] [Google Scholar]
- 37.Manning J, Indrova M, Lubyova B, Pribylova H, Bieblova J, Hejnar J, Simova J, Jandlova T, Bubenik J, Reinis M. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218–227. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 40.Mendez R, Rodriguez T, Del Campo A, Monge E, Maleno I, Aptsiauri N, Jimenez P, Pedrinaci S, Pawelec G, Ruiz-Cabello F, Garrido F. Characterization of HLA class I altered phenotypes in a panel of human melanoma cell lines. Cancer Immunol Immunother. 2008;57(5):719–729. doi: 10.1007/s00262-007-0411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menrad A, Speicher D, Wacker J, Herlyn M. Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res. 1993;53:1450–1455. [PubMed] [Google Scholar]
- 42.Nie Y, Yang G, Song Y, Zhao X, So C, Liao J, Wang LD, Yang CS. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogen. 2001;22:1615–1623. doi: 10.1093/carcin/22.10.1615. [DOI] [PubMed] [Google Scholar]
- 43.Paschen A, Méndez RM, Jimenez P, Sucker A, Ruiz-Cabello F, Song M, Garrido F, Schadendorf D. Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer. 2003;103:759–767. doi: 10.1002/ijc.10906. [DOI] [PubMed] [Google Scholar]
- 44.Petersson M, Charo J, Salazar-Onfray F, Noffz G, Mohaupt M, Qin Z, Klein G, Blankenstein T, Kiessling R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J Immunol. 1998;161:2099–2105. [PubMed] [Google Scholar]
- 45.Ramal LM, Maleno I, Cabrera T, Collado A, Ferron A, Lopez-Nevot MA, Garrido F. Molecular strategies to define HLA haplotype loss in microdissected tumor cells. Hum Immunol. 2000;61:1001–12. doi: 10.1016/S0198-8859(00)00171-3. [DOI] [PubMed] [Google Scholar]
- 46.Rammensee HG, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 47.Ramnath N, Tan D, Li Q, Hylander BL, Bogner P, Ryes L, Ferrone S. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–899. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, Griekspoor A, Mesman E, Verreck FA, Spits H, Schlom J, van Veelen P, Neefjes JJ. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritz U, Momburg F, Pilch H, Huber C, Maeurer MJ, Seliger B. Deficient expression of components of the MHC class I antigen processing machinery in human cervical carcinoma. Int J Oncol. 2001;19:1211–1220. doi: 10.3892/ijo.19.6.1211. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez T, Mendez R, Del Campo A, Jimenez P, Aptsiauri N, Garrido F, Ruiz-Cabello F. Distinct mechanisms of loss of IFN-gamma mediated HLA class I inducibility in two melanoma cell lines. BMC Cancer. 2007;7:34. doi: 10.1186/1471-2407-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero JM, Jiménez P, Cabrera T, Cózar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–610. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
- 52.Santos SG, Campbell EC, Lynch S, Wong V, Antoniou AN, Powis SJ. Major histocompatibility complex class I-ERp57-tapasin interactions within the peptide-loading complex. J Biol Chem. 2007;282:17587–17593. doi: 10.1074/jbc.M702212200. [DOI] [PubMed] [Google Scholar]
- 53.Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, van Endert PM. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol. 2005;6:689–697. doi: 10.1038/ni1208. [DOI] [PubMed] [Google Scholar]
- 54.Seliger B, Hohne A, Knuth A, Bernhard H, Ehring B, Tampe R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res. 1996;2:1427–1433. [PubMed] [Google Scholar]
- 55.Seliger B, Hohne A, Knuth A, Bernhard H, Meyer T, Tampe R, Momburg F, Huber C. Analysis of the major histocompatibility complex class I antigen presentation machinery in normal and malignant renal cells: evidence for deficiencies associated with transformation and progression. Cancer Res. 1996;56:1756–1760. [PubMed] [Google Scholar]
- 56.Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61:8647–8650. [PubMed] [Google Scholar]
- 57.Seliger B. Strategies of tumor immune evasion. BioDrugs. 2005;19:347–354. doi: 10.2165/00063030-200519060-00002. [DOI] [PubMed] [Google Scholar]
- 58.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int J Cancer. 2006;118:129–138. doi: 10.1002/ijc.21312. [DOI] [PubMed] [Google Scholar]
- 59.Sigalotti L, Fratta E, Coral S, Tanzarella S, Danielli R, Colizzi F, Fonsatti E, Traversari C, Altomonte M, Maio M. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2´-deoxycytidine. Cancer Res. 2004;64:9167–9171. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]
- 60.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 61.van Endert PM, Saveanu L, Hewitt EW, Lehner P. Powering the peptide pump: TAP crosstalk with energetic nucleotides. Trends Biochem Sci. 2002;27:454–461. doi: 10.1016/S0968-0004(02)02090-X. [DOI] [PubMed] [Google Scholar]
- 62.Varona A, Blanco L, Lopez JI, Gil J, Agirregoitia E, Irazusta J, Larrinaga G. Altered levels of acid, basic, and neutral peptidase activity and expression in human clear cell renal cell carcinoma. Am J Physiol Renal Physiol. 2007;292:F780–F788. doi: 10.1152/ajprenal.00148.2006. [DOI] [PubMed] [Google Scholar]
- 63.Vertuani S, De Geer A, Levitsky V, Kogner P, Kiessling R, Levitskaya J. Retinoids act as multistep modulators of the major histocompatibility class I presentation pathway and sensitize neuroblastomas to cytotoxic lymphocytes. Cancer Res. 2003;63:8006–8013. [PubMed] [Google Scholar]
- 64.Vertuani S, Dubrovska E, Levitsky V, Jager MJ, Kiessling R, Levitskaya J. Retinoic acid elicits cytostatic, cytotoxic and immunomodulatory effects on uveal melanoma cells. Cancer Immunol Immunother. 2007;56:193–204. doi: 10.1007/s00262-006-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vitale M, Pelusi G, Taroni B, Gobbi G, Micheloni C, Rezzani R, Donato F, Wang X, Ferrone S. HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res. 2005;11:67–72. [PubMed] [Google Scholar]
- 66.Yewdell JW. The seven dirty little secrets of major histocompatibility complex class I antigen processing. Immunol Rev. 2005;207:8–18. doi: 10.1111/j.0105-2896.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 67.Zeidler R, Eissner G, Meissner P, Uebel S, Tampe R, Lazis S, Hammerschmidt W. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin–10. Blood. 1997;90:2390–2397. [PubMed] [Google Scholar]
- 68.Zhu K, Wang J, Zhu J, Jiang J, Shou J, Chen X. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene. 1999;18:7740–7747. doi: 10.1038/sj.onc.1203235. [DOI] [PubMed] [Google Scholar]