Abstract
Regulatory NK cell receptors can contribute to antigen-specific adaptive immune responses by modulating T cell receptor (TCR)-induced T cell activation. We investigated the potential of the NK cell receptor 2B4 (CD244) to enhance tumor antigen-induced activation of human T cells. 2B4 is a member of the CD2 receptor subfamily with both activating and inhibitory functions in NK cells. In T cells, its expression is positively associated with the acquisition of a cytolytic effector memory phenotype. Recombinant chimeric receptors that link extracellular single-chain Fv fragments specific for the tumor-associated surface antigens CD19 and GD2 to the signaling domains of human 2B4 and/or TCRζ were expressed in non-specifically activated peripheral blood T cells by retroviral gene transfer. While 2B4 signaling alone failed to induce T cell effector functions or proliferation, it significantly augmented the antigen-specific activation responses induced by TCRζ. 2B4 costimulation did not affect the predominant effector memory phenotype of expanding T cells, nor did it increase the proportion of T cells with regulatory phenotype (CD4+CD25hiFoxP3+). These data support a costimulatory role for 2B4 in human T cell subpopulations. As an amplifier of TCR-mediated signals, 2B4 may provide a powerful new tool for immunotherapy of cancer, promoting sustained activation and proliferation of gene-modified antitumor T cells.
Keywords: Costimulation, T cells, NK cell receptors, Immunotherapy
Introduction
Chimeric T cell receptors (CARs) that combine tumor antigen-specific recognition domains and T cell signaling elements in a single molecule are promising tools for cellular immunotherapy of cancer [1]. They allow to generate T cells with engineered specificities, thereby overcoming the lack of immunogenic tumor antigens and allowing tumor cell recognition in an HLA-independent manner. The original CARs consisted of an antigen-specific single chain Fv fragment fused to the T cell receptor (TCR) ζ chain. T cells transduced with these CARs failed to induce complete activation responses and did not persist in the circulation beyond 2–3 weeks after infusion in vivo [2–8]. Increased awareness of the importance of costimulatory signaling in T cell activation has subsequently led to the design of “second-generation” CARs with costimulatory signaling components derived from CD28 [9, 10], 4-1BB [11], or OX-40 [12] receptors. Indeed, these CARs promoted superior T cell proliferation, persistence and tumor control in vivo.
In addition to classical costimulatory T cell receptors, receptors that act as modulators of innate immunity have now been found to be expressed in T cells [13]. The physiological significance of these components in T cells and their potential to enhance TCRζ-mediated activation signals are largely unresolved. One example is the signaling lymphocyte activation molecule (SLAM)-related receptor 2B4 (CD244) which has emerged as an important immune regulator in NK cells [14–20]. The human 2B4 homolog is expressed on all NK cells and a subset of CD8+ T cells [18, 19, 21, 22], while its ligand CD48 is broadly expressed on all nucleated hematopoietic cells [23]. 2B4 expression in T cells was found to be associated with differentiation stage markers of a highly cytotoxic subpopulation, such as perforin, granzyme B, and IFN-γ, in the absence of the naïve T cell markers CD45RA, CD62L, CD28, and CCR7. This pattern suggests progressive acquisition of 2B4 during generation of cytotoxic T cell (CTL) memory [19, 22, 24]. Investigations addressing the functional role of 2B4 signaling in antigen-specific CD8+ T cell responses are limited. Indirect evidence for an important regulatory role of 2B4 in human CTL function derives from studies in patients affected by X-linked lymphoproliferative disease (XLP) [25]. XLP is a severe immunodeficiency caused by inherited mutations in the SH2D1A gene and subsequent deficiency of SLAM-associated protein (SAP) function which is required for 2B4 signaling. Indeed, blockade of 2B4 in normal CTLs efficiently reproduces the defective lytic function of CTLs in these patients against EBV-positive targets [25]. Accordingly, in CD8+ T cells from TCR transgenic mice, triggering of 2B4 augmented MHC-restricted cytolytic function [16]. By contrast, antigen-induced proliferation and cytokine production was not found to be affected in SAP-deficient human CD8+ CTLs, and 2B4 receptor crosslinking experiments have failed to demonstrate a direct stimulatory effect of 2B4 on human T cell activation and proliferation [18].
To study the direct consequences of 2B4 signaling on the functional activation responses of human T cells to tumor cells, we generated CARs with specificities for the tumor-associated surface antigens CD19 and GD2, linked to either the combined or individual signal transduction domains of human 2B4 and/or the TCRζ chain. Following genetic engineering of T cells with the recombinant receptors, we compared their antigen-specific effector functions as well as their activation and proliferative responses to interaction with antigen-expressing tumor target cells. Specifically, we addressed the question whether the 2B4 endodomain can enhance tumor antigen-specific activation of in vitro stimulated peripheral blood effector memory T cells.
Materials and methods
Constructs
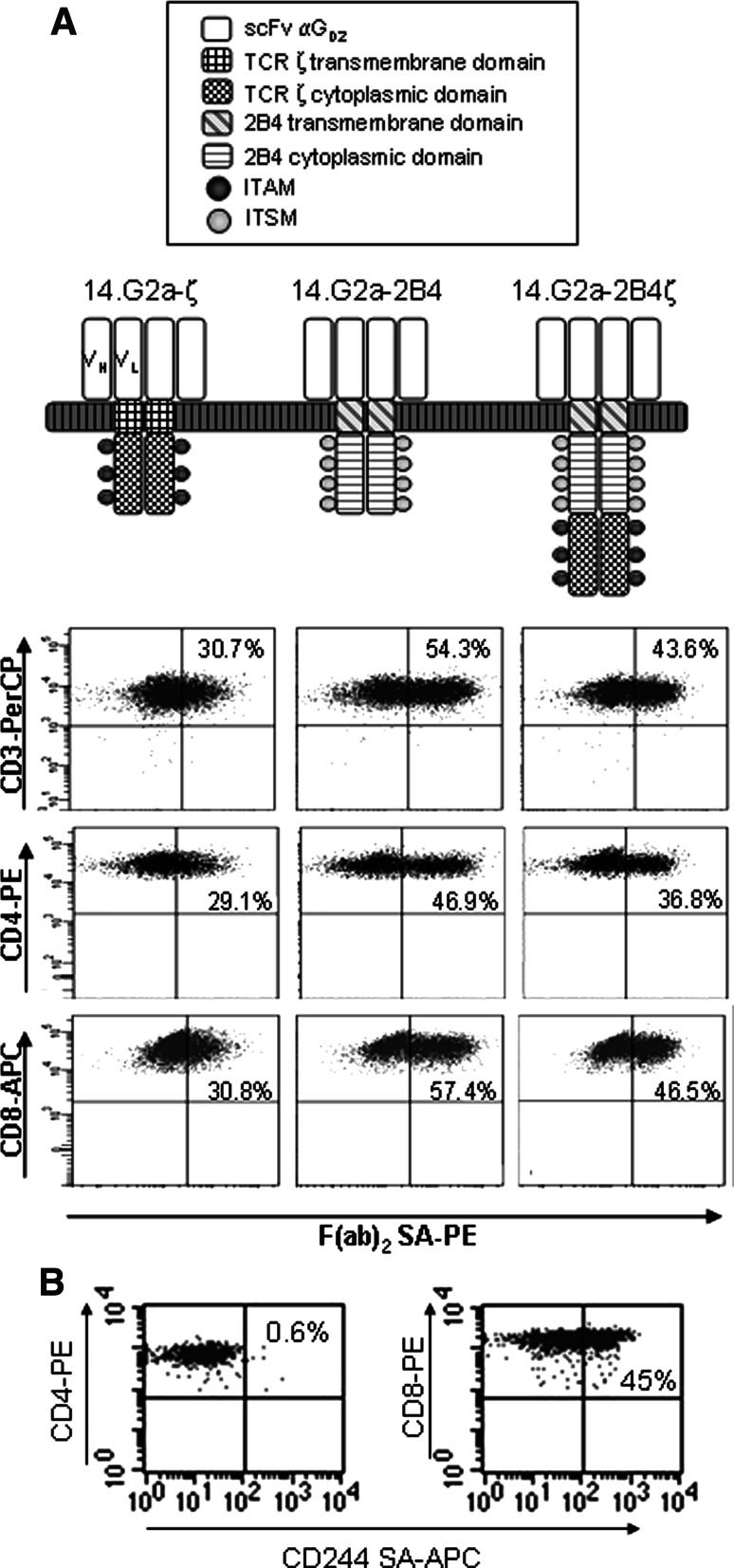
14.G2a-ζ [26] contains the single-chain antibody domain of the GD2-specific antibody 14.G2a. The antigen-binding domain of CD19-ζ is derived from the CD19-specific antibody FMC-63 [27]. The gene fragment encoding the transmembrane and cytoplasmic domains of the 2B4 receptor was cloned from cDNA obtained from peripheral blood mononuclear cells of a healthy donor. To generate 14.G2a-2B4ζ and CD19-2B4ζ, the 2B4-gene fragment was subcloned upstream of the cytoplasmic domain of the TCRζ genes while replacing the transmembrane domains of TCRζ. For 14.G2a-2B4 and CD19-2B4, the entire TCRζ fragment was replaced by the transmembrane and cytoplasmic domains of 2B4. All 14.G2a-based CAR genes (Fig. 1) were subcloned into the BamHI and NcoI sites of the retroviral vector SFG [28] (provided by R. C. Mulligan, Cambridge, MA). The CD19-specific receptors were subcloned into the AgeI and NotI sites of the retroviral vector SFG-IRES-GFP which was generated by M. P. by inserting the IRES-GFP expression cassette into SFG.
Fig. 1.
CAR transduction efficencies and natural 2B4 expression. a The various CAR constructs are depicted schematically. Following retroviral transduction of non-specifically activated peripheral blood mononuclear cells with 14.G2a-specific T cells, gene expression was determined by flow cytometric quantification of F(ab′)2-positive cells. To quantify the transduction efficiencies of CD3+ T cells (upper panel), the gate included all vital lymphocytes. For CD4+ and CD8+ T cells, gates were set on CD3+/CD4+ or CD3+/CD8+ cells, respectively. b Expression of 2B4 on CD4+ and CD8+ T cells measured by flow cytometry on day 14 after non-specific stimulation. Shown is one representative experiment of three
Cell lines
The packaging cell line Phoenix-ampho [29] was provided by Gary P. Nolan (Stanford, CA). FLYRD 18 (provided by E. Vanin, Houston, TX, USA) is an amphotrophic retrovirus packaging cell line that provides viral recombinants with the RD114 envelope. LAN-5 (R. Seeger, Los Angeles, CA) is a neuroblastoma cell line, A-204 (ATCC) is a rhabdomyosarcoma cell line, and K-562 (ATCC) is a human erythroleukemia line that is sensitive to lysis by lymphokine-activated killer cells. REH and SupB15 (ATCC) are human acute lymphoblastic leukemia cell lines, and ML-2 (ATCC) is a human myeloid leukemia cell line.
Production of recombinant retrovirus
To generate stable retroviral producer cell lines, fresh retroviral supernatants collected from transiently transfected Phoenix ampho cells were used to infect the packaging cell line FLYRD18 by overnight incubation at 37°C in the presence of polybrene (4 μg/ml). The cells were subjected to multiple rounds of infection under the same conditions. Viral supernatants were generated on the resulting bulk producer cell lines by adding Isocoves modified Dulbecco medium (IMDM; BioWhittaker) supplemented with 20% FCS. After 24 h of incubation at 32°C, the supernatants were filtered through a 0.45 μm filter and used to transduce the T cells.
Generation and transduction of T cell cultures
T cells were non-specifically prestimulated by 48 h culture of peripheral blood mononuclear cells (PBMCs) at 1 × 106/ml on 24-well plates precoated with OKT-3 (1 μg/ml; Ortho Pharmaceuticals, Raritan, NJ) and anti-CD28 antibody (1 μg/ml; Pharmingen, San Diego, CA). For retroviral transductions, prestimulated T cells were transferred to 24-well non-tissue culture-treated plates (Becton Dickinson, Franklin Lakes, New Jersey) coated with recombinant fibronectin fragment FN CH-296 [30] (Retronectin, Takara Shuzo, Otsu, Japan) at 4 μg/cm2, and coincubated with viral supernatant for 48 h, followed by expansion in the presence of rhIL-2 (50 IU/ml).
Flow cytometry
For immunophenotyping, cells were stained with fluorescein-conjugated monoclonal antibodies directed against CD3, CD4, CD8, CD25, CD56, CD45RO, CCR7, (Becton Dickinson, San Jose, CA), CD244 (eBioscience, San Diego, CA), and CD48 (Immunotools, Friesoythe, Germany) surface proteins. Surface expression of 14.G2a-ζ, 14.G2a-2B4, and 14.G2a-2B4ζ was analyzed by staining with a biotinylated goat anti-mouse antibody specific for IgG F(ab′)2 fragment (Jackson ImmunoResearch, Cambridgeshire, UK) for 10 min at room temperature, followed by incubation with phycoerythrin (PE)-labeled streptavidin antibody (Becton Dickinson, San Jose, CA). Expression of CD19-specific CAR genes cloned into SFG-IRES-GFP was quantified by flow cytometric identification of GFP-expressing T cells. To ensure comparability of the gene-modified T cells, the majority of functional data, as indicated in the respective figure legends, were obtained with either FACS purified F(ab)2+ or GFP+ cells, or cells within the gate of F(ab)2+ or GFP+ cells. For intracellular staining with FoxP3 antibody (Becton Dickinson), the cells were fixed with fixation/permeabilization buffer (eBioscience, San Diego, CA) for 30 min, then washed twice in permeabilization buffer (eBioscience) and resuspended in 100 μl permeabilization buffer containing 2% rat serum. After 15 min of blocking, FoxP3 antibody (eBioscience) was added for 30 min. For each sample, 20,000 cells were analyzed with FACSCalibur and Cell Quest Software (Becton Dickinson, San Jose, CA).
Intracellular cytokine assay
T cells were seeded at 1 × 106 cells per well in a 24-well plate and were stimulated with 1 × 106 irradiated tumor target cells. Controls consisted of T cells cultured without target cells for 4 h. Cytokine secretion was blocked with 10 μg brefeldin A (Sigma) per 2 × 106 cells. Permeabilization of the cells was performed using a proprietary solution (Becton Dickinson, San Jose, CA). Cells were stained according to the manufacturer’s recommendations, and isotype-matched negative controls were used for all antibodies.
Cytotoxicity assay
Cytotoxic specificity was determined in standard 51Cr release assays. Various numbers of T effector cells were co-incubated in triplicate with 2,500 target cells labeled with 100 μCi 51Cr/0.5 × 106 cells (PE Applied Biosystems) in a total volume of 200 μl in a V-bottomed 96-well plate. At the end of a 4-h incubation period at 37°C and 5% CO2, supernatants were harvested, and radioactivity was counted in a gamma counter. Maximum release was determined by lysis of target cells with Triton X.
Proliferation and expansion assay
Transduced polyclonal T cells underwent positive selection by FACS sorting after staining with F(ab′)2 specific antibody (14.G2a CARs) or based on GFP expression (CD19 CARs). T cells were then coincubated at 0.5 × 105 cells/well with various irradiated (30 Gy) tumor cell targets at a 1:1 stimulator to responder ratio for 7 days. The cells were then harvested and the proliferative T cell response was determined by flow count enumeration using Perfect Count Microspheres (IQ Products, Groningen, Netherlands) according to the manufacturer′s recommendations. To measure the antigen-specific expansion of transduced T cells, 1 × 106 cells/well were coincubated with irradiated (30 Gy) tumor cell targets at a 1:1 stimulator to responder ratio. Weekly counts of viable cells were performed by trypan blue exclusion.
Statistical analysis
The student T test was used to test for significance in each set of values, assuming equal variance. Mean values ±SD are given unless otherwise stated.
Results
Expression of 2B4-containing CARs in human T cell populations
We generated six CARs containing either the TCR ζ chain (14.G2a-ζ, CD19-ζ) or the 2B4 signaling domain alone (14.G2a-2B4, CD19-2B4) or both combined (14.G2a-2B4ζ, CD19-2B4ζ) (Fig. 1a). The extracellular single chain Fv domains were derived from the monoclonal antibody 14.G2a, which recognizes the ganglioside antigen GD2 on tumors of neural crest origin, including neuroblastoma [26], or from an antibody specific for the B lineage antigen CD19 [31]. The CARs were expressed by retroviral gene transfer in non-specifically activated peripheral blood (PB) T cells expanded from six healthy donors. Flow cytometric analysis of T cells transduced with the GD2-specific receptors and stained with a monoclonal antibody specific for human IgG F(ab′)2 fragment revealed CAR surface expression on 51.0 ± 19.6% (14.G2a-ζ), 67.3 ± 20.6% (14.G2a-2B4), and 52.2 ± 17.0% (14.G2a-2B4ζ) of T cells, respectively (Fig. 1a). CD4+ and CD8+ T cell subpopulations were all transduced with comparable efficiencies. Since the extracellular domains of the CD19-specific CARs are undetectable by staining with anti-human Ig antibodies, an IRES-GFP vector was used for gene transfer, allowing indirect quantification of transduction efficiencies by flow cytometric identification of GFP-expressing T cells. After gene transfer of CD19-based constructs, comparable GFP autofluorescence was detected (57.3 ± 17.4% for CD19-ζ, 58.5 ± 11.8% for CD19-2B4, and 58.8 ± 14.7% for CD19-2B4ζ). The immunophenotypes of T cell cultures on day 15 consisted of both CD3+CD8+ (67.9 ± 12.2%) and CD3+CD4+ (32.4 ± 13.3%) T cells, while CD3−CD56+ NK cells were <1% in all cultures. CD3+ T cells accounted for 97.0–99.9% (mean of 99.0 ± 1.2%) of the cells in all cultures. 2B4 was naturally expressed on 34.8 ± 6.9% of CD3+ T cells (Fig. 1b). CD4+ T cells were 2B4-negative. The cellular phenotypes of the cultures were maintained after transduction with either of the CAR genes (not shown).
Antigen-specific TCRζ and combined 2B4 and TCRζ signaling induces comparable tumor cytolysis and IFN-γ secretion by human T cells
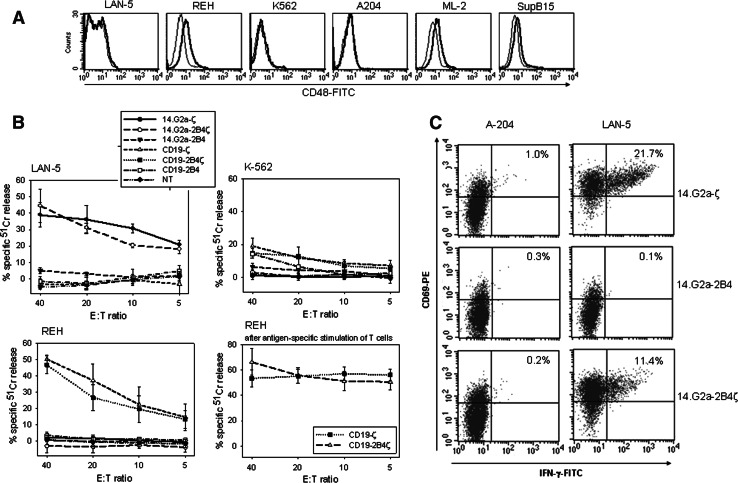
To compare the capacity of the various CARs to induce antigen-specific effector functions, we investigated the cytolytic activity of CAR-transduced T cells against antigen-expressing tumor targets. To appropriately consider potential interactions between 2B4 expressed on the effector T cells and its natural ligand CD48, we assessed expression of CD48 on all target cells by flow cytometry. While all neuroblastoma cell lines were CD48-negative (not shown), the leukemia cell lines ML-2 and REH had low surface expression of the 2B4 ligand (Fig. 2a). In a 51Cr release assay, comparable cytolysis of GD2+ LAN-5 tumor cells was observed by T cells engineered with 14.G2a-ζ or −2B4ζ, respectively (Fig. 2b), while antigen-specific signaling via 2B4 alone failed to induce target cytolysis. The antigen specificity of the response was preserved in the presence of the 2B4 signal, as demonstrated by the lack of significant background lysis of antigen-negative target cells (K562, and A-204 (not shown) by the transduced T cells. Accordingly, specific and efficient cytolysis of CD19-expressing leukemia cells was observed after coincubation with CD19-ζ or CD19-2B4ζ (Fig. 2b). Neither of the CD19-specific CARs mediated background cytolysis of GD2+ target cells, and vice versa, the GD2-specific CARs failed to interact with CD19+ tumor cells. Importantly, in spite of their low surface expression of the 2B4 ligand CD48, neither REH (Fig. 2b) nor ML-2 (not shown) were natural targets of T cell mediated cytotoxicity in the absence of the transgene, confirming the limited capacity of CD48-2B4 interaction alone in inducing T cell activation. Repeated assessment of antigen-specific cytotoxicity following CAR stimulation with REH cells demonstrated an increased cytotoxic function of the expanded CD8+ T cells (Fig. 2b) mediated by both CD19ζ and CD19-2B4ζ CARs.
Fig. 2.
CAR transduced T cells functionally interact with antigen-expressing tumor targets. a Expression of the 2B4 ligand CD48 on tumor target cell lines by flow cytometry (fine line isotype control, bold line CD48-specific antibody). b Specific cytolysis of GD2+, CD19-negative (LAN-5), CD19+, GD2-negative (REH), and CD19-negative, GD2-negative (K-562) tumor cells by CAR transduced T cells was assessed in a 51Cr-release assay. Shown is one representative experiment each of two. Assessment of REH cytolysis was repeated after 14 day antigen-specific stimulation of CD19-ζ and CD19-2B4ζ transduced T cells (lower right panel). c Intracellular interferon (IFN)-γ secretion by 14.G2a-ζ, 14.G2a-2B4 and 14.G2a-2B4ζ transduced T cells in response to GD2-negative rhabdomyosarcoma cells (A204) and GD2+ neuroblastoma cells (Lan-5) at stimulator-to-responder ratios of 1:1. To exclude nontransduced PBMC within the cultures from analysis, the gate was set on F(ab′)2+, CAR-expressing cells. Shown is one representative experiment of three
In further experiments, we analyzed intracellular cytokine production in response to antigenic stimulation using flow cytometry. To compensate for differences in transduction efficiencies, the gate excluded CAR-negative T cells. Coincubation of 14.G2a-ζ or -2B4ζ transduced T cells with GD2-expressing tumor cell targets resulted in antigen-specific secretion of IFN-γ, confirming the functionality of the two receptors (Fig. 2c). Stimulation via the two receptors further induced high proportions of cells secreting IFN-γ at comparable fluorescence intensities. As consistently observed with CARs containing CD28 costimulatory domains [32], and not shown), the IFN-γ response in this assay was slightly inferior compared to CARs containing TCRζ alone. Analogous to the cytolytic responses, the cytoplasmic domain of 2B4 alone failed to transmit a sufficient signal for inducing antigen-specific cytokine secretion (Fig. 2c).
Thus, 2B4 fails to provide a direct stimulatory signal in human peripheral blood T cells in the absence of the TCRζ chain. Its integration into tumor antigen-specific CARs does not substantially alter the phenotype, tumor specificity or antigen-specific effector properties of the gene-transduced T cells.
2B4 costimulation via GD2-specific CARs enhances tumor antigen-specific T cell proliferation
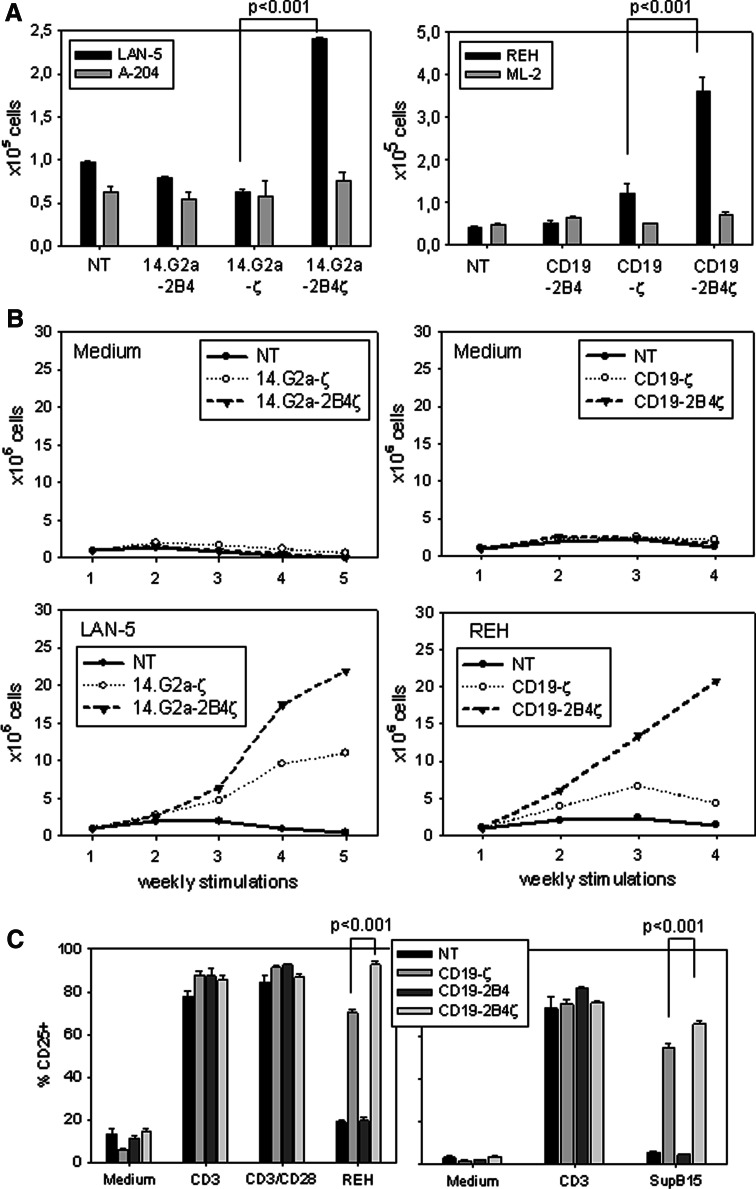
As opposed to a direct stimulatory role, the costimulatory function of an immune receptor generally emerges with its capacity to induce or enhance T cell proliferation and expansion in response to a primary activating signal, as provided by TCRζ. Therefore, we compared the proliferative responses mediated by the various receptor constructs in a flow cytometry based bead assay. To allow for a quantitative comparison between the populations, CAR-expressing cells within the bulk populations of transduced T cells were positively selected by flow cytometric cell sorting based on direct staining of 14.G2a CARs with IgG F(ab′)2 specific monoclonal antibody, or GFP-mediated autofluorescence of CD19 CAR-transduced cells. 7 day stimulation with the GD2+ tumor target LAN-5 revealed a significant (P < 0.001) proliferative advantage of T cells transduced with 14.G2a-2B4ζ over those transduced with 14.G2a-ζ (Fig. 3a). Likewise, CD19-mediated 2B4-signaling significantly augmented the proliferative responses of PB T cells to specific antigen stimulation compared to TCRζ signaling alone (Fig. 3a). Both CD19-2B4 and 14.G2a-2B4 transduced T cells failed to proliferate in response to antigen-expressing irradiated tumor target cells above the background levels obtained with non-transduced T cells (Fig. 3a). Importantly, the proliferation of T cells in cultures containing antigen-negative tumor target cells was not increased by 2B4ζ CAR expression.
Fig. 3.
CAR-specific T cell expansion and activation is enhanced by 2B4 signaling. a Proliferative responses of nontransduced (NT) and CAR-transduced T cell cultures to 7 days of stimulation with irradiated GD2- (LAN-5) or CD19- (REH) expressing tumor cell targets at a 1:1 stimulator-to-responder ratio were assessed by flow cytometric enumeration of triplicate wells. Prior to stimulation, the transduced T cells underwent positive selection by FACS based on staining with F(ab′)2 specific antibody or GFP expression. The GD2- and CD19-negative cell lines A-204 and ML-2 were used as controls. The counts refer to initial cell numbers of 0.5 × 105 each. Shown is one representative experiment of two. b 1 × 106/well of nontransduced (NT) and transduced T cell cultures were weekly restimulated with irradiated GD2 (LAN-5) or CD19 (REH) expressing tumor cell targets at a 1:1 stimulator-to-responder ratio, and their growth kinetics were assessed by weekly counting using the trypan blue exclusion assay. The graph shows cell counts prior to subsequent stimulation. Shown is one representative experiment of five (GD2) or two (CD19). c Upregulation of surface IL-2 receptor (CD25) expression on nontransduced (NT) and transduced T cells 24 h after stimulation with CD19+ (REH, SupB15) tumor cells at a 1:1 stimulator-to-responder ratio, or after non-specific stimulation with monoclonal antibodies against CD3 and/or CD28. Shown is one representative experiment each of four (REH) or two (SupB15)
To investigate whether the proliferative advantage of T cells receiving antigen-specific 2B4 costimulatory signals was reflected in prolonged in vitro expansion, we assessed the growth characteristics of bulk populations of cells in response to antigen stimulation by weekly trypan blue staining of viable cells. Indeed, for both CD19- and 14.G2a-specific receptors, the combined signaling by 2B4 and ζ substantially increased antigen-specific T cell expansion over that obtained in response to the receptor containing TCR ζ alone (Fig. 3b). Thus, in the presence of signal 1 provided by the TCR ζ chain, human T cells are efficiently costimulated by the 2B4 signal for proliferation and expansion in a strictly antigen-dependent manner.
Since REH cells express CD48 (Fig. 2a), and activated T cells upregulate 2B4 (Fig. 1b), native CD48/2B4 ligand/receptor interactions may contribute to the proliferative response. However, the lack of an adequate activation response of CD19ζ transduced T cells to stimulation with REH cells excludes relevant costimulation via 2B4 engagement by CD48 on REH (Fig. 3a, b).
As an alternative parameter for T cell activation, CD25 upregulation was determined on non-transduced and transduced T cells in response to interaction with antigen-expressing tumor targets. In contrast to cellular proliferation as a read-out for T cell activation, CD25 upregulation via ζ-based chimeric receptors did not require a costimulatory signal (Fig. 3c). However, the percentage of cells expressing the activation marker CD25 significantly (P < 0.0001) further increased in T cells stimulated with tumor cells via 2B4ζ CARs compared to ζ alone, while no CD25 upregulation was observed in CD19-2B4-transduced T cells. Stimulation of transduced T cells with the CD48-negative cell line SupB15 (Fig. 2a) reproduced the results obtained with the CD48+ cell line REH, again arguing against a major role of native CD48/2B4 interactions in the stimulation of CAR gene-modified T cells. Thus, while we cannot exclude a marginal contribution of CD48/2B4 or other costimulatory ligand/receptor interactions to the functional outcome of transduced T cell stimulation, we demonstrate a significant advantage by the presence of endogenous, CAR-mediated 2B4 signaling regarding both proliferation and CD25 upregulation.
Antigen-specific T cell expansion via combined 2B4 and TCR ζ signals results in selective enrichment of an effector memory T cell population
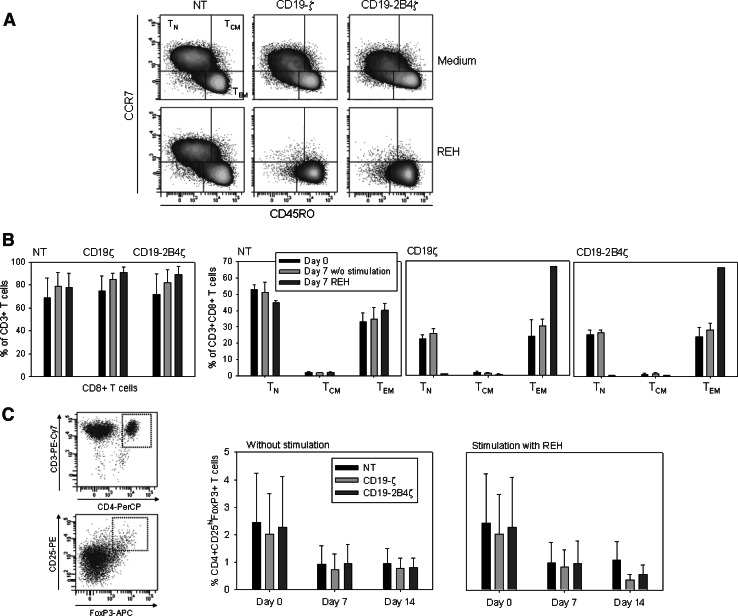
Activation of specific costimulatory pathways has been shown to have major effects on the selective in vitro differentiation of individual T cell subsets [33]. Therefore, we determined whether the superior T cell expansion induced by 2B4 costimulation results in an altered distribution of T cell phenotypes reflecting divergent antigen experience, differentiation stage and effector or regulatory function. The culture conditions favored selection of CD8+ T cells which dominated the cultures even in nontransduced T cell cultures, whereas CD4+ T cells were continuously eliminated from the cultures (Fig. 4b). Within the CD8+ T cell subset, the relative proportions of effector memory T cells (TEM), central memory T cells (TCM), and naïve T cells (TN) were quantified by staining with CD45RO and CCR7-specific antibodies (Fig. 4a). During expansion in IL-2 containing medium in the absence of specific stimulation, both non-transduced and transduced T cell cultures maintained stable proportions of these subpopulations (Fig. 4a, b). By contrast, antigen-specific stimulation of T cells via both CD19ζ and CD19-2B4ζ CARs resulted in phenotypic maturation of polyclonal T cells, with an increasing proportion of TEM, which dominated the cultures on day 7 (Fig. 4a, b). While T cells with TEM phenotype have been associated with high cytolytic potency, the increase of antigen-specific cytolysis by CAR-transduced T cells following 2 weeks of antigen-specific expansion (Fig. 2b) indeed argues towards selection of T cells with potent cytotoxic function.
Fig. 4.
Selective enrichment of an effector memory T cell population via chimeric receptor signaling. The effector phenotypes of nontransduced (NT) or CD19-ζ and CD19-2B4ζ transduced T cells were determined by surface expression of CCR7 and CD45RO 7 days after stimulation with irradiated CD19-expressing tumor cells (REH) at a 1:1 stimulator-to-responder ratio or in the absence of stimulation. To exclude nontransduced PBMC within the cultures from analysis, the gate was set on GFP expressing cells. Shown is one representative example (a), and the mean percentages of CD8+ T cells among CD3+ T cells as well as the percentages of naïve (TN), central memory (TCM), and effector memory (TEM) cells among CD8+ T cells in three individual donors (b). c The proportions of CD4+CD25hiFoxP3+ regulatory T cells within the T cell cultures were determined by flow cytometry after stimulation with irradiated CD19-expressing tumor cells (REH) at a 1:1 stimulator-to-responder ratio for 7 and 14 days, respectively. Shown is the mean proportion of T cells with Treg phenotype cells in three individual donors
The lack of 2B4 expression on CD4+ T cells argues against a natural role of this molecule in the expansion of CD4+ CD25hi T cells with regulatory (Treg) phenotype. Following non-specific T cell stimulation, CD4+CD25hiFoxP3+ T cells were transduced at similar efficiencies to CD8+ and total populations of CD4+ T cells. Specifically, among FoxP3+ T cells, transduction efficiencies ranged between 45.7 and 63.1% for 14.G2aζ, 49.1 and 71.4% for 14.G2a-2B4, and between 43.3 and 67.1% for 14.G2a-2B4ζ. To exclude that nonphysiological stimulation of T cells with Treg phenotype cells by artificially expressed 2B4 and TCRζ chains induces an enrichment of this T cell subset, we assessed the percentage of T cells coexpressing CD4, CD25hi and FoxP3+ in response to antigen-specific stimulation via 2B4 and ζ. At the initiation of the cultures, T cells with Treg phenotype cells identified by these markers accounted for 1.1–4.5% (2.3 ± 1.5%) of T cells. During both ζ- and 2B4ζ-induced, CD19-specific expansion, the proportion of T cells with Treg phenotype maintained stable or decreased in the cultures (Fig. 4c), thus ruling out a selective growth advantage of natural Treg cells mediated by 2B4 costimulation. In conclusion, 2B4 provides a potent costimulus for the tumor antigen-induced expansion of T cells undergoing phenotypic maturation towards CD8+ effector memory T cells.
Discussion
The discovery that many of the receptors that regulate NK cells are also expressed by T cells has raised interest regarding their role in adaptive immunity which may be exploited for specific immunotherapy of cancer. By applying CAR technology, we demonstrate here for the first time that the cytoplasmic signaling domain of the NK cell receptor 2B4 can efficiently enhance activation of human T cells in a strictly antigen-dependent manner, augmenting TCRζ-induced functional responses to both neuroblastoma and leukemia target cells.
Previously, the effects of 2B4 triggering on the proliferative function of T cells have only been addressed in a single study. By receptor crosslinking, the investigators have failed to demonstrate a role of 2B4 in mediating or enhancing antigen-specific cytokine production or proliferation [18]. The contrasting results may be related to differences in signal strength under the two experimental conditions. Compared to crosslinking with 2B4- and CD3-specific antibodies, the engagement of the CAR by its specific ligand may have induced stronger concomitant 2B4 and TCRζ signals which were sufficient to overcome the high threshold required for inducing T cell proliferation. Beyond establishing a T cell costimulatory capacity for the 2B4 receptor endodomain, our study contributes to elucidating the controversial question whether the activating function of CD48/2B4 interactions is mediated by signaling via either CD48 or 2B4 [34]. In one study using influenza-specific TCR-transgenic mice, 2B4 was found to increase proliferation of CD8+ T cells by acting as a stimulatory ligand for CD48 on the neighboring T cells, rather than inducing the necessary activation signals to the cell on which it is expressed [35]. While we cannot exclude that CD48 may also provide costimulatory signals, our experimental system allowed us to unequivocally confirm the direct effects of 2B4 signaling in T cells.
Other previous attempts at defining the functional role of 2B4-mediated signals in T cells have focused exclusively on the contribution of 2B4 engagement to target cytolysis. In contrast to our own report, two studies in murine and human T cells, respectively, have revealed an augmenting function of 2B4 on MHC-restricted cytolysis [25, 36]. In both studies, 2B4 receptor triggering was achieved by coincubation with CD48-expressing target or adjacent cells, offering a potential explanation for the controversial findings: Compared to endogenous 2B4 signaling provided by CARs, interactions between 2B4 on cytolytic T cells and its ligand CD48 may result in prolonged cellular interaction and facilitate signaling by other molecules, thereby contributing to target cell lysis even in the absence of a direct effect of 2B4 signaling.
While inhibitory effects of 2B4 have been reported in NK cells [37–39], neither we nor others [16, 18, 40] have found any evidence for a functional inhibition of T cell activation by 2B4. Recently, the differential and even opposing roles of 2B4 in NK cells have been attributed to varying degrees of receptor expression, extent of receptor ligation, and the relative abundance of adaptor molecules [39]. We cannot exclude that under specific conditions or in distinct subpopulations or activation states of T cells 2B4 signaling may induce varying and even opposing functions.
Detailed exploration of the role of this receptor will require resolution of the molecular mechanisms by which 2B4 costimulates T cell activation. Although several of the 2B4-associated downstream molecules are known, the signaling pathways triggered by 2B4 in both NK cells and T cells are not fully understood. Upon phosphorylation of SLAM-related tyrosine motifs within the cytoplasmic domain of 2B4, they associate with the protein tyrosine phosphatase SHP-2 and with the signaling adaptor molecule SAP [41]. In addition, 2B4 was shown to interact with Src family kinases [42], and immunoprecipitation experiments revealed a constitutive association with linker of activation of T cells (LAT) [14]. Based on the hypothesis that these pathways may converge into activation of the transcription factor NFκB which has proven essential for other costimulatory signaling pathways such as CD28 [43] and OX40 [44], we explored the potential role of NFκB activation in CAR-mediated T cell activation. In response to antigen-specific CAR triggering, no NFκB activation was observed in a luciferase assay (unpublished results). In a future study, the chimeric 2B4 and 2B4ζ receptors described here will be used as tools for elucidating 2B4-mediated downstream signaling pathways in T cells.
Altogether, our work provides support for a contributory role of NK cell receptors in adaptive immunity. The abundant expression of the 2B4 ligand CD48, the upregulation of 2B4 in memory CTL populations [45], the failure of patients with genetic deficiency of 2B4-induced signaling pathways to control EBV infection [42, 46], as well as our new finding that the 2B4 endodomain can provide a powerful costimulatory signal in T cells point towards a role of 2B4 in the sustained activation of memory CD8+ T cells. By upregulating 2B4 and other NK cell activating receptors, antigen-experienced, CD28-negative effector memory T cells may acquire potent additional pathways that help to meet their costimulatory requirements and contribute to their fate at advanced stages of the immune response.
Due to its capacity to enhance antigen-specific activation of gene-modified T cells without negatively affecting tumor-antigen induced cytolytic effector responses, 2B4 is a promising candidate molecule for the generation of optimized CARs for immunotherapy of cancer. Current receptor designs have accounted for the costimulatory requirement of gene-modified T cells by including the signaling domain of the well-characterized costimulatory molecule CD28 into the cytoplasmic portion of CAR [47]. In direct comparison of 2B4ζ and CD28ζ CARs, both antigen-specific cytolysis and IFN-γ responses of transduced T cells were comparable (data not shown). However, while the proliferative response to CD28ζ stimulation was characterized by substantial background proliferation in vitro in the absence of antigen-expressing cells ([32] and not shown), inclusion of the 2B4 endodomain strictly maintains the antigen-specificity of the CAR (Fig. 3b) and is thus unlikely to cause unwanted side effects by non-specific in vivo proliferation of gene-modified T cells. Importantly, we show that 2B4 fails to provide a selective growth advantage to T cells with Treg phenotype, which have emerged as an important obstacle to successful immunotherapy by counteracting antitumor effector responses. Instead, combined 2B4 and ζ signaling induces predominant expansion of a T cell subset with an effector memory phenotype that has been associated with a high cytolytic potential [48]. Thus, tumor-antigen specific 2B4ζ CAR may promote improved survival and sustained activation of therapeutic T cells for the targeting of both hematological and solid malignancies.
Acknowledgments
We thank Guido Bisping for helpful discussions. This work was supported by a grant from the Dr. Mildred-Scheel-Stiftung der Deutschen Krebshilfe (to C. R.) and EU funding provided for the “CHILDHOPE” network program under the terms of an EU Framework 6 grant (to C. R. and M. P.).
References
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 3.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 4.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamers CHJ, Langeveld SCL, Ruijven CMGV, Debets R, Sleijfer S, Gratama JW. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56:1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–793. [PubMed] [Google Scholar]
- 7.Park JR, DiGiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg JR, Jensen MC. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 8.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161:2791–2797. [PubMed] [Google Scholar]
- 10.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 11.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 12.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 14.Chuang SS, Kumaresan PR, Mathew PA. 2B4 (CD244)-mediated activation of cytotoxicity and IFN-gamma release in human NK cells involves distinct pathways. J Immunol. 2001;167:6210–6216. doi: 10.4049/jimmunol.167.11.6210. [DOI] [PubMed] [Google Scholar]
- 15.Garniwagner BA, Purohit A, Mathew PA, Bennett M, Kumar V. A novel function-associated molecule related to non-Mhc-restricted cytotoxicity mediated by activated natural-killer-cells and T cells. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 16.Lee KM, Bhawan S, Majima T, Wei BR, Nishimura MI, Yagita H, Kumar V. Cutting edge: the NK cell receptor 2B4 augments antigen-speciflic T cell cytotoxicity through CD48 ligation on neighboring T cells. J Immunol. 2003;170:4881–4885. doi: 10.4049/jimmunol.170.10.4881. [DOI] [PubMed] [Google Scholar]
- 17.Mathew PA, Garniwagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-Mhc-restricted killing mediated by activated natural-killer-cells and T Cells. J Immunol. 1993;151:5328–5337. [PubMed] [Google Scholar]
- 18.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol. 1999;29:1676–1683. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–1406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivori S, Falco M, Marcenaro E, Parolini S, Biassoni R, Bottino C, Moretta L, Moretta A. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc Natl Acad Sci USA. 2002;99:4526–4531. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boles KS, Nakajima H, Colonna M, Chuang SS, Stepp SE, Bennett M, Kumar V, Mathew PA. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- 22.Speiser DE, Colonna M, Ayyoub M, Cella M, Pittet MJ, Batard P, Valmori D, Guillaume P, Lienard D, Cerottini JC, Romero P. The activatory receptor 2B4 is expressed in vivo by human CD8+ effector alpha beta T cells. J Immunol. 2001;167:6165–6170. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 23.Yokoyama S, Staunton D, Fisher R, Amiot M, Fortin JJ, Thorley-Lawson DA. Expression of the Blast-1 activation/adhesion molecule and its identification as CD48. J Immunol. 1991;146:2192–2200. [PubMed] [Google Scholar]
- 24.Peritt D, Sesok-Pizzini DA, Schretzenmair R, Macgregor RR, Valiante NM, Tu X, Trinchieri G, Kamoun M. C1.7 antigen expression on CD8(+) T cells is activation dependent: Increased proportion of C1.7(+)CD8(+) T cells in HIV-1-infected patients with progressing disease. J Immunol. 1999;162:7563–7568. [PubMed] [Google Scholar]
- 25.Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, Aiuti A, Roncarolo MG. SAP controls the cytolytic activity of CD8(+) T cells against EBV-infected cells. Blood. 2005;105:4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- 26.Rossig C, Bollard CM, Nuchtern JG, Merchant DA, Brenner MK. Targeting of G(D2)-positive tumor cells by human T lymphocytes engineered to express chimeric T cell receptor genes. Int J Cancer. 2001;94:228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 27.Rossig C, Pscherer S, Landmeier S, Altvater B, Jurgens H, Vormoor J. Adoptive cellular immunotherapy with CD19-specific T cells. Klin Padiatr. 2005;217:351–356. doi: 10.1055/s-2005-872521. [DOI] [PubMed] [Google Scholar]
- 28.Riviere I, Brose K, Mulligan RC. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc Natl Acad Sci USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinsella TM, Nolan GP. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum Gene Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- 30.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2:876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 31.Rossig C, Bar A, Pscherer S, Altvater B, Pule M, Rooney CM, Brenner MK, Jurgens H, Vormoor J. Target antigen expression on a professional antigen-presenting cell induces superior proliferative antitumor T cell responses via chimeric T cell receptors. J Immunother. 2006;29:21–31. doi: 10.1097/01.cji.0000175492.28723.d6. [DOI] [PubMed] [Google Scholar]
- 32.Altvater B, Pscherer S, Landmeier S, Niggemeier V, Juergens H, Vormoor J, Rossig C. CD28 co-stimulation via tumour-specific chimaeric receptors induces an incomplete activation response in Epstein-Barr virus-specific effector memory T cells. Clin Exp Immunol. 2006;144:447–457. doi: 10.1111/j.1365-2249.2006.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waller ECP, McKinney N, Hicks R, Carmichael AJ, Sissons JGP, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28(−) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 34.Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren HG. 2B4/CD48-mediated regulation of lymphocyte activation and function. J Immunol. 2005;175:2045–2049. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]
- 35.Kambayashi T, Assarsson E, Chambers BJ, Ljunggren HG. Regulation of CD8(+) T cell proliferation by 2B4/CD48 interactions. J Immunol. 2001;167:6706–6710. doi: 10.4049/jimmunol.167.12.6706. [DOI] [PubMed] [Google Scholar]
- 36.Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 38.Schatzle JD, Sheu S, Stepp SE, Mathew PA, Bennett M, Kumar V. Characterization of inhibitory and stimulatory forms of the murine natural killer cell receptor 2B4. Proc Natl Acad Sci USA. 1999;96:3870–3875. doi: 10.1073/pnas.96.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V. Molecular basis of the dual functions of 2B4 (CD244) J Immunol. 2008;180:8159–8167. [Google Scholar]
- 40.Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG, Chambers BJ. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol. 2004;173:174–180. doi: 10.4049/jimmunol.173.1.174. [DOI] [PubMed] [Google Scholar]
- 41.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol. 1999;162:6981–6985. [PubMed] [Google Scholar]
- 42.Nakajima H, Cella M, Bouchon A, Grierson HL, Lewis J, Duckett CS, Cohen JI, Colonna M. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur J Immunol. 2000;30:3309–3318. doi: 10.1002/1521-4141(200011)30:11<3309::AID-IMMU3309>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 44.Chen AI, Mcadam AJ, Buhlmann JE, Scott S, Lupher ML, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. Ox40-ligand has a critical costimulatory role in dendritic cell: T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/S1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 45.Rey J, Giustiniani J, Mallet F, Schiavon V, Boumsell L, Bensussan A, Olive D, Costello R. The co-expression of 2B4 (CD244) and CD160 delineates a subpopulation of human CD8(+) T cells with a potent CD160-mediated cytolytic effector function. Eur J Immunol. 2006;36:2359–2366. doi: 10.1002/eji.200635935. [DOI] [PubMed] [Google Scholar]
- 46.Parolini S, Bottino C, Falco M, Augugliaro R, Giliani S, Franceschini R, Ochs HD, Wolf H, Bonnefoy JY, Biassoni R, Moretta L, Notarangelo LD, Moretta A. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–346. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eshhar Z, Waks T, Bendavid A, Schindler DG. Functional expression of chimeric receptor genes in human T cells. J Immunol Methods. 2001;248:67–76. doi: 10.1016/S0022-1759(00)00343-4. [DOI] [PubMed] [Google Scholar]
- 48.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]